ABSTRACT
Introduction
Treatment goals for ulcerative colitis (UC) are evolving from the achievement of clinical remission to more rigorous goals defined by endoscopic and histologic healing. Achievement of deeper remission targets aims to reduce the risk of colectomy, hospitalizations, and colorectal cancer.
Areas covered
This review covers histologic assessments, histologic remission as a clinical trial endpoint, and the association between histologic disease activity and clinical outcomes. Future directions are also discussed, including the use of advanced imaging and artificial intelligence technologies, as well as potential future treatment targets beyond histologic remission.
Expert opinion
Histologic assessments are used for their sensitivity in measuring mucosal inflammatory changes in UC. Due to correlation with disease activity, histologic assessments may support clinical decision-making regarding treatment decisions as such assessments can be associated with rates of clinical relapse, hospitalization, colectomy, and neoplasia. While histologic remission is limited by varying definitions and multiple histologic indices, work is ongoing to create a consensus on the use of histologic assessments in clinical trials. As research advances, aspirational targets beyond histologic remission, such as molecular healing and disease clearance, are being explored.
Plain Language Summary
Ulcerative colitis (UC) is the most common inflammatory bowel disease and often results in bloody diarrhea, frequent bowel movements, and bowel urgency. Patients with UC are at greater risk for hospitalization, surgery, and colorectal cancer. To reduce these risks, the goals of UC treatment are changing from mainly addressing symptoms to reducing inflammation at a deeper histologic, or microscopic, level. The inflammation in UC causes distinct microscopic changes in the colon, which can be assessed after collecting biopsies or tissue samples. This review provides an overview of histologic remission (when no signs of inflammation are seen in tissue samples viewed under a microscope) as a treatment goal in UC.
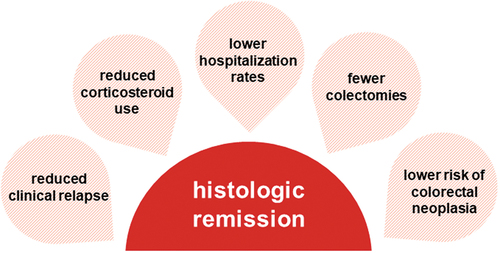
Histologic remission has been shown to be associated with lower rates of relapse, hospitalization, surgical removal of the colon, and colorectal cancer. However, using histologic remission as a treatment target can be difficult due to varying definitions and the many different scoring assessments available to healthcare providers. Updated guidance from regulatory agencies and academic organizations has helped align definitions of histologic remission and how to assess histologic healing in clinical trials.
The introduction of targeted advanced therapies has allowed for deeper healing with the potential for histologic resolution. This enables clinicians and researchers to aim for treatment targets that are harder to achieve but have a greater impact for patients in the course of their disease. New technologies such as artificial intelligence, high-resolution endoscopy, and digital pathology have also led to targets beyond histologic healing, aiming to restore the function of the colon’s mucosal barrier and disease clearance.
1. Introduction
Ulcerative colitis (UC) has a complex pathogenesis involving genetics, environment, epithelial barrier defects, dysregulation of immune responses, and gut microbiota [Citation1–4]. The mucosal inflammation present in UC may be observed in the large intestine, beginning in the rectum, and extending proximally to varying extents [Citation1,Citation3]. Histologic characteristics of UC include changes in mucosal architecture (diffuse crypt atrophy and distortion), changes in the lamina propria cellularity, immune cell infiltration (crypt abscesses and basal plasmacytosis), epithelial injury, and mucus depletion [Citation3,Citation5]. Common UC symptoms include bloody diarrhea, increased stool frequency, and urgency of bowel movements [Citation1,Citation3]. Symptoms are experienced by patients in a relapsing-remitting pattern [Citation1,Citation6–9]. UC is associated with long-term adverse outcomes, including hospitalization, colectomy, colorectal dysplasia, and cancer [Citation3,Citation6,Citation10].
To improve long-term patient outcomes, UC treatment goals are shifting from symptomatic treatment (i.e. clinical remission) to achieving mucosal healing and disease clearance [Citation3]. Mucosal healing can refer to endoscopic remission or endoscopic and histologic remission. Histologic improvement and remission in UC may indicate a ‘more complete’ or ‘deeper’ remission, which could benefit patients beyond endoscopic targets [Citation3,Citation5,Citation11,Citation12]. This review explores histologic assessments, histologic remission and combined histologic-endoscopic measures as clinical trial endpoints, associations between histologic disease activity and clinical outcomes, and areas for future research.
2. Current treatment goals in UC
The evaluation of UC therapies in clinical trials and clinical practice requires clear treatment goals [Citation11,Citation13]. The selection of a therapy for a given patient is complicated by the availability of multiple treatments with varied efficacy and safety profiles [Citation12]. Clinical decision-making is determined by a treatment’s ability to induce and maintain remission, reduce short-term colectomy risk, and patient safety [Citation11,Citation12].
Although many clinical and endoscopic parameters have been proposed, there is no unanimous definition of remission in UC [Citation14]. There is a growing consensus that the primary target in UC should be clinical and/or patient-reported remission, defined as the absence of rectal bleeding and return to normal bowel habits combined with endoscopic remission (Mayo endoscopic subscore, MES ≤ 1) [Citation5,Citation10]. Histologic remission is an additional evolving target. While there is no standardized definition, histologic remission is an endpoint in some recent UC clinical trials. A systematic review of UC cohort studies by Yoon et al. identified that the 12-month risk of clinical relapse was 28.7% for patients with an MES of 1 and 13.7% for an MES of 0, compared to 5.0% for patients achieving histologic remission [Citation15].
2.1. From treat-to-target to treat-to-clearance
The ‘treat-to-target’ approach, focusing on remission, was most notably developed and implemented in the context of rheumatoid arthritis and has been applied in other areas [Citation16]. In UC, a treat-to-target approach aims to resolve underlying inflammation, leading to the healing of the mucosa [Citation17,Citation17–19]. The definition of mucosal healing has been evolving from focusing only on endoscopy to including histology as well [Citation20]. Both components of endoscopic and histologic healing have been recognized as important due to the persistence of microscopic disease activity in the context of clinical remission in some patients [Citation21]. Targeting mucosal healing may also be cost-effective as it is associated with sustained remission and reduced hospitalization and colectomy rates [Citation17].
The 2021 Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE)-II consensus does not identify histologic remission as a treatment target but does recommend its use in supporting endoscopic remission targets and recognizes histologic remission as an aspirational therapeutic goal [Citation22]. Of note, STRIDE guidelines focus on feasibility in clinical practice rather than trials [Citation23]. However, a recent Delphi Consensus panel recommended that inflammatory histological activity should be absent in comprehensive disease control [Citation24].
Observational studies have demonstrated that endoscopic remission does not necessarily lead to histologic remission, as up to 40% of patients in endoscopic remission may have continued histologic disease activity as defined by mucosal neutrophils [Citation25–28]. Inversely, patients in histologic remission may also have endoscopic disease activity. Definitions of histologic remission are detailed in Section 3 of this review. In a meta-analysis by Gupta et al., endoscopic remission with underlying histologic disease activity is associated with a two-fold risk of relapse compared to the achievement of both endoscopic and histologic remission [Citation29]. In the Yoon et al. systematic review, a meta-analysis of 10 studies in patients with MES 0 reported that persistent histologic disease activity was associated with a three-fold risk of clinical relapse compared to the achievement of histologic remission (Risk Ratio 0.37, 95% CI: 0.24–0.56) [Citation15]. Histology may be a more accurate indicator of underlying inflammation compared to endoscopy, especially in cases with nonexistent or mild endoscopic evidence of mucosal inflammation [Citation26,Citation30,Citation31]. Additionally, achieving histologic remission has a stronger negative association with corticosteroid use, hospitalization, and colorectal cancer risk than endoscopic or clinical remission [Citation19].
Histologic healing is gaining traction as a target in UC clinical trials, often as a secondary or exploratory endpoint [Citation17–19,Citation29]. Disease clearance, the emergent concept of a ‘complete’ remission encompassing clinical, endoscopic, and histologic remission, is becoming an aspirational target in UC. For the purposes of this review, disease clearance is defined as the prevention of relapse and complications of UC as a result of achieving mucosal healing that includes histologic remission. Current evidence supports the feasibility of disease clearance, but fewer drugs may meet stringent clinical trial endpoints [Citation18,Citation19,Citation23].
2.2. Clinical and endoscopic remission targets
The 2021 STRIDE-II consensus confirmed the importance of clinical response as an immediate target and clinical remission as an intermediate target but noted they were insufficient as long-term treatment targets [Citation16,Citation22]. STRIDE-II assigned endoscopic remission, which implies the absence of friability, ulcerations, and erosions, as a long-term target [Citation22,Citation32].
While patient-reported outcomes are valuable for determining clinical remission, there is often a disconnect between symptoms and objective signs of inflammation. Clinical remission with normalization in stool frequency and/or rectal bleeding can be accompanied by underlying endoscopic disease activity [Citation33–35]. Conversely, some patients may have ongoing symptoms despite the absence of mucosal inflammation demonstrated by endoscopy or histology and endoscopic remission is not always indicative of absence of bowel urgency.
3. Relevance of histology in ulcerative colitis to date
The use of histologic remission in assessing UC is briefly described in this section to provide important context but has been more thoroughly covered in several previous review articles [Citation36,Citation37].
3.1. Histologic remission definitions
Histopathology can confirm the UC diagnosis, exclude other conditions or comorbidities, assess disease activity and extent, identify dysplasia, and evaluate response to therapy [Citation38–40]. The use of histology as a disease activity measure in clinical practice is a relatively recent development with no widely accepted criteria for the achievement of histologic remission [Citation27]. The term refers both to histologic quiescence (with some lingering microscopic inflammation and architectural distortion) and to complete normalization (resolution of microscopic inflammation) [Citation16,Citation28,Citation31,Citation34,Citation38,Citation41]. Histologic remission definitions are dependent on accurate and consistent histopathology evaluations using samples stained with hematoxylin and eosin. A harmonized and consistent definition of histologic remission – and how to assess it via biopsy and index scores – remains an unmet need in UC management [Citation31,Citation37,Citation42,Citation43].
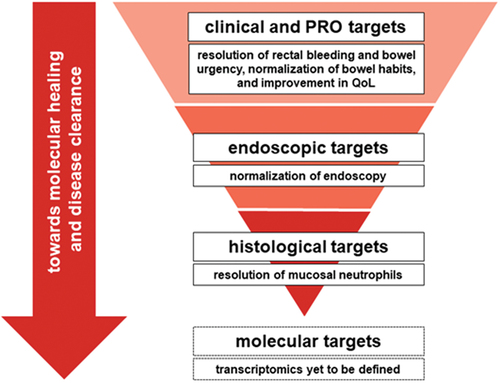
Microscopic-level healing in UC progresses from histologic improvement to histologic remission and normalization (). Inflammatory responses in mucosal epithelial cells engage neutrophils, which release mediators needed for degranulation and/or phagocytosis and recruit other immune cells. However, excess neutrophil accumulation leads to mucosal injury and inflammatory bowel disease (IBD) symptoms [Citation33], with any mucosal intraepithelial neutrophils being abnormal [Citation49]. For this reason, the most accepted definition for histologic remission is the absence of neutrophilic inflammation of the mucosa, which includes the absence of neutrophils in both the lamina propria and epithelium [Citation30,Citation31]. Fecal calprotectin levels are related to histologic remission and are associated with the presence of epithelial neutrophils [Citation50]. The minimum threshold for histologic remission has recently been defined as a lack of intraepithelial neutrophils, erosion, and ulceration [Citation30,Citation31].
Table 1. Definitions of histologic disease activity targets in ulcerative colitis.
The sensitivity of histology-based treatment targets adds predictive value to endoscopic targets, as basal plasmacytosis and epithelial neutrophils serve as independent risk factors for relapse [Citation30,Citation31,Citation51]. Future aspirational targets of molecular healing may use molecular studies in addition to biopsies to evaluate microscopic disease activity in UC [Citation21]. A prospective study of patients with IBD in clinical remission found that barrier healing assessed by confocal laser endomicroscopy (CLE) was more predictive of major adverse outcomes than histologic remission [Citation52]. provides a conceptual overview of current UC treatment targets and their relative predictive value from clinical remission to molecular healing.
3.2. Histologic assessments
Histologic assessments from biopsies collected 2 to 3 months after the initiation of therapeutic intervention may help determine the feasibility of histologic remission as the rate of histologic healing in UC is unknown [Citation27]. Histologic assessments are becoming a common secondary endpoint in clinical trials [Citation53], and indices used for determining histologic disease activity should be robust in reproducibility and responsiveness [Citation36,Citation41,Citation49,Citation54].
While endoscopic and histologic disease activity are not always directly correlated, mucosal appearance on endoscopy should influence the location of biopsies. A standardized biopsy protocol should be developed to allow comparison between different studies. In order to reflect changes in histologic disease activity, biopsies should be taken at 8 to 12 weeks after randomization in induction studies and at 52 weeks in maintenance studies; taking 3- or 5-segment biopsies and 2 to 4 biopsies per segment can mitigate heterogeneity in histologic disease activity [Citation42]. However, in clinical practice, the invasive and costly nature of frequent biopsies could contribute to patient burden and negative impacts on quality of life.
01w?>No standard procedure for histological assessment exists for use in clinical trials [Citation31]. Common assessments of histological disease activity include the Geboes Score (GS), Robarts Histopathology Index (RHI), and the Nancy Histological Index (NHI), which are summarized in . There are at least 30 histologic indices available for use in UC, but only a few (e.g. GS, RHI, and NHI) have been validated [Citation27,Citation36,Citation41,Citation56,Citation58]. illustrates a summary timeline of key histologic index development alongside relevant regulatory guidance.
Figure 2. Timeline of selected UC histology assessments and regulatory guidance.
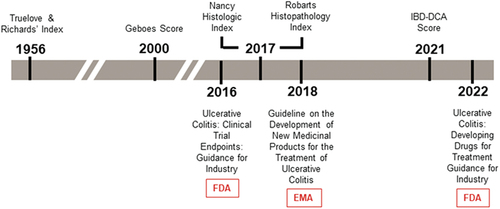
Table 2. Scoring of selected histologic assessments.
The multiple definitions and scores used to assess histology in UC may play a part in its underapplication as a treatment target [Citation41,Citation59]. The need for validated, reproducible, and responsive histologic assessments that facilitate the interpretation of histologic improvement in UC clinical trials has been well recognized [Citation41,Citation42,Citation56,Citation57,Citation60]. A 2021 systematic literature review found histologic response or remission is a feasible and appropriate treatment target and that collected biopsies should be scored for crypt architectural distortion, lamina propria chronic inflammation, basal plasmacytosis, lamina propria and epithelial neutrophils, epithelial damage, and erosions/ulcerations [Citation42].
The GS, RHI, and NHI are comparable and similarly determine histologic remission (); in particular, RHI and NHI are highly correlated. A 2019 study of patients with UC (N = 377) found that all those classified as being in remission with NHI were also in remission with RHI [Citation50]. A subsequent study of 422 patients compared NHI with a continuous GS and demonstrated agreement in histologic remission and response (correlation coefficient: 0.882, p < 0.001) [Citation61]. Post hoc analyses of TOUCHSTONE data reported that inter-rater reliability of the GS, modified Riley score, RHI, NHI, and a visual analogue scale ranged from substantial to almost perfect. The responsiveness of those indices was considered moderate-to-large [Citation49].
The European Crohn’s and Colitis Organization (ECCO) recommends RHI and NHI for evaluating UC histologic disease activity [Citation41]. RHI and NHI are recommended for randomized controlled trials; NHI is recommended for observational studies and clinical practice [Citation62].
3.3. Histologic endpoints in UC clinical trials
Histopathology has been identified as a core outcome domain in UC [Citation63]. In particular, the emergence of immunotherapies has led to the inclusion of histologic assessments in clinical trials due to their sensitivity in detecting mucosal inflammatory changes and, by proxy, treatment efficacy. The 2016 Food and Drug Administration (FDA) guidance on UC clinical trial endpoints recommends assessing treatment efficacy through a primary endpoint combining endoscopy and histopathological findings. The 2016 FDA guidance discourages defining mucosal healing by endoscopic findings alone and suggests the use of endoscopy in tandem with histology [Citation20]. At the time, this recommendation had limited evidence that histologic-endoscopic healing provided long-term benefits. The 2022 FDA draft guidance on developing drugs for UC treatment further discouraged the use of the term ‘mucosal healing,’ as the concept remains undefined [Citation64]. The 2022 guidance also noted the lack of consensus on definitions and scoring for histologic response and remission, recommending that sponsors justify their definitions of histologic endpoints and assessments in clinical trials [Citation64].
The 2018 update of the European Medicines Agency (EMA) guideline addressing the development of UC treatments recommends histologic assessments of mucosal inflammation, including the number of patients achieving histologic normalization as a secondary endpoint in UC clinical trials [Citation65]. In 2021, a public-private partnership was established between the Foundation for the National Institutes of Health (FNIH), the FDA, pharmaceutical companies, and academic centers. The goals of this Biomarkers Consortium are to establish harmonized definitions and evaluations of mucosal healing, influencing future regulatory guidance [Citation66]. After FDA guidance on UC clinical trial endpoints and drug development encouraged sponsor development of combined endoscopic and histologic assessment scales and exploratory histologic endpoints, more trials have designated histologic response and/or remission as part of a histologic-endoscopic or standalone endpoint [Citation20,Citation64].
summarizes recent UC trials with histologic endpoints and compares clinical, endoscopic, and histologic results. A substantial limitation in making such comparisons is the heterogeneity in reported endpoints across different studies. This heterogeneity reflects both the evolution of the concepts and the stringency of how the endpoints were evaluated in each trial.
Table 3. Histologic disease activity endpoints in clinical trials of treatments for UC.
Histo-endoscopic mucosal improvement (HEMI) was the most commonly reported combined endpoint in UC trials and incorporated an MES of less than or equal to 1 and a GS of less than 3.1 (defined as neutrophil infiltration in < 5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue) [Citation68,Citation70,Citation75,Citation76,Citation78]. The importance of absence of neutrophils, defined as a GS of less than 2B (indicating no neutrophils in the epithelial crypts or lamina propria, and no crypt destruction, erosions, ulcerations, or granulation tissue) is reflected in how histologic remission and combined histo-endoscopic mucosal remission (HEMR) endpoints were defined in the mirikizumab trials [Citation78].
Absence of any increase in eosinophils (GS < 2) is required for histologic remission in the vedolizumab and ozanimod trials and is included in the combined mucosal healing definition in the ozanimod, upadacitinib, and etrasimod trials [Citation46,Citation68,Citation73,Citation75]. In the upadacitinib trials, mucosal healing needed an MES of 0 (endoscopic remission or normalization) in addition to a GS of less than 2 [Citation75]. The variance in definitions highlights the need for harmonization and consensus on reporting histologic and combined histo-endoscopic endpoint data to facilitate understanding and recognition of their clinical significance.
3.4. Histologic remission and clinical outcomes
Histologic remission is more strongly associated with clinical outcomes, including rates of clinical relapse, hospitalization, colectomy, and neoplasia, than endoscopic remission [Citation26,Citation31]. Most evidence supporting the association between histologic remission and improved clinical outcomes comes from retrospective and prospective observational studies. illustrates these potential associations. Multiple studies have found associations between the achievement of histologic remission and improved clinical outcomes, including lower risks and/or rates of relapse, hospitalization, colectomy, and dysplasia (). Early achievement of histo-endoscopic mucosal improvement served as a stronger predictor of outcomes than histologic improvement or endoscopic remission alone as demonstrated in LUCENT trials with patients who achieved histo-endoscopic mucosal improvement at Week 12 showing significantly greater rates of corticosteroid-free remission, clinical remission, and symptomatic remission at Week 40 compared with those who did not achieve histologic improvement or endoscopic remission [all p < 0.05] [Citation84]. Conversely, histologic disease activity is associated with worse clinical outcomes along these same metrics [Citation26,Citation79]. The recent UNIFI trial identified residual histologic inflammation even in the presence of endoscopic improvement as an early indication of long-term inflammatory burden [Citation44]. Prolonged histologic disease activity (i.e. uncontrolled mucosal inflammation) can also contribute to carcinogenesis [Citation27].
Table 4. Selected studies analyzing associations between histologic remission and clinical outcomes.
Clinical, endoscopic, and histologic targets are imperfectly correlated; therefore, the results of studies reporting their correlation can be subject to discrepancies and vary in interpretation [Citation30,Citation50,Citation54]. The APOLLO trial reported that histo-endoscopic inactive disease is associated with reduced but not completely absent disability from IBD [Citation85]. A post hoc analysis of VARSITY trial data found that improvement in the presence of epithelial neutrophils was the only histologic parameter associated with endoscopic and histo-endoscopic mucosal improvement [Citation86]. An ongoing randomized, controlled trial (VERDICT) aims to assess the suitability and strength of clinical-, endoscopic-, histologic-, and biomarker-defined treatment targets in patients with active UC and may provide more insight into whether histologic remission is an ideal treatment target [Citation87]. Further evidence from randomized, controlled trials is still needed to validate that histologic remission is associated with improved long-term outcomes compared to endoscopic remission only.
3.5. Relationship of histology to fecal calprotectin in ulcerative colitis
While advances in histologic and endoscopic assessments are providing new insights into UC, additional biomarkers are important complementary diagnostic tools. Compared with histology and endoscopy procedures, fluid biomarkers provide the opportunity for less invasive disease evaluations at a greater frequency, essential to the tracking of UC progression [Citation88].
Calprotectin is a highly abundant protein in neutrophils, and thus highly indicative of macroscopic inflammation [Citation89]. Fecal calprotectin can distinguish between inflammatory and non-inflammatory intestinal diseases and shows a strong correlation with IBD activity [Citation90]. In a systematic review, D’Amico et al examined 12 studies that all found a high correlation between fecal calprotectin and histologic disease activity and could discriminate histologic remission [Citation91]. However, high variability in collection and laboratory procedure contributes to high variability in cutoff levels and thus a low reliability as an accepted diagnostic biomarker capable of distinguishing histologic remission from histologically active disease [Citation91,Citation92]. Interpretation of patient levels is further limited by potential genetic and ethnic variabilities in fecal calprotectin which have yet to be fully characterized [Citation90]. Intramucosal calprotectin can serve as an independent marker of disease activity. This biomarker can be evaluated by performing immunohistochemistry on colonic biopsies, allowing for retrospective analyses in studies where fecal calprotectin was not initially assessed [Citation93]. This method could provide further insight into correlations between histologic endpoints and calprotectin levels without the need for new sample collection.
4. Future directions
4.1. Addressing current limitations of histologic assessments
As new UC treatments target deeper healing, the limitations of histologic remission as a clinical trial endpoint and treatment target must be addressed.
Accurate histologic assessments also rely on the availability and opinion of specialist pathologists. Discrepancies between histologic assessments performed during routine clinical practice can be mitigated by training on different indices (particularly NHI due to its simplicity) and the utilization of central reading facilities or consulting a second specialist gastrointestinal pathologist [Citation94]. GS, RHI, and NHI are all suitable indices for measuring histologic disease activity and have been comprehensively validated [Citation14,Citation27,Citation36,Citation41,Citation42,Citation50]. The CORE-IBD panel achieved a consensus on the use of RHI or GS for scoring histopathology, with histologic remission defined as RHI less than 3 without neutrophils or GS less than 3.0 without neutrophilic inflammation in the epithelium [Citation63]. However, independent of the histologic index used, architectural changes, lamina propria chronic inflammation, basal plasmacytosis, lamina propria neutrophils, epithelial neutrophils, and epithelial damage as well as evaluating the presence or absence of erosions and ulcers (and distinguishing between erosions and ulcers) should be evaluated [Citation42]. Emphasis should be placed on the presence or absence of epithelial and lamina propria neutrophils.
A lack of evidence from randomized clinical trials supporting the association of histologic remission with clinical outcomes further limits the use of histologic assessments in evaluating treatment efficacy [Citation42]. To generate further evidence supporting histologic remission as a treatment target, the use of validated histologic indices in clinical trials (and clinical practice) should be implemented in a consistent, reproducible way [Citation41]. Despite mentions of histologic endpoints in new FDA and EMA guidance, recommendations on the use of histologic remission as a clinical trial endpoint could be elaborated upon further [Citation20,Citation42,Citation64,Citation65]. In order to assess histologic remission as a UC clinical trial endpoint, inclusion criteria should include a minimum histologic disease activity score at baseline [Citation42]. In recent trials, histologic endpoints have been included in protocols, which supports the growing recognition of histologic healing as an important outcome. The ongoing VERDICT trial is assessing a composite endpoint of corticosteroid-free histological remission, endoscopic remission, and symptomatic remission [Citation87]. Despite its current limitations, histologic remission is a feasible and appropriate endpoint in UC clinical trials [Citation18,Citation23,Citation42].
4.2. Advanced imaging and artificial intelligence
The combination of advanced imaging technologies, such as endocytoscopy, virtual electronic chromoendoscopy (VEC), and CLE, and tools utilizing artificial intelligence (AI) can aid in the detection and monitoring of histologic disease activity without repeated biopsies. Conventional endoscopy may not determine histologic disease activity accurately, but it is possible to detect inflammation at this level with endocytoscopy, which can image cellular features with up to 1390-fold magnification [Citation95,Citation96]. A 2019 study assessed the development of a computer-aided diagnosis tool that was able to predict persistent histologic disease activity in patients with UC (74% sensitivity, 97% specificity, 91% accuracy with perfect reproducibility) [Citation95]. Multiple tools using AI to assess endoscopic images are in development for clinical use. Examples include Red Density (RD), EndoBRAIN, Satisfai, and Paddington International virtual ChromoendoScopy ScOre (PICaSSO) [Citation97,Citation98]. PICaSSO was the first validated endoscopic score using the new generation of virtual chromoendoscopy endoscopes in UC [Citation97].
While MES and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) are common endoscopic indices, they were developed for earlier, lower-resolution endoscopic images. VEC can visually detail specific mucosal and vascular features. The RD score uses an algorithm that analyzes images from VEC to assess UC disease activity; RD correlated with RHI (r = 0.74, p < 0.0001) MES (r = 0.76, p < 0.0001), and UCEIS (r = 0.74, p < 0.0001) [Citation99]. PICaSSO is an endoscopic index that uses VEC [Citation100]. A 2022 international, multicenter study of patients with UC (N = 307) evaluated PICaSSO’s correlation with other endoscopic indices against histologic indices and clinical outcomes. PICaSSO had almost perfect interobserver agreement and better correlation with histologic scores compared to MES and UCEIS and was more predictive of histologic remission. A PICaSSO score of 3 or less points to endoscopic remission and is reliably associated with histologic remission [Citation100].
The PICaSSO Histologic Remission Index (PHRI) is a histologic index developed to apply PICaSSO scores to a computer-aided diagnosis of histologic disease activity. PHRI is a binary score based on the presence or absence of mucosal neutrophils, and an algorithm based on PHRI could predict histologic remission (differentiation of active vs quiescent UC: 78% sensitivity, 91.7% specificity, 86% accuracy) [Citation101]. Results from a 2023 study evaluating an artificial intelligence computer-aided diagnostic tool designed for use with the PHRI demonstrated acceptable sensitivity and specificity in detecting histologic activity versus remission (PHRI: 89% and 85%; RHI 94% and 76%; NHI 89% and 79%) [Citation102].
CLE is another tool capable of imaging mucosal structures at the cellular and subcellular levels in real time at 1000-fold magnification, allowing evaluations of barrier function [Citation52,Citation96,Citation103]. A small 2016 study of Danish patients with relapsing UC (N = 22) and healthy subjects (N = 7) evaluated their mucosa via colonoscopy, histopathology, and CLE and found that the detection of mucosal changes via CLE was reproducible [Citation103]. A 2021 observational, multicenter study of French patients with UC in remission (N = 100) developed a noninvasive histologic assessment using CLE. This confocal laser ENdomicroscopy for histological HeAliNg in ulCErative colitis (ENHANCE) index used NHI as a benchmark and had 79.6% accuracy, 57.8% sensitivity, and 82.8% specificity [Citation104]. An additional study focusing on the aspirational target of intestinal barrier healing in UC as assessed by CLE is further detailed in Sections 3.4 and 4.3.
4.3. Future aspirational treatment targets
The emergence of novel technologies and immunotherapies to assess and treat UC, respectively, has established molecular healing and disease clearance as aspirational targets beyond histologic healing, which have the potential to further prevent long-term adverse outcomes [Citation21,Citation22]. Molecular targets selected for their ability to repair immune dysregulation can restore mucosal barrier function; molecular studies may then serve as an adjunct to biopsy- and imaging-based assessments of microscopic disease activity to not only evaluate efficacy but also elucidate treatments’ mechanisms of action at a molecular level [Citation21,Citation105,Citation106]. Molecular targets for UC therapies include epithelial cells and their regulators, receptors and transporters, atypical lymphocytes, antigen-presenting cells, cytokines, interferons, tumor necrosis factors, and signaling pathways [Citation105,Citation106].
The Erlangen Remission in IBD (ERIca) trial, a prospective study of German patients with IBD in clinical remission (Crohn’s disease: N = 100; UC: N = 81), evaluated barrier dysfunction through 2 years using CLE [Citation52]. While 53.1% of the patients with UC in clinical remission were also in endoscopic remission at study inclusion, and 44.4% were in both endoscopic and histologic remission, only 25.9% of patients had intact barrier function (no fluorescein leakage into crypt lumen) as determined by CLE. The accuracy of barrier healing in predicting survival free of major adverse outcomes was 85% [Citation52]. Functional assessments of the intestinal barrier as measured by CLE may further support the predictive value of combined endoscopic-histologic measures and serve as an additional endpoint in future UC trials alongside histologic remission.
5. Expert opinion
Utilization of histologic evaluation by clinicians is currently aspirational and has not yet been applied to therapeutic decision-making. Results from the ongoing VERDICT trial will be necessary to determine the risk-benefit of treatment escalation in patients with symptomatic, biomarker, and/or endoscopic remission in order to achieve histologic remission. In the interim, ongoing treatments should not stop and could be further optimized in patients with persistent histologic activity even in the presence of endoscopic mucosal healing. Assessments of histologic remission may improve the accuracy and precision of treatment decisions and serve as predictors of improved clinical outcomes, including decreased rates of hospitalization, clinical relapse, colectomy, and neoplasia. Compared to histologic remission or quiescent histologic disease activity, histologic normalization (i.e. healing beyond histologic remission) is associated with incremental benefits in clinical outcomes.
Combining treatment targets in UC could be a strategy to improve long-term patient outcomes, and histologic remission could be used as adjunct to endoscopic remission, representing a deeper level of healing. Significant barriers to the adoption of histologic targets remain. Firstly, histologic assessments are often used in clinical trials with biopsies read by expert pathologists, but histologic assessments in clinical practice may have different levels of accuracy and/or reliability. Secondly, multiple, inconsistent definitions of histologic remission limit its use as a treatment target in both clinical trials and clinical practice. In addition, the number of histologic indices available further complicates consistent definitions of histologic remission and other histologic-based targets. Thirdly, standardized endoscopic and biopsy procedures are needed, requiring collaboration between gastroenterologists and pathologists. Lastly, histologic assessments in clinical practice can increase patient burden as they require an invasive procedure.
The treat-to-target strategy in UC has rapidly evolved from aiming for symptom control to endoscopic remission and now exploring the feasibility of histologic remission. Rather than using histologic remission as a primary target in clinical practice, it should be incorporated into a broader treat-to-target strategy. The use of combined endpoints has created new target definitions such as disease clearance, the simultaneous achievement of clinical, endoscopic, and histologic remission. While updated FDA and EMA guidance has touched on the use of histologic-based targets in clinical trials, regulatory guidance on the use of histologic remission as an endpoint could be further elaborated upon. Correlations between clinical, endoscopic, and histologic targets are unaligned, and the results of studies comparing them may not be easily interpreted.
The existence of consensus statements such as the 2020 European Crohn’s and Colitis Congress (ECCO) has helped to harmonize definitions of histologic remission and mucosal healing. In addition, ECCO has provided recommendations on which of the many histologic indices to use for randomized controlled trials, RHI and NHI, and for observational studies or clinical practice, NHI. The need for specialist pathologists for histologic assessments can be addressed by trainings on the use of simpler indices, such as NHI, as well as sending biopsies to central reading facilities. The recent establishment of the FNIH Biomarkers Consortium with the express goal of influencing future regulatory guidance is a step forward for harmonizing histologic endpoints. As most data supporting the association of histologic remission with improved outcomes are from observational studies, consistent and reproducible use of histologic assessments in clinical trials should be continued to generate more substantive evidence on the association of histologic remission with improved long-term clinical outcomes.
The rise of immunotherapies has influenced the increasing use of histologic assessments for their sensitivity in detecting changes in mucosal inflammation and established molecular healing and disease clearance as aspirational UC targets. Successful future research and targeted therapies selected for repairing immune dysregulation may then lead to the inclusion of combined histo-endoscopic endpoints, mucosal healing, and disease clearance as treatment targets alongside histologic remission. The next decade may bring about the feasibility of the restoration of mucosal barrier function and the use of artificial intelligence in creating indices to measure it based on endocytoscopy, VEC, and/or digital pathology.
Article highlights
The rise of immunotherapies has influenced the increasing recognition of histology for its sensitivity in detecting “deeper” mucosal healing and established disease clearance and molecular healing as aspirational treatment targets.
Assessment of histologic remission may improve the accuracy and precision of treatment decisions and serve as predictor of improved clinical outcomes, including decreased rates of hospitalization, clinical relapse, colectomy, and neoplasia.
Significant barriers to the adoption of histology remain including inconsistent histologic target definitions, lack of standardized endoscopic and histologic recommendations, variability in biopsy interpretation, and high patient burden due to invasive biopsy collection.
Declaration of interest
RK Pai has received consulting fees from: AbbVie, Alimentiv, Allergan, Eli Lilly and Company, Genentech, and PathAI. G D’Haens has been a consultant for AbbVie, Agomab, AstraZeneca, AM Pharma, AMT, Arena Pharmaceuticals, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Exeliom Biosciences, Exo Biologics, Galapagos, Index Pharmaceuticals, Kaleido, Roche, Gilead, GlaxoSmithKline, Gossamerbio, Pfizer, Immunic, Johnson & Johnson, Origo, Polpharma, Procise Diagnostics, Prometheus Laboratories, Prometheus Biosciences, Progenity, and Protagonist; and has received speaker’s bureau fees from AbbVie, Arena Pharmaceuticals, Galapagos, Gilead, Pfizer, BMS, and Takeda. T Kobayashi has received grants and/or contracts from: AbbVie, Activaid, Alfresa Pharma Corporation, Bristol-Myers Squibb, EA Pharma, Eli Lilly Japan K.K., Gilead Sciences, Google Asia Pacific, Janssen Japan K.K., JIMRO, JMDC, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Pfizer Japan, Takeda, and Zeria Pharmaceutical; has received lecture payment or honoraria from: AbbVie, Activaid, Alfresa Pharma, Galapagos, Janssen Japan K.K., JIMRO, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Nippon Kayaku, Pfizer Japan, Takeda, ThermoFisher Diagnostics, Zeria Pharmaceutical; has received payment for expert testimony from: AbbVie, Activaid, Alfresa Pharma, EA Pharma, Janssen Japan K.K., KISSEI Pharmaceutical, Kyorin Pharmaceutical, Mitsubishi Tanabe, Mochida Pharmaceutical, Nippon Kayaku, Pfizer Japan, and Takeda; and has participated on data safety monitoring boards or advisory boards for: Bristol-Myers Squibb, EA Pharma, Eli Lilly and Company, and Janssen. BE Sands has received fees for consulting and speaking from Abivax, Bristol Myers Squibb, Janssen, Lilly, Pfizer, and Takeda; for consulting from AbbVie; Adiso Therapeutics; Alimentiv; Amgen; Arena Pharmaceuticals; Artizan Biosciences; Artugen Therapeutics; AstraZeneca; Bacainn Therapeutics; Boehringer-Ingelheim; Boston Pharmaceuticals; Calibr; Celltrion Healthcare; ClostraBio; Cytoki Pharma; Connect Biopharma; Entera; Evommune; Fresenius Kabi; Galapagos; Genentech; Gilead Sciences; GlaxoSmithKline; Gossamer Bio; Imhotex; Immunic; Index Pharmaceuticals; Inotrem; Innovation Therapeutics; Ironwood Pharmaceuticals; Kaleido; Kallyope; MiroBio; Morphic Therapeutics; MRM Health; OSE Immunotherapeutics; Progenity; Prometheus Biosciences; Protagonist Therapeutics; Q32 Bio; Redhill Biopharma; Sun Pharma; Surrozen; Synlogic; Target RWE; Teva; Theravance Biopharma; TLL Pharmaceutical; USWM Enterprises; VielaBio: VTA Labs; and personal fees and stock options for consulting from Ventyx Biosciences.
S Travis has served as a consultant for Abacus, AbbVie, Actial, ai4gi, Alcimed, Allergan, Amgen, Apexian, Aptel, Arena, Asahi, Aspen, Astellas, Atlantic, AstraZeneca, Barco, Biocare, Biogen, BLPharma, Boehringer Ingelheim, BMS, Buhlmann, Calcico, Celgene, Cellerix, Cerimon, ChemoCentryx, Chiesi, CisBio, ComCast, Coronado, Cosmo, Ducentis, Dynavax, Elan, Enterome, EQrX, Equillium, Falk, Ferring, FPRT Bio, Galapagos, Genentech/Roche, Genzyme, Gilead, Glenmark, Grunenthal, GSK, GW Pharmaceuticals, Immunocore, Immunometabolism, Indigo, Janssen, Lexicon, Lilly, Medarex, Medtrix, Merck, Merrimack, Mestag, Millenium, Neovacs, Novartis, Novo Nordisk, NPS-Nycomed, Ocera, Optima, Origin, Otsuka, Palau, Pentax, Pfizer, Pharmaventure, Phesi, Phillips, P&G, Pronota, Protagonist, Proximagen, Resolute, Robarts, Sandoz, Santarus, Satisfai, Sensyne Health, Shire, SigmoidPharma, Sorriso, Souffinez, Syndermix, Synthon, Takeda, Theravance, Tigenix, Tillotts, Topivert, Trino Therapeutics with Wellcome Trust, TxCell, UCB Pharma, Vertex, VHsquared, Vifor, Warner Chilcott, and Zeria; as a speaker for AbbVie, Amgen, Biogen, Falk; Ferring, Janssen, Pfizer, Shire, Takeda, and UCB; and received grant support from AbbVie, Buhlmann, Celgene, ECCO, Helmsley Trust, IOIBD, Janssen, Lilly, Pfizer, Takeda, UCB, UKIERI, Vifor, and Norman Collisson Foundation.
V Jairath has received grants or contracts from: AbbVie, Adare, Atlantic, Boehringer Ingelheim, Celgene/Bristol-Myers Squibb, Eli Lilly and Company, Janssen, Seres, Takeda, UCB Pharma, and VH Squared; has received consulting fees from: AbbVie, Alimentiv, Arena, Asahi, Asieris, Bristol-Myers Squibb, Celltrion, Eli Lilly and Company, Ferring, Fresenius, Galapagos, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Kabi, Kasei Pharma, Landos Biopharma, Merck, Mylan, Organon Pandion, Pendopharm, Pfizer, Prometheus Biosciences, Protagonist Therapeutics, Reistone Biopharma, Roche, Sandoz, Second Genome, Takeda, Teva, Ventyx Biosciences, and Vividion Therapeutics; has received payment or honoraria from: AbbVie, Ferring, Galapagos, Janssen, Pfizer, and Takeda; and has participated on data safety monitoring or advisory boards for: AbbVie, Arena, Bristol-Myers Squibb, Fresenius, Gilead Sciences, Janssen, Kabi, Mylan, Roche, and Takeda.
FR has received consulting or advisory board fees from: AbbVie, Adnovate, Agomab, Allergan, Arena, Boehringer-Ingelheim, Celgene/BMS, CDISC, Celsius, Cowen, Ferring, Galapagos, Galmed, Genentech, Gilead Sciences, Gossamer, Guidepoint, Helmsley, Horizon Therapeutics, Image Analysis Limited, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Mopac, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB Pharma, Ysios, and 89Bio.
G De Hertogh has no conflicts of interest to declare.
B Park, K McGinnis, I Redondo, and TH Gibble are employees and shareholders of Eli Lilly and Company.
NG Lipitz is an employee of Syneos Health.
F Magro has served as a speaker and received honoraria from: AbbVie, Biogen, Falk, Ferring, Hospira, Laboratórios Vitória, Merck Sharp & Dohme, and Vifor.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Deborah Fisher, Eli Lilly and Company, for medical review.
Additional information
Funding
References
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet (London, England). 2017 Apr 29;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2
- Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohn’s & Colitis. 2012 Dec;6(10):965–990.
- Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020 Sep 10;6(1):74. doi: 10.1038/s41572-020-0205-x
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011 Nov 3;365(18):1713–1725. doi: 10.1056/NEJMra1102942
- Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019 Dec;68(Suppl 3):s1–s106.
- Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014 Nov;89(11):1553–1563. doi: 10.1016/j.mayocp.2014.07.002
- Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016 Mar;14(3):348–354.e17.
- Yarlas A, Willian MK, Nag A. The impact of clinical symptoms and endoscopic and histologic disease activity on health-related quality of life in patients with ulcerative colitis following treatment with multimatrix mesalazine. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. Qual Life Res. 2021 Jul;30(7):1925–1938. doi: 10.1007/s11136-021-02787-4
- Yarlas A, Rubin DT, Panés J, et al. Burden of ulcerative colitis on functioning and well-being: a systematic literature review of the SF-36® health survey. J Crohn’s & Colitis. 2018 Apr 27;12(5):600–609. doi: 10.1093/ecco-jcc/jjy024
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical Guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019 Mar;114(3):384–413.
- Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020 Apr;158(5):1450–1461.
- Singh S, Allegretti JR, Siddique SM, et al. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020 Apr;158(5):1465–1496.e17.
- Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology. 2015 Jan;148(1):37–51.e1.
- Mosli MH, Parker CE, Nelson SA, et al. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2017 May 25;5(5):Cd011256. doi: 10.1002/14651858.CD011256.pub2
- Yoon H, Jangi S, Dulai PS, et al. Incremental benefit of achieving endoscopic and histologic remission in patients with ulcerative colitis: a systematic review and meta-analysis. Gastroenterology. 2020 Oct;159(4):1262–1275.e7.
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015 Sep;110(9):1324–38.
- Colombel JF, D’Haens G, Lee WJ, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohn’s & Colitis. 2020 Feb 10;14(2):254–266. doi: 10.1093/ecco-jcc/jjz131
- Dal Buono A, Roda G, Argollo M, et al. Treat to target or ‘treat to clear’ in inflammatory bowel diseases: one step further? Expert review of gastroenterology & hepatology. Exp Rev Gastroenterol Hepatol. 2020 Sep;14(9):807–817.
- Wetwittayakhlang P, Lontai L, Gonczi L, et al. Treatment targets in ulcerative colitis: is it time for all In, including histology? J Clin Med. 2021 Nov 26;10(23):5551. doi: 10.3390/jcm10235551
- Ulcerative Colitis: Clinical Trial Endpoints. Draft Guidance for Industry. (CDER) CfDEaR. editor. 2016.
- Ungaro R, Colombel JF, Lissoos T, et al. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019 Jun;114(6):874–883.
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021 Apr;160(5):1570–1583.
- Danese S, Roda G, Peyrin-Biroulet L. Evolving therapeutic goals in ulcerative colitis: towards disease clearance. Nat Rev Gastroenterol Hepatol. 2020 Jan;17(1):1–2. doi: 10.1038/s41575-019-0211-1
- Schreiber S, Danese S, Dignass A, et al. Defining comprehensive disease control for use as a treatment target for ulcerative colitis in clinical practice: international delphi consensus recommendations. J Crohn’s & Colitis. 2023 Aug 16;17(Supplement_1):i311–i312. doi: 10.1093/ecco-jcc/jjac190.0281
- Bhattacharya S, Cross RK. Is endoscopic remission in ulcerative colitis still good enough? Inflammatory bowel diseases. Inflamm Bowel Dis. 2019 Oct 18;25(11):1729–1730.
- Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016 Mar;65(3):408–414.
- Pai RK, Lauwers GY, Pai RK. Measuring histologic activity in inflammatory bowel disease: why and how. Advances in anatomic pathology. Adv Anat Pathol. 2022 Jan 1;29(1):37–47.
- Bessissow T, Kron CM, Marcus V, et al. Impact of endoscopic and histologic activity on disease relapse in ulcerative colitis. Am J Gastroenterol. 2022 Oct 1;117(10):1632–1638. doi: 10.14309/ajg.0000000000001912
- Gupta A, Yu A, Peyrin-Biroulet L, et al. Treat to target: the role of histologic healing in inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021 Sep;19(9):1800–1813.e4.
- Pai RK, Geboes K. Disease activity and mucosal healing in inflammatory bowel disease: a new role for histopathology? Virchows Arch. 2018 Jan;472(1):99–110. doi: 10.1007/s00428-017-2156-5
- Magro F, Doherty G, Peyrin-Biroulet L, et al. ECCO position paper: harmonization of the approach to ulcerative colitis histopathology. J Crohn’s & Colitis. 2020 Nov 7;14(11):1503–1511. doi: 10.1093/ecco-jcc/jjaa110
- D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007 Feb;132(2):763–786.
- de Jong MJ, Huibregtse R, Masclee AAM, et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018 May;16(5):648–663.e3.
- Dubinsky M, Bleakman AP, Panaccione R, et al. Bowel urgency in ulcerative colitis: current perspectives and future directions. Am J Gastroenterol. 2023 Aug 4;118(11):1940–1953. doi: 10.14309/ajg.0000000000002404
- Pakpoor J, Travis S. Why studying urgency is urgent. Gastroenterol & Hepatol. 2023 Feb;19(2):95–100.
- Pai RK, Jairath V, Vande Casteele N, et al. The emerging role of histologic disease activity assessment in ulcerative colitis. Gastrointest Endosc. 2018 Dec;88(6):887–898.
- Rath T, Atreya R, Neurath MF. Is histological healing a feasible endpoint in ulcerative colitis? Expert review of gastroenterology & hepatology. Exp Rev Gastroenterol Hepatol. 2021 Jun;15(6):665–674. doi: 10.1080/17474124.2021.1880892
- Feakins RM. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013 Dec;66(12):1005–1026. doi: 10.1136/jclinpath-2013-201885
- Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohn’s & Colitis. 2013 Nov;7(10):827–851.
- DeRoche TC, Xiao SY, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol Rep (Oxf). 2014 Aug;2(3):178–192. doi: 10.1093/gastro/gou031
- Vespa E, D’Amico F, Sollai M, et al. Histological scores in patients with inflammatory bowel diseases: the state of the art. J Clin Med. 2022 Feb 11;11(4):939. doi: 10.3390/jcm11040939
- Ma C, Sedano R, Almradi A, et al. An international consensus to standardize integration of histopathology in ulcerative colitis clinical trials. Gastroenterology. 2021 Jun;160(7):2291–2302.
- Villanacci V, Antonelli E, Geboes K, et al. Histological healing in inflammatory bowel disease: a still unfulfilled promise. World J Gastroenterol. 2013 Feb 21;19(7):968–978. doi: 10.3748/wjg.v19.i7.968
- Li K, Marano C, Zhang H, et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology. 2020 Dec;159(6):2052–2064.
- Pai RK, Khanna R, D’Haens GR, et al. Definitions of response and remission for the Robarts Histopathology index. Gut. 2019 Nov;68(11):2101–2102.
- Peyrin-Biroulet L, Loftus EV Jr., Colombel JF, et al. Histologic outcomes with vedolizumab versus adalimumab in ulcerative colitis: Results from an efficacy and safety study of vedolizumab intravenous compared to adalimumab subcutaneous in participants with ulcerative colitis (VARSITY). Gastroenterology. 2021 Oct;161(4):1156–1167.e3.
- Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut. 2017 Jan;66(1):43–49.
- Lang-Schwarz C, Angeloni M, Agaimy A, et al. Validation of the ‘inflammatory bowel disease-distribution, chronicity, activity [IBD-DCA] score’ for ulcerative colitis and Crohn´s disease. J Crohn’s & Colitis. 2021 Oct 7;15(10):1621–1630. doi: 10.1093/ecco-jcc/jjab055
- Jairath V, Peyrin-Biroulet L, Zou G, et al. Responsiveness of histological disease activity indices in ulcerative colitis: a post hoc analysis using data from the TOUCHSTONE randomised controlled trial. Gut. 2019 Jul;68(7):1162–1168.
- Magro F, Lopes J, Borralho P, et al. Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut. 2019 Apr;68(4):594–603.
- Magro F, Estevinho MM, Dias CC, et al. Clinical, endoscopic and histological outcomes in induction of moderate-to-severe ulcerative colitis: a systematic review with meta-analysis. J Crohn’s & Colitis. 2021 Apr 6;15(4):551–566. doi: 10.1093/ecco-jcc/jjaa176
- Rath T, Atreya R, Bodenschatz J, et al. Intestinal barrier healing is Superior to endoscopic and histologic remission for predicting major adverse outcomes in inflammatory bowel disease: the prospective ERIca Trial. Gastroenterology. 2022 Oct 21;164(2):241–255. doi: 10.1053/j.gastro.2022.10.014
- Kishi M, Hirai F, Takatsu N, et al. A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation. J Gastroenterol. 2022 Apr;57(4):246–266.
- Chateau T, Feakins R, Marchal-Bressenot A, et al. Histological remission in ulcerative colitis: under the microscope is the cure. Am J Gastroenterol. 2020 Feb;115(2):179–189.
- Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000 Sep;47(3):404–409.
- Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut. 2017 Jan;66(1):50–58
- Lang-Schwarz C, Agaimy A, Atreya R, et al. Maximizing the diagnostic information from biopsies in chronic inflammatory bowel diseases: recommendations from the Erlangen International Consensus Conference on inflammatory bowel diseases and presentation of the IBD-DCA score as a proposal for a new index for histologic activity assessment in ulcerative colitis and Crohn’s disease. Virchows Arch. 2021 Mar;478(3):581–594.
- Hindryckx P, Baert F, Hart A, et al. Clinical trials in ulcerative colitis: a historical perspective. J Crohn’s & Colitis. 2015 Jul;9(7):580–8.
- Pandey A, Achrafie L, Kodjamanova P, et al. Endoscopic mucosal healing and histologic remission in ulcerative colitis: a systematic literature review of clinical, quality-of-life and economic outcomes. Curr Med Res Opin. 2022 Sep;38(9):1531–1541.
- Mosli MH, Feagan BG, Zou G, et al. Reproducibility of histological assessments of disease activity in UC. Gut. 2015 Nov;64(11):1765–1773.
- Magro F, Lopes J, Borralho P, et al. Comparison of the Nancy index with continuous geboes score: histological remission and response in ulcerative colitis. J Crohn’s & Colitis. 2020 Jul 30;14(7):1021–1025. doi: 10.1093/ecco-jcc/jjaa010
- Magro F, Sabino J, Rosini F, et al. ECCO Position on harmonisation of Crohn’s disease mucosal histopathology. J Crohn’s & Colitis. 2022 Jul 14;16(6):876–883. doi: 10.1093/ecco-jcc/jjac006
- Ma C, Hanzel J, Panaccione R, et al. CORE-IBD: a multidisciplinary international consensus initiative to develop a core outcome set for randomized controlled trials in inflammatory bowel disease. Gastroenterology. 2022 Oct;163(4):950–964.
- Ulcerative Colitis: Developing Drugs for Treatment. Draft Guidance for Industry. (CBER) CfBEaR. editor. 2022.
- Guideline on the development of new medicinal products for the treatment of ulcerative colitis. (CHMP) CfMPfHU. editor. 2018.
- Biomarkers consortium - mucosal healing in UC: definition, treatment target and clinical endpoints foundation for the national institutes of Health. 2021. [cited 2022 Dec 5]. Available from: https://fnih.org/our-programs/biomarkers-consortium/programs/mucosal-healing
- Atreya R, Peyrin-Biroulet L, Klymenko A, et al. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): a phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol Hepatol. 2020 Dec;5(12):1063–1075.
- Sandborn WJ, Vermeire S, Peyrin-Biroulet L, et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet (London, England). 2023 Apr 8;401(10383):1159–1171. doi: 10.1016/S0140-6736(23)00061-2
- Feagan BG, Danese S, Loftus EV Jr., et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet (London, England). 2021 Jun 19;397(10292):2372–2384. doi: 10.1016/S0140-6736(21)00666-8
- Peyrin-Biroulet L, Allegretti JR, Rubin DT, et al. Guselkumab in patients with moderately to severely active ulcerative colitis: QUASAR phase 2b induction study. Gastroenterology. 2023 Sep 1;165(6):1443–1457.10.1053/j.gastro.2023.08.038
- An induction study of Mirikizumab in participants with moderately to severely active ulcerative colitis (LUCENT 1). Available from: https://classic.clinicaltrials.gov/show/NCT03518086
- A maintenance study of Mirikizumab in participants with moderately to severely active ulcerative colitis. Available from: https://classic.clinicaltrials.gov/show/NCT03524092
- Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021 Sep 30;385(14):1280–1291. doi: 10.1056/NEJMoa2033617
- Risankizumab (SKYRIZI®) Met primary and key secondary endpoints in 52-week phase 3 maintenance study in ulcerative colitis patients [Internet]. 2023 Jun 15. Available from: https://news.abbvie.com/2023-06-15-Risankizumab-SKYRIZI-R-Met-Primary-and-Key-Secondary-Endpoints-in-52-Week-Phase-3-Maintenance-Study-in-Ulcerative-Colitis-Patients
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet (London, England). 2022 Jun 4;399(10341):2113–2128. doi: 10.1016/S0140-6736(22)00581-5
- Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019 Sep 26;381(13):1201–1214. doi: 10.1056/NEJMoa1900750
- A study to evaluate the safety and efficacy of ustekinumab induction and maintenance therapy in participants with moderately to severely active ulcerative colitis. Available from: https://classic.clinicaltrials.gov/show/NCT02407236
- D’Haens G, Dubinsky M, Kobayashi T, et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2023 Jun 29;388(26):2444–2455. doi: 10.1056/NEJMoa2207940
- Pai RK, Hartman DJ, Rivers CR, et al. Complete resolution of mucosal neutrophils associates with improved long-term clinical outcomes of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2510–2517.e5
- Kobayashi T, Motoya S, Nakamura S, et al. Discontinuation of infliximab in patients with ulcerative colitis in remission (HAYABUSA): a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021 Jun;6(6):429–437.
- Jangi S, Yoon H, Dulai PS, et al. Predictors and outcomes of histological remission in ulcerative colitis treated to endoscopic healing. Aliment Pharmacol Ther. 2020 Sep;52(6):1008–1016.
- Christensen B, Hanauer SB, Erlich J, et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017 Oct;15(10):1557–1564.e1.
- Shaffer SR, Erondu AI, Traboulsi C, et al. Achieving histologic normalization in ulcerative colitis is associated with a reduced risk of subsequent dysplasia. Inflamm Bowel Dis. 2022 Mar 30;28(4):553–559.10.1093/ibd/izab130
- Magro F, Pai RK, Kobayashi T, et al. Resolving histologic inflammation in ulcerative colitis with mirikizumab in the LUCENT induction and maintenance trial programs. J Crohn’s & Colitis. 2023 Apr 14;17(9):1457–1470. doi: 10.1093/ecco-jcc/jjad050
- Verstockt B, Pouillon L, Ballaux F, et al. Patient-reported outcomes and disability are associated with histological disease activity in patients with ulcerative colitis: results from the APOLLO study. J Crohn’s & Colitis. 2023 Jul 5;17(7):1046–1054. doi: 10.1093/ecco-jcc/jjad015
- Narula N, Wong ECL, Colombel JF, et al. Early change in epithelial neutrophilic infiltrate predicts long-term response to biologics in ulcerative colitis. Clin Gastroenterol Hepatol. 2022 May;20(5):1095–1104.e9.
- Determination of the optimal treatment target in ulcerative colitis. Available from: https://ClinicalTrials.gov/show/NCT04259138
- Honig G, Heller C, Hurtado-Lorenzo A. Defining the path forward for biomarkers to address unmet needs in inflammatory bowel diseases. Inflamm Bowel Dis. 2020 Sep 18;26(10):1451–1462.
- D’Inca R, Sturniolo G. Biomarkers in IBD: what to utilize for the diagnosis? Diagnostics (Basel). 2023 Sep 13;13(18). doi: 10.3390/diagnostics13182931
- Jukic A, Bakiri L, Wagner EF, et al. Calprotectin: from biomarker to biological function. Gut. 2021 Oct;70(10):1978–1988.
- D’Amico F, Bonovas S, Danese S, et al. Review article: faecal calprotectin and histologic remission in ulcerative colitis. Aliment Pharmacol Ther. 2020 Apr;51(7):689–698.
- Dulai PS, Peyrin-Biroulet L, Danese S, et al. Approaches to integrating biomarkers into clinical trials and care pathways as targets for the treatment of inflammatory bowel diseases. Gastroenterology. 2019 Oct;157(4):1032–1043 e1.
- Guirgis M, Wendt E, Wang LM, et al. Beyond histological remission: Intramucosal Calprotectin as a potential predictor of outcomes in ulcerative colitis. J Crohn’s & Colitis. 2017 Apr 1;11(4):460–467.
- Römkens TEH, Kranenburg P, Tilburg AV, et al. Assessment of histological remission in ulcerative colitis: discrepancies between daily practice and expert opinion. J Crohn’s & Colitis. 2018 Mar 28;12(4):425–431. doi: 10.1093/ecco-jcc/jjx165
- Maeda Y, Kudo SE, Mori Y, et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019 Feb;89(2):408–415.
- Nakase H, Hirano T, Wagatsuma K, et al. Artificial intelligence-assisted endoscopy changes the definition of mucosal healing in ulcerative colitis. Dig Endosc. 2021 Sep;33(6):903–911.
- Ahmad HA, East JE, Panaccione R, et al. Artificial intelligence in inflammatory bowel disease endoscopy: implications for clinical trials. J Crohn’s & Colitis. 2023 Aug 21;17(8):1342–1353. doi: 10.1093/ecco-jcc/jjad029
- Byrne MF, Panaccione R, East JE, et al. Application of deep learning models to improve ulcerative colitis endoscopic disease activity scoring under multiple scoring systems. J Crohn’s & Colitis. 2023 Apr 19;17(4):463–471. doi: 10.1093/ecco-jcc/jjac152
- Bossuyt P, Nakase H, Vermeire S, et al. Automatic, computer-aided determination of endoscopic and histological inflammation in patients with mild to moderate ulcerative colitis based on red density. Gut. 2020 Oct;69(10):1778–1786.
- Iacucci M, Smith SCL, Bazarova A, et al. An international multicenter real-life prospective study of electronic chromoendoscopy score PICaSSO in ulcerative colitis. Gastroenterology. 2021 Apr;160(5):1558–1569.e8.
- Gui X, Bazarova A, Del Amor R, et al. PICaSSO histologic remission index (PHRI) in ulcerative colitis: development of a novel simplified histological score for monitoring mucosal healing and predicting clinical outcomes and its applicability in an artificial intelligence system. Gut. 2022 May;71(5):889–898.
- Iacucci M, Parigi TL, Del Amor R, et al. Artificial intelligence enabled histological prediction of remission or activity and clinical outcomes in ulcerative colitis. Gastroen. 2023 Jun;164(7): 1180–1188. doi: 10.1053/j.gastro.2023.02.031
- Karstensen JG, Săftoiu A, Brynskov J, et al. Confocal laser endomicroscopy in ulcerative colitis: a longitudinal study of endomicroscopic changes and response to medical therapy (with videos). Gastrointest Endosc. 2016 Aug;84(2):279–286.e1.
- Rahmi G, Coron E, Perrod G, et al. Probe-based confocal laser endomicroscopy for in vivo assessment of histological healing in ulcerative colitis: development and validation of the ENHANCE index. J Crohn’s & Colitis. 2021 Jun 22;15(6):994–999. doi: 10.1093/ecco-jcc/jjaa255
- Katsanos KH, Papadakis KA. Inflammatory bowel disease: updates on molecular targets for biologics. Gut Liver. 2017 Jul 15;11(4):455–463.
- Neurath MF, Leppkes M. Resolution of ulcerative colitis. Semin Immunopathol. 2019 Nov;41(6):747–756. doi: 10.1007/s00281-019-00751-6