ABSTRACT
Introduction
Asthma is a common chronic respiratory disease characterized by chronic airway inflammation, airway hyperresponsiveness, reversible airflow limitation, and airway remodeling. Mild asthma is the most common type of asthma, but it is the most neglected. Sometimes mild asthma can lead to acute severe exacerbations or even death.
Areas covered
This article reviews the epidemiology, risk factors, and possible predictors of acute severe exacerbations and disease progression in mild asthma to improve the understanding of mild asthma and its severe acute exacerbations and progression.
Expert opinion
There is a necessity to improve asthma patient categorization and redefine mild asthma’s concept to heighten patient and physician attention. Identifying mild asthma patients that are highly vulnerable to severe acute exacerbations and researching the mechanisms are future prioritizations.
1. Introduction
Asthma, a chronic respiratory disease, is a serious health and economic burden for 339 million people worldwide [Citation1]. One study in China estimated the overall prevalence of asthma to be 4.2% [Citation2]. Depending on the severity of clinical symptoms, asthma can be classified as mild, moderate, or severe. Mild asthma accounts for approximately 50–75% of asthma cases, while severe asthma accounts for only 3–5% [Citation3,Citation4]. Previous research has focused on severe asthma because of its severity, difficulty in controlling the disease and high mortality rate; however, mild asthma should not be ignored. Mild asthma is typically characterized by infrequent exacerbations and low disease severity, leading to under-recognition, low diagnostic rates, inadequate medication regimens and poor symptom control. Moreover, the presence of mild symptoms in daily life for people with asthma does not necessarily indicate that they will experience only mild exacerbations. Some patients with mild asthma, who have been asymptomatic for a long term or have mild symptoms and normal pulmonary function, can still experience severe and life-threatening exacerbations [Citation5–7]. Therefore, mild asthma is not necessarily ‘mild,’ and it is imperative to explore how to identify patients with mild asthma who are at a greater risk of severe exacerbations. In this review, we discuss the definition and epidemiology of mild asthma and summarize the risk factors and biomarkers of ‘severe’ acute exacerbations in patients with mild asthma to improve the understanding of mild asthma and its severe acute exacerbations.
2. Severe acute exacerbations and progression
Currently, mild asthma is mainly judged by the severity of its symptoms and symptom control. For patients diagnosed with mild asthma, the severity of their symptoms during the stable phase and the results of their initial pulmonary function are the main factors taken into consideration: 1. Symptoms occurring less than once a day; 2. Symptoms that could impede daily activities and disrupt sleep; 3. Nocturnal asthma symptoms occurring less than once a week; 4. Forced expiratory volume in the first second (FEV1) % of predicted value should be ≥ 80%, or peak expiratory flow (PEF) should be ≥ 80% of personal best, and PEF variability should be < 30% for patients with mild asthma [Citation8]. Patients with mild asthma are reviewed primarily on the basis of the level of medication used to control symptoms: has defined mild asthma as a condition that can be well-managed through on-demand use of inhaled corticosteroids (ICS)-formoterol (called GINA step 1) or low-dose ICS and on-demand short-acting beta-agonists (SABA) (called GINA step 2). Mild asthma has been found to account for a significant percentage, around 50–70%, of the total number of asthma patients. The prevalence of mild asthma on a global scale is approximately 3.3% [Citation4]. Statistics from an internet-based survey conducted among urban residents in China indicate that approximately 75% of the total asthma population suffers from mild asthma (per GINA step 1–2) [Citation9].
As the disease advances, some individuals with mild asthma may suffer degradation and exacerbation. However, this is not always given sufficient attention by physicians and patients, and follow-up and assessment methods may not always accurately detect and timely update changes in the condition, which affects the classification of mild asthma. A study conducted on 8,000 European patients with asthma found that 45% of asthma patients were not effectively controlled and 44% had an acute exacerbation in the previous year, but over 80% of these patients believed that their asthma was under control [Citation10]. Another study, based on 1,000 asthma patients, revealed that only 14.3% (73/510) of those who had been given therapeutic medications reported good asthma control. However, the majority of patients considered their asthma to be either completely (21.8%) or well controlled [Citation11]. In addition, a cross-sectional study showed that 75% of patients with mild asthma were considered by their physicians to have ‘good’ or ‘complete’ control, but according to the GINA guidelines criteria, only 14.2% of patients had good control [Citation12], suggesting limitations and inaccuracies in the classification of asthma patients by physicians.
Acute exacerbations, disease progression, and burden of asthma in patients with ‘mild’ symptoms still require attention [Citation13]. The incidence of severe acute exacerbations in mild asthma, which require oral or systemic corticosteroids or hospitalization, is estimated to be (0.12–0.77)/year, and such exacerbations account for 30–40% of total asthma [Citation4]. In the previous year, severe asthma exacerbation was experienced by up to 25% of patients with mild asthma [Citation10], and 9.5% of adults with mild asthma progressed to severe asthma [Citation14]. In addition, one study included 70,829 patients with incident mild asthma found that 8% of these patients progressed to moderate or severe asthma over 10 years [Citation15]. Furthermore, a systematic review of 49,743 subjects noted that deaths resulting from acute exacerbations of mild asthma (assessed as mild asthma or in patients displaying mild symptoms) are projected to constitute 5–13% of all asthma fatalities, with this rate being as high as 33% in children [Citation5].
3. Possible risk factors
Investigating potential risk factors for severe acute exacerbations in individuals with mild asthma will help to identify potentially at-risk populations and contribute to a more reliable and practical classification of mild asthma. Several commonly known risk factors have been identified. . presents an overview of conceivable factors associated with the risk of severe exacerbations in patients with mild asthma.
Figure 1. Possible risk factors for severe acute exacerbations and progression of mild asthma. These factors include genetic predisposition, older age, female sex, co-existing conditions, uncontrolled inflammation, airway remodelling, respiratory infections, persistent airflow limitation, poor medication adherence, and so forth. BAS: Basophils; EOS: Eosinophils; NEU: Neutrophils; SABA: Short-acting beta-agonists; ICS: Inhaled corticosteroids.
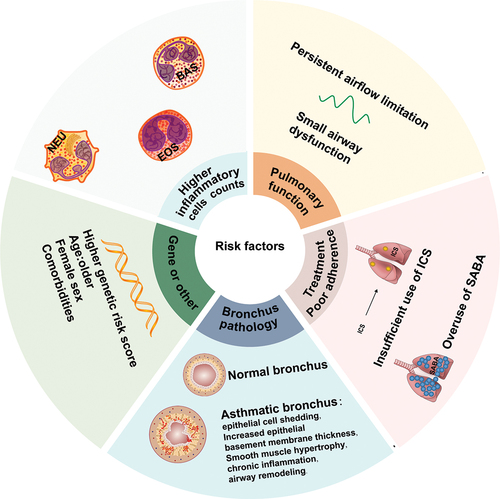
3.1. Genetic factors
Asthma exhibits genetic heterogeneity, with over 100 genes potentially influencing its development and severity. The interplay between genes and the environment can give rise to various pathogenic mechanisms and clinical manifestations [Citation16]. The application of genome-wide association studies (GWASs) and single nucleotide polymorphism (SNP) analysis identified some genetic loci associated with susceptibility to asthma [Citation17–19]. A study, based on the GWAS findings, constructed a multi-locus genetic risk profile by summing risk alleles across GWAS-identified SNPs to compute a ‘genetic risk score’ (GRS) (involving 17 SNPs located within or near genes IL17R18, IL1, HLA-DQ, IL33, SMAD3, ORMDL3, GSDMB, GSDMA and IL2RB), revealing that patients with a higher GRS are more likely to experience persistent asthma attacks, airway hyperresponsiveness, and incompletely reversible airflow obstruction [Citation20]. In addition, the genetic structure of mild asthma patients is similar to that of moderate-to-severe asthma [Citation21,Citation22], which may suggest that the difference in symptom severity is not significant, but also that mild asthma has a similar genetic basis to acute exacerbations of moderate-to-severe asthma. Although there is no concrete research evidence, it is plausible to suggest that the occurrence of severe acute exacerbations in patients with mild asthma could be attributed to their genetic idiosyncrasies. Further investigation is required to optimize the utilization of genetic factors for the prognosis of severe acute exacerbations.
The application of multi-omics has revealed asthma-related molecular markers not only in terms of genetic information but also at various molecular levels. Gautam et al. [Citation23] reviewed the transcriptomics of asthma susceptibility, disease severity and acute asthma exacerbations up to 2022, and Espuela-Ortiz et al. [Citation24] reviewed the genomic, epigenetic and transcriptomic features. However, the prediction of severe acute exacerbations in mild asthma lacks more relevant omics evidence, and future multi-omics analyses may help in the typing of mild asthma and the prediction of severe acute exacerbations in mild asthma.
3.2. Pathophysiological mechanisms
Despite experiencing mild symptoms, patients with mild asthma display significant pathophysiological changes in their bodies. Airway remodeling is closely associated with the shedding of epithelial cells, thickening of epithelial basement membranes, and hypertrophy of smooth muscle. Chronic inflammation is one mechanism responsible for airway remodeling. Additionally, research has shown a connection between small airway inflammation and the severity, risk of exacerbation, and increased airway responsiveness of asthma and chronic obstructive pulmonary disease (COPD). It has been reported that individuals with ‘mild intermittent’ asthma may have underlying airway inflammation, as well as those asymptomatic subjects with airway hyperresponsiveness asthma may experience airway inflammation and remodeling [Citation25–27]. In addition, a recent study found that patients with mild asthma display comparable levels of airway inflammation and remodeling as individuals with moderate-to-severe asthma [Citation28]. These findings indicate that the pathological basis of mild asthma is similar to that of more severe forms of the condition, such as moderate-to-severe asthma, which is the reasons that mild asthma progression [Citation15,Citation29–31].
3.3. Pulmonary function
Asthma with airflow limitation is associated with reduced quality of life, increased risk of future exacerbations, and increased need for health care resources [Citation32]. In patients with mild asthma, persistent airflow limitation is associated with a higher risk of acute exacerbations. The post hoc analysis of the ATLANTIS study aimed to explore the determinants, clinical significance, and outcomes of persistent airflow limitation in asthmatics who were enrolled in the study. The study revealed that persistent airflow limitation was evident not only in severe asthma patients but also in 21 (16%) patients categorized as GINA Step 1 and 24 (29%) as GINA Step 2 [Citation33]. According to the ATLANTIS study, there is a clinical correlation between mild asthma severity (GINA 1–2) patients and the presence of persistent airflow limitation (PAL), and PAL has a raised risk of future asthma exacerbations that is unrelated to age, gender, smoking habits, and blood eosinophil count [Citation33]. It has been observed that with an increase in the number of severe asthma exacerbations, there is a more significant decrease in FEV1 and bronchodilator reversibility [Citation34]. Patients with refractory asthma who have a smoking history or currently smoke are at a higher risk of developing PAL. Small airway obstruction (FEF 25–75%), detected through lung function, has been postulated as a potential factor in the persistent nature of asthma, leading to a poor asthma outcome independent of the effects of large airways [Citation35].
3.4. Eosinophils, basophilic or neutrophil counts
Inflammation cell counts could potentially serve as a biomarker for the future worsening of asthma [Citation36]. In a study of 4800 asthma patients followed up by testing their blood eosinophil and neutrophil counts, it was concluded that high blood eosinophil counts were associated with an increased risk of moderate and severe exacerbations of mild asthma, whereas high blood neutrophil counts were associated with an increased risk of moderate asthma exacerbations, but not with a risk of severe exacerbations of mild asthma [Citation37]. In addition, a count-response relationship existed between asthma-related outcomes and blood eosinophil count, where a count greater than 400 cells/μL resulted in more severe exacerbations and poorer asthma control. This suggested that blood eosinophil count could be used as a predictive marker for asthma risk assessment [Citation38]. Furthermore, there is an association between blood eosinophil count and SABA overuse, which can increase the risk of moderate asthma exacerbations [Citation39]. In summary, an elevated count of eosinophils or basophils in the peripheral blood may serve as a predictor of the risk of severe exacerbations in mild asthma [Citation36]. Sputum eosinophil levels also have been considered closely associated with associated with acute asthma attacks, not only in mild asthma [Citation40–42]. A study followed up with 50 mild asthma patients, conducting lung function tests and induced sputum sampling 12–15 years after initial diagnosis. It found that elevated levels of eosinophils, IL5, and IL8 in sputum were associated with accelerated decline in FEV1 (exceeding 30 ml/year) [Citation40], potentially providing predictive value for exacerbations in mild asthma patients. It’s important to note that anti-IL-5 therapy (which effectively reduces eosinophilic inflammation) can decrease eosinophil counts in severe eosinophilic asthma, acute exacerbations and corticosteroid dependence [Citation43–46]. However, in mild asthma cases, while anti-IL-5 therapy may reduce the number of eosinophils, it does not typically lead to an improvement in clinical symptoms, nor does it mitigate the decline in FEV1, FVC, fractional exhaled nitric oxide, and activation of residual eosinophils caused by rhinovirus (RV) 16 attacks [Citation47–49]. This aligns with the treatment principles outlined in the guidelines and the requirements for the use of biologics. Eosinophilic inflammation in mild asthma is often milder compared to moderate to severe asthma, so the effects that biologics can offer may indeed be less pronounced. However, it’s important to be vigilant, as an increase in eosinophilic inflammation in mild asthma may contribute to the risk of exacerbation.
3.5. Poor adherence: SABA overuse and inadequate use of ICS
SABA overuse is also a risk factor for acute exacerbations and disease progression in mild asthma [Citation15,Citation50]. Higher SABA use is related to an increased risk of exacerbations and death. SABA overuse is defined as using three or more SABA canisters per year. This is considered undesirable since it indicates overreliance on SABA. According to a report, around 20% of adults with asthma use three or more SABA canisters per year (≥12 inhalations/week) [Citation51]. A study conducted in Sweden with 365,324 patients with asthma revealed that around one-third of them had SABA overuse. Additionally, the study found that overuse of SABA was linked to a higher risk of acute exacerbations and mortality in asthma. Male gender, age, use of hypnotics and sedative medications, and a greater burden of comorbidity were identified as contributing factors to SABA overuse. Furthermore, comorbidity burden was determined to be the primary cause of SABA overuse [Citation52]. Another study included 8,351 patients across 24 countries in five continents reported that 38% of patients were prescribed ≥ 3 SABA canisters. Prescriptions of ≥ 3 SABA canisters was associated with lower adds of controlled or partly controlled asthma and higher rates of severe exacerbations [Citation53]. Additionally, it was observed that more than 53.6% of mild asthma patients receiving SABA monotherapy were prescribed ≥ 3 canisters and 29.9% were prescribed ≥ 10 SABA canisters in the past year [Citation53].
Acute exacerbations in mild asthma are also strongly associated with the underuse of ICS. Several large randomized controlled trials have indicated that low-dose ICS minimizes severe acute asthma exacerbations by 50%, enhances asthma symptoms and patients’ quality of life, and reduces severe acute exacerbations of mild asthma, which includes visits to the emergency department, hospitalization, and lung function loss in patients experiencing severe acute exacerbations [Citation54,Citation55]. Both the large randomized controlled trials, SYGMA1 and SYGMA2, have indicated a substantial decrease in the usage of budesonide-formoterol as a needed therapy, which is superior to the usage of terbutaline as needed therapy in managing asthma symptoms and minimizing exacerbations in mild asthma patients [Citation7]. It has been demonstrated that patients suffering from near-fatal asthma (NFA) display inadequate reliance on asthma medication and are not prescribed ICS therapy when using the LABA [Citation56]. A survey study showed that 49% (490/1000) of asthma patients were not using control medication [Citation11].
Poor adherence is an important reason for the underuse of ICS. Mild asthma patients may use ICS more irregularly due to their lack of attention to the disease. SABA overuse or inadequate use of ICS has been associated with an increased risk of exacerbations and emergency visits in asthma patients. It appears that the overuse of SABA is due to over-reliance on SABA or poor ICS adherence (inadequate use of ICS), leading to increasing difficulty in controlling asthma. However, a study that included 203 new-onset adult asthmatics over a longer period (12 years) found that patients with high SABA use had higher ICS adherence [Citation50]. High adherence to inhaled corticosteroids (ICS) may be due to increased knowledge about asthma from regular interaction with follow-up workers [Citation57]. There are various reasons for the overuse of SABA and inadequate use of ICS, including poor knowledge of the disease, poor adherence, general forgetfulness and inattention to disease progression, which is more likely to occur in patients with mild asthma. Additionally, properly inhaled prescription medications are associated with improved health and lung function [Citation58,Citation59]. Poor technique in using inhalation devices may also be the reason for poor symptom control in patients with mild asthma. This phenomenon is more likely to occur in patients with mild asthma due to the lack of attention paid to mild asthma by medical staff and patients. Instruction on the correct use of inhalation devices is necessary.
There is another issue to be considered: whether there is a causal relationship between the association between SABA overuse and poor asthma outcomes is actually unclear. Some mechanistic studies have shown negative effects of routine SABA use on biomarkers of airway inflammation, airway hyperresponsiveness, asthma symptom control and exacerbation risk, so a causal relationship cannot be ruled out [Citation60,Citation61], but there is no reliable evidence of causality or other direct relationships. Nevertheless, the large body of consistent epidemiological evidence supports SABA overuse as a risk factor for adverse asthma outcomes, and as mild asthmatics tend to have poor compliance and are more likely to experience SABA overuse and ICS inadequacy, it emphasizes that serious consequences of SABA overuse and ICS inadequacy may not be a bad thing in asthma management.
Furthermore, there is a view among some allergists that SABA should be eradicated from asthma management. Unrestricted access to as-needed SABA cannot overcome SABA’s current state of affairs. However, eradicating SABA from asthma management can achieve a goal of zero asthma hospitalizations [Citation62], which may be a good idea to overcome SABA’s current state of affairs and reduce asthma hospitalizations. To summarize, it is needed to improve asthma care and education and to pay more attention to combination therapy, which provides both rapid symptom control and anti-inflammatory effects, especially in mild asthma.
3.6. Other factors
Mild asthma exacerbations have several associated factors. A recent Korean cohort study examined 22,130 asthma patients, including 17,533 (79.23%) with mild asthma, to analyze the risk factors related to asthma exacerbations and severity. The study found that acute exacerbations were significantly associated with females, age ≥45 years, gastro-esophageal reflux disease and chronic rhinitis in mild and moderate asthma [Citation63]. Another study included 70,829 patients with incident mild asthma found that not only inappropriate use of SABA, but also older age and comorbidity were independently the risk factors for disease progression to moderate or severe asthma [Citation50]. Women are reported to be more susceptible to asthma exacerbations, with a study of 5,058 adults with asthma identifying female gender as a risk factor for severe asthma exacerbations [Citation36]. Overweight has also been identified as a potential risk factor in some children with fatal exacerbations [Citation64].
Given the significant heterogeneity in asthma, studies have classified patients with severe or life-threatening asthma exacerbations into five different clusters: Cluster 1 represented younger asthma patients with severe asthma symptoms at baseline. Cluster 2 was predominantly elderly females with asthma. Cluster 3 was individuals with allergic asthma who did not receive baseline ICS treatment. Cluster 4 was predominantly elderly males with asthma overlapped with COPD. Despite reporting mild symptoms at baseline, 41% of individuals in cluster 5 had previously been hospitalized for asthma, indicating that mild asthma may not necessarily be as mild as perceived. Furthermore, the present exacerbation was triggered by strain/stress, viral infection, or bacterial infection [Citation65].
4. Possible predictive biomarkers
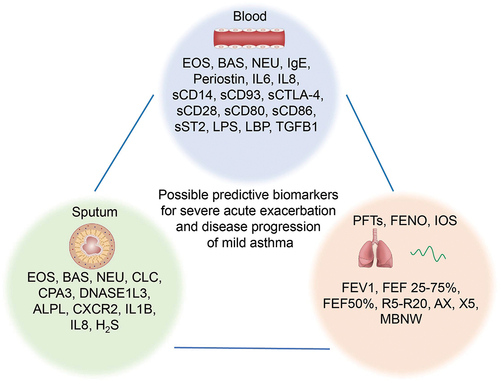
There is limited research on biomarkers to predict acute severe exacerbations and progression in mild asthma. However, one study discovered that patients with mild to severe asthma who had suffered a near-fatal asthma attack (at least one non-fatal asthma exacerbation within 5 years) had similar lung function, blood gases, atopic status, sputum, and blood eosinophil counts to patients with mild to moderate asthma at a steady state, and no significant differences were found in comparison to patients with severe asthma [Citation56]. This suggests that patients with NFA have a similar baseline status during their stabilization period as patients with mild to moderate asthma, and that prediction of fatal asthma attacks in mild asthma may be possible using common biomarkers. Some of the following biomarkers of acute asthma exacerbations may be predictive of severe asthma attacks in mild asthma. An overview of possible biomarkers for the prediction of acute attacks in mild asthma is shown in .
Figure 2. Potential biomarkers for predicting severe acute exacerbations and progression in mild asthma. ALPL: Alkaline phosphatase, liver/bone/kidney; AX: Area of reactance; BAS: Basophil; CPA3: Carboxypeptidase A3; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; CXCR2: chemokine (C-X-C motif) receptor 2; DNASEIL3: Deoxyribonuclease I‐like3; EOS: Eosinophils; FEV1: Forced expiratory volume in the first second; FEF 25–75%: The mid-expiratory flow rate; H2S: Hydrogen sulfide; IOS: Impulse oscillometry; LBP: Lipopolysaccharide binding protein; MBNW: Multiple nitrogen washout; NEU: Neutrophils; PFTs: Pulmonary function tests; R5: The resistance at 5 Hz; R20: The resistance at 20 Hz; sCD: Soluble cluster of differentiation; sST2: Soluble growth stimulation expressed gene 2; TGFB1: Transforming growth factor beta 1; X5: Respiratory system impedance at 5 Hz.
4.1. Hematological biomarkers
Studies have shown that blood eosinophil count (BEC) and immunoglobulin E (IgE) may be associated with asthma exacerbations and poor control [Citation37–39,Citation66–68]. Interestingly, one study found that among patients receiving as-needed salbutamol, the proportion of patients with severe exacerbations increased progressively with increasing blood eosinophil counts, but not in patients receiving as-needed budesonide-formoterol, suggesting that high blood eosinophil counts and as-needed SABA are associated with asthma exacerbations. In addition, patients with higher blood eosinophil counts had more benefit from maintenance inhaled budesonide than those with low counts [Citation69]. Serum periosteal proteins are also predictive of asthma exacerbations [Citation67,Citation70,Citation71]. In a study of exacerbations in severe asthma, IL-6 and eosinophils were found to predict worsening asthma [Citation72], reflecting the characteristics of exacerbation-prone asthma, and perhaps worsening/exacerbation of mild asthma is similarly related and requires further study.
Soluble CD14 (sCD14), which may act as a protective factor in asthma, was negatively correlated with asthma severity in adults, but significantly positively correlated with clinical signs and symptoms (e.g. severe dyspnea, wheezing and cyanosis) during acute asthma exacerbations [Citation73]. In addition, serum soluble CD93 (sCD93) has been found to increase significantly during acute asthma exacerbations and decrease with treatment, which may also be a predictor of acute asthma exacerbations [Citation74]. Plasma levels of soluble cytotoxic T-lymphocyte-associated protein 4, sCD28, sCD80 and sCD86 are highest in pediatric asthmatics during acute exacerbations and may also be used to assess disease severity in acute asthma [Citation75]. The expression of lipopolysaccharide (LPS)/LBP (LPS-binding protein) and TGFB1 has been shown to be significantly increased during the acute exacerbation phase of asthma in children, suggesting that it is an important regulator of the immune response during an acute asthma exacerbation and may be predictive of acute asthma exacerbation [Citation76].
The above evidence may have guiding significance in predicting acute attacks of mild asthma. More appropriate evidence is that sST2 (a soluble IL-33 receptor) has been found to be associated with severe exacerbations of mild asthma, with high levels of serum sST2 (>18 ng/ml) predicting severe exacerbations of mild asthma within 3 months [Citation77].
4.2. Sputum-related biomarkers
As mentioned above, eosinophils in sputum play a crucial role in the acute exacerbation and progression of mild asthma. Therefore, the proportion, count, and related biomarkers of eosinophils in sputum, such as IL5, Eosinophil Cationic Protein (ECP), may have predictive value [Citation40–42]. However, there are other predictive biomarkers worth noting. A study found that the sputum gene expression signature of six biomarkers (including Charcot-Leyden crystal galectin [CLC]; carboxypeptidase 3 [CPA3]; deoxyribonuclease 1-like 3 [DNASE1L3]; alkaline phosphatase, liver/bone/kidney [ALPL]; chemokine (C-X-C motif) receptor 2 [CXCR2]; and interleukin 1 beta [IL1B]) predicted acute exacerbations frequency in poorly controlled asthma and did not change with treatment, which may also suggest a pathological basis for stable acute exacerbations of asthma in the stable phase [Citation78]. Increased sputum hydrogen sulfide (H2S) has also been found to predict the risk of acute asthma exacerbations and is positively correlated with neutrophil-associated inflammation (sputum neutrophil ratio, sputum IL-8 and serum IL-8) [Citation79]. Furthermore, the viral and microbial composition in sputum may also play a predictive role in exacerbations of mild asthma. A cross-sectional study conducted viral metagenomic detection and analysis on sputum samples from healthy controls, non-severe asthma patients, and severe asthma patients. It found that the severity and exacerbation of asthma increased with the abundance of human herpesvirus (HHV), especially cytomegalovirus (CMV) and HHV-4 [Citation80]. A prospective clinical study investigating the impact of RV16 challenge on mild asthma has highlighted the role of viral attacks in acute exacerbations of mild asthma. The RV16 challenge resulted in a modest yet significant reduction in FEV1% predicted and an increase in the asthma control score (ACQ), alongside activation of remaining eosinophils [Citation49]. This underscores the importance of detecting viral-related biomarkers in predicting acute exacerbations of mild asthma and encourages further research in this area.
4.3. Respiratory-related screening indices
The level of FEV1% appears to be significantly associated with severe acute asthma exacerbations, and it has been suggested that a lower FEV1% is a predictor of the risk of severe asthma exacerbations [Citation36]. Fractional exhaled nitric oxide (FENO), as an indicator of type 2 inflammation, is also useful in predicting asthma exacerbations [Citation68,Citation70,Citation71]. Small airway dysfunction is also strongly associated with acute asthma exacerbations, and small airway parameters such as small to medium airway resistance (R5-R20) of impulse oculometry (IOS), area of reactance (AX) and airway impedance at 5 Hz (X5), multiple nitrogen washout (MBNW), FEF 25-75%, FEF 50% and FEV1 have been found to be significantly associated with acute asthma attacks [Citation81].
5. Prognosis of severe acute exacerbations in mild asthma
There are few studies on the prognosis of fatal mild asthma attacks as distinct from the prognosis of moderate to severe asthma attacks. However, some studies have found that patients who have had a fatal asthma attack have a poor prognosis (poor symptom control) due to factors such as older age, chronic severe asthma, high levels of inflammation-related laboratory markers (erythrocyte sedimentation rate, C-reactive protein), asthma exacerbated by pneumonia and relatively poor nutritional status (e.g. low albumin) [Citation82].
6. Mild asthma treatment and management objectives and future challenges
Numerous studies have shown that using inhaled budesonide-formoterol as needed is generally well-tolerated in mild asthma patients and can significantly reduce severe exacerbations [Citation55,Citation83–91]. Additionally, as-needed ICS-formoterol therapy in patients with mild to moderate asthma has been shown to have lower costs and higher quality-adjusted life years compared to regular ICS therapy [Citation92,Citation93]. The objective of treatment is to attain effective symptom control and to minimize the risk of future asthma-related deaths, exacerbations, persistent airflow limitation and treatment side effects [Citation94]. Details of treatment strategies for mild asthma are not discussed in this review, but we would like to emphasize some of the principles of the management of mild asthma and future challenges. This is because effective management and education of mild asthma patients can improve adherence and effectiveness of mild asthma treatment. Firstly, during the initial diagnosis process, attention should be paid to educating patients about asthma, helping them understand its heterogeneity, the potential for exacerbations and associated risks, the significance of defining monitoring and follow-up, and the importance of adherence. Subsequently, establishing a regular follow-up plan that is easily accessible is essential to address changes in the condition. Thirdly, to identify mild asthma patients at risk of severe exacerbations and progression, although this remains a challenge. Biomarkers may offer some assistance, but finding a reliable indicator, defining its threshold, assessing its applicability, universality, and cost-effectiveness pose significant challenges. Finally, a more standardized definition of patients with mild asthma is needed and remains a major challenge for the future. Arational definition should effectively distinguish between patients prone to severe exacerbations or rapid progression from those with milder daily symptoms and well-controlled asthma. Perhaps there should be more reasonable and individualized criteria for FEV1 as apercentage of predicted value. As previously mentioned, the current definition of mild asthma is primarily based on the severity and control of the condition, considering factors such as daily symptoms and lung function (FEV1 ≥ 80% of predicted value) for patients with initial diagnosis, and severity classification based on treatment outcomes for those with follow-up diagnoses. However, there is a need for a more standardized and rational definition due to the varying disease outcomes among patients with mild asthma and the lack of emphasis on the initial diagnosis of ‘mild’ asthma. Additionally, incorporating additional definitional conditions at the time of initial diagnosis, such as biomarker tests for predicting severe exacerbations and disease progression, could be beneficial. Further challenges lie ahead in refining these definitions for improved patient care and management.
7. Conclusion
Risk factors for acute exacerbations and progression of mild asthma include genetic factors, disease pathology, but also abnormal lung function, increased inflammatory cells, and also inappropriate use of SABA and ICS; age, gender, obesity, and comorbidities may be other risk factors for acute exacerbations and progression of mild asthma. Further research is necessary to identify biomarkers that can predict acute severe exacerbations of mild asthma. It is possible that blood and sputum markers of inflammation, as well as conditions such as lung function, could help predict acute lethal exacerbations of mild asthma and the progression of mild asthma, and could help predict this population. Mild asthma warrants serious attention, and identifying patient groups that are susceptible to acute severe exacerbations is a vital direction for future research.
8. Expert opinion
As a controllable and heterogeneous disease, prevention of allergens and standardized treatment and management of asthma can effectively control its progression. The original intention of categorizing asthma into varying degrees of severity was to enable specific and effective management, control, and treatment. Nevertheless, the term ‘mild’ necessitates reevaluation. Classifying asthma as ‘mild’ may result in insufficient awareness of the disease, inadequate treatment, failure to recognize potential risks (such as life-threatening exacerbations), and the physician underestimating the severity of the condition, which could harm disease control and management. Moreover, emerging data suggests that some individuals labeled as having ‘mild’ asthma are vulnerable to poorly controlled symptoms, acute exacerbations, declining lung function, and even mortality. Neglect and inadequate management of mild asthma can lead to disease exacerbation. Therefore, patients and physicians should not underestimate or neglect any cases of so-called ‘mild asthma.’
To improve mild asthma management, it is imperative to redefine the categorization of asthma patients. Alternatively, identifying individuals with mild asthma who are at risk of adverse outcomes and providing targeted interventions is crucial. Biomarkers could be helpful in categorizing and identifying clusters of different degrees of asthma. Secondly, it is important to improve the management of asthma treatment modalities. The on-demand use of short-acting SABAs may still lead to their overuse, as patients tend to prefer SABAs, which may be due to inadequate patient knowledge of the condition (which is actually a result of our lack of education about asthma), neglect of the condition due to intermittent symptomatic episodes of the patient, panic about ICS and so-called ‘addiction,’ other savings, etc. Eliminating the use of SABA from management could potentially contribute to better therapeutic management of asthma. Third, improving the education of primary care physicians and initial patients is necessary. A program for respiratory specialists and allergists has addressed the issue of education in asthma to enhance adherence to treatment and management. We also need to be aware of the need to focus the education population among primary care physicians and initial patients, as this will effectively advance asthma management. Additionally, further extensive research is necessary to better understand the mechanisms underlying the onset and progression of mild asthma. Why do some patients with mild asthma display mild symptoms without corresponding mild pathology? Why do some patients with mild asthma fail to prevent disease progression to moderate-to-severe asthma despite compliance with medication? Why do some patients experience mild asthma symptoms but develop severe symptoms during an acute attack? Which patients with mild asthma are at greater risk for an acute, severe attack? We still have a long way to go. A more dependable and practical categorization of asthma, investigation of the factors contributing to sudden worsening in patients with mild asthma, and a more reasonable approach to managing patients with mild asthma are the focal points for future research.
Article highlights
Mild asthma may not be truly ‘mild,’ as this label could result in neglectful treatment and management of patients with mild asthma.
Some patients with mild asthma are at risk for poor control, acute exacerbations, reduced lung function and even death.
Multiple risk factors and biomarkers have the potential to recognize individuals with asthma who may have unfavorable outcomes.
Future research should focus on improved categorization of asthma, more effective management of emergency medication usage, and redefining the classification of ‘mild asthma.’
Abbreviations
| ACQ | = | Asthma control score |
| ALPL | = | Alkaline phosphatase, liver/bone/kidney |
| AX | = | Area of reactance |
| BAS | = | Basophils |
| CPA3 | = | Carboxypeptidase A3 |
| CTLA-4 | = | Cytotoxic T-lymphocyte-associated protein 4 |
| CLC | = | Charcot-Leyden crystal galectin |
| CMV | = | Cytomegalovirus |
| CXCR2 | = | Chemokine (C‐X‐C motif) receptor 2 |
| DNASEIL3 | = | Deoxyribonuclease I‐like3 |
| EOS | = | Eosinophils |
| FEV1 | = | Forced expiratory volume in the first second |
| FEF 25–75% | = | Forced expiratory flow rate between 25% and 75% |
| GAWS | = | Genome-wide association studies |
| HHV | = | Human herpesvirus |
| H2S | = | Hydrogen sulfide |
| ICS | = | Inhaled corticosteroids |
| IL1B | = | Interleukin 1 beta |
| IOS | = | Impulse oscillometry |
| LBP | = | Lipopolysaccharide binding protein |
| MBNW | = | Multiple nitrogen washout |
| NEU | = | Neutrophils |
| NFA | = | Near-fatal asthma |
| PAL | = | Persistent airflow limitation |
| PFTs | = | Pulmonary function tests |
| R5 | = | The resistance at 5 Hz |
| R20 | = | The resistance at 20 Hz |
| RV | = | Rhinovirus |
| SABA | = | Short-acting beta-agonists |
| sCD | = | Soluble cluster of differentiation |
| SNP | = | Single nucleotide polymorphism |
| sST2 | = | Soluble growth stimulation expressed gene 2 |
| TGFB1 | = | Transforming growth factor beta 1 |
| X5 | = | Respiratory system impedance at 5 Hz |
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A peer reviewer on this manuscript has received an honorarium from Expert Review of Respiratory Medicine for their review work. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study. 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2
- Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi: 10.1016/S0140-6736(19)31147-X
- Hekking P-P, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62(6):591–604. doi: 10.1111/j.1398-9995.2007.01394.x
- Fitzgerald JM, Barnes PJ, Chipps BE, et al. The burden of exacerbations in mild asthma: a systematic review. ERJ Open Res. 2020;6(3):00359–2019. doi: 10.1183/23120541.00359-2019
- Mulgirigama A, Barnes N, Fletcher M, et al. A review of the burden and management of mild asthma in adults — implications for clinical practice. Respir med. 2019;152:97–104. doi: 10.1016/j.rmed.2019.04.024
- Tang W, Sun L, Fizgerald JM. A paradigm shift in the treatment of mild asthma? J Thoracic Dis. 2018;10(10):5655–5658. doi: 10.21037/jtd.2018.09.127
- Cloutier MM, Dixon AE, Krishnan JA, et al. Managing asthma in adolescents and adults: 2020 asthma guideline update from the national asthma education and prevention program. JAMA. 2020;324(22):2301–2317. doi: 10.1001/jama.2020.21974
- Ding B, Dibonaventura M, Karlsson N, et al. A cross-sectional assessment of the prevalence and burden of mild asthma in urban China using the 2010, 2012, and 2013 China national health and wellness surveys. J Asthma. 2017;54(6):632–643. doi: 10.1080/02770903.2016.1255750
- Price D, Fletcher M, Van Der Molen T. Asthma control and management in 8,000 European patients: the REcognise asthma and LInk to symptoms and experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9
- Colice GL, Ostrom NK, Geller DE, et al. The CHOICE survey: high rates of persistent and uncontrolled asthma in the United States. Ann Allergy Asthma Immunol. 2012;108(3):157–162. doi: 10.1016/j.anai.2011.12.017
- Ding B, Small M. Disease burden of mild asthma in China. Respirology. 2018;23(4):369–377. doi: 10.1111/resp.13189
- Mohan A, Lugogo NL, Hanania NA, et al. Questions in mild asthma: an official American thoracic society research statement. Am J Respir Crit Care Med. 2023;207(11):e77–e96. doi: 10.1164/rccm.202304-0642ST
- Ernst P, Cai B, Blais L, et al. The early course of newly diagnosed asthma. Am J Med. 2002;112(1):44–48. doi: 10.1016/s0002-9343(01)01033-6
- Chen W, Fitzgerald JM, Lynd LD, et al. Long-term trajectories of mild asthma in adulthood and risk factors of progression. J Allergy Clin Immunol Pract. 2018;6(6):2024–2032.e5. doi: 10.1016/j.jaip.2018.04.027
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107–1119. doi: 10.1016/S0140-6736(08)61452-X
- Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi: 10.1056/NEJMoa0906312
- Wang J, Zhou Y, Zhang H, et al. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):138. doi: 10.1038/s41392-023-01344-4
- Dong Z, Ma Y, Zhou H, et al. Integrated genomics analysis highlights important SNPs and genes implicated in moderate-to-severe asthma based on GWAS and eQTL datasets. BMC Pulm Med. 2020;20(1):270. doi: 10.1186/s12890-020-01303-7
- Belsky DW, Sears MR, Hancox RJ, et al. Polygenic risk and the development and course of asthma: an analysis of data from a four-decade longitudinal study. Lancet Respir Med. 2013;1(6):453–461. doi: 10.1016/S2213-2600(13)70101-2
- Schoettler N, Ober C. Genetic architecture of moderate-to-severe asthma mirrors that of mild asthma. J Allergy Clin Immunol. 2019;144(6):1521–1523. doi: 10.1016/j.jaci.2019.09.003
- Hinds DA, Mcmahon G, Kiefer AK, et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nat Genet. 2013;45(8):907–911. doi: 10.1038/ng.2686
- Gautam Y, Johansson E, Mersha TB. Multi-omics profiling approach to asthma: an evolving paradigm. J Pers Med. 2022;12(1):66. doi: 10.3390/jpm12010066
- Herrera-Luis E, Forno E, Celedón JC, et al. Asthma exacerbations: the genes behind the scenes. J Investig Allergol Clin. 2023;33(2):76–94. doi: 10.18176/jiaci.0878
- Vignola AM, Chanez P, Campbell AM, et al. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998;157(2):403–409. doi: 10.1164/ajrccm.157.2.96-08040
- Laprise C, Laviolette M, Boutet M, et al. Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodelling. Eur Respir J. 1999;14(1):63–73. doi: 10.1034/j.1399-3003.1999.14a12.x
- Spallarossa D, Battistini E, Silvestri M, et al. Steroid-naive adolescents with mild intermittent allergic asthma have airway hyperresponsiveness and elevated exhaled nitric oxide levels. J Asthma. 2003;40(3):301–310. doi: 10.1081/jas-120018629
- Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367(3):551–569. doi: 10.1007/s00441-016-2566-8
- Boulet LP, Jobin C, Milot J, et al. Five-year changes in airflow obstruction and airway responsiveness in mild to moderate asthma. Clin Invest Med. 1994;17(5):432–442.
- Wardlaw AJ, Dunnette S, Gleich GJ, et al. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137(1):62–69. doi: 10.1164/ajrccm/137.1.62
- Boulet L-P. Airway remodeling in asthma: update on mechanisms and therapeutic approaches. Curr Opin Pulm Med. 2018;24(1):56–62. doi: 10.1097/MCP.0000000000000441
- Postma DS, Rabe KF. The Asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. doi: 10.1056/NEJMra1411863
- Kole TM, Vanden Berghe E, Kraft M, et al. Predictors and associations of the persistent airflow limitation phenotype in asthma: a post-hoc analysis of the ATLANTIS study. Lancet Respir Med. 2023;11(1):55–64. doi: 10.1016/S2213-2600(22)00185-0
- Matsunaga K, Hirano T, Oka A, et al. Progression of irreversible airflow limitation in asthma: correlation with severe exacerbations. J Allergy Clin Immunol Pract. 2015;3(5):759–64.e1. doi: 10.1016/j.jaip.2015.05.005
- Siroux V, Boudier A, Dolgopoloff M, et al. Forced midexpiratory flow between 25% and 75% of forced vital capacity is associated with long-term persistence of asthma and poor asthma outcomes. J Allergy Clin Immunol. 2016;137(6):759–64.e1. doi: 10.1016/j.jaci.2015.10.029
- Ban GY, Kim SC, Lee HY, et al. Risk factors predicting severe asthma exacerbations in adult asthmatics: a real-world clinical evidence. Allergy Asthma Immunol Res. 2021;13(3):420–434. doi: 10.4168/aair.2021.13.3.420
- Vedel-Krogh S, Fallgaard Nielsen S, Lange P, et al. Association of blood eosinophil and blood neutrophil counts with asthma exacerbations in the Copenhagen General Population Study. Clin Chem. 2017;63(4):823–832. doi: 10.1373/clinchem.2016.267450
- Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3(11):849–858. doi: 10.1016/S2213-2600(15)00367-7
- Zeiger RS, Schatz M, Li Q, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2(6):741–750. doi: 10.1016/j.jaip.2014.06.005
- Marc-Malovrh M, Camlek L, Škrgat S, et al. Elevated eosinophils, IL5 and IL8 in induced sputum in asthma patients with accelerated FEV1 decline. Respir med. 2020;162:105875. doi: 10.1016/j.rmed.2020.105875
- Ciółkowski J, Mazurek H, Hydzik P, et al. Inflammatory markers as exacerbation risk factors after asthma therapy switch from inhaled steroids to montelukast. Pulm Pharmacol Ther. 2016;39:7–13. doi: 10.1016/j.pupt.2016.05.002
- Tillie-Leblond I, Montani D, Crestani B, et al. Relation between inflammation and symptoms in asthma. Allergy. 2009;64(3):354–367. doi: 10.1111/j.1398-9995.2009.01971.x
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X
- Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi: 10.1056/NEJMoa0808991
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290
- Flood-Page PT, Menzies-Gow AN, Kay AB, et al. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167(2):199–204. doi: 10.1164/rccm.200208-789OC
- Leckie MJ, Ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–2148. doi: 10.1016/s0140-6736(00)03496-6
- Sabogal Piñeros YS, Bal SM, De Pol MA V, et al. Anti-IL-5 in mild asthma alters rhinovirus-induced macrophage, B-Cell, and neutrophil responses (MATERIAL). A placebo-controlled, double-blind study. Am J Respir Crit Care Med. 2019;199(4):508–517. doi: 10.1164/rccm.201803-0461OC
- Vähätalo I, Lehtimäki L, Tuomisto LE, et al. Long-term use of short-acting β2-agonists in patients with adult-onset asthma. J Allergy Clin Immunol Pract. 2022;10(8):2074–2083.e7. doi: 10.1016/j.jaip.2022.03.027
- Amin S, Soliman M, Mcivor A, et al. Usage patterns of short-acting β2-agonists and inhaled corticosteroids in asthma: a targeted literature review. J Allergy Clin Immunol Pract. 2020;8(8):2556–2564.e8. doi: 10.1016/j.jaip.2020.03.013
- Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi: 10.1183/13993003.01872-2019
- Bateman ED, Price DB, Wang H-C, et al. Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Respir J. 2022;59(5):2101402. doi: 10.1183/13993003.01402-2021
- Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361(9363):1071–1076. doi: 10.1016/S0140-6736(03)12891-7
- O’byrne PM, Barnes PJ, Rodriguez-Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1392–1397.
- Romagnoli M, Caramori G, Braccioni F, et al. Near-fatal asthma phenotype in the ENFUMOSA cohort. Clin Exp Allergy. 2007;37(4):552–557. doi: 10.1111/j.1365-2222.2007.02683.x
- Hogan MB, Zhao W. High SABA Using and Poor Asthma Control: Blaming Poor Adherence or Missing the Phenotype? J Allergy Clin Immunol Pract. 2022;10(8):2084–2085. doi: 10.1016/j.jaip.2022.05.011
- Gregoriano C, Dieterle T, Breitenstein A-L, et al. Use and inhalation technique of inhaled medication in patients with asthma and COPD: data from a randomized controlled trial. Respir Res. 2018;19(1):237. doi: 10.1186/s12931-018-0936-3
- Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19(1):10. doi: 10.1186/s12931-017-0710-y
- Aldridge RE, Hancox RJ, Robin Taylor D, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med. 2000;161(5):1459–1464. doi: 10.1164/ajrccm.161.5.9906052
- Hancox RJ, Cowan JO, Flannery EM, et al. Bronchodilator tolerance and rebound bronchoconstriction during regular inhaled beta-agonist treatment. Respir med. 2000;94(8):767–771. doi: 10.1053/rmed.2000.0820
- Nannini LJ. It is time to end our love affair with short-acting β2-agonists in asthma? yes. ERJ Open Res. 2022;8(4):00353–2022. doi: 10.1183/23120541.00353-2022
- Kang H-R, Song HJ, Nam JH, et al. Risk factors of asthma exacerbation based on asthma severity: a nationwide population-based observational study in South Korea. BMJ Open. 2018;8(3):e020825. doi: 10.1136/bmjopen-2017-020825
- Carroll CL, Uygungil B, Zucker AR, et al. Identifying an at-risk population of children with recurrent near-fatal asthma exacerbations. J Asthma. 2010;47(4):460–464. doi: 10.3109/02770903.2010.481344
- Sekiya K, Nakatani E, Fukutomi Y, et al. Severe or life-threatening asthma exacerbation: patient heterogeneity identified by cluster analysis. Clin Exp Allergy. 2016;46(8):1043–1055. doi: 10.1111/cea.12738
- Woo S-D, Park HS, Jang J-H, et al. Biomarkers for predicting type 2-high and uncontrolled asthma in real-world practice. Ann Allergy Asthma Immunol. 2023;131(2):209–216.e2. doi: 10.1016/j.anai.2023.05.011
- Buhl R, Korn S, Menzies-Gow A, et al. Prospective, single-arm, longitudinal study of biomarkers in real-world patients with severe asthma. J Allergy Clin Immunol Pract. 2020;8(8):2630–2639.e6. doi: 10.1016/j.jaip.2020.03.038
- Kraft M, Brusselle G, Fitzgerald JM, et al. Patient characteristics, biomarkers and exacerbation risk in severe, uncontrolled asthma. Eur Respir J. 2021;58(6):2100413. doi: 10.1183/13993003.00413-2021
- Pavord ID, Holliday M, Reddel HK, et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open-label, parallel-group, randomised controlled trial. Lancet Respir Med. 2020;8(7):671–680. doi: 10.1016/S2213-2600(20)30053-9
- Hanibuchi M, Mitsuhashi A, Kajimoto T, et al. Clinical significance of fractional exhaled nitric oxide and periostin as potential markers to assess therapeutic efficacy in patients with cough variant asthma. Respir Investig. 2023;61(1):16–22. doi: 10.1016/j.resinv.2022.10.006
- Mansur AH, Srivastava S, Sahal A. Disconnect of type 2 biomarkers in severe asthma; dominated by FeNO as a predictor of exacerbations and periostin as predictor of reduced lung function. Respir med. 2018;143:31–38. doi: 10.1016/j.rmed.2018.08.005
- Peters MC, Mauger D, Ross KR, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med. 2020;202(7):973–982. doi: 10.1164/rccm.201909-1813OC
- Zhou T, Huang X, Ma J, et al. Association of plasma soluble CD14 level with asthma severity in adults: a case control study in China. Respir Res. 2019;20(1):19. doi: 10.1186/s12931-019-0987-0
- Sigari N, Jalili A, Mahdawi L, et al. Soluble CD93 as a novel biomarker in asthma exacerbation. Allergy Asthma Immunol Res. 2016;8(5):461–465. doi: 10.4168/aair.2016.8.5.461
- Ip WK, Wong CK, Leung TF, et al. Plasma concentrations of soluble CTLA-4, CD28, CD80 and CD86 costimulatory molecules reflect disease severity of acute asthma in children. Pediatr Pulmonol. 2006;41(7):674–682. doi: 10.1002/ppul.20432
- Jones AC, Leffler J, Laing IA, et al. LPS binding protein and activation signatures are upregulated during asthma exacerbations in children. Respir Res. 2023;24(1):184. doi: 10.1186/s12931-023-02478-3
- Watanabe M, Nakamoto K, Inui T, et al. Serum sST2 levels predict severe exacerbation of asthma. Respir Res. 2018;19(1):169. doi: 10.1186/s12931-018-0872-2
- Fricker M, Gibson PG, Powell H, et al. A sputum 6-gene signature predicts future exacerbations of poorly controlled asthma. J Allergy Clin Immunol. 2019;144(1):51–60.e11. doi: 10.1016/j.jaci.2018.12.1020
- Suzuki Y, Saito J, Kikuchi M, et al. Sputum-to-serum hydrogen sulphide ratio as a novel biomarker of predicting future risks of asthma exacerbation. Clin Exp Allergy. 2018;48(9):1155–1163. doi: 10.1111/cea.13173
- Choi S, Sohn K-H, Jung J-W, et al. Lung virome: new potential biomarkers for asthma severity and exacerbation. J Allergy Clin Immunol. 2021;148(4):1007–1015.e9. doi: 10.1016/j.jaci.2021.03.017
- Kraft M, Richardson M, Hallmark B, et al. The role of small airway dysfunction in asthma control and exacerbations: a longitudinal, observational analysis using data from the ATLANTIS study. Lancet Respir Med. 2022;10(7):661–668. doi: 10.1016/S2213-2600(21)00536-1
- Kim MS, Cho YJ, Moon HB, et al. Factors for poor prognosis of near-fatal asthma after recovery from a life-threatening asthma attack. Korean J Intern Med. 2008;23(4):170–175. doi: 10.3904/kjim.2008.23.4.170
- Bateman ED, Reddel HK, O’byrne PM, et al. As-Needed Budesonide-Formoterol versus Maintenance Budesonide in Mild Asthma. N Engl J Med. 2018;378(20):1877–1887. doi: 10.1056/NEJMoa1715275
- Domingo C, Rello J, Sogo A. As-needed ICS-LABA in mild asthma: what does the evidence say? Drugs. 2019;79(16):1729–1737. doi: 10.1007/s40265-019-01202-0
- Daley-Yates P, Singh D, Igea JM, et al. Assessing the effects of changing patterns of inhaled corticosteroid dosing and adherence with fluticasone furoate and budesonide on asthma management. Adv Ther. 2023;40(9):4042–4059. doi: 10.1007/s12325-023-02585-z
- O’byrne PM, Fitzgerald JM, Bateman ED, et al. Effect of a single day of increased as-needed budesonide-formoterol use on short-term risk of severe exacerbations in patients with mild asthma: a post-hoc analysis of the SYGMA 1 study. Lancet Respir Med. 2021;9(2):149–158. doi: 10.1016/S2213-2600(20)30416-1
- Reddel HK, O’byrne PM, Fitzgerald JM, et al. Efficacy and safety of as-needed budesonide-formoterol in adolescents with mild asthma. J Allergy Clin Immunol Pract. 2021;9(8):3069–3077.e6. doi: 10.1016/j.jaip.2021.04.016
- O’byrne PM, Fitzgerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378(20):1865–1876. doi: 10.1056/NEJMoa1715274
- Tong X, Liu T, Li Z, et al. Is it really feasible to use budesonide-formoterol as needed for mild persistent asthma? A systematic review and meta-analysis. Front Pharmacol. 2021;12:644629. doi: 10.3389/fphar.2021.644629
- Bateman ED, O’byrne PM, Fitzgerald JM, et al. Positioning As-needed budesonide-formoterol for mild asthma: effect of prestudy treatment in pooled analysis of SYGMA 1 and 2. Ann Am Thorac Soc. 2021;18(12):2007–2017. doi: 10.1513/AnnalsATS.202011-1386OC
- Fitzgerald JM, O’byrne PM, Bateman ED, et al. Safety of As-needed budesonide-formoterol in mild asthma: data from the two phase III SYGMA studie. Drug Saf. 2021;44(4):467–478. doi: 10.1007/s40264-020-01041-z
- Fitzgerald JM, Arnetorp S, Smare C, et al. The cost-effectiveness of as-needed budesonide/formoterol versus low-dose inhaled corticosteroid maintenance therapy in patients with mild asthma in the UK. Respir med. 2020;171:106079. doi: 10.1016/j.rmed.2020.106079
- Buendía JA, Patiño DG. Cost-utility of as-needed ICS-formoterol versus to maintenance ICS in mild to moderate persistent asthma. BMC Pulm Med. 2021;21(1):397. doi: 10.1186/s12890-021-01775-1
- Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. doi: 10.1183/09031936.00138707

