Abstract
Background and purpose Antibiotic‐impregnated cement is used as a spacer or during re‐implantation surgery for the treatment of infected total hip arthroplasties. The routine use of antibiotic‐impregnated cement during primary or uninfected revision total hip arthroplasty remains controversial. With this meta‐analysis of the published literature, we intended to assess efficacy and safety in the use of antibiotic‐impregnated cement for uninfected arthroplasty.
Methods Following a detailed literature search, only studies reporting on the outcome of total hip replacement performed with antibiotic cement were included. Strict inclusion criteria were used and studies lacking sufficient sample size or critical data were excluded. 19 studies reporting on 36,033 hip replacements in 35,659 patients met the initial inclusion criteria. The main aim of the meta‐analysis was to determine the rate of deep infection with and without the use of antibiotic cement, and to assess the revision rate and the ultimate survivorship of arthroplasty using antibiotic cement.
Results The rate of deep infection following primary total hip arthroplasty, at 1.2%, was significantly lower when antibiotic cement was used than when cement without antibiotics was used (2.3%). Similarly, the rate of deep infection following revision total hip arthroplasty when a standard combination of cement and antibiotic—or a custom‐made combination of cement and antibiotic, depending on the results of culture—was used, was almost half of the rate of deep infection when no antibiotics were present in the cement. Overall, the survivorship was 98% (101 failures in 5,178 hips) for primary arthroplasty and 88% (100 failures in 855 hips) for revision arthroplasty. There were no reported adverse events or complications associated with the use of antibiotic‐impregnated cement.
Interpretation The use of antibiotic‐impregnated cement lowered the infection rate by approximately 50% in primary hip arthroplasty. For revisions of previously infected hips, combinations or culture‐dependent antibiotics lowered infection rates by approximately 40%.
The use of acrylic bone cement impregnated with antibiotic agents in joint arthroplasty has been a common practice in Europe for many years (Josefsson et al. Citation1990, Espehaug et al. Citation1997). Several studies have also reported widespread clinical use of antibiotic‐impregnated cement in US hospitals for many years (Fish et al. Citation1992, Heck et al. Citation1995), despite the fact that there has only been recent approval of antibiotic‐impregnated cement for commercial use by the Food and Drug Administration. Although studies evaluating the elution of various antibiotics from methylmethacrylate cement have demonstrated effective tissue concentrations of antibiotic (Wahlig et al. Citation1984, Penner et al. Citation1999), debate about the efficacy and the role of antibiotic‐impregnated cement in minimizing periprosthetic infection continues (Bourne Citation2002, Jiranek Citation2003). A previous study from the Norwegian joint registry has suggested that the use of antibiotic‐impregnated cement may confer benefits in terms of reducing the rate of periprosthetic infection (Espehaug et al. Citation1997). If true, such a finding has marked clinical implications as periprosthetic infection confers a substantial social, psychological, and economic burden on patients and society (Zimmerli et al. Citation2004).
There has also been concern regarding the addition of antibiotics to cement and routine use of this procedure in joint arthroplasty. The adverse effect on the mechanical strength of methylmethacrylate cement, which may in turn compromise the longevity of the arthroplasty, is one such concern (Moran et al. Citation1979, Davies et al. Citation1989). Other questions relate to the potential for the emergence of resistant organisms with the routine use of antibiotic‐impregnated cement (Hope et al. Citation1989). Although both the compromise in the mechanical strength of the methylmethacrylate cement and the possibility of emergence of resistant organisms remain theoretical at this stage, some surgeons refrain from routine use of antibiotic‐impregnated solutions for the above reasons.
The aim of this meta‐analysis was to determine the effectiveness of antibiotic‐impregnated cement used during primary and uninfected revision total hip arthroplasty in reducing the rate of deep infection. A secondary aim was to determine whether impregnating cement with antibiotic has any adverse effect on the long‐term survivorship of hip arthroplasties.
Material and methods
We carried out a literature search using Medline to identify all articles published between January 1966 and December 2004 that evaluated clinical outcome following the use of antibiotic‐impregnated cement in primary and revision total hip arthroplasty. No restrictions were placed on the origin or language of the publications. Reports relating to both primary and revision hip arthroplasty were included, and the results were analyzed separately. Key words used were infection, total hip arthroplasty/replacement, revision total hip arthroplasty/replacement, antibiotic‐impregnated cement, and cement. We performed a literature search for each author of the studies, to identify further studies on the same topic. The bibliographies of all the articles retrieved were also searched for reports that evaluated outcome following antibiotic cement use in total hip replacement. Studies that related to mechanical properties of cement, in vitro studies, and studies of joints other than the hip were excluded. We then reviewed all the articles identified in this manner to determine whether they met strict eligibility criteria, as determined by the investigators, for inclusion in the meta‐analysis (as described below). Although we acknowledge that publication bias may be a source of inaccuracy in meta‐analysis, no attempt was made to include unpublished research.
While it is clearly preferable to include only randomized controlled trials in meta‐analyses, the paucity of such studies precluded the use of this standard as a criterion for inclusion. Reports were considered eligible for inclusion in the meta‐analysis if they were comparative trials of antibiotic‐loaded vs. non‐antibiotic cement, if they included data on 100 or more primary hip replacements or 20 or more revision hip replacements, and if they included outcome data at specified follow‐up times. Outcome data required for inclusion were the incidence of deep infection and the overall survival rate at the specified interval after surgery. Finally, each eligible study was assessed for scientific quality based on “Systems to rate the strength of scientific evidence” AHRQ Publication No. 02 E016, April 2002 (http://www.ahcpr.gov/news/ pubcat/c_evid.htm). This assessment was made by 2 of the authors who were blinded regarding the source institution, the journal, and the authors for each included publication.
To ensure that our assessment of the literature was as complete as possible, we performed an additional review of a further group of studies that described outcome of antibiotic cement use without a control group. The following criteria were specified for eligibility for inclusion in this group: non‐comparative cohort studies which reported outcome in terms of infection rates or revision rates at a minimum follow‐up of 2 years.
Although we performed detailed data extraction from the selected papers, at the outset of the study the exact questions to be answered by the meta‐analysis were already clearly defined. This included the following 2 specific questions. 1. Does the use of antibiotic cement reduce the risk of deep infection in total hip replacement? 2. Does the use of antibiotic cement reduce the rate of failure as determined by revision surgery following total hip replacement?
Data analysis
As the studies related to different populations in different countries, and the data on comparability of surgical technique, choice of implant, and use of systemic antibiotics were limited, a random‐effects model was used for statistical analysis of the data. All statistical analysis was performed using the Comprehensive Meta‐analysis software package (Biostat Inc., Englewood, NJ). Homogeneity of the effectiveness of antibiotic cement across the studies was tested by use of the Q statistic. We used relative risk to express the effectiveness of antibiotic cement in preventing deep infection and lowering the risk of revision. Finally, the results of our analyses were expressed as forest plots, which showed weighted mean reduction (or, in 1 study, increase) in the relative risks of infection and revision with 95% confidence intervals for each study and a cumulative weighted mean effect for all the studies in the analysis. In our plots, values less than 1 indicate increasing effectiveness of antibiotic cement and values greater than 1 indicate greater effectiveness of non‐antibiotic cement.
Weighted mean relative risk of infection with 95% confidence limits is shown for each individual study, and the overall cumulative relative risk is shown on the lowest row. In this type of analysis, when the 95% confidence intervals are entirely within the negative range, this corresponds to statistical significance at the p < 0.05 level in the cumulative meta‐analysis.
Results
3,575 reports concerning the use of methylmeth‐acrylate cement in hip arthroplasty were found in the literature. After exclusion of non‐clinical studies and non‐outcome clinical studies, 71 studies pertaining to clinical use of antibiotic cement were identified. Following elimination of reports that did not include data on clinical outcome or had insufficient sample size (as defined above), 19 studies met the initial inclusion criteria. 13 of the latter 19 studies did not include a control group and were mostly historical reports. These were thus excluded from the main meta‐analysis. 6 studies (Lynch et al. Citation1987, Josefsson et al. Citation1990, Josefsson and Kolmert Citation1993, Havelin et al. Citation1995, Espehaug et al. Citation1997), comprising 24,661 total hip replacements, formed the basis of this meta‐analysis.
In these studies, 1,081 hips (4.4%) were lost to follow‐up or the patients died and were excluded. In order to maximize homogeneity of the study population, 1,990 hips that had been inserted with low‐viscosity “Boneloc” cements in the study by Havelin et al. (Citation1995) were excluded.
In an additional effort to improve homogeneity, 145 hips in the study by Espehaug et al. (Citation1997) that had been performed using Simplex cement containing erythromycin and colistin were also excluded, as there were no separate data reported for this subgroup. This subgroup represented only 2.5% of the cohort in that study and only 0.7% of the total population of the meta‐analysis. Similarly, data in the study performed by Havelin et al. (Citation1995) contained 8 hips that had been inserted with Simplex cement containing erythromycin. Again, it was believed that this number was sufficiently small that the exclusion would not affect the outcome of the meta‐analysis. The remaining 21,445 hips in 6 studies that were included in the meta‐analysis had been performed with or without the use of gentamicin as the sole antibiotic.
The analysis of effectiveness of antibiotic cement in reducing infection rate in primary total hip arthroplasty is shown in . The forest plot shows that in relation to prevention of infection, statistical significance was reached by only 1 of the individual studies. While the other studies did not reach statistical significance, the trend was clearly in favor of antibiotic cement in all but 1 study. Meta‐analysis of the cumulative data from all of the studies confirmed the efficacy of antibiotic cement in reducing the rate of deep infection in primary total hip replacement—from 2.3% when no antibiotic was present in the cement to 1.2% with the use of antibiotic cement.
Figure 1. Forest plot showing the weighted mean effect (with 95% confidence intervals) of antibiotic cement in reducing the risk of infection in primary total hip arthroplasty. In the graph, values of less than 1 indicate increasing effectiveness of antibiotic cement and values greater than 1 indicate greater effectiveness of non‐antibiotic cement. When the 95% confidence intervals are entirely within the negative range, this corresponds to statistical significance at the p < 0.05 level in the cumulative meta‐analysis. The forest plot shows that statistical significance was reached by only 1 of the individual studies in relation to prevention of infection. While the other studies did not reach statistical significance, the trend was clearly in favor of antibiotic cement.
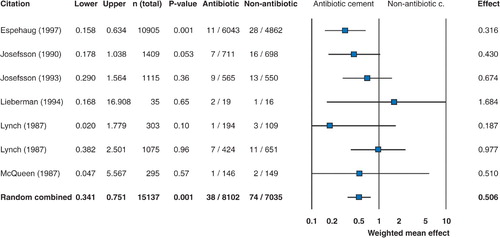
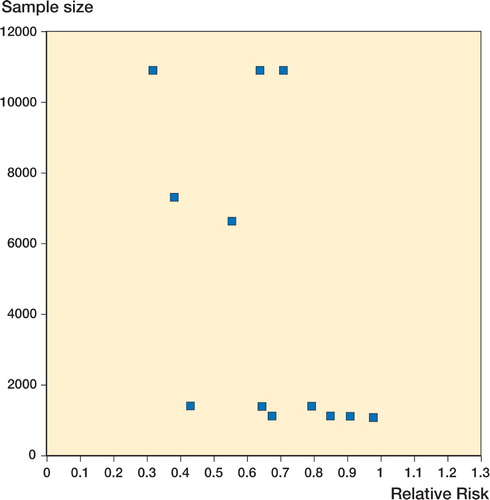
The cumulative incidence of revision for failure was statistically significantly lower following primary arthroplasty when antibiotic cement was used (). Relative risks ofr evision at the mean follow‐up period specified in each study (range 3-10 years) are illustrated with 95% confidence intervals. Overall, the survivorship was 98% (101 failures in 5,178 hips) for primary arthroplasty and 88% (100 failures in 855 hips) for revision arthroplasty. A funnel plot showing sample size vs. relative risk in the studies included in the meta‐analysis is shown in .
Figure 2. Forest plot showing a reduction in relative risk of revision of primary total hip replacements when antibiotic‐impregnated cement is used. In the graph, values of less than 1 indicate increasing effectiveness of antibiotic cement and values greater than 1 indicate greater effectiveness of non‐antibiotic cement. When the 95% confidence intervals are entirely within the negative range, this corresponds to statistical significance at the p < 0.05 level in the cumulative meta‐analysis.
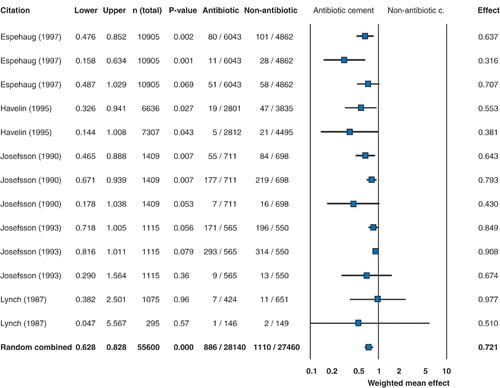
Figure 3. Funnel plot showing sample size vs. relative risk in the studies included in the meta‐analysis.
The data relating to the use of antibiotic cement during uninfected revision total hip replacements comprised 2 studies, with 213 patients. No statistically significant difference in the infection rates was seen. Outcome data relating to long‐term survival were not reported in either of these studies.
The non‐comparative studies that met our inclusion criteria contained data on a total of 655 revision hips (Carlsson et al. Citation1985, Weber and Lauten‐bach Citation1986, Sanzen et al. Citation1988, Hope et al. Citation1989, Marti et al. Citation1990, Garvin et al. Citation1994, Younger et al. Citation1997, Ure et al. Citation1998, Koo et al. Citation2001) with follow‐up ranging from 1 to 10 years. The 11 studies that reported data on antibiotic cement use in revisions formed an inhomogeneous group of studies, which rendered group analysis difficult. 5 brands of cement were used in the constituent studies. 7 types of antibiotics were reported overall. In some studies, a single type of antibiotic was used empirically; in others, antibiotic type was determined by bacteriological sensitivity. All papers reported recurrence of infection as a measure of outcome. The rates of re‐infection ranged from 0 to 24% for one‐stage revisions and from 0 to 17% for two‐stage revisions. Only 9 of the latter studies reported an overall revision rate, which varied from 0 to 31%. As there was such a wide variety of treatment techniques and follow‐up times, it was not possible to draw clinically relevant conclusions from this group of studies.
Discussion
The use of prophylactic antibiotics, body exhaust systems, laminar airflow, and other precautions have helped reduce the incidence of clinically recognized periprosthetic infection from nearly 10% in the early years of arthroplasty to less than 1% in some series (Fitzgerald Citation1992, Peersman et al. Citation2001). Despite this decline, periprosthetic infection is still one of the most challenging complications of total joint arthroplasty.
Addition of antibiotics to methylmethacrylate cement with demonstrable elution over a period of time has been shown to be very effective in the treatment of established periprosthetic infections (Josefsson et al. Citation1990, Josefsson and Kolmert Citation1993, Espehaug et al. Citation1997). The role of antibiotic cement in prevention of periprosthetic infection remains unclear, however (Trippel Citation1986). One of the major problems in evaluating the influence of any factor such as the addition of antibiotics to methylmethacrylate relates to the low incidence of periprosthetic infection. In order to confirm or refute the beneficial effects of antibiotic‐impregnated cement on the incidence of periprosthetic infection, a large number of patients must be recruited into any such study.A previous observational study from the Nor- wegian registry has suggested that the incidence of this complication may be reduced with the routine use of antibiotic cement (Espehaug et al. Citation1997). In the literature, there is a paucity of randomized controlled studies evaluating the role of antibiotic cement in elective total joint arthroplasty.
With the recent Federal Drug Administration approval, one brand of antibiotic cement—namely Simplex P containing tobramycin (Stryker, Mahwah, NJ)—has become commercially available for use in the United States. Although the strict indication for the use of this product is during re‐implantation surgery for periprosthetic infection, a large number of surgeons have begun to employ the product for off‐label use during uninfected primary and revision arthroplasty. It is not known whether the use of antibiotic cement does indeed confer any benefits in reducing the incidence of periprosthetic infection following elective uninfected total joint arthroplasty. Furthermore, it is not known whether the routine use of antibiotic could have deleterious effects, particularly related to longevity of the arthroplasty. The lack of consensus on the latter issues provided the impetus for our meta‐analysis to investigate the effect of antibiotic‐impregnated cement on the rate of periprosthetic infection. Our analysis of the available comparative studies has shown a clear benefit of antibiotic cement preparations in primary total hip arthroplasty, with a statistically significant reduction in the rate of periprosthetic infection following primary total hip arthroplasty. Furthermore, the meta‐analysis revealed that the incidence of revision total hip arthroplasty following a primary hip replacement was also significantly lower when antibiotic cement was used. This perhaps reflects the lower incidence of revisions that may have occurred for treatment of proven or suspected infections. It is also possible that some of the so‐called “aseptic” failures are in fact due to unrecognized occult infections or to the presence of endotoxins (Chimento et al. Citation1996, Fehring and Cohen Citation1996, Greenfield et al. Citation2005). The use of antibiotic cement could possibly have reduced the incidence of failures occurring secondary to unrecognized infection.
Our study did, however, have all the limitations inherent in any meta‐analysis. As is the case with many meta‐analyses, the greatest difficulty faced was the limited number of published controlled and comparative studies eligible for inclusion. There were few randomized controlled trials; an overwhelming majority of the studies were retrospective reviews lacking the comparative data required to perform a clinically useful evaluation. We found only 6 comparative studies that examined the use of prophylactic antibiotic cement in primary total hip replacements and 2 retrospective studies relating to outcomes in revision total hip arthroplasty with use of antibiotic cement.
Several reports have shown the benefit of antibiotic‐impregnated cement in direct‐exchange and two‐stage revision arthroplasty (Weber and Laut‐enbach Citation1986, Sanzen et al. Citation1988, Marti et al. Citation1990, Younger et al. Citation1997, Koo et al. Citation2001). However, the lack of well‐designed comparative studies with adequate statistical power precludes drawing any clear conclusions about the use of antibiotic cement in uninfected revision total hip arthroplasty. When industrially manufactured cement has not been available, surgeons have used bone cement mixed intraoperatively with heat‐stable antibiotics at concentrations left to the discretion of the particular surgeon. This factor, in conjunction with the use of different antibiotics and different cements, may inherently lead to varied results in any application—thus making comparative studies difficult. Our analysis has again highlighted the benefits of large‐scale prospectively recorded total hip arthroplasty registries.
We found a reduction in infection and overall revision rates for primary total hip arthroplasty when antibiotic‐impregnated cement was used. This finding lends weight to the argument of surgeons advocating routine use of commercially available antibiotic‐impregnated cement. We believe that the evidence in favor of antibiotic‐impregnated cement in primary cemented hip replacement is compelling.
Contributions of authors
JP: main investigator, first and final draft. KJS, PSR, MAM: co‐investigators, first draft; AEP: co‐investigator, data collection, first draft, final draft.
- Bourne R B. Antibiotic bone cement approval: fuss-in at the Feds! Orthopedics. 2002; 25: 913–4
- Carlsson A S, Egund N, Gentz C F, Hussenius A, Josefs-son G, Lindberg L. Radiographic loosening after revision with gentamicin-containing cement for deep infection in total hip arthroplasties. Clin Orthop. 1985; 194: 271–9
- Chimento G F, Finger S, Barrack R L. Gram stain detection of infection during revision arthroplasty. J Bone Joint Surg (Br) 1996; 78: 838–9
- Davies J P, O'Connor D O, Burke D W, Harris W H. Influence of antibiotic impregnation on the fatigue life of Simplex P and Palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res 1989; 23: 379–97
- Espehaug B, Engesaeter L B, Vollset S E, Havelin L I, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg (Br) 1997; 79: 590–5
- Fehring T K, Cohen B. Aspiration as a guide to sepsis in revision total hip arthroplasty. J Arthroplasty 1996; 11: 543–49
- Fish D N, Hoffman H M, Danziger L H. Antibiotic-impregnated cement use in U.S. hospitals. Am. J Hosp Pharm 1992; 49: 2469–74
- Fitzgerald R H, Jr. Total hip arthroplasty sepsis. Prevention and diagnosis. Orthop Clin North Am. 1992; 23: 259–64
- Garvin K L, Evans B G, Salvati E A, Brause B D. Palacos gentamicin for the treatment of deep periprosthetic hip infections. Clin Orthop. 1994; 298: 97–105
- Greenfield E M, Bi Y, Ragab A A, Goldberg V M, Nalepka J L, Seabold J M. Does endotoxin contribute to aseptic loosening of orthopedic implants?. J Biomed Mater Res B Appl Biomater 2005; 72: 179–85
- Havelin L I, Espehaug B, Vollset S E, Engesaeter L B. The effect of the type of cement on early revision of Charnley total hip prostheses. A review of eight thousand five hundred and seventy-nine primary arthroplasties from the Norwegian Arthroplasty Register. J Bone Joint Surg (Am) 1995; 77: 1543–50
- Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty 1995; 10: 470–5
- Hope P G, Kristinsson K G, Norman P, Elson R A. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg (Br) 1989; 71: 851–5
- Jiranek W A. Antibiotic loaded cement in aseptic joint replacement: whys, wherefores and caveats. Hanssen A D and Greenwald A S. American Association of Orthopaedic Surgeons, Committee on Biological Implants. 200
- Josefsson G, Kolmert L. Prophylaxis with systematic antibiotics versus gentamicin bone cement in total hip arthroplasty. A ten-year survey of 1,688 hips. Clin Orthop. 1993; 292: 210–4
- Josefsson G, Gudmundsson G, Kolmert L, Wijkstrom S. Prophylaxis with systemic antibiotics versus gentamicin bone cement in total hip arthroplasty. A five-year survey of 1688 hips. Clin Orthop. 1990; 253: 173–8
- Koo K H, Yang J W, Cho S H, Song H R, Park H B, Ha Y C, Chang J D, Kim S Y, Kim Y H. Impregnation of vanco-mycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty 2001; 16: 882–92
- Lynch M, Esser M P, Shelley P, Wroblewski B M. Deep infection in Charnley low-friction arthroplasty. Comparison of plain and gentamicin-loaded cement. J Bone Joint Surg (Br) 1987; 69: 355–60
- Marti R K, Schuller H M, Besselaar P P, Vanfrank Haasnoot E L. Results of revision of hip arthroplasty with cement. A five to fourteen-year follow-up study. J Bone Joint Surg (Am) 1990; 72: 346–54
- Moran J M, Greenwald A S, Matejczyk M B. Effect of gentamicin on shear and interface strengths of bone cement. Clin Orthop. 1979; 141: 96–101
- Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop. 2001; 392: 15–23
- Penner M J, Duncan C P, Masri B A. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty 1999; 14: 209–14
- Sanzen L, Carlsson A S, Josefsson G, Lindberg L T. Revision operations on infected total hip arthroplasties. Two-to nine-year follow-up study. Clin Orthop. 1988; 229: 165–72
- Trippel S B. Antibiotic-impregnated cement in total joint arthroplasty. J Bone Joint Surg (Am) 1986; 68: 1297–302
- Ure J K, Amstutz H C, Nasser S, Schmalzried T P. Direct-exchange arthroplasty for the treatment of infection after total hip replacement. An average ten-year follow-up. J Bone Joint Surg (Am) 1998; 80: 961–8
- Wahlig H, Dingeldein E, Buchholz H W, Buchholz M, Bach-mann F. Pharmacokinetic study of gentamicin-loaded cement in total hip replacements. Comparative effects of varying dosage. J Bone Joint Surg (Br) 1984; 66: 175–9
- Weber F A, Lautenbach E E. Revision of infected total hip arthroplasty. Clin Orthop. 1986; 211: 108–15
- Younger A S, Duncan C P, Masri B A, McGraw R W. The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip. J Arthroplasty 1997; 12: 615–23
- Zimmerli W, Trampuz A, Ochsner P E. Prosthetic-joint infections. N Engl. J Med 2004; 351: 1645–54
