1. Cancer increases the risk of venous thromboembolism
Cancer is known to be a powerful risk factor for Venous Thromboembolism (VTE), but this risk varies depending on the primary cancer site [Citation1]. For example, the relative risk for VTE varies between 2.87 for breast cancer to 15.56 for pancreatic cancer, while the relative risk was 7.24 for lung cancer, compared to patients without cancer [Citation2]. As a result, the combination of this increased risk of VTE with a high frequency of lung cancer in the general population explains why lung cancer has gradually become the leading cause of cancer-associated thrombosis (CAT) [Citation3].
Besides lung cancer being diagnosed at the time of VTE initial management, CAT may occur during the journey of patients with non-resectable lung cancer, but also after thoracic oncology surgery. For them, thromboprophylaxis is indicated during the hospital stay. However, up to one-third of CAT may occur after discharge [Citation4]. Hence, investigators are evaluating the relevance of extending thromboprophylaxis after discharge [Citation5].
However, until recently, there has been no significant distinction in the therapeutic management of CAT among the various primary cancer types. Nevertheless, recent data indicate that the clinical course under anticoagulant treatment varies according to the cancer’s primary site. This, combined with the enriched therapeutic options available for both cancer and VTE, justifies the need for specialized evaluation of the optimal antithrombotic coverage in patients with VTE associated with lung cancer [Citation6].
2. Lung cancer: a cancer with high risk of thrombosis, but also of VTE recurrence despite anticoagulant treatment
We know for two decades that the presence of cancer at the time of VTE diagnosis exposes patients to a three-fold increase in the risk of recurrence despite anticoagulant treatment compared to patients without cancer. Furthermore, these patients also have more than a two-fold increase in the risk of major bleeding while on anticoagulation therapy [Citation7].
For several years, the cancer’s primary site has been thought to have an impact on both the risk of VTE recurrence as well as the bleeding risk. In an analysis of nearly 4000 patients treated for VTE associated with the four most common cancers worldwide (breast, lung, colorectal, and prostate), the clinical course differed significantly between primary cancer sites. In lung cancer, the primary risk was death (occurring in more than 50% of patients at 6 months), followed by a recurrence risk of more than 10%, and a risk of major bleeding at 4%, all under anticoagulant treatment. This clinical profile contrasted with colorectal and prostate cancers, where the risk of major bleeding was higher than the risk of recurrence in the first 6 months of treatment [Citation8]. Thus, the lung cancer site was associated with the highest increase in recurrence risk (hazard ratio of 3.8 (2.6–5.6)) compared to other primary sites.
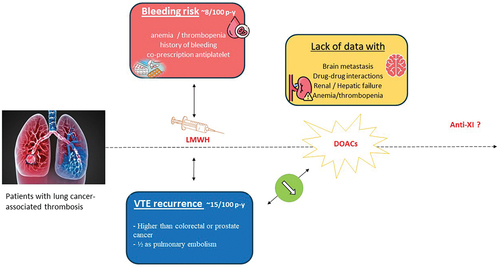
Very recently, epidemiological data obtained from a nationwide medico-administrative database analysis, confirmed the high-risk profile of VTE recurrence in patients with lung cancer, despite treatment with low molecular weight heparin (LMWH) [Citation9]. Among 31,771 patients admitted in France between 2013 and 2018 for VTE associated with active cancer, 7,899 had lung cancer (24.86%, the main provider). The mortality rate remained very high, around 44% at 6 months. The rate of VTE recurrence was elevated, reaching 15 per 100 patient-years (with half of the recurrences being pulmonary embolisms), despite continued anticoagulant treatment with LMWH.
It is noteworthy to mention that lung cancer patients are particularly susceptible to the diagnosis of incidental pulmonary embolism, due to their regular thoracic CT scan surveillance. These patients are candidates for conventional anticoagulant therapy; however, it seems that the risk of recurrence is comparatively lower than in patients presenting with symptomatic pulmonary embolism [Citation6].
3. Anticoagulation in patients with lung cancer-associated thrombosis: a significant bleeding risk
Lung cancer patients also suffer from a significant risk of bleeding under anticoagulant therapy. Indeed, bleeding requiring hospitalization occurred with an incidence rate of 8 per 100 patient-years, which was similar to the risk of recurrent pulmonary embolism [Citation9]. Several patient-related factors may explain this unexpected high rate of bleeding events. At inclusion, 25% of patients presented with anemia, and 11% with recent history of active bleeding. Additionally, co-prescription of antiplatelet agents was found in over 20% of lung cancer patients. These real-world data highlight that, while the risk of recurrence despite anticoagulant treatment remains the main concern, the risk of hemorrhage is not negligible.
4. Towards a tailored anticoagulant therapy of patients with lung cancer and CAT?
Numerous trials have demonstrated the superiority of low molecular weight heparins (LMWHs) over vitamin K antagonists (VKAs) in patients with CAT, with a 40–50% reduction in the risk of VTE recurrence without an increased bleeding risk [Citation10,Citation11]. More recently, direct oral anticoagulants (particularly apixaban [Citation12] and edoxaban [Citation13]) have shown non-inferiority in terms of efficacy compared to LMWH (specifically, dalteparin prescribed following cancer-adapted modalities). However, DOACs have been associated with a potentially higher risk of bleeding, particularly clinically relevant bleeding events [Citation14]. The increased bleeding risk seems to be mainly driven by mucosal tumors, especially those persisting in an endoluminal conduit, such as gastrointestinal and genitourinary tumors. In the two main therapeutic trials evaluating DOACs for VTE treatment, the proportion of patients with lung cancer ranged from 15% to 17%, and no unfavorable signals were detected [Citation15,Citation16].
Thus, DOACs appear to be a promising option for reducing the risk of VTE recurrence in lung cancer patients. However, it is essential to note that the proportion of patients eligible for DOACs trials in real-world clinical practice, especially lung cancer patients, may be relatively modest, possibly around 50% or more [Citation17]. Main ineligibility criteria mainly included the aforementioned bleeding risk factors (anemia, thrombocytopenia, active bleeding, etc.), brain metastasis, and specific contraindications for DOAC use (severe renal or hepatic insufficiency, co-prescription associated with a major drug–drug interaction) [Citation18]. The question of brain metastases is crucial as these lesions increase both the risk of thrombotic events and bleeding, particularly intracranial bleeding. These considerations have led some colleagues to prefer the use of LMWHs for patients with brain metastasis for the initial several months of treatment, followed by DOACs after that [Citation19]. The question of the clinical impact of potential drug–drug interactions with targeted therapies requires the development of appropriate tools. Therefore, the extrapolation of phase III trial results necessitates data from phase IV studies, particularly in lung cancer patients ().
5. Expert opinion
Lung cancer is nowadays the first provider of cancer-associated thrombosis, accounting for 20–25% of patients diagnosed with cancer-associated thrombosis. Clinical course under anticoagulant therapy varies widely with the type of cancer. Patients with lung cancer-associated thrombosis have a high risk of VTE recurrence despite anticoagulant therapy.
Recent data indicate that the risk of bleeding leading to hospitalization is higher than anticipated and occurs as frequently as recurrent pulmonary, when using low molecular weight heparin. The use of DOACs may reduce the rate of VTE recurrence. However, further data will be required to determine the best strategy in situations that have not been evaluated in therapeutic trials, or have been evaluated only to a limited extent.
In the absence of contraindications or situations where there is a very high risk of bleeding, treatment with a direct oral anticoagulant seems to be the preferred option, particularly apixaban, which has the highest level of evidence.
Further data are needed to determine strategies with a lower risk of hemorrhage (such as anti-factor XI for the initial therapy, and as a reduced dose of apixaban for the extended therapy), and to determine the best management of recurrences under anticoagulant therapy, in particular under DOACs.
Significant progress can be hoped for by improving thromboprophylaxis strategies, both by better targeting ambulatory patients who could benefit from thromboprophylaxis and by evaluating the possibility of extending the duration of post-operative thromboprophylaxis.
Declaration of interest
L Bertoletti reports personal fees and non-financial support from Viatris; grant, personal fees and non-financial support from Bayer; personal fees and non-financial support from BMS-Pfizer; personal fees and non-financial support from Léo-Pharma; grants, personal fees and non-financial support from MSD; non-financial support from Johnson and Johnson; outside the submitted work. S Accassat reports non-financial support from Léo-Pharma and BMS-Pfizer. P Corbaux reports non-financial support from Astra-Zeneca and Novartis; outside the submitted work. G Poenou reports personal fees and non-financial support from Viatris; personal fees and non-financial support from Bayer; personal fees and non-financial support from BMS-Pfizer; grant, personal fees and non-financial support from Léo-Pharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–464. doi: 10.1001/archinte.166.4.458
- Horsted F, West J, Grainge MJ, et al. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLOS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275
- Bertoletti L, Madridano O, Jiménez D, et al. Cancer-Associated thrombosis: trends in clinical features, treatment, and outcomes from 2001 to 2020. JACC Cardio Oncol. 2023;5(6):758–772. doi: 10.1016/j.jaccao.2023.09.003
- Eckardt JL, Wanek MR, Udeh CI, et al. Evaluation of prophylactic antibiotic use for delayed sternal closure after cardiothoracic operation. Ann Thorac Surg. 2018;105(5):1365–1369. doi: 10.1016/j.athoracsur.2017.12.012
- Shargall Y, Schneider L, Linkins LA, et al. Double blind pilot randomized trial comparing extended anticoagulation to placebo following major lung resection for cancer. Semin Thorac Cardiovasc Surg. 2021;33(4):1123–1134. doi: 10.1053/j.semtcvs.2021.02.032
- Poenou G, Dumitru Dumitru T, Lafaie L, et al. Pulmonary embolism in the cancer associated thrombosis landscape. J Clin Med. 2022 sep 25;11(19):5650.
- Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100(10),3484–3488. doi: 10.1182/blood-2002-01-0108
- Mahé I, Chidiac J, Bertoletti L, et al. The clinical course of venous thromboembolism may differ according to cancer site. Am J Med 2017;130(3),337–347. doi: 10.1016/j.amjmed.2016.10.017
- Bertoletti L, Gusto G, Quignot N, et al. Low molecular weight heparin treatment patterns and outcomes in cancer patients with acute venous thromboembolism: a nationwide cohort study in France. Cancers (Basel). 2023;15(11):3011. doi: 10.3390/cancers15113011
- Carrier M, Cameron C, Delluc A, et al. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: a systematic review and meta-analysis. Thromb Res 2014;134(6),1214–1219. doi: 10.1016/j.thromres.2014.09.039
- Laporte S, Bertoletti L, Romera A, et al. Long-term treatment of venous thromboembolism with tinzaparin compared to vitamin K antagonists: a meta-analysis of 5 randomized trials in non-cancer and cancer patients. Thromb Res 2012;130(6),853–858. doi: 10.1016/j.thromres.2012.08.290
- Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med 3821599–1607 (2020). 17 10.1056/NEJMoa1915103
- Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 378615–624 (2017). 7 10.1056/NEJMoa1711948
- Planquette B, Bertoletti L, Charles-Nelson A, et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism: a randomized trial. Chest. 2022;161(3),781–790. doi: 10.1016/j.chest.2021.09.037
- Agnelli G, Muñoz A, Franco L, et al. Apixaban and Dalteparin for the treatment of venous thromboembolism in patients with different sites of cancer. Thromb Haemost. 2022;122(5):796–807. doi: 10.1055/s-0041-1735194
- Mulder FI, van Es N, Kraaijpoel N, et al. Edoxaban for treatment of venous thromboembolism in patient groups with different types of cancer: results from the Hokusai VTE cancer study. Thromb Res. 2020;185, 13–19. doi: 10.1016/j.thromres.2019.11.007
- Petit B, Soudet S, Poenou G, et al. Cancer-associated thrombosis: how many patients seen in clinical practice would be eligible for a direct oral anticoagulant randomized controlled trial? Respir Med Res. 2023;85101069. doi: 10.1016/j.resmer.2023.101069
- Lafaie L, Hodin S, Saïb S, et al. Tyrosine kinase inhibitors and direct oral anticoagulants: in vitro evaluation of drug–drug interaction mediated by P‐glycoprotein. Fundam Clin Pharmacol. 2022;36(5):860–868.
- Carrier M, Blais N, Crowther M, Kavan P, Le Gal G, Moodley O, et al. Treatment algorithm in cancer-associated thrombosis: updated Canadian expert consensus. Curr Oncol. 2021;28(6):5434–5451. doi: 10.3390/curroncol28060453