Abstract
Background and purpose Previous studies on shoulder arthroplasty have usually described small patient populations, and few articles have addressed the survival of shoulder implants. We describe the results of shoulder replacement in the Norwegian population (of 4.7 million) during a 12-year period. Trends in the use of shoulder arthroplasty during the study period were also investigated.
Patients and methods 1,531 hemiprostheses (HPs), 69 total shoulder replacements (Neer type TSR), and 225 reversed total shoulder replacement (reversed TSR) operations were reported to the Norwegian Arthroplasty Register between 1994 and 2005. Kaplan-Meier failure curves were drawn up for particular subgroups of patients, and revision rates were calculated using Cox regression analysis.
Results The 5- and 10-year failure rates of hemiprostheses were 6% (95% CI: 5–7) and 8% (95% CI: 6–10), and for reversed total shoulder replacements they were 10% (95% CI: 5–15) and 22% (95% CI: 10–33), respectively. For hemiprostheses, the risk of revision for patients who were 70 years or older was half that of those who were younger (RR = 0.47, CI: 0.28–0.77), while the risk of revision was highest for patients with sequelae after fracture compared to those with acute fractures (RR = 3.3, CI: 1.5–7.2). No differences in prosthesis survival were found between the different hemiprosthesis brands. The main reasons for revision of hemiprostheses were pain and luxation. For reversed total prostheses, the risk of revision was less for women than for men (RR = 0.26, CI: 0.11–0.63), and the main cause of revision was aseptic loosening of the glenoid component. During the study period, the incidence of shoulder arthroplasty increased for all diagnostic groups except inflammatory arthritis, for which a decrease was seen.
Interpretation We found good results in terms of 5-year prosthesis failure rate, with the use of hemiarthroplasty for patients with inflammatory arthritis, osteoarthritis, and acute fractures. Reversed total shoulder replacement was associated with a rather poor prognosis.
Shoulder replacement surgery was introduced as a treatment method for 3- or 4-part proximal humerus fractures by Neer in the 1950s (Neer et al. 1953). During the 1970s, the use of shoulder arthroplasty was extended to include patients with osteoarthritis (Neer 1974), and this treatment method was eventually used with success in other diagnostic groups such as inflammatory arthritis.
Loosening of the glenoid component has been shown to be a major problem in total shoulder arthroplasty (Cofield and Edgerton 1990, Torchia et al. 1997, Bohsali et al. 2006). However, pain due to glenoid arthritis has been described after hemiarthroplasty (Parsons et al. 2004, Haines et al. 2006). Previous studies on revision after shoulder arthroplasty have usually included small samples, and they have rarely compared survival between groups. In a study of total shoulder arthroplasty, the survival rates were 95% at 5 years and 85% at 10 years (Martin et al. 2005). Haines et al. (2006) found different survival rates according to the primary diagnosis, the 10-year survival being 86% for patients with osteoarthritis and 33% for posttraumatic arthritis. In proximal humerus fractures, survival of the prosthesis has been reported to be 83–94% at 10 years (Robinson et al. 2003, Kwon and Zuckerman 2005), and a study of patients with rheumatoid arthritis reported prosthesis survival at 8 years to be 92% (Trail and Nuttall 2002).
We evaluated prosthesis failure after shoulder replacement in a large national series of patients, and we investigated the effect of prosthesis type and shoulder disease on the outcome. Furthermore, we assessed trends in the use of shoulder arthroplasty over a 12-year period.
Patients and methods
The Norwegian Arthroplasty Register (NAR) was established in 1987, initially as a hip prosthesis register. In January 1994, it was extended to include all arepsicial joints (Havelin 1999). Individual reports of joint replacements were received from all the hospitals in Norway that were performing arthroplasty surgery. Data concerning identity of the patient, diagnosis, date of surgery, type of prosthesis, whether cement was used, whether the operation was primary or a revision, cause of revision, and procedure at revision were taken from the form filled in by the operating surgeon (Furnes et al. 2002). The completeness of registration was evaluated in a recent study and 89% of all primary shoulder arthroplasty operations were reported to the NAR (Espehaug et al. 2006). In the present study involving 1,825 shoulder arthroplasties, the main outcome variable was whether or not a revision was performed after primary surgery. No information on function or patient satisfaction was registered.
From 1994 through 2005, 1,531 hemiprosthesis procedures and 294 total shoulder replacement procedures were reported to the NAR. A reversed total prosthesis was used in 225 cases and a Neer-type total prosthesis was used in 69 cases. Mean age of the patients operated with a reversed prosthesis was 69 years, which was practically the same as in the total material (). There were 1,428 women and 397 men, and the mean age at surgery was significantly higher in women (70 years for women as opposed to 64 years for men) (p < 0.001).
Table 1. General characteristics and diseases leading to shoulder replacement
During the study period, 13 different brands of shoulder prostheses were used (). The most commonly used type, the “Neer-type”, consisted of a stem and a caput and, for total shoulder replacements, a glenoid component. They were inserted with or without cement. The Delta I prosthesis differs from the others in having a hydroxyapatite-coated surface.
Table 2. Prosthesis brands
The Delta III total shoulder replacement (DePuy International Ltd. Leeds, England) represents a separate entity using the reversed design with a glenoid head and humeral cup. The Copeland prostheses (Biomet Merck Limited, South Wales, UK) as well as 42 of the Scan prostheses were resurfacing prostheses.
132 patients were given shoulder replacements bilaterally during the study period. In these patients, each replacement procedure was considered a separate case. A revision (or failure) was defined as the removal or exchange of part of or the whole implant. The follow-up time was the time from primary shoulder replacement until revision, or until the end of the study or death. The date of death for the patients was obtained from Statistics Norway (www.ssb.no/english/). Median follow-up time was 4.0 years (range: 2 days to 12 years).
Major causes of surgery were rheumatoid arthritis (RA), osteoarthritis (OA), acute fracture, and fracture sequelae (). Only 4 patients with acute fractures had a TSR, while TSR was used more commonly for the other major diagnostic groups.
In some analyses (Table 4, and ), rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis were grouped together and called inflammatory arthritis (IA). Several causes of revision could be given for the same patient (). For the purpose of this study, however, pain was only registered as the cause of revision in cases where this was the only recorded cause.
Statistics
The Student t-test was used to compare continuous variables. The significance level was set at 5% and all p-values were two-tailed. Median follow-up (observation) time was calculated using the reverse Kaplan-Meier method. Kaplan-Meier survival tables were used to find 5- and 10-year failure rates, calculated as 1 minus the survival rate. Failure curves based on the Kaplan-Meier method with revision for any reason as endpoint were given for several subgroups of patients. The failure curves were discontinued when the number of patients at risk was less than 20. In failure curves for different brands of hemiprosthesis, only brands that had been used in at least 30 shoulders were included in the analyses (). The curve for Global Fx was not shown because the follow-up time was short (median 1.3 years).
Cox multiple regression analysis was used to calculate relative risk (RR) of revision according to shoulder disease, age, sex, and year of primary operation. Separate analyses were done for hemiprostheses and reversed total shoulder replacements (). All relative risks were adjusted for the other variables in the analysis. A Cox regression analysis was also done to compare resurfacing hemiprostheses to other hemiprostheses. The analysis is not shown, but the p-value given in the text was obtained from this analysis.
For revision due to specific causes (such as aseptic loosening or infection), the number of events was small with occasional zeros (Table 6), which is why models for exact Cox regression were set up. The statistical program LogXact (Cytel Inc., Cambridge, MA) was used according to Samuelsen (2003). The models were adjusted for shoulder disease, cement, sex, and age.
Poisson regression analysis was used to analyze trends in the incidence of shoulder arthroplasties for all shoulder arthroplasties and for HP and TSR separately (), and for the 4 major diagnostic groups (). These analyses were performed based on annual population rates for the Norwegian population obtained from Statistics Norway. The p-values given in and in the legend to were derived from these Poisson analyses. All analyses, except for the exact regression analyses (LogXact), were performed using SPSS software version 13.0.
Figure 1. Shoulder arthroplasties in 4 major diagnostic groups from 1994 through 2005. IA: inflammatory arthritis; OA: osteoarthritis.
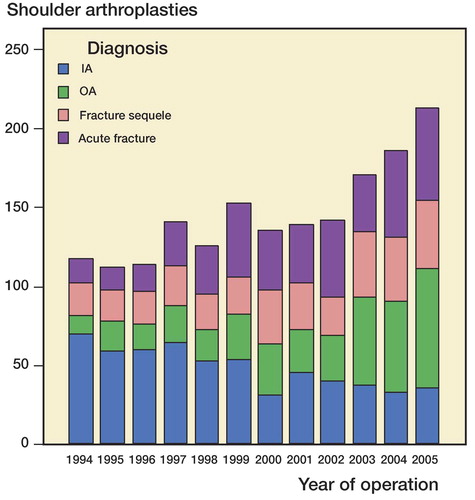
Table 3. Primary shoulder arthroplasties per annum. Overall incidence (per 100,000 inhabitants), total annual number, and annual number according to prosthesis type
Results
Time trends
An increase in the overall incidence of shoulder arthroplasties took place during the 12-year study period, and this trend was seen for HP but not for TSR (p < 0.001 and p = 0.3) (). The increasing trend was seen in all the major diagnostic groups except inflammatory arthritis, for which the trend was opposite (p < 0.001) ().
Operation volume
The shoulder replacements were performed at 54 hospitals. On average, 4 procedures were done per hospital per year, and only 2 hospitals had 10 or more procedures annually. The mean number of HP operations performed per hospital during the study period was 28 (1–176) while for reversed TSR the mean was 13 (1–74) procedures per hospital that performed TSR (p = 0.01). 7 HP brands and 1 TSR brand had a relatively high volume in at least one hospital (> 30 cases during the study period) ().
Revision procedures
The procedures performed at revision after TSR were exchange of parts of or the whole prosthesis in 22 patients, and removal of prosthetic parts without replacement in 14 cases. In 2 cases, the procedure performed during revision was not reported. In patients with HP, removal of prosthesis or prosthesis components was performed in 11 cases, while in 61 patients the whole prosthesis or parts were exchanged. The procedure was not reported in 3 cases with HP.
Revision after hemiarthroplasty
The cumulative 5- and 10-year failure rates for HP were 6% and 8% (). When analyzing resurfacing hemiprostheses separately, there was no statistically significant difference between these prostheses and other HPs (p = 0.9) (). No statistically significant difference in failure rate was found between the different brands of hemiprosthesis (). Shoulder disease did, however, influence the failure rate, and for hemiprostheses the failure rate was lowest for patients with an acute fracture and highest after fracture sequelae (p = 0.01) (). Cox regression analysis showed that the relative risk of revision was 3.3 (CI: 1.5–7.2) for patients with sequelae after fractures compared to those with acute fractures ().
Figure 2. Kaplan-Meier failure curve (with 95% CI shown in color) for all hemiprostheses, resurfacing hemiprostheses, reversed total shoulder replacement, and Neer-type total shoulder replacement.
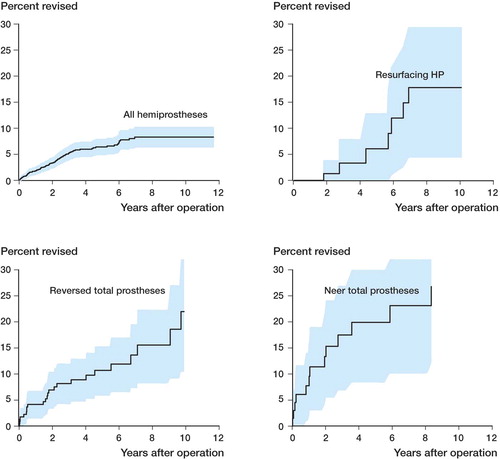
Figure 3. A. Kaplan-Meier failure curves for different hemiprosthesis brands. Groups with less than 20 patients were excluded. B. Kaplan-Meier failure curves according to shoulder disease.
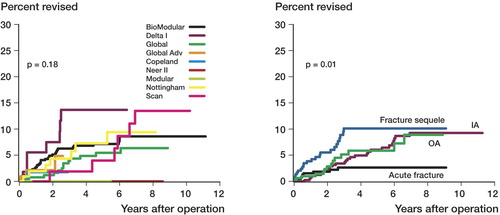
Table 4a. Cox regression analysis for revision after hemiarthroplasty, according to potential explanatory factors. 5- and 10-year failure rates estimated using the Kaplan-Meier method
The median follow-up time was longer for patients with IA (6.1 years) than for those with OA (2.9 years), acute fracture (3.3 years), and sequelae after fracture (3.5 years) (p < 0.001).
Furthermore, patients who were 70 years old or more had half the risk of revision compared to those who were younger (RR = 0.47, CI: 0.28–0.77) (), while the risk of revision was independent of gender and year of operation. Cementation of the humeral component had no influence on revision rate (RR = 1.1, CI = 0.67–1.8) (not presented in the table).
Revision after total shoulder arthroplasty
The 5- and 10-year failure rates for the reversed TSR were 10% and 22% (). The failure curve for Neer-type TSR shows 5- and 10-year revision rates of 20% and 27% (). The Cox regression analysis did not reveal any difference in revision rates between the 3 major diagnostic groups for reversed TSR (). Women had a lower risk of revision than men (RR = 0.26, CI: 0.11–0.63) ().
Table 4b. Cox regression survival analysis for revision after reversed TSR, according to potential explanatory factors. 5- and 10-year failure rates estimated using the Kaplan-Meier method
Causes of revision
The main cause of revision after TSR was aseptic loosening of the glenoid component (). For hemiprostheses, the major cause of revision was pain, seen in 15 of 439 cases with RA but in none of the 422 cases with acute fractures. Altogether, this was given as the only cause of revision in 32 cases. Other major causes of revision are listed in .
Table 5. Causes of revision, according to type of prosthesis
For hemiprostheses, revision due to pain was less frequent in patients with acute fractures (RR = 0.12, p = 0.02) and in patients > 70 years of age (RR = 0.25, p = 0.005) (). In HP patients with sequelae after fractures, revision due to instability and luxation was more common (RR = 11, p = 0.04 and RR = 8.8, p = 0.04, respectively) ().
Table 6a. Risk of revision due to specific causes, for hemiprostheses (HPs), according to shoulder disease and fixation. The results were obtained using an exact regression model
For reversed TSR, women had a smaller risk of revision due to deep infections than men (RR = 0.006, p = 0.01) (). Except for this, no statistically significant differences in revision due to the causes given in Table 6, were found between patients with different shoulder diseases and fixation methods.
Table 6b. Risk of revision due to specific causes, after reversed TSR, according to shoulder disease and fixation. The results were obtained using an exact regression model
Discussion
Our patient series is among the largest in studies on hemiprostheses of the shoulder. The major finding was that the survival after insertion of a hemiprosthesis was satisfactory, with 6% and 8% revision within 5 and 10 years, respectively. For the reversed TSR, however, the results were more disappointing with more than 20% revision at 10 years.
The age and sex distribution was similar to that in some other large series (Jain et al. 2004, Sharma and Dreghorn 2006). In our study, there were 84% hemiprostheses, which is similar to the findings from the Scottish and Swedish registries (88% and 87%, respectively) (Rahme et al. 2001, Sharma and Dreghorn 2006). Ravenscroft and Calvert (2004) reported twice as many HPs as TSRs in the UK, but there were more TSRs in a large American study (Jain et al. 2004).
Time trends
There was an increase in the overall incidence of shoulder arthroplasty during the 12-year period. A similar trend was recently described in a review by Bohsali et al. (2006). In the Norwegian population, the increased incidence only applied to hemiprostheses while no significant change in the incidence of total shoulder replacement (TSR) was detected. Jain et al., investigating time trends for TSR in the United States, found a minor increase in the use of TSR during the period 1990–2000 (Jain et al. 2006).
We found an increase in the use of shoulder replacement for patients with osteoarthritis, fracture sequelae, and acute fractures, but the opposite trend was seen for patients with IA. This corresponds well with the trend in the US population reported by Jain et al. (2006), showing an increase in the use of TSR due to osteoarthritis and a minor decline for rheumatoid arthritis. The findings correspond well with the general trend towards reduced need for surgical procedures in patients with rheumatic diseases (Fevang et al. 2007).
Operation volume
In a recent study of knee replacements, the revision rate was found to be lower at hospitals having a high volume of operations (Furnes et al. 2007). Jain et al. (2004) found better outcome in terms of in-hospital mortality and complications, length of stay, and disposition of patients on discharge, after shoulder arthroplasty performed in high-volume hospitals. In our study, none of the hospitals had a high volume of shoulder surgery, and the procedures at the “high-volume hospitals” may have been performed by several different surgeons. Still, the average number of procedures was lower for the reversed TSRs and this may have contributed to the inferior results.
Revision after hemiarthroplasty
The overall 5-year failure rate for hemiprostheses (6%) in our study is acceptable; it is similar to the 4% overall 5-year failure rate for knee prostheses (Havelin et al. 2000) and 4% for hip prostheses (in patients with osteoarthritis) (Furnes et al. 2001). However, the threshold for revising a hip prosthesis may be different from that for shoulder prostheses. We have no information concerning the number of patients suffering from pain without being revised. Consequently, comparison of failure rates for different joints is difficult. Use of revision as outcome factor does, however, allow comparison of results for subgroups of patients with arthroplasties of the same joint.
The disease leading to the hemiarthroplasty influenced the outcome. Patients who were operated due to sequelae after a previous fracture had worse prognosis than patients with IA and acute fractures. Haines et al. (2006) also found the highest revision rate following shoulder arthroplasty in patients with posttraumatic arthritis. Sperling et al. (2007) reported a 5-year survival rate of 90% in 108 patients with RA who had a hemiarthroplasty, and Trail and Nuttall (2002) reported an 8-year survival of 92% in a study of 105 patients with rheumatoid arthritis who were treated with HP or TSR. Robinson et al. (2003) reported 94% prosthesis survival at 10 years in 163 patients treated with hemiarthroplasty due to proximal humeral fractures.
We found no statistically significant difference in failure rates for the different brands of hemiprostheses. Different brands would be best compared in randomized studies, but as far as we know, no such studies have been published. Register studies, however, have been proven useful in pointing out inferior brands of prosthesis or cement, due to the long observation time and large number of patients (Furnes et al. 1997, Espehaug et al. 2002). Thus, we may conclude that no particular brand of prosthesis had markedly inferior or superior results.
We found that the risk of revision was dependent on age. The failure rate was doubled in patients younger than 70 years, compared to those who were older. This has been shown previously for patients with hip and knee prostheses (Havelin et al. 1994, 2000), but not in shoulder arthroplasty.
Revision after total shoulder arthroplasty
Previous studies on TSR have reported 10-year survival rates of between 93% and 97% (Torchia et al. 1997, Sperling et al. 2004, 2007, Deshmukh et al. 2005), which is much better than the 78% for reversed TSR in our study. Only Neer prostheses were used in these studies, as opposed to the reversed TSRs in our study. The survival results were, however, no better for the 69 Neer-type TSRs in our study. In a study of reverse total shoulder replacements, the 5-year survival was 91%, with replacement of the prosthesis as endpoint (Guery et al. 2006). These results are similar to ours, in which the 5-year survival for reversed total prostheses was 90%. The failure rate was higher for men than for women. A similar finding has been reported previously for total hip replacement (Havelin et al. 1994).
The indication for choosing one or other prosthesis is unknown in register studies, but as hemiarthroplasty was “the treatment of choice”, we cannot exclude the possibility that it was used in most “uncomplicated” cases—possibly contributing to the superior results with HP compared to TSR. Furthermore, the volume of TSR surgery at each hospital was lower than for HP. Surgeons’ experience in performing TSR is most probably less, and this may contribute to the inferior results for TSR. The use of reversed TSR is recommended for older patients, preferably 70 years or older (Matsen et al. 2007). In our study, the mean age at surgery was the same for reversed TSRs as for HPs (69 years). The use of reversed TSR in younger patients may, to some degree, have influenced the results adversely.
Cause of revision
Glenoid loosening was the most common cause of revision in patients with reversed TSR (). This corresponds well with that reported in other studies of reversed TSR (Nwakama et al. 2000, Rittmeister and Kerschbaumer 2001, Sirveaux et al. 2004, Bohsali et al. 2006). In our study, the major cause of revision after insertion of hemiprostheses was pain. This was seen in 15 of 439 cases with RA, while none of the 422 cases with acute fracture had a revision due to pain. The reason for this is most likely that patients with IA often have some involvement of the glenoid, which may cause pain after insertion of a hemiprosthesis. The opposite is seen with acute proximal humeral fractures in which only the humerus is involved and the glenoid is unaffected.
Strength and weaknesses of the study
One weakness of our study is that the use of different types and brands of prostheses might not have been randomly assigned. We have, however, adjusted for known possible confounding factors such as age, sex, year of surgery, and diagnosis, using regression analysis. Comparison of different subgroups should be made with caution, knowing that unknown factors may have influenced the results. The register has no data on function after shoulder arthroplasty. Thus, a follow-up study of postoperative function and pain in our patients is planned.
The use of registry data provides some advantages, in that we have almost complete data over a particular time period for a whole country, which represents a large study population. In addition, the results were obtained from all types of institutions—small and large—for all surgeons and for all diagnoses. Most other studies present the results of a single institution and often of a few specialized surgeons using one or a small number of prosthesis brands. The results of our study may be more easily generalized to the average hospital and surgeon. In register studies, revision is used as the outcome factor after joint replacement surgery. However, patients may suffer from pain but may still not have their implant replaced due to known difficulties during replacement surgery. Such situations may be different for different joints (i.e. hips versus shoulders), and also in patients with different diseases. Even so, a close relationship between rate of revision and the rate of radiographic failure has been shown previously (Fender et al. 1999), supporting the use of registry data (with revision as the outcome factor) for the surveillance of joint replacement surgery.
In summary, the failure rate after insertion of primary hemiprostheses was low. Patients with sequelae after fracture had a significantly higher risk of revision after hemiarthroplasty than patients with acute fracture or inflammatory arthritis. This might advocate more frequent use of HP for acute fractures, possibly reducing the need for arthroplasty due to sequelae after fractures. Reversed TSR was used in patients younger than 70 years of age, but this could not fully explain the relatively disappointing results with this type of prosthesis.
Acknowledgements
All authors contributed to the conception and design of the study and to preparation of the manuscript. In addition, individual authors contributed with the following. BTF: data processing, statistics, and writing of the manuscript. SAL: data processing and statistics. LIH, AS, and ONF: data collection and interpretation.
We thank the Norwegian surgeons for providing data for the register.
No competing interests declared.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty.”. J Bone Joint Surg (Am) 2006; 88(10)2279–92
- Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect 1990; 39: 449–62
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg 2005; 14: 5–471
- Espehaug B, Furnes O, Havelin LI, Engesaeter LB, Vollset SE. The type of cement and failure of total hip replacements. J Bone Joint Surg (Br) 2002; 84: 6–832
- Espehaug B, Furnes O, Havelin LI, Engesaeter LB, Vollset SE, Kindseth O. Registration completeness in the Norwegian Arthroplasty Register. Acta Orthop 2006; 77(1)49–56
- Fender D, Harper WM, Gregg PJ. Outcome of Charnley total hip replacement across a single health region in England: the results at five years from a regional hip register. J Bone Joint Surg (Br) 1999; 81(4)577–81
- Fevang BT, Lie SA, Havelin LI, Engesaeter LB, Furnes O. Reduction in orthopedic surgery among patients with chronic inflammatory joint disease in Norway, 1994-2004. Arthritis Rheum 2007; 57(3)529–32
- Furnes O, Lie SA, Havelin LI, Vollset SE, Engesaeter LB. Exeter and charnley arthroplasties with Boneloc or high viscosity cement. Comparison of 1,127 arthroplasties followed for 5 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 1997; 68(6)515–20
- Furnes O, Lie SA, Espehaug B, Vollset SE, Engesaeter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg (Br) 2001; 83(4)579–86
- Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Early failures among 7,174 primary total knee replacements: a follow-up study from the Norwegian Arthroplasty Register 1994-2000. Acta Orthop Scand 2002; 73(2)117–29
- Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Failure mechanisms after unicompartmental and tricompartmental primary knee replacement with cement. J Bone Joint Surg (Am) 2007; 89(3)519–25
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg (Am) 2006; 88(8)1742–7
- Haines JF, Trail IA, Nuttall D, Birch A, Barrow A. The results of arthroplasty in osteoarthritis of the shoulder. J Bone Joint Surg (Br) 2006; 88(4)496–501
- Havelin LI. The Norwegian Joint Registry. Bull Hosp Jt Dis 1999; 58(3)139–47
- Havelin LI, Espehaug B, Vollset SE, Engesaeter LB. Early failures among 14,009 cemented and 1,326 uncemented prostheses for primary coxarthrosis. The Norwegian Arthroplasty Register, 1987-1992. Acta Orthop Scand 1994; 65(1)1–6
- Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand 2000; 71(4)337–53
- Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg (Am) 2004; 86(3)496–505
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum 2006; 55(4)591–7
- Kwon YW, Zuckerman JD. Outcome after treatment of proximal humeral fractures with humeral head replacement. Instr Course Lect 2005; 54: 363–9
- Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg (Am) 2005; 87(6)1284–92
- Matsen FA, 3rd, Boileau P, Walch G, Gerber C, Bicknell RT. The reverse total shoulder arthroplasty. J Bone Joint Surg (Am) 2007; 89(3)660–7
- Neer CS, 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg (Am) 1974; 56(1)1–13
- Neer CS, Brown TH, Jr, McLaughlin HL. Fracture of the neck of the humerus with dislocation of the head fragment. Am J Surg 1953; 85(3)252–8
- Nwakama AC, Cofield RH, Kavanagh BF, Loehr JF. Semiconstrained total shoulder arthroplasty for glenohumeral arthritis and massive rotator cuff tearing. J Shoulder Elbow Surg 2000; 9(4)302–7
- Parsons IMT, Millett PJ, Warner JJ. Glenoid wear after shoulder hemiarthroplasty: quantitative radiographic analysis. Clin Orthop 2004, 421: 120–5
- Rahme H, Jacobsen MB, Salomonsson B. The Swedish Elbow Arthroplasty Register and the Swedish Shoulder Arthroplasty Register: two new Swedish arthroplasty registers. Acta Orthop Scand 2001; 72(2)107–12
- Ravenscroft M, Calvert P. Utilisation of shoulder arthroplasty in the UK. Ann R Coll Surg Engl 2004; 86(1)25–8
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg 2001; 10(1)17–22
- Robinson CM, Page RS, Hill RM, Sanders DL, Court-brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg (Am) 2003; 85(7)1215–23
- Samuelsen SO. Exact inference in the proportional hazard model: possibilities and limitations. Lifetime Data Anal 2003; 9(3)239–60
- Sharma S, Dreghorn CR. Registry of shoulder arthroplasty - the Scottish experience. Ann R Coll Surg Engl 2006; 88(2)122–6
- Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg (Br) 2004; 86(3)388–95
- Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg 2004; 13(6)604–13
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: Results of 303 consecutive cases. J Shoulder Elbow Surg 2007; 16(6)683–90
- Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg 1997; 6(6)495–505
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg (Br) 2002; 84(8)1121–5
