ABSTRACT
Introduction
There has been a paradigm shift in the treatment of chronic lymphocytic leukemia (CLL) over the past decade. With the advent of self-administered targeted oral anticancer agents (OAAs), the treatment of CLL has begun to shift from the infusion clinic to the patient’s home. This introduced new challenges including patient non-adherence, class-specific adverse effects, and financial toxicity to treatment. In this paper, we discuss a structured approach to identifying and addressing barriers to optimal patient outcomes.
Areas covered
We will ground our discussion using the five dimensions of adherence as defined by the World Health Organization (WHO): therapy factors, health-system factors, condition-related factors, social/economic factors, and patient factors. We discuss how each of these domains present in patients with CLL. We will also discuss how we can prevent and address these barriers in through the various phases of treatment.
Expert opinion
A multidisciplinary program to support patients on OAAs is critical for patients with CLL. This team should involve pharmacists and social workers in addition to nursing, advanced practitioner and physician colleagues. The program should aim to identify, prevent, and address patient-specific barriers by offering individualized solutions. We describe how such a program can be designed and implemented.
1. Introduction
The last ten years have marked a large shift in the standards of care in the treatment of chronic lymphocytic leukemia (CLL). Standards of care have shifted from cytotoxic chemotherapies to treatments that target small molecules, reducing the treatment toxicity profile [Citation1]. Novel small molecule agents have allowed CLL treatment to be self-administered in oral dosage forms. The availability of oral dosage forms has moved CLL treatment into the home setting, providing patients increased autonomy over treatment, but introducing a myriad of new challenges such as financial toxicity, reduced adherence, and the possibility of drug–drug interactions with home medication regimens. Treatment of CLL with oral agents requires a high rate of adherence to maintain progression free survival (PFS). Patients with CLL have been shown to require >90% adherence to achieve major cytogenic response [Citation2,Citation3]. Unfortunately, adherence to oral anticancer agents (OAA) in practice has been found as low as 40% due to medication access barriers, interruptions in treatment due to unmanaged regimen toxicities, forgetfulness, and fear [Citation4]. Health systems can develop and implement multidisciplinary OAA adherence programs to improve outcomes of patients with CLL by addressing both social barriers and addressing treatment toxicities.
OAAs have become a large part of the standard of care for CLL and are interwoven into the National Comprehensive Cancer Network’s (NCCN) treatment algorithm since the approval of ibrutinib, the first targeted OAA approved for use in patients with CLL [Citation5]. Ibrutinib, as well as other Bruton tyrosine kinase inhibitors (BTKi) on the market, have been found to be superior to chemoimmunotherapy as a first-line treatment of CLL [Citation6]. Ibrutinib (2014), acalabrutinib (2019), and zanubrutinib (2023) are Bruton kinase inhibitors (BTKis) used in CLL treatment to prevent B cell surface receptor signaling to prevent B-cell trafficking, chemotaxis, adhesion, proliferation, and survival [Citation5,Citation6]. Pirtobrutinib (2023) is a BTKi that bonds in a non-covalent manner to BTK (unlike other agents in class) and thus can be effective in patients who have failed the other (covalent) BTKis [Citation7]. The use of all BTKis is marked by an increased risk of bleeding, cardiovascular effects (atrial fibrillation, arrhythmias, hypertension (covalent BTKis only)), and cytopenia (infection) [Citation6]. The administration of BTKi inhibitors is complicated by inhibition of CYP3A, making consideration of drug–drug interactions (DDIs) and hepatic function essential during care. Regimens are fairly uncomplicated, requiring daily (ibrutinib, zanubrutinib, pirtobrutinib) or twice daily oral dosing (acalabrutinib, zanubrutinib) with or without food. Certain BTKis have data in combination with monoclonal antibodies, although the additional benefit of monoclonal antibodies may be marginal [Citation5].
Venetoclax (2019) is a B-cell lymphoma 2 (BCL-2) inhibitor that restores apoptosis in tumor cells, preventing tumor survival [Citation8]. Venetoclax is used in combination with obintuzumab or ibrutinib as first-line therapy and approved with rituximab as second-line therapy. Venetoclax can be used as monotherapy as a third line option [Citation5]. The use of venetoclax is associated with tumor lysis syndrome (TLS), cytopenia, and infection. Patients begin venetoclax by following a 5-week oral dose-ramp up schedule designed to gradually debulk the tumor without increasing the risk of tumor lysis syndrome, complicating the process of initiating treatment [Citation8]. Following the initial ramp up, venetoclax dosing is daily, but must be taken at the same time each day with a meal. CYP3A metabolism further complicates venetoclax administration, requiring DDI examination and dose reduction in patients with hepatic impairment [Citation8].
Anti-CD20 monoclonal antibodies obinutuzumab (2013) and rituximab (2009) are used in patients with CLL in combination with BTKis, BCL-2 inhibitor (venetoclax), or as monotherapy (Obinutuzumab) [Citation5]. Anti-CD20 antibodies bind with the CD20 cell surface antigen on B lymphocytes, control the rate of B-cell lysis by causing direct cell death, activate the complement cascade, and modify immune response [Citation9]. These agents are generally infused on Day 1 of a 28-day cycle for six cycles (Obinutuzumab patients have four daily infusions during the first cycle while rituximab patients are given a lower dose intensity for the first infusion) [Citation9]. These agents are associated with infusion reactions (requiring pretreatment prophylaxis), hypersensitivity, Hepatitis B reactivation, arrhythmia, bowel obstruction, and cytopenia (infection) [Citation9].
Duvelisib (2018) and Idelalisib (2014) are phosphoinositide 3 kinase (PI3K) inhibitors, used with rituximab (idelalisib) or as monotherapy (duvelisib) in patients with CLL resistant to BTKi and venetoclax-based regimens [Citation5]. PI3K inhibitors induce apoptosis and prevent chemotaxis by inhibiting PI3K (expressed in malignant B-cells), and binding to several different cell signaling pathways (B-cell receptor, multiple chemokine CXC receptors) [Citation10]. CYP3A interactions complicate treatment with consideration needed for drug–drug interactions and reduction in dose for patients with hepatic impairment. Treatment with PI3K inhibitors could possibly cause severe cutaneous reactions, cytopenia, hepatotoxicity, infection, colitis, or pneumonitis [Citation10]. The dosing regimen for PI3K inhibitors is straightforward, twice daily with or without food [Citation10].
2. Reasons for nonadherence with oral anticancer agents
As we elucidate the reasons for patient nonadherence to CLL therapy, we will utilize the World Health Organization (WHO)’s framework conceptualizing five ‘dimensions’ previously identified to affect patient ability to adhere to therapy [Citation11]. These dimensions include (1) health system/team factors, (2) social/socioeconomic factors, (3) condition-related factors, (4) patient-related factors, and (5) therapy-related factors [Citation11]. We will identify changes in these barriers throughout the patient’s journey through two phases of treatment: (1) Pre-Treatment Initiation and (2) Post-Treatment Initiation. For the purposes of this paper, pre-treatment initiation will begin at the writing of the prescription and end with the patient receiving medication. Post-treatment initiation will begin after receiving medication, ending when treatment is discontinued. Common reasons for patient non-adherence (barriers) to therapy are listed in separated by the WHO adherence dimension the barrier is classified into. categorizes the barriers into the phase in treatment where they are expected to have the greatest effect on adherence to OAA therapy.
Table 1. Interventions needed to support OAA adherence throughout the course of treatment.
Table 2. Reasons for oral anticancer agent (OAA) nonadherence.
2.1. Health system/health care team factors
2.1.1. Lack of coordination of care
Treatment initiation can be delayed due to delays in receiving standard of care prophylaxis, appropriate laboratory monitoring, and delays in entry of clearance for treatment to the Risk Evaluation Mitigation Strategies (REMS) programs of the respective medications, if needed [Citation11,Citation13,Citation16,Citation19].
Treatment initiation may be also be delayed if the prescription is sent to a pharmacy that is contracted to fill for the patient’s insurance [Citation19]. Most OAAs are dispensed by mail order specialty pharmacies. For certain patients, delivery coordination may prove difficult due to the need for a phone call between the pharmacy and patient to arrange medication shipment (i.e. if the pharmacy does not confirm address and speak with the patient, the drug will not be sent to the patient) [Citation19]. Utilization of external specialty care pharmacies – as opposed to medically integrated dispensing pharmacies affiliated with health systems – is thought to contribute more to delays in treatment largely due to the lack of system integration and lack of the clinical team’s inability to easily track timely drug delivery and treatment initiation [Citation16].
Treatment initiation may be delayed or interrupted by lack of care coordination for complicated regimens. For example, during initiation of the venetoclax/Obinutuzumab regimen for CLL, the 5-week ramp up schedule in Cycle 1 to prevent TLS consists of one week of an initial 20 mg dose followed by ramping up the daily dose weekly in the following four weeks (50 mg, 100 mg, 200 mg and 400 mg) [Citation8]. This must be carefully coordinated with administering increasing doses of Obinutuzumab intravenously [Citation8]. Careful coordination by the care team is required to schedule infusions, educate about the changing venetoclax doses, and administer the proper dose of obinutuzumab.
2.1.2. Lack of communication with care team
Treatment with OAA can be marked by significant side effect burden, and patient reported outcome measures (PROM) have been developed to assess symptom burden; however, systems must be established for the outcomes to be reported to healthcare providers to provide treatment optimization and side effect mitigation strategies [Citation13,Citation20]. Patients taking OAA reporting a high side effect burden (>4 severe side effects) have been found to have reduced adherence rates of <70% (optimal for CLL ≥ 90%) [Citation2,Citation3,Citation13,Citation20].
Many medications that have changed the therapeutic landscape of CLL including PI3K inhibitors (idelalisib, duvelalisib) and BTkis (ibrutinib, Zanubrutinib, acalabrutinib, pirtobrutinib) have significant side effect profiles as outlined in . If adverse effects to these medications are not reported and addressed promptly, it could lead to worsening symptoms and decreased adherence rates [Citation21]. Unmanaged adverse events often lead to dose reductions and discontinuations due to immune mediated disorders and infections. Optimization of efforts to manage side effects of during treatment with therapeutic agents such as BTkis and PI3Kis has not been achieved and represents an unmet need in CLL treatment. There is often a lack of established communication plans for dissemination of practical side effect management recommendations in CLL treatment [Citation21].
Table 3. Adverse events associated with targeted anticancer therapies.
Structured patient monitoring models such as the Pharmacists Coordinated Care Oncology Model (PCOM) allow intervention starting at 2 weeks following OAA initiation, the collection of patient reported outcome measures, and scheduled comprehensive medication review [Citation13]. Patients with comorbidities are at high risk for drug–drug interactions (DDIs) (complicated medication regimens) and drug–disease interactions (changing organ function) so they can benefit from structured interventions such as PCOM.
2.1.3. Unmanaged drug–drug and drug–disease interactions (DDIs)
DDIs that are not identified and managed lead to patients receiving sub or supratherapeutic doses of medication that can result in unintentional reduction in dose intensity or excessive toxicities that result in premature medication discontinuation or gaps in therapy. DDIs with QTc prolonging agents, anticoagulants, enzyme inducers and inhibitors, antidepressants, and acid suppressants are commonly encountered with anticancer therapies [Citation22]. Comprehensive medication reviews (CMRs) with healthcare providers, are used to obtain a complete medication list, identify, and resolve various medication-related problems [Citation13].
As outlined in , many of the medications used to treat CLL (BTKis, PI3Kis, venetoclax), undergo metabolism with CYP3A enzymes, leading to significant potential for interactions with other prescribed medications, over the counter (OTC) medications, and dietary supplements such as St. John’s wort. Identification of newly added CYP3A inducers or inhibitors to the medication regimen of patients with CLL, and medication regimen optimization to address potential DDIs is crucial to maintaining the optimal dose intensity, and thus the efficacy of BTKis, PI3Kis, and venetoclax.
Table 4. Oral anticancer agent dosing considerations.
BTKis have been shown to cause atrial fibrillation and arrhythmias while also causing thrombocytopenia, as shown in . Direct oral anticoagulants (DOACs), or at a minimum, aspirin, are often recommended as prophylaxis for patients with CLL at risk for experiencing arrhythmia, taking lenalidomide, or exhibiting another thrombogenic factor [Citation5]. Patients on warfarin were excluded from clinical trials for CLL efficacy and safety for acalabrutinib and ibrutinib, so it must be ensured that patients taking those medications are offered alternative antiplatelet or anticoagulation medications [Citation5]. Benefit-risk must be considered when using anticoagulants in patients with CLL on BTKis due to their increased risk of bleeding secondary to platelet dysfunction [Citation5].
2.1.4. Lack of access to social services
Patients with social barriers that are not addressed may delay treatment initiation or remain non-adherent to treatment due to competing priorities. In the social determinants of health domains of social context, economic stability, and neighborhood/physical environment, prevalent needs causing medication nonadherence have been found to include lack of support to perform daily activities, lack of funds to pay bills, food insecurity, housing instability, employment instability, transportation barriers, and isolation [Citation17]. Social needs surveys will need to be included in patient care support models to assess and address psychosocial barriers [Citation17]. Tools such as the Health-Related Social Needs Screening (HRSN) tool developed by the Centers for Medicare and Medicaid Services (CMS) used to systematically find and deal with the health-related social needs of healthcare beneficiaries including housing instability, food insecurity, transportation problems, utility help needs, and interpersonal safety [Citation26]. HRSN is being used to inform patients’ treatment plans and make referrals to community services to deal with unmet need [Citation26]. Once identified, a multidisciplinary adherence initiative must be designed to connect patients with social support systems to address identified barriers including social workers, assistance with obtaining insurance coverage, and patient assistance programs.
Patients with CLL have an increased likelihood of financial toxicity due to the high cost of the agents used for CLL treatment. Ibrutinib (provided as 420 mg tablet) has an average wholesale price (AWP) of $680.72USD per tablet. This results in an average monthly cost of $20,422USD [Citation27]. Venetoclax (provided as a 100 mg tablet for maintenance dosing) has AWP of $152.66 per tablet. Once treatment is initiated, patients require 400 mg daily, resulting in an average monthly cost of $18,319 USD [Citation27]. Idelalisib (provided as a 150 mg tablet) has an AWP of $276.16USD. This results in an average monthly cost of $16,570USD due to 150 mg twice daily dosing. Even with insurance coverage, there is often a large co-pay that is required for patients to obtain the medication – particularly for patients with Medicare Part D. Patients without insurance likely will not have access to these medications due to the large financial access barriers outside of manufacturer-sponsored free drug programs for drugs.
2.2. Therapy-related factors
2.2.1. Treatment toxicities
In past studies, OAAs have been associated with significant symptom burden with 56% of patients taking OAAs reporting moderate-to-severe symptoms and 23% reporting 4 or more severe symptoms [Citation11,Citation13,Citation20]. Common adverse events to the medications typically used in the treatment of CLL (BTKis, anti CD20, antibodies, BCL2 inhibitors, and PI3Kis, and anti CD52 antibodies) are listed in . Patients may intentionally skip or reduce doses of medications to avoid side effects without reporting changes to their healthcare team [Citation28].
Patients who do not receive education to effectively self-manage or timely report side effects are more likely to suffer unnecessary toxicities and become nonadherent [Citation29]. Adherence rates have been found not to be significantly correlated to the mean severity of the total side effects of OAA at any time point, but instead was correlated with the mean effectiveness of self-management side effects [Citation29]. The multidisciplinary intervention must provide effective self-management strategies to patients to decrease the likelihood of nonadherence. Standard of care prophylaxis for treatment toxicities can reduce the likelihood of side effects and the reduction or skipping of doses [Citation5].
2.3. Social/economic factors
2.3.1. Lack of understanding about treatment (low health literacy)
Misunderstanding about the timing of drug, requirement to take the drug with food and difficulty understanding the labeling instructions have been shown to be some of the most patient-identified barriers associated with the use of oral chemotherapies [Citation11,Citation12,Citation28]. As previously discussed, patients who have not been educated to expect and self-manage common side effects may skip or reduce doses of medication [Citation29]. Patients’ expectations for treatment outcomes and their health status may affect their adherence to OAA treatment [Citation29]. Those who reported better knowledge of the impact of non-adherence on their disease and treatment are more likely to be adherent [Citation15].
It has been found that patient reported adherence to OAA can be improved through education to increase comprehension of OAA treatment (leading to increased molecular response rate) when combined with scheduled provider interactions that allowed for reporting and intervention of adverse events [Citation12].
Patients diagnosed with CLL have a median age at diagnosis of 65–70 years of age [Citation30]. Advanced age could contribute to cognitive decline and increase the likelihood of nonadherence due to a lack of understanding of how to properly take OAAs. CLL and other lymphoproliferative diseases are thought to cause cognitive decline in patients, also making nonadherence more likely due to lack of understanding of how a medication must be taken [Citation31]. Additional support is needed to assist with ensuring understanding of how medication should be taken to increase adherence to OAAs.
2.3.2. Financial toxicity of OAA
The high cost of oral chemotherapy medications used to treat CLL, even after insurance payment, can be an access barrier for patients. As mentioned earlier, the costs of many classes of oral medications used for the treatment of CLL impose a large financial burden on patients with CLL, serving as a major access barrier for many patients [Citation17]. >80% of patients report severe or moderate financial toxicity with OAA treatment [Citation32]. Lack of insurance, underinsurance, lack of employment, or inability to enroll in a medication assistance program are financial barriers leading to nonadherence to treatment. Changes to employment status, changes in insurance (i.e. aging into Medicare), and changes in financial status throughout treatment can also increase nonadherence if an established payment arrangement for a medication becomes obsolete. Households with income <$50K are associated with reduced adherence to OAA in patients with hematologic malignancies [Citation14].
Due to the high cost of medication, there are patient assistance programs (PAPs) that have been developed and are sponsored by pharmaceutical companies or foundations to provide free or affordable medication. Barriers to accessing PAPs include eligibility criteria, having Medicare insurance benefits (industry sponsored PAPs), lack of pharmacy/physician office staffing, and difficulty of financial navigators identifying patients who would benefit from each program [Citation33]. Many insurance companies require prior authorization processes to be completed for covered, high cost OAAs, which can be time-intensive for staff and lead to substantial delays in treatment initiation.
A multidisciplinary intervention must address access barriers created through financial toxicity of OAA through access to PAPs, timely processes for completing prior authorization processes, and social support to obtain adequate insurance coverage.
2.3.3. Socioeconomic disparity
As was discussed in the ‘Lack of Access to Social Services’ section; factors affecting the social determinants of health domains of social context, economic stability, and neighborhood/physical environment can serve as barriers to treatment. Lack of stable housing could create barriers to receiving medication shipment from specialty pharmacy. Patients may have reduced adherence as transportation, social support, or food and housing stability continue to be barriers the patient must overcome [Citation17].
Additionally, living arrangements and social support were associated with level of adherence to OAA in patients with hematological malignancy, with higher levels of social support associated with higher adherence [Citation14].
2.3.4. Low health literacy
Patients with low health literacy may not understand how to follow complicated treatment regimens or instructions for side effect self-management, pertinent to maintaining adherence with medications with many known toxicities [Citation29]. Patients with a secondary level of education or higher report higher levels of adherence compared to those with a lower level of education [Citation14]. The primary barriers identified with OAA use are difficulties with understanding directions for taking medication as aforementioned [Citation28]. Health literacy is also associated with important self-management behaviors in cancer care including the receipt of prescribed chemotherapy [Citation34].
2.4. Patient-related factors
2.4.1. Forgetfulness
Forgetting to take OAA medication as prescribed is a common reason for patient non-adherence in hematological cancers [Citation11,Citation14]. Patients often struggle to make taking medications a part of their routine, may not be knowledgeable about medication reminder techniques, and may not have caregivers or family/friends to assist with medication reminders.
Effective reminder techniques for reducing OAA forgetfulness include using pill dosage boxes and building medication adherence into the daily routine, storing the medication in a visual and commonly used area, and the use of clear and monitored treatment schedules to prompt medication taking behavior [Citation14]. Patients can be trained to utilize these strategies as a part of the structured multidisciplinary intervention.
2.4.2. Medication hesitancy/fear
Patients are often nonadherent to treatment due to fear of chemotherapy medication and its potential side effects. Fear of oral chemotherapy can have a strong influence on the behaviors surrounding taking the therapy [Citation15]. Patients who perceive themselves as having a higher functional status were also more likely to be nonadherent, not perceiving themselves as needing treatment [Citation14]. The only way to improve the patients’ fear of the medication and provide prospective about the potential for the worsening of prognosis without therapy is through educational efforts [Citation15]. Side effect self-management education has been previously discussed. Both side effect self-management and the role of treatment in prevention of disease prevention must be incorporated into the educational portion of the multidisciplinary intervention.
2.5. Condition-related factors
Patients with active CLL often have disease-related symptoms that can affect activities of daily living and self-sufficiency including unintentional weight loss of >10% in a six-month period, significant fatigue interfering with work and usual activities, prolonged fevers ≥100.5°F for longer than 2 weeks and night sweats lasting ≥1 month in the absence of infection [Citation11,Citation18]. Early in the treatment course, the condition-related factors may exacerbate medication-related toxicities and potentially increase OAA non-adherence.
3. Expert opinion: mitigating adherence barriers
3.1. A multidisciplinary intervention is needed
A multidisciplinary intervention must be developed that addresses the barriers identified above which are known to prevent adherence to oral anticancer agents used in the treatment of patients with CLL. The program should aim to identify, prevent, and address patient-specific barriers by offering individualized solutions. Because this initiative must help patients mitigate barriers across five domains of adherence, the structure must interweave the workflows of multiple care providers who represent different disciplines of oncology care providers (i.e. pharmacy, social workers, nursing, physicians).
The intervention must be specific to the complications and challenges associated with CLL treatment including CYP3A interactions with many oral agents AND nonclinical barriers, such as financial toxicity, low health literacy, and other socioeconomic barriers faced by patients with CLL.
A possible structure for such an intervention is outlined in , which details strategies for supporting patients with barriers that arise throughout the course of treatment.
Figure 1. A possible structure for a multidisciplinary intervention to support patients with CLL with oral anticancer agent (OAA) adherence. In the pRE-TREATMENT INitiation phase (writing of the prescription until patient receives medication), support begins with referrals to social services as needed, detailed medication education, management of OAA financial toxicity, and ensuring care coordination (ordering of labs, referral to specialty care pharmacy, ordering prophylactic medications, REMS compliance when needed, and scheduling follow ups). In the post- treatment initiation phase (patient receipt of medication until discontinuance of medication), support begins with a check-in 2 weeks after the first dose of medication taken: PROs, CMR and social needs assessment conducted. Patients found nonadherent due to forgetfulness or hesitancy will be provided education. Toxicities will be managed, potential DDIs will be addressed, and medication regimen will be simplified as needed. Patients found to have SDoH barriers will be referred to social services and financial toxicities will be managed. Following the initial check in 2 weeks after initiating therapy, follow ups will be scheduled every 2–4 weeks until patient is stable on treatment. Patients stable on treatment will have follow ups every 3–6 months until discontinuation of treatment.
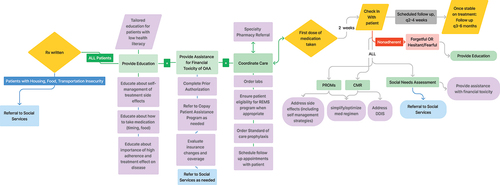
3.2. Components of the initiative
3.2.1. Pre-treatment initiation phase
During treatment initiation support must be provided beginning during the visit in which treatment is prescribed and spanning until the patient obtains medication, to overcome adherence barriers.
The health system/health care team will need to provide access to social services to overcome Social Determinants of Health (SDoH) barriers, facilitate coordination of care between the care team and specialty care pharmacy, facilitate the collection of labs for prescription initiation, clear patients in the appropriate REMS systems, provide standard of care prophylaxis, and ensure completion of a CMR to reconcile pretreatment medications/comorbidities for DDIs.
Clinicians must provide education to patients about the medication’s effect on disease state, education on effective side-effect self-management strategies, and education about the effects of adherence on disease progression to proactively address social/economic barriers. To address the socioeconomic barriers before treatment initiation, the healthcare team must assist patients with addressing financial toxicity of treatment; providing prior authorization for therapy, facilitating enrollment in patient assistance programs, and assisting with obtaining adequate insurance coverage when possible. Clinicians should provide referrals to social services and additional education to patients identified as having low health literacy to address SDoH barriers.
3.2.2. Post-treatment initiation phase
After treatment initiation and throughout the duration of therapy, starting the first day that a patient takes the medication, the clinical team should provide sustained support focused on identifying and addressing medication nonadherence.
During the post-treatment initiation phase, the health system/health care team must eliminate barriers such as coordination of care for laboratory monitoring, ensuring maintenance of REMS requirements (if relevant), and maintaining scheduled communication with the patient. Scheduled communications with the care team can utilize structured assessment approaches such as PROs and CMRs throughout treatment to uncover bothersome symptoms and DDIs, respectively. SDoH barriers can be identified using social needs surveys and can be incorporated into scheduled medication reviews.
To address the therapy-related factors in the treatment of CLL, the high side effect/toxicity burden barrier can be overcome through scheduled communications with the care team during which reeducation on self-management can be given as needed and frequent regimen optimization can occur.
The healthcare team can address social/economic barriers of financial toxicity by providing assistance program access, timely prior authorization, and help with insurance changes as needed. The care team should support patients with SDoH barriers of socioeconomic disparity through providing referrals to social services and support patients with low health literacy through providing additional supportive education.
Clinicians can address patient-related factors of fear/hesitancy and forgetfulness through education about side effect self-treatment, strategies to reduce OAA forgetfulness, and education about disease prognosis with treatment.
3.3. Structure of the initiative
The adherence support provided will need to be structured to allow the action needed by the care team identified to resolve each cause of nonadherence. It is thought that 100% of the patients should be counseled in a standardized way upon starting oral anticancer agents and that there should be periodic check-ins at prespecified increments throughout treatment [Citation20]. Suggested timing of intervention throughout treatment is outlined below. As outlined in the Pharmacist Care in Oncology (PCOM) model, follow-up communications with a member of the care team begin within 2 weeks of beginning therapy [Citation20].
Article highlights
Elucidates barriers to oral anticancer agent (OAA) adherence in patients with chronic lymphocytic leukemia (CLL) in the context of the WHO five dimensions of adherence
Highlights treatment toxicities and access barriers unique to CLL patients that prevent treatment adherence
Barriers to adherence separated by phase in treatment (pre- and post-treatment initiation)
Objectives of multidisciplinary intervention needed to support medication adherence in patients with CLL explained
Provides possible structure of a multidisciplinary intervention to support OAA adherence in patients with CLL
Declaration of interest
B Muluneh serves as a consultant for Servier Pharmaceuticals. B Muluneh’s spouse is an employee and stockholder of Novartis Pharmaceuticals. M Upchurch is currently completing a postdoctoral fellowship sponsored by GSK, plc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Rivera D, Ferrajoli A. Managing the risk of infection in chronic lymphocytic leukemia in the era of new therapies. Curr Oncol Rep. 2022;24(8):1003–1014. doi:10.1007/s11912-022-01261-9
- Collins J 4th, Stump SE, Heiling H, et al. Impact of adherence to ibrutinib on clinical outcomes in real-world patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2022;63(8):1823–1830. doi: 10.1080/10428194.2022.2045597
- Barr PM, Brown JR, Hillmen P, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;129(19):2612–2615. doi: 10.1182/blood-2016-12-737346
- Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncology. 2016;21(3):354–376. doi: 10.1634/theoncologist.2015-0405
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. chronic lymphocytic leukemia/small lymphocytic lymphoma: version 3.2023. National Comprehensive Cancer Network; 2023 Sep 15. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf
- Imbruvica (ibrutinib). Package insert. Sunnyvale, CA: Janssen Biotech, Inc and Pharmacyclics; 2015.
- Jaypirca (pirtobrutinib). Package insert. Indianapolis, IN: Eli Lilly; 2023.
- Venclexta (venetoclax). Package insert. South San Francisco, CA: Genentech; 2016.
- Gazyva(Obintuzumab). Package insert. South San Francisco, CA: Genentech; 2013.
- Zydelig (idelalisib). Package insert. Foster City, CA: Gilead; 2014.
- Burkhart PV, Sabaté E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35(3):207.
- Muluneh B, Schneider M, Faso A, et al. Improved adherence rates and clinical outcomes of an integrated, closed-loop pharmacist-led oral chemotherapy management program. J Oncol Pract. 2018;14(6):e324–e334. doi: 10.1200/JOP.17.00039
- Farris KB, Cadwallader T, Farley J, et al. Implementation of a model integrating primary and oncology pharmacists’ care for patients taking oral anticancer agents (OAA). Explor Res Clin Soc Pharm. 2022;7:100163. doi:10.1016/j.rcsop.2022.100163
- Hall AE, Paul C, Bryant J, et al. To adhere or not to adhere: rates and reasons of medication adherence in hematological cancer patients. Crit Rev Oncol Hematol. 2016;97:247–262. doi: 10.1016/j.critrevonc.2015.08.025
- Iacorossi L, Gambalunga F, Fabi A, et al. Adherence to oral administration of endocrine treatment in patients with breast cancer: a qualitative study. Cancer Nurs. 2018;41(1):E57–E63. doi: 10.1097/NCC.0000000000000452
- Oncology Pharmacy Education Network. Care coordination: the role of pharmacy to help manage patients with cancer on oral oncolytics. Association Of Community Cancer Centers (ACCC). 2021;36(5):70–74.
- Almodóvar AS, Ross E, Nahata MC, et al. Social needs of dual-enrolled medicare-medicaid patients with medication nonadherence in a telehealth medication therapy management program. J Manag Care Spec Pharm. 2023;29(2):210–215. doi:10.18553/jmcp.2023.29.2.210
- Hallek M, Cheson BD, Catovsky D, et al. CLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398
- Anders B, Shillingburg A, Newton M. Oral antineoplastic agents: assessing the delay in care. Chemother Res Pract. 2015;2015:1–4. doi: 10.1155/2015/512016
- Mackler E, Petersen L, Severson J. Implementing a method for evaluating patient-reported outcomes associated with oral oncolytic therapy. J Oncol Pract. 2017;13(4):e395–e400. doi: 10.1200/JOP.2016.018390
- Cuneo A, Barosi G, Danesi R, et al. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: a multidisciplinary position paper. Hematol Oncol. 2019;37(1):3–14. doi: 10.1002/hon.2540
- Rogala BG, Charpentier MM, Nguyen MK, et al. Oral anticancer therapy: management of drug interactions. J Oncol Pract. 2019;15(2):81–90. doi:10.1200/JOP.18.00483
- Brukinsa (zanubrutinib). Package insert. BeiGene USA: Inc.; 2019.
- Calquence (acalabrutinib). Package insert. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2022.
- Copiktra (duvelisib). Package insert. Las Vegas, NV: Secura Bio; 2018.
- Billioux A, DPhil MD, Verlander K, et al. Standardized screening for health-related social needs in clinical settings: the accountable health communities screening tool. Nat Acad Med Persp. 2017;7(5):1–9. https://nam.edu/wpcontent/uploads/2017/05/Standardized-Screening-for-Health-Related-Social-Needs-in-Clinical-Settings.pdf
- Lexicomp Online. Pediatric and neonatal lexi-drugs online. Waltham (MA): UpToDate, Inc.; 2021 Jul 30 [cited 2024 Jan 24]. Available from: https://online.lexi.com
- Muluneh B, Deal A, Alexander MD, et al. Patient perspectives on the barriers associated with medication adherence to oral chemotherapy. J Oncol Pharm Pract. 2018;24(2):98–109. doi: 10.1177/1078155216679026
- Jiang Y, Wickersham KE, Zhang X, et al. Side effects, self-management activities, and adherence to oral anticancer agents. Patient Prefer Adherence. 2019;13:2243–2252. doi: 10.2147/PPA.S224496
- Parikh SA, Rabe KG, Kay NE, et al. Chronic lymphocytic leukemia in young (≤ 55 years) patients: a comprehensive analysis of prognostic factors and outcomes. Haematologica. 2014;99(1):140–147. doi: 10.3324/haematol.2013.086066
- Teregulova DR, Bakirov BA, Akhmadeeva LR. Vliianie affektivnykh i kognitivnykh narusheniĭ na kachestvo zhizni u patsientov s limfoproliferativnymi zabolevaniiami [An impact of affective and cognitive impairment on the quality of life in patients with lymphoproliferative diseases]. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119(4):5–8. [ Russian]. doi: 10.17116/jnevro20191190415
- Chen Y, Chen Z, Jin H, et al. Associations of financial toxicity with symptoms and unplanned healthcare utilization among cancer patients taking oral chemotherapy at home: a prospective observational study. BMC Cancer. 2023;23(1):140. doi:10.1186/s12885-023-10580-4
- Yezefski T, Schwemm A, Lentz M, et al. Patient assistance programs: a valuable, yet imperfect, way to ease the financial toxicity of cancer care. Semin Hematol. 2018;55(4):185–188. doi:10.1053/j.seminhematol.2017.07.004
- Papadakos JK, Hasan SM, Barnsley J, et al. Health literacy and cancer self-management behaviors: a scoping review. Cancer. 2018;124(21):4202–4210. doi: 10.1002/cncr.31733

