Abstract
Background and purpose Few economic or quality-of-life studies have investigated the long-term consequences of fragility fractures. This prospective observational data collection study assessed the cost and quality of life related to hip, vertebral, and wrist fracture 13–18 months after the fracture, based on 684 patients surviving 18 months after fracture.
Patients and methods Data regarding resource use and quality of life related to fractures was collected using questionnaires at 7 research centers in Sweden. Information was collected using patient records, register sources, and by asking the patient. Quality of life was estimated using the EQ-5D questionnaire. Direct and indirect costs were estimated from a societal standpoint.
Results The mean fracture-related cost 13–18 months after a hip, vertebral, or wrist fracture were estimated to be €2,422, €3,628, and €316, respectively. Between 12 and 18 months after hip, vertebral, and wrist fracture, utility increased by 0.03, 0.05, and 0.02, respectively. Compared to prefracture levels, the mean loss in quality of life between 13 and 18 months after fracture was estimated to be 0.05, 0.11, and 0.005 for hip, vertebral, and wrist fracture.
Interpretation The sample of vertebral fracture patients was fairly small and included a high proportion of fractures leading to hospitalization, but the results indicate higher long-term costs and greater loss in quality of life related to vertebral fracture than previously believed.
Osteoporosis-related fractures are associated with a high degree of morbidity and mortality. In Sweden, more than 70,000 osteoporosis-related fractures occur annually. Of these, approximately 27%, 23%, and 23% occur at the hip, spine, and wrist, respectively. The burden these fractures impose on society, both in terms of cost and in terms of reduction in survival and quality of life, makes osteoporosis a major public health issue (SBU 2003, Borgstrom et al. Citation2007). The risk of osteoporotic fractures can be reduced by different treatment strategies such as behavioural changes, hip protectors, and drugs. However, preventive measures cost money and the cost-effectiveness of different intervention strategies must be documented. To assess the cost-effectiveness of different interventions, it is important to have good estimates of costs and quality of life related to osteoporotic fractures that may be avoided by the treatment strategy.
Previous studies have estimated reduction in costs and/or quality of life related to osteoporotic fractures (Liljegren et al. Citation1997, Zethraeus et al. Citation1997, Dolan and Torgerson Citation1998, Zethraeus and Gerdtham Citation1998, Reginster et al. Citation1999, Haentjens et al. Citation2001, Koeck et al. Citation2001, Wiktorowicz et al. Citation2001, Gabriel et al. Citation2002, Wong et al. Citation2002, Nurmi et al. Citation2003, Lippuner et al. Citation2005), but only a few of them are appropriate for use in economic evaluations and only one of them investigated the long-term consequences of fragility fractures (De Laet et al. Citation1999). Not surprisingly, it is the hip fracture—the type of fracture associated with the highest mortality and most severe morbidity—that has been investigated most frequently in these studies. This is mainly because in most cases of hip fracture patients are hospitalized; thus, the data are readily available from hospital records.
A study involving prospective observational data collection was launched in July 2002 with the purpose of obtaining relevant estimates of costs and health-related quality-of-life consequences associated with osteoporotic fractures of the hip, vertebrae, and wrist in Sweden. The costs and effects of osteoporosis-related fractures study (the KOFOR study) continuously enrolled patients with fracture at 7 hospitals in Sweden and followed them over a period of 18 months after the fracture event. Use of resources was determined from the point of view of society and the quality of life of patients after fracture was estimated using the EQ-5D questionnaire. The fracture-related costs and the effect on quality of life during the first year after a sustained fracture (based on an interim analysis including 635 patients with either a hip, vertebral,or a wrist fracture) have already been published (Borgstrom et al. Citation2006b). We now present the fracture-related costs and quality of life for the first 13–18 months after fracture and present an update of the estimates from the first 12 months, with all 684 patients who survived for 18 months. The update has been included since comparison between 12 and 18 months would otherwise have to be viewed as two different populations. Other aims were to compare the 0–4-month estimates for patients who died during the first year with those of patients who survived for 18 months, and to obtain cost-per-month estimates over all the phases in the study.
Patients and methods
The materials and methods used in the KOFOR study have also been described by Borgstrom et al. (Citation2006b). When estimating the costs related to fractures, it is important that only the extra cost incurred by the fracture is counted. In this study, fracture-related resource use was determined pro-spectively after the fracture. Patients and healthcare staff were questioned about resource use directly related to the fracture event rather than using a control group. The advantages of this approach are that it is possible to include all relevant cost items and that no information has to be collected before the fracture, thus reducing the time period for the data collection. A potential disadvantage could be that it might be difficult to determine whether or not a resource is directly related to the fracture. To our knowledge, the KOFOR study has been the first study to use this data-collection methodology. To test and validate the methodology prior to the KOFOR study, we first performed a pilot study at Malmö University Hospital in Sweden (Zethraeus et al. Citation2002).
Study design and data collection
The KOFOR study, which is currently still running, includes patients who have suffered an osteoporosis-related fracture of the hip, vertebrae, or wrist and who have been treated at the orthopedics departments of one of 7 Swedish hospitals. The participating centers were: Södersjukhuset in Stockholm, Malmö University Hospital, Lund University Hospital, Norrland's University Hospital, Ystad Hospital, Helsingborg Hospital, and Hässleholm Hospital. To be included, a patient had to be diagnosed with a fracture caused by low-energy trauma and had to be at least 50 years old. Vertebral fractures had to be confirmed by radiographs. Patients seeking care for multiple fractures were not eligible for the study. Fractures caused by other diseases such as cancer were not included. Also, patients who were judged to be unable to complete the questionnaires due to dementia or other psychological problems were excluded from the study. Patients had to give informed consent and had to have been included and interviewed within 4 weeks of the fracture event. The study was approved by the ethics committees associated with each hospital.
Data regarding resource use and quality of life related to fractures were collected using questionnaires at baseline and 4 months, 12 months, and 18 months after the fracture event. The data collection questionnaire used was tested at Malmö University Hospital (Zethraeus et al. Citation2002). At baseline, patient characteristics, background information, and perceived health status (EQ-5D) just before and after the occurrence of the fracture were collected. At the 4-month, 12-month, and 18-month follow-up, resource use since the last visit and current health status were determined. If the interview could not be conducted at the same time as the hospital visit, the information was collected by telephone. In the study of the first 12 months after fracture, 98% of the hip and vertebral fracture patients lived at home before the fracture (Borgstrom et al. Citation2006b). In this study, patients not living at home were excluded from the analysis.
Differences in cost between deceased and surviving patients
To investigate whether fracture patients who died during the study incurred more costs or suffered a greater loss in quality of life (QoL) than patients who survived, the total cost and loss in QoL for 0–4 months after fracture for patients who died during months 5–18 of the study (n = 38) were compared to the corresponding cost and loss in QoL for those who survived more than 18 months (n = 684).
Use of resources
Use of resources was determined from patient records, register sources, and by asking the patient. The resources were categorized into medical costs (hospitalizations, outpatient care, and pharmaceu-ticals), non-medical costs (community care and informal care) and indirect costs (e.g. loss regarding production). By community care we a referring to special living arrangements, home care, and transportation. Patient-reported use of resources (community care and informal care) and resources lost, i.e. indirect costs, were recorded for a shorter time period (1 month) in order to minimize recollection bias.
Hospitalizations and outpatient care
For each fracture-related hospitalization, the number of bed days was recorded along with the ICD-10 main and other diagnoses and operation codes. Outpatient care consisted of outpatient surgery, visits by a physician, nurse visits, physiotherapist visits and occupational therapist visits at the hospital, primary care visits and at home, radio-graphic examinations, and help by telephone.
Pharmaceuticals
The use of pharmaceuticals deemed to be relevant for treatment of osteoporosis and fractures was recorded. The patient was considered to be a user of a drug if it had been prescribed due to the fracture some time during the study period. If the patient had been noted as a user of a particular drug, then it was assumed that the drug had been taken during the whole period. The categories of pharmaceuticals included were: calcium and vitamin D, estrogens, bisphosphonates, glucocorticoids, and pain medication.
Community care, informal care, and loss of production
Each patient's living accommodation before fracture was recorded at baseline. At the 4-, 12-, and 18-month follow-up the patient was asked how many days had been spent in any special living arrangements (e.g. nursing home or group living) due to the fracture, any time during the 4 weeks prior to the interview. The patients also reported the average number of hours of home help they received per week and the number of transportations in the previous 4 weeks that were related to the fracture. To obtain an estimate of the total use of resources regarding community care, the use during the 4 last weeks was extrapolated over the whole period.
The patients were asked about the average number of hours of fracture-related care given by relatives in the 4 weeks prior to the interviews at 4-, 12-, and 18-month follow-up. To obtain an estimate of the total amount of informal care given in each period, the resource use during the 4 last weeks was extrapolated over the whole period.
At the baseline interview, the patients gave information concerning their working status before the fracture (full-time work/part-time work/not employed). At the 4-, 12- and 18-month follow-up interviews, they described their current working situation and—if they were working—stated how many days they had been on sick leave in the previous 4 weeks due to the fracture. The sick leave stated was assumed to be the average monthly sick leave for the whole period.
Costing
The cost of fractures was estimated by multiplying the degree of use of the different resources by a corresponding value for the resource, i.e. a unit cost. All costs are given in 2005 prices, inflated, where necessary, using the Swedish consumer price index. The costs have been converted from Swedish kroner (SEK) to euros (€) using the yearly average exchange rate for 2005 (SEK 9.3 to €1).
Medical care and pharmaceutical costs
Costs of inpatient stays were assessed by multiplying the number of hospital bed days by the corresponding daily departmental cost per bed day. Unit costs for inpatient bed days, outpatient hospital visits, outpatient operations, radiographic examinations, primary care visits, physiotherapist and occupational therapist visits, and telephone help were calculated as averages from price lists for inter-regional care from 11 hospitals (Borgstrom et al. Citation2006b). The costs for home visits (physician, nurse, and therapists) were derived from a study by Janzon et al. (Citation2002). The cost of pharmaceuticals was based on the cost for the average daily dose of each drug, obtained from the Swedish national pharmacy.
Community care costs. Costs for special living arrangements (nursing home, elderly home, and group living) were obtained from the Stockholm municipality annual budget report of 2003 (Stad-sledningskontoret 2003). It was assumed that living at home was not associated with any extra costs. Transportation costs for the disabled were based on the mean cost of transportation for the disabled in the Skäne (Scania) Region.
Indirect costs and informal care. The value of lost production, stratified by sex, was estimated using the average hourly pre-tax salary, including social insurance contributions in the private sector, in 2003 (SCB 2004). Because information concerning the employment status of informal caregiv-ers was not collected, informal care was conservatively valued at the cost of lost leisure time in the base estimations. Lost leisure time was valued at €3 per hour, which is the opportunity cost of travel time estimated in a contingent valuation study conducted by the Swedish Road Authority (Vägverket Citation1997). In a sensitivity analysis, we also valued informal care using the replacement cost method with the cost of home help (€25) to value informal care.
Quality of life
The quality of life was estimated using the EQ-5D questionnaire. This is a general quality of life instrument that divides health status into five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (Brooks Citation1996). Each dimension is divided into 3 degrees of severity: no problem, some problems, or major problems. The 5 health dimensions divide health status into 243 (35) possible health states. Social tariff values for these health states, estimated as TTO utility values, were applied to the health states observed in the study (Dolan Citation1997).
For the calculations of fracture-related loss in quality of life, it was assumed that the patient would have remained at the prefracture level of quality of life stated, for the first 12 months, if the fracture had not occurred. Between 13 and 18 months, the prefracture QoL was reduced by the same interpolated 1-year proportion that QoL decreases by in the general population (Burstrom et al. Citation2001). The annual loss in quality of life related to fracture was then calculated by subtracting the estimated quality of life the year after fracture from the adjusted prefracture quality of life. The prefracture quality of life was obtained from patients after the fracture and not before. This could possibly lead to some recollection bias, since the prefracture health status may have been perceived to have been better than it actually was. Thus, the annual loss in quality of life was also estimated using Swedish age-differentiated EQ-5D population norms (Burstrom et al. Citation2001) as a proxy for the quality of life of patients before fracture. Since no information was available after 18 months, 2 different methods were used to estimate utility loss for the whole second year after fracture. In one scenario (linear extrapolation), utility was assumed to increase at the same rate during the whole year. In the second scenario (flat extrapolation), utility was assumed to stay at the same level as that after 18 months, for the rest of the second year after fracture. 1 patient had not completed the EQ-5D questionnaire and was therefore removed from all QoL analyses.
Statistics
Cost data are often skewed, and thus not normally distributed. The Shapiro-Wilk test was used for testing the normality of the cost and quality-of-life distributions (Wackerly et al. Citation1996). If the data were not found to be normally distributed, the nonparametric Kruskal-Wallis one-way analysis of variance test was used to test the difference in costs and quality of life between different subgroups (e.g. age, sex); if shown to be normally distributed, the regular t-test was used. If the data were not normally distributed, confidence intervals were obtained by using the bias-corrected accelerated (bca) percentile bootstrapping method (Briggs et al. Citation1997). Differences in QoL between 12 and 18 months after fracture were tested with the Wilcoxon sign test of matched pairs.
Because cost data are often heavily skewed, they are not appropriate for use in regression modeling in their original form. Thus, in an analysis investigating the relationship between costs and other variables, the Box-Cox method (Neter et al. Citation1996) was used to find the best transformation of the cost data to fit a normal distribution. The Box-Cox procedure suggested that the most appropriate transformation parameter value was close to 0, which is equivalent to a logarithmic transformation of the dependent variable. Thus, a log-linear model was chosen for the estimations, i.e. the natural logarithm of the costs was regressed on the untransformed independent variables. The transformed cost variable was then used as the dependent variable in a multivariate ordinary least-squares (OLS) regression model. The Breusch-Pagan test was used to test for het-eroscedasticity (Gujarati Citation1995). In the presence of heteroscedasticity, White's corrected standard errors were applied (Gujarati Citation1995). All analyses were conducted using the statistical software package STATA 9.0 for Windows, with 5% chosen as the level of significance.
Results
Patient characteristics and baseline information
Most patients (86%) were women and 23%, 21%, and 14% had had a previous osteoporotic fracture during the previous 5 years (hip, vertebral, or wrist fracture) (). All hip fracture patients were admitted to hospital when seeking care for the fracture, whereas 70% of the vertebral fracture patients and 9% of the wrist fracture patients were admitted.
Table 1. Patient characteristics and baseline information
Resource use
As expected, the number of hospitalizations decreased considerably since the first year after fracture. The same is true of most of the fracture-associated resource use collected in the study. For the average fracture patient, the most frequent visit to a health professional was to a physiotherapist. (Table 2; see Supplementary data).
Vertebral fracture was associated with statistically significantly more transportations and a general trend towards greater use of community care and informal care than hip fracture. Wrist fracture patients used relatively little municipality care and informal care (Table 3; see Supplementary data).
Vertebral fracture patients used more drugs (1.72 vs. 0.99 drugs per patient) than hip fracture patients during the paeriod 13–18 months after fracture (p < 0.001) and a larger proportion of patients were taking pain relievers (0.55 vs. 0.33; p < 0.005). Calcium and vitamin D supplements and pain relievers were the most common medications. 15%, 30%, and 17% of hip, vertebral, and wrist fracture patients, respectively, were prescribed bisphospho-nates (Table 4; see Supplementary data).
Costs
Re-estimation summary of costs in the first year after fracture. Table 5 (see Supplementary data) provides an update of the cost findings during the first year after fracture. The costs are based on the 684 patients who survived for at least 18 months after fracture. The estimates in this analysis are similar to the previous estimates based on 635 patients who survived for 12 months (Borgstrom et al. Citation2006b). It is noteworthy that the cost of special living accommodation associated with vertebral fractures was lower than in the previous estimates, while outpatient care costs for hip fracture and indirect costs for wrist fractures were higher (Borgstrom et al. Citation2006b). In a subanalysis of all 115 wrist fracture patients below the age of 65, mean indirect costs amounted to €796 per patient. Vertebral fracture was associated with the highest community care costs, but because of higher costs of inpatient care, hip fracture had the highest total annual fracture-related cost (€14,360), followed by vertebral fracture (€11,901) and wrist fracture (€2,316).
All types of fracture were associated with relatively modest medical care costs (). Costs related to special living accommodation were about the same for hip and vertebral fractures, while wrist fractures were not associated with any costs for special living accommodation. Costs for other community care (i.e. transportation and home help) were considerably higher for vertebral fractures. When considering the whole patient sample, the estimated indirect cost was fairly low because few of the patients were below 65 years of age and working. In a subanalysis of the 104 wrist fracture patients below the age of 65, mean indirect costs amounted to €423 per patient. Vertebral fracture was found to have the highest cost regarding informal care, community care, and informal care. Accordingly, vertebral fractures were associated with the highest total fracture-related cost (€ 3,628), followed by hip fractures (€ 2,422) and wrist fractures (€316).
Table 6. Average (95% CI) cost 13–18 months after fracture (€, 2005)
The estimated cost of hip fracture varied significantly over the age groups (p < 0.005) in women and ranged from €527 (50–64 years) to €4,000 (85 years and above). Also, hip fracture cost (at all ages) was higher for men than for women (p < 0.005) ().
Table 7. Average age- and sex-differentiated annual cost 13–18 months after hip fracture (€, 2005)
Wrist fracture costs were not found to vary significantly with age (p = 0.1) or between the sexes (p = 1). Also, the cost of vertebral fractures was not significantly different for men and women (p = 0.4).
The cost for hospitalized patients was not significantly higher than for patients who were not hospitalized (p = 0.1). The same was true when comparing patients who were 65 years or older to those who were younger (p < 0.2) ().
Table 8. Average age-differentiated annual cost 13–18 months after vertebral fracture (€, 2005)
There was a difference in the cost of total hip fracture in the first 4 months between patients who had died during months 5–18 of the study and those who had survived for 18 months (€14,115 vs. €11,350; p = 0.04). No significant difference was found for vertebral or wrist fractures alone but when all patient fractures were pooled, the group that died showed 47% higher total costs in the first 4 months after fracture than the group that survived for 18 months (p = 0.02). Analyses were adjusted for age, previous fracture, sex, and rate of hospi-talization.
An econometric analysis was carried out to identify factors that could explain the difference in total costs 13–18 months after fracture for each fracture type (Table 9; see Supplementary data). The independent variables that were considered for inclusion in the model were quality of life before fracture, age at fracture occurrence, sex, and sustained fracture within 5 years prior to the study fracture. Regressions were estimated based on all patients and each fracture type separately. The logarithm of the cost variable was used in the regression (see Methods section). An example of interpretation of the regression estimates based on all patients would be that costs decreased significantly by about 10% for each 0.1 unit increase in QoL before fracture, and increased significantly (4.4% per year) with increasing age at occurrence of fracture. The change in costs due to a 1-unit increase in the explanatory variable was calculated as: per cent change = (ecoefficient−1) × 100 (Gujarati Citation1995). Vertebral fracture-related costs were higher than hip fracture-related costs in the period 13–18 months after fracture (p = 0.03). The fracture-related cost was relatively stable between 5 and 18 months after all types of fracture ().
Quality of life
Between 13 and 18 months after fracture, utility increased by 0.03, 0.05, and 0.02 for hip fracture, vertebral fracture, and wrist fracture, respectively (). The increase was significant for hip fracture (p = 0.01) and wrist fracture (p = 0.05), but not for vertebral fracture (p = 0.1). No significant difference was found in the QoL-loss for patients who were hospitalized and those who were not. Wrist fractures did not have any significant effect on quality of life beyond the first year after fracture. There was no significant difference in QoL-loss for any of the fracture types investigated in the first 4 months between patients who died during months 5–18 of the study and those who survived for 18 months. QoL-loss estimates were adjusted for age, previous fracture, sex, and rate of hospitalization.
Table 10. Estimated health-related utility (EQ-5D social tariff values)
If utility was assumed to continue to increase linearly over the year (linear extrapolation), the loss was (as expected) smaller than if utility recovery was assumed to cease after 18 months (flat extrapolation). The absolute loss in utility 13–18 months after fracture was significantly higher for vertebral fractures than for either hip fractures or wrist fractures ().
Discussion
Our study presents estimates of fracture-related costs and quality of life from prospectively collected data for the 3 most common osteoporotic fracture types. The study that preceded the present study estimated hip-, vertebral-, and wrist fracture-related costs and quality of life during the first 12 months after occurrence of the fracture (Borgstrom et al. Citation2006). This paper deals with the period 13–18 months after fracture.
70% of the vertebral fracture patients in our sample were hospitalized at some point because of their fracture and can thus be assumed to have sustained more severe fractures than the average vertebral fracture patient. Thus, the patient sample under study cannot be said to be representative of fracture patients in general. The selection bias in the vertebral fracture sample is due to the fact that patients were not included through the usual primary care settings, but through hospitals where the likelihood of hospitalization is higher. Hip and wrist fractures are more likely to have been representative of the population they were sampled from since post-fracture care is more homogenous for these types of fracture. All the hip fracture patients studied were admitted from their own home while 65–90% of the Swedish hip fracture population (depending on age) reside at home at the time of fracture (Johnell et al. Citation2005) and the remaining patients live in some form of institution before fracture. A previous study estimated that there was no extra cost related to hip fracture for patients admitted from institutions (Zethraeus et al. Citation1997). This suggests that the hip fracture-related cost estimated in our study may have been overestimated somewhat in relation to the cost of hip fracture for the average Swedish hip fracture patient.
When using results from the KOFOR study in health-economic evaluations, these issues of representativeness should be kept in mind—and adjustments for dying patients, other distributions regarding hospitalization, and patients who were institutionalized at time of fracture may be necessary. The literature covering cost and consequences regarding quality of life beyond the first year after osteoporotic fractures is almost non-existent. The long-term costs of hip and vertebral fractures have previously been assessed by De Laet et al. (Citation1999) in the Rotterdam study, but wrist fractures were not included. The cost was estimated to be approximately $1,300 (adjusted to 2005) during the second year after hip fracture, which is considerably lower than the estimates presented here. These authors compared cost during the year preceding the hip fracture to the cost for the 2 years following the hip fracture. Apart from the fact that the studies were carried out at different times, the differences between the results can be attributed to several factors, for example differences in prices and resource use between Sweden and the Netherlands and cost items that were missing in the Dutch study. Home care, home help, paramedical care, ambulatory physiotherapy, and transportation costs were not included, and would thus explain the lower cost of hip fractures to some extent. Long-term vertebral fracture costs were estimated to be approximately $715 per year (adjusted to 2005), but these were for patients diagnosed with morphometry whereas our study only included clinical verterbral fractures. Also, the exact time of occurrence of vertebral fracture was unknown in the Dutch study. Acute costs after the fracture may therefore have been missing in the cost estimates.
In health-economic models of interventions dealing with fracture prevention, the assumed long-term cost of a fracture will usually have a substantial effect on the incremental cost- effectiveness ratio. This is because the cost will often follow the patient in the model for several years or until death. The long-term costs of hip fractures have often been based on the proportion of patients institutionalized after fracture (Jonsson et al. Citation1996, Kanis et al. Citation2005, Borgstrom et al. Citation2006a, Zethraeus et al. Citation2006, Strom et al. Citation2007). The annual long-term costs for Sweden used in these models are comparable to the total hip fracture cost of €2,422 for 6 months found in this study. However, these figures are solely based on rates of institutionalization, whereas a broader range of cost items has been measured in this study. For vertebral fractures, on the other hand, substantial long-term costs of any kind are seldom included and use of the long-term cost of vertebral fractures found in our study (€3,628 for 6 months) over one or several years would considerably improve the cost effectiveness of interventions preventing vertebral fractures. Costs of wrist fracture amounted to €316 for 13–18 months after fracture and amounted to 42% of indirect costs. In a subanaly-sis of wrist fracture patients who were less than 65 years old, indirect costs amounted to €796 for the first year after fracture and €423 for between 13 and 18 months. Compared to the other fracture types studied, a higher proportion of wrist fracture patients had been working at the time of fracture and the indirect costs of wrist fractures should not be neglected. In females in the 50–64-year age group, the incidence of wrist fractures in Sweden is 1.5 times higher than that of hip and vertebral fractures combined (Kanis et al. Citation2000).
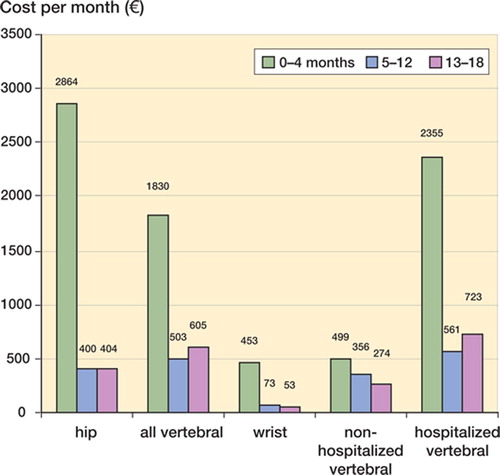
In the first 4 months after fracture, medical care represented 87%, 78%, and 87% of total costs for hip, vertebral, and wrist fractures, respectively. Community care represented 7%, 13%, and 4% of total costs during the same period. 5–12 months after fracture, the picture was different; 19%, 9%, and 56% of total costs were for medical care while 47%, 60%, and 14% were associated with community care. In the period of 13–18 months after fracture, there was further progression of this trend; only 17%, 11%, and 32% of total costs were for medical care and 78%, 83%, and 54% of costs were associated with community care. Although resource use is, unsurprisingly, shifting from medical care to community care as time passes, the cost level seems to be relatively stable between 5 and 18 months after fracture (Figure). Hip fracture costs appear to be higher during the first 4 months after fracture while vertebral fracture costs are higher in the longer term, 5–18 months after fracture.
Health-related quality of life improved by 0.03, 0.05, and 0.02 between 12 and 18 months for patients with hip, vertebral, and wrist fractures. In previous health-economic evaluations, a reduction in utility of 10% has often been used (Borgstrom et al. Citation2004a, Zethraeus et al. Citation2004, Kanis et al. Citation2005, Strom et al. Citation2007) for the second and following years after hip fracture. 10% is also the reduction that can be calculated from the results in this study if we assume that health-related quality of life after hip fracture will continue to increase at the same rate between 19 and 24 months as between 13 and 18 months after fracture. Loss in QoL associated with vertebral fracture is considerable, and if QoL recovery is assumed to continue during the whole second year after fracture the total loss would still amount to 0.20—which could be compared to 0.08 for hip fracture. The question is: for how long will the improvement continue? A case control study of patients enrolled in the Multiple Outcomes of Ral-oxifene Evaluation (MORE) showed that quality of life was reduced by approximately 9% when the clinical vertebral fracture may have occurred at a previously unknown time (Oleksik et al. Citation2000). If we apply a 9% reduction to the results of this study, the average vertebral fracture patient would eventually reach a QoL value of 0.67. This level would be reached approximately 3 years after the vertebral fracture if QoL were to continue to increase at the same rate as between our measurements at 12 and 18 months. It is also possible that patients adapt to their new health state as time passes, so that a response shift occurs. 70% of the vertebral fracture patients in our sample were hospitalized and recovery in QoL might be different in this group than in a group of normal vertebral fracture patients where approximately 25% of clinical fractures warrant hospitalization. Loss in QoL 13–18 months after a vertebral fracture was more than twice as great as after a hip fracture. This gives further support to the conclusion that the effect of vertebral fractures on quality of life has been underestimated. In agreement with previous assumptions (Borgstrom et al. Citation2004b, Zethraeus et al. Citation2004, Kanis et al. Citation2005, Strom et al. Citation2007), wrist fractures did not have any significant effect on quality of life beyond the first year after fracture.
We used a methodology in which the protocol is designed to capture only those costs that are related to the fracture under investigation. The potential cost savings of avoiding a fracture could either be estimated by relating all costs for the year before fracture to all costs for the year after fracture, or by only including the costs relevant to the fracture. The first alternative requires large numbers of patients at risk of fracture who are studied pro-spectively. Using a retrospective study design and selecting people with a fracture is more practical, since the risk of fracture for any particular person is very small. This approach has also been used in previous Swedish studies involving fracture costs (Zethraeus et al. Citation1997, Zethraeus and Gerdtham Citation1998) where costs before and after a fracture have been estimated based on register data. The main disadvantage of such studies is that it is difficult to determine all relevant uses of resources. For example, it is unusual for information about indirect costs and informal care to be available in any register. The advantage is that register studies can often provide fairly large sample sizes. Other studies have used a matched-case design (Melton et al. Citation2003), or a combination of both (De Laet et al. Citation1999). The benefits and limitations of the present design have been thoroughly discussed by Borgström et al. (Citation2006b), and will not be discussed in depth here. It would have been desirable to collect data for an even longer period of time, but when data are collected using the present methodology it is important that the use of resources registered should be a direct result of the actual fracture, and this would be increasingly difficult for both healthcare professionals and patients to assess with the passage of time. When these estimates are used in cost-effectiveness modeling, one must take into account that the estimates only relate to patients who have survived 18 months after fracture. Patients who died during the study period were associated with higher costs during the first 4 months after fracture, so the patients in the sample being analyzed were to some extent less dependent on care than those who died earlier in the study. However, this difference would partly be compensated for by the fact that dying patients not will have time to accumulate as much fracture related costs as those who survive.
The KOFOR study further emphasizes the importance of preventing osteoporotic fractures, and future evaluations of preventive strategies are likely to reach the conclusion that even younger and less fragile patients can be treated in a cost-effective way.
Whether or not QoL data that apply to local conditions should be used in other countries is a question that requires more research. However, one multinational study has shown a similar degree of loss in QoL after hip fracture to that found in the present study (Brazier et al. Citation2002). Cost data are not directly transferable to other countries since the use of resources, prices, and patterns of treatment can vary substantially between countries. There is thus an increasing demand for country-specific data, and we intend to launch a larger multinational study using the same methodology. Several European countries and the US will be involved in this study, which will be known by the acronym ICUROS (International Costs and Utilities Related to Osteoporotic Fractures Study).
In summary, we have presented results on fracture-related costs and reduction in quality of life 13–18 months after a fracture using a previously published method of prospective data collection. Long-term fracture-related costs for hip, spinal, and wrist fractures in Sweden have been estimated, including all relevant items of cost. The most significant finding has been the persistently high cost and large effect on quality of life associated with vertebral fractures. This suggests that the long-term consequences of vertebral fractures have been underestimated in health-economic evaluations assessing the cost-effectiveness of measures aimed at treating or reducing the risk of osteoporotic fractures.
Financial support for this study was obtained from the Alliance for Better Bone Health. We are most grateful to the research nurses who collected the data. Without your help, this study could not have been and cannot be conducted. Thanks to Ulla-Britt Ohlsson, Anita Söderqvist, Eva Ekström, Helen Wikdahl, Susanne Thomas, Rut Äkerberg, Eva Theander, Ulf Borgqvist, and Karin Önnby, and to the Foundation for Assistance to the Disabled in Skåne and the Medical Faculty, Lund University, Sweden.
Contributions of authors
OSt: planning of study, main author, data management, plan of analysis, analysis, and statistics. FB: planning of study, writing of manuscript, plan of analysis, monitoring of study. NZ, BJ: planning of study/initiative, editing of manuscript, plan of analysis. LL, SP, OSv, PA, EO, LC, KGT, and IS: data collection and writing of manuscript.
Supplementary data
Tables 2, 3, 4, 5, and 9 are available on the Acta Orthopae-dica website (www.actaorthop.org), identification number 0802.
- Borgstrom F, Johnell O, Jonsson B, et al. Cost effectiveness of alendronate for the treatment of male osteoporosis in Sweden. Bone 2004a; 34(6)1064–71
- Borgstrom F, Johnell O, Kanis J A, et al. Cost effectiveness of raloxifene in the treatment of osteoporosis in Sweden: an economic evaluation based on the MORE study. Phar-macoeconomics 2004b; 22(17)1153–65
- Borgstrom F, Jonsson B, Strom O, et al. An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting : Based on the results of the SOTI and TROPOS trials. Osteoporos Int 2006a; 17(12)1781–93
- Borgstrom F, Zethraeus N, Johnell O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 2006b; 17(5)637–50
- Borgstrom F, Sobocki P, Strom O, et al. The societal burden of osteoporosis in Sweden. Bone 2007; 40(6)1602–9
- Brazier J E, Green C, Kanis J A. Asystematic review of health state utility values for osteoporosis-related conditions. Osteoporos Int 2002; 13(10)768–76
- Briggs A H, Wonderling D E, Mooney C Z. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997; 6(4)327–40
- Brooks R. EuroQol: the current state of play. Health Policy 1996; 37(1)53–72
- Burstrom K, Johannesson M, Diderichsen F. Swedish population health-related quality of life results using the EQ-5D. Qual Life Res 2001; 10(7)621–35
- De Laet C E, van Hout B A, Burger H, et al. Incremental cost of medical care after hip fracture and first vertebral fracture: the Rotterdam study. Osteoporos Int 1999; 10(1)66–72
- Dolan P. Modeling valuations for EuroQol health states. MedCare 1997; 35(11)1095–108
- Dolan P, Torgerson D J. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int 1998; 8(6)611–7
- Gabriel S E, Tosteson A N, Leibson C L, et al. Direct medical costs attributable to osteoporotic fractures. Osteoporos Int 2002; 13(4)323–30
- Gujarati D N. Basic Econometrics. McGraw Hill, Singapore 1995
- Haentjens P, Autier P, Barette M, et al. The economic cost of hip fractures among elderly women. A one-year, prospective, observational cohort study with matched-pair analysis. Belgian Hip Fracture Study Group. J Bone Joint Surg (Am) 2001; 83(4)493–500
- Janzon M, Levin L A, Swahn E. Cost-effectiveness of an invasive strategy in unstable coronary artery disease; results from the FRISC II invasive trial. The fast revas-cularisation during in stability in coronary artery disease. Eur Heart J 2002; 23(1)31–40
- Johnell O, Kanis J A, Jonsson B, et al. The burden of hospitalised fractures in Sweden. Osteoporos Int 2005; 16(2)222–8
- Jonsson B, Christiansen C, Johnell O, et al. Cost-effectiveness of fracture prevention in established osteoporosis. Scand J Rheumatol (Suppl) 1996; 103: 30–8
- Kanis J A, Johnell O, Oden A, et al. Long-term risk of osteo-porotic fracture in Malmo. Osteoporos Int 2000; 11(8)669–74
- Kanis J A, Borgstrom F, Johnell O, et al. Cost-effectiveness of raloxifene in the UK: an economic evaluation based on the MORE study. Osteoporos Int 2005; 16(1)15–25
- Koeck C M, Schwappach D L, Niemann F M, et al. Incidence and costs of osteoporosis-associated hip fractures in Austria. Wien Klin Wochenschr 2001; 113(10)371–7
- Liljegren G, Karlsson G, Bergh J, et al. The cost-effectiveness of routine postoperative radiotherapy after sector resection and axillary dissection for breast cancer stage I. Results from a randomized trial. Ann Oncol 1997; 8(8)757–63
- Lippuner K, Golder M, Greiner R. Epidemiology and direct medical costs of osteoporotic fractures in men and women in Switzerland. Osteoporos Int (Suppl 2) 2005; 16: S8–S17
- Melton L J, 3rd, Gabriel S E, Crowson C S, et al. Cost-equivalence of different osteoporotic fractures. Osteoporos Int 2003; 14(5)383–8
- Neter J, Kutner M H, Nachtsheim C J, et al. Applied Linear Statistical Models. McGraw-Hill, Chicago 1996
- Nurmi I, Narinen A, Luthje P, et al. Cost analysis of hip fracture treatment among the elderly for the public health services: a 1-year prospective study in 106 consecutive patients. Arch Orthop Trauma Surg 2003; 123(10)551–4
- Oleksik A, Lips P, Dawson A, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 2000; 15(7)1384–92
- Reginster J Y, Gillet P, Ben Sedrine W, et al. Direct costs of hip fractures in patients over 60 years of age in Belgium. Pharmacoeconomics 1999; 15(5)507–14
- SBU. Osteoporos-prevention, diagnostik och behandling, rapport Nr 165. Stockholm 2003
- SCB Average hourly prices in the private sector,Statistiska Centralbyån (Statistical Sweden). 2004 cited 2005–01–11, from. http://www.ssd.scb.se/databaser/makro/MainTable.asp?yp=tansss&xu=C9233001&omradekod=AM&omradetext=Arbetsmarknad&lang=1
- Stadsledningskontoret Stockholms stads budgetavräkning. 2003 cited 2005-05-20, from www.stockholm.se
- Strom O, Borgstrom F, Sen S S, et al. Cost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries - an economic evaluation based on the fracture intervention trial. Osteoporos Int (epub ahead of print). Copenhagen 2007
- Wackerly D, Mendenhall W, Schaeffer R. Mathematical Statistics with Applications. Duxbury Press, New York 1996
- Wiktorowicz M E, Goeree R, Papaioannou A, et al. Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int 2001; 12(4)271–8
- Wong M K, Arjandas, Ching L K, et al. Osteoporotic hip fractures in Singapore—costs and patient's outcome. Ann Acad Med Singapore 2002; 31(1)3–7
- Vägverket. Väg verkets samhällsekonomiska kalkymodell. Ekonomisk teori och värderingar. Stockholm 1997
- Zethraeus N, Gerdtham U G. Estimating the costs of hip fracture and potential savings. Int J Technol Assess Health Care 1998; 14(2)255–67
- Zethraeus N, Stromberg L, Jonsson B, et al. The cost of a hip fracture. Estimates for 1,709 patients in Sweden. Acta Orthop Scand 1997; 68(1)13–7
- Zethraeus N, Borgström F, Johnell O, et al. Costs and Quality of Life Associated with Osteoporosis Related Fractures -Results from a Swedish Survey. Stockholm 2002
- Zethraeus N, Borgström F, Jönsson B, et al. A reassessment of the cost-effectiveness of hormone replacement therapy in Sweden - results based on the Women's Health Initiative randomised controlled trial. Working Paper Series in Economics and Finance at the Stockholm School of Economics, 2004, Working paper No. 571. 2004; http://swopec.hhs.se/hastef/papers/hastef0571.pdf
- Zethraeus N, Strom O, Borgstrom F. What is the risk of institutionalization after hip fracture?. Poster at ECCEO Vienna Volume 17 Supplement 1 / March 2006