 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
Low- and middle-income countries (LMICs) bear a high proportion of the global morbidity and mortality caused by COPD. Increased exposure to risk factors throughout life (e.g. malnutrition, indoor and outdoor air pollution, and smoking) is associated with higher COPD prevalence in LMICs and the lack of treatment availability increases avoidable harm.
Areas covered
This review covers the epidemiology and burden of COPD in LMICs, and challenges and recommendations related to health-care systems, prevention, diagnosis, and treatment. Main challenges are related to under-resourced health-care systems (such as limited availability of spirometry, rehabilitation, and medicines). Lack of policy and practical local guidelines on COPD diagnosis and management further contribute to the low diagnostic and treatment rates. In the absence of, or limited number of respiratory specialists, primary care practitioners (general practitioners, nurses, pharmacists, physiotherapists, and community health workers) play an even more pivotal role in COPD management in LMICs.
Expert opinion
Raising awareness on COPD, educating health-care workers, patients, and communities on cost-effective preventive measures as well as improving availability, affordability and proper use of diagnostic and pharmacological and non-pharmacologic treatment in primary care are the key interventions needed to improve COPD prevention, diagnosis, and care in LMICs.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most prevalent and potential life threatening noncommunicable diseases (NCDs). According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), COPD is defined as a common, preventable, and treatable disorder [Citation1]. The pathogenesis and pathophysiology of COPD is considered multifactorial while the course of the disease varies for each patient and is often life limiting. The most common characteristics of the disorder are breathlessness, cough, and sputum production, along with multiple respiratory infections and cardiovascular complications that dramatically increase mortality from the disease [Citation1].
Globally, COPD is the third leading cause of death, and accounted for 6% of total deaths in 2019, of which more than 80% occurred in low- and middle-income countries (LMICs) [Citation2,Citation3]. Apart from increased mortality and high morbidity, COPD also results in significant socio-economic burden in LMICs due to its impact on work productivity [Citation4,Citation5].
The aim of this review is to portray the current situation and strategies for COPD prevention, diagnosis, and treatment in LMICs (as defined by the World Bank [Citation6]). In parallel, we highlight the challenges that impede implementation of proven COPD burden mitigation strategies and make further recommendations that meet current needs, focusing on the role of primary care. To inform this review, we searched the literature on COPD in LMICs, and added the authors’ international and multidisciplinary experience on this topic.
2. Epidemiology and burden of disease
A recent review on global diagnosis of COPD reported large variations in the prevalence of COPD across different geographical regions (i.e. 3% to 21%) and stressed the significant variation between and within individual countries in terms of, for example, urban and rural populations, poverty levels, risk factors, and those at highest risk of disadvantage [Citation7].
The lack of spirometric confirmation is a major impediment in establishing comparable epidemiological data. In addition, most of the data on disease burden are modeled (e.g. the Global Burden of Disease study) and the modeling is influenced by the available literature which is mainly based on studies from high-income countries (HICs) studies [Citation8].
2.1. Epidemiology of COPD in LMICs
Most LMICs are located in Africa, Latin America, and Asia, including Eurasia. In the absence of routine data in primary care in these countries, our knowledge of COPD epidemiology in LMICs mostly comes from prevalence surveys. The majority of prevalence surveys on chronic respiratory diseases in LMICs have been conducted in Asia, and the most common methods used to determine COPD prevalence are as follows: a) spirometry, with FEV1/FVC ratio, or b) based on the GOLD guideline definition (i.e. clinical manifestations with history of risk factors, confirmed by spirometry) [Citation1,Citation9].
Asia is the world’s largest tobacco producer and half of the world's current smokers live in three middle-income Asian countries: India, China, and Indonesia [Citation10]. Smoking rates are high in Bangladesh as well [Citation10]. COPD prevalence data from East and South-East Asian countries are still sparse and estimates based on the prevalence of certain risk factors and epidemiological relationships vary, with 3.5% in Hong Kong and Singapore, 6.7% in Vietnam, 4.7% in Malaysia, 5% in Thailand, 5.6% in Indonesia, 6.3% in the Philippines, 5.4% in Taiwan, 5.9% in South Korea and 6.1% in Japan [Citation7,Citation11]. In India, the BOLD study in 2014 used standardized spirometry and reported overall COPD prevalence estimates of 5.7% to 17.3% in several Indian cities (i.e. Pune, Mumbai, and Srinagar) for males and 6.8% to 14.8% for females [Citation12]. In Bangladesh, the prevalence of COPD was 13.5% by GOLD definition criteria and 10.3% by lower limit of normal (LLN) criteria, assessed using hand-held spirometers. The prevalence of COPD was higher among rural than urban residents and in males than females; more than half of the COPD cases were stage II (‘moderate’) COPD by both criteria [Citation13].
In Africa, COPD has been recognized as a silent epidemic posing a large public health problem. In 2010, there were 26.3 million cases of COPD in Africa in 2010, an increase of 31.5% over a decade (mainly attributable to aging of the African population) [Citation14]. Although the prevalence of COPD in sub-Saharan Africa has been poorly studied, a meta-analysis of nine cross-sectional, spirometry-based, studies (including 3673 people from South Africa, Nigeria, Malawi, and Cape Verde) have shown a prevalence ranging from 4% to 25%, depending on the definition or diagnostic criterion used [Citation15]. Studies and data based on spirometry generally report a higher prevalence rate. Indeed, the median prevalence of COPD in persons aged 40 years based on spirometry data (13.4%) was significantly higher than those based on non-spirometry data (4.0%) such as using questionnaires to investigate exposures, population characteristics, and symptoms. Moreover, Ho et al. [Citation7] reported that the prevalence of chronic airflow obstruction differs significantly not only among different countries but also between different communities within a country.
In Latin America, the prevalence of smoking in Central and South America is high (30%), contributing significantly to the burden of respiratory diseases [Citation16]. The PLATINO and PREPOCOL population-based have estimated the prevalence of COPD in several large urban centers that ranged between 6.2% and 19.6% in individuals ≥40 years of age, with substantial rates of underdiagnosis (up to 89%) but also overdiagnosis, mostly due to the lack of spirometric confirmation [Citation16,Citation17]. These findings were confirmed in a recent review of studies assessing the prevalence of COPD by using both questionnaires and portable spirometry in a small number of cities across the Latin America region where the prevalence was reported to be between 7.8% and 19.7% [Citation16,Citation18]. However, larger studies and those representative of the different communities across countries (including isolated areas) have not been performed to date [Citation7].
Worldwide, COPD prevalence seems to be increasing. Although the highest formal incidence and prevalence are observed in HICs, it is estimated that official data from LMICs, that show relatively lower rates, do not reflect the real situation. In 2019, the prevalence of COPD in HICs was 5.4%, whereas in LMICs it was 1.1% () [Citation19]. Lack of awareness, underdiagnosis and underreporting of COPD cases are considered some of the factors that underestimate the actual COPD incidence in LMICs [Citation7]. Moreover, most estimates are not necessarily based on actual epidemiological data, but rather on projections based on risk factor exposure and hence do not reflect the true prevalence of COPD in LMICs. As a result, policymakers do not have accurate data to plan effective strategies to reduce the burden of disease. Despite the overall reported low rates in LMIC, there is an upward trend forecast due to increasing life expectancy and reduction of childhood mortality, paired with heightened COPD awareness [Citation20].
Figure 1. Prevalence (a), Incidence (b), Mortality (c) and Burden of COPD (d) in countries according to income level during 2000–2020 [Citation19,Citation21]
![Figure 1. Prevalence (a), Incidence (b), Mortality (c) and Burden of COPD (d) in countries according to income level during 2000–2020 [Citation19,Citation21]](/cms/asset/32d0de8f-fa87-48c8-8178-11350b20236c/ierx_a_1985762_f0001_oc.jpg)
2.2. Burden of COPD in LMIC
Although there is limited robust epidemiological data on COPD burden in LMICs, it is estimated that the burden of COPD is greatest in lower-middle-income settings [Citation3]. Burden of a disease comprises symptom, healthcare, and societal burden [Citation22]. The direct impact to the patient is expressed by morbidity and mortality. Although COPD-related mortality has decreased over the last 20 years, mainly in higher and upper-middle-income settings, the vast majority of COPD-related deaths occur in LMICs [Citation3]. In addition, the highest number of Disability Adjusted Life Years (DALYs) and Years Lost due to Disability (YLDs) is seen in LMICs ().
Currently, COPD is the third leading cause of death worldwide and is estimated to rise to the top of the list in the next decade [Citation23]. In the PURE study, the 1-year case-fatality rates for COPD after an event (e.g. hospital admission) were found to be 8 times higher in low-income compared to HICs [Citation24].
COPD, a public health problem, has also posed a negative financial impact. In 2010, the global cost of COPD was US$ 2.1 trillion, which is expected to rise to US$4.8 trillion by the year 2030 [Citation25]. This is an extremely conservative estimate, as the actual economic burden due to COPD underdiagnosis, the long list of comorbidities, such as cardiovascular disease, lung cancer, and mental health problems that COPD is associated with, would further strain health-care systems [Citation26].
3. Healthcare systems in LMICs
Skilled health services and workforces along with access to affordable interventions including medication are among the drivers for good COPD care according to the World Health Organization (WHO)’s six building blocks to universal healthcare () [Citation27]. Individuals in LMICs lack access to health-care and medical facilities in a timely manner [Citation28,Citation29]. Proactive care is not the norm and is often episodic, driven by immediate need and influenced by personal financial status. Services may be fragmented and inadequate due to overcrowding, lack of equipment, deficient infrastructure, poor referral systems, and accessibility [Citation30].
Figure 2. Drivers of good COPD care (Adapted by IPCRG from WHO Health Systems 6 Building Blocks Framework for Universal Coverage) S. Williams 2021
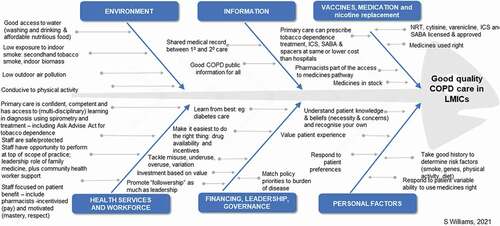
Primary care is the first contact of care for most patients. It offers continuous, preventive, comprehensive, and coordinated care and provides the means to achieving universal health coverage [Citation31]. However, countries vary in maturity of their primary health-care systems. Challenges include an insufficiently trained and competent workforce, particularly in rural and remote communities, with deficits in coordination within primary health-care systems, as well as between primary and secondary care [Citation32]. In many cases, there is no system for monitoring, evaluating, and promoting health status including respiratory health. The lack of preventive strategies and measures results in an increased diseased population. As the prevalence grows, the burden becomes greater; a higher demand is placed on an already deficient system and the local economies struggle to cope [Citation33]. To reduce inequalities and improve people’s health, international organizations such as the United Nations and WHO regularly provide funding to support LMICs, offering grants designated for several specific diseases. Unfortunately, these funds are rarely allotted to healthcare system infrastructure [Citation34].
The ratio of medical doctors to population is very low in many LMICs. This implies a lack of human resources especially in African countries such as Somalia where the corresponding ratio is as low as 0.23 medical doctors per 10,000 people (2014) [Citation35]. As such, there is a need to involve a wide range of health-care providers, beyond (specialized) physicians alone. Primary care can play a key role with the involvement of general practitioners, nurses, pharmacists, physiotherapists, and non-medically educated personnel, including community health workers.
Respiratory NCDs have not received attention in tandem with the disease burden. These conditions are often excluded from national NCDs reports and guidelines and overlooked by policy and miscoded in clinical practice, leading to under-investment in research and clinical provision. Hence, there is a need to strengthen and empower primary care in the diagnosis, prevention, and management of COPD, beyond respiratory physicians.
4. Prevention
COPD is preventable if the risk factors are identified and addressed. However, mitigating risk factors is complex in practice. The following sections detail the main risk factors associated with COPD.
4.1. Risk factors and current management
4.1.1. Behavioral risk factors: tobacco smoking
Tobacco smoking is widely acknowledged as the most causative factor for COPD [Citation36]. Worldwide, there are 1.3 billion smokers, more than 80% of whom live in LMICs [Citation37]. Smoking alone was responsible for almost 1.5 million COPD-related deaths in 2015 [Citation38]. Beyond the health impact, smoking further increases poverty and inequity, leading to a development trap [Citation37]. Most nations participate in the WHO Framework Convention on Tobacco Control (FCTC) [Citation39] (). However, the vast majority of non-parties are in LMICs [Citation39,Citation40]. Quitting smoking reduces personal risk of developing COPD and family risk due to secondhand smoke. However, it should not only be regarded as a preventive measure. Quitting smoking is the only intervention for COPD known to slow progression of COPD [Citation41]. Helping people quit smoking, i.e. Article 14 of the FCTC, is a core target in the WHO’s MPOWER strategy and Sustainable Development Goal (SDG) 3 [Citation42,Citation43]. In many countries, the majority of smokers can access support to quit smoking from primary care.
Figure 3. World map showing countries with (Yes) or without (No) operational policy/ strategy/ action plan to decrease tobacco use according to income (2019) [Citation42]
![Figure 3. World map showing countries with (Yes) or without (No) operational policy/ strategy/ action plan to decrease tobacco use according to income (2019) [Citation42]](/cms/asset/876226c3-e970-4e04-9fc6-5fcb552e47f2/ierx_a_1985762_f0003_oc.jpg)
4.1.2. Environmental risk factors: air pollution
In 2015, an approximate 1.8 million COPD-related deaths were attributed to air pollution [Citation38]. Additionally, 354,000 COPD-related deaths were attributed to occupational exposures to air pollution (i.e. particulate matter, fumes, and gases) [Citation38]. Air pollution can be categorized into household (indoor) and ambient (outdoor) pollution.
The major source of household air pollution is the use of biomass fuels (e.g. wood, crop waste, and animal dung) for cooking and heating. Other examples include kerosene lamps, burning incense sticks in religious places and burning mosquito repellent coils [Citation44]. Three billion people worldwide, accounting for 40% of households, make use of solid fuels and 90% of rural households located in LMICs rely on them [Citation45]. The epicenter of solid fuel consumption are LMICs and especially Africa [Citation46,Citation47]. Exposure to household air pollution is a serious health hazard. In a recent study focusing on household air pollution in 13 LMICs, exposure increased the risk of COPD by 41% [Citation45]. The population that is most at risk for developing COPD due to biomass exposure are women living in rural areas, possibly due to extended hours spent indoors. Interestingly, it appears that different etiologies of COPD influence the phenotype of the disease. For example, biomass-induced COPD is often observed in younger patients and is associated with more acute symptoms compared to smoking-induced COPD. In addition, biomass-induced COPD is characterized by more frequent acute exacerbations and greater number of hospitalizations [Citation44].
The highest exposure rates of ambient air pollution (i.e. forest fires, dust storms, industrial and traffic exhausts [Citation44]) are seen in developing countries [Citation48]. In the last 10 years, a significant increase in DALYs attributed to ambient air pollution has been documented in areas with a low- and low-middle socio-demographic index. Therefore, air pollution remains a leading risk factor for DALYs in LMICs, especially in eastern sub-Saharan Africa and South Asia [Citation49].
The strategic solution outlined in the Sustainable Development Goals 7 is switching to affordable cleaner energy. Indonesia and India have programs in place for transition to cleaner fuels [Citation50,Citation51]. Meanwhile, the use of improved cookstoves, or improving ventilation, are feasible interim steps where switching is not possible. However, numerous initiatives for supplying improved cookstoves have not demonstrated long-term effects [Citation52].
Notably, the attribution of COPD to biomass exposure is an issue of ongoing discussion and research. Several studies, including the BOLD, have shown contradictory and inconsistent links between airflow obstruction and indoor pollution [Citation53]. Similarly, data are suggestive, but not conclusive, of a role for ambient air pollution in the etiology of COPD [Citation54]. However, the Global Burden of Disease study has identified environmental pollutant exposure as the largest contributor to COPD burden in low-income countries [Citation55]. Yet, long-term exposure to indoor air pollution is difficult to measure and may also be associated with other risk factors such as lower socio-economic status and poverty, resulting in poorer nutritional status and smaller lung development during pregnancy and infancy [Citation56,Citation57].
4.1.3. Genetic, early childhood, and physiological risk factors: host characteristics
A relatively new area that has attracted attention is the influence of genetics and early life risk factors for COPD, starting from pre-conception. Parental and especially maternal health and lifestyle (e.g. asthma, smoking, and nutrition) influence embryonic lung formation and lung function growth and trajectory [Citation58]. Early life disadvantages such as low birth weight, prematurity, malnutrition, childhood respiratory infections, and allergic diseases are powerful determinants of lung function [Citation58,Citation59]. Similarly, chronic exposure to air pollution reduces attainment of maximal lung function in childhood, accelerates lung function decline later in life and impairs pulmonary innate immunity [Citation60]. This is significant because the lung-function value reached in early adulthood appears to have an impact on COPD development [Citation61]. Similarly, a lower Body Mass Index (BMI), stemming usually from malnutrition, is a risk factor for COPD and impaired lung function, as well as a poor prognostic factor [Citation62].
Regarding infections, HIV and tuberculosis are both highly prevalent in many LMICs, have detrimental effects on the lungs, and are therefore COPD risk factors [Citation63]. The immunization coverage against tuberculosis in the first year of life is 78% in low-income and 88% in lower-middle-income countries [Citation64].
Noninfectious diseases can also be a risk factor for COPD. For example, asthma is associated with impaired lung function and, consequently, with COPD [Citation59]. Indeed, the prevalence of asthma is increasing in LMICs and extensive severity of the disease is documented in these countries despite the fact that asthma is considered not well diagnosed and its understanding is poor in LMICs [Citation65]. There is an apparent need for clinical tools to aid accurate diagnosis, as well as to incentivize systems that enable regular reviews to ensure disease control. This underlines the importance of equipping primary care with the skills to differentiate between chronic respiratory diseases, to ensure correct diagnosis and treatment.
The most well-established genetic risk factor for COPD is alpha-1 antitrypsin deficiency. Although mostly encountered in Western and European countries, it is also seen in some sub-Saharan countries [Citation26].
4.1.4. Demographic risk factors: socioeconomic status (SES)
Income and poverty aside, characteristics such as education, occupation, housing situation, living environments conducive to physical activity, and social life have been linked to COPD [Citation66]. Many of these characteristics are associated with other known risk factors, such as daily exposure to biomass fuels for cooking, and may be confounding the relationship between SES and COPD [Citation57].
In general, COPD prevention efforts have focused on modifiable risk factors, i.e. environmental and behavioral risk factors. In contrast, host characteristics and SES are typically less easily modifiable, meaning that primordial prevention targeting the former, primarily provided by trained primary care providers such as general practitioners and family doctors, and untrained health-care workers such as community health workers needs to be put in place.
4.2. Ongoing challenges in prevention
Preventive interventions are key to reducing the burden of COPD in LMICs. However, they have often been proved unwelcome, ineffective or not appropriately implemented especially when referred to behavioral elements that need tailoring to local traditions and culture, for instance, the programs for improved cookstoves and heaters. Stakeholder engagement is essential in the implementation of a program that respects cultural norms. Combining respect for cultural norms requires an active program of stakeholder engagement [Citation67]. Primary care, which embeds within the communities, plays an important role to coordinate this.
Some of the challenges in LMIC include the lack of a structured primary care system and trained primary care physicians for continuous care and a good medical record system, the lack of public awareness of the risks, the lack of support/sustained policy for smoking cessation, and the revenue from the tobacco industry that undermines the efforts [Citation68].
4.3. Recommendations for prevention
With regards to prevention of COPD and given its multifactorial pathogenesis, multiple policy, practical and clinical actions are required as specified below.
4.3.1. Policy and legislations
It is imperative to establish legislations targeting the control of risk factors. The WHO proposes several ‘Best Buys’, which are cost-effective interventions including increasing taxation on tobacco products, controlling tobacco advertisements and banning smoking in public places to reduce secondhand exposure. Sales of tobacco to minors should be prohibited [Citation69]. SDG 3 sets out the specific tobacco-related target of a 30% relative reduction in prevalence of current tobacco use by 2025. Anti-smoking campaigns such as the WHO’s Commit to Quit campaign that educate the public on the dangers of smoking and promote a healthy lifestyle should be implemented [Citation70]. Measures need to be urgently established in countries that lack tobacco policies, while reinforcing and reevaluating their corresponding strategies in other countries. Improved cookstoves programs should be reevaluated, taking into account the local context. Local communities should be informed about the correct use and health benefits of improved cookstoves and be offered maintenance services to preserve its use [Citation71].
Moving forward, we should support implementation of the SDGs outside the health field, such as Goal 1 (reduction in poverty), Goal 4 (inclusive and equitable quality education and lifelong opportunities for all), Goal 7 (access to affordable, reliable sustainable and modern energy for all) and Goal 11 (make cities and human settlements inclusive, safe, resilient, and sustainable). In fact, if SDG 7 could be achieved, a substantial amount of COPD could be prevented by universal access to electricity or renewables and a doubling in the rate of improvement in energy efficiency. However, where this is not yet feasible, primary care plays an important role to educate people diagnosed with COPD on how to reduce risk exposure, such as cooking outdoors, letting children play outside, improve ventilation, and use cleaner cookstoves, as long as these are maintained and chimneys cleaned regularly. Finally, household air pollution is associated with lower SES, which again makes primary care the best strategy for these communities [Citation66]. We should support efforts to eradicate socioeconomic disparities, such as access to education and clean energy.
4.3.2. Practical and clinical
The WHO defines COPD as an ambulatory care-sensitive condition which can be managed in the community [Citation72]. Primary care leadership in respiratory health needs strengthening in order to raise risk factor awareness in communities and health-care workers. In countries where there is a family medicine program, family physicians are well placed to champion prevention, diagnosis, and treatment of COPD. In countries where there is no family medicine program, leadership may come from district health teams, supported by community health workers who can be trained by respiratory physicians to educate communities about COPD and potentially detect COPD. Lung health education programs for primary care settings such as the Massive Open Online Course (MOOC) developed by charitable organizations such as the International Primary Care Respiratory Group (IPCRG) and RESPIRE at the University of Edinburgh [Citation73] can be offered.
Furthermore, governments should invest more resources and facilities for primary medical care. Access to better obstetric, neonatal and pediatric care can contribute to reduction of early life risks. Better provision of maternal health and nutrition and reducing exposure to pollutants and smoke during pregnancy should be prioritized [Citation59]. Approaches that optimize lung development both in utero and in early childhood have a large potential cumulative effect, such as reducing prematurity and HIV vertical transmission. We propose to strengthen the vaccination programs in primary care and make them widely available, including in remote and rural areas [Citation20,Citation26]. Traditionally, all these services have been offered through primary care and family doctors who may advise families about the risk, and manage each of the treatable risk factors, and guide communities on how to reduce their exposure. A greater concerted effort is needed from all stakeholders to support primary care in preventing COPD risk.
4.3.3. Research
Most COPD research takes place in HICs. In the last decade, only 0.2% of major grants for NCDs funded respiratory research in low-income countries [Citation74]. LMICs populations have different lifestyle and genetic features which expose them to varied risk factors. We cannot expect HICs findings to apply universally; more research in LMICs is needed. Moreover, studies should have an international and multi-institutional character [Citation75]. There is a gap in the field of poor lung development that results in lower maximum lung function. It is crucial to identify the populations at risk, such as indigenous populations [Citation76], and emphasize the importance of prevention. Lastly, the effectiveness of current interventions should be assessed.
Summarizing points
The two most prevalent risk factors of COPD in LMICs are smoking and household air pollution, especially from biomass fuels.
The majority of smokers worldwide live in LMICs; however, many LMICs lack tobacco control policies.
Many risk factors are associated with lower SES.
Key recommendations:
Implement enhanced programs for cleaner fuels and improved appliances.
Raise awareness regarding COPD prevention.
Strategies need to take into consideration local stakeholders’ views and culture.
5. Diagnosis
5.1. Techniques, availability, and unmet needs
Spirometry is fundamental for the diagnosis of COPD. Unfortunately, in many LMICs, spirometry is not available at primary care level. As seen in , in the Africa and South-East Asia spirometry is not available at the primary health-care level in the majority of countries. For example, in Pakistan, spirometry is usually not performed in primary care and is mainly available in urban hospitals, access to which can be difficult [Citation77]. In Uganda, a high-burden country with 16% COPD prevalence [Citation78], spirometry was available to the majority of urban providers but not available in the rural districts, diagnosis is consequently made based on physical findings [Citation79].
Figure 4. World map showing countries with (Yes) or without (No) availability of peak flow measurement spirometry at the primary health care level according to income (2019) [Citation80]
![Figure 4. World map showing countries with (Yes) or without (No) availability of peak flow measurement spirometry at the primary health care level according to income (2019) [Citation80]](/cms/asset/8731f53c-30f9-407c-a210-0370b580042e/ierx_a_1985762_f0004_oc.jpg)
Health-care workers need to be trained to operate a spirometer; nevertheless, in Africa, most official guidelines do not elaborate on the training of (primary care) physicians on spirometry [Citation33,Citation81]. However, the Pan African Thoracic Society has developed a spirometry training program, which teaches and evaluates spirometry usage [Citation33]. IPCRG has developed an e-learning module on spirometry addressing primary care professionals [Citation82] and is currently developing a globally relevant curriculum and proposals for licensing.
Screening based on risk factors is a potentially helpful diagnostic approach; however, most case-finding instruments (i.e. questionnaires) have not been validated in LMICs. Similarly, prognostic tools such as indices that are widely used in practice have been validated only in HICs [Citation26].
WHO has a clear vision on diagnosing COPD. According to the 2013–2020 global action plan for the prevention and control of NCDs, achieving 80% availability of affordable basic technologies for diagnosis is key [Citation83]. The strategy needs to be specific, elaborating on the type of disease and the appropriate diagnostic technologies. Also, the capacity of every health-care system differs and will require a tailored plan.
5.2. Ongoing challenges in diagnosis
COPD’s main diagnostic challenge lies in the scarce availability and accessibility of diagnostic devices and skills. This problem pertains to both human and material resources and disparities are found between rural and urban settings.
Early detection is a serious challenge. More than 50% of people with airway obstruction are not diagnosed with COPD although most of them have mild COPD, meaning that they could benefit the most from early interventions since they may be more manageable and there are more quality-adjusted-life years to be saved [Citation84]. Identifying these individuals will enable strategies such as smoking cessation and immunization to be taken to reduce risk [Citation26]. Both health-care workers and patients lack education on COPD [Citation82], which influences patients’ health seeking behavior [Citation85]. Patients often underestimate their symptoms and only seek help when the disease has progressed or limitations have become intolerable, or if they experience an exacerbation. The delayed or absent healthcare-seeking behavior can also be attributed to the stigma associated with respiratory diseases [Citation67]. Some health-care workers find it challenging to differentiate between COPD, asthma and tuberculosis, leading to misdiagnosis [Citation79]. This misconception is even more pervasive among the general public, where the distinction is confusing and hard to explain.
Regarding spirometry, besides the lack of appropriate training, even if the clinicians are trained and familiar with the use of spirometers, there are other obstacles such as outdated or defective devices as well as lack of equipment maintenance and technical support that discourage the use of spirometry in favor of other rather empirical or unreliable diagnostic means [Citation33]. Another challenge is the lack of electricity in some health facilities, encountered mostly in rural settings [Citation33].
5.3. Recommendations for diagnosis
Several policies, clinical and research actions for diagnosis can be identified.
5.3.1. Policy and legislations
At a national level, local task forces with national coordinators should be established and supported by both national governments and international organizations by means of funding and advice. Collaboration between government bodies, health ministries, and local societies will leverage their knowledge of the healthcare system and allow for better use of financial resources. Spirometers should be included in the WHO list of priority medical devices as well as in the Packages of Essential NCDs (PEN) Intervention Toolkit. Declaring spirometers as essential devices will not only raise awareness of respiratory disease but also affect policy making. As a result, investments and government spending should be directed accordingly [Citation86].
Αctive targeted approaches for case-finding have been proposed as a cost-effective way to identify undiagnosed patients and reduce the burden associated with COPD [Citation87,Citation88]. Under the same concept, screening programs that will target the most common risk factors in LMICs can be implemented in order to find those who are tobacco dependent, or those exposed to household air pollution and offer spirometry testing for early diagnosis. Existing screening programs for lung cancer or tuberculosis could potentially be utilized to identify individuals at risk.
5.3.2. Practical and clinical
To standardize making a diagnosis of COPD, diagnostic algorithms and guidelines need to be developed and followed to facilitate easier and faster diagnosis [Citation89]. In primary care, pooling resources to provide spirometry services in a clinic that serves multiple clinics could be an option such as ‘hub and spoke’ models.
Health-care professionals should be better educated on COPD at undergraduate, postgraduate, and continuing professional development levels. The education can be delivered by peers, for example by primary care for primary care, using Teach the Teacher programs for cascade training to strengthen teaching capacity [Citation90]. Consideration should be given to incentivize attendance and learning. Brochures and seminars can be used for teaching the clinical features of COPD and help them differentiate from other respiratory problems. In this manner, awareness will also be raised. Standardized training on spirometry usage and interpretation should be introduced to primary health-care professionals. Regular education programs should be evaluated and widely endorsed. Taking into account characteristics of the local healthcare system, e-learning modules and workshops can be adapted in different countries. Training can be offered in different languages and means (e.g. paper instructions, guiding, and videos). National respiratory and thoracic societies should be included in the development and dissemination process.
Spirometry remains the gold standard for COPD diagnosis; however, as it can be costly and not widely available, interim measures might be beneficial. Validated case-finding instruments tailored to LMICs, which take into account unique topical risk factors and population characteristics, can be used as the first diagnostic step in resource-constrained settings. Notably, a survey-based COPD-assessment score for LMICs has been developed [Citation91]. Combined with PEF measurement, the assessment score appears to be a promising cost-effective instrument to detect COPD or at least prioritize a smaller group of individuals at high risk who are most important to target for confirmatory spirometry.
Portable hand-held spirometers are a revolutionary advance in early detection and screening of chronic pulmonary disorders [Citation92]. These devices can be valuable for resource-constrained health-care systems serving large populations, including rural areas. Although they are not interchangeable with laboratory spirometers, they are small, inexpensive, and simple to use [Citation93,Citation94]. Performing hand-held spirometry can detect potential obstruction, which can then be referred to the hospital for further examination. Hence, referral to secondary or tertiary centers with limited capacity can be streamlined [Citation92]. Technical support should be made easily available and consistent across all health-care levels. Local manufacturing of consumables may be a solution since it will increase availability and decrease shipping cost.
5.3.3. Research
Currently, most standard spirometric reference values are modeled based on HICs data. As the risk factors and subsequent pathogenesis and morphology of COPD is distinct in LMICs, HICs’ values may not apply to all settings. Moreover, race and lifestyle influence lung function, the normal ranges may differ among different ethnicities and countries [Citation26]. For example, it is known that a low FVC can reflect poor lung growth and, as previously mentioned, early-life disadvantages affect lung development [Citation58]. A decreased post-bronchodilator FEV1/FVC ratio (<0.7 or below the lower limit of normal) confirms COPD diagnosis, so in a patient with decreased FVC the ratio may appear normal [Citation20]. We emphasize on the importance of creating and validating diagnostic instruments and having a local reference value for spirometry measurements in order to avoid under- or over-diagnosis of COPD. This requires a better understanding of how COPD differs in these countries. Exploring the etiology and clinical picture will allow for custom and directed diagnostic techniques. Studies determining spirometry reference standards, taking into account the genetic and physiological characteristics for different populations are needed. Initiatives such as the GOLD and the BOLD studies, which aim to advance diagnosis of COPD patients globally and to develop reports and strategies for improving diagnosis, should be further supported and continued.
Summarizing points
There are urban-rural disparities in COPD diagnostic opportunities.
Underdiagnosis and misdiagnosis of COPD is common.
Many COPD risk factors are associated with lower SES.
Key recommendations:
Improve spirometry availability and accessibility in primary care.
Implement operational (primary care) guidelines and training programs for health-care workers and community health workers.
Where spirometry is not available, consider alternatives such as validated questionnaires and tool, particularly those researched in LMICs.
Develop spirometry standard values for different populations.
6. Treatment
6.1. Techniques, availability, and unmet needs
First and foremost treatment is always exposure reduction. Smoking cessation is a fundamental part of COPD management and appears to be the only intervention that has been shown to reduce mortality regardless of COPD severity level [Citation26]. Globally, only 15% of the population has access for treating tobacco dependence [Citation41]. Despite Nicotine Replacement Therapy (NRT) being included in the WHO model list of essential medicines, only 17 countries have NRT on their national formulary [Citation95]. Tertiary prevention is a necessary part of treatment in order to preserve lung function and prevent exacerbations.
In addition, standard treatment of COPD consists of both non-pharmacological and pharmacological options [Citation67,Citation96]. Pulmonary rehabilitation is one of the key non-pharmacologic treatments, consisting of graded exercise and patient education. It is a very effective method that has shown symptom alleviation, quality of life improvement and cost-effectiveness [Citation97]. The main barriers to wide use of pulmonary rehabilitation are the lack of awareness, shortage of trained health-care workers on pulmonary rehabilitation techniques and the lack of funds [Citation98]. Palliative, end-of-life care for patients with very severe COPD comprises person-centered care focused on the alleviation of symptoms to improve quality of life often using low cost simple interventions such as mindfulness, breathing positions, and techniques can be introduced from the point of diagnosis. Palliative care is typically not accessible nor available in LMICs, and therefore people are hospitalized and their preferences for where they die are not taken into account [Citation99,Citation100]. Examples of adaptation to the local system and culture would need to include how the subject of both living and dying with COPD can be raised and addressed.
Regarding pharmacologic treatment, GOLD and national guidelines advise the use of pharmacotherapy with inhaled agents, such as bronchodilators and corticosteroids [Citation96]. Most LMICs do not have national guidelines for COPD, however, even in those who have, the quality of the guidelines is poor and implementation gaps remain. COPD guidelines can be found in only 30 LMICs, which corresponds to almost 22% of LMICs [Citation76]. In some of the remaining countries, the GOLD strategy guidelines are used, which in some instances have been translated to the native language without taking context and resources into account.
Treatment consists of maintenance therapy and exacerbation management. In LMICs, the focus lies mostly on the latter; treating acute episodes is prioritized and the majority of patients are not on appropriate maintenance therapy. Care services and resources are directed at acute interventions while the health-care systems fail to meet the treating requirements of chronic disorders [Citation33].
A list of medications for both symptomatic and exacerbation management is included in the latest WHO model list of essential medicines, as shown in . However, inconsistencies are found in the corresponding national lists. For example, long-acting bronchodilators and inhaled corticosteroids are absent from Nepal’s national list of essential medicines [Citation81].
Table 1. WHO model list of essential medicines for COPD [Citation101]
The strategy of WHO for NCDs by 2025 targets availability and affordability of essential medicines. In LMICs, the target is not met and shortages are seen in both the public and private sectors [Citation102].
In the public sector, low-income countries and lower middle-income countries buying the lowest price generic medicines for COPD cost an average of 0.7 and 1.4 days wage, respectively (). Large variations are seen depending on the medication, brand, and country [Citation103]. Budgets for medication of individual countries may deter availability.
Table 2. Percentage of countries meeting the WHO medicine requirements for COPD (adapted from Ewen et al. (2017)) [Citation102]
6.2. Ongoing challenges
Most governments do not offer adequate social insurance coverage and the economy is not strong enough to offer reimbursement for treatment. This means that COPD treatment cost burdens individuals and discourages them from seeking care. Indeed, some countries have public and private systems where most care is fully funded by the government, and universal access is achieved. However, approximately 48% of health-care funds in lower-income countries and 30% in lower-middle-income countries come from out-of-pocket payments [Citation34]. It is worth mentioning that some countries have initiated plans for improving health insurance. In the Philippines and Vietnam, social health insurance programs with voluntary enrollment are in place [Citation34]. A similar strategy in Rwanda has achieved satisfactory results and high coverage [Citation34].
Improving knowledge among local health-care providers remains a key challenge. Notably, the knowledge deficit in primary care providers stems from insufficient, or non-existing, training and education on COPD management. Paired with the absence of protocols for standardized therapy, many issues arise. Even when guidelines do exist, they are underused in several LMICs. In Latin America, non-adherence to guidelines and under-prescription of long-acting bronchodilators, despite adequate availability, has been reported [Citation20,Citation104]. In rural Nepal, intravenous corticosteroids and antibiotics for exacerbations were prescribed inappropriately [Citation81]. While in other countries, dissemination of the guidelines has been suboptimal and many health-care workers are not aware of their existence [Citation76,Citation79]. Most patients are treated by a single health-care worker, who usually is not specialized in respiratory diseases [Citation105]. This demonstrates that primary care physicians with special training can manage patients with COPD. We do not always need a specialist; rather, we need specialized training.
Certain risk factors for the development of COPD influence the course of the disease and potentially impede treatment. Malnutrition – a problem encountered in low-income settings – is a determinant of overall worse health and comorbidities, and it poses a challenge to treatment. Poverty and illiteracy affect patients’ health and their treatment expectations. Societal beliefs and culture, paired with lack of awareness, lead many to seek alternative medicine and faith healing [Citation20].
As previously mentioned, COPD is frequently misdiagnosed as other respiratory diseases [Citation79]. This can be a serious threat because, for example, misdiagnosing COPD as asthma may result in the condition being mistreated with inappropriate medication and poor follow-up.
6.3. Recommendations for treatment
Regarding optimization of COPD treatment in LMICs, a wide range of policy, clinical, and research actions are recommended.
6.3.1. Policy and legislations
Strengthening health-care systems to ensure affordability and accessibility to medication should be prioritized. National public health insurance programs need to be implemented. All efforts toward universal health coverage should be encouraged and countries need to undertake rapid actions [Citation106]. Developing simplified and accessible protocols for COPD treatment will facilitate the implementation. An alternative solution is to adapt and integrate existing international guidelines. As we aim at community acceptance, patient associations, expert patients, and community organizations should be included in the development of guidelines. Essential medicines, as declared by the WHO, should be included in the national lists of essential medicines to ensure availability [Citation107].
Action plans are key for multi-sectoral engagement. In Pakistan, a successful system of collaboration between national and provincial bodies for TB control is in place. This program has adapted to deliver care for other respiratory diseases such as COPD [Citation77]. The original strategy was designed to support primary care facilities with case management. Improved availability of guidelines and management tools, as well as training for health-care workers, were among the steps taken.
Evidence-based smoking cessation strategies should be incorporated including services for advising patients, offering counseling and medication for tobacco dependence. Smoking cessation interventions provided by general practitioners to COPD patients are effective [Citation77]. Additionally, it could be beneficial to consider gender-specific interventions for quitting smoking [Citation41]. Lastly, medicines for tobacco use disorder, such as varenicline and cytisine, should be included in the WHO model list of essential medicines.
6.3.2. Practical and clinical
There is strong evidence of cost-effective interventions in primary care yet many opportunities to put these into practice are missed [Citation41].
Education is the cornerstone for disease management. Training health-care workers on the use of guidelines will promote adherence. The problem of over-prescription of corticosteroids and antibiotics should be addressed. Doctors should be trained on how to treat mild and moderate COPD and on the appropriate use of medication for acute exacerbations. Apart from prescribing medication and advising the public, emphasis needs to be put on educating and empowering the patients as well. Self-management plans can be recommended along with patient training [Citation108]. After ensuring availability of medicine, it is important to teach patients the correct use of inhalers and spacers [Citation26].
Non-pharmacological interventions, for instance pulmonary rehabilitation programs and a palliative approach that supports people with life-limiting conditions to cope with symptoms should also be more widely available [Citation99]. A palliative approach should be an important component of an integrated primary care service [Citation109]. We must recognize that a palliative approach is beyond a service to which patients are referred at the end of life. It is also about optimizing symptom relief – which arguably should begin at the time of diagnosis. Since there is limited palliative care provision in LMICs, to begin at the time of diagnosis may not be possible. However, it is crucial to offer palliative care at least for patients with severe COPD for symptom relief.
In many LMICs, informal healthcare, e.g. community health workers/volunteers, are commonly seen. For some communities, the informal health sector is the only option. These providers can be a huge asset; they reach in rural areas and are engaged with the local communities. Their understanding of the local lifestyle and tradition enables them to engage readily with patients and communities. They can potentially become a bridge between the community and the healthcare system [Citation110].
Developing an improved referral system begins with strengthening primary healthcare and implementing task shifting. Village and community health workers are closer to the public and have a relationship of trust and sustainability. Educating them through workshops and supporting them (by means of equipment and advise) will bring the healthcare system to the people [Citation108]. Specifically for medication availability, the public systems of supply should be reinforced while also utilizing the private retail systems. Also, introducing new supply mechanisms should be considered. In Kenya, a successful initiative had been taken to train pharmacists in the provision of antimalarial drugs with the adequate dose and instructions [Citation34]. Such initiatives can open up new possibilities to integrate community pharmacists into the COPD care pathway, for example in offering teaching on inhaler technique and support for self-management.
The Practical Approach to Lung health (PAL) strategy is a concept suggested by the WHO to create guidelines and improve healthcare delivery focusing on primary care settings. Implementing the PAL strategy is a cost-effective approach to standardize treatment and facilitate linkage among the different health-care levels [Citation110]. In El Salvador and Malawi, among other countries, PAL implementation has shown advances in patient’s management and quality of life [Citation111].
Due to the chronic nature of the disorder, continuity of healthcare delivery is essential. Follow-up should transform from passive to active. Instead of the patient contacting the doctor when needed, regular scheduled reviews can be provided and a letter/text message can be actively sent to defaulters [Citation77]. General practitioners and family doctors have the most frequent contact with patients and can offer continuous care through regular follow-up.
Tailoring the treatment by taking personal circumstances into account, advising individuals on what would work best for them and utilizing shared decision-making could improve adherence to the treatment course. All these can be best carried out in primary care as the provider has established a trusting relationship with the patient.
In addition, vaccination programs for COPD patients in both urban and rural areas need to be established as part of tertiary prevention; in particular, influenza vaccination has been assessed as a potentially cost-effective measure [Citation20,Citation26].
6.3.3. Research
The particular etiology and pathogenesis of COPD in LMICs generates different courses of disease and consequently different response to treatment. Research on endotyping will set the ground for, firstly a correct diagnosis and secondly, improved and precise treatment since precision medicine may target the unique clinical and biological characteristics of COPD in LMICs [Citation112]. Therefore, studies are needed on the performance of the different existing medications in LMICs’ populations. Similarly, most palliative care plans have been developed in high-income settings; research on the effective implementation of these strategies in LMICs is required [Citation99]. Furthermore, the cost-effectiveness of management plans needs to be assessed. Also, behavioral and cultural patterns in relation to medical adherence should be studied.
LMICs, with their unique mosaic of risk factors and host characteristics, are a key setting for studying the pathophysiology and treatment of the various endotypes and phenotypes of COPD. Treatment of COPD caused by exposure to HAP and COPD caused by childhood disadvantages is not fully evidence-based yet.
Summarizing points
In LMICs, the focus is on exacerbation management, few people are on maintenance therapy.
There is a glaring absence and inadequate implementation of COPD guidelines.
Misuse or underuse of medication is common.
Key recommendations:
Improve medicine availability and affordability.
Improve referral systems and task-shifting.
Engage community health-care workers, pharmacists, and informal health-care providers.
Ensure continuity of healthcare delivery.
Invest in non-pharmacological treatments and smoking cessation support.
7. Conclusion
In LMICs, steps should be taken to accurately diagnose and determine the true burden of COPD. COPD prevention measures, as well as cost-effective treatment strategies, must be implemented. Given the scale of the burden and the need for providing locally based, culturally adaptive and holistic approaches, creating a strong primary care system is the most valuable and feasible approach to achieving these goals, and is of utmost importance.
8. Expert commentary
Although it has been long acknowledged by some pioneers, it is only in the last 5 years that we have seen an increased awareness of the importance of COPD in LMICs in the wider respiratory community. Observations that underpinned these theories mostly stemmed from epidemiologic research on COPD in LMICs such as the BOLD and FRESH AIR studies. These studies highlighted that COPD is highly prevalent in nonsmoking people including many people below the age of 40 years old in LMICs. This led us to expand our focus on COPD risk factors beyond tobacco use. Factors such as maternal and childhood malnutrition and environmental risk factors like ambient and indoor air pollution are now gaining heightened attention. Tackling these risk factors remain key challenges in LMICs. However, while studies in LMICs have greatly helped us in understanding the development and course of COPD, they also revealed the high burden of COPD in LMICs and ongoing challenges related to prevention, diagnosis, and treatment. Indeed, the Global Burden of Disease studies ranked COPD as the 3rd leading cause of death, with 80% of these deaths occurring in LMICs. Since 2021, this has also been recognized in the international GOLD strategy document. Further in-depth studies in LMIC settings highlighted that challenges are at the complete system level ranging from lack of awareness, education, and training on risk factors and disease management to limited availability of staff, diagnostic, and treatment resources. Notably, there seems to be a lack of investment in chronic respiratory services and research compared to disease burden especially when compared to communicable diseases (HIV, malaria, and TB).
The current situation requires coordinated action, and engagement with wider stakeholders including governments and policymakers. We believe that on a global scale, organizations such as the World Health Organization should acknowledge the invisibility of COPD and call for it to receive appropriate focus, yet true change can only be realized when national and local governments also put COPD higher on their policy agendas. The main priorities are prevention, better diagnosis, and greater access to affordable interventions especially in primary care as part of the implementation of universal health coverage. Global policy and goals may further help strengthen policy shifts and strategies such as the UN Sustainable Development Goals (SDGs) may be a useful resource to persuade local governments. Local LMIC communities and policymakers can all help to support implementation of the SDGs – several of which are relevant to COPD prevention. In particular, primary care can play a more prominent role in the prevention, diagnosis, and management of COPD, and more support, training, and empowerment is needed from the government and policymakers to enable them to do so. However, while with sufficient training (on e.g. spirometry) primary care can help in prevention and better diagnosis, we would like to stress that primary care would need the means to provide treatment as well. Regarding non-pharmacologic treatment options, low-cost pulmonary rehabilitation is among the most (cost) effective options, though training and capacity building remains necessary. On the pharmacological level, the availability and implementation of national essential medicines lists, including sufficient pharmacotherapy for tobacco dependence and COPD, is crucial.
In terms of future research, more studies and local evidence are needed to guide the aforementioned policy shifts and inform local guidelines. This is true for middle-income countries and even more so for low-income countries, where current evidence is even more scarce. Notably, cost-effectiveness and budget impact regarding interventions for the prevention, diagnosis, and treatment of COPD are available for HIC settings, but generally not well studied in LMICs. While the clinical need for better COPD treatment seems evident, most LMICs face an immense scarcity of resources, requiring careful choices and allocation of these limited resources. Therefore, in order to persuade policymakers to place more emphasis on COPD, solid economic business cases are required. There is an argument for the implementation of more preventive measures because the clinical and economic benefits may stretch beyond COPD alone. For example, prioritizing treating tobacco dependence is not only impacting the COPD burden but also prevents many other conditions such as cardiovascular disease, diabetes, depression, and cancers.
Looking ahead, the momentum for a change in COPD management in LMICs seems to be there, especially now that awareness has been created over the past 5 years. Beyond ongoing economic challenges, the major challenges to overcome are related to implementation of cost-effective best practices by strengthening local capacities. A relatively feasible and low-cost option is investing more in family medicine as system leaders, by increasing their education and leadership skills. Here, some tailoring will certainly be needed and we highlight the need for developing local COPD guidelines as well as asset-based pathways, depending on availability of local health workers including community pharmacists, nurses, physiotherapists, community health workers, volunteers, faith organizations, and community groups. Within these local pathways, strong local champions need to be identified and/or trained. Community engagement and awareness on disease prevention, diagnosis, and treatment is key.
Article highlights
The World Health Organization (WHO) ranks COPD as the third leading cause of death globally. In 2019, 80% of COPD-related deaths occurred in LMICs.
Smoking and household air pollution from burning biomass fuels are major risk factors that are very common in lower-income settings.
Limited availability of spirometers, as well as a lack of training in their use and interpretation, lead to many cases remain undiagnosed or misdiagnosed and therefore opportunities to support self-management and slow the rate of lung function decline are missed.
Investing in primary care by recruiting, reimbursing, and educating health-care workers about COPD, raising awareness among patients and communities, improving availability and affordability of diagnostic and non-pharmacological and pharmacological treatments are key interventions that can reduce the burden of COPD in LMICs.
All approaches and recommendations should take into account the local contexts such as culture and genetic characteristics of the populations, and the capabilities and resources of local health-care systems.
Collaboration across local and international associations, policymakers, health-care workers, patients, and the scientific community is essential.
Additional information
Funding
References
- GOLD. POCKET GUIDE TO COPD DIAGNOSIS, MANAGEMENT, AND PREVENTION A guide for health care professionals [Internet]. 2020 [cited 2021 May 7]. Available from: www.goldcopd.org Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- WHO. The top 10 causes of death. [cited 2021 Sep 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death . 2020.
- Chronic obstructive pulmonary disease (COPD) [Internet]. [cited 2021 Sep 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- Iheanacho I, Zhang S, King D, et al. Economic burden of chronic obstructive pulmonary disease (COPD): a systematic literature review. Int J COPD. 2020;15:439–460.
- Brakema EA, Tabyshova A, Rmjj VDK, et al. The socioeconomic burden of chronic lung disease in low-resource settings across the globe - An observational FRESH AIR study. Respir Res. 2019;20(1):291.
- World Bank Country and Lending Groups World Bank Data Help Desk [Internet]. [cited 2021 May 3]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- Ho T, Cusack RP, Chaudhary N, et al. Under-and over-diagnosis of COPD: a global perspective. Vol. 15, Breathe (Sheff) 2019;15(1): 24–35.
- Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
- Hanafi NS, Agarwal D, Chippagiri S, et al. Chronic respiratory disease surveys in adults in low- and middle-income countries: a systematic scoping review of methodological approaches and outcomes. J Glob Health. 2021;11:1–11.
- Yang JJ, Yu D, Wen W, et al. Tobacco smoking and mortality in Asia a pooled meta-analysis. JAMA Network Open. 2019;2(3):e191474.
- COPD Working Group R, Regional COPD Working Group. COPD prevalence in 12 Asia-Pacific countries and regions: projections based on the COPD prevalence estimation model. Respirology. 2003;8(2):192–198.
- Burney P, Jithoo A, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty-A bold analysis. Thorax. 2014;69(5):465–473.
- Alam DS, Chowdhury MA, Siddiquee AT, et al. Prevalence and determinants of chronic obstructive pulmonary disease (COPD) in Bangladesh. COPD. 2015;12(6):658–667.
- Adeloye D, Basquill C, Papana A, et al. An estimate of the prevalence of COPD in Africa: a systematic analysis. COPD. 2015; 12(1):71–81
- Finney LJ, Feary JR, Leonardi-Bee J, et al. Chronic obstructive pulmonary disease in sub-Saharan Africa: a systematic review. Int J Tuberc Lung Dis. 2013;17(5):583–589
- Menezes AM, Wehrmeister FC, Perez-Padilla R, et al. The PLATINO study: description of the distribution, stability, and mortality according to the global initiative for chronic obstructive lung disease classification from 2007 to 2017. Int J COPD. 2017;12:1491–1501.
- Perez-Padilla R, Menezes AMB Chronic obstructive pulmonary disease in Latin America. Vol. 85, Ann Glob Health. Ubiquity Press; 2019. 85 1 https://doi.org/10.5334/aogh.2418
- Menezes AMB, Victora CG, The P-PR Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol. 2004;4:15.
- GBD Results Tool | gHDx [Internet]. [cited 2021 May 3]. Available from: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/10c5ba0e4930d0696dd60d81f14e93a0
- Halpin DMG, Celli BR, Criner GJ, et al. The GOLD Summit on chronic obstructive pulmonary disease in low- And middle-income countries. Int J Tuberc Lung Dis 2019;23(11):1131–1141.
- GBD results tool | gHDx [Internet]. [cited 2021 May 3]. Available from: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/0764447007e4f1bc597831546b8c6685
- Agarwal D, Hanafi NS, Chippagiri S, et al. Systematic scoping review protocol of methodologies of chronic respiratory disease surveys in low/middle-income countries. Npj Prim Care Respir Med 2019; 29(1):17.
- Quaderi SA, Hurst JR The unmet global burden of COPD. Glob Health Epidemiol Genom 2018;3:e4
- Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395(10226):785–794.
- The Global Economic Burden of Non-communicable Diseases [Internet]. [cited 2021 May 3]. 2011. Available from: www.weforum.org/EconomicsOfNCD
- Hurst JR, and Siddharthan T Global burden of COPD Haring, Robin, Kickbusch, Ilona, Ganten, Detlev, and Moeti, Matshidiso. In: Handbook of Global Health. Springer International Publishing; 2020. p. 1–20.
- World Health Organization. Monitoring the building blocks of health systems : a handbook of indicators and their measurement strategies. World Health Org; 2010. .
- Das J The quality of medical care in low-income countries: from providers to markets. PLoS Med. 2011;8(4):4.
- Hamid H, Abid Z, Amir A, et al. Current burden on healthcare systems in low- and middle-income countries: recommendations for emergency care of COVID-19. Drugs Ther Perspect 2020; 1–3. https://doi.org/10.1007/s40267-020-00766-2
- Kruk ME, Gage AD, Arsenault C, et al. High-quality health systems in the sustainable development goals era: time for a revolution. Lancet Glob Health 2018; 6(11):e1196–e1252
- Universal Health Coverage [Internet]. [cited 2021 Jul 7]. Available from: https://www.who.int/health-topics/universal-health-coverage#tab=tab_1
- Kassai R, van Weel C, Flegg K, et al. Priorities for primary health care policy implementation: recommendations from the combined experience of six countries in the Asia-Pacific. Aust J Prim Health. 2020;26(5):351–357.
- Masekela R, Zurba L, Gray D Dealing with access to spirometry in Africa: a commentary on challenges and solutions. Int J Environ Res Public Health. 2019;16(1):62.
- Health Care MA Systems in low- and middle-income countries. N Engl J Med 2014;370(6):552–557.
- Medical doctors (per 10 000 population) [Internet]. WHO. [cited 2021 May 10]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/medical-doctors-(per-10-000-population)
- Laniado-Laborin R Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21st century. Int J Environ Res Public Health 2009; 6(1):209–224.
- Tobacco [Internet]. [cited 2021 May 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/tobacco
- Forouzanfar MH, Afshin A, Alexander LT, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016 388(10053):1659–1724.
- Parties to the WHO Framework Convention on Tobacco Control [Internet]. [cited 2021 May 2]. Available from: https://www.who.int/fctc/cop/en/
- ASH Fact Sheet. Tobacco and the Developing World. July 2019
- OCP VS, Williams S, Barchilon V, et al. Treating tobacco dependence: guidance for primary care on life-saving interventions. Position statement of the IPCRG. NPJ Prim Care Respir Med 2017; 27(10: 38.
- Existence of operational policy/strategy/action plan to decrease tobacco use [Internet]. [cited 2021 May 2]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/existence-of-operational-policy-strategy-action-plan-to-decrease-tobacco-use
- THE 17 GOALS | sustainable Development [Internet]. [cited 2021 Jun 22]. Available from: https://sdgs.un.org/goals
- Jindal S, Jindal A COPD in Biomass exposed nonsmokers: a different phenotype. Expert Rev Respir Med 2021;15(1):51–58
- Siddharthan T, Grigsby MR, Goodman D, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low- and middle-income country settings. Am J Respir Crit Care Med. 2018;197(5):611–620.
- Proportion of population with primary reliance on polluting fuels and technologies for cooking (%) [Internet]. [cited 2021 May 1]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-phe-population-with-primary-reliance-on-polluting-fuels-and-technologies-for-cooking-proportion
- Indoor Air WHO Quality guidelines: household fuel combustion. Geneva, Switzerland: World Health Organization. 2014;1–172.
- Gulia S, Khanna I, Shukla K, et al. Ambient air pollutant monitoring and analysis protocol for low and middle income countries: an element of comprehensive urban air quality management framework. Atmos Environ. 2020;222.
- Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396(10258):1223–1249
- Thoday K, Benjamin P, Gan M, et al. The Mega Conversion Program from kerosene to LPG in Indonesia: lessons learned and recommendations for future clean cooking energy expansion. Energy Sustainable Dev. 2018;46:71–81.
- Goldemberg J, Martinez-Gomez J, Sagar A, et al. Household air pollution, health, and climate change: cleaning the air. Vol. 13, environmental research letters. Institute of Physics Publishing; 2018.
- van Gemert F, de Jong C, Kirenga B, et al. Effects and acceptability of implementing improved cookstoves and heaters to reduce household air pollution: a FRESH AIR study. Npj Prim Care Respir Med. 2019;29(1):32.
- Amaral AFS, Patel J, Kato BS, et al. Airflow obstruction and use of solid fuels for cooking or heating BOLD (Burden of Obstructive Lung Disease) results. Am J Respir Crit Care Med. 2018;197(5):595–610.
- Schikowski T, Mills IC, Anderson HR, et al. Ambient air pollution: a cause of COPD? Eur Respir J 2014; 43(1): 250–263.
- Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5(9):691–706.
- Pleasants RA, Riley IL, Mannino DM Defining and targeting health disparities in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11(1):2475–2496.
- Grigsby M, Siddharthan T, Chowdhury MAH, et al. Socioeconomic status and COPD among low-and middle-income countries. Int J Chron Obstruct Pulmon Dis. 2016;11(1):2497–2507.
- Brakema EA, van Gemert FA, Rmjj VDK, et al. COPD’s early origins in low-and-middle income countries: what are the implications of a false start? Npj Prim Care Respir Med. 2019; 29(1):6
- Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018;6(7):535–544.
- Grigg J Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009; 6(7):564–569
- Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122.
- Grigsby MR, Siddharthan T, Pollard SL, et al. Low body mass index is associated with higher odds of COPD and lower lung function in low- and middle-income countries. COPD 2019; 16(1):58–65
- Siddharthan T, Grigsby M, Morgan B, et al. Prevalence of chronic respiratory disease in urban and rural Uganda. Bull World Health Organ 2019;97(5):318–327.
- GHO | by category | BCG - Immunization coverage estimates by World Bank Income Group. Geneva, Switzerland: WHO. World Health Organization;
- ÁA C, Stelmach R, Ponte EV Asthma prevalence and severity in low-resource communities. Curr Opin Allergy Clin Immunol. 2017;17(3): 188–193.
- Sahni S, Talwar A, Khanijo S, et al. Socioeconomic status and its relationship to chronic respiratory disease. Adv Respir Med 2017;85(2): 97–108.
- Meghji J, Mortimer K, Agusti A, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet. 2021; 397(10277): 928–940.
- WHO report on the global tobacco epidemic, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO
- Organization WH. Tackling NCDs: “best buys” and other recommended interventions for the prevention and control of noncommunicable diseases. World Health Organization; 2017.
- WHO launches year-long campaign to help 100 million people quit tobacco [Internet]. [cited 2021 Sep 11]. Available from: https://www.who.int/news/item/08-12-2020-who-launches-year-long-campaign-to-help-100-million-people-quit-tobacco
- Thakur M, Van Schayck CP, Boudewijns EA Improved cookstoves in low-resource settings: a spur to successful implementation strategies. Npj Prim Care Respir Med. 2019; 29(1):36.
- Purdy S, Griffin T, Salisbury C, et al. Ambulatory care sensitive conditions: terminology and disease coding need to be more specific to aid policy makers and clinicians. Public Health. 2009;123(2):169–173.
- Diagnosing and managing chronic respiratory diseases - Online Course [Internet]. [cited 2021 Jun 21]. Available from: https://www.futurelearn.com/courses/chronic-respiratory-diseases-primary-care-settings?utm_campaign=the_university_of_edinburgh_chronic_respiratory_diseases_primary_care_settings_february_2021&utm_medium=organic_email&utm_source=newsletter_broadcast
- Williams S, Sheikh A, Campbell H, et al. Respiratory research funding is inadequate, inequitable, and a missed opportunity. Lancet Respir Med 2020; 8(8): e67–e68.
- Su Y, Long C, Yu Q, et al. Global scientific collaboration in COPD research. Int J Chron Obstruct Pulmon Dis. 2017;12:215–225.
- Tabyshova A, Hurst JR, Soriano JB, et al. Gaps in COPD guidelines of low- and middle-income countries: a systematic scoping review. Chest. 2021;159(2):575–584.
- Khan MA, Ahmed M, Anil S, et al. Strengthening the delivery of asthma and chronic obstructive pulmonary disease care at primary health-care facilities: study design of a cluster randomized controlled trial in Pakistan. Glob Health Action. 2015;8(1):28225.
- van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3(1):e44–51.
- Robertson NM, Nagourney EM, Pollard SL, et al. Urban-rural disparities in chronic obstructive pulmonary disease management and access in Uganda. COPD. 2019;6(1):17–28.
- General availability of peak flow measurement spirometry at the primary health care level [Internet]. [cited 2021 May 10]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/general-availability-of-peak-flow-measurement-spirometry-at-the-primary-health-care-level
- Mehanni S, Jha D, Kumar A, et al. Implementing a quality improvement initiative for the management of chronic obstructive pulmonary disease in rural Nepal. BMJ Open Qual. 2019;8(1):e000408.
- 13th GARD general meeting report (2019) [Internet]. [cited 2021 May 2]. Available from: https://gard-breathefreely.org/wp-content/uploads/2019/12/13-GARD-General-Meeting-Report-Final-Draft-2019-12-09.pdf
- World Health Organization. Global action plan for the prevention and control of noncommunicable diseases : 2013-2020. 103.
- Polverino F, Celli B The challenge of controlling the COPD epidemic: unmet needs. Am J Med. 2018;131(9):1–6.
- Pinnock H, Steed L, Jordan R Supported self-management for COPD: making progress, but there are still challenges. Eur Respi J 2016; 48(1): 6–9.
- WHO | priority medical devices [Internet]. [cited 2021 May 2]. Available from: https://www.who.int/medical_devices/access/en/
- Van Boven JFM Costs of case-finding uncovered: time to revisit COPD’s value pyramid? Thorax 2019;74(8):727–729
- Jordan RE, Adab P, Sitch A, et al. Targeted case finding for chronic obstructive pulmonary disease versus routine practice in primary care (TargetCOPD): a cluster-randomised controlled trial. Lancet Respir Med 2016;4(9):720–730.
- Billo NE Simple diagnostic and treatment algorithms needed for COPD. Int J Tuberc Lung Dis. 2017;21(4): 365.
- Teach the teacher | IPCRG [Internet]. [cited 2021 Jul 7]. Available from: https://www.ipcrg.org/projects/education/teach-the-teacher
- Siddharthan T, Wosu AC, Pollard SL, et al. A novel case-finding instrument for chronic obstructive pulmonary disease in low-and middle-income country settings. Int J Chron Obstruct Pulmon Dis. 2020;15:2769–2777.
- Plessis ED, Swart F, Maree D, et al. The utility of hand-held mobile spirometer technology in a resource-constrained setting. S Afr Med J. 2019;109(4):219–222.
- Ching SM, Pang YK, Price D, et al. Detection of airflow limitation using a handheld spirometer in a primary care setting. Respirology. 2014;19(5):689–693.
- Derom E, van Weel C, Liistro G, et al. Primary care spirometry. Eur Respir J 2008; 31(1): 197–203.
- Williams S, Tsiligianni ICOVID-19 poses novel challenges for global primary care. Npj Prim Care Respir Med. 2020;30(1):30.
- Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019; 53(5):1900164
- Habib GM, Rabinovich R, Divgi K, et al. Systematic review of clinical effectiveness, components, and delivery of pulmonary rehabilitation in low-resource settings. Npj Primary Care Respiratory Medicine 2020;30(1):52.
- Farah R, Groot W, and Pavlova M Barriers to access to pulmonary rehabilitation in developing countries: a systematic review. In: Rehabilitation and chronic care . European Respiratory Society; 2020 56: Suppl 64;884 .
- Leng MEF, Daniel S, Munday D Respiratory problems in low-resource settings. Curr Opin Support Palliat Care. 2017; 11(3):174–178.
- Spathis A, Burkin J, Moffat C, et al. Cutting through complexity: the breathing, thinking, functioning clinical model is an educational tool that facilitates chronic breathlessness management. Npj Prim Care Respir Med. 2021;31(1):25.
- World Health Organization model list of essential medicines.
- Ewen M, Zweekhorst M, Regeer B, et al. Baseline assessment of WHO’s target for both availability and affordability of essential medicines to treat non-communicable diseases. PLoS ONE. 2017;12(2): e0171284.
- Kibirige D, Kampiire L, Atuhe D, et al. Access to affordable medicines and diagnostic tests for asthma and COPD in sub Saharan Africa: the Ugandan perspective. BMC Pulm Med. 2017;17(1):179.
- Montes de Oca M, Lopez VMV, Jardim J, et al. Bronchodilator treatment for COPD in primary care of four Latin America countries: the multinational, cross-sectional, non-interventional PUMA study. Pulm Pharmacol Ther. 2016;38:10–16.
- Boland MRS, Tsiachristas A, Kruis AL, et al. The health economic impact of disease management programs for COPD: a systematic literature review and meta-analysis. BMC Pulm Med. 2013;13(1):40.
- Beran D, Zar HJ, Perrin C, et al. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir Med; 2015; 3(2):159–170.
- Hurst JR, Buist AS, Gaga M, et al. Challenges in the implementation of chronic obstructive pulmonary disease guidelines in low- and middle-income countries: an official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2021;18(8):1269–1277.
- Siddharthan T, Pollard SL, Quaderi SA, et al. Effectiveness-implementation of COPD case finding and self-management action plans in low- and middle-income countries: global excellence in COPD outcomes (GECo) study protocol. Trials. 2018;19(1):571.
- World Health Organization Why palliative care is an essential function of primary health care. 2018. Genova: World Health Organization. https://apps.who.int/iris/handle/10665/328101. License: CC BY-NC-SA 3.0 IGO
- Gama E, Madan J, Banda H, et al. Economic evaluation of the practical approach to lung health and informal provider interventions for improving the detection of tuberculosis and chronic airways disease at primary care level in Malawi: study protocol for cost-effectiveness analysis. Implement Sci. 2015;10(1).
- Practical approach to lung health-manual on initiating PAL implementation [Internet]. 2008. [cited 2021 May 3]. Available from: www.who.int/tb
- Siddharthan T, Gupte A, Barnes PJ Chronic obstructive pulmonary disease endotypes in low-and middle-income country settings: precision medicine for all. Am J Respir Crit Care Med. 2020; 202(2): 171–172.

