?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The positive effects of combined hyperoxia and physical exercise on physiological parameters and cognitive functioning are established for normobaric laboratory contexts. Still, increased practicability exists in hyperbaric settings like underwater activities and SCUBA diving, where environmental and sport-specific factors might moderate effects. Improved cognition, reduced ventilation (V̇E), and lower blood lactate concentrations [Lac-] are highly relevant, especially during high-stress and rescue scenarios. Fifteen participants performed 3 × 8 min of continuous underwater fin-swimming at 25 % (low), 45 % (moderate), and 75 % (vigorous) heart rate reserve (HRR) in each test. Three separate test days differed solely by the inspiratory oxygen partial pressure (PIO2: 29 kPa, 56 kPa, and 140 kPa). V̇E was measured continuously, whereas breathing gas analysis, blood sampling, and Eriksen Flanker tasks for inhibitory control (100 stimuli) were performed post-exercise. Two-way ANOVAs with repeated measures on the factors PIO2 and exercise intensity analyzed physiological outcome variables and reactions times (RT) and accuracy (ACC) of inhibitory control. V̇E was significantly reduced for 140 kPa during moderate and vigorous and for 56 kPa during vigorous compared to 29 kPa. 56 kPa and 140 kPa showed no differences. [Lac-], post-exercise V̇CO2, and velocity were unaffected by PIO2. Faster RTs but lower ACC of inhibitory control were observed following exercise at 75 % HRR compared to rest, 25 %, and 45 % HRR, while PIO2 produced no effects. Underwater performance in hyperoxia presents reduced V̇E, possible by dampened chemoreceptor sensitivity, and effects on cognition that differ from laboratory results and emphasise the moderating role of sport-specific factors.
Highlights
Hyperoxia-induced reductions in V̇E with 56 and 140 kPa PIO2 during constant submaximal fin-swimming intensity compared to air might be prominently caused by peripheral chemoreceptor suppression.
No difference between 56 and 140 kPa was detected, indicating a PIO2 threshold limiting further hyperoxic influence on V̇E. O2 supply might sufficiently cover metabolic demands of submaximal exercise with 56 kPa, while further reductions in V̇E could be observed only by severely higher PIO2.
Cognitive performance by inhibitory control was unaffected by PIO2. Faster RTs but lower ACC were observed following vigorous exercise (75 % HRR) compared to rest, low, and moderate exercise.
Introduction
All underwater activities include physical exercise in hyperoxia (i.e. > 21 kPa inspiratory oxygen pressure (PIO2) in the breathing gas), based on increased ambient pressures at depth and amplified by frequently elevated fractions of oxygen in the breathing gas (Brebeck et al., Citation2018). During exercise in laboratory conditions, hyperoxia reduces heart rate (HR) and the ventilatory response with reduced blood lactate concentrations [Lac-] and a prolonged time to exhaustion (Peacher et al., Citation2010; Stellingwerff et al., Citation2006; Ulrich et al., Citation2017). However, the applied setting of SCUBA diving involves potentially influential factors like the physiological adaptations to immersion and the unique exercise modality of underwater fin-swimming (UFS). Furthermore, it offers practical relevance for the safety of all dives with a limited gas supply. Earlier work from our group confirmed reduced ventilation (V̇E) (Möller et al., Citation2022), but discrepancies to laboratory studies remained with no effects on HR and [Lac-]. In addition to the physiological effects, an elevated PIO2 might affect executive functions of cognitive performance (Scholey et al., Citation1999), potentially moderating the established interaction between exercise and cognition (Chang et al., Citation2012; Moreau & Chou, Citation2019). Therefore, both physiological and cognitive responses to hyperoxia were assessed within the unique combination of immersion effects and specific exercise offered in SCUBA diving.
Exercise performance enhancements of 3-30% are reported during hyperoxic exercise at dry sea level and are mainly attributed to improved maximum oxygen uptake (V̇O2max) of up to 10%. This effect is accompanied by reductions in V̇E, HR, and [Lac-] (Forster et al., Citation2011; Prieur et al., Citation2002; Sperlich et al., Citation2013; Sperlich et al., Citation2017; Ulrich et al., Citation2017). In these settings, hyperoxia might enhance the aerobic and delay the anaerobic metabolism's contribution during prolonged exercise, reflected by a reduction in local muscular O2 deficits (Dunworth et al., Citation2017; Mallette et al., Citation2018; Smit et al., Citation2018; Stellingwerff et al., Citation2006; Ulrich et al., Citation2017). This mechanism was also discussed in earlier work from our group to explain reductions in V̇E during hyperoxic UFS (Möller et al., Citation2022). While O2-hemoglobin saturation is nearly maximal during normoxia, arterial oxygen partial pressure (PaO2) is further increased by physically dissolved O2 from PIO2 > 21 kPa (Peacher et al., Citation2010). In turn, the O2 diffusion gradient to the working muscles and tissues can be facilitated. The concentration of physically dissolved O2 increases consistently with ambient pressure at water depth.
Wet pressure chamber experiments at 460 kPa ambient pressure with 174 kPa PIO2 revealed reductions in V̇E and HR without changes in V̇O2 during constant exercise (Fraser et al., Citation2011; Peacher et al., Citation2010). However, Peacher et al. (Citation2010) found significant butequal reductions in carbon dioxide output (V̇CO2) between hyperoxic and normoxic conditions attributed to similar metabolic processes. Conversely, Fraser et al. (Citation2011) found no changes in V̇CO2. Although these inconsistent results seem to reflect specific changes in muscle metabolism, a mitigated metabolic acidosis by hyperoxia might not be the only factor to explain lower V̇E. Instead, a direct inhibition of peripheral chemoreceptors controlling respiration by PaO2, PaCO2, and pH can be assumed. Chemoreceptor inhibition was also suspected to explain our earlier findings, where an approximately 20% reduction of V̇E for moderate fin-swimming velocities (0.6 m·s−1) was observed with 56 kPa compared to 29 kPa PIO2, whereas HR and [Lac-] were unaffected (Möller et al., Citation2022). However, limitations included a non-individualised exercise intensity prescription with possible influences on results. Generally, effects seem to depend on various influential factors, such as exercise modality, -intensity, -duration, and PIO2 (Knight et al., Citation1993; Linnarsson et al., Citation1974; Peltonen et al., Citation2001; Richardson et al., Citation1999; Welch et al., Citation1977), requiring further applied investigations to provide sport-specific recommendations for underwater activities.
In addition to physical fitness, cognition is another integral part of performance. Intact executive functions (EF) are paramount for higher-order processes underwater, including goal-directed behaviour, the inhibition of prepotent responses, and alternating attention between other divers, equipment, and environmental information (Diamond, Citation2013). A positive interaction between moderate-intensity aerobic exercise and EFs is established from laboratory studies. However, inconsistent results exist for the effects of high-intensity exercise (see Hsieh et al., Citation2021 for a review and Moreau & Chou, Citation2019 for a meta-analysis). EFs are impaired by shallow water immersion (Dalecki et al., Citation2012) and deteriorate with increasing water depth and nitrogen narcosis (Lafère et al., Citation2019; Steinberg & Doppelmayr, Citation2017). Furthermore, the positive exercise-cognition interaction seems to be altered during sport-specific underwater exercise (Möller et al., Citation2021a). Here, beneficial effects were reported for reaction times (RT) after moderate intensity exercise only and accompanied by a deterioration in task accuracy (i.e. speed-accuracy trade-off). This trade-off has also been reported as a compensatory mechanism in other stressful environments, like weightlessness in space (Strangman et al., Citation2014), where mistakes might result in severe consequences. However, the presence of hyperoxia during underwater activities leads to an increase in PaO2 and cerebral oxygenation (Calvert et al., Citation2007; Chung et al., Citation2007; Damato et al., Citation2020), possibly counteracting immersion-induced impairments and facilitating cognitive function (Brebeck et al., Citation2017; Chung et al., Citation2008; Scholey et al., Citation1999). Especially during high-intensity exercise, the cognitive and motor regions’ competition for O2 might be reduced (i.e. hypofrontality hypothesis; Davranche et al., Citation2009; Dietrich & Audiffren, Citation2011; Zimmer et al., Citation2016).
This study investigates physiological responses to varying levels of hyperbaric hyperoxia (i.e. 29–140 kPa PIO2) at three individually prescribed constant exercise intensities in the sport-specific context of underwater fin-swimming. Furthermore, EF performance (i.e. inhibitory control) is investigated following each exercise intensity and level of hyperoxia. We hypothesise (I) reduced V̇E and [Lac-] values during moderate and high exercise intensities with an increasing effect for 56 kPa PIO2 and 140 kPa PIO2 compared to breathing air and (II) improved executive functioning by hyperoxia in all exercise intensities with the best effects during high-intensity.
Methods
Participants
Ten male and five female certified autonomous divers (ISO: 24801–2 or higher; dives 376 ± 762; age: 28 ± 6.2 (mean ± SD)) participated in the study. All held a valid SCUBA diving medical examination and provided written informed consent before participation. Diving gear consisted of a provided 3 mm wetsuit, buoyancy control device, and personal mask and fins. All experimental procedures were performed following the declaration of Helsinki and its amendments. Ethical approval was granted by the German Sports University Cologne's ethics committee (Nr. 212/2021).
Physical performance assessment
All participants completed five tests separated by 2–29 days to ensure complete physical recovery. General physical fitness and peak values were assessed with an incremental laboratory performance test on a supine bicycle ergometer. The applied step protocol started with 3 min rest followed by 3 min of 50 watts (W) and increased by 25 W every 3 min until exhaustion. HR and breathing gases were measured beat-to-beat via electrocardiography belt (CustoGuard belt 3, Customed, Ottobrunn, Germany) and breath-by-breath (Metalyzer 3B®, Cortex Biophysik GmbH©, Leipzig, Germany), respectively. Capillary blood samples were taken from the earlobe at rest and within the last 15 s of each exercise step.
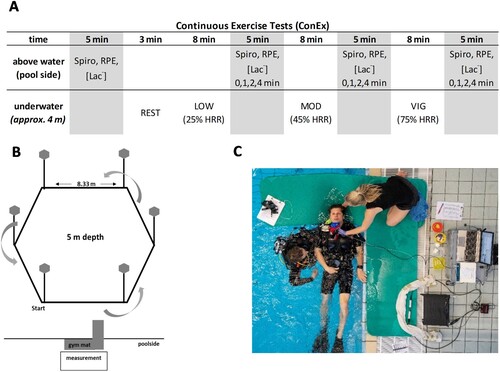
In contrast, sport-specific fitness must consider the influence of diving equipment, water resistance, and physiological adaptations to immersion. Therefore, an additional incremental underwater fin-swimming (UFS) test was conducted and subsequently used to derive individual exercise intensity prescriptions for the consecutive continuous exercise tests. A hexagonal-shaped parcours with 50 m girth was circled at about 4 m water depth (B). Fin-swimming velocity (v) was self-controlled via a stopwatch and a marching table, adapted from Steinberg et al. (Citation2017). After 3 min of rest at 5 m depth, participants started fin-swimming at 0.4 m·s−1, increasing v stepwise by 0.1 m·s−1 every 3 min until individual exhaustion (i.e. until the timing of the marching chart could not be maintained for two consecutive checkpoints). After every step, participants momentarily surfaced for capillary blood sampling and the rating of perceived exertion (RPE; Borg, Citation1982; surface time 30–45s). HR during underwater exercise was recorded continuously beat-to-beat via a Polar© chest belt and Polar© V800 watch. The diving tank and ambient pressure were recorded in 4 s intervals via a pressure transmitter and stored on a diving computer (Uwatec©, Galileo sol). Fin-swimming technique was evaluated for sufficiency during incremental exercise, excluding none of the participants for further participation. Peak values for UFS were reduced for V̇E, [Lac-], HR and RPE compared to exercise in the lab, emphasising the necessity to derive exercise prescriptions from sport-specific testing.
Figure 1. (A) Test procedure of the underwater performance test and the three continuous exercise tests (ConEx). Exercise intensity was controlled by individual percentages of heart rate reserve (HRR%) for low (low, 25% HHR), moderate (mod, 45% HRR), and vigorous exercise (vig, 75% HRR). (B) The underwater parcours setup for all underwater tests. (C) Measurement setup on the poolside for ConEx.
Continuous exercise tests
Three identical continuous exercise tests (ConEx) were performed in a cross-over, double-blind, and randomised design solely different by the fraction of O2 in the breathing gas (i.e. 21% = ConEx21, 40% = ConEx40, and 100% O2 = ConEx100). On each test day, participants performed UFS in three 8 min blocks, differentiated by the individually prescribed exercise intensities (see next section) and resulting in 24 min of total exercise time. The utilised O2 fractions resulted in approximately 29, 56, and 140 kPa PIO2 at 4 m water depth, respectively. An O2-certified filling station provided breathing gases. Mixtures were analysed again before every dive (Greisinger GOX100, Regenstauf, Germany). Despite the relative precision of currently available O2 analyzers, a deviation was unavoidable (i.e. 40.7 ± 1; 97.7 ± 1.4 kPa normobaric O2 pressure). Identical O2-clean and O2-compatible 11.1-liter aluminum tanks and breathing regulators were used.
Following the ACMS's guidelines for exercise testing and prescription, individual exercise intensity was derived as a percentage of heart rate reserve (HRR), calculated as the difference between resting HR (HRrest) and maximum HR (HRmax), as obtained by the sport-specific incremental underwater performance assessment (Riebe, Citation2018). For each test, participants performed UFS in 3 blocks of 8 min at low, 25% HRR (low), moderate, 45% HRR (mod), and vigorous, 75% HRR (vig) intensity, respectively. Before the first descent and after each exercise block, breathing gas analysis (Metalyzer 3B®, Cortex Biophysik GmbH©, Leipzig, Germany) and capillary blood sampling were performed for 5 min (A). RPE on the previous block of the underwater exercise was stated shortly after the ascent. The first 45 s of post-exercise measurements were conducted in a horizontal body position to reduce posture-induced blood shift (refer to data processing calculations for details and reasoning; C). Distance travelled was documented for each exercise block to allow the calculation of v. An experienced safety diver supervised all tests (≥ ISO Norm 24802-2).
Cognitive testing
Following every block of low, mod, and vig in all gas conditions, a tablet-based Eriksen Flanker task (B. A. Eriksen & Eriksen, Citation1974) was performed, investigating inhibitory control as an EF of cognitive performance (i.e. start: 60 s post-exercise; total duration: 4 min). Presented scenarios consisted of five arrows, from which only the middle one required a response, either left or right, depending on the direction of the middle arrow (i.e. the stimulus), with the surrounding arrows acting as distractors. The directions of the surrounding arrows were manipulated to create compatible (>>>>>) or incompatible (>><>>) settings, with the latter requiring inhibitory control for fast and correct responses. A complete trial consisted of 100 stimuli (50 compatible and 50 incompatible). A fixation cross appeared in the centre of the screen for 300 ms, thus, marking the exact spot where the target arrow would appear. Next, the fixation cross was wholly replaced by a five-arrow configuration that appeared for 100 ms and was followed by a blank screen until a response was given or the maximum response time of 2500 ms elapsed. Hand-held trigger buttons were used to enable responses, and participants were instructed to respond as fast and accurately as possible, which was established during task familiarisation (i.e. 200 stimuli initial familiarisation with additional 50 stimuli on every test day). RTs were recorded on a millisecond basis. The cognitive task was conducted upright in chest-out water immersion parallel to breathing gas analysis (see chapter continuous exercise tests). A constant distance to the screen, good visibility, and a quiet environment without auditory or visual distractions were ensured.
Data processing
Breath-by-breath and beat-to-beat data of the incremental test and all ConEx tests were interpolated to 1 s intervals. Continuous measurements of V̇O2 during underwater fin-swimming are typically complicated by locomotion, immersion, and ambient pressure (Pendergast et al., Citation2003). To receive an additional measure of exercise demands underwater, V̇O2 was predicted for UFS using backward calculation from post-exercise measurements of V̇O2 and HR during the time interval 5 - 20 s (t), as proposed by (Chaverri et al., Citation2016),
where pV̇O2(EX) [L·min−1] is the predicted V̇O2 for each of the 3 exercise intensity (low, mod, vig), V̇O2post-exercise (t) [L·min−1] and HRpost-exercise (t) [min−1] are measured during (t) post-exercise, and HRin-exercise [min−1] describes the measured HR during exercise. This formula stands under two assumptions: A relatively stable arterio-venous O2 difference short-term post-exercise, before venous return from the muscle can be measured at the mouth (Yoshida & Whipp, Citation1994), and a short-term unchanged stroke volume (SV) (M. Eriksen et al., Citation1990). Measurements were performed in a horizontal body position for the first 45 s post-exercise to prevent influences on SV by changes in posture and blood redistribution by hydrostatic pressure. V̇CO2 for the ConEx was calculated via intra-breath data using a weighted average over the breaths during 5 - 20 s post-exercise.
V̇E during UFS was calculated for ambient pressure with recordings of tank pressure and water depth,
where V̇E [L·min−1] reflects minute ventilation, ΔPtank [bar] is the change in tank pressure, Vtank [L] is the tank volume, and Pdepth−1 [bar] is the mean of depth (4 s sample rate). Means of HR and V̇E were calculated for the last 30 s of each exercise intensity for incremental UFS. For the ConEx tests, means of HR and V̇E were calculated over the last 7 min of each intensity to exclude the time necessary to adapt to the new v. The mean v of each exercise block was calculated as the dividend of distance travelled and time.
Capillary blood samples were analysed for blood lactate concentrations ([Lac-] (mmol·L−1)) (Biosen EKF-diagnostics, GmbH, Germany).
For the cognitive task, mean values and standard deviations were calculated for the RTs of correct responses, incorrect responses, and misses. Response accuracy (ACC) was calculated in percent [%] as the dividend from stimuli and correct responses. RTs and ACC are displayed separately for compatible (RTcom; ACCcom) and incompatible settings (RTincom; ACCincom). RTs > 1000 ms were excluded from the analysis.
Statistics
An a priori power analysis (G*Power 3.1.9.7; Faul et al., Citation2009) was conducted for a mixed design and α-level < 0.05, demanding 15 participants to provide a physiologically relevant effect size of f = 0.5 and a power of 1-ß = 0.9. Using SPSS statistics 28® (IBM©, USA), two-way ANOVAs with repeated measures on the factor GAS (ConEx21, ConEx40, ConEx100) and INTENSITY (low, mod, vig) were calculated to investigate differences for V̇E, [Lac-], v, HR, RPE, and post-V̇CO2.
Bonferroni corrected pairwise comparisons were used to investigate significant results. Main effect sizes were stated as partial eta squared (η2P) with values ≤ 0.10 indicating a small, ≤ 0.25 a medium, and ≤ 0.40 a large effect. Cohen's d was used for post hoc comparisons by calculating the quotient of the mean difference and the mean standard deviation. Effect sizes are depicted as small > 0.2, medium > 0.5, and large > 0.8 (Cohen, Citation2013).
Results
Continuous exercise tests
Significant main effects for V̇E were found for the factor GAS (p = 0.004, η2P = 0.356) and GAS*INTENSITY (p < 0.001, η2P = 0.302), with significantly lower values during ConEx100 compared to ConEx21 during mod (p = 0.006, d = 0.437) and vig (p = 0.006, d = 0.405), and significantly lower values during ConEx40 compared to ConEx21 during vig (p = 0.002, d = 0.525) compared to air (). [Lac-], v, HR, and RPE showed no effects for O2-content post-exercise. V̇CO2 showed no effect for O2-content (p = 0.669, η2P = 0.027) or GAS*INTENSITY (p = 0.717, η2P = 0.034) (refer to for all results).
Figure 2. Ventilation (V̇E) (L·min−1) is depicted as individual data (dots), mean values (x), medians (horizontal line), and standard errors (bars) for the continuous exercise tests (Conex21, ConEx40, ConEx100) and exercise intensities (low, mod, vig) over the last 7 min of underwater exercise. Significant post hoc effects are indicated with *.
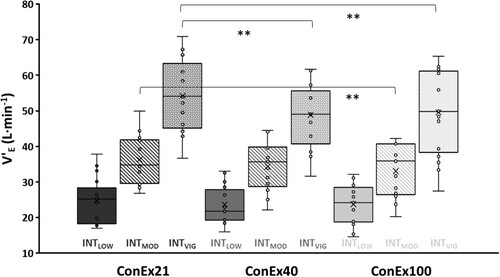
Table 1. Mean values and standard deviation are depicted for minute ventilation (V̇E) (L·min−1), heart rate (HR) (min−1), velocity (v) (m·s−1), rating of perceived exertion (RPE), blood lactate [Lac-] (mmol·L−1), and post-exercise carbon dioxide output (V̇CO2) (L·min−1) for each exercise intensity (low, mod, vig). Main effects for GAS, INTENSITY, and GAS*INTENSITY are listed below. Significant post hoc differences to 29 kPa are indicated with* and for RTincom with #.
Executive functions
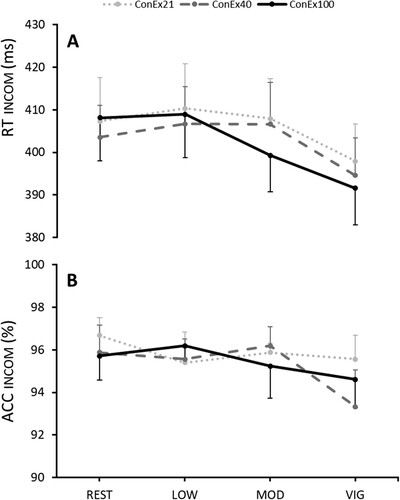
RTincom showed a significant main effect for INTENSITY (P < 0.001; ηp2 = 0.4) with significantly faster RTs after vig compared to rest (P = 0.022), low (P < 0.001), and mod (P < 0.001). No effects were observed for ACCincom. No GAS effect could be observed (see and ).
Discussion
This study investigated the effects of different exercise intensities during sport-specific UFS and different O2 fractions in the breathing gas on physiological values and post-exercise inhibitory control. Results revealed significantly lower V̇E during mod (−8.9 %) and vig (−8.5 %) exercise at 140 kPa, and during vig (−9.8 %) for 56 kPa compared to 29 kPa. No significant differences arose between 56 and 140 kPa PIO2. No effects of O2-fraction were observed for HR, [Lac-], v, RPE, and post-exercise V̇CO2. Therefore, our first hypothesis, suggesting amplified beneficial effects of elevated PIO2, is only partly confirmed. Furthermore, significantly lower RTs but not ACC for inhibitory control were observed after vig compared to all other intensities, with no influence of O2-fraction. Thus, we cannot confirm the hypothesised positive effects of hyperoxia on EFs.
The observed reduction in V̇E during UFS with an elevated O2-fraction in the breathing gas is in line with work from Fraser et al. (Citation2011) and Peacher et al. (Citation2010), reporting 15.2 % and 16.3 % reductions in V̇E during 16 min cycle exercise in a wet pressure chamber with 175 kPa PIO2 at 470 kPa ambient pressure. Compared to our earlier work (i.e. reductions in V̇E up to 20 %), the present findings show slightly lower effects that might be attributed to differences in load control (i.e. individualised vs. fixed exercise intensities) or exercise mode (i.e. 3 min steps vs. continuous exercise; Möller et al., Citation2022). It can be assumed that the effects of PIO2 on V̇E occur only up to a certain threshold, likely dependent on exercise intensity and acute metabolic O2-demands.
To date, the literature produced inconsistent results in supporting altered metabolic processes as the main reason for physiological changes during exercise, suspecting sport-specific exercise modality, -duration, and PIO2 as modulating factors for effects on HR and [Lac-] in addition to V̇E (Brugniaux et al., Citation2018; Favier et al., Citation2005; Sperlich et al., Citation2013; Stellingwerff et al., Citation2006; Welch & Pedersen, Citation1981). Based on the very limited feasibility of direct metabolic measurements during sport-specific exercise, post-exercise values of [Lac-] and V̇CO2 were utilised for metabolic estimations. End-tidal V̇CO2 can be regarded as highly representative of arterial carbon dioxide pressure (PaCO2) under normal conditions (Dunworth et al., Citation2017), enabling estimation of metabolic processes from post-exercise breathing gas analysis. Based on this assumption, our results of similar [Lac-] and post-exercise V̇CO2 values between 29 and 140 kPa PIO2 (all p > 0.669) suggest no significant hyperoxic influence on metabolic processes. Opposed to that, results from Lambertsen et al. (Citation1959) and Peacher et al. (Citation2010) question the connection between PaCO2 and end-tidal V̇CO2, reporting decreases in V̇CO2 paralleled by elevated PaCO2 during hyperoxic exercise. However, both authors used ≥ 175 kPa PIO2, which is beyond recreational diving limits (i.e. max PIO2 140 kPa). Furthermore, hyperoxia might have reduced venous CO2 solubility and thus facilitated the Haldane effect, corresponding to the absence of differences for PaCO2 in the PIO2 range between69 and 127 kPa at 470 kPa ambient pressure.
Moreover, while our earlier results and data from the present work show only descriptively lower [Lac-] values after hyperoxic exercise, possible metabolic changes at the muscular level might be too small to detect by indirect measurements at the mouth (i.e. V̇CO2) or in the periphery (i.e. capillary blood lac). Adding to that, the validity of V̇CO2 measurements might be reduced by breath-by-breath measurement errors up to 6.3% (Carter & Jeukendrup, Citation2002). Therefore, the reported effects of hyperoxia on metabolic processes during laboratory exercise might be strengthened for sport-specific contexts by more invasive measurements in the future (Brugniaux et al., Citation2018; Favier et al., Citation2005; Mallette et al., Citation2018; Sperlich et al., Citation2013; Stellingwerff et al., Citation2006). Although we discussed hyperoxia-induced decreased local O2 deficits, especially during high-intensity and transient phases during exercise, the anaerobic demands might be insufficiently high during the applied intensities of underwater fin-swimming (i.e. no incremental exercise).
Concerning hyperoxic effects on HR, inconsistent findings from the literature include a tendency to decreased resting HR by increased baroreceptor activity, altered chemoreceptor activity, or increased parasympathetic tone with no or only a slightly decreased peak HR (Lund et al., Citation1999; Mallette et al., Citation2018; Sperlich et al., Citation2017). In hyperbaric immersed conditions, Fraser et al. (Citation2011) and Peacher et al. (Citation2010) did report reduced resting and peak HR, possibly compensatory to immersion-induced blood shift and increased right-heart pre-load (Brugniaux et al., Citation2018; Godek & Freeman, Citation2023). Our previous results did not show any differences in HR between 29 kPa and 56 kPa PIO2 during immersed exercise (Möller et al., Citation2022). While the present studies’ HR-controlled underwater exercise did not allow the direct investigation of hyperoxic effects on HR, we would have expected an increase in v during hyperoxic conditions, attempting to meet the prescribed HR during exercise. We did not find any influence of the O2-fraction on v. Ultimately, a suppression of the ventilatory drive by direct chemoreceptor inhibition seems to be the superior cause for reductions in V̇E in the context of sport-specific submaximal exercise and elevated PIO2 (Fraser et al., Citation2011; Möller et al., Citation2022; Peacher et al., Citation2010).
Inhibitory control as a core function of cognitive control was assumed to be reduced by shallow water immersion but facilitated by physical exercise and increased PIO2. The first assumption was built on work by Dalecki et al. (Citation2012), observing cognitive impairments for tasks in shallow water immersion. The present study could not verify these results, as all tasks were conducted in head-out immersion without land-based control. Nevertheless, an overall dampening effect from physiological adaptations and slightly increased inspiratory nitrogen pressure might be carefully assumed. In addition, a positive influence of moderate exercise intensity on following executive functioning was reported by numerous laboratory studies (Hsieh et al., Citation2021; Moreau & Chou, Citation2019). Underlying mechanisms range from increased arousal, blood flow, and cerebral oxygenation, explaining effects during or shortly following exercise (Pontifex et al., Citation2019), to the increasing release of neurochemicals like catecholamines, brain-derived neurotrophic factor or the accumulation of lactate with rising exercise intensity (Hashimoto et al., Citation2018; Knaepen et al., Citation2010; Rasmussen et al., Citation2009).
Based on the positive effects of catecholamines on neural excitability and its long half-time (Eisenhofer et al., Citation2004; McMorris, Citation2016), the positive effects of moderate-intensity exercise are expected to persist during post-exercise cognitive testing. Furthermore, high-intensity exercise might induce similar or even superior effects. Surprisingly, our findings showed no effects of moderate-intensity exercise, which contradicts earlier results from our group (Möller et al., Citation2021b). In the present work, RTs for inhibitory control were improved only after vigorous intensity (i.e., 75 % HRR). These findings might arise from counteracting effects of water immersion and exercise, where higher intensities are necessary to produce beneficial effects on cognition. Furthermore, some studies reported an increase in cerebral blood flow and oxygenation after high-intensity and long-duration exercise (i.e. the hyperfrontality hypothesis; Sudo et al., Citation2017; Tempest et al., Citation2017), opposing the hypofrontality hypothesis established by Dietrich (Citation2006). Unfortunately, no measurements could verify these effects for the present findings. Lastly, hyperoxia is known to increase cerebral oxygenation despite vasoconstriction and improve cognition, demonstrated in work by Damato et al. (Citation2020) with a normobaric PIO2 of 100 kPa and backed up our hypothesis of positive hyperoxic effects on EFs. These results are supported by work from Scholey et al. (Citation1999), discussing an elevated PaO2 to support increased metabolic demands during cognitive workload, thus, facilitating cognition. In combination with competing motor- and cognitive demands during exercise, hyperoxia could maintain oxygenation, especially during prolonged and intense exercise. However, our findings showed no effects of hyperoxia on EF performance.
Limitations
The main limitations include post-exercise and non-invasive measurements, with the potential of missing effects during exercise. In addition, a time delay for pulmonary and peripheral measurements must be considered. Considering inhibitory control, this work concentrated on the after-effects of hyperoxic exercise, as participants breathed under normobaric and normoxic conditions during cognitive testing. Due to design complexity and pool availabilities, it was not possible to maintain a constant interval between the test days. However, a minimum of 2 days was implemented to secure full physical recovery from each test. While sufficient individual fin-swimming technique, body position in the water, and the resulting water resistance were evaluated as inclusion criteria, interindividual differences and an influence by the applied gender distribution could still be expected.
Conclusion
Results show a significantly lower V̇E for mod and vig fin-swimming at 56 kPa (vig 10.8%) and 140 kPa PIO2 (mod: 9.6 %; vig: 9.3 %) compared to air. No differences for [Lac-], post-V̇CO2, v, and RPE were observed between 29, 56, and 140 kPa PIO2, which is in line with results from our earlier study and hyperbaric exercise without sport-specific locomotion. Hyperoxia-induced V̇E reductions are seemingly caused prominently by peripheral chemoreceptor suppression during submaximal intensity, while the metabolic component might gain importance with increased anaerobic energy demand. Hyperoxia did not influence EFs, contradicting some laboratory results and emphasising the potential influence of sport-specific and environmental factors. RTs were accelerated, and ACC was reduced with increasing exercise intensity. This trade-off in demanding environments and exercise intensities might increase the risk of errors. Overall, these findings might improve safety for dives with a limited gas supply, especially in the context of incidents or rescue scenarios.
Acknowledgments
The German Sport University Cologne supported this work with an internal research fund. We thank “Poseidon Diving Systems” (Kahl am Main, Germany) and the Diving School “Magic Factory” (Wuppertal, Germany) for equipment and gas support, respectively. Special thanks to all participants for their involvement and Dennis Cetin and Tariq Koch for their help in data acquisition.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Borg, G. (1982). Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. International Journal of Sports Medicine, 3(3), 153–158. https://doi.org/10.1055/s-2008-1026080
- Brebeck, A., Deussen, A., Range, U., Balestra, C., Cleveland, S., & Schipke, J. D. (2018). Beneficial effect of enriched air nitrox on bubble formation during scuba diving. An open-water study. Journal of Sports Sciences, 36(6), 605–612. https://doi.org/10.1080/02640414.2017.1326617
- Brebeck, A., Deussen, A., Schmitz-Peiffer, H., Range, U., Balestra, C., Cleveland, S., & Schipke, J. D. (2017). Effects of oxygen-enriched air on cognitive performance during SCUBA-diving - an open-water study. Research in Sports Medicine, 25(3), 345–356. https://doi.org/10.1080/15438627.2017.1314289
- Brugniaux, J. V., Coombs, G. B., Barak, O. F., DUJIC, Z., Sekhon, M. S., & Ainslie, P. N. (2018). Highs and lows of hyperoxia: Physiological, performance, and clinical aspects. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 315(1), R1–R27. https://doi.org/10.1152/ajpregu.00165.2017
- Calvert, J. W., Cahill, J., & Zhang, J. H. (2007). Hyperbaric oxygen and cerebral physiology. Neurological Research, 29(2), 132–141. https://doi.org/10.1179/016164107X174156
- Carter, J., & Jeukendrup, A. E. (2002). Validity and reliability of three commercially available breath-by-breath respiratory systems. European Journal of Applied Physiology, 86(5), 435–441. https://doi.org/10.1007/s00421-001-0572-2
- Chang, Y. K., Labban, J. D., Gapin, J. I., & Etnier, J. L. (2012). The effects of acute exercise on cognitive performance: A meta-analysis. Brain Research, 87–101. https://doi.org/10.1016/j.brainres.2012.02.068
- Chaverri, D., Schuller, T., Iglesias, X., Hoffmann, U., & Rodríguez, F. A. (2016). A New model for estimating peak oxygen uptake based on postexercise measurements in swimming. International Journal of Sports Physiology and Performance, 11(4), 419–424. https://doi.org/10.1123/ijspp.2015-0227
- Chung, S.-C., Kwon, J.-H., Lee, H.-W., Tack, G.-R., Lee, B., Yi, J.-H., & Lee, S.-Y. (2007). Effects of high concentration oxygen administration on n-back task performance and physiological signals. Physiological Measurement, 28(4), 389–396. https://doi.org/10.1088/0967-3334/28/4/005
- Chung, S.-C., Lee, H.-W., Choi, M.-H., Tack, G.-R., Lee, B., Yi, J.-H., Kim, H.-J., & Lee, B.-Y. (2008). A study on the effects of 40% oxygen on addition task performance in three levels of difficulty and physiological signals. The International Journal of Neuroscience, 118(7), 905–916. https://doi.org/10.1080/00207450701750455
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Routledge.
- Dalecki, M., Bock, O., & Schulze, B. (2012). Cognitive impairment during 5 m water immersion. Journal of Applied Physiology (Bethesda, Md. : 1985), 113(7), 1075–1081. https://doi.org/10.1152/japplphysiol.00825.2012.
- Damato, E. G., Flak, T. A., Mayes, R. S., Strohl, K. P., Ziganti, A. M., Abdollahifar, A., Flask, C. A., La Manna, J. C., & Decker, M. J. (2020). Neurovascular and cortical responses to hyperoxia: Enhanced cognition and electroencephalographic activity despite reduced perfusion. The Journal of Physiology, 598(18), 3941–3956. https://doi.org/10.1113/JP279453
- Davranche, K., Hall, B., & McMorris, T. (2009). Effect of acute exercise on cognitive control required during an Eriksen flanker task. Journal of Sport and Exercise Psychology, 31(5), 628–639. https://doi.org/10.1123/jsep.31.5.628
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
- Dietrich, A. (2006). Transient hypofrontality as a mechanism for the psychological effects of exercise. Psychiatry Research, 145(1), 79–83. https://doi.org/10.1016/j.psychres.2005.07.033
- Dietrich, A., & Audiffren, M. (2011). The reticular-activating hypofrontality (RAH) model of acute exercise. Neuroscience & Biobehavioral Reviews, 35(6), 1305–1325. https://doi.org/10.1016/j.neubiorev.2011.02.001
- Dunworth, S. A., Natoli, M. J., Cooter, M., Cherry, A. D., Peacher, D. F., Potter, J. F., Wester, T. E., Freiberger, J. J., & Moon, R. E. (2017). Hypercapnia in diving: A review of CO2 retention in submersed exercise at depth. Undersea and Hyperbaric Medicine, 44(3), 191–209. https://doi.org/10.22462/5.6.2017.1
- Eisenhofer, G., Kopin, I. J., & Goldstein, D. S. (2004). Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacological Reviews, 56(3), 331–349. https://doi.org/10.1124/pr.56.3.1
- Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. https://doi.org/10.3758/BF03203267
- Eriksen, M., Waaler, B. A., Walløe, L., & Wesche, J. (1990). Dynamics and dimensions of cardiac output changes in humans at the onset and at the end of moderate rhythmic exercise. The Journal of Physiology, 426(1), 423–437. https://doi.org/10.1113/jphysiol.1990.sp018147
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
- Favier, F. B., Prieur, F., Grataloup, O., Busso, T., Castells, J., Denis, C., Geyssant, A., & Benoit, H. (2005). A high blood lactate induced by heavy exercise does not affect the increase in submaximal VO2 with hyperoxia. European Journal of Applied Physiology, 94(1-2), 107–112. https://doi.org/10.1007/s00421-004-1310-3
- Forster, H. V., Haouzi, P., & Dempsey, J. A. (2011). Comprehensive physiology. Comprehensive Physiology, 2(1), 743–777. https://doi.org/10.1002/cphy.c100045
- Fraser, J. A. V., Peacher, D. F., Freiberger, J. J., Natoli, M. J., Schinazi, E. A., Beck, I. V., Walker, J. R., Doar, P. O., Boso, A. E., Walker, A. J., Kernagis, D. N., & Moon, R. E. (2011). Risk factors for immersion pulmonary edema: Hyperoxia does not attenuate pulmonary hypertension associated with cold water-immersed prone exercise at 4.7 ATA. Journal of Applied Physiology (1985), 110(3), 610–618. https://doi.org/10.1152/japplphysiol.01088.2010
- Godek, D., & Freeman, A. M. (2023). StatPearls: Physiology, diving reflex. NCBI. PMID: 30855833.
- Hashimoto, T., Tsukamoto, H., Takenaka, S., Olesen, N. D., Petersen, L. G., Sørensen, H., Nielsen, H. B., Secher, N. H., & Ogoh, S. (2018). Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. The FASEB Journal, 32(3), 1417–1427. https://doi.org/10.1096/fj.201700381RR
- Hsieh, S.-S., Chueh, T.-Y., Huang, C.-J., Kao, S.-C., Hillman, C. H., Chang, Y. K., & Hung, T.-M. (2021). Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. Journal of Sports Sciences, 39(1), 10–22. https://doi.org/10.1080/02640414.2020.1803630
- Knaepen, K., Goekint, M., Heyman, E. M., & Meeusen, R. (2010). Neuroplasticity – exercise-induced response of peripheral brain-derived neurotrophic factor. Sports Medicine (Auckland, N.Z.), 40(9), 765–801. https://doi.org/10.2165/11534530-000000000-00000
- Knight, D. R., Schaffartzik, W., Poole, D. C., Hogan, M. C., Bebout, D. E., & Wagner, P. D. (1993). Effects of hyperoxia on maximal leg O2 supply and utilization in men. Journal of Applied Physiology (Bethesda, Md.: 1985), 75(6), 2586–2594. https://doi.org/10.1152/jappl.1993.75.6.2586
- Lafère, P., Hemelryck, W., Germonpré, P., Matity, L., Guerrero, F., & Balestra, C. (2019). Early detection of diving-related cognitive impairment of different nitrogen-oxygen gas mixtures using critical flicker fusion frequency. Diving and Hyperbaric Medicine Journal, 49(2), 119–126. https://doi.org/10.28920/dhm49.2.119-126
- Lambertsen, C. J., Owen, S. G., Wendel, H., Stroud, M. W., Lurie, A. A., Lochner, W., & Clark, G. F. (1959). Respiratory and cerebral circulatory control during exercise at.21 and 2.0 atmospheres inspired pO2. Journal of Applied Physiology, 14(6), 966–982. https://doi.org/10.1152/jappl.1959.14.6.966
- Linnarsson, D., Karlsson, J., Fagraeus, L., & Saltin, B. (1974). Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. Journal of Applied Physiology, 36(4), 399–402. https://doi.org/10.1152/jappl.1974.36.4.399
- Lund, V. E., Kentala, E., Scheinin, H., Klossner, J., Helenius, H., Sariola-Heinonen, K., & Jalonen, J. (1999). Heart rate variability in healthy volunteers during normobaric and hyperbaric hyperoxia. Acta Physiologica Scandinavica, 167(1), 29–35. https://doi.org/10.1046/j.1365-201x.1999.00581.x
- Mallette, M. M., Stewart, D. G., & Cheung, S. S. (2018). The effects of hyperoxia on sea-level exercise performance, training, and recovery: A meta-analysis. Sports Medicine (Auckland, N.Z.), 48(1), 153–175. https://doi.org/10.1007/s40279-017-0791-2
- McMorris, T. (2016). Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiology & Behavior, 165, 291–299. https://doi.org/10.1016/j.physbeh.2016.08.011
- Möller, F., Hoffmann, U., Dalecki, M., Dräger, T., Doppelmayr, M., & Steinberg, F. (2021a). Physical exercise intensity during submersion selectively affects executive functions. Human Factors: The Journal of the Human Factors and Ergonomics Society, 63(2), 227–239. https://doi.org/10.1177/0018720819879313.
- Möller, F., Hoffmann, U., Vogt, T., & Steinberg, F. (2021b). Exercise-Related effects on executive functions during a simulated underwater extravehicular activity. Human Factors: The Journal of the Human Factors and Ergonomics Society, 187208211032868. https://doi.org/10.1177/00187208211032868
- Möller, F., Jacobi, E., Hoffmann, U., Muth, T., & Schipke, J. D. (2022). Oxygen-enriched air decreases ventilation during high-intensity fin-swimming underwater. International Journal of Sports Medicine, 43(03), 230–236. https://doi.org/10.1055/a-1554-5093.
- Moreau, D., & Chou, E. (2019). The acute effect of high-intensity exercise on executive function: A meta-analysis. Perspectives on Psychological Science, 14(5), 734–764. https://doi.org/10.1177/1745691619850568
- Peacher, D. F., Pecorella, S. R. H., Freiberger, J. J., Natoli, M. J., Schinazi, E. A., Doar, P. O., Boso, A. E., Walker, A. J., Gill, M., Kernagis, D., Uguccioni, D., & Moon, R. E. (2010). Effects of hyperoxia on ventilation and pulmonary hemodynamics during immersed prone exercise at 4.7 ATA: Possible implications for immersion pulmonary edema. Journal of Applied Physiology (1985), 109(1), 68–78. https://doi.org/10.1152/japplphysiol.01431.2009
- Peltonen, J. E., Tikkanen, H. O., & Rusko, H. K. (2001). Cardiorespiratory responses to exercise in acute hypoxia, hyperoxia and normoxia. European Journal of Applied Physiology, 85(1-2), 82–88. https://doi.org/10.1007/s004210100411
- Pendergast, D. R., Mollendorf, J., Logue, C., & Samimy, S. (2003). Evaluation of fins used in underwater swimming. Undersea & Hyperbaric Medicine, 30(1), 57–73. PMID: 12841609.
- Pontifex, M. B., McGowan, A. L., Chandler, M. C., Gwizdala, K. L., Parks, A. C., Fenn, K., & Kamijo, K. (2019). A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychology of Sport and Exercise, 40, 1–22. https://doi.org/10.1016/j.psychsport.2018.08.015
- Prieur, F., Benoit, H., Busso, T., Castells, J., Geyssant, A., & Denis, C. (2002). Effects of moderate hyperoxia on oxygen consumption during submaximal and maximal exercise. European Journal of Applied Physiology, 88(3), 235–242. https://doi.org/10.1007/s00421-002-0707-0
- Rasmussen, P., Brassard, P., Adser, H., Pedersen, M. V., Leick, L., Hart, E., Secher, N. H., Pedersen, B. K., & Pilegaard, H. (2009). Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology, 94(10), 1062–1069. https://doi.org/10.1113/expphysiol.2009.048512
- Richardson, R. S., Grassi, B., Gavin, T. P., Haseler, L. J., Tagore, K., Roca, J., & Wagner, P. D. (1999). Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. Journal of Applied Physiology (Bethesda, Md.: 1985), 86(3), 1048–1053. https://doi.org/10.1152/jappl.1999.86.3.1048
- Riebe, D. (2018). Guidelines for exercise testing and prescription (ACSM). Wolters Kluwer/Lippincott Williams & Wilkins.
- Scholey, A. B., Moss, M. C., Neaven, N., & Wesnes, K. (1999). Cognitive performance, hyperoxia, and heart rate following oxygen administration in healthy young adults. Physiology & Behavior, 67(5), 783–789. https://doi.org/10.1016/S0031-9384(99)00183-3
- Smit, B., Smulders, Y. M., van der Wouden, J. C., Oudemans-van Straaten, H. M., & Spoelstra-de Man, A. M. E. (2018). Hemodynamic effects of acute hyperoxia: Systematic review and meta-analysis. Critical Care (London, England), 22(1), 45. https://doi.org/10.1186/s13054-018-1968-2
- Sperlich, B., Schiffer, T., Hoffmann, U., Strüder, H. K., & Hollmann, W. (2013). The spirografic oxygen deficit: Its role in cardiopulmonary exercise testing. International Journal of Sports Medicine, 34(12), 1074–1078. https://doi.org/10.1055/s-0033-1334877
- Sperlich, B., Zinner, C., Hauser, A., Holmberg, H.-C., & Wegrzyk, J. (2017). The impact of hyperoxia on human performance and recovery. Sports Medicine (Auckland, N.Z.), 47(3), 429–438. https://doi.org/10.1007/s40279-016-0590-1
- Steinberg, F., & Doppelmayr, M. (2017). Executive functions of divers are selectively impaired at 20-meter water depth. Frontiers in psychology, 8, 1000. https://doi.org/10.3389/fpsyg.2017.01000.
- Steinberg, F., Dräger, T., Steegmanns, A., Dalecki, M., Röschmann, M., & Hoffmann, U. (2017). Title: fit2dive – A field test for assessing the specific capability of underwater fin swimming with SCUBA. International Journal of Performance Analysis in Sport, 11(1), 197–208. https://doi.org/10.1080/24748668.2011.11868540.
- Stellingwerff, T., Leblanc, P. J., Hollidge, M. G., Heigenhauser, G. J. F., & Spriet, L. L. (2006). Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. American Journal of Physiology-Endocrinology and Metabolism, 290(6), E1180–E1190. https://doi.org/10.1152/ajpendo.00499.2005
- Strangman, G. E., Sipes, W., & Beven, G. (2014). Human cognitive performance in spaceflight and analogue environments. Aviation, Space, and Environmental Medicine, 1033–1048. https://doi.org/10.3357/ASEM.3961.2014
- Sudo, M., Komiyama, T., Aoyagi, R., Nagamatsu, T., Higaki, Y., & Ando, S. (2017). Executive function after exhaustive exercise. European Journal of Applied Physiology, 117(10), 2029–2038. https://doi.org/10.1007/s00421-017-3692-z
- Tempest, G. D., Davranche, K., Brisswalter, J., Perrey, S., & Radel, R. (2017). The differential effects of prolonged exercise upon executive function and cerebral oxygenation. Brain and Cognition, 113, 133–141. https://doi.org/10.1016/j.bandc.2017.02.001
- Ulrich, S., Hasler, E. D., Müller-Mottet, S., Keusch, S., Furian, M., Latshang, T. D., Schneider, S., Saxer, S., & Bloch, K. E. (2017). Mechanisms of improved exercise performance under hyperoxia. Respiration; International Review of Thoracic Diseases, 93(2), 90–98. https://doi.org/10.1159/000453620
- Welch, H. G., Bonde-Petersen, F., Graham, T., Klausen, K., & Secher, N. (1977). Effects of hyperoxia on leg blood flow and metabolism during exercise. Journal of Applied Physiology, 42(3), 385–390. https://doi.org/10.1152/jappl.1977.42.3.385
- Welch, H. G., & Pedersen, P. K. (1981). Measurement of metabolic rate in hyperoxia. Journal of Applied Physiology, 51(3), 725–731. https://doi.org/10.1152/jappl.1981.51.3.725
- Yoshida, T., & Whipp, B. J. (1994). Dynamic asymmetries of cardiac output transients in response to muscular exercise in man. The Journal of Physiology, 480(2), 355–359. https://doi.org/10.1113/jphysiol.1994.sp020365
- Zimmer, P., Stritt, C., Bloch, W., Schmidt, F.-P., Hübner, S. T., Binnebößel, S., Schenk, A., & Oberste, M. (2016). The effects of different aerobic exercise intensities on serum serotonin concentrations and their association with Stroop task performance: A randomized controlled trial. European Journal of Applied Physiology, 116(10), 2025–2034. https://doi.org/10.1007/s00421-016-3456-1