ABSTRACT
Iron is the most abundant transition metal on Earth. In this study, we have demonstrated an iron nanoparticle–loaded kaolin-catalyzed cross dehydrogenative coupling (CDC) reaction via C(sp3)–H activation. We use CDC of carboxylic acids with cyclohexenes to discuss various reaction parameters such as the amount of catalyst, oxidant, reaction temperature, and time. Under optimized reaction condition, the corresponding allylic ester was obtained with good yield of (85%). Screening of substituted benzoic acids and cyclic and acyclic olefins shows broad catalytic activities. Notably, the catalyst can be easily separated from the reaction mixture via simple centrifugation and remains active over five cycles of reuse.
1. Introduction
Numerous natural products, pharmaceutical molecules, and fine chemicals contain allylic ester structural motifs. Such motifs are precursors of bioactive molecules such as ferrudiol, (-)-zeylenol, 5-O-gallate, goniodomin A, and miliusolide (Citation1). Therefore, in recent years, the efficient, convenient, direct and atom economic methods for the synthesis of allylic esters via C-X (C, O, N, etc.) bond constructions has received increasing attention (Citation2). Further, research on the direct esterification of the allylic C(sp3)–H bond via oxidative cross dehydrogenative coupling (CDC) to synthesize allylic esters has attracted considerable attention involving the use of the metal-free catalysts such as tetrabutylammonium iodide (Citation3) and transition-metal based catalysts such as copper (Citation1–6), copper–aluminum (Citation7), cobalt (Citation8), and iron (Citation9). Hartwig et al. developed the oxidative dehydrogenative coupling synthesis of allylic esters from alkanes using a copper catalyst (Citation10). Unlike processes where raw materials have to be prefunctionalized for activation of the C–H bond, in CDC, the C–H bond is directly functionalized – which helps avoiding the prior functionalization – making it a highly atom economic and step-economic procedure.
Iron is the most abundant transition metal on Earth and has received increasing research attention owing to its low cost, easy accessibility, and low toxicity. Therefore, compared with noble-metal catalysts, iron catalysts are more cost effective and environmentally sustainable (Citation11). Multiple reviews and research papers have been reported on iron catalysts (Citation11–16). Notably, previous studies on homogeneous catalysis have not focused on the environmental or economic viewpoint, which is necessary to develop alternative reusable heterogeneous catalytic systems. We focused on transition metal–loaded solid-state heterogeneous catalysis. Previously, we prepared an iron nanoparticle–loaded kaolin catalyst that exhibited good catalytic activity for the direct esterification of benzoic acid with a cyclic ether via activation of the C(sp3)–H bond in a CDC reaction (Citation17).
Kaolin is an abundant nonmetallic clay mineral. In particular, coal-bearing kaolin is widely distributed in Inner Mongolia, China, within coal mines. According to a report, these mines contain several billion tons of coal-bearing kaolin (Citation18). However, the industrial applications of coal-bearing kaolin are limited; it is used in the coating and ceramics industry. After high-temperature calcination, its features, such as interlayer structure (1:1 silica:alumina sheets), surface area, and porosity, have limited applications (Citation19, Citation20). Thus, further in-depth research is required to improve the efficient use of kaolin. Recent reviews on kaolin for use in catalysis and pollutant removal have shown that kaolin has attracted increasing attention (Citation19–21). We have studied the preparation of modified kaolin, its adsorption property for removal of water pollutants, and its catalytic activities (Citation17, Citation22–24).
Herein, we further examine the catalytic activity of iron nanoparticle–loaded kaolin for the direct esterification of aromatic carboxylic acids with alkenes by activation of the allylic C(sp3)–H bond via CDC.
2. Results and discussion
Specific characterization methods were used to characterize the silane coupling agent–modified and iron nanoparticle–loaded kaolin. shows the Fourier-transform infrared (FT-IR) spectra of raw coal-bearing kaolin (CK), organically modified kaolin (NH2,Ph@CK) formed using two silane coupling agents with –NH2 and –Ph functional groups, and 3 wt% of iron-loaded modified kaolin (3wt%Fe/NH2,Ph@CK). The FT-IR absorption peaks of NH2,Ph@CK were observed at 2920 and 2855 cm−1, attributed to the asymmetric and symmetric C–H stretching vibrations of –CH2, respectively, and the peak observed at 1396 cm−1 was attributed to bending vibrations, suggesting successful grafting of the silane coupling agents onto kaolin. These peaks were retained in the FT-IR spectrum of 3wt%Fe/NH2,Ph@CK (i.e. after loading iron onto NH2,Ph@CK). X-ray diffraction patterns (Figure S1 in the Supporting information) suggested that the crystal structure of kaolin was unaltered, even after organic modifications and iron loading. Results of the Brunauer–Emmett–Teller (BET) surface area and iron elemental analyses are shown in . Raw and organically modified kaolin exhibited low BET surface areas of <10 m2/g (entries 1–4). However, after iron loading, the surface area increased (28 m2/g, entry 5) and remained almost unaltered after the first cycle of catalyst (3wt%Fe/NH2,Ph@CK) reuse (29 m2/g, entry 6). The inductively coupled plasma mass spectrometry (ICP-MS) analysis showed that the iron elemental content of fresh 3wt%Fe/NH2,Ph@CK was 2.8 wt% (entry 5), which became 2.5 wt% after the first cycle. This suggests that iron is stable when loaded on kaolin. The distribution, size, and shape of loaded iron particles were determined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image presented in a shows the amorphous lamellar structure of kaolin. The SEM and energy dispersive spectroscopy (SEM/EDS) analysis revealed the elemental content of iron as 2.0 wt% (b). Although this was a localized inaccurate quantitative approach, it suggested that most of the iron was distributed on the surface of kaolin. SEM elemental mapping showed that iron was uniformly distributed on the kaolin surface (c). TEM images showed that the iron particles distributed on the kaolin surface were oval shaped, similar to rice grains, with widths of 30–50 nm and lengths of 120–170 nm, with a dispersed distribution (). The X-ray photoelectron spectroscopy analysis (Figure S2 in the Supporting information) showed that the fresh and used catalysts exhibited binding energies at 710.5 and 724.4 eV, attributed to 2p3/2 and 2p1/2 of Fe(III), respectively, suggesting Fe(III) as a possible active center in the catalyst.
Table 1. BET surface area and iron elemental analysis.
Based on our previous work (Citation17), we decided to further investigate the catalytic efficiency of iron nanoparticle–loaded kaolin for C–O bond formation via CDC through the activation of C(sp3)–H. The direct synthesis of an allylic ester from benzoic acid (1) and cyclohexene was chosen as the initial test system. Previously, we tested different wt% values of iron-loaded Fe/NH2,Ph@CK catalysts in the range of 1–5 wt% and found that 3 wt% showed the best catalytic performance (Citation17). Therefore, in the test, we used 3 wt% iron-loaded catalyst (3wt%Fe/NH2,Ph@CK). Further, we discussed the effect of adding different amounts of 3wt%Fe/NH2,Ph@CK on the product yield. Results () showed that adding 1 mol% (compared with benzoic acid) of 3 wt%Fe/NH2,Ph@CK afforded the product with 33% yield (, entry 9). However, adding 3 mol% of the catalyst afforded the highest yield of 72% (, entry 11). Further increments in catalyst amount (5, 7, and 10 mol%) (, entries 13–15) did not improve the catalytic performance (yields of 57%–66%). To determine the effects of silane coupling agents (NH2–silane and Ph–silane) on catalytic efficiency, we loaded iron on different silane coupling agent (3-aminopropyltriethoxysilane and phenyltrimethoxysilane, respectively)-modified CK samples to produce various iron-loaded CK catalysts(3wt%Fe/NH2@CK, 3wt%Fe/Ph@CK, and 3wt%Fe@CK). Results showed that the catalytic efficiency of the individual iron-loaded NH2– and Ph–silane coupling agent–modified CK catalysts (, entries 7 and 8, respectively; product yields of 64% and 60%, respectively) were inferior to that of the iron-loaded CK catalyst modified by both the coupling agents (entry 11 yield 72%) but superior to that of the iron-loaded unmodified CK catalyst (entry 6, yield 50%). This suggests that CK modifications using silane coupling agents improve the catalytic performance. Interestingly, not adding the catalyst (, entry 1) or adding raw or unloaded modified CK catalysts (, entries 2 and 3–5, respectively) afforded the products with 20%–30% yields in the presence of di-tert-butyl peroxide (DTBP; 1.5 eqiuv; , entry 1). The yield was higher in absence of catalysts and with 3 equiv of DTBP (33%; , entry 7). This suggests that the oxidant DTBP also shows catalytic activity for this reaction but with low efficiency. This is consistent with the results obtained by the Liu group, which showed that using DTBP in the absence of a catalyst yielded the desired product (Citation1).
Table 2. Effect of catalysts on the reactionTable Footnotea.
Table 3. The effect of oxidants on the reactionTable Footnotea.
Further, we determined the catalytic efficiency of various homogeneous iron catalysts, including FeCl3, FeCl2, and Fe(OAc)2. Despite using 3 equiv of DTBP, the desired product of benzoic allylic ester was obtained in poor yields of <20% when homogeneous iron catalysts were employed (, entries 17–21), except for FeCl3, which afforded the product in 76% yield (, entry 16).
presents the effect of various oxidants such as tert-butyl hydroperoxide (TBHP) and different amounts of DTBP on product yields. However, for 1.5 equiv of the oxidants TBHP (in H2O and nonane; entries 10 and 11, respectively), cumene hydroperoxide (entry 16), and H2O2 (entry 14) afforded the desired product in low yields (≤53%), while oxidants yielded no product or only trace amounts of the product. In comparison, when 1–5 equiv of DTBP was used, the product was obtained in good yield (60%–85%), among which 3 equiv of DTBP afforded the best yield of 85%. Higher amounts of oxidants did not improve the product yield. The reaction temperature was optimized by performing the reaction at 80°C, 100°C, 110°C, 120°C, 130°C, and 140°C for 24 h (a). The reaction time was optimized by performing the reaction at 120°C for 6, 12, 18, 24, 32, and 48 h (b). The reaction conducted at 120°C for 24 h gave the highest product yield.
After optimizing the reaction conditions, we investigated the substrate scope using various aromatic carboxylic acids and cyclic and acyclic alkenes. As shown in , the reaction of cyclohexene with benzoic acid substituted with electron-donating groups such as Me– (2a–4a), MeO– (5a–7a), Ph– (8a), PhO– (9a), and t-Bu–- (10a) at single positions and the benzoic acid substituted with two methyl groups (11a) gave the corresponding product yields of 60%–82%. Benzoic acid substituted with three methyl groups produced a moderate yield of 45% (12a). In comparison, benzoic acid with single and two halogen substitutions at the ortho-, meta-, and para-positions produced the desired product in good yields (up to 70%) (13a–23a). However, benzoic acids substituted with –OH (24a) and –NH2 (25a) and the strong electron-withdrawing groups –NO2 (26a) and –CN (27a) did not produce the desired products. Naphthalene carboxylic acid, cinnamic acid, and heteroaryl carboxylic acids (except picolinic acid (32a) and pyrazine-2-carboxylic acid (33a), which yielded trace amounts of the products) produced the corresponding allylic esters in 78% (28a), 37% (29a), and 66% (30a) and 82% (31a) yields, respectively. The substrate scope of alkenes is presented in , showing that cyclic alkenes such as cyclopentene and cyclooctene afforded the corresponding allylic esters in 66% (1b) and 55% (1c) yields, respectively. Further, 2,3-dihydro-1H-indene and 1,2,3,4-tetrahydronaphthalene produced the corresponding α-acyloxy products in 81% (1d) and 58% (1e) yields, respectively. The acyclic alkene 2,3-dimethylbut-2-ene formed the desired product in good yield (66%, 1f). 1H-Indene did not produce the corresponding product (1 g).
Table 4. Substrate scope for CDC of various carboxylic acids with cyclohexeneTable Footnotea.
Table 5. Substrate scope for CDC of various alkenes with benzoic acidTable Footnotea.
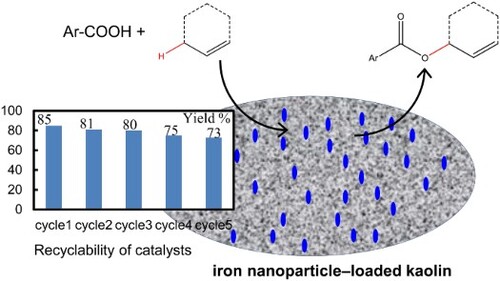
For heterogeneous catalysts, recyclability is the most critical feature. To determine recyclability, we used the same catalyst for five cycles of the reaction, i.e. the catalyst was recycled after each cycle and used in the next cycle. The separation and regeneration of the catalyst were performed using a simple procedure, where after separating the catalyst via centrifugation (centrifuged at room temperature for 10 min at 3900 rpm), it was washed successively with water and ethanol and vacuum dried at 40°C. The recyclability results are shown in . After five cycles, the yield remained 73%, indicating the stability and recyclability of the developed catalyst.
To discuss the possible reaction mechanism, we performed a free radical–capturing experiment using 2,6-di-tert-butyl-4-methylphenol (BHT). Addition of 1 equiv of BHT to the reaction mixture generated the corresponding product. Meanwhile, when 3 equiv of BHT was used, the allylic ester product was not formed and instead the allylic radical intermediate was trapped by BHT (h) (gas chromatography–mass spectrometry analysis of the reaction product obtained via radical trapping is shown in the Supporting information 3). This suggests that the reaction proceeded with the formation of an allylic radical intermediate. Based on previous studies (Citation1, Citation2, Citation8), the abovementioned results, and our previous research (Citation17), the proposed reaction mechanism for the iron nanoparticle–loaded kaolin-catalyzed esterification of carboxylic acids with alkenes is depicted in Scheme 1. First, the catalyst combines with the carboxylic acid and oxidant DTBP to form an Fe(IV) intermediate A, which then captures the hydrogen of cyclohexene to form an allylic radical intermediate (mode 1) and transforms into intermediate B. Notably, the difference between these results and those from our previous work (Citation17) is that here, the product is obtained (albeit in low yields of ∼20%) even when the oxidant (DTBP) ( entry 8) or the catalyst (3wt%Fe/NH2,Ph@CK) ( entry 7) is excluded. This suggests that in the reaction system, the allylic radical intermediate is provided through two pathways (Scheme 1, mode 1 and mode 2). Finally, the allylic radical intermediate reacts with intermediate B to form the desired product and complete the catalytic cycle. However, further studies exploring this reaction mechanism in greater detail are required, which poses a considerable challenge and will be the focus of our future work.
3. Conclusion
We built on our previous study to further prove the catalytic activities of iron nanoparticle–loaded CK for C(sp3)–H activation via CDC and established a reusable catalyst that works under environmentally benign conditions to synthesize allylic esters using the CDC reaction of carboxylic acids with cyclohexenes as a model. We optimized the oxidant, amount of reactants, reaction temperature, and time of the reaction. Iron nanoparticle–loaded kaolin showed good catalytic activity for the model reaction. When screening substituted carboxylic acids even those substituted with electron-donating groups or halogens could form the corresponding products in moderate-to-good yields. Moreover, five-membered heterocyclic carboxylic acid and various cyclic and acyclic alkenes formed the corresponding products in moderate-to-good yields. The catalyst was separated from the reaction mixture through a simple centrifugation method and did not require further treatment to be recovered. It was reused directly for five cycles with a good catalytic activity. Although we have proposed the reaction mechanism, further exploration is required for the detailed reaction mechanism.
Supporting information
Details of the experimental section, particle characterization, and 1H and 13C NMR spectra are given in the Supporting information.
Supplemental Material
Download MS Word (4.7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tian, W.; Li, M.; Yang, S.; Zhang, H.; Liu, H.; Xiao, X. Copper Corrole as an Efficient Catalyst for Esterification of Allylic sp3-C-H Bonds with Carboxylic Acids. Chin. J. Org. Chem 2021, 41, 2875–2884.
- Xiong, M.F.; Ali, A.; Akram, W.; Zhang, H.; Si, L.P.; Liu, H.Y. Copper Porphyrin as Efficient Catalysts for Esterification of Allyl sp3 C-H Bond with Carboxylic Acid. Catal. Commun 2019, 125, 93–97.
- Shi, E.; Shao, Y.; Chen, S.; Hu, H.; Liu, Z.; Zhang, J.; Wan, X. Tetrabuty lammonium Iodide Catalyzed Synthesis of Allylic Ester with tert-Butyl Hydroperoxide as an Oxidant. Org. Lett 2012, 14, 3384–3387.
- Wang, C.Y.; Song, R.J.; Wei, W.T.; Fan, J.H.; Li, J.H. Copper-catalyzed Oxidative Coupling of Acids with Alkanes Involving Dehydrogenation: Facile Access to Allylic Esters and Alkylalkenes. Chem. Commun 2015, 51, 2361–2363.
- Almasalma, A.A.; Mejia, E. Copper-Catalyzed Allylic C-H Alkynylation by Cross-Dehydrogenative Coupling. Chem. Eur. J 2018, 24, 12269–12273.
- Chen, X.Y.; Yang, S.; Ren, B.P.; Shi, L.; Lin, D.Z.; Zhang, H. Copper Porphyrin-Catalyzed Cross Dehydrogenative Coupling of Alkanes with Carboxylic Acids: Esterification and Decarboxylation Dual Pathway. Tetrahedron 2021, 96, 132377.
- Garcia-Cabeza, A.L.; Marin-Barrios, R.; Moreno-Dorado, F.J.; Ortega, M.J.; Massanet, G.M.; Guerra, F.M. Allylic Oxidation of Alkenes Catalyzed by a Copperaluminum Mixed Oxide. Org. Lett 2014, 16, 1598–1601.
- Ren, T.L.; Xu, B.H.; Mahmood, S.; Sun, M.X.; Zhang, S.J. Cobalt-catalyzed Oxidative Esterification of Allylic/benzylic C(sp3)–H Bonds. Tetrahedron 2017, 73, 2943–2948.
- Lu, B.; Zhu, F.; Wang, D.; Sun, H.; Shen, Q. Iron-catalyzed Esterification of Allylic sp 3 C–H Bonds with Carboxylic Acids: Facile Access to Allylic Esters. Tetrahedron Lett. 2017, 58, 2490–2494.
- Tran, B.L.; Driess, M.; Hartwig, J.F. Copper-catalyzed Oxidative Dehydrogenative Carboxylation of Unactivated Alkanes to Allylic Esters via Alkenes. J. Am. Chem. Soc 2014, 136, 17292–17301.
- Rana, S.; Biswas, J.P.; Paul, S.; Paik, A.; Maiti, D. Organic Synthesis with the Most Abundant Transition Metal–Iron: From Rust to Multitasking Catalysts. Chem. Soc. Rev 2021, 50, 243–472.
- Shang, R.; Ilies, L.; Nakamura, E. Iron-Catalyzed C–H Bond Activation. Chem. Rev 2017, 117, 9086–9139.
- Cera, G.; Ackermann, L. Iron-Catalyzed C–H Functionalization Processes. Top. Curr. Chem 2016, 374, 57.
- Itazaki, M.; Nakazawa, H. Iron-Catalyzed Cross-Dehydrogenative-Coupling Reactions. Top. Organomet. Chem. 2015, 50, 47–81.
- Jia, F.; Li, Z.-P. Iron-catalyzed/mediated Oxidative Transformation of C–H Bonds. Org. Chem. Front 2014, 1, 194–214.
- Sun, C-L.; Li, B-J.; Shi, Z.-J. Direct C−H Transformation via Iron Catalysis. Chem. Rev 2011, 111, 1293–1314.
- Bao, U.; Muschin, T.; Bao, A.; Bao, Y.-B.; Jia, M. FeNP-loaded Coal-bearing Kaolin Catalysts for the Direct Esterification of Benzoic Acid with Cyclic Ether via C(sp3)-H Bond Activation. Green Chem. Lett. Rev 2021, 14, 563–564.
- Ding, S-L.; Liu, Q-F.; Wang, M.-Z. Study of Kaolinite Rock in Coal Bearing Stratum, North China. Procedia Earth Planet. Sci 2009, 1, 1024–1028.
- Detellier, C. Functional Kaolinite. Chem. Rec. 2018, 18, 868–877.
- Alaba, P.A.; Sani, Y.M.; Daud, W.M.A.W. Kaolinite Properties and Advances for Solid Acid and Basic Catalyst Synthesis. RSC Adv. 2015, 5, 101127–101147.
- Cao, Z.; Wang, Q.; Cheng, H. Recent Advances in Kaolinite-Based Material for Photocatalysts, Chin. Chem. Lett 2021, 32, 2617–2628.
- Yang, P.; Bao, Y.S. Palladium Nanoparticles Supported on Organofunctionalized Kaolin as an Efficient Heterogeneous Catalyst for Directed C–H Functionalization of Arylpyrazoles. RSC. Adv 2017, 7, 53878–53886.
- Muschin, T.; Duo, X.; Bao, U.; Zulchin, H.; Agula, B. Environmentally Friendly Treatment of Coal-Bearing Kaolin by Polyhydroxy-Iron for Anionic Dye Removal. Chemistry Select 2019, 4, 13810–13816.
- Muschin, T.; Zulchin, H.; Jia, M. Adsorption Behavior of Polyhydroxy-Iron-Modified Coal-Bearing Kaolin for Fluoride Removal. Chemistry Select 2021, 6, 3075–3083.