Abstract
Background Wear particles, found at the bone-implant interface surrounding a loose prosthesis, are commonly phagocytosed by macrophages. Wear particles and wear particle-containing macrophages are also found in regional lymph nodes draining arthroplasty tissues. The means by which wear particles are transported from arthroplasty tissues to lymph nodes is uncertain, as the presence or absence of lymphatic vessels in periprosthetic tissues has not been established.
Methods We determined immunophenotypic expression of LYVE-1 and podoplanin, two highly specific lymphatic endothelial cell markers, in the hip arthroplasty pseudocapsule surrounding the false joint and the bone-implant interface of the femoral and acetabular pseu-domembrane.
Results LYVE-1+/podoplanin+ lymphatic vessels were not identified in the pseudomembrane but were found in the pseudocapsule. Normal bone did not contain lymphatic vessels.
Interpretation Our findings suggest that the wear particles shed at the bone-implant interface are not transported to draining lymph nodes by lymphatics directly from the pseudomembrane, but via the pseudocapsule. The absence of a lymphatic clearance mechanism may contribute to accumulation of wear particles at the bone-implant interface and promote periprosthetic osteolysis through stimulation of osteoclast formation and activity.
Aseptic loosening, the most common complication of prosthetic joint replacement, is often associated with periprosthetic osteolysis (Harris Citation1994, Citation1995). This is characterized histologically by deposition of wear particles in peri-implant tissue—both the pseudocapsule that surrounds the articulating structures and the fibrous pseudomembrane that lies between the implant component and surrounding bone (Revell Citation1982, Malcolm Citation1988). There is a heavy foreign body response to wear particles in periprosthetic tissues, with many of these particles being phagocytosed by macrophages and macro-phage polykaryons. Wear particles and the macrophages that have phagocytosed these particles can also be found in regional lymph nodes draining prosthetic joints (Bos et al. Citation1990, Basle et al. Citation1996, Urban et al. Citation2000). However, the precise role that the lymphatic circulation plays in transporting macrophages and the wear particles they contain from periprosthetic tissues to lymph nodes has not been established. In addition, it is not known whether the wear particles found in lymph nodes are derived from the arthroplasty pseudocapsule, the pseudomembrane, or both of these tissues.
Lymphatic vessels have been identified in the synovial membrane by various techniques, including lymphangiography, electron microscopy, his-tochemistry, and immunohistochemistry (Edwards and Wilkinson Citation1991, Fujiwara et al. Citation1995, Hu et al. Citation2003). However, the question of whether lymphatics are present in bone is more controversial; studies of fluid movement in bone using intra-osseous injection, microdissection, and histomorphological studies have provided conflicting evidence (Anderson Citation1960, Smith et al. Citation1960, Munka and Gregor Citation1965, Olszewski and Sawicki Citation1977). Lymphan-giomas have been identified in bone (Harris and Prandoni Citation1950, Bullough and Goodfellow Citation1976), but lymphatic vessels not been identified with certainty in any other neoplastic or non-neoplastic disease of bone. With regard to aseptic loosening, this consideration is of some importance as lymphatic clearance of wear particles from the bone-implant interface is one possible route whereby wear particles could be cleared from this site. Accumulation of wear particles at the bone-implant interface could have a number of effects on the macrophages that phagocytose these particles, including stimulation of the secretion of cytokines that promote bone resorption (Horowitz et al. Citation1991, Glant et al. Citation1993, Haynes et al. Citation1993). There is also evidence that macrophages isolated from periprosthetic tissues, and mouse monocytes that have phagocy-tosed wear particles, are capable of differentiating into osteoclasts (Sabokbar et al. Citation1996, Citation1997). In addition, it has been shown that mature human osteoclasts that have phagocytosed particles retain their ability to carry out acunar resorption (Wang et al. Citation1997).
In this study we have used two highly specific phenotypic markers of lymphatic endothelial cells, LYVE-1 and podoplanin (Banerji et al. Citation1999, Bre-iteneder-Geleff et al. Citation1999), to determine whether lymphatic vessels are present in the hip arthroplasty pseudocapsule and pseudomembrane. We have also examined whether lymphatics are present in bone surrounding the pseudomembrane and in normal bone specimens, in order to determine whether lymphatic clearance plays a role in the transport of fluid or cells in bone.
Material and methods
Cases studied
Specimens of periprosthetic tissues were obtained from 26 patients (age range 47–86 years; 16 females) undergoing revision hip arthroplasty surgery because of osteoarthritis (n = 22) or rheumatoid arthritis (n = 4). Ethical permission for this study was obtained from the Oxfordshire Research Ethics Committee.
In order to determine the distribution of lymphatics in normal bone and joint tissue, specimens of compact and cancellous bone and joint synovium and capsule were obtained from the femoral head and femoral neck of 3 hindquarter amputations in patients with soft tissue sarcomas of the lower limb, and from 5 osteoarthritic femoral head specimens.
Immunohistochemistry
Specimens of the hip arthroplasty pseudocapsule and femoral and acetabular pseudomembrane were fixed in buffered formalin at room temperature and embedded in paraffin wax. 5-μm sections were cut, dewaxed, and rehydrated by successive immersion in xylene, graded ethanol, and water; this was followed by microwave treatment (700 W, 2 treatments of 4 min each) in target retrieval solution (Dako, Ely, Cambridge, UK). Sections were stained by an indirect immunoperoxidase technique as previously described (Hu et al. Citation2003). Endogenous peroxidase was blocked with 3% (v/v) hydrogen peroxide and protein block serum (Dako), and the sections were then incubated with mouse monoclonal antibodies directed against human podoplanin (D2-40; Signet Laboratories, Dedham, MA, USA) or LYVE-1 (Hu et al. Citation2003), which react with lymphatic endothelial cells, as well as antibodies against CD34 (Q-Bend10; Dako) or factor VIII (Dako), which react with blood vessel endothelium. Antigens were detected by incubation with labeled polymer and diaminobenzidine. The sections were then coun-terstained with hematoxylin, dehydrated, cleared, and then mounted. Positive controls consisted of sections of lymph node and skin containing lymphatic vessels. Negative controls consisted of sections stained with primary antibody diluent alone and substitution of an irrelevant (anti-cytokeratin) antibody (MNF116; Dako).
Results
All specimens of hip joint arthroplasty pseudocapsule and femoral and acetabular pseudomembrane showed typical pathological features of aseptic loosening. The tissues contained a heavy macro-phage and giant cell response to implant-derived metal and polymer wear particles ( and ). Some specimens of the fibrous pseudomem-brane also contained underlying cancellous bone and a foreign body macrophage infiltrate in the marrow space ().
Figure 1. Low-power (A) and high-power (B) photomicrographs showing LYVE-1+ endothelial cells lining lymphatic vessels in the arthroplasty pseudocapsule. (Immunoperoxidase; 100× and 200× magnification).
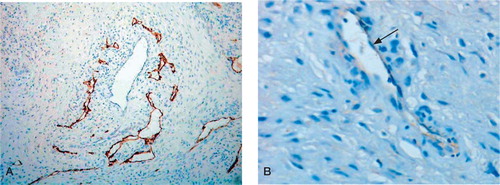
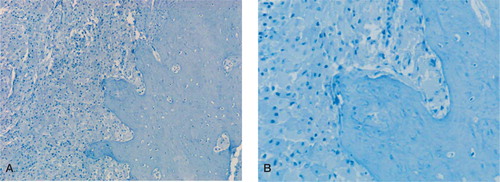
Figure 2. Low-power (A) and high-power (B) photomicrographs showing absence of LYVE-1 staining in the femoral arthroplasty membrane, which contains numerous plump (wear particle-containing) macrophages and surrounding compact cortical bone. (Immunoperoxidase; 100× and 200× magnification).
LYVE-1 and podoplanin expression in peri-prosthetic tissues
LYVE-1 +/podoplanin+ lymphatic vessels were found in all the arthroplasty pseudocapsule specimens examined. The lymphatic vessels were most prominent in the superficial zone of the pseudocapsule, but were also present in underlying fat and connective tissue (). Many of the lymphatic vessels in the superficial zone were slit-like and appeared compressed by surrounding macrophages containing wear particles, whereas lymphatics in deeper capsular tissues were more dilated. There were considerably fewer LYVE-1+/podoplanin+ lymphatic vessels than CD34+/factor VIII+ blood vessels.
The fibrous acetabular and femoral pseudomem-brane also had heavy infiltration of wear particle-containing macrophages, but no lymphatic vessels were identified by LYVE-1 or podoplanin staining of these tissues. In 5 cases, compact or cancellous bone adjacent to the arthroplasty pseudomembrane was included in the specimens received; this contained fibrous tissue and a macrophage and macrophage polykaryon infiltrate in response to wear particles, but no LYVE-1+/podoplanin+ lymphatic vessels ().
In order to determine whether lymphatic vessels are present in normal bone tissue of the femur, compact and cancellous bone derived from 3 amputation specimens and 5 osteoarthritic femoral head and neck specimens were examined for LYVE-1 and podoplanin expression. LYVE-1 +/podoplanin+ lymphatic vessels were not identified in compact or cancellous bone, but LYVE-1 +/podoplanin+ lymphatics were identified in the periosteum and the attached hip joint synovium and capsule.
Discussion
It has been estimated that as many as 40 billion polyethylene particles smaller than 10 μm are released from the artificial hip joint annually (Maloney and Jasty Citation1993). There should be a mechanism to clear the large amount of polyethylene and other polymer and metal particles generated from arthroplasty tissues. As macrophages and wear particles have been found in regional lymph nodes, it has been assumed that one means whereby wear particles are cleared from peripros-thetic tissues is via the lymphatic circulation. We found that lymphatics are only present in the hip arthroplasty pseudocapsule and not in the femoral or acetabular pseudomembrane. These results indicate that wear particles are most likely transported by lymphatics from the pseudocapsule (and not the pseudomembrane) to regional lymph nodes draining arthroplasty tissues.
After an arthroplasty is performed, a new joint capsule and synovial lining forms around the joint endoprosthesis (Revell Citation1982, Malcolm Citation1988). Implantation of the prosthesis in bone is followed by bony repair and formation of a fibrous tissue membrane that separates the bone from the implant or PMMA cement covering the implant. A consequence of implant loosening is the generation of numerous implant-derived wear particles, most of which are less than 1 μm in size (Margevicius et al. Citation1994). It has been estimated that the tissue adjacent to a failed total joint implant contains billions of particles per gram of tissue and that, when the number of particles is greater than 1 × 1010 per gram of tissue, the incidence of osteolysis is increased (Revell et al. Citation1997). Generation of wear particles and the foreign body macrophage response that it evokes are thought to play a key role in promoting the periprosthetic osteolysis that is carried out by osteoclasts. Wear particle-containing macrophages from the bone-implant interface are capable of osteoclast differentiation and wear particles stimulate osteoclastic bone resorption by promoting the release of prostaglandins, cytokines, and growth factors from activated macrophages and fibroblasts (Harris et al. Citation1976, Horowitz et al. Citation1991, Glant et al. Citation1993, Haynes et al. Citation1993). Osteolytic pseudotu-mors result from aggressive granulomatosis, where massive deposition of wear particles leads to very heavy infiltration of macrophages and an increase in osteoclast numbers and resorption (Lassus et al. Citation1998). Absence of lymphatic vessels at the bone-implant interface would exacerbate this particle-induced osteolysis, as this effectively means that particles cannot be cleared from this site via the lymphatic circulation.
Our results indicate that wear particle-containing macrophages found in regional lymph nodes are derived not from the arthroplasty pseudomembrane, but from the pseudocapsule. This raises the question of how wear particles are cleared from the bone-implant interface. Such a mechanism is likely to exist, as implant wear particles are commonly found at the bone-implant interface. It is conceivable that the absence of such a clearance mechanism would result in rapid accumulation of particles to a critical threshold at which osteoclast formation and resorption would be triggered. It has previously been shown that even in a mechanically stable prosthesis, polyethylene wear debris can be found in areas of bone resorption some distance from the articulating surfaces (Schmalzried et al. Citation1992). To explain this phenomenon, it has been proposed that wear particles are dispersed into the “effective joint space”, i.e. the sum of all periprosthetic territories accessible to the joint fluid (Schmalzried and Callaghan Citation1999). This effective joint space permits joint fluid and wear debris floating within it to penetrate between the bone and the implant. In this way, particles may be transported to sites remote from the implant component from which they were originally shed. The effective joint space may thus provide a possible route whereby wear particles shed at the bone-implant interface are carried to the pseudojoint cavity, and from there to the pseudocapsule—from where they would be transported via lymphatics to draining lymph nodes. Elevated intra-articular pressures of 700 mm Hg or more have been noted during activity after total hip arthroplasty (Robertsson et al. Citation1997). These elevated pressures may result in pumping of particles and particle-containing cells away from the bone-implant interface towards the pseudojoint cavity (Aspenberg and Van der Vis Citation1998, Van der Vis et al. Citation1998). However, in cases where implant loosening results in excessive generation of wear particles, this clearance mechanism may be overwhelmed. Consequent particle accumulation at the bone-implant interface may then result in periprosthetic osteolysis due to particle-induced macrophage-osteoclast differentiation and resorption. Increased pressure resulting from particle accumulation has been shown to induce bone resorption in animal models of implant loosening (Robertsson et al. Citation1997), and it is conceivable that particle accumulation per se could also contribute to osteolysis by a mass effect.
No competing interests declared.
Contributions of authors
JE carried out the immunostaining of most of the samples, ES reviewed the clinical cases, AS was responsible for the overall supervision of the project and writing of parts of the manuscript, HGA carried out immunostaining on some control and test cases, DJ supplied LYVE-1 and podoplanin 1, and NA planned, designed, and supervised the study.
- Anderson D W. Studies of the lymphatic pathways of bone and bone marrow. J Bone Joint Surg (Am) 1960; 42: 716–7
- Aspenberg P, Van der Vis H. Migration, particles, and fluid pressure. A discussion of causes of prosthetic loosening. Clin Orthop 1998, 352: 75–80
- Banerji S, Ni J, Wang S X, Clasper S, Su J, Tammi R, Jones M, Jackson D G. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999; 144: 789–801
- Basle M F, Bertrand G, Guyetant S, Chappard D, Lesourd M. Migration of metal and polyethylene particles from articular prostheses may generate lymphadenopathy with histiocytosis. J Biomed Mater Res 1996; 30: 157–63
- Bos I, Johannisson R, Lohrs U, Lindner B, Seydel U. Comparative investigations of regional lymph nodes and pseu-docapsules after implantation of joint endoprosthesis. PatholResPract 1990; 186: 707–16
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endo-thelium. Am J Pathol 1999; 154: 385–94
- Bullough P G, Goodfellow J W. Solitary lymphangioma of bone. A case report. J Bone Joint Surg (Am) 1976; 58: 418–9
- Edwards J C, Wilkinson L S. Demonstration of lymphatics in human synovial tissue. Rheumatol Int 1991; 11: 151–5
- Fujiwara T, Kato S, Itonaga I, Torisu T, Masumi S. Fine structure and distribution of lymphatics in the synovial membrane of monkey and human knee joints. A study using an enzyme-histochemical method. Int Orthop 1995; 19: 396–402
- Glant T T, Jacobs J J, Molnar G, Shanbhag A S, Valyon M, Galante J O. Bone resorption activity of particulate-stimu-lated macrophages. J Bone Miner Res 1993; 8: 1071–9
- Harris W H. Osteolysis and particle disease in hip replacement: a review. Acta Orthop Scand 1994; 65: 113–23
- Harris W H. The problem is osteolysis. Clin Orthop 1995, 311: 46–53
- Harris R, Prandoni A G. Generalized primary lymphangio-mas of bone. Ann Intern Med 1950; 33: 1302–13
- Harris W H, Schiller A L, Scholler J M, Freiberg R A, Scott R. Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg (Am) 1976; 58: 612–8
- Haynes D R, Rogers S D, Hay S, Pearcy M J, Howie D W. The differences in toxicity and release of bone-resorbing mediators induced by titanium and cobalt-chromium-alloy wear particles. J Bone Joint Surg (Am) 1993; 75: 825–34
- Horowitz S M, Gautsch T L, Frondoza C G, Riley L, Jr. Macrophage exposure to polymethyl methacrylate leads to mediator release and injury. J Orthop Res 1991; 9: 406–13
- Hu X, Edwards J, Banerji S, Jackson D, Athanasou N A. Distribution of lymphatics in normal and arthritic human synovial tissues. Ann Rheum Dis 2003; 62: 1227–9
- Lassus J, Salo J, Jiranek W A, Santavirta S, Nevalainen J, Matucci-Cerinic M, Horak P, Konttinen Y. Macrophage activation results in bone resorption. Clin Orthop 1998, 352: 7–15
- Malcolm A J. The pathology of long-standing cemented total hip replacement in Charnley's cases. J Bone Joint Surg (Br) 1988; 70: 153–6
- Maloney W J, Jasty M. Wear debris in total hip arthroplasty. Semin Arthroplasty 1993; 4: 125–35
- Margevicius K J, Bauer T W, McMahon J T, Brown S A, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg (Am) 1994; 76: 1664–75
- Munka V, Gregor A. Lymphatics and bone marrow. Folia Morphol 1965; 13: 404–12
- Olszewski W L, Sawicki Z. Radiological evidence of lymphatic drainage of bone marrow cavity in long bones. Lymphology 1977; 10: 1–4
- Revell P A. Tissue reactions to joint prostheses and the products of wear and corrosion. Current trends in pathology, C Berry. Springer-Verlag, Berlin 1982; 73–101
- Revell P A, Al-Saffar N, Kobayashi A. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng [H] 1997; 211: 187–97
- Robertsson O, Wingstrand H, Kesteris U, Jonsson K, Onnerfalt R. Intracapsular pressure and loosening of hip prostheses. Preoperative measurements in 18 hips. Acta Orthop Scand 1997; 68: 231–4
- Sabokbar A, Fujikawa Y, Brett J, Murray D W, Athanasou N A. Increased osteoclastic differentiation by PMMA particle-associated macrophages. Inhibitory effect by interleu-kin 4 and leukaemia inhibitory factor. Acta Orthop Scand 1996; 67: 593–8
- Sabokbar A, Fujikawa Y, Neale S, Murray D, Athanasou N A. Human arthroplasty-derived macrophages differentiate into osteoclastic bone-resorbing cells. Ann Rheum Dis 1997; 56: 414–20
- Schmalzried T P, Callaghan J J. Current concept reviews: Wear total hip and knee replacements. J Bone Joint Surg (Am) 1999; 87: 51–6
- Schmalzried T P, Jasty M, Harris W H. Periprosthetic bone loss in total hip arthroplasty. J Bone Joint Surg (Am) 1992; 72: 849–63
- Smith R J, Sage H H, Myazak M D, Kizilay D. The relationship of lymphatics to bone. Bull Hosp Joint Dis 1960; 21: 25–41
- Urban R M, Jacobs J J, Tomlinson M J, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg (Am) 2000; 82: 457–76
- Van der Vis H M, Aspenberg P, Marti R K, Tigchelaar W, Van Noorden C J. Fluid pressure causes bone resorption in a rabbit model of prosthetic loosening. Clin Orthop 1998; 350: 201–8
- Wang W, Ferguson D J P, Quinn J M W, Simpson A H R W, Athanasou N A. Biomaterial particle phagocytosis by bone-resorbing osteoclasts. J Bone Joint Surg (Br) 1997; 79: 849–56
