Abstract
Abscesses are a common problem in goat farms worldwide. In most cases, the disease is caused by Corynebacterium pseudotuberculosis that is the aetiological agent of caseous lymphadenitis (CLA). CLA causes considerable economic losses, due to reduced milk production, and to carcase damage. At present, no effective therapy for CLA is available and vaccine use is limited. The current study presents evidence for loci associated to CLA susceptibility identified using the 50K Goat SNP panel in a case-control genome wide association study. The analysis of the genotype data identified four chromosomal regions associated with disease status: on chromosomes 5, 7, 8 and 11. These results provide the first evidence for genetic loci involved in the immunological response to CLA in goat. Knowledge of genetic variations related to susceptibility will facilitate the incorporation of this information for the improvement of health status in the goat species.
Introduction
Abscesses are a common problem in goat farms all over the world: they have been reported in Africa (e.g. Ethiopia; Egypt; Oreiby et al. Citation2014, and Abebe & Sisay Tessema Citation2015), North America (e.g. Quebec; Debien et al. Citation2013), South America (e.g. Brazil; Seyffert et al. Citation2010), Asia (e.g. Korea; Jung et al. Citation2015), Australia (Peel et al. Citation1997) and Europe (e.g. Czech Republic; Skalka et al. Citation1998; Italy; Battini et al. Citation2016, and Portugal; Can et al. Citation2016). Surveys in Italy and Portugal revealed the presence of abscesses in 90% of the visited farms in both countries (Battini et al. Citation2016; Can et al. Citation2016), whereas in Brazil CLA was present in 98% of the herds (Seyffert et al. Citation2010). The problem is therefore widespread and it may jeopardise animal welfare (Ferrante et al. Citation2012) and cause economic losses, mainly due to reduced milk production, and to carcase damage (Fontaine & Baird Citation2008; Regassa et al. Citation2013).
External abscesses are usually located in superficial lymph nodes, mainly in the head, jaw, neck, shoulder and precrural regions, and contain a purulent, thick, greenish-yellowish material. In most cases, the disease is caused by Corynebacterium pseudotuberculosis that is the aetiological agent of caseous lymphadenitis (CLA; Al-Gaabary et al. Citation2009; Smith & Sherman Citation2009) and can cause necrotising granulomatous lymphadenitis also in humans, although human infections rarely occurs (Mills et al. Citation1997; Peel et al. Citation1997). CLA affects especially adult animals (Jung et al. Citation2015), is highly contagious and can be transmitted by direct contact among infected animals or by indirect contact of goats with body lesions with water, soil, straw or housing structures contaminated with the purulent contents deriving from the rupture of the abscesses (Fontaine & Baird Citation2008). Therefore, CLA can spread rapidly and its complete eradication from an infected herd is very difficult. Abscesses can be surgically excised, drained, washed and disinfected, but they often reappear in a short time. Additional procedures for the control of the disease are the culling of infected animals and the separation of kids from infected mothers (Simpson & Washburn Citation2012). However, at present, no really effective therapy is available to limit or control the spread of abscesses in goat herds. Vaccination has been suggested as a potential solution to the problem. A vaccine is produced in the US and is presently available on the market, but it proved to be effective mainly for sheep (Smith & Sherman Citation2009). Promising results have been recently achieved in a field experiment by Bencetti et al. (Citation2014) using vaccines obtained from the isolation of bacterial strains deriving from lesions of goats from a specific herd. However, vaccination should be repeated for several consecutive years, because of the high risk of having positive animals that may spread again the disease in the herd (Smith & Sherman Citation2009). Moreover, autogenous vaccines are more costly than commercial vaccines, therefore they may be viable only if utilised in large herds (Simpson & Washburn Citation2012).
For these reasons, identifying loci associated with susceptibility to abscesses in goats could represent an important step towards the control and, possibly, the help in the reduction of incidence of the disease. The current study presents evidence for loci associated to CLA susceptibility in alpine goats that were identified using the 50K Goat SNP panel (Illumina goat SNP50 Bead Chip whole genome SNP assay) in a case-control study using the presence of abscesses as the definition of susceptibility.
Materials and methods
Animals
Samples were collected from 153 secondiparous Alpine goats, daughters of 12 bucks, reared at "G.P. Guidobono Cavalchini" experimental farm of the University of Milan in Borgo Adorno (Cantalupo Ligure, Alessandria, Italy). CLA was diagnosed and confirmed by the herd veterinarian. For collecting phenotypic data, all goats were individually restrained and their whole body was accurately palpated to identify the presence and location of external abscesses. Positive cases were defined as animals having one or more abscesses and negative controls were defined as animals showing absolutely no sign of external abscesses. Confirmation of the presence of Corynebacterium pseudotuberculosis in all the animals that showed the presence of abscesses was not possible. However, previous bacteriological analysis on abscess material collected in the same herd had already confirmed the presence of bacteria at herd level. Furthermore, two goats with abscesses used for the present investigation were slaughtered shortly after the end of the experiment. At the slaughterhouse, the presence of internal abscesses in their lungs was recorded and bacteriological analysis of the lung tissues confirmed the presence of Corynebacterium pseudotuberculosis.
Blood samples were collected in EDTA vacutainers (BD Vacutainer Systems, Plymouth, UK), immediately refrigerated at 4 °C and then stored at -20 °C until analysis. DNA was extracted from whole blood samples using the NucleoSpin® Blood kit (Macherey-Nagel, Düren, Germany) starting from 200 μl of blood. Extracted DNA was analysed using a NanoDrop ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA) to assess DNA concentration and purity, whereas DNA integrity was verified by electrophoresis in 1% agarose gel. Due to economic constraints, 70 samples (25 positive and 45 negative cases), randomly selected from the whole herd, were genotyped by GeneSeek Inc (Nebraska, USA) using the Illumina GoatSNP50 BeadChip. Genotypes were assigned using BEADSTUDIO (Illumina, San Diego) software.
Genotype quality assurance
Genotype quality assurance was performed within the R statistical environment using the GenABEL package as implemented with the “check.marker” function (Aulchenko et al. Citation2007a). Data was quality controlled for marker call rate, minor allele frequency: markers missing 5% of data, or with MAF of less than 5%, were removed. Genotyping efficiency for samples was also verified and samples with more than 5% missing data were removed.
Statistical analysis
Genome-wide association analysis was performed using the GenABEL package (Aulchenko et al. Citation2007a) in R using a three step GRAMMAR-CG approach, (Genome wide Association using Mixed Model and Regression – Genomic Control), with the extension of using the genomic kinship matrix estimated through genomic marker data, instead of the pedigree (Amin et al. Citation2007, Aulchenko et al. Citation2007b). The relationship matrix used in the analysis was estimated using genomic data with the ‘‘ibs’’ (option weight = ‘‘freq’’) function of GenABEL. The Genomic Control (GC) approach was used to correct for conservativeness of the GRAMMAR test, based on the estimation of the lambda factor, which is the median of all genome-wide observed test statistics divided by the expected median of the test statistic under the null hypothesis of no association, assuming that the number of true associations is very small compared to the number of tests that are actually performed. Cases were set to 1 and negative controls to 0. Uncorrected p-values lower than 10 × 10−6 were accepted to represent strong proof of genome-wide association, while p-values lower than 10 × 10−5 were considered as moderately significant associations.
SNP location and gene names were based on the Goat Chinese Assembly CHI_1.0 assembly composed of 77,432 scaffolds and 337,495 contigs. Full details on the CHIR_1.0 goat genome assembly are available at: http://www.ncbi.nlm.nih.gov/assembly/GCA_000317765.1. All analyses were carried out within the R statistical environment (http://www.r-project.org).
Results
Phenotype
Fifty-eight out of 153 goats (37.9%) were positive to the presence of external abscesses. Forty-three (74.1%) had only one abscess, 13 (22.4%) had two abscesses and only 2 (3.5%) had three abscesses. Thirty-eight percent (37.7%) of the observed abscesses were located in the shoulders, 23.2% in the hind legs, 18.8% in the ears, 13.0% in the neck, 4.3% in the jaw and 1.4% in the udder and in the parotid. The 70 goats randomly selected for genetic analysis were representative of the whole herd, with a prevalence of abscess of 35.7% and were composed of 25 animals with at least one abscess and 45 animals with no external abscesses.
Genotype quality assurance and internal population structure analysis
Following quality control cheques, 2895 of the 53,347 markers were excluded because of low (< 95%) call rate and 1327 markers were excluded because of low minor allele frequency (MAF) < (0.05). With respect to the samples: one sample was removed because of low call rate (< 0.95).
The mean heterozygosity of the samples was 0.39 ± 0.03. Consequently, the final data set that passed the quality controls and was used in the association analysis contained 49,125 genome wide SNPs and 69 samples. Full details of quality check results are given in Table .
Table 1. Distribution of minor allele frequency, successful genotypes per marker, sample heterozygosity and mean heterozygosity.
Genome wide association analysis
Genome Wide Analysis (GWA) identified SNPs with significant association on chromosomes 5, 7, 8 and 11. Genome-wide Manhattan plot displaying the Genome wide association results with respect to their genomic position, is shown in Figure and details are given in Table . All the markers with significant associations had high call rates, ranging between 0.98 and 1 (Table ). Evidence of population substructure was estimated by the genomic inflation factor λ = 1.18 for a basic chi-square statistics test, and was completely corrected by the GRAMMAR-CG methodology that yielded λ = 1.
Figure 1. Manhattan plot displaying the results (−log10 of p-value) of the genome-wide scan using the GRAMMAR-GC method with respect to their genomic position.
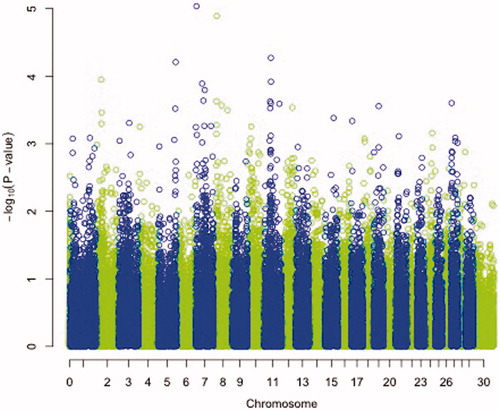
Table 2. List of SNP associated with CLA identified by Genome Wide Association study in goat.
One highly significant SNP was identified on chromosome 7 at positions 3603410 with p-value of 9.21 e-06, (Table ) namely, SNP rs268245496. In addition, 3 further SNPs were identified on chromosomes 5, 8 and 11 at positions 105527370, 18050225 and 39878893 with significant p-values of 6.12 e-05, 1.28 e-05 and 5.35 e-05, respectively. These three SNPs, namely SNPs rs268256983, rs268256651, rs268259057 also had high call rates. A graphical representation of all the chromosomal regions associated is shown in Figure . Table shows MAF and genotype distribution of the significant SNPs and samples.
Table 3. Distribution of samples per genotype and Minor allele frequencies per group.
Discussion
Surprisingly, little is known on the genetic loci involved in resistance/susceptibility to CLA. From an economical and epidemiological point of view it would be important to select for disease resistant animals in order to decrease steadily the presence of CLA in goat herds. Although the application of genomic technologies, mainly applied in dairy cattle, can decrease the generation interval and boost genetic gain compared with traditional phenotypic selection, it is still important to perform genome wide association studies for infectious diseases to pinpoint and localise the genetic variations that have an effect on health traits, in cases where no effective therapy or prophylaxis are available, as in the case of bovine tuberculosis (Allen et al. Citation2010; Finlay et al. Citation2012; Bermingham et al. Citation2014; Zare et al. Citation2014; Richardson et al. Citation2016) and bovine paratuberculosis (Settles et al. Citation2009; Kirkpatrick et al. Citation2011; Minozzi et al. Citation2012; van Hulzen et al. Citation2012; Alpay et al. Citation2014; Pauciullo et al. Citation2015), allowing the possibility to include the genetic approach as a complementary aid for disease incidence reduction.
Recent developments in the animal genome sequencing technologies have allowed the application of a series of methods designed to identify regions of the genome linked or that affect certain characters of economic interest in cattle (e.g., production and/or resistance to diseases in dairy cattle) (Minozzi et al. Citation2013) and in small ruminants, suitable when pedigree information is not available or unreliable. Genome wide association (GWA) studies are now widely used to estimate the association among single nucleotide polymorphisms distributed across the whole genome and one or more traits. This field has rapidly advanced through the availability of thousands of SNPs that can be genotyped or obtained from sequence information in a cost-effective manner, even in livestock. Furthermore, robust methodology has been developed to account for genetic background, based on a polygenic model and which is implemented in the GRAMMAR-CG approach used in the analysis presented here. This approach was able to disentangle the cryptic relatedness in our goat cohort by modelling the polygenic relatedness between pairs of samples (Amin et al. Citation2007).
In the present work, a genome wide scan using a relatively high density of markers identified significant associations between four SNPs and the presence of CLA infection on chromosomes 5, 7, 8 and 11. Although we could not have a serological confirmation of the presence of Corynebacterium pseudotuberculosis in all the animals that showed the presence of abscesses, our results support the attribution of the presence of abscesses to CLA, on the basis of several considerations: first of all, the wide spread of the disease in the herd and the localisation of the abscesses near the lymph nodes are typical of Corynebacterium pseudotuberculosis (Smith & Sherman Citation2009); besides this, all goats were secondiparous, and therefore adult animals, and the risk of CLA seems to be higher in adult animals and increases with age (Abebe & Sisay Tessema Citation2015). As previously described, the presence of CLA was further confirmed by previous bacteriological analysis in the same herd and by bacteriological analysis of the lung tissues of two culled goats. Given these considerations, we are reasonably sure that the goats with abscesses were affected by CLA.
Our results identified a SNP on chromosome 7 at position 3,603,410 which was significantly associated with CLA. The SNP, rs268245496, lies in the intronic region of the neurocan gene (NCAN). The NCAN gene, in human, is associated to schizophrenia and bipolar disorder (Schultz et al. Citation2014).
Interestingly, even the third most significant SNP identified in our study (rs268256651), situated on goat Chromosome 11 at position 39,878,893 bp lies close to two genes that have been found to be associated to schizophrenia: the FANCL and VRK2 gene (Steinberg et al. Citation2011; Schizophrenia Working Group of the Psychiatric Genomics Consortium, Citation2014).
The FANCL gene (Fanconi anaemia complementation group L) is situated in position 40,263,578 – 40,351,518 (Gene ID: 102190968). The human homologue of the FANCL gene has been found to be associated to the Fanconi anaemia, that is a genetically heterogeneous recessive disorder described by cytogenetic instability, hypersensitivity to DNA crosslinking agents, augmented chromosomal breakage, and defective DNA repair (Vetro et al. Citation2015; Ceccaldi et al. Citation2016). Interestingly, two SNPs located close to the human FANCL gene on Human Chromosome 2 (rs75575209 and rs11682175) have been found to be associated to Schizophrenia in a multi stage genome wide association study conducted on 36,989 cases and 113,075 controls (Schizophrenia Working Group of the Psychiatric Genomics Consortium, Citation2014).
Further, the VRK2 gene is located in the same region in both goats and humans. The gene, in the human genome, is located on chromosome 2 close the FANCL gene and consequently close to the above mentioned SNPs associated to schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, Citation2014). Moreover, the VRK2 gene has been further validated to be highly associated to schizophrenia (p value 1.9 × 10−9) in an association study conducted by Steinberg et al. (Citation2011).
Interestingly, in the abovementioned studies several associations were enriched among genes expressed in tissues that have main functions in immunity, offering support for the link between the immune system and schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, Citation2014).
The pathophysiology of schizophrenia is unknown; however, it is known that it is a highly heritable disorder (Sullivan et al. Citation2012). Epidemiological studies have long hypothesised the role of immune dysregulation in schizophrenia (Benros et al. Citation2012). Altered immune function, together with altered lipid metabolism, have been found to be associated or better, identified as risk factors, to several other psychiatric disorders with a genetic basis as Alzeheimer’s disease; however, until few years ago, it was not clear if they represented a causation or a revers causation (Guerreiro & Hardy Citation2011).
Indeed, an immunological hypothesis has become progressively prominent when analysing the aetiology of schizophrenia (Muller & Schwarz Citation2010). In detail, both bacterial and viral infections have been suggested to be causally linked with schizophrenia because of inflammatory mechanisms, which possibly affect the brain through different pathways that are not necessarily mutually exclusive. Stress, infections and inflammation are a set of possible events that can modify the blood brain barrier and might allow immune stimuli to disturb the central nervous system (Irani & Lang, Citation2008). In human, since 1896 bacterial infections have been identified to have a casual relationship with psychoses (Noll, Citation2007). Recent studies have identified associations between schizophrenia and Toxoplasma gondii, human herpesvirus 2, Borna disease virus, human endogenous retrovirus W, Chlamydophila psittaci and Chlamydophila pneumonia (Arias et al. Citation2012). Furthermore, a significant dose-response relationship was found between autoimmune diseases, number of severe infections and risk of schizophrenia (Benros et al. Citation2012). However, how the immunological process affects the brain is still unclear, although the hypothesis of the potential contribution of infections to the development of schizophrenia remains plausible. We can therefore possibly speculate that there could be a common genetic predisposition, although this hypothesis still has to be confirmed in goats, as neurological abnormalities still have to be addressed in this species concomitantly to immune measures.
It is worth noting that even candidate genes identified by GWAs for bovine paratuberculosis, such as the protein tyrosine phosphatase, receptor type, D (PTPRD) gene, located on chromosome 8 in cattle (Minozzi et al. Citation2010), have been found to be associated in humans to neurological disorders such as the restless leg syndrome (Schormair et al. Citation2008), linking immunological and neurological pathologies even in this case.
Additional potential candidate genes under the SNPs on chromosome 5 (rs268259057) and 8 (rs268256983) have not been identified, as the regions are not particularly gene rich. However, it must be noticed that in the future, a new assembly of the goat genome will be released that could shed light in further finding.
In conclusion, most of the genes identified by our study have been found to be associated to schizophrenia in humans. Evidence of predisposition or causation between bacterial infections and schizophrenia has long been hypothesised in human studies (Noll Citation2007). Thus, in the case of CLA that is caused by Corynebacterium pseudotuberculosis infection, these findings suggest that the genes identified could be possible positional candidates.
Conclusions
The genome wide association analysis conducted in this study identified four markers identified associated with resistance or susceptibility to CLA in goats, what when confirmed in a wider cohort, could be used in breeding strategies to reduce disease incidence. These encouraging findings represent the first insights in the role of genetics of CLA in goats. In dairy cattle, exploitation of genetic variability has already been established and used in selection for mastitis resistance (Rupp & Boichard Citation2003). Such an approach could be applied as a long-lasting and sustainable complement to sanitary controls, especially considering that no really effective therapy is presently available to limit or control the spread of CLA in goat herds.
Confirmation of the data presented here will be sought in further replication studies in a wider population that in this case was limited due to economical constrains, as well as in studies in different goat breeds together with serological confirmation. Moreover, the four markers identified could also be the starting point in identifying the genes and hence the biological pathways, associated with response to infection with Corynebacterium pseudotuberculosis, which may be useful in developing diagnostic tests, or therapeutic approaches to further control the disease.
Ethical approval
The ethical approval was given by the OPBA (Organismo Preposto al Benessere Animale) of the University of Milan.
Acknowledgments
We are grateful to Dr Pietro Riccaboni and Diana Binanti of the Dipartimento di Medicina Veterinaria of the University of Milan for the inspection of goats at the slaughterhouse and for performing the bacteriological analysis of lung tissues and to the Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna (IZSLER) – Lodi Section, for the bacteriological analysis of abscesses content. We also thank Dr Chiara Caslini and Mario Villa for their contribution in the collection of phenotypic information.
Disclosure statement
The authors declare that they have no competing interests.
References
- Abebe D, Sisay Tessema T. 2015. Determination of Corynebacterium pseudotuberculosis prevalence and antimicrobial susceptibility pattern of isolates from lymph nodes of sheep and goats at an organic export abattoir, Modjo, Ethiopia. Lett Appl Microbiol. 61:469–476.
- Al-Gaabary MH, Osman SA, Oreiby AF. 2009. Caseous lymphadenitis in sheep and goats: Clinical, epidemiological and preventive studies. Small Rumin Res. 87:116–121.
- Allen AR, Minozzi G, Glass EJ, Skuce RA, McDowell SW, Woolliams JA, Bishop SC. 2010. Bovine tuberculosis: the genetic basis of host susceptibility. Proc Biol Sci. 277:2737–2745.
- Alpay F, Zare Y, Kamalludin MH, Huang X, Shi X, Shook GE, Collins MT, Kirkpatrick BW. 2014. Genome-wide association study of susceptibility to infection by Mycobacterium avium subspecies paratuberculosis in Holstein cattle. PLoS One. 9:e111704.
- Amin N, van Duijn CM, Aulchenko YS. 2007. A genomic background based method for association analysis in related individuals. PLoS One. 2:e1274.
- Arias I, Sorlozano A, Villegas E, et al. 2012. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 136:128–136.
- Aulchenko YS, de Koning DJ, Haley C. 2007b. Genome wide rapid association using mixed model and regression: a fast and simple method for genome-wide pedigree-based quantitative trait loci association analysis. Genetics. 177:577–585.
- Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. 2007a. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 23:1294–1296.
- Battini M, Barbieri S, Vieira A, Stilwell G, Mattiello S. 2016. Results of testing the prototype of the AWIN welfare assessment protocol for dairy goats in 30 intensive farms in Italy. Italian J Anim Sci. 15(2):283–293.
- Bencetti F, Pedrini G, Gaffuri A, Martinelli N, Lombardi G. 2014. Risultati preliminari dell’uso di vaccini stabulogeni per il controllo della “malattia degli ascessi” in allevamenti di capre da latte del Nord Italia. Large Anim Rev. 20:115–116.
- Benros ME, Mortensen PB, Eaton WW. 2012. Autoimmune diseases and infections as risk factors for schizophrenia. Ann NY Acad Sci. 1262:56–66.
- Bermingham ML, Bishop SC, Woolliams JA, Pong-Wong R, Allen AR, McBride SH, Ryder JJ, Wright DM, Skuce RA, McDowell SW, et al. 2014. Genome-wide association study identifies novel loci associated with resistance to bovine tuberculosis. Heredity (Edinb). 112:543–551.
- Can E, Vieira A, Battini M, Mattiello S, Stilwell G. 2016. On-farm welfare assessment of dairy goat farms using animal-based indicators: the example of 30 commercial Portuguese farms. Acta Agriculturae Scandinavica, Section a - Animal Science. 66:43–55.
- Ceccaldi R, Sarangi P, D'Andrea AD. 2016. The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol. 17:337–349.
- Debien E, Hélie P, Buczinski S, Leboeuf A, Bélanger D, Drolet R. 2013. Proportional mortality: A study of 152 goats submitted for necropsy from 13 goat herds in Quebec, with a special focus on caseous lymphadenitis. Can Vet J. 54:581–587.
- Ferrante V, Battini M, Caslini C, Grosso L, Mantova E, Noè L, Barbieri S, Mattiello S. 2012. Presence of abscesses as a welfare indicator in dairy goats: a preliminary study. Proceeding of XLVI congress of the International Society for Applied Ethology. Wien (Austria); p 224.
- Finlay EK, Berry DP, Wickham B, Gormley EP, Bradley DG. 2012. A genome wide association scan of bovine tuberculosis susceptibility in Holstein-Friesian dairy cattle. PLoS One. 7:e30545.
- Fontaine MC, Baird GJ. 2008. Caseous lymphadenitis. Small Ruminant Res. 76:42–48.
- Guerreiro RJ, Hardy J. 2011. Alzheimer's disease genetics: lessons to improve disease modelling. Biochem Soc Trans. 39:910–916.
- Irani S, Lang B. 2008. Autoantibody-mediated disorders of the central nervous system. Autoimmunity. 41:55–65.
- Jung BY, Lee SH, Kim HY, Byun JW, Shin DH, Kim D, Kwak D. 2015. Serology and clinical relevance of Corynebacterium pseudotuberculosis in native Korean goats (Capra hircus coreanae). Trop Anim Health Prod. 47:657–661.
- Kirkpatrick BW, Shi X, Shook GE, Collins MT. 2011. Whole-Genome association analysis of susceptibility to paratuberculosis in Holstein cattle. Anim Genet. 42:149–160.
- Mills AE, Mitchell RD, Lim EK. 1997. Corynebacterium pseudotubercolosis is a cause of human necrotizing granulomatous lymphadenitis. Pathology. 29:231–233.
- Minozzi G, Buggiotti L, Stella A, Strozzi F, Luini M, Williams JL. 2010. Genetic loci involved in antibody response to Mycobacterium avium ssp. paratuberculosis in cattle. PLoS One. 5:e11117.
- Minozzi G, Nicolazzi EL, Stella A, Biffani S, Negrini R, Lazzari B, Ajmone-Marsan P, Williams JL. 2013. Genome wide analysis of fertility and production traits in Italian Holstein cattle. PLoS One. 8:e80219.
- Minozzi G, Williams JL, Stella A, Strozzi F, Luini M, Settles ML, Taylor JF, Whitlock RH, Zanella R, Neibergs HL. 2012. Meta-analysis of two genome-wide association studies of bovine paratuberculosis. PLoS One. 7:e32578.
- Muller N, Schwarz MJ. 2010. Immune system and schizophrenia. Curr Immunol Rev. 6:213–220.
- Noll R. 2007. Kraepelin's 'lost biological psychiatry'? Autointoxication, organotherapy and surgery for dementia praecox. Hist Psychiatry. 18:301–320.
- Oreiby AF, Hegazy YM, Osman SA, Ghanem YM, Al-Gaabary MH. 2014. Caseous lymphadenitis in small ruminants in Egypt. Clinical, epidemiological and prophylactic aspects. Tieraerztliche Praxis Ausgabe Grosstiere Nutztiere. 42:271–277.
- Pauciullo A, Küpper J, Brandt H, Donat K, Iannuzzi L, Erhardt G. 2015. Wingless-type MMTV integration site family member 2 (WNT2) gene is associated with resistance to MAP in faecal culture and antibody response in Holstein cattle. Anim Genet. 46:122–132.
- Peel MM, Palmer GG, Stacpoole AM, Kerr TG. 1997. Human lymphadenitis due to Corynebacterium pseudotubercolosis; report of ten cases from Australia and review. Clin Infect Dis. 24:185–191.
- Regassa A, Moje N, Megersa B, Beyene D, Sheferaw D, Debela E, Abunna F, Skjerve E. 2013. Major causes of organs and carcass condemnation in small ruminants slaughtered at Luna Export Abattoir, Oromia Regional State, Ethiopia. Prev Vet Med. 110:139–148.
- Richardson IW, Berry DP, Wiencko HL, Higgins IM, More SJ, McClure J, Lynn DJ, Bradley DG. 2016. A genome-wide association study for genetic susceptibility to Mycobacterium bovis infection in dairy cattle identifies a susceptibility QTL on chromosome 23. Genet Sel Evol. 48:19.
- Rupp R, Boichard D. 2003. Genetics of resistance to mastitis in dairy cattle. Vet Res. 34:671–688.
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511:421–427.
- Schormair B, Kemlink D, Roeske D, Eckstein G, Xiong L, Lichtner P, et al. 2008. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet. 40:946–948.
- Schultz CC, Mühleisen TW, Nenadic I, Koch K, Wagner G, Schachtzabel C, Siedek F, Nöthen MM, Rietschel M, Deufel T, et al. 2014. Common variation in NCAN, a risk factor for bipolar disorder and schizophrenia, influences local cortical folding in schizophrenia. Psychol Med. 44:811–820.
- Settles M, Zanella R, McKay SD, Schnabel RD, Taylor JF, Whitlock R, Schukke Y, Van Kessel JS, Smith JM, Neibergs H. 2009. A whole genome association analysis identifies loci associated with Mycobacterium avium subsp. paratuberculosis infection status in US holstein cattle. Anim Genet. 40:655–662.
- Seyffert N, Guimarães AS, Pacheco LGC, Portela RW, Bastos BL, Dorella FA, Heinemann MB, Lage AP, Gouveia AMG, Meyer R, et al. 2010. High seroprevalence of caseous lymphadenitis in Brazilian goat herds revealed by Corynebacterium pseudotuberculosis secreted proteins-based ELISA. Res Vet Sci. 88:50–55.
- Simpson KM, Washburn KE. 2012. Caseous lymphadenitis: realities in treatment and prevention. Bovine Practitioner. 46:94–100.
- Skalka B, Literak I, Michalik I, Skřivánek M. 1998. Corynebacterium pseudotuberculosis infection in goats in the Czech Republic. Zentralblatt Veterinarmedizin Reihe B. 45:31–35.
- Smith MC, Sherman DM. 2009. Goat medicine. Second edition. Oxford, UK: Wiley-Blackwell.
- Steinberg S, de Jong S, Andreassen OA, Werge T, Børglum AD, Mors O, et al. Irish Schizophrenia Genomics Consortium. 2011. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum Mol Genet. 20:4076–4081.
- Sullivan PF, Daly MJ, O'Donovan M. 2012. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 13:537–551.
- Van Hulzen KJ, Schopen GC, van Arendonk JA, Nielen M, Koets AP, Schrooten C, Heuven HC. 2012. Genome-wide association study to identify chromosomal regions associated with antibody response to Mycobacterium avium subspecies paratuberculosis in milk of Dutch Holstein-Friesians. J Dairy Sci. 95:2740–2748.
- Vetro A, Iascone M, Limongelli I, Ameziane N, Gana S, Della Mina E, Giussani U, et al. 2015. Loss-of-function FANCL mutations associate with severe fanconi anemia overlapping the VACTERL association. Hum Mutat. 36:562–568.
- Zare Y, Shook GE, Collins MT, Kirkpatrick BW. 2014. Genome-wide association analysis and genomic prediction of Mycobacterium avium subspecies paratuberculosis infection in US Jersey cattle. PLoS One. 9:e88380.

