 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Purpose
Fatigue in patients with acquired brain injury (ABI) is common. However, to better target fatigue, clear ways to categorize/interpret fatigue-severity in individual patients are lacking. This study aims to determine/categorize fatigue severity among children, adolescents, and young adults with ABI.
Methods
This cross-sectional study included young patients admitted to outpatient rehabilitation and their parents. To determine fatigue, the PedsQL™Multidimensional-Fatigue-Scale was used (MFS, scores 0–100, lower scores = higher fatigue, patient-/parent-reported). Based on scores from a reference population, four categories were formed: “1 = no/little fatigued” to “4 = severely-more fatigued.”
Results
All scores were lower than those from the reference population, with comparisons in the adolescent and young adult groups reaching statistical significance (p < .05). The proportions of patients in category 4 were: 9%/50%/58% among children/adolescents/young adults, showing that many patients were “severely-more fatigued”-than the reference population.
Conclusions
Measuring fatigue and categorizing fatigue severity looks promising for clinical practice and could help to better target fatigue.
Introduction
Fatigue is a common symptom with mental, emotional, and physical components among children, adolescentsCitation1 and young adultsCitation2 and it could influence their health-related quality of life (HRQoL).Citation1–4 Specifically, in young patients (5–24 years old) with “irreversible damage to the brain” due to a traumatic (TBI) or a non-traumatic cause (nTBI), that is, acquired brain injury (ABI),Citation5,Citation6 fatigue was found to be one of the most reported symptoms.Citation7–20 Furthermore, fatigue is known for its persistence over time even years after onset of ABI.Citation21,Citation22
Outpatient rehabilitation treatment could focus on fatigue-specific treatment to optimize HRQoL in young patients with ABI.Citation8,Citation9,Citation23 To date, the complex relationship between brain injury and fatigue is not entirely understood.Citation24 Only a few studies among adolescents and young adults with ABI (hospital and rehabilitation based) specifically addressed fatigue, concluding that it is relatively common,Citation8,Citation25 even five years after onset.Citation20 In clinical rehabilitation practice, a measurement of fatigue is not always part of the standardized assessment at admission and thus remains under-recognized in assessment and treatment.
One Danish study compared the patient population to healthy age-matched peers, where adolescents and young adults with ABI reported considerably higher fatigue levels.Citation25
It is known that fatigue is measured and monitored with a broad variety of outcome measures, with different feasibilities, validities, and internal consistencies.Citation3,Citation13,Citation26–28 For example, the previously described studies used the Multidimensional Fatigue Inventory-20 (MFI-20) and the Pediatric Quality of Life Inventory™ (PedsQL™) Multidimensional-Fatigue Scale (MFS).Citation8,Citation20,Citation25 The PedsQL™ MFS is the only outcome measure that has been translated in many languages, has been used among young patients with ABI (0–30 years old) and in rehabilitation-based studies.Citation1,Citation2,Citation8,Citation20,Citation25 Fatigue outcomes are often only presented on a linear scale, that is, 0–10 or 0–100, where higher scores indicate less fatigue or vice-versa.Citation3,Citation13,Citation26–28 Furthermore, when clinicians are interpreting 0–100 scores, based on Likert rating values (i.e., 100, 75, 50, 25, 0), this is not always suitable for treatment selection, nor does it automatically provide information in terms of how severe scores are compared to healthy peers. Therefore, in clinical practice, severity cutoff scores based on reference population scoresCitation1,Citation2 may be a more effective measure of fatigue severity than just pinpointing a score on a 0–100 scale.
One previous study compared fatigue (as measured with the PedsQL™ MFS) in patients with sickle cell disease to fatigue in healthy peers. They presented means, SDs, and effect sizes to compare both groups. Results of this study showed that patients were more fatigued than healthy peers (>2 SDs below the mean of healthy peers, effect size: 1.28).Citation29 However, this study did not present clear cutoff scores to categorize fatigue severity. It would be useful in clinical practice to differentiate between potential levels of fatigue severity by using cutoff scores based on outcomes from healthy peers to monitor changes in fatigue in individual patients with ABI.
Fatigue in young patients with ABI in a rehabilitation setting is commonly seen. However, a comparison of fatigue outcomes in young patients with ABI (5–24 years old) in an outpatient rehabilitation setting to fatigue outcomes in healthy peers is absent. A comparison with fatigue in healthy age-matched peers is available for patients with ABI that are older than 15 years old.Citation25 In this study, an outcome measure was used that is not suitable for patients under 15 years old (MFI-20).Citation25
To gain further knowledge on fatigue in young patients with ABI this current study has three aims. First, to describe fatigue using the PedsQL™ MFS in 5- to 24-year-old patients with ABI that were admitted to outpatient rehabilitation.
Second, to categorize the severity of fatigue in these patients using cutoff scores based on data obtained from healthy age-matched peers. Categorizing fatigue in severity cutoffs could support the interpretation of fatigue scores. Third, to examine the association between the severity of fatigue and HRQoL of patients, with the hypothesis that worse fatigue scores are associated with diminished HRQoL.
Based on the nature and severity of fatigue, treatments, such as psycho-education and/or physical fitness treatment and/or cognitive-behavioral therapy could be better tailored to a patient’s needs.Citation30–32 The insights from our study could support the interpretation of fatigue scores by clinicians, thereby enhancing its recognition and treatment in rehabilitation as well as increasing awareness of one of the major “invisible” problems after ABI in young patients: fatigue.
Methods
Design and Setting
This study was part of a larger, observational, longitudinal multi-center study on family impact, fatigue, participation, and quality of life in Dutch children, adolescents and young adults with ABI. The study was conducted from 2015 to 2019 in 10 rehabilitation centers in the Netherlands, all of which treat patients with ABI. The study protocol was reviewed by the medical ethics committee of the Leiden University Medical Center (P15.165), and an exempt from full medical ethical review was provided. In the current study, only data regarding patient and parent reported fatigue and HRQoL were used. The “Strengthening the Reporting of Observational studies in Epidemiology” (STROBE) guidelines were used for the reporting.Citation33
Population/Participants
Patients with ABI
Children, adolescents, and young adults aged 5–24 years with a diagnosis of ABI, who were referred to a participating rehabilitation center and their parents were eligible for the study. If patients and/or parents were unable or limited to understand the Dutch language, they were not invited. Patients over the age of 16 years had to give permission for their parents to participate according to the Dutch law of healthcare decision-making and vice-versa in patients below 16 years old.
Healthy Dutch Peers
Dutch reference data regarding fatigue, as measured with the Pediatric Quality of Life Inventory™ (PedsQL™) Multidimensional-Fatigue Scale (MFS),Citation3 were previously reported by Gordijn et al. and Haverman et al.Citation1,Citation2
The study by Gordijn et al. included 366 healthy 5- to 18-year-old children and/or their parents (n = 497) from day care facilities and schools in the Netherlands. They divided the participants into age groups: children 5–7 years, children 8–12 years, and adolescents 13–18 years.Citation1 The study by Haverman et al. included 512 healthy 18- to 30- year-old young adults. The study was part of a larger Dutch study aimed at establishing normative data for several questionnaires measuring various psychosocial concepts, where young adults from the general population were invited by e-mail to participate.Citation2 For the present study, only published, aggregated results, that is, mean and SD per age group were used.
Assessments
The assessment comprised a set of (digital) questionnaires that were administered at admission and as part of routine care. Questionnaires were filled out either at home or at the outpatient clinic (digitally or on paper). Unique links to the digital questionnaires were sent to the participants by e-mail by the medical health professionals. Questionnaires that were filled out on paper were literally copied and transcribed into the digital database by the data manager. Thereafter, all data were recoded anonymously, and stored in a secured central digital database at Basalt Rehabilitation Center in The Hague, The Netherlands.
For the present study on fatigue, only data gathered at admission were used.
Demographic and Injury Characteristics
Patient demographics and injury-related characteristics were extracted from the medical records. Characteristics included: date of birth, sex, date of ABI onset, date of first appointment, and cause of the ABI. The time between ABI onset and referral to rehabilitation was presented per age group as numbers (%) and median (IQR) in months and divided into two groups: time between onset and referral less (<) and more (>) than 6 months. Age was determined at time of the first appointment and further divided into three groups: children (5–12 years), adolescents (13–17 years), and young adults (18–24 years). ABI cause was divided in: TBI or nTBI and if known, the TBI severity level was reported as mild, or moderate/severe, based on the Glasgow Coma Scale at hospital admission.Citation34 NTBI causes were divided into; stroke/cerebrovascular accidents, brain tumors, meningitis/encephalitis, hypoxia/intoxication, and other.
Outcome Measures
Fatigue
To assess patient fatigue (reported by patients, parents, or both), the 18-item PedsQL™ Multidimensional Fatigue Scale (MFS) was used as outcome measure. The PedsQL™ MFS is considered a feasible, valid, and reliable tool to assess fatigue in patients with different age groups and diagnoses, including ABI.Citation3 It is translated and validated in Dutch.Citation1,Citation2 The MFS yields a total scale score, and three domain-scores: general fatigue (GF, six items), sleep/rest fatigue (SRF, six items), and cognitive fatigue (CF, six items).
All scores are calculated as the sum of the items divided by the number of items answered. Items are answered on a Likert-scale (0 = never to 4 = almost always) and thereafter linearly transformed to a 0–100 scale (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0). Lower scores indicate more fatigue.Citation3
Reference Data regarding Fatigue
Self- and parent-reported Dutch reference data is available regarding fatigue among children and adolescents. For the young adult group, only self-reported data is available.Citation1,Citation2 Regarding children and adolescents, mean total PedsQL™ MFS self-reported reference data scores were 76.8 (95% Confidence Interval, CI: 75.5–78.1) and for the domain scores: GF; 80.3 (95%CI: 78.81–81.77), SRF; 74.5 (95%CI: 72.88–76.09), and CF; 75.7 (95%CI: 73.83–77.56). Mean total PedsQL™ MFS parent-reported reference data scores were 81.2 (95% CI: 80.1–82.3, and for the domain scores: GF; 81.3 (95%CI: 80.01–82.52), SRF; 83.8 (95%CI: 82.62–85.06), and CF; 78.5 (95%CI: 76.90–80.06). For the young adult group, the mean (SD) total score was 71.8 (14.56) and for the domain scores: GF; 70.4 (18.2), SRF; 68.6 (14.6), and CF; 76.3 (18.4).
HRQoL
The PedsQL™Generic Core Scales-4.0 (PedsQL™ GCS-4.0, self- and parent-reported Dutch language version) was used to determine the HRQoL of young patients.Citation35,Citation36 Only HRQoL total scores were used in this study. The scoring system of the The PedsQL™GCS-4.0 is similar to that of the above-described PedsQL™MFS.
Statistical Analysis
Characteristics
All patient characteristics and fatigue outcomes were described per total and age group using descriptive statistics. These age-ranges correspond with the Dutch reference data from healthy peers.Citation1,Citation2
Fatigue
In this study, we compared fatigue outcomes (continuous variables) from patients with ABI with age-matched healthy children, adolescents, which was both self- and parent-reported. Regarding young adults, only self-reported reference data was available. Mean fatigue scores and standard deviations from these healthy peers were used to determine how many standard deviations the patients in our cohort differ from the mean scores from healthy peers. The study by Gordijn et al. only reported 95% Confidence Intervals (95%CI) and SDs were calculated by taking the square root of the number of participants in this study (N) and multiplying it with the upper limit of the 95% CI minus the lower limit of the 95% CI and dividing it by 3.92 (normal distribution):
For every (age)group, aggregated Z-scores (or standard scores) were calculated using the formula: “X” (the mean fatigue score from patients), minus “μ” (the mean fatigue score from healthy peers), divided by “σ” (the SD from the mean fatigue score in healthy peers). This method was also done for the parent-reported data.
X = mean fatigue score (patients with ABI)
μ=mean of the healthy peers
σ=SD of the healthy peers
To find corresponding probabilities, we used a Z-table/standard normal distribution table (a table for the values of Phi) to find p-values on the left of the mean to check whether the mean differences between the patients and the healthy peers were significant.
Negative scores in the Z-table correspond to the p-values which are less than the mean and vice-versa with positive scores.
Categorization of PedsQL™ MFS Scores
The mean total PedsQL™ MFS scores and SDs from the reference data from Dutch healthy peers were used to create four categories of fatigue severity. The cutoffs for the categorization were age-group and patient/parent-reported specific. Further, the categorization was calculated for the total and domain scores as presented below and specified in .
Figure 1. Fatigue severity classification in a normal distribution curve.
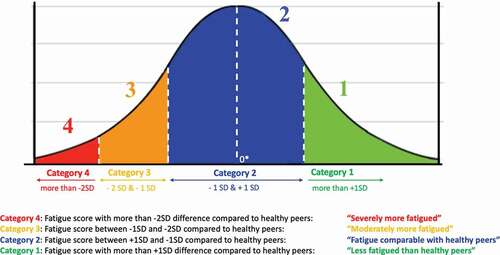
Category 1: Fatigue score with more than +1SD difference compared to healthy peers: “less fatigued than healthy peers”
Category 2: Fatigue score between +1SD and −1SD compared to healthy peers: “fatigue comparable with healthy peers”
Category 3: Fatigue score between −1SD and −2SD compared to healthy peers: “moderately more fatigued”
Category 4: Fatigue score with more than −2SD difference compared to healthy peers: “severely more fatigued”
This four-point categorization was discussed with a statistician (from the Leiden University Medical Center), and consensus was reached between the statistician and all authors before using this classification in the current analyses.
A Bonferroni correction was performed to account for multiple testing, that is, the α-value divided by the number of analyses on the dependent variable did not exceed 0.05. All p-values less than 0.05 in these analyses were considered statistically significant. All above-described analyses were performed using SPSS 25.0 for Windows (IBM, SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp).
Results
Patient Characteristics
shows the inclusion of the patients and/or parents who completed the questionnaires that were used in the analyses for the present study. Characteristics of the 260 participants are presented in . Seventy-six (29%) patients were children (5–12 years), 141 (54%) were adolescents (13–17 years), and 43 (17%) were young adults (18–24 years). Fifty-two percent of all patients were female and 74% of the patients had a traumatic brain injury. Regarding these patients with TBI, 78% had a mild TBI. Forty-two percent of patients were referred to the rehabilitation center more than six months after onset of brain injury. Regarding HRQoL, mean patient- and parent-reported total PedsQL™ GCS-4.0 mean (SD) scores of the whole population were 64.7 (17.4) and 61.4 (16.9), respectively.
Figure 2. Distribution of participants from 10 Dutch rehabilitation centers.
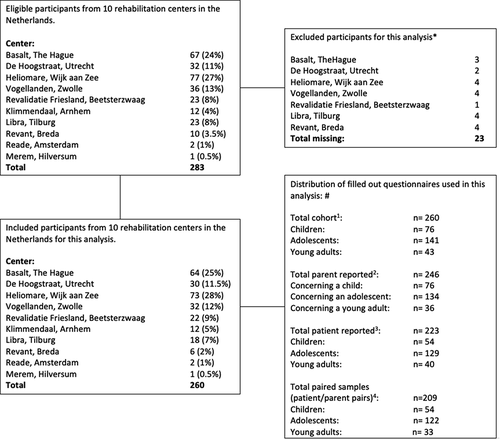
Table 1. Patient, family and injury characteristics of children, adolescents, and young adults with acquired brain injury (ABI) referred to an outpatient rehabilitation center.
Patient/parent-reported Fatigue in Young Patients with ABI, versus Healthy Peers
In , the mean (SD) PedsQL MFS total and domain scores from all children/adolescents/young adults, (both self and parent-reported) are presented. The mean (SD) total PedsQL MFS patient and parent-reported fatigue scores were 50.1 (17.3) and 53.8 (19.2), respectively.
Table 2. Patient- and parent-reported fatigue in children, adolescents, and young adults with ABI compared to healthy Dutch peers.
The lowest scores (i.e., more fatigue) were reported in the domain “cognitive fatigue” for all age groups, both patient- and parent-reported. The highest scores (i.e., less fatigue) were found in the domain sleep/rest fatigue for all groups. Considering the average total fatigue scores in the different age groups, the results show that overall, both the patient- and parent-reported fatigue scores decreased with age, indicating more severe fatigue in older children.
Total fatigue scores and almost all domain scores reported by patients with ABI and their parents were lower than those of healthy peers. Scores reported by adolescents (and their parents) and young adults were significantly lower than scores from healthy peers (p < .05), except for patient-reported sleep/rest fatigue (p = .08) and parent-reported cognitive fatigue (p = .07) in the adolescent group.
Fatigue Severity Categorization of Children, Adolescents, and Young Adults With ABI Based on Data From Healthy Peers
All results and the procedure regarding the categorization of fatigue severity levels in children/adolescents/young adults, based on Dutch reference data can be found in the supplementary table, . The supplementary table presents the calculated ranges regarding the four-group categorization based on the means and SDs from the reference data with the method described in . shows the proportions of patients per fatigue severity categorization (Category 1 to 4). The proportion of children (n = 54) assigned to categories 2 (50%) and 3 (41%) were higher than in categories 1 (0%) and 4 (9%). The proportions of children reported by their parents (n = 76) assigned to categories 2 (42%) and 3 (35%) were higher than in categories 1 (3%) and 4 (20%). The proportion of the adolescents (n = 129) assigned to categories 2 (26%) and 4 (51%) were higher than in categories 1 (0%) and 3 (23%). The proportions of the adolescents reported by their parents (n = 134) assigned to categories 3 (23%) and 4 (52%) were higher than in categories 1 (1%) and 2 (26%) The proportion of young adults (n = 40) assigned to categories 3 (28%) and 4 (60%) were higher than in categories 1 (10%) and 2 (12%).
Figure 3. Percentages of children/adolescents/young adults with ABI per fatigue severity level category reported by patients and parents.1. GF: Domain score PedsQL MFS; General fatigue.2. SRF: Domain score PedsQL MFS; Sleep/Rest fatigue.3. CF: Domain score PedsQL MFS; Cognitive fatigue.
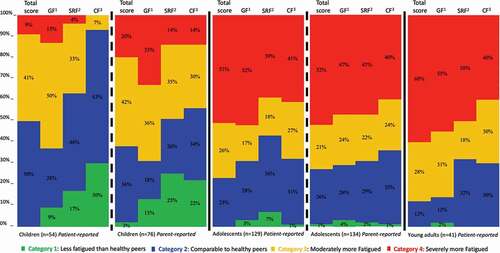
Figure 4. Patient-reported mean HRQoL total scores# per fatigue severity category in children/adolescents/young adults with ABI. # PedsQLTM Generic Core Scales-4.0 for Health-related Quality of Life (HRQoL), 0-100, with lower scores indicating diminished HRQoL.
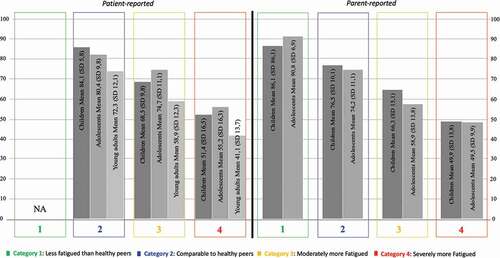
presents the HRQoL total scores per fatigue severity category. Irrespective of age group or whether its concerned patient or parent reported scores, HRQoL scores decreased with each higher level on the fatigue severity category (i.e., more fatigue, lower QoL).
Discussion
Young patients with ABI, referred for outpatient rehabilitation treatment in The Netherlands, and their parents reported high levels of fatigue. Considerably higher fatigue levels were reported compared to healthy age-matched peers in the reference population. Moreover, a large number of patients were moderately more (category 3) or severely more fatigued (category 4) than healthy peers especially in the groups with adolescent and young adult groups. Finally, HRQoL scores were consistently lower when patients scored in a higher fatigue severity category.
Fatigue in Children, Adolescents, and Young Adults with ABI in the Rehabilitation Setting
Considering the whole population of patients in our cohort, highest fatigue levels were found in the “cognitive fatigue” and “general fatigue” domain scales, which was in line with previous literature.Citation1,Citation2,Citation8,Citation20,Citation37 Higher fatigue levels were found in the groups of adolescents and young adults, which was in line with previous studies among patients with ABI,Citation8,Citation20 as well as among healthy adolescents and young adults.Citation1,Citation2
HRQoL was also found to be lower in comparison with healthy populations, in line with previous studies.Citation8,Citation20 The overall high levels of fatigue seen in patients with ABI (and their parents) and lower HRQoL warrant extra attention at admission and during outpatient rehabilitation treatment in the Netherlands.
Fatigue in Young Patients with ABI Compared to Healthy age-matched Peers
Fatigue is known to be common among healthy adolescents and young adults and tends to increase over time in transition from childhood to early adulthood based on mean group scores.Citation1,Citation2,Citation21 The fatigue scores among young patients with ABI in the current study was on average approximately 20 points lower than scores of the healthy reference population.Citation1,Citation2 Moreover, in the older age groups (adolescents and young adults), the differences were found to be even greater, which may probably indicate that these groups are at a higher at-risk for more problems in daily life functioning.
An explanation for the relationship between higher age and higher fatigue levels could be that adolescents and young adults are more capable of self-reflecting and are consistently comparing themselves with (healthy) peers.Citation1,Citation2,Citation21 Another explanation could be the increasing demands and responsibilities regarding daily life activities during the transition from childhood to adulthood.Citation11,Citation21,Citation22 Furthermore, the differences in scores between patients and parents increase per age group from children toward young adults, which was also seen among the healthy Dutch population.Citation1,Citation2 An explanation for this tendency could be that adolescents in transition to adulthood and young adults spend more time away from parents than younger children. Hence, parents have a limited perspective on their activities. Another reason could be that, despite the less overt signs of fatigue associated with cognitive fatigue, this could influence daily life functioning. Given the severity of fatigue in this rehabilitation-based population, measuring and monitoring fatigue can be an important focus at the start of- and during (rehabilitation) treatment, specifically for adolescents/young adults that are in transition to adulthood.
Categorization of Fatigue Severity: Improving Usability for Health Care Professionals
To better differentiate between fatigue severity, the fatigue scores from patients with ABI and their parents were categorized into four severity levels for both the total scores and all domain scores, allowing for an easier clinical interpretation of fatigue severity levels. Previous research only described comparisons with patients versus healthy peers with fatigue scores using means and SDs, where an interpretation of a score of −2SD’s below the mean of a healthy peer could be made.Citation29 In the population in our cohort, a large proportion of patients (and parents) reported scores that fell into category 4, with scores more than −2SD below the mean score from healthy peers as well.Citation1,Citation2
Differences regarding the four-point categorization between the total and all domain scores (general fatigue, sleep/rest fatigue, and cognitive fatigue) were found. Differentiating between domain scores could help to select specific approaches in treatment and to individualize treatment in clinical practice, since higher cognitive fatigue levels require different treatment approaches than those for higher sleep/rest fatigue during treatment.
Finally, HRQoL scores decreased with each level higher on the fatigue severity category (i.e., more fatigue, lower QoL). This trend is in line with the known multidirectional relation between fatigue and HRQoL and strengthens the fatigue severity categorization.Citation8
A limitation of Likert scales, as well as that of interpreting 0–100 scores, is that these methods do not take scores from a reference population into account. Severity cutoffs based on scores of healthy peers are probably more suitable for evaluating treatment. Hence, shifting from severity category 4 to category two after treatment facilitates better interpretation of treatment outcome. It could also help select patients for fatigue-related therapy, i.e., a patient in a ‘severely fatigued’ category could benefit from different approaches than a patient in a less severely fatigued category.
Overall, the proposed fatigue severity cutoff classification may be used for research purposes to facilitate the comparisons of the severity of fatigue among different populations of children, adolescents, and young adults. Nevertheless, it remains to be established if, and to what extent, the categorization is helpful to describe changes over time. The relatively high proportion of patients categorized in the moderate and severe fatigue categories in this rehabilitation-based population suggests that fatigue is a serious problem in these patients and needs a tailored rehabilitation treatment.
Limitations
There were some limitations to this study. First, we could not display a complete severity classification of TBI, since we only had access to GCS scores (and not in all cases, GCS scores were available). Only the GCS is commonly used in the Netherlands. Yet, it is not a foolproof predictor for the functioning of the child over time since it only gives a classification in the acute phase.Citation34,Citation38 Future Dutch research should focus on collecting additional information regarding TBI severity (e.g., the length of coma (LOC) or the duration of post-traumatic amnesia (PTA)). Furthermore, for non-traumatic brain injuries, there is no ‘golden standard’ for classification due to its complexity. Secondly, only self-reported reference data was available regarding the young adult age group.Citation1,Citation2 Therefore, it was not possible to assign parent-reported scores in this group according to the four-level fatigue severity categorization. Third, the majority (74%) of the patients in the study had a traumatic brain injury, of which 78% was ‘mild.’ Moreover, it concerned a rehabilitation setting, where only patients with serious and/or persisting symptoms are admitted. It remains unclear if this specific selection of patients impacts the generalizability of the results. Even though the majority of the study population had a mild injury, the proportions with moderate-to-severe fatigue were substantial in our study, which is in line with other TBI population studies in The NetherlandsCitation6,Citation8 ruling in favor of the generalizability of our results. It cannot be ruled out, however, that the patients who were referred to a rehabilitation facility are distinct from those with similar severity of brain injury who are not treated or treated elsewhere. Finally, as is the case with every self-report measure, the results could be influenced by lack of comprehension or motivation, or (patients/parents) moment-bound stress and mood.
Directions for Future Research
A large part of young patients with ABI in the outpatient rehabilitation setting and their parents reported high levels of fatigue, specifically, the patients that were in the age in transition to adulthood. Adolescents and young adults (and parents) reported significantly more fatigue than the healthy reference population. Taking fatigue into account in an early stage after ABI could possibly influence long-term persisting fatigue positively by appropriate interventions, based on specific domains regarding fatigue. However, future studies need to be undertaken to investigate fatigue outcomes over time and in evaluating these interventions.
Categorizing fatigue severity levels appears to be promising for use in the outpatient rehabilitation setting as a tool to better target fatigue at the start of rehabilitation treatment, and it can be used next to the initial linear 0–100 total and domain scores from the PedsQL™MFS. We also expect that categorizing fatigue could help to give health-care professionals as well as patients and their parents more insight regarding severity to optimize goal setting. The use of categorization levels and cutoff values is a first step in contextualizing and differentiating fatigue scores for research and clinical practice. The categorization could also be used as a tool to monitor fatigue over time and to evaluate the effect of (rehabilitation) treatment, that is, when a patient scores in the “severely fatigued” category at the start of treatment and in the category “comparably fatigued to healthy peers” after treatment. The next step would be to calculate the minimal clinically important difference (MCID) for this questionnaire and in this population to facilitate clinical use even more.
Supplemental Material
Download MS Word (17.3 KB)Disclosure Statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17518423.2022.2099994
Additional information
Funding
References
- Gordijn M, Cremers EM, Kaspers GJ, and Gemke RJ. Fatigue in children: reliability and validity of the Dutch PedsQL multidimensional fatigue scale. Qual Life Res. 2011;20(7):1103–08. accessed Jan 20, 2011. doi:10.1007/s11136-010-9836-9.
- Haverman L, Limperg PF, van Oers HA, van Rossum MA, Maurice-Stam H, and Grootenhuis MA. Psychometric properties and Dutch norm data of the PedsQL multidimensional fatigue scale for young adults. Qual Life Res. 2014 2014/June/14;23(10):2841–47. doi:10.1007/s11136-014-0734-4.
- Varni JW, Limbers CA. The PedsQL multidimensional fatigue scale in young adults: feasibility, reliability and validity in a University student population. Qual Life Res. 2008 2007/November/21;17(1):105–14. doi:10.1007/s11136-007-9282-5.
- Eddy L, Cruz M. The relationship between fatigue and quality of life in children with chronic health problems: a systematic review. J Spec Pediatr Nurs. 2007 2007/March/21;12(2):105–14. doi:10.1111/j.1744-6155.2007.00099.x.
- Greenwald BD, Burnett DM, Miller MA. Congenital and acquired brain injury. 1. brain injury: epidemiology and pathophysiology. Arch Phys Med Rehabil. 2003 2003/April/24;84(3B):S3–7. doi:10.1053/ampr.2003.50052.
- de Kloet AJ, Hilberink SR, Roebroeck ME, Catsman-Berrevoets CE, Peeters E, Lambregts SAM, van Markus-Doornbosch F, Vliet Vlieland TPM. Youth with acquired brain injury in The Netherlands: a multi-centre study. Brain Inj. 2013 2013/June/14;27(7–8):843–49. doi:10.3109/02699052.2013.775496.
- Wilkinson J, Marmol NL, Godfrey C, Wills H, van Eijndhoven Q, Botchway EN, Sood N, Anderson V, Catroppa C. Fatigue following paediatric acquired brain injury and its impact on functional outcomes: a systematic review. Neuropsychol Rev. 2018 2018/April/20;28(1):73–87. doi:10.1007/s11065-018-9370-z.
- van Markus-Doornbosch F, van der Holst M, de Kloet AJ, Vliet Vlieland TPM, Meesters JJL. Fatigue, participation and quality of life in adolescents and young adults with acquired brain injury in an outpatient rehabilitation cohort. Dev Neurorehabil. 2020 2019/November/21;23(5):328–35. doi:10.1080/17518423.2019.1692948.
- Lenaert B, Neijmeijer M, van Kampen N, van Heugten C, Ponds R. Poststroke fatigue and daily activity patterns during outpatient rehabilitation: an experience sampling method Study. Arch Phys Med Rehabil. 2020 2020/February/01;101(6):1001–08. doi:10.1016/j.apmr.2019.12.014.
- Ilmer EC, Lambregts SA, Berger MA, de Kloet AJ, Hilberink SR, Roebroeck ME. Health-related quality of life in children and youth with acquired brain injury: two years after injury. Eur J Paediatr Neurol. 2016 2015/October/13;20(1):131–39. doi:10.1016/j.ejpn.2015.09.003.
- Cantor JB, Ashman T, Gordon W, Ginsberg A, Engmann C, Egan M, Spielman L, Dijkers M, Flanagan S. Fatigue after traumatic brain injury and its impact on participation and quality of life. J Head Trauma Rehabil. 2008 2008/January/26;23(1):41–51. doi:10.1097/01.HTR.0000308720.70288.af.
- Saksvik SB, Karaliute M, Kallestad H, Follestad T, Asarnow R, Vik A, Håberg AK, Skandsen T, Olsen A. The prevalence and stability of sleep-wake disturbance and fatigue throughout the first year after mild traumatic brain injury. J Neurotrauma. 2020 2020/May/29;37(23):2528–41. doi:10.1089/neu.2019.6898.
- Maaijwee NA, Arntz RM, Rutten-Jacobs LC, Schaapsmeerders P, Schoonderwaldt HC, van Dijk EJ, de Leeuw F-E. Post-stroke fatigue and its association with poor functional outcome after stroke in young adults. J Neurol Neurosurg Psychiatry. 2015 2014/November/02;86(10):1120–26. doi:10.1136/jnnp-2014-308784.
- Norrie J, Heitger M, Leathem J, Anderson T, Jones R, Flett R. Mild traumatic brain injury and fatigue: a prospective longitudinal study. Brain Inj. 2010 2010/November/10;24(13–14):1528–38. doi:10.3109/02699052.2010.531687.
- Mollayeva T, Kendzerska T, Mollayeva S, Shapiro CM, Colantonio A, Cassidy JD. Fatigue in adults with traumatic brain injury: predictors and consequences. A systematic review of longitudinal study protocols. Syst Rev. 2013 2013/July/12;2(1):57. doi:10.1186/2046-4053-2-57.
- Bell KR. Fatigue and traumatic brain injury. Arch Phys Med Rehabil. 2015 2015/February/24;96(3):567–68. doi:10.1016/j.apmr.2013.06.010.
- de la Cour Fl D, Forchhammer BH, Mogensen J, Norup A. On the relation between dimensions of fatigue and depression in adolescents and young adults with acquired brain injury. Neuropsychol Rehabil. 2020 2018/September/06;30(5):872–87. doi:10.1080/09602011.2018.1517368.
- Kumar RG, Gao S, Juengst SB, Wagner AK, Fabio A. The effects of post-traumatic depression on cognition, pain, fatigue, and headache after moderate-to-severe traumatic brain injury: a thematic review. Brain Inj. 2018 2018/January/23;32(4):383–94. doi:10.1080/02699052.2018.1427888.
- Belmont A, Agar N, Hugeron C, Gallais B, Azouvi P. Fatigue and traumatic brain injury. Ann Readapt Med Phys. 2006 2006/May/24;49(6):283–288, 370–284. doi:10.1016/j.annrmp.2006.04.017.
- Greenham M, Gordon AL, Cooper A, Ditchfield, M, Coleman, L, Hunt, RW, Mackay, MT, Monagle, P, and Anderson, V, et al. Fatigue following pediatric arterial ischemic stroke: prevalence and associated factors. Stroke. 2021 2021/June/29;STROKEAHA120033000. doi:10.1161/STROKEAHA.120.033000.
- ter Wolbeek M, van Doornen LJ, Kavelaars A, Tersteeg-Kamperman MDJ, Heijnen CJ. Fatigue, depressive symptoms, and anxiety from adolescence up to young adulthood: a longitudinal study. Brain Behav Immun. 2011 2011/May/10;25(6):1249–55. doi:10.1016/j.bbi.2011.04.015.
- Lequerica AH, Botticello AL, Lengenfelder J, Chiaravalloti N, Bushnik T, Dijkers MP, Hammond FM, Kolakowsky-Hayner SA, Rosenthal J. Factors associated with remission of post-traumatic brain injury fatigue in the years following traumatic brain injury (TBI): a TBI model systems module study. Neuropsychol Rehabil. 2017 2016/September/17;27(7):1019–30. doi:10.1080/09602011.2016.1231120.
- Reuter-Rice K, Eads JK, Berndt S, Doser K. The initiation of rehabilitation therapies and observed outcomes in pediatric traumatic brain injury. Rehabil Nurs. 2018 2018/November/06;43(6):327–34. doi:10.1097/rnj.0000000000000116.
- Zgaljardic DJ, Durham WJ, Mossberg KA, Foreman J, Joshipura K, Masel BE, Urban R, Sheffield-Moore M. Neuropsychological and physiological correlates of fatigue following traumatic brain injury. Brain Inj. 2014 2014/February/26;28(4):389–97. doi:10.3109/02699052.2014.884242.
- Norup A, Svendsen SW, Doser K, Ryttersgaard T, Frandsen N, Gade L, Forchhammer H. Prevalence and severity of fatigue in adolescents and young adults with acquired brain injury: a nationwide study. Neuropsychol Rehabil. 2019 2017/September/13;29(7):1113–28. doi:10.1080/09602011.2017.1371045.
- Wrightson JG, Zewdie E, Kuo HC, Millet GY, Kirton A. Fatigue in children with perinatal stroke: clinical and neurophysiological associations. Dev Med Child Neurol. 2020 2019/June/22;62(2):234–40. doi:10.1111/dmcn.14273.
- Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment Of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile Of Fatigue (ProF), short form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res (Hoboken). 2011 2012/May/25;63(11):S263–286. doi:10.1002/acr.20579.
- Smets EM, Garssen B, Bonke B, and De Haes, JC, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–25. doi:10.1016/0022-3999(94)00125-o.
- Panepinto JA, Torres S, Bendo CB, McCavit, TL, Dinu, B, Sherman-Bien, S, Bemrich-Stolz, C, and Varni, JW, et al. PedsQL multidimensional fatigue scale in sickle cell disease: feasibility, reliability, and validity. Pediatr Blood Cancer. 2014;61(1):171–77. doi:10.1002/pbc.24776.
- Brigden A, Beasant L, Gaunt D, Hollingworth, W, Mills, N, Solomon-Moore, E, Jago, R, Metcalfe, C, Garfield, K, Wray, C, Trist, A, Vilenchik, V, Grayson, C, and Crawley, E, et al. Results of the feasibility phase of the managed activity graded exercise in teenagers and pre-adolescents (MAGENTA) randomised controlled trial of treatments for chronic fatigue syndrome/myalgic encephalomyelitis. Pilot Feasibility Stud. 2019;5(1):151. doi:10.1186/s40814-019-0525-3.
- Renaud MI, van de Port IGL, Catsman-Berrevoets CE, Kohler, S, Lambregts, SAM, and van Heugten, CM, et al. Effectiveness of the brains ahead! intervention: 6 months results of a randomized controlled trial in school-aged children with mild traumatic brain injury. J Head Trauma Rehabil. 2020;35(6):E490–E500. doi:10.1097/HTR.0000000000000583.
- Xu GZ, Li YF, Wang MD, and Cao, DY, et al. Complementary and alternative interventions for fatigue management after traumatic brain injury: a systematic review. Ther Adv Neurol Disord. 2017;10(5):229–39. doi:10.1177/1756285616682675.
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019 2019/April/02;13(5):S31–S34. doi:10.4103/sja.SJA_543_18.
- Jain S, and Iverson LM. 2020. . StatPearls. Treasure Island (FL).
- Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005 2005/May/21;3(1):34. doi:10.1186/1477-7525-3-34.
- Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 generic core scales. Health Qual Life Outcomes. 2007 2007/January/05;5(1):2. doi:10.1186/1477-7525-5-2.
- Wylie GR, Flashman LA. Understanding the interplay between mild traumatic brain injury and cognitive fatigue: models and treatments. Concussion. CNC50. 2017 2018/September/12;2(4):CNC50. doi:10.2217/cnc-2017-0003.
- Anaby D, Law M, Hanna S, Dematteo C. Predictors of change in participation rates following acquired brain injury: results of a longitudinal study. Dev Med Child Neurol. 2012 2012/January/20;54(4):339–46.doi:10.1111/j.1469-8749.2011.04204.x.

