Abstract
The unexpected high revision rates of large-diameter (femoral head sizes of 36 mm or greater) metal-on-metal hip arthroplasties (MoMHAs) have led to worldwide regulatory authorities recommending regular surveillance, even for asymptomatic individuals. However, these recommendations are not evidence-based and are very costly. The rapidly evolving evidence base requires an update regarding the investigation and management of MoMHA patients. This article is the first of 2 (the second article in this series will consider the threshold for performing revision, and the outcomes following ARMD revision surgery: Matharu et al., Revision surgery of metal-on-metal hip arthroplasties for adverse reactions to metal debris: A clinical update. Acta Orthop 2018; in press), and considers the various investigative modalities used during surveillance, with specific focus on blood metal ion sampling and cross-sectional imaging. No single investigation can universally be used during MoMHA patient surveillance. Recent studies have now provided important information on interpreting blood metal ions (effective in identifying patients at low risk of problems), clarifying the roles of cross-sectional imaging (reserve combined ultrasound and MARS-MRI for complex cases), and providing parameters to safely exclude many asymptomatic patients from regular surveillance. This information will be useful when designing future surveillance protocols for MoMHA patients
Many metal-on-metal hip arthroplasties (MoMHAs) with large femoral head diameters (36 mm or greater) have been implanted worldwide, either as hip resurfacing arthroplasty (HRA) or as total hip arthroplasty (THA) (Bozic et al. Citation2009, NJR Citation2016). In recent years high short-term failure rates have been reported for most large-diameter MoMHA designs, which has led to an almost worldwide cessation of their use (Smith et al. Citation2012a, Citation2012b, AOANJRR Citation2016, NJR Citation2016). Many MoMHA revisions have been performed for adverse reactions to metal debris (ARMD) (Langton et al. Citation2010, Citation2011a) and pseudotumor (a specific type of ARMD, defined as a cystic, solid, or mixed mass communicating with the joint) (Pandit et al. Citation2008). ARMD is the sequela of metal debris released from the bearing surface and/or other THA modular implant junctions due to wear and corrosion (Kwon et al. Citation2010, Langton et al. Citation2011b, Matthies et al. Citation2011, Langton et al. Citation2012, Jacobs et al. Citation2014). This debris can result in destruction of the local bone and soft tissues, as well as large invasive pseudotumors, which often require revision surgery (Pandit et al. Citation2008, Grammatopoulos et al. Citation2009, Langton et al. Citation2010).
To identify ARMD early, regulatory authorities worldwide have published follow-up guidance for MoMHA patients (MHRA Citation2012, FDA Citation2013, Hannemann et al. Citation2013). Patients with ARMD may be asymptomatic which can make diagnosis difficult (Hart et al. Citation2012, Fehring et al. Citation2014), therefore currently most MoMHA patients are regularly followed up for life. The main investigations used in surveillance are blood metal ions and cross-sectional imaging (ultrasound or metal artefact reduction sequence magnetic resonance imaging (MARS-MRI)) (MHRA Citation2012, FDA Citation2013, Hannemann et al. Citation2013). However, a recent review of MoMHA follow-up protocols issued by 5 worldwide authorities demonstrated that the guidance was extremely variable between authorities, not evidence-based and very costly (Matharu et al. Citation2015c). Furthermore, there are still a number of questions that need to be addressed regarding the specific roles of each investigative modality during surveillance (Matharu et al. Citation2015c).
This article is the first of 2 providing a clinical update on the investigation and management of MoMHA patients. The present article considers the various investigative modalities used during MoMHA surveillance, with specific focus on blood metal ion sampling and imaging. The second article considers the threshold for performing revision, and the outcomes following ARMD revision surgery (Acta Orthop 2018; in press).
Blood metal ions
Rationale, methods, and interpretation
Blood metal ion concentrations are a surrogate marker of in-vivo wear in MoMHAs, and therefore have been used to investigate patients (De Smet et al. Citation2008). Cobalt and chromium ions are measured, which constitute the primary elements of the MoM alloy. Wear (normal and excessive) and corrosion of MoMHAs causes release of both insoluble metal particles (found in the synovial fluid and periprosthetic tissues) and soluble metal ions (entering the bloodstream thus allowing measurement) (McMinn Citation2009).
Whole blood or serum samples are used for measuring these metal ion concentrations. Samples should be collected, stored, transported, and processed according to laboratory guidance. Analysis must be performed at an accredited laboratory with expertise in trace metal element analysis, with excellent accuracy of measurement and reproducibility reported (Harrington and Taylor Citation2012). Samples are analyzed using dynamic reaction cell or collision cell inductively coupled plasma mass spectrometry.
Serial samples should be sent to the same laboratory given that clinically significant variations can occur in blood samples from the same patients analyzed at different laboratories (Rahme et al. Citation2014). To prevent incorrect interpretation of serial results the same blood fraction must be used. Whole blood is preferred given that serum requires further preparation (centrifuging), which increases the risk of contamination (Daniel et al. Citation2007). Serum cobalt and chromium are up to 1.4 times greater than their respective values in whole blood, with stronger correlation of cobalt in serum and whole blood compared with that of chromium (Vendittoli et al. Citation2007, Engh et al. Citation2014).
Advantages of blood metal ion sampling include being relatively inexpensive (£30 per test) (Lloyd et al. Citation2013) and simple to conduct. Disadvantages relate to potential patient (renal function, diet/medication/supplements containing trace metals, occupational exposure, other metal eluting implants) and laboratory (contamination, inter-laboratory, and intra-laboratory measurement variability) factors that can influence test interpretation (Daniel et al. Citation2010, FDA Citation2013, Rahme et al. Citation2014). Therefore, management decisions should never be solely based on 1 blood test.
Blood metal ions in healthy controls and well-functioning hips
97% of healthy subjects have both blood cobalt and chromium concentrations of 2 µg/L or less, with little variation observed between individuals (Sidaginamale et al. Citation2013). In well-functioning MoMHAs, metal ions can enter the bloodstream by day 5 following implantation (Daniel et al. Citation2007). Blood metal ion concentrations then increase significantly over the first year during the bearing running-in phase (Heisel et al. Citation2008). Concentrations then either stabilize or slowly decrease from 1 year postoperatively during medium-term follow-up (Daniel et al. Citation2007, Citation2009, Amstutz et al. Citation2013). However, some authors have also observed significantly decreased blood metal ion concentrations by 10 years (Bernstein et al. Citation2012, Van Der Straeten et al. Citation2013a, Citation2013b).
Factors influencing blood metal ion concentrations
High blood metal ion concentrations in MoMHA patients have been associated with poorly functioning implants (Hart et al. Citation2011a, Van Der Straeten et al. Citation2013a, Hart et al. Citation2014), including those specifically revised for ARMD (Langton et al. Citation2010, Sidaginamale et al. Citation2013, Lainiala et al. Citation2014b, Langton et al. Citation2016). Predictors of raised blood metal ion concentrations can be divided into patient (female sex, young age, time since implantation), implant (design, small femoral HRA components, large femoral MoM THA components, bilateral MoMHAs), and surgical factors (acetabular component malposition, reduced contact patch to rim distance) (De Haan et al. Citation2008, Langton et al. Citation2009, Grammatopoulos et al. Citation2010a, Bernstein et al. Citation2011, Hart et al. Citation2011b, Langton et al. Citation2011c, Lavigne et al. Citation2011, Chang et al. Citation2013, Van Der Straeten et al. Citation2013a, Emmanuel et al. Citation2014, Matthies et al. Citation2014, Matharu et al. Citation2015a, Citation2015b, Lainiala et al. Citation2016).
A recent large study involving 1,748 patients with different MoMHA implant designs observed that MoM THAs had significantly higher blood metal ion concentrations compared with HRA patients (Lainiala et al. Citation2016). This confirms the findings from 2 randomized controlled trials of MoM THAs and HRAs with identical bearing surfaces (Garbuz et al. Citation2010, Beaule et al. Citation2011), with the higher metal ion concentrations in MoM THAs likely to be related to the additional wear and corrosion occurring at the modular implant junction(s).
Blood metal ion thresholds for clinical use
There is presently no international consensus on the acceptable metal ion threshold(s) of concern in MoMHA patients. Since 2010, blood cobalt and/or chromium concentrations above 7 µg/L have been considered a cause for concern by some authorities (MHRA Citation2010, Canada Citation2012, MHRA Citation2012, Citation2015). Initial reports suggested that this arbitrarily selected threshold had good specificity (89%) but poor sensitivity (52%) for detecting unilateral failed MoMHAs, with the optimal threshold identified as a cobalt or chromium of 5.0 µg/L (86% specificity and 63% sensitivity) (Hart et al. Citation2011a). Others have reported similar findings (Malek et al. Citation2012). A subsequent study involving 597 unilateral MoMHAs identified 7 µg/L as having nearly optimal misclassification rates, although the authors suggested blood metal ions should not be used alone for screening for failed implants (Hart et al. Citation2014).
Thresholds for poorly functioning HRAs have been proposed by Van Der Straeten et al. (Citation2013a) (cobalt 4.0 µg/L and chromium 4.6 µg/L for unilateral HRA) and others (cobalt 4.5 µg/L) (Sidaginamale et al. Citation2013) with all cut-offs having higher specificity than sensitivity. Similar findings were observed in poorly functioning bilateral HRAs (Van Der Straeten et al. Citation2013a). Blood metal ion concentrations above 20 µg/L have been associated with osteolysis at ARMD revision, therefore it was recommended that revision should be considered in patients above this threshold (Langton et al. Citation2013). More recently a US consensus statement proposed risk-stratifying MoMHA patients to assist management with blood cobalt and chromium concentrations arbitrarily grouped into low risk (< 3 µg/L), moderate risk (3–10 µg/L), and high risk (> 10 µg/L) (Kwon et al. Citation2014).
2 large cohort studies involving 783 MoMHA patients recently devised implant-specific blood metal ion thresholds for unilateral Birmingham Hip Resurfacings (BHRs, Smith & Nephew, Warwick, UK: cobalt 2.15 µg/L), bilateral BHRs (maximum cobalt or chromium 5.5 µg/L), and unilateral Corail-Pinnacle THAs (DePuy, Leeds, UK: cobalt 3.57 µg/L) (Matharu et al. Citation2016a, Citation2016b). Patients with ion concentrations below the respective newly devised thresholds were at a low risk of having ARMD. However, these new thresholds were not effective for identifying patients who had ARMD. Currently proposed fixed regulatory authority thresholds from the UK MHRA and US surgeons missed more patients with ARMD compared with the newly devised thresholds (MHRA Citation2012, Kwon et al. Citation2014). The findings from these 2 studies were successfully validated in 710 patients from 3 different European centers (Matharu et al. Citation2017a). It was therefore recommended that the newly devised implant-specific blood metal ion thresholds should be used in preference to fixed regulatory authority thresholds when managing MoMHA patients.
Repeat blood metal ion sampling
After repeating blood metal ions within 1 year in 254 unilateral Articular Surface Replacement (ASR, DePuy) patients, 1 study observed that most HRAs had concentrations below published thresholds (Van Der Straeten et al. Citation2013a) on both tests, whilst one-third of MoM THAs with normal initial cobalt concentrations experienced significant increases on repeat testing (Reito et al. Citation2014b). Similar observations were reported in bilateral ASR patients undergoing repeat blood testing (Reito et al. Citation2016). Another study repeating blood metal ions during the short term in 205 patients with HRAs observed that cobalt concentrations did not significantly change with time since implantation (Langton et al. Citation2013).
Hip imaging
Radiographs
Conventional radiographs provide important information on component position, bone quality, and implant fixation, therefore they can identify signs suggestive of MoMHA failure early (Chen et al. Citation2011). However radiographs cannot directly diagnose ARMD or pseudotumors, given that these are predominantly soft-tissue lesions (Johnston et al. Citation2007, Toms et al. Citation2008), but they can identify associated abnormalities including component loosening, osteolysis, femoral neck narrowing, component malposition, fracture, subluxation/dislocation, and in HRAs femoral neck erosion due to impingement (Grammatopoulos et al. Citation2010a, Citation2010b, Chen et al. Citation2011, De Smet et al. Citation2011, Langton et al. Citation2011b, Kwon et al. Citation2014, Matharu et al. Citation2014). Furthermore, radiographs can identify other causes of hip pain, such as heterotopic ossification (HO) (Chen et al. Citation2011).
When assessing radiographs, it is important to compare serial images, including the immediate post-primary radiograph, for subtle signs of MoMHA failure. Medial calcar erosion represents a radiographic sign suggestive of ARMD in MoM THAs (Madanat et al. Citation2016). A recent case-control study involving 384 HRAs demonstrated that radiographic factors predictive of hips with evidence of a pseudotumor included acetabular component malposition (high inclination, and anteversion below 5 degrees), acetabular osteolysis, femoral osteolysis, acetabular loosening, and the absence of HO (Matharu et al. Citation2017b). However, it was recommended that radiographs should not be considered a substitute for performing blood metal ions and cross-sectional imaging, given that 20% of HRAs revised for pseudotumors had normal radiographs (Matharu et al. Citation2017b). It remains unclear whether femoral neck narrowing represents a normal physiological process following HRA or a clinically significant finding given that neck narrowing has been reported in both well-functioning patients (Hing et al. Citation2007, Coulter et al. Citation2012, Daniel et al. Citation2014) and ARMD revisions (Chen et al. Citation2011, Matharu et al. Citation2014).
Advantages of conventional radiographs when assessing MoMHAs include their wide availability, low cost, and ease of interpretation, with the main disadvantage being pelvic radiation exposure.
Ultrasound
Ultrasound has frequently been used as the initial imaging modality for investigating MoMHA patients (Fang et al. Citation2008, Douis et al. Citation2012, Nishii et al. Citation2012). Compared with MARS-MRI, the main advantages of ultrasound include being cheaper (ultrasound £49 vs. MARS-MRI £216) (Lloyd et al. Citation2013), faster to perform, not being affected by prosthetic artefacts, and being more accessible with fewer patient contraindications (can be used in patients with pacemakers or those with claustrophobia) (Siddiqui et al. Citation2014). Ultrasound also permits dynamic imaging, hip aspirations, and biopsies (Kwon et al. Citation2011, Siddiqui et al. Citation2014). The main disadvantages of ultrasound include the technique being operator dependent, and difficulties when assessing deeper structures or examining larger patients (Kwon et al. Citation2014, Siddiqui et al. Citation2014).
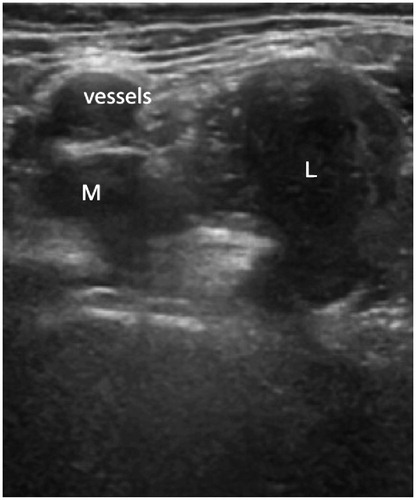
Ultrasound can identify numerous abnormalities around MoMHAs (Nishii et al. Citation2012), including pseudotumors of variable sizes, which are often cystic or solid and located anterior or posterolateral to MoMHAs (Figure) (Fang et al. Citation2008, Kwon et al. Citation2011, Williams et al. Citation2011, Nishii et al. Citation2012). A recent study of 82 MoMHAs undergoing revision concluded that ultrasound was effective for intraoperative detection of pseudotumors (Lainiala et al. Citation2015). Ultrasound has also been useful for detecting other ARMD pathology including joint effusions, iliopsoas and trochanteric bursal collections, capsular and bursal thickening, and synovitis (Douis et al. Citation2012, Nishii et al. Citation2012). Other abnormalities that ultrasound can identify, which may not always be related to ARMD, include muscle atrophy and tendon avulsions (Sofka et al. Citation2004, Garcia et al. Citation2010).
3 systems for classifying ARMD abnormalities on ultrasound have been proposed (Nishii et al. Citation2012, Siddiqui et al. Citation2013, Matharu et al. Citation2016c). It must be acknowledged that the prevalence of ARMD in MoMHAs on both ultrasound and MARS-MRI has been much higher (up to 61%) (Kwon et al. Citation2011, Williams et al. Citation2011, Hart et al. Citation2012, Fehring et al. Citation2014) than that confirmed at revision surgery (Langton et al. Citation2016, Matharu et al. Citation2016d, Citation2017c). As ARMD on imaging has now frequently been observed around well-functioning conventional THAs (Bisseling et al. Citation2015, van der Veen et al. Citation2015, Das et al. Citation2016), it is possible that current cross-sectional imaging classifications have over-diagnosed imaging ARMD in MoMHA patients (Anderson et al. Citation2011, Hart et al. Citation2012, Hauptfleisch et al. Citation2012, Nishii et al. Citation2012, Siddiqui et al. Citation2013, Matharu et al. Citation2016c).
Ultrasound has a role in identifying both the development and progression of ARMD. Repeat ultrasound in a small MoMHA cohort with asymptomatic ARMD on initial imaging demonstrated that most lesions increased in size in the short term with occasional remission of small masses (Almousa et al. Citation2013). By contrast, a recent study repeated ultrasounds in 10 HRAs with asymptomatic pseudotumors between 4.0 and 6.5 years later, with no changes occurring in pseudotumor volume or patient reported outcomes (Matharu et al. Citation2016f). This may suggest that some asymptomatic ARMD lesions can be safely left and only monitored with interval scanning.
A recent prospective longitudinal study of 152 asymptomatic HRAs demonstrated that 10% developed new pseudotumors on ultrasound within 5 years of initial assessment (Low et al. Citation2016). No asymptomatic patients with both a normal initial ultrasound and low blood metal ions (< 2 µg/L) developed new pseudotumors within 5 years of initial assessment (33% of hips studied). It was therefore concluded that these particular patients do not need regular follow-up. As large cohort studies have reported that 84% of all comprehensively investigated HRA patients were asymptomatic with well-functioning devices (Hart et al. Citation2014), it is estimated that 28% of all hip resurfacing patients would be asymptomatic with normal cross-sectional imaging and low blood metal ions. These findings have important clinical and financial implications, given that several protocols recommend intensive surveillance for asymptomatic HRA patients (Therapeutic Goods Administration Citation2012, Hannemann et al. Citation2013, MHRA Citation2015). By contrast asymptomatic HRA patients with normal initial ultrasounds alone but ions of 2 µg/L or above, and those asymptomatic HRA patients with low blood metal ions but abnormal imaging both still had a small, but potentially clinically significant, risk of developing new pseudotumors within 5 years of initial assessment (Low et al. Citation2016). Therefore it was recommended that this patient subgroup required some form of surveillance within 5 years of initial assessment.
MARS-MRI
Most centers use MARS-MRI as the initial cross-sectional imaging modality for investigating MoMHAs, which is likely to be increasingly used as techniques become refined (Kwon et al. Citation2014). MARS-MRI is not operator dependent, it provides excellent visualization of soft-tissue structures including deeper tissues, and images can be assessed retrospectively, which is helpful when obtaining further opinions or planning revision (Liddle et al. Citation2013, Siddiqui et al. Citation2014). Comparison can be made with the contralateral hip, and it is also easier to compare serial MARS-MRI images rather than serial ultrasounds (Siddiqui et al. Citation2014). The main disadvantages of MARS-MRI include the high cost (£216), periprosthetic metal artefact despite suppression techniques, longer examination times, reduced availability, and being contraindicated in patients with pacemakers (Siddiqui et al. Citation2014).
Using an optimized MARS-MRI protocol allows detection of numerous abnormalities around MoMHAs. Pseudotumors are frequently of variable sizes, located anterior or posterolateral to MoMHAs, with a cystic, solid, or mixed consistency (Wynn-Jones et al. Citation2011, Hart et al. Citation2012, Hauptfleisch et al. Citation2012, Fehring et al. Citation2014). 3 main MARS-MRI pseudotumor classification systems have been proposed (Anderson et al. Citation2011, Hart et al. Citation2012, Hauptfleisch et al. Citation2012). Comparison of these systems suggested the Anderson et al. classification was most reliable (van der Weegen et al. Citation2014). The Modified Oxford Classification has been proposed more recently (Briant-Evans et al. Citation2015), which classifies MARS-MRI scans initially into 4 groups (normal, trochanteric fluid, effusion, ARMD), with the ARMD group subsequently classified according to others (Hauptfleisch et al. Citation2012). However small periprosthetic effusions may still be missed because of prosthetic artefacts (Siddiqui et al. Citation2014).
When pseudotumors have been detected, MARS-MRI can provide detailed information regarding any soft-tissue or muscle invasion and destruction as well as neurovascular involvement, which assists when planning revision (Liddle et al. Citation2013). Furthermore, studies by Liddle et al. (Citation2013) (85% sensitivity and 59% specificity) and Lainiala et al. (Citation2014a) (71% sensitivity and 87% specificity) have demonstrated that MARS-MRI is effective for diagnosing intraoperative pseudotumors. The latter study also reported that these results varied with MARS-MRI timing, with scans performed more than 1 year before revision surgery having lower sensitivity (29%) for detecting intraoperative pseudotumors compared with scans performed within 1 year before revision (77–88% sensitivity) (Lainiala et al. Citation2014a).
Other ARMD abnormalities also detected on MARS-MRI include muscle atrophy, tendon avulsions, and osteolysis (Hayter et al. Citation2012, Siddiqui et al. Citation2014, Berber et al. Citation2015b, Matharu et al. Citation2016c). Hip abductor atrophy has frequently been reported in MoMHAs and can progress over 1 year, with some suggesting atrophy may be a precursor to irreversible muscle destruction (Berber et al. Citation2015b).
Like ultrasound, MARS-MRI can identify both the development and progression of ARMD associated with MoMHAs. Most studies repeating MARS-MRI in MoMHA patients with normal initial scans have observed that few changes occur within 2 years of repeat imaging (van der Weegen et al. Citation2013, Reito et al. Citation2014a), with any development of ARMD from normal scans occurring over several years (Ebreo et al. Citation2013). By contrast, a study of 155 MoM THAs undergoing repeat MARS-MRI within a mean time of 15 months after the initial imaging observed that the risk of disease progression was high in patients with initially normal scans (30%), and in those with isolated trochanteric fluid (47%) and effusions (58%) on initial MARS-MRI (Briant-Evans et al. Citation2015). Disease progression on imaging was associated with high blood cobalt concentrations, or an irregular pseudocapsule lining at initial MARS-MRI (Briant-Evans et al. Citation2015).
Of the 4 studies repeating MARS-MRI in MoMHA patients with asymptomatic pseudotumors, 3 reported either little change or partial regression with serial imaging (van der Weegen et al. Citation2013, Kwon et al. Citation2016b, Goldstein et al. Citation2016). The other, a small study of 24 hips, observed that pseudotumors frequently changed in size when MARS-MRI was repeated, with larger lesions most likely to increase (Hasegawa et al. Citation2014).
Ultrasound vs. MARS-MRI
A number of studies have compared ultrasound and MARS-MRI for detecting ARMD (Table) (Garbuz et al. Citation2014, Nishii et al. Citation2014, Siddiqui et al. Citation2014, Muraoka et al. Citation2015, Kwon et al. Citation2016a, Matharu et al. Citation2016e). The benchmark for diagnostic accuracy in such studies should be correlation with the findings at revision; however, most did not use intraoperative findings as the reference standard. Studies have largely demonstrated that ultrasound was effective for identifying pseudotumors on MARS-MRI, and therefore recommended ultrasound for initial screening, with MARS-MRI reserved if ultrasound abnormalities required further assessment (Garbuz et al. Citation2014, Nishii et al. Citation2014, Muraoka et al. Citation2015, Kwon et al. Citation2016a). 1 study concluded that a negative ultrasound excluded pseudotumor in asymptomatic patients; ultrasound was 100% sensitive (Garbuz et al. Citation2014). Furthermore another study repeated both the ultrasound and MARS-MRI in 42 MoMHAs after a mean time of 14 months; when ultrasound was repeated it was accurately able to detect changes in ARMD lesion size and grade, therefore suggesting ultrasound is effective in the longitudinal assessment of MoMHA patients (Kwon et al. Citation2016a). By contrast, the authors of the smallest study reported that ultrasound was inferior to MARS-MRI for detecting pseudotumors and muscle atrophy, but ultrasound was superior to MARS-MRI for diagnosing effusions and tendon pathology (Siddiqui et al. Citation2014).
A recent retrospective diagnostic accuracy study of 40 HRAs subsequently requiring revision surgery for any reason compared the imaging findings prior to revision with the intraoperative findings (Matharu et al. Citation2016e). It was concluded that ultrasound and MARS-MRI both have a role when assessing HRA patients requiring revision, which parallels other studies where either ultrasound or MARS-MRI alone was performed (Liddle et al. Citation2013, Lainiala et al. Citation2014a, Citation2015). However, combined imaging using ultrasound and MARS-MRI was most effective for both identifying and excluding intraoperative pseudotumors (Table 1). These observations suggest that combined imaging may be useful in more complex clinical cases, such as symptomatic patients with either a normal initial ultrasound or MARS-MRI.
Computed tomography
Computed tomography (CT) scanning provides useful information regarding MoMHA component positioning and fixation, and can also detect osteolysis or fractures, which may be radiographically occult (Hart et al. Citation2009, Roth et al. Citation2012, Liddle et al. Citation2013). However, CT appears unsuitable for routine MoMHA imaging with a sensitivity of 44% for diagnosing pseudotumors compared with MARS-MRI, although CT was superior for detecting osteolysis (Robinson et al. Citation2014).
Given the advantages of both ultrasound and MARS-MRI, CT should not be recommended as a first-line MoMHA imaging modality (FDA Citation2013, Hannemann et al. Citation2013). Furthermore, CT is associated with pelvic radiation, image artefact from prostheses, and high costs.
Other investigations
Infection and ARMD can have similar presentations, therefore a comprehensive assessment is required. This includes blood tests for the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Infected MoMHA revision patients have significantly higher ESR and CRP values compared with non-infected revision cases (which included ARMD failures) (Yi et al. Citation2015). However, the ESR and CRP may also be raised in aseptic ARMD patients (Pandit et al. Citation2008, Mikhael et al. Citation2009), and where ARMD and infection coexist (Watters et al. Citation2010, Judd and Noiseux Citation2011, Matharu et al. Citation2014).
Hip aspirations can help assess infection (synovial fluid culture, sensitivity, white blood cell count, and cell differential, i.e. percentage of polymorphonuclear cells) (Lombardi et al. Citation2012, Yi et al. Citation2015) and ARMD (synovial cobalt and chromium concentrations) (De Smet et al. Citation2008, Langton et al. Citation2010, Davda et al. Citation2011, Kwon et al. Citation2011). Synovial metal ion concentrations can assist in cases where blood metal ions and cross-sectional imaging have been equivocal despite clinical concerns, and may be informative when patients have problematic bilateral MoMHAs. In a recent study leukocyte esterase strip testing of synovial fluid samples was reliable at ruling out infection in MoMHAs revised for ARMD (Tischler et al. Citation2016).
Other useful investigations for unexplained symptoms include single-photon emission CT (Berber et al. Citation2015a), diagnostic hip injections, and investigating other causes (such as spinal or non-orthopedic pathology).
Symptoms and patient-reported outcome measures
When interpreting the results of any investigations performed in MoMHA patients, pain and functional limitations should be assessed. Symptoms and signs looked for are pain (groin, buttock, lateral hip, or thigh), swellings or masses, mechanical symptoms (clicking, clunking, grinding, squeaking, catching, or instability), or a limp when walking. Patient-reported outcome measures such as the Oxford Hip Score (OHS) and Harris Hip Score (HHS) are reliable and responsive instruments; however, regulatory authorities do not currently provide guidance on meaningful thresholds for stratifying MoMHA patients (Matharu et al. Citation2015c).
An OHS less than 34, or an HHS less than 80 (Kalairajah et al. Citation2005, Murray et al. Citation2007) could be considered suboptimal patient-reported outcome measures following MoMHA that warrant further investigation. Indeed, a recent large cohort study which developed a clinical scoring system for assessing the risk of revision in 1,434 MoMHAs identified the HHS and blood metal ion levels as the most important predictors of revision. The HHS was subsequently categorized into low risk (80–100), moderate risk (70–79), and high risk (< 70) groups (Hussey et al. Citation2016). Patient-reported outcome measures therefore seem important for risk stratifying MoMHA patients who otherwise may not appear to be symptomatic for surveillance and/or further investigation.
Conclusions
Many studies have assessed various investigative modalities used in MoMHA surveillance. No single investigation can universally be used during surveillance based on our literature update. We recommend that all MoMHA patients undergo a complete clinical assessment as a baseline. This assessment should include blood metal ion concentrations, cross-sectional imaging, and pelvic radiographs as well as completion of validated patient-reported outcome measures. The choice of cross-sectional imaging modality (ultrasound or MARS-MRI) used will depend on the availability of ultrasound expertise at each institution as well as any financial constraints. Subsequent patient management will depend on a combination of the results of this clinical assessment, hip symptoms, and the patient’s risk of ARMD (Grammatopoulos et al. Citation2010a, Matharu et al. Citation2016d).
Recent studies have provided information about (1) interpreting blood metal ions (effective at identifying patients at low risk of ARMD), (2) the roles of cross-sectional imaging (reserve combined ultrasound and MARS-MRI for complex cases), and (3) providing evidence-based parameters (blood metal ions <2 µg/L and normal cross-sectional imaging) to safely exclude many asymptomatic patients from regular surveillance. The latter issue is particularly important in asymptomatic HRA patients and could result in considerable financial savings, given that the current recommendations from regulatory authorities for this subgroup vary ranging from local protocol (MHRA Citation2012) to annual radiographs and blood metal ions (Hannemann et al. Citation2013). Therefore, the findings from recent studies will be useful when designing future surveillance protocols for MoMHA patients. However, the natural history of ARMD remains incompletely understood. Further longitudinal studies are thus needed to identify specifically which clinical, laboratory, and imaging findings predict patients with aggressive ARMD requiring revision surgery.
AJ has undertaken medico-legal work for Freshfields Bruckhaus Deringer, which includes work relating to metal-on-metal hip replacements. HGP provides expert testimony to Kennedys Law, which includes work relating to metal-on-metal hip replacements. None of the other authors have any conflicts of interest relating specifically to this work. No commercial companies were involved in the planning of this review, analysis, and interpretation of data, or writing of the manuscript.
GSM: literature review, and manuscript draft, revision and approval. AJ, AE, DWM, HGP: contributed to the literature review, and manuscript revision and approval.
The authors would like to thank the following organizations for funding this research: Arthritis Research UK (grant reference number 21006), the Royal College of Surgeons of England, the Orthopaedics Trust and the Royal Orthopaedic Hospital Hip Research and Education Charitable Fund.
Acta thanks Christian P. Delaunay and Ola Rolfson for help with peer review of this study.
Summary of studies comparing ultrasound and MARS-MRI for detecting adverse reactions to metal debris and pseudotumors
Pseudotumor in association with a metal-on-metal hip resurfacing arthroplasty identified on ultrasound examination. Anterior view of the hip demonstrating an enlarged mixed pseudotumor lying in the psoas bursa region. The pseudotumor lies in close proximity to the femoral vessels (marked) and has a smaller medial (M) and a larger lateral (L) component.
- Almousa S A, Greidanus N V, Masri B A, Duncan C P, Garbuz D S. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res 2013; 471 (12): 3814–21.
- Amstutz H C, Campbell P A, Dorey F J, Johnson A J, Skipor A K, Jacobs J J. Do ion concentrations after metal-on-metal hip resurfacing increase over time? A prospective study. J Arthroplasty 2013; 28 (4): 695–700.
- Anderson H, Toms A P, Cahir J G, Goodwin R W, Wimhurst J, Nolan J F. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: Reliability of an MR grading system. Skeletal Radiol 2011; 40 (3): 303–7.
- AOANJRR. Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) Hip, Knee & Shoulder Arthroplasty Annual Report. 2016; https://aoanjrr.sahmri.com/annual-reports-2016.
- Beaule P E, Kim P R, Hamdi A, Fazekas A. A prospective metal ion study of large-head metal-on-metal bearing: A matched-pair analysis of hip resurfacing versus total hip replacement. Orthop Clin North Am 2011; 42 (2): 251–7, ix.
- Berber R, Henckel J, Khoo M, Wan S, Hua J, Skinner J, Hart A. Clinical usefulness of SPECT-CT in patients with an unexplained pain in metal on metal (MOM) total hip arthroplasty. J Arthroplasty 2015a; 30 (4): 687–94.
- Berber R, Khoo M, Cook E, Guppy A, Hua J, Miles J, Carrington R, Skinner J, Hart A. Muscle atrophy and metal-on-metal hip implants: A serial MRI study of 74 hips. Acta Orthop 2015b; 86 (3): 351–7.
- Bernstein M, Walsh A, Petit A, Zukor D J, Huk O L, Antoniou J. Femoral head size does not affect ion values in metal-on-metal total hips. Clin Orthop Relat Res 2011; 469 (6): 1642–50.
- Bernstein M, Desy N M, Petit A, Zukor D J, Huk O L, Antoniou J. Long-term follow-up and metal ion trend of patients with metal-on-metal total hip arthroplasty. Int Orthop 2012; 36 (9): 1807–12.
- Bisseling P, de Wit B W, Hol A M, van Gorp M J, van Kampen A, van Susante J L. Similar incidence of periprosthetic fluid collections after ceramic-on-polyethylene total hip arthroplasties and metal-on-metal resurfacing arthroplasties: Results of a screening metal artefact reduction sequence-MRI study. Bone Joint J 2015; 97-B (9): 1175–82.
- Bozic K J, Kurtz S, Lau E, Ong K, Chiu V, Vail T P, Rubash H E, Berry D J. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am 2009; 91 (7): 1614–20.
- Briant-Evans T W, Lyle N, Barbur S, Hauptfleisch J, Amess R, Pearce A R, Conn K S, Stranks G J, Britton J M. A longitudinal study of MARS MRI scanning of soft-tissue lesions around metal-on-metal total hip arthroplasties and disease progression. Bone Joint J 2015; 97-B (10): 1328–37.
- Canada. Health Canada. Metal-on-metal hip implants—information for orthopaedic surgeons regarding patient management following surgery—for health professionals. 2012; http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/15835a-eng.php.
- Chang E Y, McAnally J L, Van Horne J R, Van Horne J G, Wolfson T, Gamst A, Chung C B. Relationship of plasma metal ions and clinical and imaging findings in patients with ASR XL metal-on-metal total hip replacements. J Bone Joint Surg Am 2013; 95 (22): 2015–20.
- Chen Z, Pandit H, Taylor A, Gill H, Murray D, Ostlere S. Metal-on-metal hip resurfacings: A radiological perspective. Eur Radiol 2011; 21 (3): 485–91.
- Coulter G, Young D A, Dalziel R E, Shimmin A J. Birmingham hip resurfacing at a mean of ten years: Results from an independent centre. J Bone Joint Surg Br 2012; 94 (3): 315–21.
- Daniel J, Ziaee H, Pynsent P B, McMinn D J. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg Br 2007; 89 (6): 736–41.
- Daniel J, Ziaee H, Pradhan C, McMinn D J. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings. J Bone Joint Surg Br 2009; 91 (2): 176–9.
- Daniel J, Ziaee H, Pradhan C, Pynsent P B, McMinn D J. Renal clearance of cobalt in relation to the use of metal-on-metal bearings in hip arthroplasty. J Bone Joint Surg Am 2010; 92 (4): 840–5.
- Daniel J, Pradhan C, Ziaee H, Pynsent P B, McMinn D J. Results of Birmingham hip resurfacing at 12 to 15 years: A single-surgeon series. Bone Joint J 2014; 96-B (10): 1298–306.
- Das D H, van der Weegen W, Wullems J A, Brakel K, Sijbesma T, Nelissen R G. Periprosthetic pathology in “at risk” ceramic-on-polyethylene total hip arthroplasty: A clinical study using MARS-MRI in 50 patients. Hip Int 2016; 26 (3): 244–8.
- Davda K, Lali F V, Sampson B, Skinner J A, Hart A J. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J Bone Joint Surg Br 2011; 93 (6): 738–45.
- De Haan R, Pattyn C, Gill H S, Murray D W, Campbell P A, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br 2008; 90 (10): 1291–7.
- De Smet K, De Haan R, Calistri A, Campbell P A, Ebramzadeh E, Pattyn C, Gill H S. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am 2008; 90(Suppl4): 202–8.
- De Smet K A, Van Der Straeten C, Van Orsouw M, Doubi R, Backers K, Grammatopoulos G. Revisions of metal-on-metal hip resurfacing: Lessons learned and improved outcome. Orthop Clin North Am 2011; 42 (2): 259–69, ix.
- Douis H, Dunlop D J, Pearson A M, O’Hara J N, James S L. The role of ultrasound in the assessment of post-operative complications following hip arthroplasty. Skeletal Radiol 2012; 41 (9): 1035–46.
- Ebreo D, Bell P J, Arshad H, Donell S T, Toms A, Nolan J F. Serial magnetic resonance imaging of metal-on-metal total hip replacements. Follow-up of a cohort of 28 mm Ultima TPS THRs. Bone Joint J 2013; 95-B (8): 1035–9.
- Emmanuel A R, Bergin K M, Kelly G E, McCoy G F, Wozniak A P, Quinlan J F. The effect of acetabular inclination on metal ion levels following metal-on-metal hip arthroplasty. J Arthroplasty 2014; 29 (1): 186–91.
- Engh C A, MacDonald S J, Sritulanondha S, Korczak A, Naudie D, Engh C. Metal ion levels after metal-on-metal total hip arthroplasty: A five-year, prospective randomized trial. J Bone Joint Surg Am 2014; 96 (6): 448–55.
- Fang C S, Harvie P, Gibbons C L, Whitwell D, Athanasou N A, Ostlere S. The imaging spectrum of peri-articular inflammatory masses following metal-on-metal hip resurfacing. Skeletal Radiol 2008; 37 (8): 715–22.
- FDA. U.S. Food and Drug Administration (FDA). Medical devices. Metal-on-metal hip implants. Information for Orthopedic Surgeons. 2013; http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241667.htm.
- Fehring T K, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res 2014; 472 (2): 517–22.
- Garbuz D S, Tanzer M, Greidanus N V, Masri B A, Duncan C P. The John Charnley Award: Metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: A randomized clinical trial. Clin Orthop Relat Res 2010; 468 (2): 318–25.
- Garbuz D S, Hargreaves B A, Duncan C P, Masri B A, Wilson D R, Forster B B. The John Charnley Award: Diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res 2014; 472 (2): 417–23.
- Garcia F L, Picado C H, Nogueira-Barbosa M H. Sonographic evaluation of the abductor mechanism after total hip arthroplasty. J Ultrasound Med 2010; 29 (3): 465–71.
- Goldstein J M, Fehring T K, Fehring K A. Cystic adverse local tissue reactions in asymptomatic modular metal-on-metal total hips may decrease over time. J Arthroplasty 2016; 31 (7): 1589–94.
- Grammatopoulos G, Pandit H, Kwon Y M, Gundle R, McLardy-Smith P, Beard D J, Murray D W, Gill H S. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br 2009; 91 (8): 1019–24.
- Grammatopoulos G, Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gill H S, Murray D W. Optimal acetabular orientation for hip resurfacing. J Bone Joint Surg Br 2010a; 92 (8): 1072–8.
- Grammatopoulos G, Pandit H, Oxford H, Knee G, Murray D W, Gill H S. The relationship between head-neck ratio and pseudotumour formation in metal-on-metal resurfacing arthroplasty of the hip. J Bone Joint Surg Br 2010b; 92 (11): 1527–34.
- Hannemann F, Hartmann A, Schmitt J, Lutzner J, Seidler A, Campbell P, Delaunay C P, Drexler H, Ettema H B, Garcia-Cimbrelo E, Huberti H, Knahr K, Kunze J, Langton D J, Lauer W, Learmonth I, Lohmann C H, Morlock M, Wimmer M A, Zagra L, Gunther KP. European multidisciplinary consensus statement on the use and monitoring of metal-on-metal bearings for total hip replacement and hip resurfacing. Orthop Traumatol Surg Res 2013; 99 (3): 263–71.
- Harrington C F, Taylor A. Metal-on-metal hip implants: UK quality assurance of blood cobalt and chromium after hip implants. BMJ 2012; 344: e4017.
- Hart A J, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner J A. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br 2009; 91 (6): 738–44.
- Hart A J, Sabah S A, Bandi A S, Maggiore P, Tarassoli P, Sampson B, J A S. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br 2011a; 93 (10): 1308–13.
- Hart A J, Skinner J A, Henckel J, Sampson B, Gordon F. Insufficient acetabular version increases blood metal ion levels after metal-on-metal hip resurfacing. Clin Orthop Relat Res 2011b; 469 (9): 2590–7.
- Hart A J, Satchithananda K, Liddle A D, Sabah S A, McRobbie D, Henckel J, Cobb J P, Skinner J A, Mitchell A W. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: A case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am 2012; 94 (4): 317–25.
- Hart A J, Sabah S A, Sampson B, Skinner J A, Powell J J, Palla L, Pajamaki K J, Puolakka T, Reito A, Eskelinen A. Surveillance of patients with metal-on-metal hip resurfacing and total hip prostheses: A prospective cohort study to investigate the relationship between blood metal ion levels and implant failure. J Bone Joint Surg Am 2014; 96 (13): 1091–99.
- Hasegawa M, Miyamoto N, Miyazaki S, Wakabayashi H, Sudo A. Longitudinal magnetic resonance imaging of pseudotumors following metal-on-metal total hip arthroplasty. J Arthroplasty 2014; 29 (12): 2236–8.
- Hauptfleisch J, Pandit H, Grammatopoulos G, Gill H S, Murray D W, Ostlere S. A MRI classification of periprosthetic soft tissue masses (pseudotumours) associated with metal-on-metal resurfacing hip arthroplasty. Skeletal Radiol 2012; 41 (2): 149–55.
- Hayter C L, Koff M F, Potter H G. Magnetic resonance imaging of the postoperative hip. J Magn Reson Imaging 2012; 35 (5): 1013–25.
- Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer J P. Characterization of the running-in period in total hip resurfacing arthroplasty: An in vivo and in vitro metal ion analysis. J Bone Joint Surg Am 2008; 90Suppl3: 125–33.
- Hing C B, Young D A, Dalziel R E, Bailey M, Back D L, Shimmin A J. Narrowing of the neck in resurfacing arthroplasty of the hip: A radiological study. J Bone Joint Surg Br 2007; 89 (8): 1019–24.
- Hussey D K, Madanat R, Donahue G S, Rolfson O, Bragdon C R, Muratoglu O K, Malchau H. Scoring the current risk stratification guidelines in follow-up evaluation of patients after metal-on-metal hip arthroplasty: A proposal for a metal-on-metal risk score supporting clinical decision-making. J Bone Joint Surg Am 2016; 98 (22): 1905–12.
- Jacobs J J, Cooper H J, Urban R M, Wixson R L, Della Valle CJ . What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty 2014; 29 (4): 668–9.
- Johnston C, Kerr J, Ford S, O’Byrne J, Eustace S. MRI as a problem-solving tool in unexplained failed total hip replacement following conventional assessment. Skeletal Radiol 2007; 36 (10): 955–61.
- Judd K T, Noiseux N. Concomitant infection and local metal reaction in patients undergoing revision of metal on metal total hip arthroplasty. Iowa Orthop J 2011; 31: 59–63.
- Kalairajah Y, Azurza K, Hulme C, Molloy S, Drabu K J. Health outcome measures in the evaluation of total hip arthroplasties: A comparison between the Harris hip score and the Oxford hip score. J Arthroplasty 2005; 20 (8): 1037–41.
- Kwon Y M, Glyn-Jones S, Simpson D J, Kamali A, McLardy-Smith P, Gill H S, Murray D W. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg Br 2010; 92 (3): 356–61.
- Kwon Y M, Ostlere S J, McLardy-Smith P, Athanasou N A, Gill H S, Murray D W. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: Prevalence and metal ion study. J Arthroplasty 2011; 26 (4): 511–18.
- Kwon Y M, Lombardi A V, Jacobs J J, Fehring T K, Lewis C G, Cabanela M E. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: Consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopedic Surgeons, and the Hip Society. J Bone Joint Surg Am 2014; 96 (1): e4.
- Kwon Y M, Dimitriou D, Liow M H, Tsai T Y, Li G. Is ultrasound as useful as metal artifact reduction sequence magnetic resonance imaging in longitudinal surveillance of metal-on-metal hip arthroplasty patients? J Arthroplasty 2016a; 31 (8): 1821–7.
- Kwon Y M, Liow M H, Dimitriou D, Tsai T Y, Freiberg A A, Rubash H E. What is the natural history of “asymptomatic” pseudotumours in metal-on-metal hip arthroplasty? Minimum 4-year metal artifact reduction sequence magnetic resonance imaging longitudinal study. J Arthroplasty 2016b; 31(9Suppl): 121–6.
- Lainiala O, Elo P, Reito A, Pajamaki J, Puolakka T, Eskelinen A. Comparison of extracapsular pseudotumors seen in magnetic resonance imaging and in revision surgery of 167 failed metal-on-metal hip replacements. Acta Orthop 2014a; 85 (5): 474–9.
- Lainiala O, Eskelinen A, Elo P, Puolakka T, Korhonen J, Moilanen T. Adverse reaction to metal debris is more common in patients following MoM total hip replacement with a 36 mm femoral head than previously thought: Results from a modern MoM follow-up programme. Bone Joint J 2014b; 96-B (12): 1610–17.
- Lainiala O, Elo P, Reito A, Pajamaki J, Puolakka T, Eskelinen A. Good sensitivity and specificity of ultrasound for detecting pseudotumors in 83 failed metal-on-metal hip replacements. Acta Orthop 2015; 86 (3): 339–44.
- Lainiala O S, Moilanen T P, Hart A J, Huhtala H S, Sabah S A, Eskelinen A P. Higher blood cobalt and chromium levels in patients with unilateral metal-on-metal total hip arthroplasties compared to hip resurfacings. J Arthroplasty 2016; 31 (6): 1261–6.
- Langton D J, Sprowson A P, Joyce T J, Reed M, Carluke I, Partington P, Nargol A V. Blood metal ion concentrations after hip resurfacing arthroplasty: A comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br 2009; 91 (10): 1287–95.
- Langton D J, Jameson S S, Joyce T J, Hallab N J, Natu S, Nargol A V. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br 2010; 92 (1): 38–46.
- Langton D J, Jameson S S, Joyce T J, Gandhi J N, Sidaginamale R, Mereddy P, Lord J, Nargol A V. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br 2011a; 93 (8): 1011–16.
- Langton D J, Joyce T J, Jameson S S, Lord J, Van Orsouw M, Holland J P, Nargol A V, De Smet K A. Adverse reaction to metal debris following hip resurfacing: The influence of component type, orientation and volumetric wear. J Bone Joint Surg Br 2011b; 93 (2): 164–71.
- Langton D J, Joyce T J, Mangat N, Lord J, Van Orsouw M, De Smet K, Nargol A V. Reducing metal ion release following hip resurfacing arthroplasty. Orthop Clin North Am 2011c; 42 (2): 169–80, viii.
- Langton D J, Sidaginamale R, Lord J K, Nargol A V, Joyce T J. Taper junction failure in large-diameter metal-on-metal bearings. Bone Joint Res 2012; 1 (4): 56–63.
- Langton D J, Sidaginamale R P, Joyce T J, Natu S, Blain P, Jefferson R D, Rushton S, Nargol A V. The clinical implications of elevated blood metal ion concentrations in asymptomatic patients with MoM hip resurfacings: A cohort study. BMJ Open 2013; 3 (3).
- Langton D J, Sidaginamale R P, Avery P, Waller S, Tank G, Lord J, Joyce T, Cooke N, Logishetty R, Nargol A V. Retrospective cohort study of the performance of the Pinnacle metal on metal (MoM) total hip replacement: A single-centre investigation in combination with the findings of a national retrieval centre. BMJ Open 2016; 6 (4): e007847.
- Lavigne M, Belzile E L, Roy A, Morin F, Amzica T, Vendittoli P A. Comparison of whole-blood metal ion levels in four types of metal-on-metal large-diameter femoral head total hip arthroplasty: The potential influence of the adapter sleeve. J Bone Joint Surg Am 2011; 93(Suppl2): 128–36.
- Liddle A D, Satchithananda K, Henckel J, Sabah S A, Vipulendran K V, Lewis A, Skinner J A, Mitchell A W, Hart A J. Revision of metal-on-metal hip arthroplasty in a tertiary center: A prospective study of 39 hips with between 1 and 4 years of follow-up. Acta Orthop 2013; 84 (3): 237–45.
- Lloyd J, Starks I, Wainwright T, Middleton R. Metal-on-metal resurfacing and the cost to the nation: A conservative estimate of the unexpected costs required to implement the new metal-on-metal follow-up programme in the UK. In: Knahr K, editor. Total Hip Arthroplasty Tribological Considerations and Clinical Consequences. Berlin Heidelberg: Springer; 2013. p. 45–51.
- Lombardi A V, Jr, Barrack R L, Berend K R, Cuckler J M, Jacobs J J, Mont M A, Schmalzried T P. The Hip Society: Algorithmic approach to diagnosis and management of metal-on-metal arthroplasty. J Bone Joint Surg Br 2012; 94(11SupplA): 14–18.
- Low A K, Matharu G S, Ostlere S J, Murray D W, Pandit H G. How should we follow-up asymptomatic metal-on-metal hip resurfacing patients? A prospective longitudinal cohort study. J Arthroplasty 2016; 31 (1): 146–51.
- Madanat R, Rolfson O, Donahue G S, Hussey D K, Potter H G, Wallace R, Muratoglu O K, Malchau H. Medial calcar erosion is associated with synovial thickness in patients with ASR XL total hip arthroplasty. J Arthroplasty 2016; 31 (11): 2588–92.
- Malek I A, King A, Sharma H, Malek S, Lyons K, Jones S, John A. The sensitivity, specificity and predictive values of raised plasma metal ion levels in the diagnosis of adverse reaction to metal debris in symptomatic patients with a metal-on-metal arthroplasty of the hip. J Bone Joint Surg Br 2012; 94 (8): 1045–50.
- Matharu G S, Pynsent P B, Sumathi V P, Mittal S, Buckley C D, Dunlop D J, Revell P A, Revell M P. Predictors of time to revision and clinical outcomes following revision of metal-on-metal hip replacements for adverse reaction to metal debris. Bone Joint J 2014; 96-B (12): 1600–9.
- Matharu G S, Berryman F, Brash L, Pynsent P B, Treacy R B, Dunlop D J. Influence of implant design on blood metal ion concentrations in metal-on-metal total hip replacement patients. Int Orthop 2015a; 39 (9): 1803–11.
- Matharu G S, Berryman F, Brash L, Pynsent P B, Treacy R B, Dunlop D J. Predicting high blood metal ion concentrations following hip resurfacing. Hip Int 2015b; 25 (6): 510–19.
- Matharu G S, Mellon S J, Murray D W, Pandit H G. Follow-up of metal-on-metal hip arthroplasty patients is currently not evidence based or cost effective. J Arthroplasty 2015c; 30 (8): 1317–23.
- Matharu G S, Berryman F, Brash L, Pynsent P B, Dunlop D J, Treacy R B. Can blood metal ion levels be used to identify patients with bilateral Birmingham hip resurfacings who are at risk of adverse reactions to metal debris? Bone Joint J 2016a; 98-B (11): 1455–62.
- Matharu G S, Berryman F, Brash L, Pynsent P B, Treacy R B, Dunlop D J. The effectiveness of blood metal ions in identifying patients with unilateral Birmingham hip resurfacing and Corail-Pinnacle metal-on-metal hip implants at risk of adverse reactions to metal debris. J Bone Joint Surg Am 2016b; 98 (8): 617–26.
- Matharu G S, Janardhan S, Brash L, Pynsent P B, Dunlop D J, James S L. The utility of repeat ultrasound imaging in the follow-up of metal-on-metal hip arthroplasty patients. Ann R Coll Surg Engl 2016c; 98 (2): 143–9.
- Matharu G S, Judge A, Murray D W, Pandit H G. Prevalence of and risk factors for hip resurfacing revision: A cohort study into the second decade after the operation. J Bone Joint Surg Am 2016d; 98 (17): 1444–52.
- Matharu G S, Mansour R, Dada O, Ostlere S, Pandit H G, Murray D W. Which imaging modality is most effective for identifying pseudotumours in metal-on-metal hip resurfacings requiring revision: Ultrasound or MARS-MRI or both? Bone Joint J 2016e; 98-B (1): 40–8.
- Matharu G S, Ostlere S J, Pandit H G, Murray D W. What is the natural history of asymptomatic pseudotumours in metal-on-metal hip resurfacing patients? Hip Int 2016f; 26 (6): 522–30.
- Matharu G S, Berryman F, Judge A, Reito A, McConnell J, Lainiala O, Young S, Eskelinen A, Pandit H G, Murray D W. Blood metal ion thresholds to identify patients with metal-on-metal hip implants at risk of adverse reactions to metal debris: An external multicenter validation study of Birmingham Hip Resurfacing and Corail-Pinnacle implants. J Bone Joint Surg Am 2017a; 99(18): 1532–9.
- Matharu G S, Blanshard O, Dhaliwal K, Judge A, Murray D W, Pandit H G. Patient and radiographic factors help to predict metal-on-metal hip resurfacings with evidence of a pseudotumor. J Bone Joint Surg Am 2017b; 99 (3): 214–22.
- Matharu G S, Nandra R S, Berryman F, Judge A, Pynsent P B, Dunlop D J. Risk factors for failure of the 36 mm metal-on-metal Pinnacle total hip arthroplasty system: A retrospective single-centre cohort study. Bone Joint J 2017c; 99-B (5): 592–600.
- Matthies A, Underwood R, Cann P, Ilo K, Nawaz Z, Skinner J, Hart A J. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing. J Bone Joint Surg Br 2011; 93 (3): 307–14.
- Matthies A K, Henckel J, Cro S, Suarez A, Noble P C, Skinner J, Hart A J. Predicting wear and blood metal ion levels in metal-on-metal hip resurfacing. J Orthop Res 2014; 32 (1): 167–74.
- McMinn D. Modern hip resurfacing. London: Springer; 2009. xvi, 430 pp.
- MHRA. Medicines & Healthcare products Regulatory Agency (MHRA). Medical device alert: ASR hip replacement implant manufactured by DePuy International. 2010; http://www.mhra.gov.uk.
- MHRA. Medicines & Healthcare products Regulatory Agency (MHRA). Medical device alert: All metal-on-metal (MoM) hip replacements. MDA/2012/036. 2012; http://www.mhra.gov.uk/.
- MHRA. Medicines & Healthcare products Regulatory Agency (MHRA). Medical device alert: Metal-on-metal (MoM) hip replacements: Birmingham Hip Resurfacing (BHR) system (Smith & Nephew Orthopaedics). 2015; https://assets.digital.cabinet-office.gov.uk/media/558bf80e5274a1559000002/MDA-2015-024.pdf.
- Mikhael M M, Hanssen A D, Sierra R J. Failure of metal-on-metal total hip arthroplasty mimicking hip infection: A report of two cases. J Bone Joint Surg Am 2009; 91 (2): 443–6.
- Muraoka K, Naito M, Nakamura Y, Hagio T, Takano K. Usefulness of ultrasonography for detection of pseudotumors after metal-on-metal total hip arthroplasty. J Arthroplasty 2015; 30 (5): 879–84.
- Murray D W, Fitzpatrick R, Rogers K, Pandit H, Beard D J, Carr A J, Dawson J. The use of the Oxford hip and knee scores. J Bone Joint Surg Br 2007; 89 (8): 1010–14.
- Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Ultrasound screening of periarticular soft tissue abnormality around metal-on-metal bearings. J Arthroplasty 2012; 27 (6): 895–900.
- Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Is ultrasound screening reliable for adverse local tissue reaction after hip arthroplasty? J Arthroplasty 2014; 29 (12): 2239–44.
- NJR. National Joint Registry (NJR) for England, Wales, Northern Ireland and the Isle of Man 13th Annual Report. 2016; http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/13th Annual Report/07950 NJR Annual Report 2016 ONLINE REPORT.pdf.
- Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons C L, Ostlere S, Athanasou N, Gill H S, Murray D W. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br 2008; 90 (7): 847–51.
- Rahme M, Lavigne M, Barry J, Cirtiu C M, Belanger P, Vendittoli P A. Whole blood metal ion measurement reproducibility between different laboratories. J Arthroplasty 2014; 29 (11): 2214–18.
- Reito A, Elo P, Puolakka T, Pajamaki J, Nieminen J, Eskelinen A. Repeated magnetic resonance imaging in 154 hips with large-diameter metal-on-metal hip replacement. Acta Orthop 2014a; 85 (6): 570–6.
- Reito A, Moilanen T, Puolakka T, Pajamaki J, Eskelinen A. Repeated metal ion measurements in patients with high risk metal-on-metal hip replacement. Int Orthop 2014b; 38 (7): 1353–61.
- Reito A, Lainiala O, Nieminen J, Eskelinen A. Repeated metal ion measurement in patients with bilateral metal on metal (ASR) hip replacements. Orthop Traumatol Surg Res 2016; 102 (2): 167–73.
- Robinson E, Henckel J, Sabah S, Satchithananda K, Skinner J, Hart A. Cross-sectional imaging of metal-on-metal hip arthroplasties: Can we substitute MARS MRI with CT? Acta Orthop 2014; 85 (6): 577–84.
- Roth T D, Maertz N A, Parr J A, Buckwalter K A, Choplin R H. CT of the hip prosthesis: Appearance of components, fixation, and complications. Radiographics 2012; 32 (4): 1089–107.
- Sidaginamale R P, Joyce T J, Lord J K, Jefferson R, Blain P G, Nargol A V, Langton D J. Blood metal ion testing is an effective screening tool to identify poorly performing metal-on-metal bearing surfaces. Bone Joint Res 2013; 2 (5): 84–95.
- Siddiqui I A, Sabah S A, Satchithananda K, Lim A K, Henckel J, Skinner J A, Hart A J. Cross-sectional imaging of the metal-on-metal hip prosthesis: The London ultrasound protocol. Clin Radiol 2013; 68 (8): e472–8.
- Siddiqui I A, Sabah S A, Satchithananda K, Lim A K, Cro S, Henckel J, Skinner J A, Hart A J. A comparison of the diagnostic accuracy of MARS MRI and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop 2014; 85 (4): 375–82.
- Smith A J, Dieppe P, Howard P W, Blom A W, National Joint Registry for England and Wales. Failure rates of metal-on-metal hip resurfacings: Analysis of data from the National Joint Registry for England and Wales. Lancet 2012a; 380 (9855): 1759–66.
- Smith A J, Dieppe P, Vernon K, Porter M, Blom A W, National Joint Registry for England and Wales. Failure rates of stemmed metal-on-metal hip replacements: Analysis of data from the National Joint Registry for England and Wales. Lancet 2012b; 379 (9822): 1199–204.
- Sofka C M, Haddad Z K, Adler R S. Detection of muscle atrophy on routine sonography of the shoulder. J Ultrasound Med 2004; 23 (8): 1031–4.
- Therapeutic Goods Administration DoH, Australian Government. Metal-on-metal hip replacement implants—information for general practitioners, orthopaedic surgeons and other health professionals. 2012; http://www.tga.gov.au/hp/information-devices-mom-hip-implants.htm.
- Tischler E H, Plummer D R, Chen A F, Della Valle C J, Parvizi J. Leukocyte esterase: Metal-on-metal failure and periprosthetic joint infection. J Arthroplasty 2016; 31 (10): 2260–3.
- Toms A P, Marshall T J, Cahir J, Darrah C, Nolan J, Donell S T, Barker T, Tucker J K. MRI of early symptomatic metal-on-metal total hip arthroplasty: A retrospective review of radiological findings in 20 hips. Clin Radiol 2008; 63 (1): 49–58.
- Van Der Straeten C, Grammatopoulos G, Gill H S, Calistri A, Campbell P, De Smet K A. The 2012 Otto Aufranc Award: The interpretation of metal ion levels in unilateral and bilateral hip resurfacing. Clin Orthop Relat Res 2013a; 471 (2): 377–85.
- Van Der Straeten C, Van Quickenborne D, De Roest B, Calistri A, Victor J, De Smet K. Metal ion levels from well-functioning Birmingham Hip Resurfacings decline significantly at ten years. Bone Joint J 2013b; 95-B (10): 1332–8.
- van der Veen H C, Reininga I H, Zijlstra W P, Boomsma M F, Bulstra S K, van Raay J J. Pseudotumour incidence, cobalt levels and clinical outcome after large head metal-on-metal and conventional metal-on-polyethylene total hip arthroplasty: Mid-term results of a randomised controlled trial. Bone Joint J 2015; 97-B (11): 1481–7.
- van der Weegen W, Brakel K, Horn R J, Hoekstra H J, Sijbesma T, Pilot P, Nelissen R G. Asymptomatic pseudotumours after metal-on-metal hip resurfacing show little change within one year. Bone Joint J 2013; 95-B (12): 1626–31.
- van der Weegen W, Brakel K, Horn R J, Wullems J A, Das H P, Pilot P, Nelissen R G. Comparison of different pseudotumor grading systems in a single cohort of metal-on-metal hip arthroplasty patients. Skeletal Radiol 2014; 43 (2): 149–55.
- Vendittoli P A, Mottard S, Roy A G, Dupont C, Lavigne M. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J Bone Joint Surg Br 2007; 89 (4): 441–8.
- Watters T S, Eward W C, Hallows R K, Dodd L G, Wellman S S, Bolognesi M P. Pseudotumor with superimposed periprosthetic infection following metal-on-metal total hip arthroplasty: A case report. J Bone Joint Surg Am 2010; 92 (7): 1666–9.
- Williams D H, Greidanus N V, Masri B A, Duncan C P, Garbuz D S. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am 2011; 93 (23): 2164–71.
- Wynn-Jones H, Macnair R, Wimhurst J, Chirodian N, Derbyshire B, Toms A, Cahir J. Silent soft tissue pathology is common with a modern metal-on-metal hip arthroplasty. Acta Orthop 2011; 82 (3): 301–7.
- Yi P H, Cross M B, Moric M, Levine B R, Sporer S M, Paprosky W G, Jacobs J J, Della Valle C J. Do serologic and synovial tests help diagnose infection in revision hip arthroplasty with metal-on-metal bearings or corrosion? Clin Orthop Relat Res 2015; 473 (2): 498–505.

