 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose: To study the occurrence of speech features commonly associated with Childhood Apraxia of Speech (CAS) in Swedish children with suspected CAS (sCAS) or Speech Sound Disorder (SSD) related to Cleft Palate and/or Lip (CP ± L).
Method: Thirty-four children (4.10–5.11) with SSD related to sCAS (n = 15) or repaired CP ± L (n = 19) participated. Consensus judgement of presence/absence of CAS features in single words were based on a checklist with operationalised definitions. Speech sound production measures were based on semi-narrow phonetic transcription. Intra- and inter-transcriber agreement was determined.
Result: Twelve participants (ten with sCAS (67%) and two with CP ± L (11%)) shared a CAS profile of phonemic speech inconsistency for consonants and vowels and a set of four features: vowel error, voicing error, difficulty achieving initial articulatory configurations or transitionary movement gestures and stress errors. The most frequent speech difficulties in children with non-CAS CP ± L (n = 17) were consonant distortion (88%) and hypernasal resonance (76%). Prosodic impairment was rare.
Conclusion: A distinct CAS speech feature profile was found for children with CAS, differing in number and distribution compared to children with CP ± L and SSD. CAS was found more frequently in CP ± L and SSD compared to reported estimates of clinical prevalence.
Introduction
Errors in speech sound production and prosody, owing to impairment in planning and/or programming spatiotemporal parameters of movement sequences, are the core problems in the Speech Sound Disorder (SSD) Childhood Apraxia of Speech (CAS) (ASHA, Citation2007). According to the definition by ASHA, the category of CAS comprises children with isolated CAS at one end of the diagnostic continuum and children with CAS combined with complex neurobehavioural disorders of known or unknown origins at the other end. The prevalence of CAS in English speaking children presenting with a speech disorder is estimated to 2.4% for the idiopathic form and 4.3% when associated with complex neurodevelopmental disorder (Shriberg, Strand, Jakielski, & Mabie, Citation2019). Following research advances within genetics and neurobiology, knowledge about the origin of CAS is rapidly growing. Awareness about the phenotypic heterogeneity of CAS has resulted in research in broader populations of the disorder (e.g. Liégeois et al., Citation2019; Morgan & Webster, Citation2018). However, for clinical speech-language pathologists (SLPs), the differential diagnosis of children within the broader category of SSD remains challenging because of overlapping symptoms.
The possibility of different expressions of CAS speech difficulties in children has not been fully explored, although comorbidity has been addressed in speech sound disordered populations (e.g. Iuzzini, Citation2012; Shriberg et al., Citation2019; Zuk, Iuzzini-Seigel, Cabbage, Green, & Hogan, Citation2018). SSD is the umbrella term for childhood speech and motor speech disorders. SSD can be divided into branches of either known or unknown developmental disorders affecting cognitive, structural, sensory, motor, and/or affective development, i.e. CAS can occur in both branches of the classification dichotomy (Shriberg et al., Citation2019). Regardless of origin there is agreement that there are three subgroups of SSD related to, respectively, problems with articulation, motor planning/programming or phonology. Difficulties with classification arise primarily in children with speech profiles that do not fit exclusively into just one of these subgroups (Waring & Knight, Citation2013). The SSD associated with a known genetic syndrome and cleft palate as one possible symptom (22q11.2 deletion syndrome) (D’Antonio, Scherer, Miller, Kalbsfleisch, & Bartley, 2001; Kummer, Lee, Stutz, Maroney, & Brandt, Citation2007; Murray, McCabe, Heard, & Ballard, Citation2015) is a combination of structural and neurological origin. Consequently, overlap of speech difficulties commonly seen in children with Cleft Palate and/or Lip (CP ± L) and CAS could be expected owing to shared underlying similarities. The highest prevalence of CAS among complex neurodevelopmental disorders was reported for 22q11.2 deletion syndrome (11.8%) (Shriberg et al., Citation2019). Furthermore, it may be noted that Murray and colleagues (2015) excluded three children with submucous cleft palate from their sample with suspected CAS (sCAS), and Lohmander and Persson (Citation2008) identified a patient with sCAS and a unilateral cleft lip and palate (UCLP) in their longitudinal study of speech development and difficulties in children born with UCLP. These findings raise interest in the co-occurrence of CAS in children born with CP ± L.
In general, children born with CP ± L form a heterogeneous group - the extent and aetiology of the cleft differs, and speech may normalise after surgical repair for some, others may need speech intervention, while despite these efforts some will not reach the goal of normalised speech (Peterson-Falzone, Citation2011). In Sweden the incidence of some type of cleft lip and palate is about 2/1000, i.e. 150–200 new-borns per year (Hagberg, Larson, & Milerad, Citation1998). Research on speech in CP ± L has mainly focussed on speech outcome related to timing and type of surgical method, and cleft type (e.g. Lohmander et al., Citation2017a; Nyberg, Neovius, & Lohmander, Citation2018; Sell et al., Citation2015; Willadsen et al., Citation2017). Interestingly, not all children attain normal speech development or improved speech after surgical intervention. Between 20% and 60% of children with CP ± L display speech difficulties of varying degree at 5 years. Whereas a larger cleft has been associated with more severe speech difficulties, no clear association between surgical protocol and persistent speech difficulties has been demonstrated (Lohmander, Citation2011). Few studies have included different cleft types or children with CP ± L and additional syndromes or combined structural and neurodevelopmental disorders (e.g. Malmenholt, McAllister, & Lohmander, Citation2019; Persson, Elander, Lohmander-Agerskov, & Söderpalm, Citation2002). Recently, Malmenholt and colleagues studied an unselected group of 5-year-olds with CP ± L with or without additional syndromes and found no significant relationship between orofacial dysfunction and speech proficiency (Malmenholt et al., Citation2019).
Speech difficulties in children born with CP ± L are primarily attributed to the altered anatomical relationships in the oral cavity and vocal tract. If there is a dysfunction between the oral and nasal cavities even after cleft palate repair, there could be nasality symptoms such as hypernasality, audible nasal emission, weak articulation and nasal realisation. These cleft speech characteristics are directly caused by limited structure or function and are regarded as passive (Harding & Grunwell, Citation1998). Active compensatory behaviours are commonly used to reduce nasality symptoms. These compensatory speech characteristics are thought to reflect early mislearning and may persist after surgery despite an evidently adequate velopharyngeal function (Harding & Grunwell, Citation1998). As stated by Kuehn and Moller (Citation2000) every child born with CP ± L will find its own individual way of solving the challenges related to speech sound production based on anatomical structures and speech motor control abilities. Efforts to explain the variable outcome, by exploring different aspects, are needed.
Since ASHA’s position statement (ASHA, Citation2007) three consensus-based diagnostic criteria are central for a diagnosis of CAS. These are defined as “(a) inconsistent errors on consonants and vowels in repeated productions of syllables or words, (b) lengthened and disrupted coarticulatory transitions between sounds and syllables, and (c) inappropriate prosody, especially in the realisation of lexical or phrasal stress” (ASHA, Citation2007, p. 3). For clinical and research purposes, several observable speech symptoms have been combined and checklists constructed to reflect the three primary criteria. Iuzzini-Seigel and Murray (Citation2017) promoted a 12-feature checklist to aid in clinical diagnosis of CAS, adapted from the Strand 10-sign checklist (Shriberg, Potter, & Strand, Citation2011) used in research. They added operationalised definitions to each feature and made speech sound inconsistency a primary, mandatory CAS feature. The diagnostic criteria for CAS have been modified somewhat over time. Thus, the speech features included in diagnostic checklists and the criteria for their presence in different speech materials have varied. At present both feature checklists and/or the three primary ASHA diagnostic criteria are in use (e.g. Liégeois et al., Citation2019; Murray et al., Citation2015, Iuzzini-Seigel & Murray, Citation2017; Shriberg et al., Citation2011; Zuk et al., Citation2018). In contrast, Shriberg and colleagues (Citation2019) proposed an acoustic-aided perceptual sign, the Pause marker (PM), to identify children with CAS. The analysis of PM requires a conversational speech sample with a minimum of 24 useable utterances (Shriberg et al., Citation2019) excluding less verbal participants. Currently, no single feature or small set of core CAS features has been validated for differential diagnosis of children of all ages and severity levels.
By excluding participants and refining study populations, confounding factors are thought to be limited or controlled (e.g. Dodd, Reilly, Ttofari Eecen, & Morgan, Citation2018) at the cost of decreased clinical representativity. The heterogeneity of children with SSD at age 5 in SLP clinics is seldom reported (Malmenholt et al., Citation2019). According to normative data, 5-year-old Swedish-speaking children master the full Swedish phoneme inventory, except for /r/, /s/ and /ɕ/. About 85% of 5-year-olds also master /s/ and /r/ and 96% master s-clusters (Lohmander, Lundeborg, & Persson, Citation2017b; /ŋ, ɧ/ were not included). At age 5 children with SSD are not hard to identify on a generic level, but the differential diagnosis into subgroups of SSD (articulatory, motor programming/planning, phonological) is more challenging because of the overlap of speech features between CAS and other SSDs (McCabe, Rosenthal, & McLeod, Citation1998). Differential diagnosis is the prerequisite for providing relevant intervention for remediation of the disorder. The question therefore arises whether current diagnostic criteria for CAS can be used to differentiate CAS from other SSDs in 5-year-olds, and whether CAS is associated with some specific set of speech features in this age range.
Aim
The aim was to study the occurrence of CAS speech features in two Swedish-speaking, age-matched clinical groups with SSD: children with sCAS and children with repaired CP ± L. The study sought to investigate if the number and distribution of CAS speech features differ between 5-year-old children with CAS and children with repaired CP ± L and SSD by using a checklist developed for English speakers exploring cross-linguistic applicability.
Method
Participants
The 34 participants were Swedish-speaking 5-year-olds with SSD. They came from two different patient groups: group sCAS included children, who had suspected CAS or had been diagnosed with CAS by community based SLPs (n = 15; 5 girls, 10 boys) and group CP ± L children born with Cleft Palate ± Lip followed at a tertiary service (n = 19; 7 girls, 12 boys). Study inclusion criteria were: (a) mild to severe, suspected or diagnosed CAS or a repaired cleft palate, the latter with an SSD defined by a score at least two standard deviation units below the norm-referenced mean on a standardised word naming test (The Swedish Articulation and Nasality Test (SVANTE)) (Lohmander et al., Citation2017b) (b) being 5 years at enrolment or attending the routine speech registration at 5 years, and (c) having at least one native Swedish speaking parent. Comorbid language disorder was not an exclusionary factor.
Group sCAS was recruited through community-based SLPs in the Stockholm region. Sixteen participants (5.0–5.11) were referred for study inclusion and second opinion about diagnosis of CAS. All referred children were assessed but one was excluded due to low cooperation. Group CP ± L (n = 19) (4.10–5.4) came from a consecutive, unselected cohort of 52 verbal children, born with CP ± L (bilateral cleft lip and palate n = 4, unilateral cleft lip and palate n = 9, cleft of the hard and soft palate n = 1, cleft of the soft palate only n = 3 and submucous cleft n = 2). They also took part in a study of orofacial function, articulation proficiency, and intelligibility (Malmenholt et al., Citation2019). Detailed information about language ability was available for all children in group sCAS and a subset (42%) of group CP ± L. Language disorder was based on a score at least two standard deviation units below the norm-referenced mean on the Receptive and/or Expressive Language Index scores (CELF-4) (Semel, Wiig, & Secord, Citation2013; Swedish version). For the remaining participants in group CP ± L (n = 11), information about presence or absence of language disorder, as reported to the Swedish National Quality Registry for Cleft Lip and Palate, was available. Unspecified language disorder was found in 60% (n = 9) in group sCAS and in 16% (n = 3) in group CP ± L. Information about different aspects of orofacial function based on a screening (Nordic Orofacial Test - Screening (NOT-S)) (Bakke, Bergendal, McAllister, Sjögreen, & Åsten, Citation2007) was available for all participants. Sixty percent of participants in group sCAS and 68% in group CP ± L were found to have an orofacial dysfunction. For group sCAS, the most frequently observed difficulties were within the domains facial expression (e.g. close your eyes tightly; trying to whistle/blow), and speech, that is speech errors during counting and the inability to correctly sequencing three repetitions of /pataka/. For group CP ± L, the predominant difficulties were with habits (e.g. sucking fingers), chewing and swallowing, facial expression, oral motor function (e.g. lick your lips: “blow up” your cheeks and hold for at least 3 seconds) and speech. Hearing was tested on the day of speech assessment in 67% of the sCAS group, and none had hearing impairment. For children in group CP ± L hearing assessment was available for 68% on the day of speech assessment. Four children (31%) with CP ± L presented with a mild conductive hearing loss, three of which had otitis media with effusion. For individual participant background data, see .
Table I. Background information on concomitant impairment for all participants.
Assessment
All participants completed the standardised test SVANTE (Lohmander et al., Citation2017b). The test comprises 74 pictures for elicitation of single words including 49 monosyllabic words (three CV words, 33 CVC words, two CVCC words, one CCV word, three CCVC words, two VC words and five VCV words) and 25 disyllabic words (one VCCV word, one VCVC word, nine CVCV words, eight CVCVC, three CCVCV and three CCVCVC words). In addition, there is a sentence repetition task and a picture description for elicitation of connected speech included in SVANTE. Group CP ± L completed SVANTE during the routine speech registration at age 5, and group sCAS after inclusion. SVANTE was administered by the first author for all participants, except for 16% (n = 3) in group CP ± L, which were assessed by a SLP involved in the study as a second transcriber. The test was carried out in a quiet room and was video and/or audio recorded using a Digital Video Camrecorder (Canon FS100) and Zoom Handy Recorder H4n for group sCAS and JVC Pro HD Camera (GY-HM100E), Zoom Handy Recorder H4n and RØDE Microphone NT4-P48 for group CP ± L. According to the manual, semantic prompting was used choosing from two presented alternatives when a child needed assistance during picture naming. If this failed, the SLP modelled the targeted word for imitation. Multiple repetitions for token-to-token analysis are not included in SVANTE. On the other hand, SVANTE offers up to seven possible realisations of targeted consonants (three in initial, two in medial and two in final position). Vowels are not targeted specifically in the test. Normative data for consonants in SVANTE are available for six ages from 3 to 19 years including age 5 (Lohmander et al., Citation2017b).
Data analysis
For CAS feature assessment in this study, the 12-features checklist and operationalised definitions proposed by Iuzzini-Seigel and Murray (Citation2017) were applied on the single word speech material, as seen in . All 12 CAS features were rated as present or absent. As cut-off for a positive diagnosis of CAS, speech sound inconsistency in consonants plus any combination of at least five other features had to be present as proposed by Iuzzini-Seigel and Murray (Citation2017). A CAS speech feature was considered as present if at least three incorrect productions, in different words, were registered and agreed upon by the two raters in this study. Ratings of ten features were based on perceptual judgement and two features were calculated based on semi-narrow phonetic transcription. Results above 0% were interpreted as the feature being present.
Table II. Operational definitions for the 12-feature checklist of CAS proposed by Iuzzini-Seigel and Murray (Citation2017) and assessment procedure used in this study.
For perceptual analysis of CAS features, the first author and an external SLP, a clinician and researcher within the same area, served as listeners, each with more than 15 years’ experience in differential diagnosis of SSDs. The two SLPs jointly judged auditory and visual CAS features from the recorded single words.
For calculation of the two CAS features, difficulty with multisyllabic words and speech sound inconsistency, semi-narrow phonetic transcription of the whole single words (consonants and vowels) was performed by the first author, according to IPA and ExtIPA conventions (IPA., Citation2005). Speech sound inconsistency was based on phonemic inconsistency only.
The feature “increased difficulty with multisyllabic words” was calculated using the magnitude of change score, based on a subset of 24 single words from SVANTE, selected according to syllable structure. The most complex word syllable structure in SVANTE is CCVCVC. The magnitude of change score is the ratio of the number of phonemes correct in 12 simple monosyllabic words (VC, CV, CVC) and the phonemes correct in 12 more complex disyllabic words (VCCV, VCVC, CCVCV, CCVCVC). Participants with a quotient of >1 were judged to have increased difficulties with multisyllabic words (Murray et al., Citation2015).
For calculation of the phonemic speech sound inconsistency, 112 consonant opportunities were included. Of the 18 possible consonant phonemes from the Swedish consonant inventory (McAllister, Citation1998), 16 were represented: the six plosives /p, b, t, d, k, ɡ/, two nasals /m, n/, two approximants /l, j/, one trill or tap/flap /r/ and five fricatives, whereof two labiodental /f, v/, one dental /s/, one alveolo-palatal /ɕ/ (the latter both sibilants) and the glottal /h/. Phonemes were represented in seven possible elicitations (initial, medial and final position) except /v/ with six, /n/ with five, /j, ɕ, h/ with three and /m/ with two possible elicitations. Word initial s-clusters (/sp, st, sk, sv, sn/) had two possible elicitations each. Missing consonants in this study were the nasal /ŋ/ and the fricative /ɧ/.
For calculation of the Inconsistency Severity Percentage (ISP) the sum of the number of different error types for each phoneme minus one, allowing for consistent substitution behaviours, was calculated and divided by the sum of the total number of targeted opportunities (Iuzzini-Seigel, Hogan, & Green, Citation2017). This phonemic inconsistency measure was chosen since it has been shown to differentiate suspected CAS from phonological disorder in pre-school children (Iuzzini, Citation2012; Iuzzini & Forrest, Citation2010). In previous studies speech inconsistency has been assessed by calculating inconsistency in consonant production only (e.g. Iuzzini, Citation2012; Iuzzini-Seigel et al., Citation2017), excluding vowel inconsistency. Because inconsistent consonant and vowel production is a prerequisite for a CAS diagnosis (ASHA, Citation2007), we applied the same quantified method for vowels. The Swedish vowel inventory consists of nine distinct vowel phonemes with contrast for length (McAllister, Citation1998). The nine long and six short vowels assessed in this study, with one to seven possible elicitations, include all cardinal vowels /i:, e:, ɛ, a, ɑ:, ɔ, o:, u:/ and three complementary regarding lip-rounding /y:, ʉ̟ː, ø:/. In addition, the single words in SVANTE included words for elicitation of the long /ɛ:/ and the central short vowel /ɵ/. To include as many Swedish vowels as possible, eight vowel elicitations from sentence initial words from the SVANTE sentence repetition task were added as well, targeting /ɪ/ and /ʊ/. Vowels not assessed were short vowels /ʏ/ and /ø/. Thus, 15 vowel phonemes in 69 opportunities were used for calculations of vowel inconsistency in analogy with the Inconsistency Severity Percentage for consonants (ISPc). Phonemic speech inconsistency for vowels was calculated as
Here a calculation of ISP is provided using vowel-data from the present study. A child produced all words from the wordlist providing 74 vowel target opportunities and produced three different error types for /ɵ/ (ø, ʉ̟ː, ʉ̟), two for /ø/ (/æː, æ/), and two for /ɪ/ (i:, ɛ). Next, 1 is subtracted from the number of the error types for each vowel, allowing for consistent substitution behaviours resulting in two, one, and one error types for /ɵ/, /ø/ and /ɪ/ respectively. When summed up across all vowel opportunities this results in four and divided by the total number of target opportunities on the assessment which was 74 results in .05. Thus, the child’s ISP for vowels is 5% (4/74 * 100).
Reliability
Intra- and inter-transcriber reliability of the consonant and vowel transcriptions were calculated as percentage agreement, point by point. The analysis was based on randomly chosen re-transcriptions of ten children (29%), with five children from group sCAS and five from group CP ± L. The consonants compared had to be identically transcribed for place, manner and voicing. Vowels had to be identical in order to be considered as agreed. The median intra-transcriber agreement for the first author was 97.3% with a range of 89.0–100% for the ten participants, based on re-transcriptions of the 112 consonants (range 108–112) for every participant. The corresponding median intra-transcriber agreement for the 69 vowels (range 67–69) was 98.6% with a range of 88.1–100%. The median inter-transcriber agreement based on re-transcription of the same 29% of the material by the second transcriber was 91.1% with a range of 76.6–94.6% for consonants and 94.3% with a range of 72.5–100% for vowels. Inter-transcriber disagreements for consonants were found mainly within voicing (e.g. /p/b/, /t/d/, /k/ɡ/) (27%), place (e.g. /t/k/, /d/ɡ/) (22%) and place and manner (e.g. /l/j/, /r/j/) (21%). For vowels, inter-transcriber disagreements showed no common pattern. The participants with lowest inter-transcriber agreement on both consonants and vowels had the most severely disordered and inconsistent speech.
Ethical approval
The study was approved by the Regional Ethical Review Board in Stockholm (reg. no. 2014/609-31/2, 2015/251-32, 2015/1305-32).
Result
Shared CAS features
The speech feature profiles of all participants based on the 12 CAS features proposed by Iuzzini-Seigel and Murray (Citation2017) are presented in . The calculated inconsistency measure for production of vowels (ISPv) is added. Frequently observed and shared features in all participants were consonant distortions and inconsistent productions of consonants (ISPc). Seldom observed CAS features were slow rate, increased difficulty with multisyllabic words and intrusive schwa, present in less than 10%.
Table III. Speech features assessed according to the checklist and operational definitions proposed by Iuzzini-Seigel and Murray (Citation2017) presented individually
Number and frequency of CAS features across groups
Based on the observed ≥3 occurrences of each of the 12 CAS speech features, the number of CAS features was determined for all participants, ranging from one to eight out of the 12 features. The cut-off for a positive diagnosis of CAS, set at five features plus the inconsistent production of consonants (Iuzzini-Seigel & Murray, Citation2017), initially divided participants into four subgroups. Children from group sCAS were divided into group CAS (n = 10) (mean 6.3 CAS features, range of 5-8) and group non-CAS SSD (n = 5) (mean 2.2 CAS features, range 1–3). Children from group CP ± L formed subgroup non-CAS CP ± L (n = 17) (mean 2.4 CAS features, range 1–4) and subgroup CAS + CP ± L (n = 2) (mean 5.5 CAS features, range 5–6).
Subgroups CAS and CAS + CP ± L were merged as both were fulfilling CAS diagnostic criteria and subsequently analysed as one group in feature distribution, labelled CAS ± CP ± L (n = 12). Subgroup non-CAS SSD (n = 5) and non-CAS CP ± L (n = 17) were not merged, although both included children presenting with a SSD other than CAS. Participants in the small non-CAS SSD group showed great heterogeneity concerning both CAS speech features and concomitant impairments and were not further analysed on a group level. The individual speech profiles for these participants (4, 10, 12, 13, 15) can be found in .
Distribution of CAS features across groups
A distinct CAS feature profile emerged when analysing the CAS feature distribution across the two distinct subgroups of CAS ± CP ± L and non-CAS CP ± L. This grouping made it possible to describe CAS symptoms in CP ± L in contrast to CP ± L without CAS. Participants in group CAS ± CP ± L all demonstrated the specific combination of the four CAS features: vowel errors, voicing errors, difficulty achieving initial articulatory configurations or transitionary movement gestures, stress errors plus speech inconsistency for both consonants and vowels (ISPc and ISPv). The mean ISPc was 12.1%, range 2–34% and the mean ISPv was 3.2%, with a range of 1–7%. The most frequently seen additional features to this CAS profile were consonant distortion (75%) and syllable segregation (67%), see . Matching the speech feature profiles of subgroup CAS ± CP ± L against the three primary ASHA diagnostic criteria, as seen in , all 12 participants fulfilled all three criteria.
Figure 1. Distribution of CAS features in percentage across the two compared groups. CAS ± CP ± L: Childhood apraxia of speech with/without cleft palate with/without cleft lip; non-CAS CP ± L: speech sound disorder other than CAS and cleft palate with/without cleft lip, *Groping could not be assessed for all participants from patient group CP ± L.
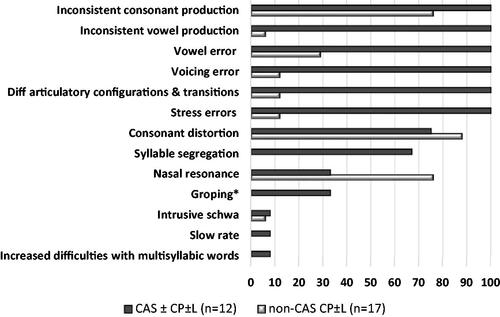
For group non-CAS CP ± L (n = 17), no distinct feature profile was found. The most frequently observed speech features were consonant distortion (88%) and difficulties with hypernasal resonance (76%). Consonant inconsistency was found in 71% (n = 12) with a mean ISPc of 4.1% and a range of 2–14%. Vowel inconsistency was found in one participant (6%) with a ISPv of 3%. When sorting the speech feature profiles of subgroup non-CAS ± CP ± L into the three primary ASHA diagnostic criteria, as seen in , only one participant fulfilled all three criteria. The ASHA criterion inappropriate prosody was rarely found.
Discussion
The aim of the present study was to determine the occurrence and distribution of CAS features in 5-year-old Swedish-speaking children with suspected CAS or in children with SSD related to CP ± L, by using definitions and operationalisations of CAS features for English-speaking children and to discuss diagnostic criteria for CAS.
Occurrence and distribution of CAS features
The diagnostic procedure used in this study (Iuzzini-Seigel & Murray, Citation2017) focussed on the number of CAS features present in a child, although it did not specify the frequency of error productions within features as a measure of severity. Thus, children with three incorrect productions were equalled to children with multiple incorrect productions within the same CAS feature. The procedure could therefore, to a varying degree, explain the occurrence of some CAS features seen also in the non-CAS CP ± L participants. The interpretation of overlapping CAS features is of special interest, since participant groups may share CAS features based on different underlying difficulties (McCabe, Rosenthal, & McLeod, Citation1998) or compensatory strategies (Harding & Grunwell, Citation1998). As expected, the frequency of observed difficulties with inappropriate nasal resonance was higher in children with CP ± L, 79%, compared to 13% in children with CAS without CP ± L. This most likely reflects velopharyngeal inadequacy or an anatomic defect such as a fistula rather than CAS.
Shared and discriminative CAS features
The most frequently observed and shared CAS feature in this study was consonant distortion, found in 75% in group CAS and 88% in group non-CAS CP ± L. Semi-narrow phonetic transcription revealed that the consonant distortions were a combination of active and passive characteristics within group CP ± L. Passive consonant distortions, that is the nasality symptoms audible nasal emission and weak articulation, were common in the group CP ± L but not present in group CAS. However, other compensatory consonant distortions found in children with structural anomalies are also commonly found in children with SSD or speech delay, limiting their specificity in the differential diagnosis of CAS (Iuzzini-Seigel & Murray, Citation2017). Speech sound inconsistency, on the other hand, has been reported to be able to differentiate children with CAS from children with phonological disorder (Iuzzini, Citation2012; Iuzzini & Forrest, Citation2010). All children with CAS had inconsistent speech sound production, whereas in children with non-CAS CP ± L the corresponding prevalence was 76%. The difference between ISPc group means was large, however, and so was the range in both groups. This resulted in a wide overlap and no cut-off point could be found indicating group assignment. Research on CAS in English-speaking children has used a cut-off of 18% (e.g. Iuzzini, Citation2012; Iuzzini-Seigel, Citation2019; Iuzzini-Seigel et al., Citation2017) based on results on a normed test (GFTA), not available in Swedish. Although quite similar to the test used in this study (SVANTE), the GFTA test includes multisyllabic and more complex words. This makes using the 18% cut-off unsuitable for this study. To explore the ISPc further, investigating a larger group of unselected children with SSDs could be one way of validating the ISPc as a marker for CAS and providing a discriminative cut-off for Swedish-speaking children with and without CAS. The same would be beneficial for the ISPv, that is, investigating an unselected group and validating the ISPv proposedly being a discriminative measure for CAS in this study of Swedish speakers.
Vowel errors were found in 29% of children with non-CAS CP ± L. In part it reflected the specific difficulty with lip-pursing for the Swedish /y:/ which could be explained by the anatomical limitation of the repaired cleft lip. However, vowel errors in this subgroup were not uniform, although mainly consistent, the erroneous productions revealed difficulties with tongue height, tongue advancement, lip rounding and vowel duration. Similar findings have been reported in 39% of 5-year-old Thai children with unilateral/bilateral cleft lip and palate (Prathanee, Pumnum, Seepuaham, & Jaiyong, Citation2016). While a more detailed analysis of vowel errors is beyond the scope of this study, this could be of future interest. There are additional CAS speech features, such as voicing errors or syllable segregation, that do not necessarily indicate CAS in children with CP ± L. These errors could be compensatory due to the inability to build up intraoral air pressure and influenced by the degree of velopharyngeal insufficiency (e.g. Harding & Grunwell, Citation1998). Thus, the CAS features seen in our non-CAS CP ± L participants are presumably attributed to the cleft condition. However, in 21% (n = 4) of the participants from group CP ± L, we observed motor speech difficulties reflected in difficulty achieving initial articulatory configurations or transitionary movement gestures, affecting consonant and/or vowel production and inappropriate prosody. The overlap of speech features in children with SSDs calls for an extra close look at the speech feature profile, which, for children with CAS should be based on the three primary ASHA criteria.
Discriminative CAS features, i.e. features that separated between children with and without CAS, did not include prosodic impairment (stress errors, syllable segregation and slow rate) in most non-CAS CP ± L participants. This is in line with results from research by Shriberg and colleagues, indicating prosodic features to be core markers for CAS (Shriberg et al., Citation2017). In addition, phonemic speech inconsistency for vowels (ISPv) was found in all children with CAS ± CP ± L, but just in one child classified as non-CAS CP ± L.
Diagnosis of CAS based on ASHA’s primary criteria
The speech feature profiles of all participants were also reviewed using ASHA’s three primary criteria for diagnosis of CAS (ASHA, 2007). For this, the 12 analysed CAS features (Iuzzini-Seigel & Murray, Citation2017) plus the vowel inconsistency measure, were matched against the three primary ASHA criteria as outlined in . Group assignment was compared resulting in a 100% match between the two diagnostic procedures. The shared CAS speech profile found in all participants with CAS thus fulfilled these criteria. In contrast, the speech profile in the non-CAS CP ± L group did not fulfil the three primary ASHA criteria except in one case.
Differential diagnostics of CAS
Two thirds (67%) of the participants from group sCAS fulfilled the criteria for CAS diagnosis according to Iuzzini-Seigel and Murray (Citation2017). Their profile contained a distinct set of four CAS features (vowel error, voicing error, difficulties achieving articulation configurations or transitionary movement gestures and stress error) and speech inconsistency for consonants and vowels. This speech feature profile was constant despite the variation among the ten participants in the number and severity of concomitant impairments within language or orofacial functions. The remaining 33% (n = 5) in the sCAS group were classified as non-CAS SSD, again displaying varying additional impairments that could explain the speech difficulties initially suspected to be CAS. The proportion of children with CAS versus non-CAS is identical to proportions reported by Murray and colleagues (2015). One third (32%) of their sample of 47 sCAS children, referred by community based SLPs as in this study, were thus diagnosed as non-CAS. At the same time, all participants with sCAS in the present study had voicing errors and 87% had vowel errors, possibly leading SLPs to suspect or diagnose CAS despite the lack of prosodic impairment. As stated by Waring and Knight (Citation2013), differential diagnosis is challenging in children with SSD presenting with speech behaviours not fitting into just one specific diagnostic label. The sCAS term has been used to indicate a SSD profile including difficulties associated with speech motor planning/programming, thus displaying that some SLPs hesitate to use the CAS diagnosis. This could be due to a lack of experience regarding CAS in community-based SLPs who reported seeing one new patient with CAS per year (Malmenholt, Lohmander, & McAllister, Citation2017). The previously formulated gold standard for a diagnosis of CAS, emphasising the need for an experienced and specialised SLP’s judgement for making a valid diagnosis (e.g. Murray et al., Citation2015) may have contributed to the use of the sCAS term, signalling uncertainty. In addition, there is still no diagnostic checklist with broad consensus to aid diagnostics.
Including larger numbers of children with a variety of SSDs should be prioritised in future studies, given the known overlap of several CAS speech features. This could challenge the CAS speech profile and cross-linguistic validity proposed in this study. Overdiagnosis of CAS within the general SSD population has been repeatedly reported (e.g. Murray et al., Citation2015; Shriberg et al., Citation2011). On the other hand, CAS may be underdiagnosed in children with genetic disorders, for example Down syndrome (e.g. Cleland, Wood, Hardcastle, Wishart, & Timmins, Citation2010). There are known conditions of combined structural and neurological aetiology (e.g. 22q11.2 deletion syndrome, Van der Woude syndrome) presenting with severe speech difficulties (Persson et al., Citation2002). Results from the present study with two (11%) participants with CP ± L meeting criteria for CAS could point in the same direction and co-occurring difficulties with speech motor control or even CAS should be considered and assessed in children with severely disordered speech at age 5. To further investigate this finding, studies including larger cohorts of children born with CP ± L of all types and syndromic populations are needed.
Limitations
The group sizes in the present study were small, particularly after subgrouping and the inclusion of children with SSD could have been wider, resulting in a third group of children with non-CAS SSD for comparison. In this study this third group was small (n = 5; participants 4, 10, 12, 13, 15) and both speech features () and concomitant impairments () were heterogeneous, thus of interest for further examination but unfeasible on a group level. To examine a larger sample of non-CAS SSD cases would also be of interest for differential diagnostics. The prevalence for concurrent childhood dysarthria (CD) and CAS in children with complex neurodevelopmental disorders has been estimated to 4.9% (Shriberg et al., Citation2019). In a study including 19 Swedish-speaking school-aged children with CD two participants had CD + CAS (Nordberg, Miniscalco, & Lohmander, Citation2014). Nordberg and colleagues (2014) also used speech material from the SVANTE test and reported voicing error as the most frequent consonant error type in CD (89%), compared to 100% in CAS reported in the present study. Voicing errors could hence signal motor speech disorder rather than CAS specifically. On the other hand, deviant prosody in children with CD without CAS was reported in 24%, making prosodic impairment less likely to be found in non-CAS SSD, as proposed in this study for non-CAS CP ± L.
Previous studies have included a wider age range. In the present study a narrow age range was applied in order to increase generalisability.
The feature groping could not be assessed in all participants with CP ± L, which could have affected the results. Half of the participants within the CAS group evidenced groping in contrast to none in the non-CAS SSD group. When looking closer at participants with groping (n = 4) all had a total of seven or eight CAS features, i.e. thus, excluding groping for all participants in this study would not have changed numbers within subgroups.
Murray and colleagues (Citation2015) calculated increased difficulties with multisyllabic words as the ratio between the number of phonemes correct in 12 monosyllabic and 12 multisyllabic words. This was not possible in this study due to the lack of multisyllabic words, also requiring a certain level of speech proficiency, not met by all participants. It could be hypothesised that there is less difference between the used complex disyllabic words and monosyllabic words, resulting in less of a difference in complexity, impacting this features’ occurrence. However, the feature has been reported not to discriminate children with and without CAS effectively (Iuzzini-Seigel & Murray, Citation2017).
Both intra- and inter-transcriber agreement were determined in this study. Though the median inter-transcriber agreement for consonants and vowels was good, agreement was low for some participants. The two cases in the lower range for consonants and vowels came from children with a more severe degree of speech deviance. The analysis of severely disordered speech in children with sCAS and in children with CP ± L is a well-known challenge (e.g. Iuzzini-Seigel, Citation2019; Kuehn & Moller, Citation2000; Lohmander, Citation2011).
Conclusions and clinical implications
All children with CAS, regardless of having comorbid CP ± L or not, shared a distinct set of CAS features and a feature distribution fulfilling the three primary ASHA criteria (ASHA, Citation2007). This indicates that the checklist proposed by Iuzzini-Seigel and Murray (Citation2017) has the ability to distinguish between Swedish-speaking 5-year-olds with CAS and non-CAS supporting its cross-linguistic applicability. Noticeable is that prosodic impairment was found almost exclusively in children with CAS. In addition, a measure of vowel inconsistency showed promising potential in discriminating between Swedish-speaking children with and without CAS. CAS was found more frequently in CP ± L and SSD compared to reported estimates of clinical prevalence.
Clinical SLPs need to consider the co-occurrence of CAS in children with CP ± L and persisting SSD to ensure specific and evidence-based intervention approaches beyond those targeting cleft speech characteristics when needed.
Acknowledgements
We are grateful to Susanne Rex, SLP, for her contribution during consensus judgement and Emilie Hagberg, SLP, for participation in assessment and transcription.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- American Speech-Language-Hearing Association, Ad Hoc Committee on Apraxia of Speech in Children. (2007). Childhood Apraxia of Speech [Technical Report]. Rockville (MD): ASHA, Retrieved from: http://www.asha.org/policy/TR2007-00278/
- Bakke, M., Bergendal, B., McAllister, A., Sjögreen, L., & Åsten, P. (2007). Development and evaluation of a comprehensive screening for orofacial dysfunction. Swedish Dental Journal, 31, 75–84.
- Cleland, J., Wood, S., Hardcastle, W., Wishart, J., & Timmins, C. (2010). Relationship between speech, oromotor, language and cognitive abilities in children with Down’s syndrome. International Journal of Language & Communication Disorders, 45, 83–95. doi:https://doi.org/10.3109/13682820902745453
- D’Antonio, L.L., Scherer, N.J., Miller, L.L., Kalbfleisch, J.H., & Bartley, J.A. (2001). Analysis of speech characteristics in children with velocardiofacial syndrome (VCFS) and children with phenotypic overlap without VCFS. Cleft Palate-Craniofacial Journal, 38, 455–467. doi:https://doi.org/10.1597/1545-1569_2001_038_0455_aoscic_2.0.co_2
- Dodd, B., Reilly, S., Ttofari Eecen, K., & Morgan, A.T. (2018). Articulation or phonology? Evidence from longitudinal error data. Clinical Linguistics and Phonetics, 32, 1027–1041. doi:https://doi.org/10.1080/02699206.2018.1488994
- Hagberg, C., Larson, O., & Milerad, J. (1998). Incidence of cleft lip and palate and risks of additional malformations. Cleft Palate-Craniofacial Journal, 35, 40–45. doi:https://doi.org/10.1597/1545-1569_1998_035_0040_ioclap_2.3.co_2
- Harding, A., & Grunwell, P. (1998). Active versus passive cleft-type speech characteristics. International Journal of Language and Communication Disorders, 33, 329–352. doi:https://doi.org/10.1080/136828298247776
- IPA. (2005). The International Phonetic Alphabet. Retrieved from https://www.internationalphoneticassociation.org/content/full-ipa-chart
- Iuzzini, J. (2012). Inconsistency of speech in children with childhood apraxia of speech, phonological disorders and typically developing speech [Unpublished doctoral dissertation].
- Iuzzini, J., & Forrest, K. (2010). Evaluation of a combined treatment approach for childhood apraxia of speech. Clinical Linguistics and Phonetics, 24, 335–345. doi:https://doi.org/10.3109/02699200903581083
- Iuzzini-Seigel, J. (2019). Motor performance in children with childhood apraxia of speech and speech sound disorders. Journal Speech, Language, and Hearing Research, 62, 3220–3233. doi:https://doi.org/10.1044/2019_JSLHR-S-18-0380
- Iuzzini-Seigel, J., & Murray, E. (2017). Speech assessment in children with childhood apraxia of speech. Perspectives of the ASHA Special Interest Groups, 2, 47–60. doi:https://doi.org/10.1044/persp2.SIG2.47
- Iuzzini-Seigel, J., Hogan, T.P., & Green, J.R. (2017). Speech inconsistency in children with childhood apraxia of speech, language impairment, and speech delay: Depends on the stimuli. Journal of Speech, Language, and Hearing Research, 60, 1194–1210. doi:https://doi.org/10.1044/2016_JSLHR-S-15-0184
- Kuehn, D.P., & Moller, K.T. (2000). Speech and language issues in the cleft palate population: The state of the art. Cleft Palate-Craniofacial Journal, 37, 1–35. doi:https://doi.org/10.1597/1545-1569_2000_037_0348_saliit_2.3.co_2
- Kummer, A.W., Lee, L., Stutz, L.S., Maroney, A., & Brandt, J.W. (2007). The prevalence of apraxia characteristics in patients with velocardiofacial syndrome as compared with other cleft populations. Cleft Palate-Craniofacial Journal, 44, 175–181. doi:https://doi.org/10.1597/05-170.1
- Liégeois, F.J., Turner, S.J., Mayes, A., Bonthrone, A.F., Boys, A., Smith, L., … Morgan, A.T. (2019). Dorsal language stream anomalies in an inherited speech disorder. Brain, 142, 966–977. doi:https://doi.org/10.1093/brain/awz018
- LKG-registret [The Swedish National Quality Registry for Cleft Lip and Palate]. Lund, Sweden: https://lkg-registret.se
- Lohmander, A. (2011). Surgical intervention and speech outcomes in cleft lip and palate. In S. Howard, & A. Lohmander (Eds.), Cleft palate speech: Assessment and intervention (pp. 55–82). Chichester, UK: Blackwell Publishing.
- Lohmander, A., Lundeborg, I., & Persson, C. (2017b). SVANTE - The Swedish articulation and nasality test - normative data and a minimum standard set for cross-linguistic comparison. Clinical Linguistics and Phonetics, 31, 137–154. doi:https://doi.org/10.1080/02699206.2016.1205666
- Lohmander, A., & Persson, C. (2008). A longitudinal study of speech production in Swedish children with unilateral cleft lip and palate and two-stage palatal repair. Cleft Palate-Craniofacial Journal, 45, 32–41. doi:https://doi.org/10.1597/06-123.1
- Lohmander, A., Persson, C., Willadsen, E., Lundeborg, I., Alaluusua, S., Aukner, R., … Semb, G., et al. (2017a). Scandcleft randomised trials of primary surgery for unilateral cleft lip and palate: 4. Speech outcomes in 5-year-olds - velopharyngeal competency and hypernasality. Journal of Plastic Surgery and Hand Surgery, 51, 27–36. doi:https://doi.org/10.1080/2000656X.2016.1254645
- Malmenholt, A., Lohmander, A., & McAllister, A. (2017). Childhood apraxia of speech: A survey of praxis and typical speech characteristics. Logopedics Phoniatrics Vocology, 42, 84–92. doi:https://doi.org/10.1080/14015439.2016.1185147
- Malmenholt, A., McAllister, A., & Lohmander, A. (2019). Orofacial function, articulation proficiency, and intelligibility in 5-year-old children born with cleft lip and palate. Cleft Palate-Craniofacial Journal, 56, 321–330. doi:https://doi.org/10.1177/1055665618783154
- McAllister, R. (1998). Talkommunikation [Speech communication] (pp. 193–200). Lund, Sweden: Studentlitteratur. [in Swedish]
- McCabe, P., Rosenthal, J.B., & McLeod, S. (1998). Features of developmental dyspraxia in the general speech-impaired population? Clinical Linguistics & Phonetics, 12, 105–126. doi:https://doi.org/10.3109/02699209808985216
- Morgan, A.T., & Webster, R. (2018). Aetiology of childhood apraxia of speech: A clinical practice update for paediatricians. Journal of Paediatrics and Child Health, 54, 1090–1095. doi:https://doi.org/10.1111/jpc.14150
- Murray, E., McCabe, P., Heard, R., & Ballard, K.J. (2015). Differential diagnosis of children with suspected childhood apraxia of speech. Journal of Speech, Language, and Hearing Research, 58, 43–60. doi:https://doi.org/10.1044/2014_JSLHR-S-12-0358
- Nordberg, A., Miniscalco, C., & Lohmander, A. (2014). Consonant production and overall speech characteristics in school-aged children with cerebral palsy and speech impairment. International Journal of Speech-Language Pathology, 16, 386–395. doi:https://doi.org/10.3109/17549507.2014.917440
- Nyberg, J., Neovius, E., & Lohmander, A. (2018). Speech outcomes at 5 and 10 years of age after one-stage palatal repair with muscle reconstruction in children born with isolated cleft palate. Journal of Plastic Surgery and Hand Surgery, 52, 20–29. doi:https://doi.org/10.1080/2000656X.2017.1316282
- Persson, C., Elander, A., Lohmander-Agerskov, A., & Söderpalm, E. (2002). Speech outcomes in isolated cleft palate: Impact of cleft extent and additional malformations. Cleft Palate-Craniofacial Journal, 39, 397–408. doi:https://doi.org/10.1597/1545-1569_2002_039_0397_soiicp_2.0.co_2
- Peterson-Falzone, S.J. (2011). Types of clefts and multianomaly craniofacial conditions. Seminars in Speech and Language, 32, 93–114. doi:https://doi.org/10.1055/s-0031-1277713
- Prathanee, B., Pumnum, T., Seepuaham, C., & Jaiyong, P. (2016). Five-year speech and language outcomes in children with cleft lip-palate. Journal of Craniomaxillofacial Surgery, 44, 1553–1560. doi:https://doi.org/10.1016/j.jcms.2016.08.004
- Sell, D., Mildinhall, S., Albery, L., Wills, A.K., Sandy, J.R., & Ness, A.R. (2015). The Cleft Care UK study. Part 4: Perceptual speech outcomes. Orthodontics and Craniofacial Research, 18 Suppl 2, 36–46. doi:https://doi.org/10.1111/ocr.12112
- Semel, E., Wiig, E.H., & Secord, W.A. (2013) Clinical Evaluation of Language Fundamentals-Forth Edition (CELF-4), Swedish version (4th ed.). Stockholm, Sweden: Pearson Assessment.
- Shriberg, L.D., Potter, N.L., & Strand, E.A. (2011). Prevalence and phenotype of childhood apraxia of speech in youth with galactosemia. Journal of Speech, Language, and Hearing Research, 54, 487–519. doi:https://doi.org/10.1044/1092-4388(2010/10-0068)
- Shriberg, L.D., Strand, E.A., Fourakis, M., Jakielski, K.J., Hall, S.D., Karlsson, H.B., … Wilson, D.L., et al. (2017). A diagnostic marker to discriminate childhood apraxia of speech from speech delay: III. Theoretical coherence of the Pause Marker with speech processing deficits in childhood apraxia of speech. Journal of Speech, Language, and Hearing Research, 60, S1135–S1152. doi:https://doi.org/10.1044/2016_JSLHR-S-15-0298
- Shriberg, L.D., Strand, E.A., Jakielski, K.J., & Mabie, H.L. (2019). Estimates of the prevalence of speech and motor speech disorders in persons with complex neurodevelopmental disorders. Clinical Linguistics and Phonetics, 33, 707–736. doi:https://doi.org/10.1080/02699206.2019.1595732
- Waring, R., & Knight, R. (2013). How should children with speech sound disorders be classified? A review and critical evaluation of current classification systems. International Journal of Language and Communication Disorder, 48, 25–40. doi:https://doi.org/10.1111/j.1460-6984.2012.00195.x
- Willadsen, E., Lohmander, A., Persson, C., Lundeborg, I., Alaluusua, S., Aukner, R., … Semb, G., et al. (2017). Scandcleft randomised trials of primary surgery for unilateral cleft lip and palate: 5. Speech outcomes in 5-year-olds - consonant proficiency and errors. Journal of Plastic Surgery and Hand Surgery, 51, 38–51. doi:https://doi.org/10.1080/2000656X.2016.1254647
- Zuk, J., Iuzzini-Seigel, J., Cabbage, K., Green, J.R., & Hogan, T.P. (2018). Poor speech perception is not a core deficit of childhood apraxia of speech: preliminary findings. Journal of Speech, Language, and Hearing Research, 61, 583–592. doi:https://doi.org/10.1044/2017_JSLHR-S-16-0106

