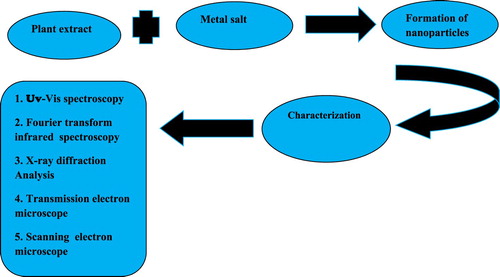
ABSTRACT
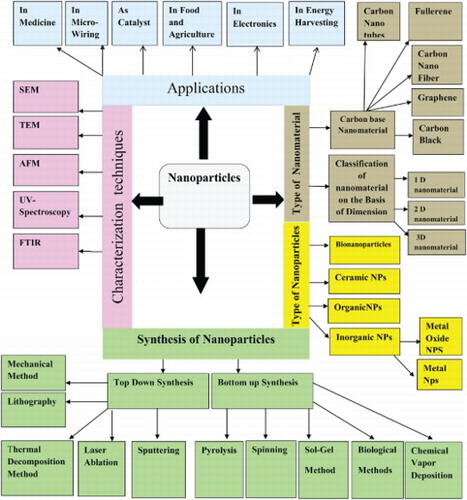
Nanotechnology is an emerging field of science. The base of nanotechnology is nanoparticles. The size of nanoparticles ranges from 1 to 100 nm. The nanoparticles are classified into different classes such as inorganic nanoparticles, organic nanoparticles, ceramic nanoparticles and carbon base nanoparticles. The inorganic nanoparticles are further classified into metal nanoparticles and metal oxide nanoparticles.similarly carbon base nanoparticles classified into Fullerene, Carbon nanotubes, Graphene, Carbon nanofiber and carbon black Nanoparticles are also classified on the basis of dimension such as one dimension nanoparticles, two-dimension nanoparticles and three-dimension nanoparticles. The nanoparticles are synthesized by using two approaches like top-down approach and bottom-up approach. In this review chemical, physical and green synthesis of nanoparticles is reported. The synthesized nanoparticles are synthesized using different qualitative and quantitative techniques. The Qualitative techniques include Fourier Transform Infrared Spectroscopy (FT-IR), UV-Vis spectrophotometry, Scanning electron microscope (SEM), X.ray diffraction (XRD) and Atomic Force Microscopy (AFM). The Quantitative techniques include Transmission Electron Microscopy (TEM), Annular Dark-Field Imaging (HAADF) and Intracranial pressure (ICP). The nanoparticles have different application which is reported in this review.
GRAPHICAL ABSTRACT
KEYWORDS:
1. Introduction
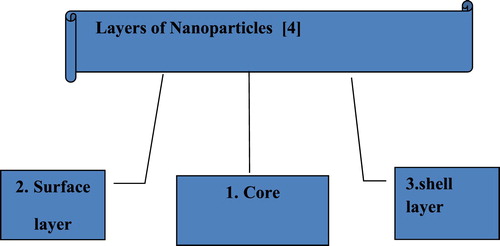
Nanotechnology is well-known area of research since the last century. Richard P. Feynman presented the word nanotechnology during his well-known lecture (1959) “There is a Plenty of Room at the Bottom” ( Citation1). Nano-scale Material of various types had been produced by nanotechnology. There are various class of nanoparticles. Nanoparticles are those particles whose size ranges between 1 and 100 nm ( Citation2). The nanoparticles can be 0D, 1D, 2D and 3D depend on the shape ( Citation3). The significance of these nanoparticles realized when researchers saw that size can affect the physio-chemical properties of substances such as optical properties. Nanoparticles are not simple molecules and consist of three layers shown in .
Figure 1. Different layer of nanoparticles ( Citation4).
2. History of nanoparticles
Although, it is considered that nanoparticles are finding of modern science but they consist of a lengthy history. From thousands of years, ancient people use nanotechnology. But nobody can clearly say when they first began to use advantages of nanoparticles in different fields.
Optical property is one of the major and fundamental properties of nanoparticles.
For example:
A silver NP has yellowish gray color.
A 20 nm-sized gold NP contains a typical red wine color.
The Platinum (Pt) and palladium (Pd) nanoparticles have black color.
Even before the fourth century AD, nanoparticles have been used in painting due to their optical characteristics. The most well-known example is the Lycurgus cup shown in .
Figure 2. Lycurgus cup that displays different colors ( Citation5).

This cup is placed in British Museum in London. This amazing cup is the complete historical example. It is made of an extraordinary type of glass that is known as the dichroic glass. It can change colors when the light falls on it. When the light incidents on the cup at 90° then the opaque green color of cup change into the glowing translucent.
This cup consists of a very small amount of crystals of gold and silver in the molar ratio of 1:14. These metal crystals give it infrequent optical properties. This Lycurgus Cup displays special color due to the presence of these nanocrystals.
Up to the Middle Ages, gold in soluble form was used as excellent healing powers of many diseases such as dysentery, epilepsy and heart diseases, dysentery, epilepsy. It was also helpful in the identification of disease like syphilis. Daniel and Astruc Ages has been summarized the nanoparticles,s history since ancient times to the Middle age( Citation6).
In 1618, Francisci Antonii was a philosopher and a medical doctor. He published a book on colloidal gold. This book has material on the methods of formation of colloidal gold sols and their uses in medical. In 1676, Kunckel established industry for manufacturing of stained glass by colloidal particles. He also published a book. In this book, he described “drinkable gold that contains metallic gold in a neutral, slightly pink solution that exerts curative properties for several diseases” ( Citation7).
In the seventeenth century, a colorant was used in glasses, which is, the “Purple of Cassius”, it was prepared by gold particles and tin dioxide. In 1718, Helcher published a complete article on colloidal gold ( Citation7).
In 1794, Fuhlame used colloidal gold to dye silkre. She reported it in her book ( Citation8). Jeremias Benjamin Richters explained in 1818 that various pink or purple gold solutions were drinkable and shows differences in color because gold particles were present in the best degree of subdivision in these solutions. Although, when fine particles were aggregated then the solutions were found in yellow color. In a famous publication, in 1857, Michael Faraday ( Citation9) described that colloidal gold gives deep red solutions when the reduction of aqueous solution of chloroaurate (AuCl4) by phosphorus in the presence of CS2 is completed. He also explored that thin films also show optical properties. He also observed that films can change reversible color from bluish-purple to green. Later on, various scientific papers have published on modification, properties, synthesis and assembly of metal nanoparticles. Then metal NPs used as different solvents and other substrates.
In various old churches, evidences of nanotechnology are easily available. A distinguished application of early nanotechnology was a ruby red color. During the middle ages, it had applications in stained glass windows.
But the medieval artisans did not know that they were using nanotechnology. Jin and coworkers recognized a particular process to produce a very beautiful color effect ( Citation10).
They explained the relation of particles and their colors that were associated with specific particles. They summarized the relationship between the color of the stained glass, shape and size of NPs.
Faraday prepared the first nanoparticle of gold. This gold particles is preserved in Royal Institution London.
In the German journal “Annalen der physic” (1908), Gustav Mie describes “how color of glass vary with size of metal”.
James Clerk Maxwell in 1867 gave a different concept of nanotechnology and proposed a tiny entity called “Maxwell Demon”. Color photography has been produced by him.
According to Maxwell “the color photography depends on the production of light sensitive nanoparticles”.
Actually, Chemical Catalysis is known as “Old nanotechnology”.
During the first decade of the twentieth century, ultra microscope with darkfield method was used by Richard Adolf Zsigmondy, through which he observed and measured the size of gold and other nanoparticles. First of all, he used the term of nanometer. He got Noble prize in 1925 in chemistry due to his unique work. In 1974, the word “Nanotechnology” was introduced by Nario Taniguchi. He explained the material whose size is less than millimeter ( Citation11).
Eric Drexler popularizes ( Citation12) is a popular scientist ( Citation13) in the field of nanotechnology ( Citation14).
He wrote the book “Engine of creation” in 1986. In this book, he firstly used a world of tiny mechanics and assemblers. With atomic-level precision, these tiny mechanics and assemblers construct a new structure in 1981, Gred Bining and Heinrich Rohrer discovered a scanning Tunneling microscope (STM) and they got the Noble prize in 1986. The position of individual surface atom can be determined by using (STM). In 1980 Richard E.Smalley and coworker discovered a Buckminsterfullerene. It is the molecule of carbon in soccer ball shaped. Buckminster fullerene is a buck ball which was man-made allotropes of carbon. Richard E. Smalley won the noble prize in 1996, because he discovered Bucminster. SumioIijima discovered carbon nanotubes ( Citation15).
3. Classification of nanoparticles
The nanoparticles are classified into different types on the basis of morphology, size and shape. some of the important classes of nanoparticles are mention in this review.
3.1. Organic nanoparticles
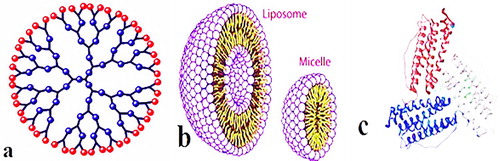
The organic nanoparticles include ferritin, micelles, dendrimers and liposomes show in . The organic nanoparticles are not toxic, biodegradable and some organic nanoparticles have a hallow sphere i.e. micelles and Liposomes. It is also familiar with name of nanocapsules which are heat and light sensitive ( Citation16). Organic nanoparticles are an ideal choice for drugs delivery due to these characteristics. Then nanoparticles are also widely used in target drug delivery. The organic nanoparticles are also known as polymeric nanoparticles. The most known shape of organic or polymeric nanoparticles is nanosphere or nanocapsule ( Citation17). The matrix particles are former overall mass of which is solid and outer boundary of spherical surface adsorb other molecules. In the later case, particles encapsulated the solid mass ( Citation18).
3.2. Inorganic nanoparticles
Carbon is not present in inorganic nanoparticles. The inorganic nanoparticles are not toxic. The inorganic nanoparticles are biocomaptiable and hydrophilic. The inorganic nanoparticles are highly stable than organic. The inorganic nanoparticles are classified into metal and metal oxide nanoparticles.
3.2.1. Metal nanoparticles
Metals are used to synthesized Metallica nanoparticles by using destructive or constructive methods. The metal precursors are used to make the pure metal nanoparticles. The metal nanoparticles possess unique optoelectrical properties due plasma on resonance characteristics. The synthesis of metal nanoparticles are controlled by shape, facet and size ( Citation19). The nanoparticles of all metals can be synthesized ( Citation20). The nanoparticles of aluminum, gold, iron, lead, silver, cobalt, zinc, cadmium and copper are well-known metal nanoparticles. Nanoparticles exhibited distinctive properties due to the small size (10–100 nm), surface properties such as surface area to volume ratio, surface charge, pore size, surface charge density, structure (crystalline and amorphous), shapes (spherical, rod, hexagonal, tetragonal, cylindrical and irregular), color and environmental factor (sunlight, moisture, air and heat).
3.2.2. Metal oxide nanoparticles
The purpose of the synthesis of metal oxide nanoparticles is to modify the property of their respective metals nanoparticles such as iron nanoparticles are oxidized to iron oxide nanoparticles. The reactivity of iron oxides nanoparticles is increased as compared to the iron nanoparticles. Due to an increase in reactivity and efficiency of metal oxide, the nanoparticles of metal oxides are synthesized ( Citation21). The example of metal oxide nanoparticles are zinc oxide, silicon dioxide, iron oxide, aluminum oxide, cerium oxide, titanium oxide and magnetite.
3.3. Ceramic nanoparticles
Ceramic nanoparticles are also known as nonmetallic solid. The ceramics nanoparticles are synthesized via heating or successive cooling. The ceramic nanoparticles may polycrystalline, amorphous, porous, dens or hollow form ( Citation22). The researcher focus on these nanoparticles due to their wide application such as photodegradation of dye, photocatalysis, catalysis and imaging applications ( Citation23).
3.4. Biological nanoparticles or bionanoparticles
Biological or Bio-nanoparticles is an assembly of atom or molecules which is prepared in the biological system having at least one dimension in the range of 1–100 nm. All bionanoparticles are naturally occurring nanoparticles. These nanoparticles are divide into two categories intracellular structure and extracellular structure. Magnetosomes is an example of intracillular structure and lipoproteins and viruses are examples of extracellular structure. Magnetosomes, exosomes, ferritin, lipoproteins and viruses are examples of bionanoparticles.
4. Nanomaterial
Nanomaterial is such material which have a structural component or unit less than one micrometer in at least one dimension. Building blocks (∼0.2 nm) of matter are considered nanomaterial.
4.1. Carbon-based nanomaterial
The carbon-based nanomaterials completely composed of carbon ( Citation24). Fullerenes, carbon nanotubes (CNT), graphene, carbon black and carbon nanofibers are the classes of carbon-based nanoparticles.
4.1.1. Fullerene
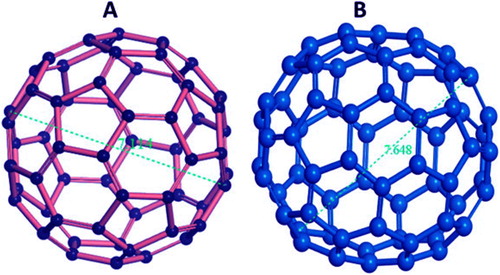
The fullerenes may be C60 or C70 Show in . The fullerenes consist of nanomaterial in the form of hollow cage. Due to their electrical conductivity, electron affinity, structure, strength and versatility, it becomes noteworthy commercial interest ( Citation25). Fullerenes consist of carbon units in the form of pentagonal and hexagonal shape. The carbon atoms in fullerenes are linked to each other by sp2 hybridized. The fullerenes made of C60 or C70 having diameter 7.114 and 7.648 nm. The fullerene may be single layer or multilayer.
4.1.2. Carbon nanotubes (CNT)
Carbon Nano Tubes are tubular elongated structure having 1–2 nm diameter ( Citation26). The carbon nanotube can be predicted as semiconducting or Metallica base on diameter ( Citation27). The structure of CNT resembles with graphite sheet rolling upon itself. On the basis of rolling CNT categorize into single walled (SWNTs), double walled (DWNTs) and multiwalled (MWNTs) show in .
Figure 5. Graphene sheet rolled into single wall nanotube, double wall nanotubes and multiple wall nanotubes.
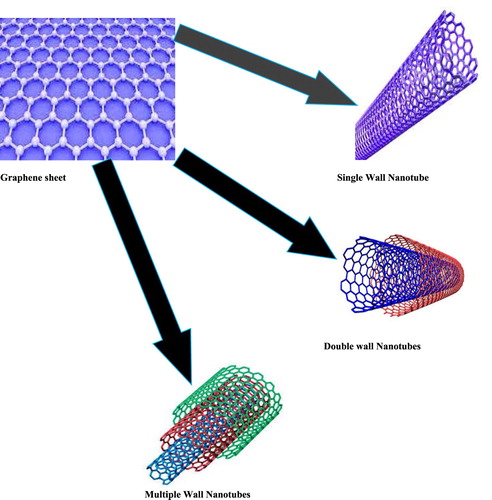
4.1.2.1. Single-walled carbon nanotubes (SWTs)
Single-walled Nanotubes (SWTs) consist of single rolled sheet. The least diameter of nanotubes for single walled is 0.7 nm.
4.1.2.2. Double-walled carbon nanotubes (DWNTs)
Double Walled Nanotubes (DWNTs) consist of double rolled sheet.
4.1.2.3. Multiwalled carbon nanotubes (MWNTs)
Multiwalled Nanotubes (MWNTs) consist of multiple rolled sheet. The least diameter of nanotubes for Multi-walled is 100nm.
The nanofoil of graphene having honeycomb carbon lattice is wound into a hollow cylinder to form nanotubes. The length of carbon tubes ranges from few micrometers to several millimeters. CNT is strong ( Citation28). CNT can be easily bent and regain its original shape without brittle when released. CNT shows various structure and shape, different thickness, length and number of layers ( Citation29) but the characteristics of CNT base on sheets of graphene.
4.1.3. Graphene
Graphene is an allotropic form of carbon. It is a hexagonal network of honeycomb carbon atom lattice having two dimensiona planar surface. The graphene is 1 nm in thickness.
4.1.4. Carbon nanofiber
The nanofoils of same graphene are transferred into carbon nanofiber as carbon nanotubes but nanofoils are wound into cup or cone instead of elongate cylindrical tubes.
4.1.5. Carbon black
It is the amorphous material which is made up of carbon. The shape of carbon black is spherical. The diameter ranges from 20 to 70 nm. High interaction between the particles is present and so bound in an aggregate show in .
4.2. Classification of nanomaterial on the basis of dimension
Nanomaterials are classified into one dimension (1D) nanomaterials, two-dimension (2d) nanomaterials and three Dimension (3D) nanomaterials.
4.2.1. One-dimension nanomaterial
The word Nano exhibited the number 109 ( Citation30) which shows the one billionth of any unit that results in the formation of one dimension nanomaterial such as thin film. These nanomaterials have various applications in the field of chemistry, pharmaceutics electronics and engineering( Citation31). The monolayers or thin-film size ranges from 1 nm to 100 nm. These nanomaterials have much importance in research and used in the field of fabricating electronics, storage system, LEDs with nanoscale dimension ( Citation32), optoelectronic, chemical and biosensing ( Citation33,Citation34), magnetooptics ( Citation35,Citation36), fiber optic system and optical devices. The one dimension nanomaterial play role in the construction of important materials at nanoscales such as nanotubes, nanobelt, nanowires ( Citation37), nano-ribbons ( Citation38,Citation39) and hierarchical nanostructures ( Citation40,Citation41).
4.2.2. Two-dimension nanomaterial
2D nanostructures have two dimension which is outside the size range of nanometric with peculiar shape and 2D nanomaterials are used as building blocks for key components of nanodevices ( Citation42). Two-dimension nanomaterials have applications in nanocontainer, sensorphotocatalysts, nanoreactor and template for 2D structure. Carbon nanotube is an example of two-dimension nanoparticles.
4.2.3. Three-dimension nanomaterial
The behavior of nanomaterials based on the shape size dimension and morphology which are the basic parameter for the application and performance of nanostructures ( Citation43). From the last 10 years, Three dimension nanomaterials have gain an interest in medical science and research. These nanomaterials have a wide range of applications in the field of catalysis, batteries, magnetic materials transport of reactant and product. The examples of three-dimension nanoparticles include Fullerenes, Dendrimers and Quantum dots.
5. Synthesis of nanoparticles
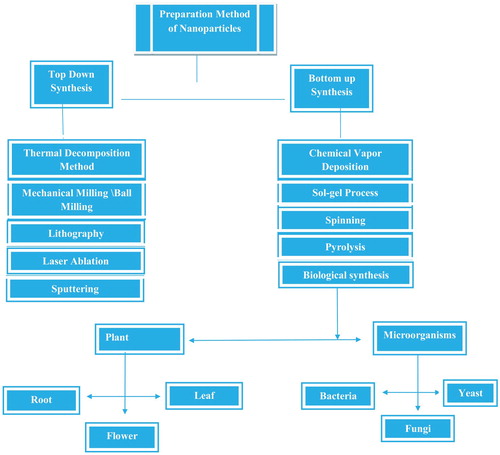
There are different methods for the synthesis of nanoparticles and these methods are divide into two main classes show in .
5.1. Top-down synthesis
In this synthesis, destructive method is used. The larger molecule (bulk material) decomposed into a smaller molecule and then these smaller molecules transform into the nanoparticles. Grinding or milling, physical vapor deposition and other destructive approaches are the example of Top-down synthesis ( Citation44). Coconut Shell nanoparticles are synthesized by using this method. Coconut Shell nanoparticles were synthesized using the milling method and raw coconut shell are finely ground for the different interval of time by using the planetary mill and ceramic ball. They observed that the overall size of nanoparticles affected by milling time through different characterizations techniques. It was observed that crystal-lite size of nanoparticles decreased with an increase in milling time as calculated by using Soherer equation. X-ray indicates that the particle size decrease with time. SEM result was also agreed with the X.ray pattern ( Citation45). One study showed the synthesis of magnetite having spherical shape from iron oxide by using top-down method ( Citation46). The top-down approach was used to synthesize colloidal carbon spherical particles having size range from 20 to 50 nm. The synthesis method was depended on chemical adsorption of polyoxometalates on the surface of interfacial carbon. Carbon black aggregates into smaller particles having spherical shape by adsorption shown in .
Figure 8. (a) The untreated carbon, (b) 10 mint (c) 1 h ultrasonication in POM solution ( Citation47).
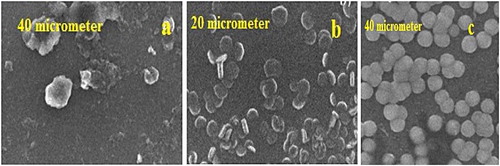
The micrograph showed that carbon particle size becomes smaller with sonication time. By the combination of grinding a sonication techniques, a series of transitional metal dichalcoenide nano dot (TMD-ND,s) from their bulk material. It showed that all the size of transitional metal dichalcoenide nano dot (TMD-ND,s) was smaller than 10 nm. The transitional metal dichalcoenide nano dot (TMD-ND,s) represent excellent dispersion due to narrow distribution of size ( Citation48).
5.1.1. Thermal decomposition method
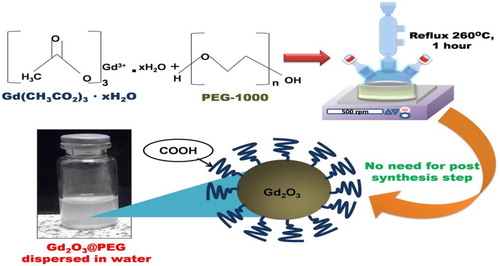
It is an endothermic process in which chemical decomposition is produced by heat. The chemical bond in compound is broken by this heat ( Citation20). The decomposition temperature may be defined as specific temperature at which element is chemically decomposed. The nanoparticles are consequences of decomposition of meta at specific temperature. Ahab, Atika, et al. Synthesized gadolinium oxide nanoparticles functionalized by paramagnetic polyethylene glycol by using thermal decomposition method shown in .
Figure 9. A simple straightforward thermal decomposition synthesis of PEG-covered Gd2O3 (Gd2O3@ PEG) nanoparticles ( Citation49).
5.1.2. Mechanical method/ball-milling method
It is inexpensive method to produce nanoparticles from bulk. Ball milling is simplest mechanical method. By attrition, ball milling produce nanoparticles. It is the method in which kinetic energy is transferred from medium of grind to the material which is under reduction. Material with enhanced properties is formed by using consolidation and compaction which is industrial scale process wherein nanoparticles are “put back together”. Alloys of different metals are made by this way. Different type of nanoparticles synthesized by using ball milling method shown in .
Table 1. Synthesis of different nanoparticles using ball milling method.
5.1.3. Lithographic methods
Lithographic methods are top-down methods which are capable of making for most part micron size feature but are energy intensive and requiring costly equipment. Lithography is used to make printed circuit and computer for several decade now. Nanoimprint lithography is type of lithography which is different from typical lithography. It is similar to the template synthesis. Firstly a template material is made and then soft polymeric material is stamped to form pattern. Top down method is used to make stamped material. Latex sphere is utilized by nanosphere lithography to form templated matric.
There are different type of lithography techniques, for instance, photo-lithography, electron beam lithography, soft lithography, focused ion lithography, nano-imprint lithography and dip pin lithography.photo-lithography involved contact an proximity printing and projection printing.
5.1.4. Laser ablation
Laser ablation synthesis in solution is simple techniques for the synthesis of nanoparticles from different solvent. The irradiation of different metal submerge in solution by laser beam condense a plasma to produce nanoparticles ( Citation59). It is very useful top down approach which is different from conventional chemical methods for the reduction of metal to nanoparticles. The stable nanoparticles synthesize using laser ablation techniques and do not require any stabilizing agent or chemical. Different types of nanoparticles synthesized by using Laser ablation shown in .
Table 2. Synthesis of different nanoparticles using laser ablation method.
5.1.5. Sputtering
Sputtering is the phenomenon of deposition of nanoparticles by means of ejection particles from it ( Citation76). Annealing is very useful for the deposition of nanoparticles thin layer. The size and shape of nanoparticles is determined from fallowing factors mention below
(i) Temperature
(ii) Thickness of the layer
(iii) Duration of annealing
(iv) Substrate etc (Citation77).
Different types of nanoparticles synthesized by using Sputtering show in .
Table 3. Different type of nanoparticles synthesized by using Sputtering.
5.2. Bottom-up method
Bottom-up method is also known as constructive method. It is the reverse of top-down method. In this method, nanoparticles are formed from relatively simpler substances. Bottom-up method includes Chemical vapor deposition (CVD), Sol–gel, spinning, pyrolysis and biological synthesis.
5.2.1. Chemical vapour deposition (CVD) method
It is the method in which a thin film of gaseous reactant is deposited on the substrate. The deposited thin film is carried out in a reaction chamber. A chemical reaction takes place as combining gas in contact with heated substrate ( Citation24). Thin film of product produced on the surface of substrate as a result of this reaction. This thin film is recovered and used. Hard, strong, uniform and highly pure nanoparticles are advantages of the CVD method. Requirement of special apparatus and formation of highly toxic gaseous as by-products are disadvantages of the CVD method ( Citation89).
Lee, Heejin, et al.synthesized and coated TiO2 by using the CVD method. They used titanium tetra iso-peroxide (TTIP) as precursor. SEM micrograph shown in and . illustrates the results of CVD coated beads, dip-coated beads and non coated beads. The dip-coated bead consisted of partially coated and un-coated areas and CVD coated bead had regular and uniform surface.
Figure 10. SEM Image of TiO2 coated beads (a) non-coated, (b) dip-coated, (c) CVD-coated with 10 min, (d) CVD-coated with 30 min, (e) CVD-coated with 60 min and (f) CVD-coated with 120 min ( Citation90).
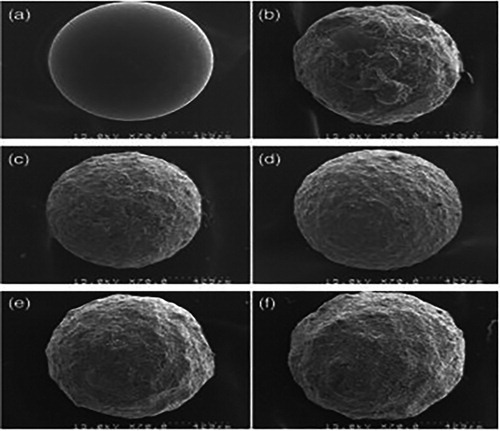
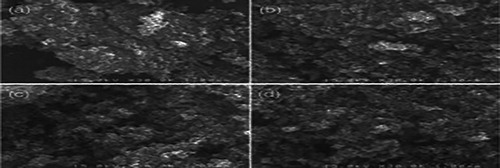
Figure 11 . SEM Image of CVD-coated bead with (a) 10 min, (b) 30 min, (c) 60 min and (d) 120 min. ( Citation90).
5.2.2. Sol–gel method
Sol–gel method is a combination of two words sol and gel. Sol is a colloid formed from solid particles suspended particles in continuous liquid. Gel is a solid macro-molecule which is dissolved in solvent. Due to simplicity, sol–gel method is the most preferred bottom-up method for the synthesis of nanoparticles. It is the method in which suitable chemical solution act as precursor. The typical precursors are metal oxide and chloride used in sol–gel method ( Citation91). By using different methods such as stirring, sonication and shaking, the precursor is dispersed in host liquid. The resultant solution consists of solid phase and liquid which is separated by using different techniques such as filtration, sedimentation and centrifugation to recover the nanoparticles show in .
Figure 12. The hydrolysis, condensation and calcination process of the sol-gel method in synthesizing the crystalline jnanatase, rutile and brookite TiO2 nanoparticles. ( Citation92).
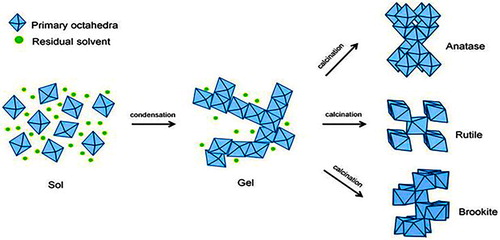
It is the transformation process of sol into gel. Its involve condensation and hydrolysis reaction shown in fig. The nanoparticles of TiO 2 is synthesized using different precursor such asTiCl3 ( Citation93), Ti[OCH(CH3)2]4 (TTIP) ( Citation94), TiCl4 ( Citation95) and Ti(OBu)4 ( Citation96)
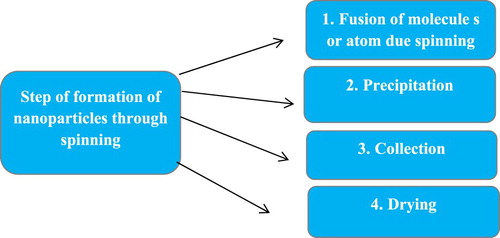
5.2.3. Spinning
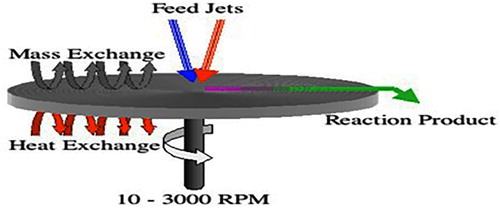
Nanoparticles are synthesized by spinning. The nanoparticles are synthesized by using spinning disc reactor (SDR) which consist of rotating disc where physical parameter can be controlled such temperature. In order to avoid chemical reaction and remove oxygen, the reactor is filled with nitrogen or inert gases. The liquid such as water and precursor is pumped inside the chamber or reactor. Step of formation of nanoparticles through spinning show in .
The characteristics of nanoparticles synthesize from SDR is determined by various factors such as disc surface, liquid/precursor ration, disc rotation speed, liquid flow rate and location of feed. Smith, Nigel, et al synthesized Magnetic Nanoparticles using Spinning Disc Processing show in . The particles size ranged from size range 3 to 12nm.
Figure 14. Synthesis magnetic nanoparticles using spinning disc processing ( Citation97).
5.2.4. Pyrolysis
The mostly commonly industrial method for synthesis of nanoparticles is pyrolysis. The precursor is burn with flame in this method. The precursor may be in state of liquid or vapor. The precursor is transferred into furnace at high pressure and recover nanoparticles ( Citation98).
Some time laser or plasma use instead of flame to produce high temperature. High temperature make easy evaporation ( Citation99).
5.2.4.1. Advantages of pyrolysis
Efficient
Cost effective
Simple
Continuous process with high yield
5.2.5. Biological synthesis
Biological synthesis involves the synthesis of nanoparticles by using plant extract and microorganisms such as bacteria and fungi.
Phytonanotechnology has shown a new field for the synthesis of nanoparticles which is eco-friendly, simple, cost effective. Scalability, bio-compatibilty and synthesis of nanoparticles via universal solvent (water) as reducing agents are advantages of phytonanotechnology. Phytonanotechnology use plants for synthesis of nanoparticles. Nanoarticles are synthesizes using different part of plant such as root, fruit, stem, seed and leaf. The exact mechanism for synthesis of nanoparticles using plant remain to be elucidated. It has been illustrated that organic acid, proteins vitamins and secondary metabolites such as alkaloids, flavonoids, terpenoids, polysaccharides and heterocyclic compound are responsible for synthesis of various types nanoparticles.
Microorganism is consider nanofactories that hold wide potential as cost effective tool, eco-friendly, avoid toxic and harsh chemicals and energy demand for the synthesis of nanoparticles. Various reductase enzymes present in the microorganism due to which microorganism have ability to accumulate and detoxify heavy metals. These reductase enzymes play vitol role in reduction of metal salt into the nanoparticles. Over past few year, various microorganism such yeast, fungi and bacteria. Proteins, reducing cofactor, metal resistant gene, enzyme and organic material play important role in synthesis of nanoparticles as reducing and capping agent.
Advantages of green or biological synthesize
There are following advantages of green synthesis
Energy Efficient
Low cost production
Fewer accident
Safe product
Economical
Lesser waste therefore it is also called as environmental friendly
Competitive advantages
protect human health and communities
Use in pharmaceutical industry and other biomedical application.
5.2.5.1. Plant extract
Different plant can be used to reduce and stabilized the nanoparticles. Many researcher have used the biological method for the synthesis of metal or metal oxide nanoparticles by using different part of plant like leaf, stem, root and fruit show in .
Various bio-molecules such proteins, co-enzymes and carbohydrates are presented in plant that reduce the metal salt into nanoparticles.
Different nanoparticles are synthesized using different plant show in .
Table 4. Synthesis of different nanoparticles using different pants.
5.5.2.2. Bacteria
Bacteria has ability to reduce the metal ion and thus utilized for the synthesis of nanoparticles show in ( Citation114). A variety of bacterial species are used for the synthesis metallic and other novel nanoparticles
Table 5. Synthesis of different nanoparticles using different bacteria.
5.5.2.3. Fungi
Biological synthesis of metal or metal oxide nanoparticles using fungi is also very efficient method with well-defined morphology. Due to the presence of inter acellular enzyme, fungi act as biological agent for synthesis of nanoparticles show in ( Citation124). Bacteria produce less amount of nanoparticles than fungi.
Table 6. Synthesis of different nanoparticles using different fungi.
5.5.2.4. Yeast
Yeast are unicellular microorganisms. 1500 species of yeast are reported. Synthesis of nanoparticles using yeast has been reported by number of scientists. Innumerable nanoparticles or nanoparticles are synthesized using yeast.
6. Comparatives study of biological, chemical and physical methods
Several techniques are developed day by day for the synthesis of nanoparticles. There are three basic categories of these techniques which are biological methods, physicals method and chemical methods. The best method for synthesis of nanoparticles is the Biological method due to simplicity, nontoxic and cost effectiveness. The capping and reducing agents are play important role in the synthesis of nanoparticles. Hazardous and highly toxic chemicals are used in the chemical and physical method of nanoparticl,s synthesis which are responsible for defect in the environment. The reducing or capping agent used in chemical and physical methods are expensive. Biologically developedkn chemicals are used in biological methods which is not harmful for our environment. Thus the most preferable process for synthesis of nanoparticles is biological method.
7. Characterization of nanoparticles
The nanoparticles can be categorized into quantitative and qualitative. These methods include dynamic light scattering (DLS), scanning electron microscope (SEM), energy dispersive spectroscopy (EDS), UV-Vis spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), surface-enhanced remain spectroscopy (SERS), Atomic force microscopy (AFM), high angle annular dark field (HAADF), atomic absorption spectroscopy (AAS) and.ray photoelectron spectroscopy (XPS) ( Citation136,Citation137)
7.1. Qualitative analysis
7.1.1. Fourier transform infrared spectroscopy (FT-IR)
Biomolecules those are responsible for capping, reduction and stabilizer, are identify by using FT-IR.
7.1.2. UV–Vis spectrophotometry
The nanoparticles of various metal ranges in size between 2 and 100 nm are characterized by using UV–Vis. The wavelength of 300 to 800 nm is generally used. The formation and stability of nanoparticles in aqueous solution are determined by using this technique.
7.1.3. Scanning electron microscope (SEM)
Output image is formed by using SEM in which electron is used instead of light. Shape, size, morphology and distribution of synthesized nanoparticles are characterized by using SEM.
7.1.4, X-ray diffraction (XRD)
Atomic structure of materials is determined by using XRD. It is used for both qualitative and quantitative analysis. It is used for the determination of crystal structure, calculation of crystalline nanoparticles size and for confirmation of nanoparticles.
7.1.5. Atomic force microscopy (AFM)
AFM is used for study of shape, size and surface area of synthesized nanoparticles.
7.2. Quantitative analysis
7.2.1. Transmission electron microscopy (TEM)
It is used for the study of particle size of material in nano-scale and crystal structure.
7.2.2. Annular dark-field imaging (HAADF)
The mechanism by which nanoparticles interact with bacteria can be studied by using HAADF. HAADF image gave the information about the size distribution of nanoparticles interacting with each type of bacteria.
7.2.3. Intracranial pressure (ICP)
ICP spectrometry is used to determine metal concentration in deionized and the original nanoparticles solutions. Inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled emission spectroscopy (ICP-ES) are used to measure the resulting metal concentration.
8. Applications of nanoparticles in biological fields
There are following applications of NPs in various biological fields are as follow
8.1. Applications in medicine
Nanoparticles have very useful applications in medicine like:
Used in Fluorescent biological labeling (Citation138).
In Drugs(Citation139) and gene delivery (Citation140).
In Biological detection of pathogens (Citation141).
Useful in Finding of proteins (Citation142).
In Probing of the DNA structure (Citation143).
For Tissue and cell engineering (Citation144).
Used for tumor destruction through heating (hyperthermia)(Citation145).
Helpful for separation and purification of biological cells and molecules(Citation146).
In MRI improvement (Citation147).
By using many kind of biosensor that are based on nanoparticles diagnosis of different disease.
In Phagokinetic studies (Citation148).
Cellular imaging (Citation149).
The best feature of nanoparticles can see in metal nanoparticles in the form of surface plasmon resonance. Whereas, the scattering intensity of nanoparticles is increase with an increase in their quantity ( Citation150). By the add of this feature, the specific molecule can have many expected applications in a living body tissue ( Citation151).
For example, it is possible now to differentiate between a healthy and cancer cell by covering cancer cell surface. It is done with the presence of antibodies which should be joined by the gold nanoparticle. Even though the junction of the gold nanoparticle with the antibody is properly distributed in the healthy cell as shown in .
Figure 16. (A) Dispersion of gold nanoparticle in healthy cells, (B) With a cancer cell concentrated gold nanoparticles.
The imaging of NPs with different shapes is performed at various wavelengths ( Citation152).
Moreover, there is another way could also be used for imaging other cells. In this method, gold nanoparticles is joined by protein and other functional molecules.
8.2. Application as chemical catalyst
In chemical reactions, nickel, lead, silver and platinum have been used as distinctive metal catalysts. Though, below the 200 °C, the dissociative adsorption of molecule of hydrogen and oxygen can not be carried out on the surface of gold. ( Citation153).
In hydrogenation and oxidation reactions the gold material is used as catalyst because gold nanoparticles are not reactive during such reaction. However, Haruta discovered that gold nanoparticles work successfully as catalyst. ( Citation154)
Metals that have the ability to form clusters that are un-stabled to the atmosphere. However, clusters of gold are highly stable that is why, gold particles can be used as catalysts. In catalyzed oxidation reactions, the catalytic activity increase with the decrease in size of the gold nanoparticle.
8.3. Application of nanoparticles in food and agriculture
The nanotechnology provided new techniques for water filtration and desalination but it will be more economical. By using nanotechnology food industry can also be developed. For example, by using nanotechnology new functional material and new instruments design for food preservation and bio-security are developed. Bayer Company introduced airtight plastic packing with the help of nanotechnology. Food is preserved in this plastic packing. From nanotechnology, genetics modifications in the constitution of the crop plant can be made.
8.4. Applications in energy harvesting
Fossil fuels are nonrenewable nature ( Citation155), that’s why recent approach informed about the limitations and inadequacy of fossil fuels in the coming years ( Citation156).
Therefore, scientists are trying to shift their research approaches to
The resources that can easily available
Generate renewable resources
Low cost material
For these purposes scientists found the NPs as the best aspirant due to the following characteristics
large surface area
photo catalytic applications
catalytic nature
optical behavior
NPs are widely used for the production of energy from splitting of electrochemical water and photo electrochemical (PEC) ( Citation157).
Solar cells ( Citation158) and piezoelectric generators ( Citation159) also very advance options ( Citation160) to produce energy other than reduction, water splitting ( Citation161), the electrochemical CO2 ( Citation162) and from fuels ( Citation163).
At nanoscale level ( Citation164), NPs can also store the energy ( Citation165) into different forms ( Citation166). That is why they also use in energy storage ( Citation167) applications.
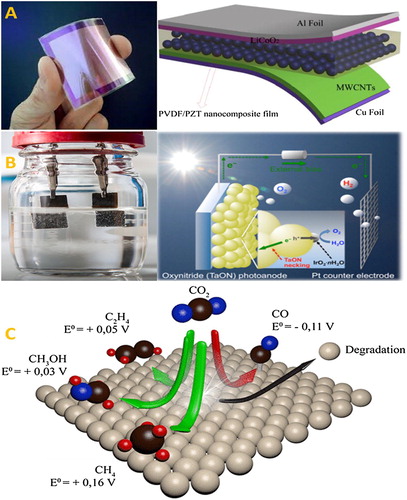
Recently, nanogenerators have the ability to convert the mechanical energy into electrical energy by using piezoelectric but it is an unconventional approach for the production of energy Show in ( Citation168).
Figure 17. Approaches of production of energy from (A) Piezoelectrics actuators (B) the Splitting of water (C) and Reduction of CO2 ( Citation169).
9. Application of nanoparticles in other fields
There are following applications of nanoparticles in other fields of science.
9.1. Application of nanoparticles in micro-wiring
In the electronic industry, the paste of metal nanoparticles is best candidate for the manufacturing of a printed wired board ( Citation170).
The metal nanoparticles have less melting point than the bulk metals. By the conventional electric conduction paste, circuit formation is possible on polymer base material. Whenever particles are used at the nano scale, the thickness of wire is decreased to a nano level. The ink-jet method is very important for the formation of wiring at nano level. The ink-jet method is not very expensive and it does not require long times like conventional techniques such as photolithographic methods and vacuum evaporation that are generally used. Metal nanoparticles paste can be prepared by gold. Gold is expensive that is why Copper is used as the substitute of it. Copper nanoparticles used as anti-oxidants.
9.2. Applications of nanoparticles in electronics
The interest in the progress of printed electronics has been growing from last few years because of high potential for low cost than the traditional silicon printed techniques. It is expected that electronics which are printed with different inks may rapidly flow. ks. These inks may also contain CNTs, organic and ceramics nanoparticles ( Citation171).
One dimensional semiconductor and metals have distinctive structural,electrical ( Citation172) andoptical properties ( Citation173) which provide the key structural blocks for the development of electronic, photonic materials and sensors( Citation174).
Nowadays, in electronic industry, new semiconducting materials are discovered gradually. However,diodes and transistors even that miniature chips are in use instead of vacuumed tubes ( Citation175).
10. Conclusion
In this review we revealed detail over view about nanoparticles, classification, synthesis, characterization and applications of various nanoparticles. The size of nanoparticles ranges from few nanometer to 500 nm shown by SEM, TEM and XRD. Nanoparticles have various application in different field of science such in medicine, micro-wiring, Food and agriculture, electronics and in energy harvesting. In simple words nanoparticles made our life easy and comfort. The future of nanotechnology is bright due its application in various field of science.
Acknowledgement
The authors thank the chemistry Department of Minhaj University Lahore.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Irfan ijaz M.phil in analytical chemistry from Minhaj University Lahore Pakistan since 2017. Area of research /specialization is Green chemistry, Nanoparticles and nanocomposites and Analytical techniques.
Ezaz Gilani M.phil in applied chemistry from University of Engineering and Technology. Lecturer and department coordinator MS/M.phil focal person in department of chemistry, Minhaj University Lahore since 2014. Area of research/specialization Green chemistry, Nanoparticles, MOF and Analytical techniques.
Ammara Nazir M.phil chemistry in inorganic chemistry from Lahore College for Women since 2009. She is assistant professor in Minhaj University Lahore since 2010. Area of research/specializations Green Chemistry, Nanocomposites and nanoparticles, heavy metals and Analytical techniques.
Aysha Bukhari she did M.phil from Forman Chirstian College Lahore (FCCU). She is Lecturer in department of chemistry Minhaj University Lahore (MUL) since 2009. Area of research/ specialization Oxidative stress status evaluation at chemical and biochemical level of biological and a biological samples, green synthesis of nanoparticles and characterizations.
References
- Feynman, R.P. “There’s Plenty of Room at the Bottom.” California Institute of Technology, Engineering and Science Magazine 1960.
- Laurent, S., et al. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108 (6), 2064–2110.
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, One-dimensional, Two-dimensional and Three-dimensional Nanostructured Materials for Advanced Electrochemical Energy Devices. Prog. Mater. Sci. 2012, 57 (4), 724–803.
- Shin, W.-K., et al. Cross-linked Composite Gel Polymer Electrolyte Using Mesoporous Methacrylate-Functionalized SiO 2 Nanoparticles for Lithium-ion Polymer Batteries. Sci. Rep. 2016, 6, 26332.
- Abbasi, E., et al. Silver Nanoparticles: Synthesis Methods, Bio-applications and Properties. Crit. Rev. Microbiol. 2016, 42 (2), 173–180.
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104 (1), 293–346.
- Horikoshi, S. A. T. O. S. H. I., and N. I. C. K. Serpone. “Introduction to Nanoparticles.” Microwaves Nanopart. Synth. Fundam. Appl. (2013): 1–24.
- Fulhame, Mrs. An Essay on Combustion: With a View to a New Art of Dying and Painting. Wherein the Phlogistic and Antiphlogistic Hypotheses Are Proven Erroneous. Printed for the author, by J. Cooper, 1794.
- Brust, M., et al. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802.
- Jin, R., et al. Photoinduced Conversion of Silver Nanospheres to Nanoprisms. Science 2001, 294 (5548), 1901–1903.
- Komanduri, R.; Lucca, D.A.; Tani, Y. Technological Advances in Fine Abrasive Processes. CIRP Ann. 1997, 46 (2), 545–596.
- Drexler, K.E. Nanosystems: Molecular Machinery, Manufacturing, and Computation; Wiley: New York, 1992.
- Drexler, K.E. Molecular Nanomachines: Physical Principles and Implementation Strategies. Annu. Rev. Biophys. Biomol. Struct. 1994, 23 (1), 377–405.
- Ulman, A. An Introduction to Ultrathin Organic Films: From Langmuir–Blodgett to Self-Assembly; Academic Press, 2013.
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354 (6348), 56.
- Tiwari, D.K.; Behari, J.; Sen, P. “ Application of Nanoparticles in Waste Water Treatment 1.” (2008).
- Mansha, M., et al. Synthesis, Characterization and Visible-Light-Driven Photoelectrochemical Hydrogen Evolution Reaction of Carbazole-Containing Conjugated Polymers. Int. J. Hydrogen Energy 2017, 42 (16), 10952–10961.
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36 (7), 887–913.
- Dreaden, E.C., et al. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41 (7), 2740–2779.
- Salavati-Niasari, M.; Davar, F.; Mir, N. Synthesis and Characterization of Metallic Copper Nanoparticles via Thermal Decomposition. Polyhedron 2008, 27 (17), 3514–3518.
- Tai, C.Y., et al. Synthesis of Magnesium Hydroxide and Oxide Nanoparticles Using a Spinning Disk Reactor. Ind. Eng. Chem. Res. 2007, 46 (17), 5536–5541.
- Sigmund, W., et al. Processing and Structure Relationships in Electrospinning of Ceramic Fiber Systems. J. Am. Ceram. Soc. 2006, 89 (2), 395–407.
- Thomas, S.C.; Kumar Mishra, P.; Talegaonkar, S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21 (42), 6165–6188.
- Bhaviripudi, S., et al. CVD Synthesis of Single-walled Carbon Nanotubes From Gold Nanoparticle Catalysts. J. Am. Chem. Soc. 2007, 129 (6), 1516–1517.
- Astefanei, A.; Núñez, O.; Galceran, M.T. Characterisation and Determination of Fullerenes: A Critical Review. Anal. Chim. Acta 2015, 882, 1–21.
- Ibrahim, K.S. Carbon Nanotubes-Properties and Applications: A Review. Carbon Lett. 2013, 14 (3), 131–144.
- Aqel, A., et al. Carbon Nanotubes, Science and Technology Part (I) Structure, Synthesis and Characterisation. Arab. J. Chem. 2012, 5 (1), 1–23.
- Winkin, N., et al. Nanomaterial-modified Flexible Micro-Electrode Array by Electrophoretic Deposition of Carbon Nanotubes. Biochip. Tissue Chip 2016, 6 (115), 2153–0777.
- Zhao, Q.; Gan, Z.; Zhuang, Q. Electrochemical Sensors Based on Carbon Nanotubes. Electroanalysis 2002, 14 (23), 1609–1613.
- Hickey, R.J., et al. Low-dimensional Nanoparticle Clustering in Polymer Micelles and Their Transverse Relaxivity Rates. ACS Nano 2013, 7 (7), 5824–5833.
- Gopi, S.; Amalraj, A.; Thomas, S. Effective Drug Delivery System of Biopolymers Based on Nanomaterials and Hydrogels – A Review. Drug Des. 2016, 5 (129), 2169–0138.
- Arroyo, C.R., et al. Reliable Tools for Quantifying the Morphological Properties at the Nanoscale. Biol. Med. 2016, 8 (3), 1.
- Alivisatos, P. The Use of Nanocrystals in Biological Detection. Nat. Biotechnol. 2004, 22 (1), 47.
- Kong, J., et al. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287 (5453), 622–625.
- Kong, J.; Dai, H. Full and Modulated Chemical Gating of Individual Carbon Nanotubes by Organic Amine Compounds. J. Phys. Chem. B 2001, 105 (15), 2890–2893.
- Cui, Y., et al. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293 (5533), 1289–1292.
- Hangarter, C.M., et al. Conducting Polymer Nanowires for Chemiresistive and FET-based Bio/Chemical Sensors. J. Mater. Chem. 2010, 20 (16), 3131–3140.
- Wang, Z.L. Characterizing the Structure and Properties of Individual Wire-like Nanoentities. Adv. Mater. 2000, 12 (17), 1295–1298.
- Duan, X., et al. Indium Phosphide Nanowires as Building Blocks for Nanoscale Electronic and Optoelectronic Devices. Nature 2001, 409 (6816), 66.
- Cui, Y.; Lieber, C.M. Functional Nanoscale Electronic Devices Assembled Using Silicon Nanowire Building Blocks. Science 2001, 291 (5505), 851–853.
- Xia, Y., et al. One-dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15 (5), 353–389.
- Jibowu, T. The Formation of Doxorubicin Loaded Targeted Nanoparticles Using Nanoprecipitation, Double Emulsion and Single Emulsion for Cancer Treatment. J. Nanomed. Nanotechnol. 2016, 7 (379), 2.
- Sreelakshmy, V.; Deepa, M.K.; Mridula, P. Green Synthesis of Silver Nanoparticles From Glycyrrhiza Glabra Root Extract for the Treatment of Gastric Ulcer. J. Develop. Drugs 2016, 5 (152), 2.
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13 (10), 2638–2650.
- Bello, S.A.; Agunsoye, J.O.; Hassan, S.B. Synthesis of Coconut Shell Nanoparticles via a Top Down Approach: Assessment of Milling Duration on the Particle Sizes and Morphologies of Coconut Shell Nanoparticles. Mater. Lett. 2015, 159, 514–519.
- Priyadarshana, G., et al. Synthesis of Magnetite Nanoparticles by Top-Down Approach From a High Purity Ore. J. Nanomater. 2015, 16 (1), 317.
- Garrigue, P., et al. Top-Down Approach for the Preparation of Colloidal Carbon Nanoparticles. Chem. Mater. 2004, 16 (16), 2984–2986.
- Zhang, X., et al. A Facile and Universal Top-Down Method for Preparation of Monodisperse Transition-Metal Dichalcogenide Nanodots. Angew. Chem. Int. Ed. 2015, 54 (18), 5425–5428.
- Ahab, A., et al. A Simple Straightforward Thermal Decomposition Synthesis of PEG-Covered Gd2O3 (Gd2O3@ PEG) Nanoparticles. Adv. Powder Technol. 2016, 27 (4), 1800–1805.
- Salah, N., et al. High-energy Ball Milling Technique for ZnO Nanoparticles as Antibacterial Material. Int. J. Nanomed. 2011, 6, 863.
- Damonte, L.C., et al. Nanoparticles of ZnO Obtained by Mechanical Milling. Powder Technol. 2004, 148 (1), 15–19.
- Giri, P.K., et al. Correlation Between Microstructure and Optical Properties of ZnO Nanoparticles Synthesized by Ball Milling. J. Appl. Phys. 2007, 102 (9), 093515.
- Yang, B.; Chen, D. Synthesis of CuO Nanoparticles for Catalytic Application via Ultrasound-assisted Ball Milling. Process. Appl. Ceram. 2017, 11 (1), 39–44.
- Khayati, G.R., et al. Synthesis of Cuprous Oxide Nanoparticles by Mechanochemical Oxidation of Copper in High Planetary Energy Ball Mill. Adv. Powder Technol. 2013, 24 (1), 301–305.
- Yadav, B.C.; Singh, S.; Yadav, T.P. Titania Prepared by Ball Milling: Its Characterization and Application as Liquefied Petroleum Gas Sensor. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2015, 45 (4), 487–494.
- Salari, M., et al. Effect of Milling Time on Mechanochemical Synthesis of TiO2 Nanoparticles. Int. J. Mod. Phys. B 2008, 22 (18n19), 2955–2961.
- Carneiro, J.O., et al. Synthesis of Iron-doped TiO2 Nanoparticles by Ball-Milling Process: The Influence of Process Parameters on the Structural, Optical, Magnetic, and Photocatalytic Properties. J. Mater. Sci. 2014, 49 (21), 7476–7488.
- Khayati, G.R.; Janghorban, K. Preparation of Nanostructure Silver Powders by Mechanical Decomposing and Mechanochemical Reduction of Silver Oxide. Trans. Nonferrous Met. Soc. China 2013, 23 (5), 1520–1524.
- Amendola, V.; Meneghetti, M. Laser Ablation Synthesis in Solution and Size Manipulation of Noble Metal Nanoparticles. Phys. Chem. Chem. Phys. 2009, 11 (20), 3805–3821.
- Gondal, M.A., et al. Synthesis of ZnO2 Nanoparticles by Laser Ablation in Liquid and Their Annealing Transformation Into ZnO Nanoparticles. Appl. Surf. Sci. 2009, 256 (1), 298–304.
- Al-Dahash, G.; Khilkala, W.M.; Abd Alwahid, S.N. Preparation and Characterization of ZnO Nanoparticles by Laser Ablation in NaOH Aqueous Solution. Iran. J. Chem. Chem. Eng. (IJCCE) 2018, 37 (1), 11–16.
- Mintcheva, N., et al. Laser-ablated ZnO Nanoparticles and Their Photocatalytic Activity Toward Organic Pollutants. Materials (Basel) 2018, 11 (7), 1127.
- Abdulateef, S.A., et al. Preparation of CuO Nanoparticles by Laser Ablation in Liquid. AIP Conference Proceedings. Vol. 1733. No. 1. AIP Publishing, 2016.
- Gondal, M.A., et al. Synthesis of Cu/Cu2O Nanoparticles by Laser Ablation in Deionized Water and Their Annealing Transformation Into CuO Nanoparticles. J. Nanosci. Nanotechnol. 2013, 13 (8), 5759–5766.
- Abdulateef, S.A., et al. Preparation of CuO Nanoparticles by Laser Ablation in Liquid. AIP Conference Proceedings. Vol. 1733. No. 1. AIP Publishing, 2016.
- Boutinguiza, M., et al. Synthesis of Titanium Oxide Nanoparticles by Ytterbium Fiber Laser Ablation. Phys. Proc. 2013, 41, 787–793.
- Singh, A., et al. Pulsed Laser Ablation-induced Green Synthesis of TiO2 Nanoparticles and Application of Novel Small Angle X-ray Scattering Technique for Nanoparticle Size and Size Distribution Analysis. Nanosc. Res. Lett. 2016, 11 (1), 447.
- Maciulevičius, M., et al. On-line Characterization of Gold Nanoparticles Generated by Laser Ablation in Liquids. Phys. Procedia. 2013, 41, 531–538.
- Al-Azawi, M.A.; Bidin, N. Gold Nanoparticles Synthesized by Laser Ablation in Deionized Water: Influence of Liquid Layer Thickness and Defragmentation on the Characteristics of Gold Nanoparticles. Chin. J. Phys. 2015, 53 (4), 201–209.
- Khumaeni, A.; Budi, W.S.; Sutanto, H. Synthesis and Characterization of High-Purity Gold Nanoparticles by Laser Ablation Method Using Low-Energy Nd: YAG Laser 1064 nm. J. Phys. Conf. Ser. 2017, 909 (1).
- Wender, H., et al. Synthesis of Gold Nanoparticles by Laser Ablation of an Au Foil Inside and Outside Ionic Liquids. Nanoscale. 2011, 3 (3), 1240–1245.
- Hajiesmaeilbaigi, F., et al. Preparation of Silver Nanoparticles by Laser Ablation and Fragmentation in Pure Water. Laser Phys. Lett. 2005, 3 (5), 252.
- Pyatenko, A., et al. Synthesis of Silver Nanoparticles by Laser Ablation in Pure Water. Appl. Phys. A 2004, 79 (4–6), 803–806.
- Tajdidzadeh, M., et al. Synthesis of Silver Nanoparticles Dispersed in Various Aqueous Media Using Laser Ablation. Sci. World J. 2014, 2014.
- Valverde-Alva, M.A., et al. Synthesis of Silver Nanoparticles by Laser Ablation in Ethanol: A Pulsed Photoacoustic Study. Appl. Surf. Sci. 2015, 355, 341–349.
- Shah, P.; Gavrin, A. Synthesis of Nanoparticles Using High-pressure Sputtering for Magnetic Domain Imaging. J. Magn. Magn. Mater. 2006, 301 (1), 118–123.
- Lugscheider, E., et al. Magnetron-sputtered Hard Material Coatings on Thermoplastic Polymers for Clean Room Applications. Surf. Coat. Technol. 1998, 108, 398–402.
- Rashid, J., et al. ZnO-nanoparticles Thin Films Synthesized by RF Sputtering for Photocatalytic Degradation of 2-Chlorophenol in Synthetic Wastewater. J. Ind. Eng. Chem. 2015, 23, 134–139.
- Das, A., et al. Copper Oxide Nano-particles Film on Glass by Using Sputter and Chemical Bath Deposition. (2016).
- Jaiswal, J.; Chauhan, S.; Chandra, R. Influence of Sputtering Parameters on Structural, Optical and Thermal Properties of Copper Nanoparticles Synthesized by dc Magnetron Sputtering. Int. J. Sci. Technol. Manage 2015, 4 (01), 678–688.
- Nakagawa, K, et al. Preparation of Copper Nanoparticles in Liquid by Matrix Sputtering Process. Journal of Physics: Conference Series. Vol. 417. No. 1. IOP Publishing, 2013.
- Gunnarsson, R.; Helmersson, U.; Pilch, I. Synthesis of Titanium-Oxide Nanoparticles with Size and Stoichiometry Control. J. Nanopart. Res. 2015, 17 (9), 353.
- Simionescu, O.-G., et al. RF Magnetron Sputtering Deposition of TiO2 Thin Films in a Small Continuous Oxygen Flow Rate. Coatings 2019, 9 (7), 442.
- González, L.G., et al. Synthesis and Characterization of Nanostructured TiO2 and TiO2/W Thin Films Deposited by co-Sputtering. Matéria (Rio de Janeiro) 2018, 23 (2).
- Peng, Y.; Park, C.; Laughlin, D.E. Fe 3 O 4 Thin Films Sputter Deposited From Iron Oxide Targets. J. Appl. Phys. 2003, 93 (10), 7957–7959.
- Xing, L., et al. Synthesis and Morphology of Iron–Iron Oxide Core–Shell Nanoparticles Produced by High Pressure gas Condensation. Nanotechnology 2016, 27 (21), 215703.
- Hu, X.L.; Takai, O.; N. Saito. Synthesis of Gold Nanoparticles by Solution Plasma Sputtering in Various Solvents. Journal of Physics: Conference Series. Vol. 417. No. 1. IOP Publishing, 2013.
- Asanithi, P.; Chaiyakun, S.; Limsuwan, P. Growth of Silver Nanoparticles by DC Magnetron Sputtering. J. Nanomater. 2012, 2012, 79.
- Adachi, M.; Tsukui, S.; Okuyama, K. Nanoparticle Synthesis by Ionizing Source gas in Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2003, 42 (1A), L77.
- Lee, H., et al. The Synthesis and Coating Process of TiO2 Nanoparticles Using CVD Process. Powder Technol. 2011, 214 (1), 64–68.
- Ramesh, S. Sol-Gel Synthesis and Characterization of Nanoparticles. J. Nanosci. 2013, 2013.
- Yahaya, M.Z., et al. Recent Characterisation of Sol-Gel Synthesised TiO2 Nanoparticles. Recent Appl. Sol–Gel Synthesis. 2017, 109.
- Kumar, S.G.; Koteswara Rao, K.S.R. Polymorphic Phase Transition Among the Titania Crystal Structures Using a Solution-based Approach: From Precursor Chemistry to Nucleation Process. Nanoscale. 2014, 6 (20), 11574–11632.
- Liu, A.R., et al. Low-temperature Preparation of Nanocrystalline TiO2 Photocatalyst with a Very Large Specific Surface Area. Mater. Chem. Phys. 2006, 99 (1), 131–134.
- Fang, C.-S.; Chen, Y.-W. Preparation of Titania Particles by Thermal Hydrolysis of TiCl4 in n-Propanol Solution. Mater. Chem. Phys. 2003, 78 (3), 739–745.
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27 (14), R713–R715.
- Smith, N., et al. “Synthesis of Magnetic Nanoparticles Using Spinning Disc Processing.” Technical Proceedings of the 2006 NSTI Nanotechnology Conference and Trade Show. Nano Science and Technology Institute, 2006.
- Kammler, H.K.; Mädler, L.; Pratsinis, S.E. Flame Synthesis of Nanoparticles. Chem. Eng. Techno. Ind. Chem. Plant Equip. Process Eng. Biotechnol. 2001, 24 (6), 583–596.
- D’Amato, R., et al. Synthesis of Ceramic Nanoparticles by Laser Pyrolysis: From Research to Applications. J. Anal. Appl. Pyrolysis 2013, 104, 461–469.
- Chandran, S.P., et al. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloevera Plant Extract. Biotechnol. Prog. 2006, 22 (2), 577–583.
- Raghunandan, D., et al. Rapid Biosynthesis of Irregular Shaped Gold Nanoparticles From Macerated Aqueous Extracellular Dried Clove Buds (Syzygium Aromaticum) Solution. Colloids Surf. B 2010, 79 (1), 235–240.
- Prathna, T.C., et al. Biomimetic Synthesis of Silver Nanoparticles by Citrus Limon (Lemon) Aqueous Extract and Theoretical Prediction of Particle Size. Colloids Surf. B 2011, 82 (1), 152–159.
- Marchiol, L. Synthesis of Metal Nanoparticles in Living Plants. Ital. J. Agron. 2012, 7 (3), e37–e37.
- Bussamara, R., et al. Controlled Synthesis of Mn 3 O 4 Nanoparticles in Ionic Liquids. Dalton Trans. 2013, 42 (40), 14473–14479.
- Chen, Y., et al. Augmented Biosynthesis of Cadmium Sulfide Nanoparticles by Genetically Engineered Escherichia Coli. Biotechnol. Prog. 2009, 25 (5), 1260–1266.
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of Zinc Oxide Nanoparticles Using Albizia Lebbeck Stem Bark, and Evaluation of its Antimicrobial, Antioxidant, and Cytotoxic Activities on Human Breast Cancer Cell Lines. Int. J. Nanomed. 2019, 14, 87.
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles Using Trifolium Pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23 (4), 517–523.
- Elumalai, K.; Velmurugan, S. Green Synthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles From the Leaf Extract of Azadirachta Indica (L.). Appl. Surf. Sci. 2015, 345, 329–336.
- Sharma, P., et al. Green Synthesis of Colloidal Copper Nanoparticles Capped with Tinospora Cordifolia and its Application in Catalytic Degradation in Textile Dye: An Ecologically Sound Approach. J. Inorg. Organomet. Polym. Mater. 2018, 28 (6), 2463–2472.
- Shende, S., et al. Green Synthesis of Copper Nanoparticles by Citrus Medica Linn.(Idilimbu) Juice and Its Antimicrobial Activity. World J. Microbiol. Biotechnol. 2015, 31 (6), 865–873.
- Yugandhar, P., et al. Cost Effective, Green Synthesis of Copper Oxide Nanoparticles Using Fruit Extract of Syzygium Alternifolium (Wt.) Walp., Characterization and Evaluation of Antiviral Activity. J. Cluster Sci. 2018, 29 (4), 743–755.
- Rao, A., et al. Removal of Hexavalent Chromium Ions by Yarrowia Lipolytica Cells Modified with Phyto-Inspired Fe0/Fe3O4 Nanoparticles. J. Contam. Hydrol. 2013, 146, 63–73.
- Venkateswarlu, S., et al. Biogenic Synthesis of Fe3O4 Magnetic Nanoparticles Using Plantain Peel Extract. Mater. Lett. 2013, 100, 241–244.
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Notices. 2014, 2014.
- Korbekandi, H.; Iravani, S.; Abbasi, S. Optimization of Biological Synthesis of Silver Nanoparticles Using Lactobacillus Casei Subsp. Casei. J. Chem. Technol. Biotechnol. 2012, 87 (7), 932–937.
- Sunkar, S.; Valli Nachiyar, C. Biogenesis of Antibacterial Silver Nanoparticles Using the Endophytic Bacterium Bacillus Cereus Isolated From Garcinia Xanthochymus. Asian. Pac. J. Trop. Biomed. 2012, 2 (12), 953–959.
- Du, L., et al. Biosynthesis of Gold Nanoparticles Assisted by Escherichia Coli DH5α and its Application on Direct Electrochemistry of Hemoglobin. Electrochem. Commun. 2007, 9 (5), 1165–1170.
- Southam, G.; Beveridge, T.J. The in Vitro Formation of Placer Gold by Bacteria. Geochim. Cosmochim. Acta 1994, 58 (20), 4527–4530.
- Philipse, A.P.; Maas, D. Magnetic Colloids From Magnetotactic Bacteria: Chain Formation and Colloidal Stability. Langmuir 2002, 18 (25), 9977–9984.
- Mann, S. Structure, Morphology, and Crystal Growth of Bacterial Magnetite. In Magnetite Biomineralization and Magnetoreception in Organisms. Boston, MA: Springer, 1985; pp. 311–332.
- Marshall, M.J., et al. c-Type Cytochrome-Dependent Formation of U (IV) Nanoparticles by Shewanella oneidensis. PLoS Biol. 2006, 4 (8), e268.
- Holmes, J.D., et al. Energy-dispersive X-ray Analysis of the Extracellular Cadmium Sulfide Crystallites of Klebsiella aerogenes. Arch. Microbiol. 1995, 163 (2), 143–147.
- Wadhwani, S.A., et al. Biogenic Selenium Nanoparticles: Current Status and Future Prospects. Appl. Microbiol. Biotechnol. 2016, 100 (6), 2555–2566.
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanopart. Res. 2008, 10 (3), 507–517.
- Pavani, K.V.; Sunil Kumar, N.; Sangameswaran, B.B. Synthesis of Lead Nanoparticles by Aspergillus Species. Pol. J. Microbiol. 2012, 61 (1), 61–63.
- Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological Synthesis of ZnO Nanoparticles Using C. albicans and Studying Their Catalytic Performance in the Synthesis of Steroidal Pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536.
- Chandrasekaran, R., et al. Formulation of Carica Papaya Latex-Functionalized Silver Nanoparticles for its Improved Antibacterial and Anticancer Applications. J. Mol. Liq. 2016, 219, 232–238.
- Fayaz, A.M., et al. Fungal Based Synthesis of Silver Nanoparticles – An Effect of Temperature on the Size of Particles. Colloids Surf. B 2009, 74 (1), 123–126.
- Garg, H. An Approach for Solving Constrained Reliability-Redundancy Allocation Problems Using Cuckoo Search Algorithm. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4 (1), 14–25.
- Xue, B., et al. Biosynthesis of Silver Nanoparticles by the Fungus Arthroderma fulvum and Its Antifungal Activity Against Genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016, 11, 1899.
- Shankar, S.S., et al. Bioreduction of Chloroaurate Ions by Geranium Leaves and its Endophytic Fungus Yields Gold Nanoparticles of Different Shapes. J. Mater. Chem. 2003, 13 (7), 1822–1826.
- Chauhan, A., et al. Fungus-mediated Biological Synthesis of Gold Nanoparticles: Potential in Detection of Liver Cancer. Int. J. Nanomed. 2011, 6, 2305.
- Rajakumar, G., et al. Fungus-mediated Biosynthesis and Characterization of TiO2 Nanoparticles and Their Activity Against Pathogenic Bacteria. Spectrochim. Acta Part A 2012, 91, 23–29.
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 Nanoparticle Biosynthesis and Its Physiological Effect on Mung Bean (Vigna radiata L.). Biotechnol. Reports 2015, 5, 22–26.
- Govender, Y., et al. Bioreduction of Platinum Salts Into Nanoparticles: A Mechanistic Perspective. Biotechnol. Lett. 2009, 31 (1), 95–100.
- Chanda, S. Silver Nanoparticles (Medicinal Plants Mediated): A New Generation of Antimicrobials to Combat Microbial Pathogens – A Review. Microbial Pathogens and Strategies for Combating Them: Science Technology and Education. FORMATEX Research Center, Badajoz, Spain (2014): 1314–1323.
- Mohammadlou, M.; Maghsoudi, H.; Jafarizadeh-Malmiri, H.J.I.F.R.J. A Review on Green Silver Nanoparticles Based on Plants: Synthesis, Potential Applications and Eco-friendly Approach. Int. Food Res. J. 2016, 23 (2), 446–463c.
- Bruchez, M., et al. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281 (5385), 2013–2016.
- Mah, C.S., et al. Microsphere-mediated Delivery of Recombinant AAV Vectors In Vitro and In Vivo. (2000).
- Pantarotto, D., et al. Immunization with Peptide-functionalized Carbon Nanotubes Enhances Virus-specific Neutralizing Antibody Responses. Chem. Biol. 2003, 10 (10), 961–966.
- Edelstein, R.L., et al. The BARC Biosensor Applied to the Detection of Biological Warfare Agents. Biosens. Bioelectron. 2000, 14 (10–11), 805–813.
- Nam, J.-M.; Shad Thaxton, C.; Mirkin, C.A. Nanoparticle-based Bio-bar Codes for the Ultrasensitive Detection of Proteins. Science 2003, 301 (5641), 1884–1886.
- Mahtab, R.; Rogers, J.P.; Murphy, C.J. “Protein-sized Quantum Dot Luminescence Can Distinguish Between “Straight”, “Bent”, and “Kinked” Oligonucleotides. J. Am. Chem. Soc. 1995, 117 (35), 9099–9100.
- Ma, J., et al. Biomimetic Processing of Nanocrystallite Bioactive Apatite Coating on Titanium. Nanotechnology 2003, 14 (6), 619.
- Matsuoka, F., et al. Hyperthermia Using Magnetite Cationic Liposomes for Hamster Osteosarcoma. Biomagn. Res. Technol. 2004, 2 (1), 3.
- Molday, R.S.; Mackenzie, D. Immunospecific Ferromagnetic Iron-Dextran Reagents for the Labeling and Magnetic Separation of Cells. J. Immunol. Methods 1982, 52 (3), 353–367.
- Weissleder, R., et al. Ultrasmall Superparamagnetic Iron Oxide: Characterization of a New Class of Contrast Agents for MR Imaging. Radiology 1990, 175 (2), 489–493.
- Parak, W.J., et al. Cell Motility and Metastatic Potential Studies Based on Quantum Dot Imaging of Phagokinetic Tracks. Adv. Mater. 2002, 14 (12), 882–885.
- Roco, M.C.; Stanley Williams, R.; Alivisatos, P., Eds. Nanotechnology Research Directions: IWGN Workshop Report: Vision for Nanotechnology in the Next Decade. Springer Science & Business Media, 2000.
- Hayat, A. Colloidal Gold: Principles, Methods, and Applications. Elsevier, 2012.
- Wang, L., et al. Biomolecular Sensing via Coupling DNA-based Recognition with Gold Nanoparticles. J. Phys. D: Appl. Phys. 2009, 42 (20), 203001.
- Jain, P.K., et al. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41 (12), 1578–1586.
- Saliba, N.; Parker, D.H.; Koel, B.E. Adsorption of Oxygen on Au (111) by Exposure to Ozone. Surf. Sci. 1998, 410 (2–3), 270–282.
- Haruta, M. When Gold Is Not Noble: Catalysis by Nanoparticles. Chem. Rec. 2003, 3 (2), 75–87.
- Avasare, V., et al. Room-Temperature Synthesis of TiO2 Nanospheres and Their Solar Driven Photoelectrochemical Hydrogen Production. Int. J. Energy Res. 2015, 39 (12), 1714–1719.
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42 (12), 4447–4453.
- Ning, F., et al. TiO 2/Graphene/NiFe-Layered Double Hydroxide Nanorod Array Photoanodes for Efficient Photoelectrochemical Water Splitting. Energy Environ. Sci. 2016, 9 (8), 2633–2643.
- Fang, X.-Q.; Liu, J.-X.; Gupta, V. Fundamental Formulations and Recent Achievements in Piezoelectric Nano-Structures: A Review. Nanoscale. 2013, 5 (5), 1716–1726.
- Gawande, M.B., et al. Cu and Cu-based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116 (6), 3722–3811.
- Lei, Y.-M., et al. Electrochemiluminescence Resonance Energy Transfer System: Mechanism and Application in Ratiometric Aptasensor for Lead Ion. Anal. Chem. 2015, 87 (15), 7787–7794.
- Nagarajan, P.K., et al. Nanofluids for Solar Collector Applications: A Review. Energy Proc. 2014, 61, 2416–2434.
- Li, D., et al. Efficient Water Oxidation Using CoMnP Nanoparticles. J. Am. Chem. Soc. 2016, 138 (12), 4006–4009.
- Zhou, Y., et al. Top-down Preparation of Active Cobalt Oxide Catalyst. ACS Catal. 2016, 6 (10), 6699–6703.
- Greeley, J.; Markovic, N.M. The Road From Animal Electricity to Green Energy: Combining Experiment and Theory in Electrocatalysis. Energy Environ. Sci. 2012, 5 (11), 9246–9256.
- Liu, D., et al. Rapid Synthesis of Monodisperse Au Nanospheres Through a Laser Irradiation-Induced Shape Conversion, Self-Assembly and Their Electromagnetic Coupling SERS Enhancement. Sci. Rep. 2015, 5 (1), 1–9.
- Liu, J., et al. Metal-free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-electron Pathway. Science 2015, 347 (6225), 970–974.
- Wang, D.-W.; Su, D. Heterogeneous Nanocarbon Materials for Oxygen Reduction Reaction. Energy Environ. Sci. 2014, 7 (2), 576–591.
- Wang, Z., et al. Piezoelectric Nanowires in Energy Harvesting Applications. Adv. Mater. Sci. Eng. 2015, 2015.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12 (7), 908–931.
- Karim, M.E. Nanotechnology Within the Legal and Regulatory Framework: An Introductory Overview. Malayan Law J. 2014, 3.
- Kosmala, A., et al. Synthesis of Silver Nano Particles and Fabrication of Aqueous Ag Inks for Inkjet Printing. Mater. Chem. Phys. 2011, 129 (3), 1075–1080.
- Hu, Y., et al. Two-dimensional Transition Metal Dichalcogenide Nanomaterials for Biosensing Applications. Mater. Chem. Front. 2017, 1 (1), 24–36.
- Millstone, J.E., et al. Synthesis, Properties, and Electronic Applications of Size-controlled Poly (3-Hexylthiophene) Nanoparticles. Langmuir 2010, 26 (16), 13056–13061.
- Shaalan, M., et al. Recent Progress in Applications of Nanoparticles in Fish Medicine: A Review. Nanomed. Nanotechnol. Biol. Med. 2016, 12 (3), 701–710.
- Cushing, B.L.; Kolesnichenko, V.L.; O'Connor, C.J. Recent Advances in the Liquid-phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104 (9), 3893–3946.