ABSTRACT
Introduction
Central Serous Chorioretinopathy (CSC), a leading cause of vision loss, presents diverse treatment challenges based on its acute or chronic manifestation.
Areas covered
In acute CSC, treatment typically involves observation, reserving intervention for cases where optimal vision is crucial for professional activities. Emerging evidence advocates the use of half-dose or half-fluence Photodynamic Therapy (PDT), directed by Indocyanine Green Angiography (ICGA) or Fluorescein Angiography (FA), to hasten subretinal fluid (SRF) resolution, improve vision, and minimize recurrence. Chronic CSC, on the other hand, increasingly favors half-dose PDT, a recommendation strengthened by studies such as the PLACE trial. CSC complicated by Choroidal Neovascularization (CNV) necessitates management through intravitreal anti-Vascular Endothelial Growth Factor (anti-VEGF) injections and/or half-dose PDT. In cases of polypoidal choroidal vasculopathy (PCV), a combination of anti-VEGF therapy with PDT proves beneficial. For scenarios where standard treatments are impractical, Eplerenone emerges as the primary oral medication.
Methods
A literature search of all original articles was performed using the major online databases. The following keywords ‘Central Serous Chorioretinopathy’ and ‘Treatment,’ were searched in combination with ‘Photodynamic therapy,’ ‘Focal Laser Photocoagulation,’ ‘Subthreshold Micropulse Laser’ and ‘Systemic treatments.’
Expert opinion
Managing CSC requires a patient-centered approach. In acute CSC, careful monitoring and lifestyle modifications may suffice, while in chronic CSC, PDT is a primary therapeutic option.
1. Introduction
Central serous chorioretinopathy (CSC) is a pathology involving choroid and retina layers characterized by areas of leakage through an altered retinal pigment epithelium (RPE), causing idiopathic serous detachment of the retina. The mean age of onset is 40–50 years old with a wide range 7 to 83 years [Citation1–3]. On average, males are more affected than females [Citation4]. CSC was first described in 1866 by Albrecht von Graefe as ‘central recurring retinitis’ [Citation5]. The term ‘central serous chorioretinitis’ was introduced in 1936 by Kitahara, postulating that was a condition secondary to Tuberculosis infection and describing the clinical manifestation in the Japanese population [Citation6]. The following year, Horniker named the pathology as ‘capillaro-spastic central retinitis’ hypothesizing a vascular origin [Citation1,Citation7]. Duke-Elder in 1940 renamed this condition in ‘central serous retinopathy’ [Citation8]. Important advancements were introduced in 1955 by Bennett who reported a correlation of this condition with ‘stress’ stimuli faced by the patient during the life [Citation9]. Originally, it was believed that the source of macular fluid was leakage from retinal vessels. In 1965, Maumenee and colleagues identified that the leakage causing macular fluid actually originated at the level of the RPE, as revealed through fluorescein angioscopy [Citation10]. The current theory was proposed by Gass in 1967 before the introduction in the clinical practice of Indocyanine Green Angiography (ICGA) and optical coherence tomography (OCT). The detachment of the neuroepithelium was hypothesized to stem from the hyperpermeability of the choriocapillaris, a condition thought to be induced by an increase in hydrostatic pressure within the choroid [Citation11]. The hypothesis of the choroid as leading cause was also supported by other studies that highlighted choroidal hyperfluorescence on ICGA [Citation12].
CSC is a prevalent ocular disease predominantly affecting middle-aged men, significantly impacting patients’ quality of life. The objective of this review is to offer a comprehensive overview of therapeutic options available for CSC.
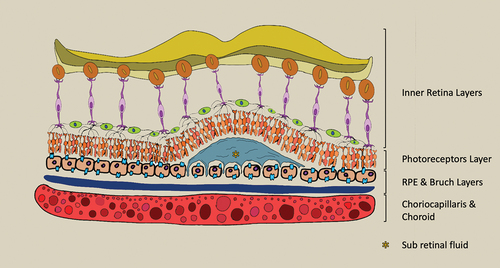
According to Gass theory, the increased hydrostatic pressure causes choroid hyperpermeability leading alterations in RPE and consequent subretinal fluid (SRF) formation [Citation11,Citation13] ( and ). Initially, various theories posited that alterations in the RPE were the principal cause of this pathology. Subsequent clarifications, however, have indicated that these RPE changes are in fact secondary, resulting from the prolonged effects of choroidal hyperpermeability [Citation14]. Additionally, the RPE changes observed can range from focal to widespread areas of degeneration, depending on the onset of CSC [Citation15]. In CSC, several choroidal alterations have been identified, including an increased thickness of the choroid, atrophy in its inner layers, and a higher choroidal vascularity index (CVI) [Citation16]. In patients with CSC, OCT angiography (OCTA) revealed increased choriocapillary hypoperfusion, caused by compression from a thickened choroid, which in turn leads to RPE ischemia and subsequent SRF accumulation [Citation17,Citation18]. Nowadays, CSC is considered part of pachychoroid spectrum disease which also count Pachychoroid Pigment Epitheliopathy (PPE), Pachychoroid Neovasculopathy (PNV) and Polypoidal Choroidal Vasculopathy (PCV) [Citation19]. The word ‘pachychoroid’ describes an increasing thickness of the choroid layer. This is marked by persistent enlargements of the large vessels in Haller’s layer, which in turn press against the adjacent Sattler’s layer and choriocapillaris [Citation20]. Margolis and Spaide in 2008 first measured the choroidal thickness using Enhanced Depth Imaging OCT [Citation21]. Moreover, the definition of pachychoroid could also indicate a dilation of the vessels in Haller’s layer, referred to as pachyvessels [Citation22]. According to the latest theory, all the aforementioned conditions present in pachychoroid spectrum disease are considered different stages of the same pathology. The first stage is only presenting a thicker choroid without any RPE changes. Many patients remain with these findings without evolving in the advanced stages for years. PPE may represent the second stage due to appearance of RPE alterations. The third stage is characterized by SRF due to leakage from choroid, typically observed the CSC [Citation22]. Conversely, it is recognized that CSC can develop without any choroidal thickening. In its advanced phase, typically the fourth stage, choroidal neovascularization (CNV) can manifest beneath the RPE, leading to either PNV or, in the presence of a polyp, PCV [Citation23]. Deepening the concept of increased hydrostatic pressure, it was seen that eyes affected by CSC exhibit intrinsic abnormalities in venous outflow. Moreover, several studies highlight that intervortex venous anastomoses are observed in the macular region, contributing to the accumulation of SRF [Citation24].
A well-documented risk factor in the development of CSC is the elevation of cortisol levels in the bloodstream. Conditions characterized by endogenous hypercortisolism, such as Cushing’s syndrome, pregnancy, stress, and A-type personality, have been linked to the development of CSC [Citation25]. Hypercortisolism induces the activation of the mineralocorticoid receptor, which exhibits expression within vascular endothelial cells, pericytes, and smooth muscle cells. Upon excessive activation, this receptor contributes pathogenically through mechanisms involving oxidative stress, inflammation, endothelial dysfunction, vascular remodeling, stiffening, and fibrosis. Within the retina, mineralocorticoid receptor expression is evident in both retinal and choroidal vessels, as well as in cells originating from neural and glial lineages. Recent experimental studies investigating mineralocorticoid receptor pathway activation, employing either pharmacological agents or transgenic manipulation, have unveiled its propensity to promote diverse pathological processes within the retinal and choroidal compartments, such as CSC [Citation26]. Patients with insomnia, anxiety and depression also appear to have an increased predisposition to developing CSC [Citation27,Citation28].
CSC is globally recognized in both acute and chronic forms, based on the duration of SRF presence. Nonetheless, the manifestations and timing of disease presentation vary, including the occurrence of recurrent forms. A recent classification by the Central Serous Chorioretinopathy International Group has proposed a categorization of CSC based on multimodal imaging use. Major criteria have been established, requiring both the presence or history of SRF as noted in OCT and at least one area of RPE alteration in infrared, autofluorescence, or OCT imaging. At least one of the following minor criteria must also be met: placoide hyperfluorescence in mid-phase in ICGA, at least one point of leakage in Fluorescein Angiography (FA), or a subfoveal choroidal thickness (SFCT) of 400 µm or more. Based on these criteria, the presence of at least two-disc diameters of RPE alteration classifies the condition as Complex; otherwise, it is considered Simple. Each of these forms can further be divided into primary, recurrent, and resolved, based on the presence or absence of SRF. If any of these forms persists for at least six months, it is considered persistent [Citation29].
2. Methods
The methodology used for this review consisted of research of all available articles reporting the treatment for CSC. A literature search of all original articles published until December 2023 was performed using PubMed, Google Scholar and Scopus databases. The following keywords ‘Central Serous Chorioretinopathy’ and ‘Treatment,’ were searched in combination with ‘Photodynamic therapy,’ ‘Focal Laser Photocoagulation,’ ‘Subthreshold Micropulse Laser’ and ‘Systemic treatments.’ The bibliographies of the selected articles were thoroughly examined. Furthermore, the references cited in these papers were manually inspected to identify any relevant studies that may have been overlooked in the electronic searches. Subsequently, a comprehensive list of electronically sourced data was compiled. Two independent reviewers (MB and AC) then reviewed the titles and abstracts to identify relevant articles. Articles published in languages other than English were not considered for inclusion.
3. Results
3.1. Search strategy
The search for relevant papers was conducted within the principal online databases such as PubMed and EMBASE, considering publications to February 2024. The PUBMED database search strategy involved the use of search filters (Central Serous Chorioretinopathy) AND (Treatment), yielded 3054 articles. Similarly, EMBASE, utilizing the search filter (‘central serous chorioretinopathy’/exp OR ‘central serous chorioretinopathy’ OR ((‘central’/exp OR central) AND serous AND (‘chorioretinopathy’/exp OR chorioretinopathy))) AND (‘treatment’/exp OR treatment), identified 2200 corresponding articles. The articles added to the initial list were identified independently by one reviewer (M.B.). Disagreements were resolved through negotiation between the two authors (D.C. and M.B.) or by consulting the senior author (A.C.) if necessary. Additionally, a backward citation search was conducted by manually screening the reference lists of included studies for additional relevant references.
3.2. Inclusion and exclusion criteria
We reviewed all published studies that presented data regarding outcomes of treatment in CSC patients. Inclusion criteria encompassed: (a) original research published in a peer-reviewed journal; (b) written in the English language; (c) inclusion of outcome of the study treatment. Exclusion criteria comprised: (a) non-human studies; (b) non-original research; (c) non-English language publications; (d) absence information about the treatment. Two independent reviewers conducted the literature screening process, which involved de-duplication, title and abstract screening to eliminate irrelevant studies, and full-text assessment of potentially relevant studies based on the inclusion criteria.
A total of 148 potentially relevant articles were identified from the two databases up to 2024. After excluding duplicates, 122 studies remained. Subsequently, 25 articles were excluded based on the title and abstract. Finally, 97 observational studies met the described inclusion criteria.
4. Discussion
4.1. Treatments
CSC has seen the development of various treatment strategies over time, tailored to its acute and chronic forms. For acute CSC, a conservative approach is often adopted. This is based on the understanding that most acute cases are self-limiting and typically resolve without intervention. However, in specific scenarios, particularly when a patient’s professional demands necessitate rapid visual recovery, active treatment may be considered.
Treatment for chronic CSC, on the other hand, is more interventionist due to the persistent nature of the condition. Chronic cases are more likely to require medical intervention to prevent long-term damage to the retina. The treatment options range from pharmacological approaches, such as the use of anti-Vascular Endothelial Growth Factor (anti-VEGF) agents, to laser therapies. Photodynamic Therapy (PDT), for instance, has been widely recognized for its effectiveness in treating chronic CSC. Additionally, lifestyle modifications and managing underlying stress factors are also crucial aspects of managing CSC, particularly in its chronic form. Patients are often advised to make changes in their lifestyle that could mitigate stress, as stress is a known risk factor for CSC.
4.1.1. Focal laser photocoagulation
The cornerstone of laser therapy for CSC involves targeting the points of leakage evident on.
FA to restore the integrity of the outer blood-retina barrier. Both green (495–570 nm) and yellow (570–590 nm) laser wavelengths are absorbed by melanin and hemoglobin, rendering each wavelength suitable for photocoagulation of macular disorders. Yellow laser may offer theoretical advantages. Its longer wavelength results in reduced scatter by ocular structures and deeper penetration into vascular and pigmented structures compared to the green wavelength. However, current data indicate that there are unlikely to be significant clinical differences related to wavelength choice. Nonetheless, yellow laser has been demonstrated to require less average power than green laser to achieve similar retinal burns during retinal photocoagulation [Citation30]. Various lasers, including Argon, diode, krypton, and xenon, have been employed in the treatment of CSC. While the Argon laser was historically the predominant choice for CSC management, the Diode laser has demonstrated superior outcomes in terms of best-corrected visual acuity (BCVA) [Citation31]. Focal photocoagulation is recommended exclusively for extrafoveal leakage points. The Argon laser, utilizing a green wavelength, produces the gray areas of retinal atrophy. Typically, the spot size measures 100 micrometers, with a duration of ≤0.1 seconds, and a power output between 70–120 milliwatts [Citation32]. However, there are associated risks with this approach, including decreased visual acuity and contrast sensitivity, as well as the potential development of CNV [Citation33].
A prospective randomized trial with a 12-year follow-up indicated that argon laser photocoagulation for CSC neither affected the visual outcome nor decreased the recurrence rate or prevalence of chronic disease. The treatment’s efficacy seems primarily related to the symptomatic relief resulting from the expedited resolution of serous detachment [Citation34]. Navigated laser photocoagulation may be a beneficial alternative for patients with chronic CSCR who are not candidates for PDT. Post-treatment, BCVA showed significant improvement, along with enhancements in central macular thickness (CMT) and SFCT and SRF was fully resolved in approximately 70% of the cases [Citation32,Citation35]. Given the lack of consensus on the use of Focal Laser Photocoagulation, it’s crucial to appropriately select the patients for treatment. Those who haven’t responded to PDT, with chronic neuroepithelial detachments and factors associated with SRF resolution, appear to be the most likely responders to focal laser treatment, especially when using a navigated micropulse laser.
A recent study compared the outcomes of conventional laser photocoagulation and Subthreshold Micropulse Laser (SML) treatments, each applied based on FA. At the 12-month mark, BCVA, SRF absorption, and Central Retinal Thickness (CRT) improved in both groups. The absorption of SRF was comparable in both, with a marginally better result seen in the conventional laser group than in SML, indicating that both treatments are essentially equivalent. However, when the leakage is in or near the foveal zone, SML is deemed a safer procedure [Citation36].
4.1.2. Subthreshold micropulse laser
The micropulse diode laser, or SML, emerged in the late ’90s and became an alternative therapeutic option for retinal venous occlusion and macular edema in the 21st century [Citation37,Citation38]. With the Argon laser being more aggressive towards the retina, the micropulse diode laser did not cause cellular damage at the neuroretina level, being selective for the RPE. The rationale behind utilizing SML involves maintaining the delivered temperature below the threshold for denaturing cellular proteins, thereby avoiding cellular damage. This treatment approach spares photoreceptors from direct harm, preserving vision [Citation1,Citation39]. The first applications in eyes with CSC date back to 2008. In a study by Chen et al., the Subthreshold diode laser treatment proved effective for cases of idiopathic CSC with point source leakage. However, an adequate response wasn’t observed in patients with diffuse leakage [Citation40]. In the same year, another prospective study was conducted with an average follow-up of 14 months. By the end of the follow-up, a reduction in both SRF and average retinal thickness was noted. In the majority of the eyes, there were no discernible changes to the RPE or retina attributable to the laser treatment. Moreover, the median BCVA significantly improved [Citation41]. Many studies have been performed in the past years with the disparate outcomes confirming the efficacy of SML in CSC treatment.
Consistent data were released by a multicenter, randomized controlled clinical trial named The PLACE Trial for chronical CSC treatment. The High-Density Sub-Threshold Micropulse Laser (HSML) treatment entails directing laser spots toward hyperfluorescent abnormalities identified on ICGA, systematically arranged within the target zone to ensure adjacent spots are non-overlapping. One-hundred seventy-nine patients were assigned randomly to half-dose PDT and HSML treatment. At the first follow up visit, the PDT group showed a significant improvement in BCVA and retinal sensitivity at microperimetry respect for HSML group. Moreover, at the end of treatment the PDT group showed a significant improvement in SRF reduction respect to the HSML group [Citation39]. However, several authors have critiqued the execution method of the HSML in the PLACE trial: the deficiency in treatment area and density of laser spots employed in the study may have led to a reduction in treatment efficacy, thus highlighting the significantly greater efficacy reported for PDT. The authors indicate that the effectiveness of laser treatment primarily depends on the overall recruitment of large areas of RPE. Conversely, in the PLACE Trial, laser action was focused solely on abnormal areas identified in ICGA [Citation42–45].
A multicenter, retrospective comparative study by the PACORES study group evaluated the efficacy of SML (577 nm) versus half-dose verteporfin PDT in eyes with chronic CSC over a 12-month period. The SML group demonstrated an overall enhancement in visual acuity. In contrast, the half-dose verteporfin PDT group showed a marginal, statistically non-significant alteration in visual acuity. A considerable number of eyes in the PDT group either improved or remained stable in their visual outcomes, while a minority experienced a decrease. Notably, there were no adverse events reported with the SML treatment [Citation46]. In other studies, the primary parameters that showed significant improvements in patients with chronic CSC following SML treatment include BCVA, SRF, maximum macular thickness (MMT), height, contrast sensitivity (CS), CMT, central macular volume (CMV), total macular volume (TMV), and SFCT [Citation46–50]. However, in other studies, the BCVA did not demonstrate any significant changes [Citation51,Citation52]. The efficacy of SML have been demonstrated mainly in patients with chronic CSC with focal leakage [Citation1,Citation40]. However, a study by Van Rijssen et al. showed that SML is inferior to half-dose PDT in terms of both focal and diffuse leakage [Citation53]. A prospective study conducted by Schworm et al. assessed the use of a standardized titration procedure and the Endpoint Management™ technique in patients with chronic CSC. A standardized pattern was applied to treat a 3000 µm area in the macula. Most patients achieved complete resolution of SRF and improvements in key retinal parameters such as CMT and SFCT, confirming the safety and reproducibility of the procedure [Citation54]. Data from the PLACE trial were examined to evaluate the resolution of retinal pigment epithelial detachments (PEDs ()) height in patients with chronic CSC. HSML resulted in a minor reduction in the height of macular PEDs compared to half-dose PDT [Citation55].
4.1.3. PDT
PDT was initially utilized in ophthalmology as a treatment for CNV [Citation56]. The underlying principle of this therapy involved using a low-intensity light beam at the same wavelength as the injected photosensitizing dye. This combination induces a cytotoxic effect on the irradiated tissue. The cytotoxicity is mediated by reactive oxygen species, which damage cellular layers, cytoplasmic organelles and the cell nucleus [Citation1,Citation56]. The primary effect was observed in neovascular structures, and the therapy was also initially used for certain ocular tumors [Citation57,Citation58]. The same approach has proven to be effective in treating patients with CSC, particularly in chronic forms. The effect on the choriocapillaris leads to decreased leakage from the affected vessels beneath the altered RPE. This results in a reduction of fluid production without adversely affecting the photoreceptors [Citation59]. A dosage of 6 mg/m2 was initially considered to be the full dose for the treatment, but some side effects, such as vein occlusion leading to reduced filtration, were observed. Subsequent studies have validated the use of half-dose and/or half-fluence (3 mg/m2; 25 J/cm2) PDT as an effective alternative to the original treatment protocol [Citation60,Citation61]. In the last years, also a one third dose (2 mg/m2) was used in selected cases with interesting results [Citation62]. The variable parameters in PDT treatment include dose, fluence, and duration. By adjusting these parameters, it’s possible to tailor specific indications and adapt the treatment according to the preferences of the physician or the patient. Comparing these different PDT techniques, it was observed that half-time PDT seemed to be superior or equivalent to other treatments for both acute and chronic CSC. However, there is no universal consensus, as some studies suggest that a full dose is more effective than a half dose in reducing SRF and improving subjective ocular measurements [Citation63]. Moreover, in some studies Half-dose PDT was found to be superior to one-third dose PDT in reducing SRF [Citation64]. In general, patients treated with PDT may exhibit an increase in choroidal thickness, SRF and visual symptoms during the initial months following treatment. In some cases, PDT could induce an acute exudative maculopathy, defined as an increase in CRT of 15% or more measured by OCT on day 3 after PDT compared with baseline. This maculopathy is a condition of serous retinal detachment due to an acute fibrinous inflammatory process that has recently attracted much attention [Citation65].
Subsequently, these effects tended to diminish, leading to an improvement in signs and symptoms [Citation66]. The treatment aims to direct the spots of treatment on the leaking area enhanced through ICGA or FA. Some studies suggest that OCT-A guided PDT is non inferior to ICGA guided PDT for detecting leaking spots [Citation67] (). The patients were treated with topical anesthesia prior to the application of a contact fundus lens for retinal examination. The examination typically lasted about 10 minutes. Following the treatment, it was necessary for patients to wear specific eyeglasses for 36 to 48 hours to minimize UV light exposure to the retina [Citation1,Citation67]. According to the PLACE trial, in patients with chronic CSC Half-dose PDT improved SRF, BCVA and retinal sensitivity on microperimetry respect for HSML [Citation39]. In addition, it was seen that both PDT and HSML do not significantly affect CVI [Citation68]. Crossover treatment can be considered an option following unsuccessful initial therapies, such as PDT or others. A study comparing PDT with HSML therapy involved patients undergoing crossover treatment if the first treatment was ineffective. The results indicated that patients who received PDT as a crossover treatment after initial HSML therapy experienced higher rate of resolution of SRF, in contrast to those who underwent the reverse sequence. Furthermore, there was no significant change in mean visual acuity or mean visual quality-of-life questionnaire scores in either group [Citation69]. However, in a comparative study, Parodi and colleagues noted a good efficacy of SML and emphasized its multitude of advantages over PDT, including ease of administration, laser treatment safety, and reduced costs [Citation45].
In comparing PDT with other treatments for CSC, The SPECTRA study, a multicenter and randomized controlled trial, demonstrated that Half-dose PDT is superior to oral eplerenone for chronic CSC with respect SRF reduction, BCVA and retinal sensitivity on microperimetry to both short-term safety and efficacy outcomes [Citation70]. Regarding intravitreal injections as treatment, Low-fluence PDT was found to be more effective than intravitreal ranibizumab in terms of resolving SRF, achieving a greater mean decrease in CRT from baseline within the first six months, and reducing choroidal hyperpermeability as seen in ICGA [Citation71].
In cases of acute CSC, spontaneous resolution of SRF is expected in the majority of patients, with approximately 70% experiencing resolution [Citation72]. A randomized controlled trial demonstrated that half-dose PDT compared to sham treatment resulted in the resolution of SRF in about 95% of patients with acute symptomatic CSC, as opposed to a 58% complete resolution rate in patients receiving sham treatment [Citation73]. Twelve-months results concerning BCVA and SRF resolution as outcomes revealed a high-resolution rate in both the half-dose PDT group and the placebo group, with resolution rates of 90% and 64%, respectively [Citation72]. Comparing the recurrence rates between patients with spontaneously resolved acute CSC and those treated with half-fluence PDT, the recurrence rate was higher in the group with spontaneous resolution (51.2%) compared to the PDT group (25%). The rate of acute CSC recurrence was lower in patients treated with PDT than in those whose condition resolved spontaneously [Citation74]. Among the conditions characterized by choroidal thickening, named pachychoroid, are listed PPE, CSC, PNV and PCV. According to a recent theory, these conditions may represent progressive stages of the same underlying disease process. Therefore, the presence of a pachychoroid with RPE alterations or RPE detachment could signify an early stage of CSC, even in the absence of SRF [Citation20]. A study enrolled patients with symptomatic PED in the macular area accompanied by angiographic evidence of choroidal hyperpermeability. Some of the enrolled patients exhibited CSC in the fellow eye or progression to CSC during the follow-up period. A single session of PDT resulted in complete resolution of the PED in 78% of the patients. Of the group that was only followed up, 38% experienced spontaneous resolution of the PED [Citation75].
4.1.4. Anti-VEGF
In vitro and in vivo experimentations evidenced the effects against proliferation and permeability of anti-VEGF on choroidal endothelial cells [Citation76,Citation77]. Clinically, anti-VEGF have shown to be effective against fibrovascular proliferation and leakage, and to lead to a significant reduction in SFCT, as demonstrated in studies of various pathologies such as age-related macular degeneration (AMD), diabetic macular edema (DME), PCV or PNV [Citation78–82]. Considering the ability to target the choroid, which is identified as the pathogenic source of subfoveal retinal fluid in CSC, intravitreal administration of anti-VEGF agents was first proposed as a new treatment option in the management of CSC patients in a pilot study by Torres-Soriano and colleagues in 2008 [Citation83]. Over the years, various authors have demonstrated that anti-VEGF agents such as bevacizumab, ranibizumab and aflibercept are effective in reducing choroidal permeability and thickness in CSC patients. For instance, in 2013, Kim and colleagues showed in a prospective, randomized study that in a group of acute CSC patients treated with ranibizumab injections achieved complete resolution of SRF nine months earlier than the untreated control group [Citation84]. The research team demonstrated in 2015 that choroidal thickness in 42 eyes with chronic CSC decreased by an average of 22 μm following intravitreal injections of bevacizumab at a mean follow-up of 9 months after starting treatment. Notably, this decrease is comparable to that reported by studies investigating the use of aflibercept and bevacizumab for AMD, which both resulted in a reduction of choroidal thickness by roughly 36 μm [Citation85]. In a prospective, comparative clinical study of 30 eyes, Artunay and colleagues obtained similar results of Bevacizumab efficacy [Citation86]. Given that these studies were conducted before the OCTA became available, it remains unclear whether the resolved SRF was caused by CSC or secondary CNV, which is very difficult to distinguish on FA and ICGA images alone. Despite these positive reports, in the following years comparative studies of the efficacy of anti-VEGF injections versus PDT in CSC patients reported better responses to the second treatment. In a prospective, randomized study by So Hyun Bae and colleagues, low-fluence PDT was compared with intravitreal injections of ranibizumab for treatment of chronic CSC patients [Citation87]. Sixteen eyes with chronic CSC were enrolled in the study and received randomized treatment with Ranibizumab or PDT. After a 3-month follow-up, 6 eyes (75%) in the low-fluence PDT group achieved complete resolution of SRF and reduction of choroidal hyperpermeability after a single treatment session, whereas only 2 eyes (25%) in the ranibizumab group achieved this after consecutive ranibizumab injections. Four eyes (50%) in the ranibizumab group underwent additional low-fluence PDT and achieved complete resolution. A similar small-scale, prospective randomized study was conducted by Semeraro et al. for intravitreal bevacizumab injection, yielding comparable results [Citation88]. For this reason, PDT is now strongly recommended as the primary treatment option for both acute and chronic CSC. However, in case of CNV complications, treatment with anti-VEGF injections comes back with a leading role in chronic CSC patients [Citation1]. A large-scale, international and prospective randomized study, named the MINERVA study, evaluated the effectiveness of ranibizumab 0.5 mg among 178 adult patients with CNV associated with any cause other than neovascular AMD and myopic CNV. At the end of the 12-month follow-up, the treatment group appeared to have improved in BCVA respect for the sham treatment group. Specifically, the treatment effect of ranibizumab demonstrated a significant increase of + 5.0 letters at Month 2 for patients with CNV caused by CSC. These results indicate that anti-VEGF is highly effective against CNV induced by CSC, and furthermore, suggest considering a combination therapy of ranibizumab and PDT for CSC patients to enhance treatment efficacy [Citation89]. Further research has shown that anti-VEGF treatment is effective in managing CSC complicated by active subretinal CNV [Citation90,Citation91]. In a recent study, cases of CSC complicated by CNV underwent either monotherapy (with anti-VEGF or PDT) or combination therapy. After 12 months of follow-up, all three groups showed significant improvement in terms of BCVA, CMT and CNV regression, while no statistically significant differences in therapeutic effects were found between anti-VEGF injections, PDT or combination therapy [Citation91]. In a study conducted by Sonoda, PDT, whether utilized in combination with anti-VEGF therapy or as a standalone treatment for CSC with CNV, exhibited a significant improvement in CRT and reduction in subretinal fluid accumulation. However, the author notes the indispensability of anti-VEGF therapy in reducing the CNV area [Citation92].
In conclusion, intravitreal injections of anti-VEGF are recommended as primary treatment for CSC patients with subretinal CNV. In uncomplicated forms of CSC, the use of PDT is preferred. Half-dose or half-fluence PDT may be used as an adjuvant treatment, as combined therapy is considered by some authors to be the current gold standard therapy for chronic CSC complicated by CNV [Citation1,Citation93] ().
4.1.5. Systemic treatments
Hypercortisolism induces the activation of the mineralocorticoid receptor, which upon excessive activation, it promotes diverse pathological processes within the retinal and choroidal compartments, such as the development of CSC [Citation94]. Specifically, it was noted that CSC can be induced by the exogenous administration of corticosteroids, whether applied topically, intravitreally, or systemically [Citation95,Citation96]. In light of these findings, antagonists of mineralocorticoid and glucocorticoid receptors may be considered for CSC treatment [Citation97,Citation98].
4.1.5.1. Spironolactone
Spironolactone acts as a mineralocorticoid receptor antagonist, inhibiting aldosterone’s action. This leads to decreased reabsorption of sodium and water in the renal tubules, promoting diuresis and reducing extracellular fluid volume. Additionally, it has mild antiandrogenic activity [Citation99]. Different studies enhanced the improving in BCVA and reduction in SRF in patient suffering from acute CSC treated with oral spironolactone [Citation100]. The use of spironolactone improved reabsorption of SRF and improving in BCVA in case of acute CSC more rapidly than standard observation. It may be considered as an alternative to observation in case of acute event of CSC [Citation101]. Moreover, the same positive effects were found in patients suffering from chronic CSC characterized with chronic SRF. These patients improved either in BCVA and SRF after the treatment [Citation102]. On the other hand, it was seen that in around 10% of patients are present recurrency in SRF and decreasing in BCVA, especially in older patients who underwent other eye treatment in the lifetime [Citation103].
Two recent meta-analyses have reported poorer outcomes, failing to demonstrate any efficacy of mineralocorticoid inhibitors across the patient cohort [Citation104,Citation105].
Spironolactone may find use in patient with chronic CSC without complete response to other treatments such as PDT [Citation106], however, its utilization is presently limited due to its severe side effects.
4.1.5.2. Eplerenone
Eplerenone selectively inhibits the mineralocorticoid receptors, primarily blocking aldosterone’s effects. This action results in decreased sodium reabsorption and increased potassium retention in the renal tubules, leading to diuresis and reduced blood pressure without significant antiandrogenic effects [Citation107,Citation108]. A recent study found that a brief course of eplerenone treatment in CSC patients leads to a decrease in both choroidal and central macular thickness. However, this treatment did not result in a reduction of SRF nor improving in BCVA. Mild side effects observed during the treatment Ied hypertension, abdominal cramps, and migraines [Citation109]. On the contrary, eplerenone used from 1 up to 3 months in patients with chronic CSC improved either SRF, BCVA and CMT [Citation110]. A randomized, multicenter, double-masked, placebo-controlled trial, named VICI trial, was performed to evaluate the safety and superiority of eplerenone against placebo in patients suffering from chronic CSC. Eplerenone was administered in dose of 25 mg/day for 1 week (increased to 50 mg/day for up to 12 months). This drug was found to be safe but not superior to placebo in patients with chronic CSC after a follow up of 12 months [Citation111]. A study with 3 years follow-up highlighted that the main rates of improving in BCVA and SRF reduction are found in the first year of treatment [Citation112]. In a study of 48 eyes from patients with CSC, half received PDT and the other half received eplerenone treatment. OCTA revealed significant enhancements in the vessel density (VD) of the deep capillary plexus and choriocapillaris in both groups post-treatment compared to baseline. However, the superficial capillary plexus (SCP) showed no significant change in VD. The PDT group exhibited a statistically significant increase in VD in both the DCP and choriocapillaris compared to the eplerenone group. A marked reduction in SFCT was observed in the PDT group compared to baseline, as well as a reduction in central foveal thickness. Notably, the SFCT was significantly thinner in the PDT group compared to the eplerenone group [Citation113]. A multicenter, open-label, randomized controlled trial, the SPECTRA trial, compared half-dose PDT versus eplerenone for treating chronic CSC. The SRF reabsorption at 3 months was greater in the PDT group with respect to the eplerenone group (78% vs 17%). Also, sensitivity in microperimetry and mean vision-related quality of life scores were higher in the same group. Instead, BCVA did not show significant differences. Among these two treatments for chronic CSC, HD-PDT is considered superior to eplerenone [Citation70].
4.1.5.3. Mifepristone
Among the glucocorticoid receptor antagonist, Mifepristone showed interesting results in treating CSC. It was seen an improvement in BCVA and SRF after treatment in patients with chronic CSC; while no adverse events were recorded [Citation114]. Goldberg and Heier conducted a randomized, double-masked, placebo-controlled study involving 30 patients with chronic or recurrent CSC. Patients were randomly assigned to receive either mifepristone 300 mg daily, mifepristone 900 mg daily, or placebo for 4 weeks, followed by a further 4 weeks of observation. In both treatment groups, there was a statistically significant reduction in CRT by 82 μm (p < 0.05) and an improvement in BCVA by 3.6 letters (p < 0.05). In contrast, the placebo group showed a non-statistically significant reduction in CRT (47 μm, p = 0.45) and BCVA improvement (0.7 letters, p = 0.64). There was no statistically significant difference in outcomes between the two treatment groups. Notably, there were no significant side effects reported [Citation115].
4.1.6. Other treatments
Other systemic treatments have been used for treatment of CSC without consistent data present in literature. Beta-blockers decrease heart rate and contractility by blocking beta-adrenergic receptors, reducing cardiac output and lowering blood pressure, thus alleviating cardiac stress and workload [Citation116]. Several studies have demonstrated that oral beta-blockers, Nadolol or Metoprolol, exhibit inconsistent efficacy in the treatment of chronic or acute CSC when compared to placebo [Citation117,Citation118]. In 2004, a double-blind randomized controlled clinical trial tested the effect of propranolol 20 mg (twice a day) versus placebo in 60 CSC patients. No differences were found in both group in terms of BCVA [Citation119]. A prospective double-blind study, 48 patients which were treated with metipranolol or placebo did not show any difference on the outcome of the duration of acute CSC [Citation120].
Carbonic anhydrase inhibitors, such as Acetazolamide, inhibits carbonic anhydrase IV, and it has been postulated that they have a beneficial effect in activating the RPE for SRF reabsorption. Acetazolamide was already tested in reducing macular edema [Citation121,Citation122]. This could prove useful in treating patients with CSC. In a prospective, non-randomized, comparative trial, patients treated with acetazolamide were compared with untreated patients [Citation122]. After 24 months of follow-up, no significant differences were observed in BCVA and recurrence rates. Furthermore, over 70% of the patients receiving treatment reported adverse effects, such as paresthesia, nervousness, and gastric discomfort [Citation107].
A retrospective study involving 37 patients treated with acetazolamide observed a significant reduction in SRF following treatment. However, it also noted that some patients did not respond to this treatment [Citation123]. Another retrospective study involving 45 patients demonstrated that subfoveal choroidal thickness and CVI tended to decrease more rapidly in patients treated with acetazolamide, accompanied by a swifter resolution of SRF. While in this instance too, acetazolamide did not affect the final functional status, it is intriguing to observe its effects on choroidal and retinal anatomical structures [Citation124].
Aspirin, or acetylsalicylic acid, is known to inhibit the aggregation of platelets and potentially decrease the levels of plasminogen activator inhibitor I in the bloodstream, which is often found at increased levels in cases of CSC. Elevated plasminogen activator inhibitor has been observed in several chronic CSC cases when compared to controls [Citation125]. This has led to the hypothesis that a state of increased blood coagulability might be involved in the development of chronic CSC [Citation126]. In a study, patients suffering from either the classical or multifocal form of chronic CSC were administered 100 mg of Acetylsalicylic acid daily for the initial month, followed by a regimen of 100 mg on every other day for an additional five months. This patient group was then compared with a control group for evaluation. The results indicate that this low dose Acetylsalicylic acid regimen could potentially expedite visual improvement and reduce the frequency of recurrence in patients with chronic CSC respect for control group [Citation127].
Ketoconazole, an agent predominantly known for its antifungal properties within the imidazole class, has also been observed to exhibit anti-glucocorticoid effects [Citation128,Citation129]. One study assessed the use of ketoconazole as a therapeutic intervention for CSC, administering a daily dose of 600 mg over a four-week period. Although there was a noted reduction in serum cortisol levels, this did not translate into significant improvements in BCVA or SRF reduction [Citation130]. A more recent study involving 41 patients with chronic CSC observed that high dosage range of Ketoconazole correlated with changes in 24-hour urinary free cortisol levels and choroidal thickness. This regimen led to an increased median time to resolution in chronic CSC cases, thereby reducing the necessity for additional rescue therapy [Citation131]. However, further research is necessary to establish a stable clinical application of Ketoconazole in the treatment of CSC.
Finasteride is a 5alpha-reductase inhibitor used for benign prostatic hypertrophy, androgenic alopecia and showed a benefit in reducing prevalence of prostate cancer [Citation132]. In a prospective study, patients diagnosed CSC underwent a treatment regimen of 5 mg of Finasteride daily for a duration of three months. The results indicated no significant changes in BCVA and SRF. However, a reduction in CMT was observed at the three-month mark. Notably, upon discontinuation of the treatment, 4 out of 5 patients exhibited an increase in either CMT or SRF [Citation133]. A study conducted in 2016, evaluating the effectiveness of Finasteride in patients with CSC demonstrated a reduction in SRF in approximately 76% of the participants. Furthermore, a comparatively lower recurrence rate of about 38% was observed after discontinuing the treatment. Notably, the study reported no additional adverse events associated with the use of Finasteride [Citation134]. Despite some positive results, the current evidence in the literature is too limited and contradictory to form a definitive evaluation of the effectiveness of Finasteride as a treatment for CSC. Therefore, further studies are required.
A randomized, placebo-controlled trial was conducted to assess the effectiveness of high-dose antioxidants in the treatment of acute CSC. In this study, it was observed that around 76% of the patients treated with high-dose antioxidants experienced a complete resolution of SRF, at a significantly higher rate compared to the 48% resolution observed in the placebo group [Citation135]. In a separate investigation, the administration of a curcumin-phospholipid compound, recognized for its antioxidant and anti-inflammatory effects, resulted in a decrease in the extent of SRF in around 78% of the patients with CSC [Citation136]. Shinojima reported compelling findings from a multicenter randomized controlled study investigating antioxidant supplementation with lutein for CSC. Among 100 patients with chronic CSC, the mean BCVA demonstrated significant improvement (p = 0.003), while no significant change was observed in the placebo group (p = 0.589). Moreover, in the supplementation group, the mean subfoveal fluid height exhibited a significant reduction of 28.6% (p = 0.028), compared to a mere 3.3% reduction in the placebo group (p = 0.898). Further investigation is warranted to fully elucidate the impact of antioxidant supplementation on BCVA in future studies [Citation137].
5. Conclusions
In acute CSC treatment is generally deferred except in cases demanding optimal vision, such as professional needs. Current evidence favors half-dose or half-fluence PDT, guided by ICGA or FA, to expedite SRF resolution, enhance vision, and reduce recurrence risk. However, some studies have shown that patients with CSC lasting six months or less, when treated with SML, achieved favorable functional outcomes with prompt implementation of the procedure [Citation138,Citation139].
For chronic CSC, half-dose (or half-fluence) PDT is recommended based on data from studies like the PLACE trial, and may support its use in reimbursement scenarios. CSC cases with CNV should be managed with intravitreal anti-VEGF injections and/or half-dose PDT. For PCV, anti-VEGF therapy alone or combined with PDT is effective. In situations where primary treatments are unsuitable, eplerenone is regarded as the first choice among oral treatment options. As research advances, new treatments continue to emerge, offering hope for more effective management of this condition.
6. Expert opinion
CSC is a retinal disorder primarily affecting the central macula. Epidemiologically, it most commonly presents in middle-aged individuals, particularly men, with a noted prevalence in those aged 30 to 50 years. The incidence rate of CSC varies globally, but it has been observed more frequently in individuals with a history of steroid use, type-A personality traits or systemic hypertension [Citation2,Citation3]. On OCT, CSC is characterized by the presence of serous detachment of the neurosensory retina. In the case of an initial episode of acute CSC with moderate SRF and no or slightly reduced visual symptoms, the correct approach is observation to monitor the case’s progression [Citation15,Citation18]. Conversely, a recurrence of acute CSC with the presence of SRF and visual impairment, or a severe case of first episode acute CSC necessitates PDT guided by ICGA/FA [Citation39,Citation46]. If PDT is not feasible due to the absence of instrumentation or other patient-specific reasons, HSML treatment may serve as a viable alternative, as emerging from our clinical experience and confirmed by the literature [Citation39]. Another option is focal laser photocoagulation, suitable in fewer and specific instances, such as when a focal leakage point is located far from the macula [Citation32,Citation35]. In our opinion, this treatment is less frequently applied due to the potential risk of retinal damage and the requirement for a certain level of expertise in laser treatment, special in young ophthalmologist. Furthermore, the availability of other treatment options reduces the necessity of this approach in contemporary clinical practice. Regarding systemic treatments, spironolactone or eplerenone has been identified as the most adequate [Citation100,Citation102]. However, we experienced some ‘rebound effect’ in SRF formation post-discontinuation of mineralocorticoid receptor antagonists, nevertheless among the systemic treatments these are the most useful. The same treatment approach remains viable if, after the observation period, the initial episode of acute CSC persists with visual impairment and SRF. Cases that do not show signs of resolution despite these treatments necessitate further diagnostic procedures using OCTA to exclude complications with CNV requiring anti-VEGF therapy, or in rarer cases, a reconsideration of the initial diagnosis [Citation84,Citation85]. Chronic CSC, lasting over six months, is associated with RPE impairment and persistent visual symptoms. Signs of hyperfluorescence due to hyperperfusion are observed in ICGA [Citation1,Citation3]. According to prevailing literature, PDT is the frontline and most suitable treatment due to its robust scientific foundation and because it offers the best clinical outcomes. PDT not only reduces SRF accumulation but also improves visual acuity and lowers recurrence rates [Citation39]. Our experience aligns with findings from authoritative studies. Nevertheless, careful evaluation of candidates for PDT is necessary, considering the severity of the disease and potential complications. Even if there is a recurrence of SRF after the initial PDT treatment, retreatment should be considered [Citation39]. SML is regarded as a valid second-line treatment, especially in patients with recurrences or in simple CSC cases, offering safe treatments, reduced costs and beneficial effects on patient signs and symptoms [Citation39]. In selected cases, alternatives like mineralocorticoid receptor antagonists may provide benefits in specific patient subpopulations, or anti-VEGF therapy is considered only in cases of CNV secondary to CSC [Citation84,Citation85]. In conclusion, our clinical experience suggests that managing CSC requires a patient-centered approach. In acute CSC, careful monitoring and lifestyle modifications may suffice, while in chronic CSC, PDT is a primary therapeutic option. However, ensuring that patients are fully informed about the potential risks and benefits of different treatment options is crucial. It would be beneficial to conduct multicentric clinical trials focusing on systemic treatments to establish a solid foundation for treating patients experiencing relapses. This approach would help standardize the diagnostic and therapeutic processes, avoiding the variability in patient management. Such standardization is essential to manage patients effectively without frequently subjecting them to invasive instrumental examinations or treatments that could further impact their quality of life, in addition to the burden of the pathology itself. Moreover, there is a need for further research to optimize treatment protocols and explore new therapeutic strategies to enhance long-term management of CSC.
Article highlights
Central serous chorioretinopathy (CSC) is characterized by idiopathic serous detachment of the retina due to leakage through an altered RPE.
The CSC International Group has categorized CSC based on multimodal imaging, utilizing minor and major criteria.
The treatment is generally deferred in acute CSC.
Half-dose (or half-fluence) PDT is recommended in chronic CSC.
Intravitreal anti-VEGF injections and/or half-dose PDT are recommended in CSC cases complicated by CNV.
Eplerenone is the most used among oral treatment options, in situations where primary treatments are unsuitable.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution
All authors have substantially contributed to the conception and design of the review article and interpreting the relevant literature, and have been involved in writing the review article or revised it for intellectual content.
Acknowledgments
All the figures present in the manuscript are original for this publication.
Additional information
Funding
References
- Van Rijssen TJ, van Dijk EHC, Yzer S, et al. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog Retin Eye Res. 2019 Nov;73:100770. doi: 10.1016/j.preteyeres.2019.07.003
- Fine SL, Owens SL. Central serous retinopathy in a 7-year-old girl. Am J Ophthalmol. 1980 Jun;90(6):871–873. doi: 10.1016/S0002-9394(14)75205-5
- Castro-Correia J, Coutinho MF, Rosas V, et al. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992;81(4):379–386. doi: 10.1007/BF00169099
- Breukink MB, Dingemans AJM, Den Hollander AI, et al. Chronic central serous chorioretinopathy: long-term follow-up and vision-related quality of life. Clin Ophthalmol. 2017 Jan;11:39–46. doi: 10.2147/OPTH.S115685
- Von Graefe A. Nachträgliche Bemerkungen über die modificirte Linearextraction. Graefes Arch Clin Exp Ophthalmol. 1866;12(1):211–215. doi: 10.1007/BF02720786
- Kitahara S. Ueber klinische beobachtungen bei der in Japan häufig vorkommenden chorioretinitis centralis serosa. Klin Monatsbl Augenheilkd. 1936;97:345–362.
- Horniker EK. Monatsblätter Augenheilkd. 1937;98:487.
- Duke-Elder S. Text-book of ophthalmology. 1940;3:2592.
- Bennett G. Central serous retinopathy. Br J Ophthalmol. 1955 Oct;39(10):605–618. doi: 10.1136/bjo.39.10.605
- Maumenee AE. Macular diseases: clinical manifestations. Trans Am Acad Ophthalmol Otolaryngol. 1965 Jul;69:605–613.
- Gass JD, Gass M. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967 Mar;63(3):1–139. doi: 10.1016/0002-9394(67)90026-8
- Hayashi K, Hasegawa Y, Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986;9(1):37–41. doi: 10.1007/BF00225936
- Negi A, Marmor MF. Experimental serous retinal detachment and focal pigment epithelial damage. Arch Ophthalmol. 1984 Mar;102(3):445–449. doi: 10.1001/archopht.1984.01040030359038
- Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16(3):203–213. doi: 10.1097/00006982-199616030-00004
- Mohabati D, van Rijssen TJ, van Dijk EHC, et al. Clinical characteristics and long-term visual outcome of severe phenotypes of chronic central serous chorioretinopathy. Clin Ophthalmol. 2018 Jun;12:1061–1070. doi: 10.2147/OPTH.S160956
- Agrawal R, Chhablani J, Tan K-A, et al. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016 Aug 23;36(9):1646–1651. doi: 10.1097/IAE.0000000000001040
- Gal-Or O, Dansingani KK, Sebrow D, et al. Inner choroidal flow signal attenuation in pachychoroid disease: optical coherence tomography angiography. Retina. 2018 Oct;38(10):1984–1992.
- Teussink MM, Breukink MB, Van Grinsven MJ, et al. Oct angiography compared to fluorescein and indocyanine green angiography in chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2015 Sep;56(9):5229–5237.
- Cheung CMG, Lee WK, Koizumi H, et al. Pachychoroid disease. Eye. 2019 Jan;33(1):14–33.
- Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015 Jan;35(1):1–9. doi: 10.1097/IAE.0000000000000331
- Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009 May;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008
- Carnevali A, Capuano V, Sacconi R, et al. OCT angiography of treatment-naïve quiescent choroidal neovascularization in pachychoroid neovasculopathy. Invest Ophthalmol Vis Sci. 2017 Jun 1;1(4):328–332. doi: 10.1016/j.oret.2017.01.003
- Fung A, Yannuzzi L, Freund KB. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Clin Exp Ophthalmol. 2012 Dec;32(Suppl. 9):1829–1837. doi: 10.1097/IAE.0b013e3182680a66
- Spaide RF, Gemmy Cheung CM, Matsumoto H, et al. Venous overload choroidopathy: a hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res. 2022 Jan;86:100973. doi: 10.1016/j.preteyeres.2021.100973
- Giovansili I, Belange G, Affortit A. Cushing disease revealed by bilateral atypical central serous chorioretinopathy: case report. Endocr Pract. 2013 Sep;19(5):e129–33. doi: 10.4158/EP12389.CR
- Behar-Cohen F, Zhao M. Mineralocorticoid pathway in retinal health and diseases. Br J Pharmacol. 2022 Jul 1;179(13):3190–3204. doi: 10.1111/bph.15770
- Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmol. 2004 Feb;111(2):244–249.
- Genovese G, Meduri A, Muscatello MRA, et al. Central serous chorioretinopathy and personality characteristics: a systematic review of scientific evidence over the last 10 years (2010 to 2020). Medicina (Kaunas). 2021 Jun 16;57(6):628. doi: 10.3390/medicina57060628
- Chhablani J, Cohen FB, Central Serous Chorioretinopathy International Group; Multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol Retina. 2020 Nov;4(11):1043–1046. doi: 10.1016/j.oret.2020.07.026
- Bressler S, Susan B, Almukhtar, et al. Green or yellow laser treatment for diabetic macular edema. Retina. 2013;33(10):2080–2088. doi: 10.1097/IAE.0b013e318295f744
- Verma L, Sinha R, Venkatesh P, et al. Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: a pilot, randomized controlled trial [ISRCTN84128484]. BMC Ophthalmol. 2004 Oct 29;4(1):15. doi: 10.1186/1471-2415-4-15
- Gupta A, Tripathy K. Central serous chorioretinopathy. Treasure Island (FL): StatPearls Publishing;2023 Dec 11.
- Daruich A, Matet A, Dirani A. Central serous chorioretinopathy: recent findings and new physio- pathology hypothesis. Prog Retin Eye Res. 2015;48:82–118. doi: 10.1016/j.preteyeres.2015.05.003
- Ficker L, Vafidis G, While A, et al. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988 Nov;72(11):829–834.
- Zhou F, Yao J, Jiang Q, et al. Efficacy of navigated laser photocoagulation for chronic central serous chorioretinopathy: a retrospective observational study. Dis Markers. 2022;2022:1–8. doi: 10.1155/2022/7792291
- Sun Z, Huang Y, Nie C, et al. Efficacy and safety of subthreshold micropulse laser compared with threshold conventional laser in central serous chorioretinopathy. Eye (Lond). 2020 Sep;34(9):1592–1599.
- Amoroso F, Pedinielli A, Cohen SY, et al. Navigated micropulse laser for central serous chorioretinopathy: efficacy, safety, and predictive factors of treatment response. Eur J Ophthalmol. 2022 Sep;32(5):2810–2818.
- Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmol. 1997;104(12):2030–2038. doi: 10.1016/S0161-6420(97)30061-X
- Van Dijk EHC, Fauser S, Breukink MB, et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmol. 2018 Oct;125(10):1547–1555. doi: 10.1016/j.ophtha.2018.04.021
- Chen S-N, Hwang J-F, Tseng L-F, et al. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmol. 2008 Dec;115(12):2229–2234.
- Lanzetta P, Furlan F, Morgante L, et al. Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy. A pilot study. Eur J Ophthalmol. 2008;18(6):934–940. doi: 10.1177/112067210801800613
- Luttrull JK. Comment on: focal and diffuse chronic central serous chorioretinopathy treated with half-dose photodynamic therapy or subthreshold micropulse laser: PLACE trial report No. 3. Am J Ophthalmol. 2020 Apr 1;212:186–187. doi: 10.1016/j.ajo.2019.10.037
- van Dijk EH, Fauser S, Breukink MB. et al. Half-dose photodynamic therapy versus high-density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy: the PLACE trial. Ophthalmol. 2019 Apr 1;126(4):e29–e30. doi: 10.1016/j.ophtha.2018.11.004
- Iacono P, Da Pozzo S, Varano M, et al. Photodynamic therapy with verteporfin for chronic central serous chorioretinopathy: a review of data and efficacy. Pharmaceuticals. 2020;13(11):349. doi: 10.3390/ph13110349
- Battaglia Parodi M, Arrigo A, Iacono P, et al. Central serous chorioretinopathy: treatment with laser. Pharmaceuticals. 2020;13(11):359. doi: 10.3390/ph13110359
- Roca JA, Wu L, Fromow-Guerra J, et al. Yellow (577 nm) micropulse laser versus half-dose verteporfin photodynamic therapy in eyes with chronic central serous chorioretinopathy: results of the pan-american collaborative retina study (PACORES) group. Br J Ophthalmol. 2018 Dec;102(12):1696–1700.
- Park YG, Kang S, Kim M, et al. Selective retina therapy with automatic real-time feedback-controlled dosimetry for chronic central serous chorioretinopathy in Korean patients. Graefes Arch Clin Exp Ophthalmol. 2017 Jul;255(7):1375–1383.
- Arsan A, Kanar HS, Sonmez A. Visual outcomes and anatomic changes after sub-threshold micropulse yellow laser (577-nm) treatment for chronic central serous chorioretinopathy: long-term follow-up. Eye (Lond). 2018 Apr;32(4):726–733. doi: 10.1038/eye.2017.293
- Scholz P, Altay L, Fauser S. Comparison of subthreshold micropulse laser (577 nm) treatment and half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Eye. 2016;30(10):1371–1377. doi: 10.1038/eye.2016.142
- Scholz P, Ersoy L, Boon CJF, et al. Subthreshold micropulse laser (577 nm) treatment in chronic central serous chorioretinopathy. Ophthalmologica. 1 Oct 2015;234(4):189–194. doi: 10.1159/000439600
- Altınel MG, Acikalin B, Gunes H, et al. Optical coherence tomography parameters as predictors of treatment response to a 577-nm subthreshold micropulse laser in chronic central serous chorioretinopathy. Lasers Med Sci. 2021;36(7):1505–1514. doi: 10.1007/s10103-020-03225-6
- Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, et al. Functional and morphological outcome in patients with chronic central serous chorioretinopathy treated by subthreshold micropulse laser. Graefes Arch Clin Exp Ophthalmol. 2017;255(12):255, 2299–2306. doi: 10.1007/s00417-017-3783-x
- Van Rijssen TJ, van Dijk EHC, Scholz P, et al. Focal and diffuse chronic central serous chorioretinopathy treated with half-dose photodynamic therapy or subthreshold micropulse laser: PLACE trial report No. 3. Am J Ophthalmol. 2019 Sep;205:1–10. doi: 10.1016/j.ajo.2019.03.025
- Schworm B, Siedlecki J, Keidel LF, et al. Subthreshold laser therapy with a standardized macular treatment pattern in chronic central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2021 Nov;259(11):3271–3281.
- Feenstra HMA, Hahn LC, van Rijssen TJ, et al. Efficacy of half-dose photodynamic therapy versus high-density subthreshold micropulse laser for treating pigment epithelial detachments in chronic central serous chorioretinopathy. Retina.2022 Apr;42(4):721–729.
- Kramer M, Miller JW, Michaud N, et al. Liposomal benzoporphyrin derivative verteporfin photodynamic therapy: selective treatment of choroidal neovascularization in monkeys. Ophthalmol. 1996;103(3):427–438. doi: 10.1016/S0161-6420(96)30675-1
- Kessel D. Porphyrin-lipoprotein association as a factor in porphyrin localization. Cancer Lett. 1986;33(2):183–188. doi: 10.1016/0304-3835(86)90023-6
- Roberts WG, Hasan T. Role of neovasculature and vascular permeability on the tumor retention of photodynamic agents. Cancer Res. 1992;52(4):924–930.
- Chan W-M, Lam DSC, Lai TYY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003 Dec;87(12):1453–1458.
- Alkin Z, Perente I, Ozkaya A, et al. Comparison of efficacy between low-fluence and half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Clin Ophthalmol. 2014 Apr 5;8:685–690. doi: 10.2147/OPTH.S58617
- Stewart JM. Half dose verteporfin PDT for central serous chorioretinopathy. Br J Ophthalmol. 2006 Jul;90(7):805–806. doi: 10.1136/bjo.2006.093328
- Kumashiro S, Takagi S, Itokawa T, et al. Decrease in choroidal blood flow after half and one-third dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. BMC Ophthalmol. 2021 May 31;21(1):241. doi: 10.1186/s12886-021-01980-w
- Nicoló M, Eandi CM, Alovisi C, et al. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2014 May;157(5):1033–1037.e2.
- Dang Y, Sun X, Xu Y, et al. Subfoveal choroidal thickness after photodynamic therapy in patients with acute idiopathic central serous chorioretinopathy. Ther Clin Risk Manag. 2014;10:37–43. doi: 10.2147/TCRM.S54213
- Honda S, Kohno T, Yamamoto M, et al. Early anatomical changes and association with photodynamic therapy induced acute exudative maculopathy in patients with macular diseases. 2022. Sci Rep. 2022;12(1):9105. doi: 10.1038/s41598-022-13208-y
- Van Dijk EHC, Dijkman G, Theelen T, et al. Short-term findings on optical coherence tomography and microperimetry in chronic central serous chorioretinopathy patients treated with half-dose photodynamic therapy. Retin Cases Brief Rep. 2018 Sep;12(4):S266–S271.
- Hu J, Qu J, Li M, et al. Optical coherence tomography angiography-guided photodynamic therapy for acute central serous chorioretinopathy. Retina. 2021 Jan;41(1):189–198.
- Van Rijssen TJ, Singh SR, van Dijk EHC, et al. Prospective evaluation of changes in choroidal vascularity index after half-dose photodynamic therapy versus micropulse laser treatment in chronic central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2020 Jun;258(6):1191–1197.
- van Rijssen TJ, van Dijk EHC, Scholz P, et al. Crossover to photodynamic therapy or micropulse laser after failure of primary treatment of chronic central serous chorioretinopathy: the REPLACE trial. Am J Ophthalmol. 2020 Aug;216:80–89. doi: 10.1016/j.ajo.2020.04.007
- Van Rijssen TJ, van Dijk EHC, Tsonaka R, et al. Half-dose photodynamic therapy versus eplerenone in chronic central serous chorioretinopathy (SPECTRA): a randomized controlled trial. Am J Ophthalmol. 2022 Jan;233:101–110. doi: 10.1016/j.ajo.2021.06.020
- Bae SH, Heo J, Kim C, et al. Low-fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one-year results of a randomized trial. Ophthalmol.2014 Feb;121(2):558–565.
- Kim KS, Lee WK, Lee SB. Half-dose photodynamic therapy targeting the leakage point on the fluorescein angiography in acute central serous chorioretinopathy: a pilot study. Am J Ophthalmol. 2014 Feb;157(2):366–373.e1. doi: 10.1016/j.ajo.2013.10.013
- Chan W-M, Lai TYY, Lai RYK, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy. One-year results of a randomized controlled trial. Ophthalmol. 2008 Oct;115(10):1756–1765.
- Ozkaya A, Alkin Z, Ozveren M, et al. The time of resolution and the rate of recurrence in acute central serous chorioretinopathy following spontaneous resolution and low-fluence photodynamic therapy: a case-control study. Eye (Lond). 2016 Jul 1;30(7):1005–1010. doi: 10.1038/eye.2016.79
- Arif F, Pryds A, Larsen M, et al. Isolated pigment epithelium detachment: evidence for relation to central serous chorioretinopathy and effect of photodynamic therapy. Acta Ophthalmol. 2018 Dec 1;96(8):821–827. doi: 10.1111/aos.13838
- Gragoudas ES, Adamis AP, Cunningham ET Jr., et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816.
- Peters S, Julien S, Heiduschka P, et al. Antipermeability and antiproliferative effects of standard and frozen bevacizumab on choroidal endothelial cells. Br J Ophthalmol. 2007;91(6):827–831.
- Koizumi H, Kano M, Yamamoto A, et al. Subfoveal choroidal thickness during aflibercept therapy for neovascular age-related macular degeneration twelve-month results. Ophthalmol. 2016 Mar 1;123(3):617–624. doi: 10.1016/j.ophtha.2015.10.039
- Nourinia R, Ahmadieh H, Nekoei E, et al. Changes in central choroidal thickness after treatment of diabetic macular edema with intravitreal bevacizumab correlation with central macular thickness and best-corrected visual acuity. Retina. 2018 May 1;38(5):970–975. doi: 10.1097/IAE.0000000000001645
- Cheung CMG, Lai TYY, Ruamviboonsuk P, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmol. 2018 May 1;125(5):708–724. doi: 10.1016/j.ophtha.2017.11.019
- Gabriel C, Lupidi M, Coscas F, et al. Toward a specific classification of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degeneration. Invest Ophthalmol Visual Sci. 2015;56(5):3187–3195.
- Cheung CMG, Lee WK, Koizumi H, et al. Pachychoroid disease. Eye (Lond). 2019 Jan 1;33(1):14–33. doi: 10.1038/s41433-018-0158-4
- Torres-Soriano ME, García-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy. Graefe’s Arch Clin Exp Ophthalmol. 2008;246(9):1235–1239.
- Kim M, Lee S-C, Lee S-J. Intravitreal ranibizumab for acute central serous chorioretinopathy. Ophthalmologica. 2013;229(3):152–157. doi: 10.1159/000345495
- Kim GA, Rim TH, Lee SC, et al. Clinical characteristics of responders to intravitreal bevacizumab in central serous chorioretinopathy patients. Eye (Lond). 2015 Jun 12;29(6):732–741. doi: 10.1038/eye.2015.58
- Artunay O, Yuzbasioglu E, Rasier R, et al. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010;35(2):91–98.
- Bae SH, Heo JW, Kim C, et al. A randomized pilot study of low-fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol. 2011 Nov;152(5):784–792.e2.
- Semeraro F, Romano MR, Danzi P, et al. Intravitreal bevacizumab versus low-fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2012 Nov;56(6):608–612.
- Lai TYY, Staurenghi G, Lanzetta P, et al. Efficacy and safety of ranibizumab for the treatment of choroidal neovascularization due to uncommon cause: twelve-month results of the MINERVA study. Retina. 2018 Jan 1;38(8):1464–1477. doi: 10.1097/IAE.0000000000001744
- Peiretti E, Caminiti G, Serra R, et al. Anti-vascular endothelial growth factor therapy versus photodynamic therapy in the treatment of choroidal neovascularization secondary to central serous chorioretinopathy. Retina. 2018 Aug 1;38(8):1526–1532. doi: 10.1097/IAE.0000000000001750
- Chhablani J, Kozak I, Pichi F, et al. Outcomes of treatment of choroidal neovascularization associated with central serous chorioretinopathy with intravitreal antiangiogenic agents. Retina. 2015;35(12):2489–2497. doi: 10.1097/IAE.0000000000000655
- Sonoda N, Araki T, Komuku Y, et al. Photodynamic therapy for central serous chorioretinopathy with choroidal neovascularization on optical coherence tomography angiography combined with intravitreal anti-vascular endothelial growth factor therapy or alone. Invest Ophthalmol Vis Sci. 2019 Jul 1;60:(9).
- Smretschnig E, Hagen S, Glittenberg C, et al. Intravitreal anti-vascular endothelial growth factor combined with half-fluence photodynamic therapy for choroidal neovascularization in chronic central serous chorioretinopathy. Eye (Lond). 2016 Jun 1;30(6):805–811. doi: 10.1038/eye.2016.41
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. doi: 10.1210/er.2010-0013
- Tsai D-C, Chen S-J, Huang C-C, et al. Risk of central serous chorioretinopathy in adults prescribed oral corticosteroids: a population-based study in Taiwan. Retina. 2014 Sep;34(9):1867–1874.
- Imasawa M, Ohshiro T, Gotoh T, et al. Central serous chorioretinopathy following vitrectomy with intravitreal triamcinolone acetonide for diabetic macular oedema. Acta Ophthalmol Scand. 2005;83(1):132–133. doi: 10.1111/j.1600-0420.2005.00379.x
- Zhao M, Valamanesh FC, Celerier I, et al. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Muller glial cells. FASEB J. 2010;24(9):3405–3415. doi: 10.1096/fj.09-154344
- Bousquet E, Beydoun T, Zhao M, et al. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013;33(10):10 (2096–2102). doi: 10.1097/IAE.0b013e318297a07a
- Carone L, Oxberry SG, Twycross R, et al. Spironolactone. J Pain Symptom Manage. 2017 Feb 1;53(2):288–292. doi: 10.1016/j.jpainsymman.2016.12.320
- Yavuz S, Balsak S, Karahan M, et al. Investigating the efficacy and safety of oral spironolactone in patients with central serous chorioretinopathy. J Fr Ophtalmol. 2021 Jan;44(1):13–23.
- Sun X, Shuai Y, Fang W, et al. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol. 2018 Aug 1;102(8):1060–1065. doi: 10.1136/bjophthalmol-2017-311096
- Falavarjani KG, Amirsardari A, Habibi A. Visual and anatomical outcomes of spironolactone therapy in patients with chronic central serous chorioretinopathy. J Ophthalmic Vis Res. 2017 Jul;12(3):281–289. doi: 10.4103/jovr.jovr_139_16
- Han JY, Kim YJ, Choi EY, et al. Therapeutic efficacy of spironolactone for central serous chorioretinopathy. Yonsei Med J. 2022 Apr 1;63(4):365–371. doi: 10.3349/ymj.2022.63.4.365
- Felipe CQ, Biancardi AL, Civile VT, et al. Mineralocorticoid receptor antagonists for chronic central serous chorioretinopathy: systematic review and meta-analyses. Int J Retina Vitreous. 2022 Jun 7;8(1):34. doi: 10.1186/s40942-022-00385-1
- Venkatesh R, Prabhu V, Joshi A, et al. Real-world practice patterns of eplerenone use for central serous chorioretinopathy. Int J Retina Vitreous. 2023 Oct 2;9(1):61. doi: 10.1186/s40942-023-00500-w
- Sinawat S, Thongmee W, Sanguansak T, et al. Oral spironolactone versus conservative treatment for non-resolving central serous chorioretinopathy in real-life practice. Clin Ophthalmol. 2020;14:1725–1734. doi: 10.2147/OPTH.S260998
- Tam TS, Wu MH, Masson SC, et al. Eplerenone for hypertension. Cochrane Database Syst Rev. 2017 Feb 28;2017(2): CD008996. doi: 10.1002/14651858.CD008996.pub2
- Sacconi R, Borrelli E, Querques G, et al. Eplerenone for chronic central serous chorioretinopathy. Lancet. 2020 Nov 14;396(10262):1556. doi: 10.1016/S0140-6736(20)31610-X
- Iqbal F, Iqbal K, Inayat B. Eplerenone treatment in chronic central serous chorioretinopathy. Cureus. 2021 Oct 1;13(10):e18415. doi: 10.7759/cureus.18415
- Fraenkel D, Suffo S, Langenbucher A. Eplerenone for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2021 Jul;31(4):1885–1891. doi: 10.1177/1120672120952648
- Lotery A, Sivaprasad S, O’Connell A, et al. Eplerenone versus placebo for chronic central serous chorioretinopathy: the VICI RCT. Efficacy And Mech Eval. 2021 Jan;8(2):1–82. Southampton (UK): NIHR Journals Library. doi: 10.3310/eme08020
- Petkovsek DS, Cherfan DG, Conti FF, et al. Eplerenone for the treatment of chronic central serous chorioretinopathy: 3-year clinical experience. Br J Ophthalmol. 2020 Feb;104(2):182–187.
- Clemente L, Cennamo G, Montorio D, et al. OCT-A in chronic central serous chorioretinopathy treated with oral eplerenone and half-fluence photodynamic therapy: a comparative study. Eur J Ophthalmol. 2023 Mar;33(2):1090–1096.
- Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina. 2011 Oct;31(9):1928–1936. doi: 10.1097/IAE.0b013e31821c3ef6
- Fusi-Rubiano W, Saedon H, Patel V, et al. Oral medications for central serous chorioretinopathy: a literature review. Eye (Lond). 2020 May;34(5):809–824.
- Ogrodowczyk M, Dettlaff K, Jelinska A. Beta-blockers: Current state of knowledge and perspectives. Mini Rev Med Chem. 2016;16(1):40–54. doi: 10.2174/1389557515666151016125948
- Browning DJ. Nadolol in the treatment of central serous retinopathy. Am J Ophthalmol. 1993 Dec 15;116(6):770–771. doi: 10.1016/S0002-9394(14)73483-X
- Fabianová J, Porubská M, Cepilová Z. Central serous chorioretinopathy–treatment with beta blockers. Cesk Slov Oftalmol. 1998 Nov;54(6):401–404.
- Kianersi F, Fesharaki F. Effects of propranolol in patients with central serous chorioretinopathy. J Res Med Sci. 2008 May;13(3):103–107.
- Chrapek O, Jirkova B, Kandrnal V, et al. Treatment of central serous chorioretinopathy with beta-blocker metipranolol. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015 Mar;159(1):120–123.
- Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988 Sep;106(9):1190–1195. doi: 10.1001/archopht.1988.01060140350030
- Tripathi RC, Fekrat S, Tripathi BJ, et al. A direct correlation of the resolution of pseudophakic cystoid macular edema with acetazolamide therapy. Ann Ophthalmol. 1991 Apr;23(4):127–129.
- Pikkel J, Beiran I, Ophir A, et al. Acetazolamide for central serous retinopathy. Ophthalmol. 2002 Sep;109(9):1723–1725. doi: 10.1016/S0161-6420(02)01157-0
- Kwak JH, Hong SW, Ra H, et al. Effect of Acetazolamide on choroidal morphology in central serous chorioretinopathy. Korean J Ophthalmol. 2019;33(6):493. doi: 10.3341/kjo.2019.0063
- Yamada R, Yamada S, Ishii A, et al. Evaluation of tissue plasminogen activator and plasminogen activator inhibitor-1 in blood obtained from patients of idiopathic central serous chorioretinopathy. Nippon Ganka Gakkai Zasshi. 1993 Aug;97(8):955–960.
- Iijima H, Iida T, Murayama K, et al. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. Am J Ophthalmol. 1999 Apr;127(4):477–478.
- Caccavale A, Romanazzi F, Imparato M, et al. Low-dose aspirin as treatment for central serous chorioretinopathy. Clin Ophthalmol. 2010;4:899–903. doi: 10.2147/OPTH.S12583
- Chou SC, Lin JD. Long-term effects of ketoconazole in the treatment of residual or recurrent Cushing’s disease. Endocr J. 2000;47(4):401–406. doi: 10.1507/endocrj.47.401
- Meyerle CB, Freund KB, Bhatnagar P, et al. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007 Sep;27(7):943–946.
- Golshahi A, Klingmüller D, Holz FG, et al. Ketoconazole in the treatment of central serous chorioretinopathy: a pilot study. Acta Ophthalmol. 2010 Aug;88(5):576–581. doi: 10.1111/j.1755-3768.2008.01467.x. Epub 2009 May 12. PMID: 19456313.
- Chantarasorn Y, Rasmidatta K, Pokawattana I, et al. Effects of ketoconazole on the clinical recovery in central serous chorioretinopathy. Clin Ophthalmol. 2022;16:1871–1882. doi: 10.2147/OPTH.S368427
- Chaudhary UB, Turner JSF. Finasteride. Expert Opin Drug Metab Toxicol. 2010 Jul;6(7):873–881. doi: 10.1517/17425255.2010.495944
- Forooghian F, Meleth AD, Cukras C, et al. Finasteride for chronic central serous chorioretinopathy. Retina. 2011 Apr;31(4):766–771.
- Moisseiev E, Holmes AJ, Moshiri A, et al. Finasteride is effective for the treatment of central serous chorioretinopathy. Eye (Lond).2016 Jun;30(6):850–856.
- Ratanasukon M, Bhurayanontachai P, Jirarattanasopa P. High-dose antioxidants for central serous chorioretinopathy; the randomized placebo-controlled study. BMC Ophthalmol. 2012;12(1):20. doi: 10.1186/1471-2415-12-20
- Mazzolani F. Pilot study of oral administration of a curcumin-phospholipid for- mulation for treatment of central serous chorioretinopathy. Clin Ophthalmol. 2012;6:801–806. doi: 10.2147/OPTH.S31859
- Shinojima A, Sawa M, Sekiryu T, et al. A multicenter randomized controlled study of antioxidant supplementation with lutein for chronic central serous chorioretinopathy. Ophthalmologica. 2017;237(3):159–166. doi: 10.1159/000455807
- Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, et al. Transfoveal micropulse laser treatment of central serous chorioretinopathy within six months of disease onset. J Clin Med. 2019 Sep 1;8(9):1398. doi: 10.3390/jcm8091398
- Luttrull D. Low-intensity/high-density subthreshold diode micropulse laser for central serous chorioretinopathy. Retina. 2016 Aug 23;36(9):1658–1663. doi: 10.1097/IAE.0000000000001005