ABSTRACT
Mammalian prions are composed of misfolded aggregated prion protein (PrP) with amyloid-like features. Prions are zoonotic disease agents that infect a wide variety of mammalian species including humans. Mammals and by-products thereof which are frequently encountered in daily life are most important for human health. It is established that bovine prions (BSE) can infect humans while there is no such evidence for any other prion susceptible species in the human food chain (sheep, goat, elk, deer) and largely prion resistant species (pig) or susceptible and resistant pets (cat and dogs, respectively). PrPs from these species have been characterized using biochemistry, biophysics and neurobiology. Recently we studied PrPs from several mammals in vitro and found evidence for generic amyloidogenicity as well as cross-seeding fibril formation activity of all PrPs on the human PrP sequence regardless if the original species was resistant or susceptible to prion disease. Porcine PrP amyloidogenicity was among the studied. Experimentally inoculated pigs as well as transgenic mouse lines overexpressing porcine PrP have, in the past, been used to investigate the possibility of prion transmission in pigs. The pig is a species with extraordinarily wide use within human daily life with over a billion pigs harvested for human consumption each year. Here we discuss the possibility that the largely prion disease resistant pig can be a clinically silent carrier of replicating prions.
Abbreviations
| PrP | = | prion protein |
| HuPrP | = | human prion protein |
| BoPrP | = | bovine prion protein |
| PoPrP | = | porcine prion protein |
| recPrP | = | recombinant prion protein |
| TSE | = | transmissible spongiform encephalopathy |
| BSE | = | bovine spongiform encephalopathy |
| PSE | = | porcine spongiform encephalopathy |
| CJD | = | Creutzfeldt-Jakob disease |
| vCJD | = | variant Creutzfeldt-Jakob disease |
Mammalian prions are composed of misfolded aggregated prion protein (PrP) with amyloid-like features. Prions are zoonotic disease agents that infect a wide variety of mammalian species including humans. Mammals and by-products thereof which are frequently encountered in daily life are most important for human health. It is established that bovine prions (BSE) can infect humans while there is no such evidence for any other prion susceptible species in the human food chain (sheep, goat, elk, deer) and largely prion resistant species (pig) or susceptible and resistant pets (cat and dogs respectively). PrPs from these species have been characterized using biochemistry, biophysics and neurobiology. Recently we studied PrPs from several mammals in vitro and found evidence for generic amyloidogenicity as well as cross-seeding fibril formation activity of all PrPs on the human PrP sequence regardless if the original species was resistant or susceptible to prion disease. Porcine PrP amyloidogenicity was among the studied. Experimentally inoculated pigs as well as transgenic mouse lines overexpressing porcine PrP have, in the past, been used to investigate the possibility of prion transmission in pigs. The pig is a species with extraordinarily wide use within human daily life with over a billion pigs harvested for human consumption each year. Here we discuss the possibility that the largely prion disease resistant pig can be a clinically silent carrier of replicating prions.
Transmissibility of Prp Amyloids
Prions are self-replicating proteinaceous pathogens composed of prion protein, PrP. Albeit the exact mechanisms of prion toxicity is not known, prion diseases are believed to correlate with the rate of prion replication.Citation1,2 Prions are known to be composed of aggregates of misfolded PrP molecules stacked in a beta-pleated-sheet structure with noticeable similarities to amyloid fibrils,Citation3,4 also known as scrapie associated fibrils first discovered by Merz.Citation5
Prion protein amyloid, APrP, is linked to a number of human prion diseases most notably inherited diseases such as Gerstmann- Straussler-Scheinker disease with mutations in the PrP sequence.Citation6 Importantly, in humans infected by BSE, vCJD (bovine prions) or Kuru (human prions) the disease is associated with large amounts of amyloid plaque. Hence these diseases likely represent strains of prions with APrP conformational polymorphs which may mature to amyloid plaque. The significance of amyloid plaques in prion diseases has been subject to considerable discussions.Citation7,8 Amyloid fibrils are defined by pathology as aggregates of misfolded protein forming insoluble non-branched fibrils stabilized by cross-beta-sheet secondary structure with tinctorial properties of Congo red affinity exhibiting birefringence under cross-polarized light.Citation9 Amyloid fibrils are also ThT/ThS positive and represent a mature phase of misfolded protein amyloidogenesis.Citation10 Amyloid fibril formation is a chemical reaction similar to crystallization where a preformed fibril functions as a nucleus, nidus, or seed that accelerates the process of further fibrillation of soluble precursor protein. It is well established that amyloid fibrils show extensive structural polymorphism and is similarly believed to manifest as different pathogenic states representing prion strains in prion diseases.Citation11 The plethora of molecular structures of PrP in infectious and transmissible prions has recently been reviewed by Baskakov and co-workers.Citation8 A consensus structural feature from several studies of APrP is a cross-beta-sheet structure of the C-terminal sequence stretching from a variable starting point from the N-terminus (residue 90–140) all the way toward the final residue 231 within PrP fibrils. Considerable variations in the alignment of inter- and intramolecular beta-strands, packing of sidechains as well as quaternary filament assemblies are believed to compose a vast variability defining the structure of prion strains.
Seeding studies to produce APrP in vitro can mimic the molecular concept of the mechanism of replicative prion transmission. Replication is sustained by access to soluble PrP substrate to feed the reaction of self-replicating APrP fibrils which is accelerated by fragmentation of fibrils.Citation2 Recently we showed that 7 diverse mammalian PrPs are amyloidogenic under near native conditions in vitro.Citation12 Hence we should treat these PrPs as a class of proteins which with ease can transform into amyloid fibrils. We purified native folded recombinant full length prion proteins (recPrP) with human (HuPrP), bovine (BoPrP) and porcine (PoPrP) sequence (among several others) and subjected them to an in vitro fibrillation assay NCCA (native condition conversion assay) employing near native conditions (50 mM phosphate (pH 7.4), 100 mM NaCl, 50 mM KCl, 37°C, vigorous shaking). In this assay all 3 protein sequences were readily converted into amyloid-like fibrils as displayed by ThT kinetics, Congo red birefringence, LCO fluorescence, and transmission electron microscopy.Citation12 Furthermore, seeds (1%, i.e. 50 nM PrP on a monomer basis) from the end point of unseeded reactions were added to freshly prepared recPrP and in all instances the lag times were significantly reduced upon seeding (). Importantly cross-seeding efficiency was essentially refractory to seed-substrate sequence heterology ()
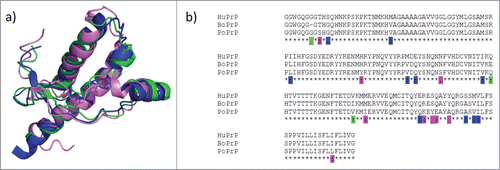
FIGURE 1. Recombinant prion protein from with human, bovine and porcine sequence was fibrillated in vitro under near native conditions as described in Nyström & Hammarström (2015).Citation12 The bars represent lag times in minutes for unseeded (black columns) and seeded with 1% preformed fibrils of all the included sequences (blue for human, green for bovine and pink for porcine).
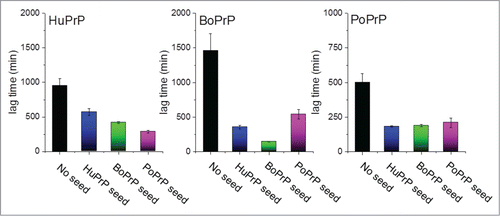
In line with the protein-only hypothesis of prion disease transmission, PrP sequence mismatches of diverse mammalian PrPs have been put forward as one likely modulator of the known species barriers for prion transmission. The overall 3 dimensional fold is well conserved as determined by NMR spectroscopy.Citation13-15 (). Sequence alignments of the human, bovine and porcine PrP sequences show that they are 88–93 % pairwise identical. Between BoPrP, HuPrP and PoPrP there are 10 amino acid substitutions that are unique to human, 3 that are unique to bovine and 9 that are unique to porcine PrP ().
Porcine Prp Amyloidogenicity
From our seeding data ( and reference.Citation12) it is evident that the nucleation dependent polymerization process of fibrillation of these PrPs is not directly dependent of sequence identity between seed and substrate. The main message therefrom is that pure mammalian PrPs are compatible to efficiently cross-seed each other. What mainly attracted our attention was the striking amyloidogenicity of porcine PrP. RecPoPrP spontaneously and rapidly converted in vitro to amyloid fibrils, even faster than human or bovine sequence ().
Despite numerous attempts to infect pigs with prions, hogs appear largely resistant to prion disease even when challenged by prions intracerebrally.Citation16 It is known that incubation time in human disease can stretch over 10 y if the contaminated material is of bovine origin and transmitted via the oral route. Incubation times can be somewhat shorter (6.5–8 years) if the route of transmission is secondary to BSE via blood transfusion.Citation17,18 In Kuru, incubation periods are estimated to be between 4 and 40 y although no species barrier exists in this case.Citation17 Since PrP misfolding appears to be causative in initiating TSEs this implicates that prion disease resistant species such as pigs could harbor replicating prions without showing symptoms. Hence, the notion of separated fibril formation and neurotoxicity signaling merits further studies also in putatively disease resistant species
Prion Strain Adaptation and Possibilities of Porcine Prion Transmission
The need of time and serial passages to allow prion strain adaptation when jumping between species is a well-known fact within the prion field. Numerous transmission experiments using transgenic mice expressing a range of prion sequences from different species have been conducted over the last decades aiming at delineating the species barrier and how this is modulated depending on homology between donor and recipient species as well as prion strain/isolate properties. Importantly transmission has almost exclusively been defined as selection by clinical disease, i.e., based on neurotoxicity. Over 50 mammalian species have been sporadically afflicted by and experimentally or accidentally infected with prions with neurotoxic outcome.Citation19 Evidence for harboring replicating and infectious prions in absence of clinical disease was shown by hamster-mouse experiments exhibiting established species transmission barriers in terms of neurotoxicity.Citation20,21 Obviously there is no simple answer to the question of species barrier modulators and perhaps there is no such thing as a prion resistant species.Citation22 Cross-species strain adaptation of BSE and scrapie in wild type mice was investigated by the Fraser group.Citation23 A number of TSE isolates from cattle and sheep were inoculated into C57BL/6 wild type mice expressing endogenous mouse PrP at 1x level. Cattle BSE was transmitted in the first generation with incubation times spanning between 400 and 500 d (). At third passage the life span of the experimental animals dropped to around 250 d. When using scrapie isolates, the initial inoculation resulted in clinical disease resulting in death between 350 and 850 d post inoculation depending on isolate. Some scrapie isolates failed to transmit to the experimental animals. By the third passage the life spans for all active scrapie isolates converged at around 200–250 d (). These experiments demonstrated that prion strain adaptation is relatively rapid also following host species jump even when PrP expression levels are normal, if the initial inoculum is transmissible.
FIGURE 3. Schematic model of prion strain adaptation. (Model adapted from Collinge and Clarke 2007 and Sandberg et al 2011, 2013.Citation31,49,50) The red horizontal line indicates the tolerance threshold for prion toxicity for the respective model, the green vertical line indicates normal lifespan/experimental termination for the mice. The black curves indicate increase in prion titer over time upon prion inoculation. (a) BSE and classical scrapie in wild type mice according to Bruce et al.Citation23 (b) BSE, classical scrapie and Nor98 scarpie in PoTg001 mice according to Espinosa, Torres et al. (2009, 2014).Citation25,26
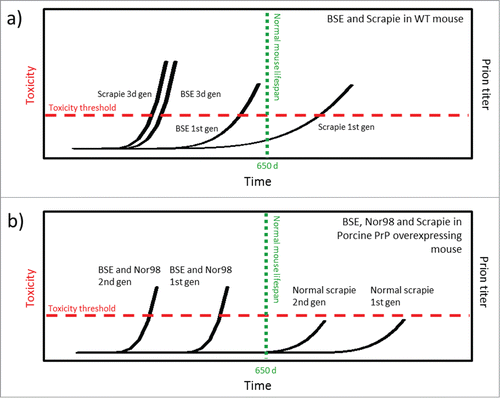
What about pigs? In several recent papers which in our view have not received sufficient attention the notion of prion resistant pigs was challenged by generation of transgenic mice with knocked out endogenous PrP and overexpressed PoPrP. Different lines of tgPoPrP mouse were proven to be susceptible to clinical disease triggered by a variety of prion strains, suggesting that the surrogate host species (mouse) and prion strain are more important than what PrP sequence it expresses for neurotoxicity to commence. In more detail, Torres and colleagues experimentally subjected transgenic mouse lines expressing porcine PrP to a number of different TSE isolates.Citation24-26 Their studies demonstrate that prion infection is strain specific when porcine PrP is overexpressed (4x) and used as in vivo substrate. PoTg001 mice inoculated with classical scrapie, regardless of donor genotype, resisted prion disease both at first and second passage (). On the other hand, Nor98 scrapie (Atypical scrapie) as well as BSE from both cattle and BoTg mouse model resulted in clinical disease in the PoTg001 mice. However, in the first generation, disease progression was slow. Incubation time until death was as long as 600 d and the hit rate was low. This indicates that disease has barely developed by the time the mice reach their natural life span limit which in this study was set to 650 d Already in the second passage the hit rate was 100 % and the incubation time was cut in half (). No further shortening of incubation time was observed upon third passage. This shows that PoPrP is capable of forming infectious and neurotoxic prions in vivo if triggered by a compatible prion strain and if given enough time to develop. Both BSE and Nor98 rapidly adapts to the PoPrP host sequence, resulting in higher penetrance as well as in markedly shorter life span already in the second passage, well within the limits of normal life span for a mouse.
There are several crucial variables which impact the susceptibility of prion diseases and transmission studies.Citation27 PrP sequence of host, PrP sequence of prion, prion strain, prion dosage, PrP expression level of host, host genetic background, route of transmission and neuroinvasiveness if peripherally infected.Citation28 Importantly the PrP expression level corresponds to the rate of prion disease onset.Citation1 This likely reflects 2 converging variables: a) PrP as a substrate to the prion misfolding reaction i.e. self-catalyzed conversion and b) PrP as a mediator of neurotoxicity through interactions with misfolded PrP within prions.
The non-homologous recPrPs presented here and in,Citation12 easily adapt to each other and form amyloid fibrils in accordance with what is seen in vivo when inoculum composed of BoPrP used to challenge mice expressing PoPrP ().Citation24-26 A review of the literature showed that BSE strains have a high degree of penetrance in both experimental and accidental transmission. Over 50% of the species reported to be susceptible to prion disease were infected by a BSE strain.Citation19 Recent data form our lab shows that the promiscuity of BoPrP fibrils holds true also in the case of recombinant in vitro experiments. When cross-seeding human, bovine, porcine, feline and canine PrPs with any of the other, the recBoPrP seed outcompetes the other seeds in all instances except when the HuPrP acted as substrate (Data not shown). In this case recPoPrP fibrils have the highest seeding efficiency (). These findings in combination with the Torres experiments,Citation24-26 implicate that a PoPrP substrate in vivo (in pigs) could adapt to an amyloidogenic prion strain of bovine or ovine prion disease and hence replicate in the new host.
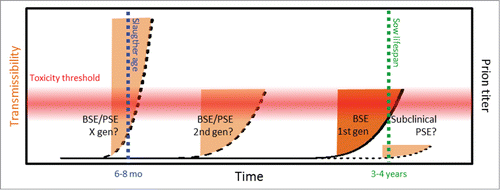
For adaptation of experimental strains through multiple passages, donors are selected based on neurotoxicity (that is on TSE disease phenotype) not on basis of amyloid fibril formation. Hence the traits of transmissible amyloidotypic prion strains may be largely unexplored if these strains require more time to transform to neurotoxic strains e.g. as proposed by Baskakov's model of deformed templating.Citation8 There is experimental evidence for BSE transmission into pig via parenteral routes.Citation16 with an incubation period of 2–3 years, well within what is to be considered normal lifespan. For a breeding sow in industrial scale pig farming that is 3–5 y (Bojne Andersson, personal communication).Citation29,30 In small scale and hobby farming both sows and boars may be kept significantly longer. Collinge and Clarke.Citation31 describe how prion titers reach transmissibility levels well before the prion burden is high enough to be neurotoxic and cause clinical disease. It is known that prion strains need time and serial passages to adapt. Knowing that pigs in modern farming are rarely kept for enough time for clinical signs to emerge in prion infected pigs it is important to be vigilant if there is a sporadic porcine spongiform encephalopathy (PSE) as has been seen in cattle (BASE) and sheep (Nor98). Hypothetically such a sporadic and then infectious event could further adapt and over a few generations have reached the point where clinical PSE is established within the time frame where pigs are being slaughtered for human consumption ()
FIGURE 4. Potential prion strain adaptation in pig. The red horizontal gradient indicates the hietherto unkown prion toxicity tolerance threshold for pigs, the blue vertical line indicates normal slaughter age for industrial pig farming, the green vertical line indicates the normal lifespan of a breeding sow in industrial scale pig farming, orange areas indicate window of neurotoxic prions before onset of clinical disease (dark orange indicates subclinical BSE as reported by Wells et al,Citation16 pale orange indicate hypothetic outcome of PSE and strain adaptation. On the outmost right a potential subclinical sporadic PSE.
Use of Materials Derived From Pig in View of Porcine Prp Amyloid
The pig is the most versatile species used by humans for food and other applications. Over 1,5 billion pigs are slaughtered each year worldwide for human use.Citation32 Besides juicy pork sirloin other parts from pig are used for making remarkably diverse things such as musical instruments, china, leather, explosives, lubricants etc. Pig offal is used for human medicine, e.g., hormone preparations such as insulin and cerebrolysin, in xenographs, sutures, heparin and in gelatin for drug capsules.Citation33,34
While the late Carleton Gajdusek had strong views in diverse areas of prion biology, according to journalist Richard Rhodes,Citation35 he was correct on his prediction on BSE prions (vCJD) in the blood supplyCitation18 (see text box above). An opinionated scientist can sometimes be ignored due to a judgment of character and Gajdusek was certainly provocative. Notwithstanding society should remain vigilant on the possibility that Gajdusek was also prophetic on porcine prions given the exceptionally wide spread use of pigs in everyday human life and medicine. As discussed previously it is currently not established what relations transmissible neurotoxic prion strains and amyloid morphotypic mature APrP strains have. Given the hypotheses that amyloidotypic PrP conformations can transmit with low neurotoxicity.Citation7,36 it is interesting to reflect on possible implications. Pigs are slaughtered at 6–8 months of age. Because amyloid deposition is associated with old age, this is likely far too young for spontaneous development of APrP amyloid from PoPrP as well as other amyloidogenic proteins. From the perspective of seeded amyloidogenesis it is however a potential ideal case for highly transmissible titers of APrP (). In such a scenario the potential of porcine prions constitutes the perfect storm, clinically silent due to neurotoxic resistance and with high titers of transmissibility. When it comes to prions CNS material is most heavily infected. In addition, however, fat tissue (to make lard and tallow) is known to harbor extraordinary amounts of amyloid in systemic amyloidoses.Citation37 Amyloid fibrils of misfolded large proteins (AA, AL, ATTR) are notoriously hydrophobic due to the abnormal exposure of hydrophobic residues which normally in the folded structure being hidden in the protein core. The amyloid accumulation in fat tissue is likely a phase-separation from a rather hydrophilic environment in circulation toward the hydrophobic environment provided by adipocytes. Adipose tissue could in addition represent an in vivo environment well suitable for fibril formation. What about APrP?
In analysis of mice expressing Glycophosphatidylinositol, (GPI)-anchorless PrP, abdominal fat contains appreciable amounts of infectious prions in APrP isoform stained with ThS.Citation38 Notably mice overexpressing anchorless PrP provides a silent carrier status for a long time prior to presenting symptoms and is severely afflicted by amyloid fibril formation following scrapie (RML) infection.Citation39 Recall that this study showed that GPI-anchored PrP is needed to present clinical neurotoxicity. Evidently circulating anchorless-PrP (analogous to recPrP) is more amyloidogenic compared to GPI-anchored PrP and is poorly neuroinvasive.Citation28 Amyloidosis is systemic in anchorless-PrP mice and is not limited to fat but is also found as extensive cardiac amyloid deposits.Citation39 Interestingly cardiac APrP was recently reported in one BSE inoculated rhesus macaque which showed symptoms of cardiac distress prior to death from prion neurotoxicity.Citation40 It is noteworthy that transgenic mice expressing PoPrP appear sensitive to strains with biochemical features of amyloidogenic prion strains i.e., BSE and Nor98.Citation25,26,36 (). We recently adopted the parallel in-register intermolecular β-sheet structural model of the APrP fibril from the Caughey lab to rationalize cross-seeding between various PrP sequences.Citation12,41 It is tempting to use this structural model to speculate on the adaptation of mono-N-glycolsylated PoPrP at the expense of double-N-glycolylated PrP in the original BSE inoculum reported in the Torres experiments.Citation25,26 In this APrP model monoglycosylated PrP at N197 is structurally compatible while N181 is not, due to burial in the in-register intermolecular cross-beta sheet ()
FIGURE 5. Model of APrP fibrillar structure displayed as 8 in register parallel beta-sheet conformations as suggested by Groveman et al 2014 based on hamster PrP 90–231.Citation41 Glycosylations (complex glycans) were added in silico using GlyProt.Citation54 Both N197 and N181 were distinguished as putative glycosylation sites by GlyProt. However, N181 was only accessible for glycosylations for one PrP chain at the end of the “elongating fibril” as shown in the back side of the model. N197 was accessible for glycosylation in all 8 PrP chains. Glycans are indicated in orange. Amino acid positions unique for PoPrP relative to HuPrP and BoPrP () are indicated in pink.
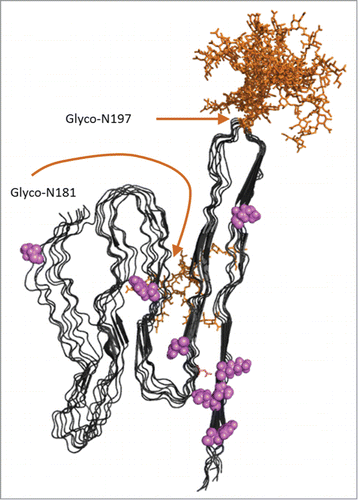
It appears that amyloidotypic prion strains, APrP, are transmissible but associated with lower neurotoxicity compared to diffuse aggregated PrP associated with synaptic PrP accumulations. It is possible that the amino acid substitutions in PoPrP compared to HuPrP and BoPrP are important for neurotoxic signal transmission (). The main issue hereby is that transmissibility of APrP will remain undetected unless used for surveillance. AA amyloidosis is frequent in many animals (e.g. cattle and birds) but is exceptionally rare in pigs.Citation42 suggesting that APrP should it reside in pig fat would be traceable using newly developed screening methods.Citation37
Concluding Remarks
Should the topic of porcine PrP amyloid be more of a worry than of mere academic interest? Well perhaps. Prions are particularly insidious pathogens. A recent outbreak of peripheral neuropathy in human, suggests that exposure to aerosolized porcine brain is deleterious for human health.Citation43,44 Aerosolization is a known vector for prions at least under experimental conditions.Citation45-47 where a mere single exposure was enough for transmission in transgenic mice. HuPrP is seedable with BoPrP seeds and even more so with PoPrP seed (), indicating that humans could be infected by porcine APrP prions while neurotoxicity associated with spongiform encephalopathy if such a disease existed is even less clear. Importantly transgenic mice over-expressing PoPrP are susceptible to BSE and BSE passaged through domestic pigs implicating that efficient downstream neurotoxicity pathways in the mouse, a susceptible host for prion disease neurotoxicity is augmenting the TSE phenotype.Citation25,26 Prions in silent carrier hosts can be infectious to a third species. Data from Collinge and coworkers.Citation21 propose that species considered to be prion free may be carriers of replicating prions. Especially this may be of concern for promiscuous prion strains such as BSE.Citation19,48 It is rather established that prions can exist in both replicating and neurotoxic conformations.Citation49,50 and this can alter the way in which new host organisms can react upon cross-species transmission.Citation51 The naïve host can either be totally resistant to prion infection as well as remain non-infectious, become a silent non-symptomatic but infectious carrier of disease or be afflicted by disease with short or long incubation time. The host can harbor and/or propagate the donor strain or convert the strain conformation to adapt it to the naïve host species. The latter would facilitate infection and shorten the incubation time in a consecutive event of intra-species transmission. It may be advisable to avoid procedures and exposure without proper biosafety precautions as the knowledge of silence carrier species is poor. One case of iatrogenic CJD in recipient of porcine dura mater graft has been reported in the literature.Citation52 The significance of this finding is still unknown. The low public awareness in this matter is exemplified by the practice of using proteolytic peptide mixtures prepared from porcine brains (Cerebrolysin) as a nootropic drug. While Cerebrolysin may be beneficial for treatment of severe diseases such as vascular dementia,Citation53 a long term follow-up of such a product for recreational use is recommended.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
FUNDING
This work was supported by Göran Gustafsson foundation, the Swedish research council Grant #2011-5804 (PH) and the Swedish Alzheimer association (SN).
REFERENCES
- Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 1996; 15:1255-64; PMID:8635458
- Knowles TP, Waudby CA, Devlin GL, Cohen SI, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM. An analytical solution to the kinetics of breakable filament assembly. Science 2009; 326:1533-7; PMID:20007899
- Scott M, Foster D, Mirenda C, Serban D, Coufal F, Walchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, et al. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 1989; 59:847-57; PMID:2574076
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, Groth DF, Glenner GG. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 1983; 35:349-58; PMID:6418385
- Merz PA, Somerville RA, Wisniewski HM, Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathologica 1981; 54:63-74; PMID:7195134
- Sikorska B, Liberski PP, Sobow T, Budka H, Ironside JW. Ultrastructural study of florid plaques in variant Creutzfeldt-Jakob disease: a comparison with amyloid plaques in kuru, sporadic Creutzfeldt-Jakob disease and Gerstmann-Straussler-Scheinker disease. Neuropatholo Appl Neurobiol 2009; 35:46-59; PMID:18513219
- Piccardo P, Manson JC, King D, Ghetti B, Barron RM. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci USA 2007; 104:4712-7; PMID:17360589
- Klimova N, Makarava N, Baskakov IV. The diversity and relationship of prion protein self-replicating states. Virus Res 2014; S0168–1702(14):00406-7; PMID:25312451
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 2014; 21:221-4; PMID:25263598
- Nystrom S, Psonka-Antonczyk KM, Ellingsen PG, Johansson LB, Reitan N, Handrick S, Prokop S, Heppner FL, Wegenast-Braun BM, Jucker M, et al. Evidence for age-dependent in vivo conformational rearrangement within Abeta amyloid deposits. ACS Chem Biol 2013; 8:1128-33; PMID:23521783
- Magnusson K, Simon R, Sjolander D, Sigurdson CJ, Hammarstrom P, Nilsson KP. Multimodal fluorescence microscopy of prion strain specific PrP deposits stained by thiophene-based amyloid ligands. Prion 2014; 8:319-29; PMID:25495506
- Nystrom S, Hammarstrom P. Generic amyloidogenicity of mammalian prion proteins from species susceptible and resistant to prions. Sci Rep 2015; 5:10101; PMID:25960067
- Lopez Garcia F, Zahn R, Riek R, Wuthrich K. NMR structure of the bovine prion protein. Proc Natl Acad Sci USA 2000; 97:8334-9; PMID:10899999
- Lysek DA, Schorn C, Nivon LG, Esteve-Moya V, Christen B, Calzolai L, von Schroetter C, Fiorito F, Herrmann T, Guntert P, et al. Prion protein NMR structures of cats, dogs, pigs, and sheep. Proc Natl Acad Sci USA 2005; 102:640-5; PMID:15647367
- Zahn R, Liu A, Luhrs T, Riek R, von Schroetter C, Lopez Garcia F, Billeter M, Calzolai L, Wider G, Wuthrich K. NMR solution structure of the human prion protein. Proc Natl Acad Sci USA 2000; 97:145-50; PMID:10618385
- Wells GA, Hawkins SA, Austin AR, Ryder SJ, Done SH, Green RB, Dexter I, Dawson M, Kimberlin RH. Studies of the transmissibility of the agent of bovine spongiform encephalopathy to pigs. J Gen Virol 2003; 84:1021-31; PMID:12655106; http://dx.doi.org/10.1099/vir.0.18788-0
- Collee JG, Bradley R, Liberski PP. Variant CJD (vCJD) and bovine spongiform encephalopathy (BSE): 10 and 20 years on: part 2. Folia neuropathol 2006; 44:102-10; PMID:16823692
- Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, Linehan JM, Brandner S, Wadsworth JD, Hewitt P, et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet 2006; 368:2061-7; PMID:17161728; http://dx.doi.org/10.1016/S0140-6736(06)69835-8
- Nystrom S, Hammarstrom P. Is the prevalent human prion protein 129M/V mutation a living fossil from a Paleolithic panzootic superprion pandemic? Prion 2014; 8:2-10; PMID:24398570; http://dx.doi.org/10.4161/pri.27601
- Race R, Chesebro B. Scrapie infectivity found in resistant species. Nature 1998; 392:770; PMID:9572135; http://dx.doi.org/10.1038/33834
- Hill AF, Joiner S, Linehan J, Desbruslais M, Lantos PL, Collinge J. Species-barrier-independent prion replication in apparently resistant species. Proc Natl Acad Sci USA 2000; 97:10248-53; PMID:10963685; http://dx.doi.org/10.1073/pnas.97.18.10248
- Fernandez-Borges N, Chianini F, Erana H, Vidal E, Eaton SL, Pintado B, Finlayson J, Dagleish MP, Castilla J. Naturally prion resistant mammals: a utopia? Prion 2012; 6:425-9; PMID:22954650; http://dx.doi.org/10.4161/pri.22057
- Bruce ME, Boyle A, Cousens S, McConnell I, Foster J, Goldmann W, Fraser H. Strain characterization of natural sheep scrapie and comparison with BSE. J Gen Virol 2002; 83:695-704; PMID:11842264
- Castilla J, Gutierrez-Adan A, Brun A, Doyle D, Pintado B, Ramirez MA, Salguero FJ, Parra B, Segundo FD, Sanchez-Vizcaino JM, et al. Subclinical bovine spongiform encephalopathy infection in transgenic mice expressing porcine prion protein. J Neurosci 2004; 24:5063-9; PMID:15163699; http://dx.doi.org/10.1523/JNEUROSCI.5400-03.2004
- Espinosa JC, Herva ME, Andreoletti O, Padilla D, Lacroux C, Cassard H, Lantier I, Castilla J, Torres JM. Transgenic mice expressing porcine prion protein resistant to classical scrapie but susceptible to sheep bovine spongiform encephalopathy and atypical scrapie. Emerg Infect Dis 2009; 15:1214-21; PMID:19751582; http://dx.doi.org/10.3201/eid1508.081218
- Torres JM, Espinosa JC, Aguilar-Calvo P, Herva ME, Relano-Gines A, Villa-Diaz A, Morales M, Parra B, Alamillo E, Brun A, et al. Elements modulating the prion species barrier and its passage consequences. PloS one 2014; 9:e89722; PMID:24608126; http://dx.doi.org/10.1371/journal.pone.0089722
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 2001; 24:519-50; PMID:11283320; http://dx.doi.org/10.1146/annurev.neuro.24.1.519
- Bett C, Kurt TD, Lucero M, Trejo M, Rozemuller AJ, Kong Q, Nilsson KP, Masliah E, Oldstone MB, Sigurdson CJ. Defining the conformational features of anchorless, poorly neuroinvasive prions. PLoS Pathog 2013; 9:e1003280; PMID:23637596; http://dx.doi.org/10.1371/journal.ppat.1003280
- Stalder K, Knauer JM, Baas TJ, Rothschild MF, Mabry JW. Sow longevity. Pig News and information 2004; 25:53-74.
- Koketsu Y, Takahashi H, Akachi K. Longevity, lifetime pig production and productivity, and age at first conception in a cohort of gilts observed over six years on commercial farms. J Vet Med Sci 1999; 61:1001-5; PMID:10535505; http://dx.doi.org/10.1292/jvms.61.1001
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science 2007; 318:930-6; PMID:17991853; http://dx.doi.org/10.1126/science.1138718
- FAOSTAT. FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS, Statistics Division, Economic and Social Development Department, 2015. Rome, Italy: FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS.
- Dunk M. Bullets, bread and beer, tambourines and toothpaste… and the 180 other things you can to do with a PIG. Daily Mail/Mail online, 2009. Available from http://www.dailymail.co.uk/sciencetech/article-1217794/From-bullets-bread-beer-tambourines-toothpaste--plus-180-things-pig.html
- Meindertsma C. Pig 05049. Asperen, The Netherlands: Flocks; 2008.
- Rhodes R. Deadly Feasts: The “Prion” Controversy and the Public's Health. New York: Simon & Schuster; 1998.
- Pirisinu L, Nonno R, Esposito E, Benestad SL, Gambetti P, Agrimi U, Zou WQ. Small ruminant nor98 prions share biochemical features with human gerstmann-straussler-scheinker disease and variably protease-sensitive prionopathy. PloS One 2013; 8:e66405; PMID:23826096; http://dx.doi.org/10.1371/journal.pone.0066405
- Sjolander D, Bijzet J, Hazenberg BP, Nilsson KP, Hammarstrom P. Sensitive and rapid assessment of amyloid by oligothiophene fluorescence in subcutaneous fat tissue. Amyloid 2015; 22:19-25; PMID:25847117; http://dx.doi.org/10.3109/13506129.2014.984063
- Race B, Meade-White K, Oldstone MB, Race R, Chesebro B. Detection of prion infectivity in fat tissues of scrapie-infected mice. PLoS Pathogens 2008; 4:e1000232; PMID:19057664; http://dx.doi.org/10.1371/journal.ppat.1000232
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 2005; 308:1435-9; PMID:15933194; http://dx.doi.org/10.1126/science.1110837
- Krasemann S, Mearini G, Kramer E, Wagenfuhr K, Schulz-Schaeffer W, Neumann M, Bodemer W, Kaup FJ, Beekes M, Carrier L, et al. BSE-associated prion-amyloid cardiomyopathy in primates. Emerg Infect Dis 2013; 19:985-8; PMID:23735198; http://dx.doi.org/10.3201/eid1906.120906
- Groveman BR, Dolan MA, Taubner LM, Kraus A, Wickner RB, Caughey B. Parallel in-register intermolecular β-sheet architectures for prion-seeded prion protein (PrP) amyloids. J Biol Chem 2014; 289:24129-42; PMID:25028516; http://dx.doi.org/10.1074/jbc.M114.578344
- Niewold TA, Murphy CL, Toussaint MJ, Solomon A, Gruys E. Chemical typing of porcine systemic amyloid as AA-amyloid. Amyloid 2005; 12:164-6; PMID:16194871; http://dx.doi.org/10.1080/13506120500231806
- Tracy JA, Dyck PJ. Auto-immune polyradiculoneuropathy and a novel IgG biomarker in workers exposed to aerosolized porcine brain. J Peripher Nerv Syst 2011; 16 Suppl 1:34-7; PMID:21696495; http://dx.doi.org/10.1111/j.1529-8027.2011.00303.x
- Lachance DH, Lennon VA, Pittock SJ, Tracy JA, Krecke KN, Amrami KK, Poeschla EM, Orenstein R, Scheithauer BW, Sejvar JJ, et al. An outbreak of neurological autoimmunity with polyradiculoneuropathy in workers exposed to aerosolised porcine neural tissue: a descriptive study. Lancet Neurology 2010; 9:55-66; PMID:19945916; http://dx.doi.org/10.1016/S1474-4422(09)70296-0
- Haybaeck J, Heikenwalder M, Klevenz B, Schwarz P, Margalith I, Bridel C, Mertz K, Zirdum E, Petsch B, Fuchs TJ, et al. Aerosols transmit prions to immunocompetent and immunodeficient mice. PLoS Pathogens 2011; 7:e1001257; PMID:21249178; http://dx.doi.org/10.1371/journal.ppat.1001257
- Stitz L, Aguzzi A. Aerosols: an underestimated vehicle for transmission of prion diseases? Prion 2011; 5:138-41; PMID:21778819; http://dx.doi.org/10.4161/pri.5.3.16851
- Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mathiason CK, et al. Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 2013; 87:1890-2; PMID:23175370; http://dx.doi.org/10.1128/JVI.02852-12
- Cassard H, Torres JM, Lacroux C, Douet JY, Benestad SL, Lantier F, Lugan S, Lantier I, Costes P, Aron N, et al. Evidence for zoonotic potential of ovine scrapie prions. Nat Commun 2014; 5:5821; PMID:25510416; http://dx.doi.org/10.1038/ncomms6821
- Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nat 2011; 470:540-2; PMID:21350487; http://dx.doi.org/10.1038/nature09768
- Sandberg MK, Al-Doujaily H, Sharps B, De Oliveira MW, Schmidt C, Richard-Londt A, Lyall S, Linehan JM, Brandner S, Wadsworth JD, et al. Prion neuropathology follows the accumulation of alternate prion protein isoforms after infective titre has peaked. Nat Commun 2014; 5:4347; PMID:25005024; http://dx.doi.org/10.1038/ncomms5347
- Beringue V, Vilotte JL, Laude H. Prion agent diversity and species barrier. Vet Res 2008; 39:47; PMID:18519020; http://dx.doi.org/10.1051/vetres:2008024
- Heath CA, Barker RA, Esmonde TF, Harvey P, Roberts R, Trend P, Head MW, Smith C, Bell JE, Ironside JW, et al. Dura mater-associated Creutzfeldt-Jakob disease: experience from surveillance in the UK. J Neurol Neurosurg Psychiatry 2006; 77:880-2; PMID:16627534; http://dx.doi.org/10.1136/jnnp.2005.073395
- Chen N, Yang M, Guo J, Zhou M, Zhu C, He L. Cerebrolysin for vascular dementia. Cochrane Database Syst Rev 2013; 1:CD008900; PMID:23440834
- Justus-Liebig University Gießen. Glycosciences. Available from www.glycosciences.de/modeling/glyprot/php/main.php