Abstract
Carcinoma invasion is a complex process regulated by genetic and epigenetic factors as well. A relevant supportive condition for cancer cell migration is the reorganization of the extracellular matrix (ECM), which is realized in an orchestrated multicellular manner including carcinoma cells and stromal fibroblasts. An important key player in the process of ECM reorganization is Tenascin-C (Tn-C). The molecule occurs as different isoforms generated by alternative splicing and de novo glycosylation. Large variants of Tn-C are abundantly re-expressed in the invasive front of many carcinoma types. A special role for initiating migration and accompanied epithelial to mesenchymal transition has been suggested. Here, we review the current knowledge concerning the tumor biological importance of Tn-C, the synthesis and alternative splicing during the invasive process in general, and give an overview on the impact of Tn-C in urothelial carcinoma of the urinary bladder (UBC) and oral squamous cell carcinoma (OSCC).
Abbreviations
| 3D | = | 3 dimensional |
| BM | = | basement membane |
| CAF | = | cancer associated fibroblast |
| ECM | = | extracellular matrix |
| EMT | = | epithelial – mesenchymal transition |
| FGF2 | = | fibroblast growth factor 2 |
| Fn | = | fibronectin |
| FNIII | = | fibronectin type III like repeats |
| hnRNPs | = | heterogeneous nuclear ribonucleoproteins |
| Ln | = | laminin |
| Lnγ2 | = | laminin gamma 2 chain |
| MMP | = | matrix metalloproteinase |
| mRNA | = | messenger RNA |
| oncFn | = | oncofetal fibronectin |
| oncTn-C | = | oncofetal tenascin-C |
| OSCC | = | oral squamous cell carcinoma |
| PDGF | = | platelet derived growth factor |
| RNA | = | ribonucleic acid |
| TGFβ1 | = | transforming growth factor beta 1 |
| Tn-C | = | tenascin-C |
| TPA | = | tetradecanoylphorbol acetate |
| UBC | = | urothelial carcinoma of the urinary bladder |
Introduction
Migration into preexisting normal surrounding tissues is one of the hallmarks of malignant tumor cells. The invasion of tumor cells of epithelial origin (carcinomas) is a complexly regulated process including a loss of epithelial cell-cell contacts, the obtainment of a migratory phenotype, the penetration of the basement membrane and the infiltration of the neighboring connective tissue. This process is accompanied by an intensive cross-talk between the carcinoma cells and cells of the tumor microenvironment like fibroblasts, endothelial cells, and inflammatory cells.
In carcinomas, the invasive process is tightly associated with the development of a tumor stroma, also known as desmoplastic stroma reaction. The carcinoma stroma is mainly formed by resident or attracted fibroblasts or fibroblast precursor cells gaining the myofibroblast phenotype as a result of activation by tumor derived cytokines – also designated as carcinoma associated fibroblasts (CAFs).Citation1 Currently, the carcinoma cell – myofibroblast (CAF) interaction is intensively studied in the light of phenotype transition of carcinoma cells (epithelial to mesenchymal transition (EMT)), tumor progression and modulation of therapeutic efficacy.Citation2,3
One of the most important steps enabling carcinoma cells to invade is the reorganization of the extracellular matrix (ECM). This process entails proteolysis of preexisting matrix structures, de novo synthesis of migration promoting matrix proteins, as well as a novel quality of structural 3D organization. Both, CAFs and carcinoma cells contribute to the remodeling of the ECM in a concerted manner. Within the last decades it could be evidenced that the newly formed tumor ECM exhibits a composition and organization showing many similarities to the situation occurring in embryonic tissues or healing wounds. This “provisional” matrix composition is characterized by the re-occurrence of matrix protein variants generated by alternative splicing, glycosylation or alternative chain assembly which are over-expressed in early development but are virtually absent in healthy adult organs.
With respect to the regulation of cell behavior, extracellular cell adhesion modulating proteins like fibronectin, the laminins or tenascins play a critical role. It is hypothesized that the reexpressed “provisional” isoforms of these adhesion proteins modulate ECM properties toward a more flexible and migration promoting state by generating a) new cell-matrix contacts via an altered integrin expression pattern and b) new interactions with other matrix proteins modulating their functional attitudes and/or 3D organization.
In this review, we will recapitulate the current knowledge in matters of the tumor biological importance of tenascin-C, its synthesis and alternative splicing during the invasive process in general. In addition, Tn-C is discussed in detail in 2 clinically important tumor types originating from different stratified epithelium: the urothelial carcinoma of the urinary bladder (UBC) and the oral squamous cell carcinoma (OSCC). For both carcinoma entities, carcinoma cell invasion into subepithelial connective or muscle tissue is well investigated and is of high predictive value for patient outcome. Furthermore, therapeutic strategies for both tumor types are rare so that understanding of the invasive process is of high clinical interest.
Tenascin-C and Alternative Splicing
The large hexameric extracellular glycoprotein Tenascin-C (Tn-C) was discovered in the early 80s by several laboratories.Citation4-7 Citation8 It is a member of a protein family comprising at least 4 different molecules in humans: Tenascin-C, Tenascin-R, Tenascin-W, and Tenascin-X.Citation9 It was shown that, in contrast to normal brain tissue, Tn-C expression is especially high in human glioma tissue and glioma cell culture supernatant highlighting this molecule as an interesting tumor marker.Citation4,10 Indeed, in the last 2 decades it was conclusively demonstrated that in many tumorous pathologies Tn-C is associated with neoplastic transformation and tumor progression.Citation11
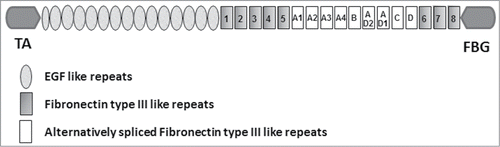
Today, the organization of the Tn-C gene and the molecular structure of the protein are well described. The entire protein with a maximal length of 2385 amino acids includes structurally different domains which are known as epidermal growth factor (EGF)-like repeats, fibronectin type III like repeats (FNIII) and a terminal fibrinogen like globular domain (FBG) (). The number of FNIII repeats included in the “mature” protein is defined by alternative splicing: in human, 9 of the 17 FNIII domains can be included or omitted by RNA splicing.Citation12,13 In addition to the known overall increase in Tn-C in tumor tissue, there are also changes in the pattern of alternatively spliced Tn-C isoforms associated with embryonic development, wound healing, neoplastic transformation and progression as well as reorganization of the tumor microenvironment. In contrast to stable adult tissues, under these conditions the reexpressed Tn-C variants contain more or less all of the FNIII domains A1 to D. By analogy with the concept of oncofetal fibronectin variants (e.g., ED-B+ fibronectin or O-glycosylated fibronectin) in the following these isoforms will be designated as oncofetal Tn-C variants (oncTn-C).
Figure 1. Schematic representation of the structure of a single chain of human tenascin-C (TA = Tenascin-C assembly domian, FBG = terminal fibrinogen like globular domain).
In general, Tn-C expression can be induced by several growth factors like TGFβ1, FGF2 and the phorbol ester tetradecanoylphorbol acetate (TPA). Furthermore, in accordance with its occurrence in early development, regulation of Tn-C synthesis is related to homeobox gene products and is functionally linked to the expression of MMPs and integrins.Citation14,15 Although it is known that the expression of Tn-C splicing variants is differentially regulated during development and seems to be of crucial functional relevance for correct organogenesis, the specific regulation of Tn-C alternative splicing is not well understood up to now. Alternative splicing of mRNA is a complex molecular mechanism. The process is catalyzed by the spliceosome and is regulated by the serine/arginine rich (SR) family of proteins and heterogeneous nuclear ribonucleoproteins (hnRNPs).Citation16 Furthermore, tissue specificity of alternative splicing is mediated by tissue related splicing factors and their posttranslational modification like phosphorylation.Citation17 Tn-C alternative splicing seems to be cell cycle dependent and epigenetically regulated by extracellular pH at least in normal nonmalignant cells.Citation18-20 Furthermore, also growth factors like TGFβ1 and PDGF-BB seem to influence the splicing in a tissue specific manner.Citation21,22 Currently, the splicing factor SRSF6 was shown to be involved in the regulation of Tn-C alternative splicing and that there is a correlation between SRSF6 and Tn-C expression with skin hyperproliferation and neoplastic transformation.Citation23
Invasion Associated Reorganization of Tenascin-C – a Collaboration of Different Cell Types
It is well known from a large number of immunohistochemical studies that carcinoma invasion is associated with a stromal deposition of de novo synthesized tenascin-C especially around the invading carcinoma cell complexes. However, it seems to be still a matter of debate, if stromal cells or the carcinoma cells themselves are the tumor biological relevant source of oncTn-C and if there are differences in the provided splicing variants. However, numerous mRNA in situ hybridization studies evidenced a multicellular origin of Tn-C at all or oncTn-C for instance in breast cancer,Citation24 prostatic adenocarcinoma,Citation25 skin tumors,Citation26 and oral squamous cell carcinoma (OSCC)Citation27 with epithelial tumor cells and stromal fibroblasts as the main producing cell types. Hindermann and coworkers were able to show conclusively that in OSCC carcinoma cell derived oncTn-C is deposited in the tumor-stroma interface. A comparable situation was evidenced for prostatic adenocarcinoma where the carcinoma cells show an abundant mRNA synthesis of oncTn-C with special pronunciation of the invasive front. Comparison of mRNA in situ hybridization and immunohistochemistry again revealed a deposition of carcinoma derived oncTn-C in the carcinoma stroma.Citation25 Furthermore, oncTn-C was also detected in association to newly formed tumor vessels and seems to be synthesized by myoepithelial cells, endothelial cells as well as by pericytes.Citation28-30 To the authors best knowledge, there is no study available from the literature that functionally and structurally compares stromal and tumor cell derived Tn-C and focuses in the question, if there are differences with respect to the provision of alternatively spliced variants. Against the background that there is also a reexpression of oncTn-C during fibrosis, wound healing, and tissue remodeling, the synthesis of Tn-C by fibroblasts / myofibroblasts / CAFs can be explained as a sign of cellular activation and may be linked also to the migratory capability of stromal cells.Citation31-34 Since Tn-C critically modulates cell adhesion to fibronectin and modulates fibronectin assembly by fibroblasts, stromal and tumor derived Tn-C may have the same function and the final situation is more or less determined by the quantity and site-specific modulation of the deposited protein. Recently, it was indeed shown that the capability of Tn-C to prevent fibrillogenesis of fibronectin by fibroblasts depends on proteolytic processing and demasking of cryptic domains.Citation35 With respect to Tn-C synthesis by carcinoma cells, different in situ situations were evidenced: no mRNA synthesis in carcinoma tissue but positivity in cell lines of the same tumor type,Citation30 mRNA synthesis in tumor cells but preferential deposition in the carcinoma stroma,Citation25,27 or cytoplasmic positivity for Tn-C immunohistochemistry in the carcinoma cells themselves.Citation36 These differences may be tissue specific but also due to the application of varying detection methods, antibodies, and probes. More likely, the pattern of Tn-C expression by carcinoma cells seems to depend on the status of de- or transdifferentiation. This may also explain the repeatedly demonstrated correlation of tenascin-C positivity to the grade of malignancy.Citation11,37 With respect to this, it was evidenced in situ and in vitro that oncTn-C reexpression is linked to an EMT phenotype of carcinoma cells.Citation38-42 Although Tn-C upregulation could be discussed as a secondary phenomenon of EMT or tumor cell dedifferentiation, there are multiple effects of Tn-C on tumor cells mediated by outside-in-signaling leading to increased proliferation, migration and invasion.Citation11
Tenascin-C in Urinary Bladder Carcinoma Invasion
The urothelial carcinoma of the urinary bladder (UBC) is the most frequently occurring cancer type of the lower urinary tract. Up to now, there are only limited therapeutic options and biological markers for noninvasive monitoring the disease progression, especially the transition from a non-invasive to an invasive state of the tumor, are rare. Therefore, increasing our knowledge on the modality of the invasive process is of great clinical importance. It is well known that, also in UBC, tumor cell invasion is accompanied by a complex reorganization of the laminin, collagen, fibronectin, as well as tenascin-C matrix.Citation43
Early in the 90s, Tiitta and coworkers already described Tn-C in the epithelial-mesenchymal interphase of the urinary bladder wall and an abundant increase in Tn-C deposition in relation to inflammation and UBC invasion. For immunohistochemistry, the authors used the antibody 143DB7 detecting Tn-C independent of alternative splicing.Citation44 Tn-C in UBC was predominantly deposited or localized within the carcinoma stroma. Results were confirmed by further immunohistochemical studies describing Tn-C expression in the context with other matrix proteins and integrins.Citation45,46 Later on, a relationship between the expression of Tn-C and TGFβ1 in UBC was described in vitro and in situ, supporting the hypothesis that invasion associated Tn-C de novo expression and deposition is linked to tumor-stroma cross talk and also EMT.Citation47,48 The tumor biological and clinical importance of stromal Tn-C reorganization is underlined by the fact, that the extent of immunohistochemical Tn-C positivity in the tumor stroma shows a correlation to grade of malignancy, stage, and proliferative activity and is a prognostic factor for worse survival. Interestingly, immunohistochemically detected cytoplasmic Tn-C positivity of the carcinoma cells or the detection of circulating Tn-C mRNA in low stage diseases is indicative for a better survival.Citation36,49,50 The tumor biological background of these contradicting findings is not fully clear up to now and should be the object of further studies. However, it underlines the hypothesis that the extracellular incorporation of Tn-C is mandatory for the development of an invasive carcinoma cell phenotype.
With the availability of splicing domain specific antibodies in combination with RT-PCR it becomes feasible to investigate the differential expression and deposition of Tn-C splicing variants in correlation to the disease progress. Using monoclonal and recombinant antibodies against the Tn-C A1, A1/A4, B, D, and C domains, we were able to show that there was a deposition of A1, B, and / or D containing Tn-C associated with invasive growth, muscle destruction and vessel formation. mRNA analysis revealed a higher variability in the B to D region among the investigated carcinomas and a restricted expression of the AD1 to compact invasion type.Citation51 Although there are reports on the functional importance of Tn-C domains and changes in the Tn-C splicing pattern,Citation14 the meaning of these findings is not clear. It is known that Annexin II is a ligand of the A-D domains and that the Annexin II / Tn-C interaction may play a role in wound healing.Citation52 This interaction may also have functional importance in UBC invasion because it was shown that in UBC annexin II is upregulated in association to invasion, metastasis and lower survival rate.Citation53 Interestingly, Tn-C A-D / Annexin II interaction exerts a comparable effect on endothelial cell mitogenesis and migration.Citation54 This goes in line with the observation that also in UBC vessel neoformation is associated with a perivascular deposition of large Tn-C.Citation28 Interestingly, in accordance with our immunohistochemical results, Hancox and colleagues were able to demonstrate an invasion promoting effect of Tn-C B+ – and Tn-C B/D + – isoforms in breast cancer cells.Citation55
20 years ago, Siri and colleagues reported on differences in the susceptibility of large and small Tn-C to the degradation by MMP´s. The greater sensitivity of the large isoform was interpreted as a sign of the provisional character of the ECM during tissue reorganization enabling proliferation and migration.Citation56 The hypothesis that the inclusion of additional domains leads to a higher degradable Tn-C ECM is supported by the fact, that indeed there is a relationship between UBC progress, immunohistochemical B domain expression, and the concentration of B-domain containing Tn-C in the urine of UBC patients. Urinary B+ Tn-C / Tn-C fragments may be the results of secretion by the carcinoma and / or stroma cells or most likely of proteolytic liberation during the invasive process.Citation57,58
Tenascin-C in Oral Squamous Cell Carcinoma Invasion
Oral squamous cell carcinoma is the most common entity within the Head and Neck tumors. Although the biology of OSCC development, invasion, and metastasis is investigated in detail and novel therapeutic concepts came up recently, the prognosis of patients could not be improved during the last years. With respect to tumor biology and also impact for diagnosis and therapy, the tumor-stroma interaction and ECM reorganization more and more gets in the focus of research.
Again, early in the 90s, the histological distribution of Tn-C in OSCC was extensively investigated by several groups worldwide. It was reported that the de novo deposition of Tn-C is strongly increased in the stroma of highly invasive tumors and metastases implicating a role of Tn-C in stroma reorganization supporting tumor cell proliferation and migration.Citation59-61 Although Tn-C expression is increased in invasive and metastatic OSCC, interestingly, the predictive value of Tn-C is critically discussed. Atula and co-workers were not able to evidence a correlation of Tn-C to survival and patient characteristics in an immunohistochemical study including 65 cases of primary oral and pharyngeal SCC.Citation62 In contrast, on mRNA expression level, Tn-C seems to be helpful to predict lymph node metastasis and prognosis and was suggested as a cancer biomarker.Citation63-65 The discrepancies in the assessment of the predictive value of Tn-C may be, at least in part, caused by methodological differences in the estimation of Tn-C matrix reorganization in OSCC. Most of the studies were performed using different antibodies detecting all variants with varying specificity and sensitivity. Furthermore, it has to be considered, that extracellular stromal deposition and cytoplasmic positivity of OSCC cells may have different tumor biological significance.Citation66 Additionally, quantification of mRNA and protein expression may have different prognostic impact. With respect to this, Fialka and colleagues observed that Tn-C mRNA was up-regulated especially in early stage and not in late stage OSCC.Citation67
Concerning the role in cancer invasion, the mode of the 3dimensional organization of Tn-C and it´s structural re-association with other provisional matrix proteins may play a critical role. Ramos and colleagues were able to show that indeed the interaction of carcinoma cells with peritumoral fibroblasts is necessary to organize a Tn-C matrix.Citation68 Although it is a matter of debate also in OSCC which cell type provides the relevant stromal Tn-C, Tn-C matrix organization is dependent on fibronectin reorganization as a result of tumor-stroma cross talk.Citation69 Furthermore, we were able to show that Tn-C matrix reorganization in OSCC seems to be associated with the formation of new quality extracellular multi-protein complexes at least including oncofetal fibronectin and the migration promoting laminin gamma2 chain (Lnγ2) suggested as guides for invasion.Citation70,71 That indeed the “collaboration” of especially oncTn-C with the basement membrane (BM) protein laminin 332 plays a pivotal role in OSCC invasion was evidenced by a quantitative colocalization analysis of oncTn-C/Lnγ2 in the OSCC basement membrane region. We were able to show that with raising malignancy grade and mode of invasion, there is an increased incorporation of oncTn-C into the OSCC BM region colocalized to laminin 332. This finding suggests that the incorporation of oncTn-C modulates the flexibility of the BM structure with increased accessibility for proteolytic degradation, and may provide new integrin binding sites to promote tumor cell proliferation and migration.Citation72
A possible invasion relevant receptor for Tn-C in OSCC is the integrin αvβ6.Citation73 Indeed, a concordant de novo expression of Tn-C and the β6 integrin in invasive OSCC could be shown.Citation74 There is increasing evidence that the Tn-C – β6 integrin interaction modulates MMP composition in the invasive front by a TGFβ1 associated activation of the uPA / MMP3 / MMP9 axis and a down regulation of MMP13.Citation75,76 MMP9 is multiply evidenced to be a key molecule in OSCC cell invasion. Furthermore, the β6 integrin may also be a link between the known EMT promoting properties of Tn-C. Ramos and coworkers were able to show that an overexpression of β6 in OSCC cells leads to EMT like phenomena Citation42 and indeed EMT in OSCC is regulated and / or perpetuated by Tn-C and MMP9 expression.Citation77,78 Interestingly, in concordance with invasion of neoplastic keratinocytes, a comparable co-organization of oncTn-C and the integrins α9 and αvβ6 was shown in healing wounds speaking well for a comprehensive mechanism during epithelial migration.Citation31
Up to now there are only few reports on the differential expression and possible function of Tn-C splicing variants in OSCC. In 1997, Mighell and coworkers published an interesting study describing the mRNA expression of splicing domains in normal, malignant, and reactive oral mucosae.Citation79 The group describes several new Tn-C splice variants which exist in parallel to the abundantly expressed large Tn-C which includes more or less all splicing domains. Interestingly, like in UBC, the Tn-C C domain is rarely expressed. A limitation of this study was that the authors could not distinguish between tumor cell and fibroblast / myofibroblast derived Tn-C mRNA. However, it demonstrates the complexity of Tn-C reorganization during oral carcinogenesis and associated inflammation and desmoplasia. That indeed large unspliced variants may play a role during OSCC invasion could be demonstrated by our group. Using antibodies specific to the Tn-C slicing domains A1, A1/A4, and C, immunohistochemistry revealed a de novo deposition of oncTn-C in the invasive front as a tumor specific process.Citation27,28 By means of mRNA in situ hybridization, it was further demonstrated that the synthesis of oncTn-C at least including the A3-A4-B domains is allocated to invading carcinoma cells and shows an association to the grade of malignancy and therefore to the mode of invasion. Combining in situ hybridization with immunohistochemistry evidenced that the tumor cell derived oncTn-C is deposited in the invasive front.Citation27 This OSCC cell specific oncTn-C synthesis is shown to be an excellent additional marker to discriminate tumor cells in brush biopsies of oral lesions.Citation80,81
Conclusion
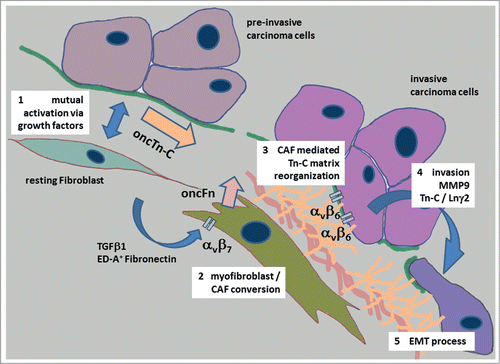
Summarizing the available data, Tn-C reorganization with the de novo synthesis of large, low spliced isoforms is an invasion associated phenomenon in general and could also be evidenced in UBC and OSCC. The process seems to be a precondition for epithelial cell migration and comprises 1) mutual activation of stromal and cancer cells, 2) increased synthesis and secretion of oncTn-C by carcinoma cells as well as stromal fibroblasts, 3) fibronectin / laminin associated structural organization of Tn-C by cancer associated fibroblasts – modulation of adhesive properties of the ECM, 4) integrin dependent support of EMT and modulation of the expression of EMT associated proteins like MMP9 and Tn-C itself, and 5) in consequence, promotion of cancer cell migration and invasion (). There is increasing evidence that some alternatively spliced variants may have a crucial biological function during cancer progression. Variants especially including the B domain may therefore have the potential to serve as diagnostic markers and / or therapeutic targets in UBC and OSCC.
Figure 2. Tn-C and its proposed role during invasion and epithelial to mesenchymal transition of carcinoma cells in oral cancer. The process of the development of an invasive carcinoma cell phenotype starts with the mutual activation of stromal and cancer cells, followed by an increased synthesis and secretion of oncofetal Tn-C variants (oncTn-C) by carcinoma cells and stromal fibroblasts (1) This process is accompanied by growth factor mediated fibro-/myofibroblast phenotype transition co-activated by an autocrine ED-A+ fibronectin signaling via αvβ7 integrin Citation82,83 (2). Activated myofibroblasts / cancer associated fibroblasts (CAF´s) produce oncofetal fibronectin variants (oncFn) and reorganize the oncTn-C / oncFn matrix together with other adhesion proteins like Laminins in a provisional manner (3). This provisional matrix mediates invasive phenotype conversion of cancer cells via β6 integrin signaling associated with up regulation of for instance MMP9, Tn-C itself and the migration promoting laminin γ2 chain (Lnγ2) (4). Finally, the carcinoma cells develop an epithelial to mesenchymal transition (EMT) phenotype (5).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’–more than meets the eye. Trends Mol Med 2013; 19:447-53; PMID:23769623; http://dx.doi.org/10.1016/j.molmed.2013.05.004
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-96; PMID:24556840; http://dx.doi.org/10.1038/nrm3758
- Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Trans Med 2014; 3:17; PMID:25050175; http://dx.doi.org/10.1186/2001-1326-3-17
- Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res 1983; 43:2796-805; PMID:6342760
- Chiquet M, Fambrough DM. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol 1984; 98:1937-46; PMID:6202699; http://dx.doi.org/10.1083/jcb.98.6.1937
- Erickson HP, Inglesias JL. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature 1984; 311:267-9; PMID:6482952; http://dx.doi.org/10.1038/311267a0
- Grumet M, Hoffman S, Crossin KL, Edelman GM. Cytotactin, an extracellular matrix protein of neural and non-neural tissues that mediates glia-neuron interaction. Proc Natl Acad Sci U S A 1985; 82:8075-9; PMID:2415980; http://dx.doi.org/10.1073/pnas.82.23.8075
- Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 1986; 47:131-9; PMID:2428505; http://dx.doi.org/10.1016/0092-8674(86)90374-0
- Chiquet-Ehrismann R, Tucker RP. Tenascins and the importance of adhesion modulation. Cold Spring Harbor Perspect Biol 2011; 3; PMID:21441591; http://dx.doi.org/10.1101/cshperspect.a004960
- Aukhil I, Slemp CC, Lightner VA, Nishimura K, Briscoe G, Erickson HP. Purification of hexabrachion (tenascin) from cell culture conditioned medium, and separation from a cell adhesion factor. Matrix 1990; 10:98-111; PMID:1695709; http://dx.doi.org/10.1016/S0934-8832(11)80176-9
- Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett 2006; 244:143-63; PMID:16632194; http://dx.doi.org/10.1016/j.canlet.2006.02.017
- Jones FS, Hoffman S, Cunningham BA, Edelman GM. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A 1989; 86:1905-9; PMID:2467292; http://dx.doi.org/10.1073/pnas.86.6.1905
- Taylor HC, Lightner VA, Beyer WF Jr, McCaslin D, Briscoe G, Erickson HP. Biochemical and structural studies of tenascin/hexabrachion proteins. J Cell Biochem 1989; 41:71-90; PMID:2482292; http://dx.doi.org/10.1002/jcb.240410204
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dynam: Off Pub Am Assoc Anatomists 2000; 218:235-59; PMID:10842355; http://dx.doi.org/10.1002/(SICI)1097-0177(200006)218:2%3c235::AID-DVDY2%3e3.0.CO;2-G
- Jones PL, Jones FS. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol: J Int Soc Matrix Biol 2000; 19:581-96; PMID:11102748; http://dx.doi.org/10.1016/S0945-053X(00)00106-2
- Chen J, Weiss WA. Alternative splicing in cancer: implications for biology and therapy. Oncogene 2014.
- Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol 2009; 10:741-54; PMID:19773805
- Borsi L, Allemanni G, Gaggero B, Zardi L. Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int J Cancer J Int Du Cancer 1996; 66:632-5; PMID:8647625; http://dx.doi.org/10.1002/(SICI)1097-0215(19960529)66:5%3c632::AID-IJC9%3e3.0.CO;2-U
- Borsi L, Balza E, Castellani P, Carnemolla B, Ponassi M, Querze G, Zardi L. Cell-cycle dependent alternative splicing of the tenascin primary transcript. Cell Adhesion Commun 1994; 1:307-17; PMID:7521758; http://dx.doi.org/10.3109/15419069409097262
- Borsi L, Balza E, Gaggero B, Allemanni G, Zardi L. The alternative splicing pattern of the tenascin-C pre-mRNA is controlled by the extracellular pH. J Biol Chem 1995; 270:6243-5; PMID:7534307; http://dx.doi.org/10.1074/jbc.270.11.6243
- Zhao Y, Young SL. TGF-beta regulates expression of tenascin alternative-splicing isoforms in fetal rat lung. Am J Physiol 1995; 268:L173-80; PMID:7532367
- LaFleur DW, Fagin JA, Forrester JS, Rubin SA, Sharifi BG. Cloning and characterization of alternatively spliced isoforms of rat tenascin. Platelet-derived growth factor-BB markedly stimulates expression of spliced variants of tenascin mRNA in arterial smooth muscle cells. J Biol Chem 1994; 269:20757-63; PMID:7519614
- Jensen MA, Wilkinson JE, Krainer AR. Splicing factor SRSF6 promotes hyperplasia of sensitized skin. Nat Struct Mol Biol 2014; 21:189-97; PMID:24440982; http://dx.doi.org/10.1038/nsmb.2756
- Ishihara A, Yoshida T, Tamaki H, Sakakura T. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res: Off J Am Assoc Cancer Res 1995; 1:1035-41; PMID:9816077
- Katenkamp K, Berndt A, Hindermann W, Wunderlich H, Haas KM, Borsi L, Zardi L, Kosmehl H. mRNA expression and protein distribution of the unspliced tenascin-C isoform in prostatic adenocarcinoma. J Pathol 2004; 203:771-9; PMID:15221936; http://dx.doi.org/10.1002/path.1589
- Tuominen H, Pollanen R, Kallioinen M. Multicellular origin of tenascin in skin tumors–an in situ hybridization study. J Cutan Pathol 1997; 24:590-6; PMID:9449485; http://dx.doi.org/10.1111/j.1600-0560.1997.tb01089.x
- Hindermann W, Berndt A, Borsi L, Luo X, Hyckel P, Katenkamp D, Kosmehl H. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J Pathol 1999; 189:475-80; PMID:10629546; http://dx.doi.org/10.1002/(SICI)1096-9896(199912)189:4%3c475::AID-PATH462%3e3.0.CO;2-V
- Berndt A, Kollner R, Richter P, Franz M, Voigt A, Berndt A, Borsi L, Giavazzi R, Neri D, Kosmehl H. A comparative analysis of oncofetal fibronectin and tenascin-C incorporation in tumour vessels using human recombinant SIP format antibodies. Histochem Cell Biol 2010; 133:467-75; PMID:20237793; http://dx.doi.org/10.1007/s00418-010-0685-y
- Martina E, Degen M, Ruegg C, Merlo A, Lino MM, Chiquet-Ehrismann R, Brellier F. Tenascin-W is a specific marker of glioma-associated blood vessels and stimulates angiogenesis in vitro. FASEB J: Off Pub Fed Am Soc Exp Biol 2010; 24:778-87; PMID:19884327; http://dx.doi.org/10.1096/fj.09-140491
- Adams M, Jones JL, Walker RA, Pringle JH, Bell SC. Changes in tenascin-C isoform expression in invasive and preinvasive breast disease. Cancer Res 2002; 62:3289-97; PMID:12036947
- Hakkinen L, Hildebrand HC, Berndt A, Kosmehl H, Larjava H. Immunolocalization of tenascin-C, alpha9 integrin subunit, and alphavbeta6 integrin during wound healing in human oral mucosa. J Histochem Cytochem: Off J Histochem Soc 2000; 48:985-98; PMID:10858276; http://dx.doi.org/10.1177/002215540004800712
- Imanaka-Yoshida K. Tenascin-C in cardiovascular tissue remodeling: from development to inflammation and repair. Circ J: Off J Jpn Circ Soc 2012; 76:2513-20; PMID:23064399; http://dx.doi.org/10.1253/circj.CJ-12-1033
- Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol 2011; 301:G950-5; PMID:21512158; http://dx.doi.org/10.1152/ajpgi.00132.2011
- Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans 2007; 35:695-7; PMID:17635125; http://dx.doi.org/10.1042/BST0350695
- To WS, Midwood KS. Cryptic domains of tenascin-C differentially control fibronectin fibrillogenesis. Matrix Biol: J Int Soc Matrix Biol 2010; 29:573-85; PMID:20708078; http://dx.doi.org/10.1016/j.matbio.2010.08.003
- Brunner A, Mayerl C, Tzankov A, Verdorfer I, Tschorner I, Rogatsch H, Mikuz G. Prognostic significance of tenascin-C expression in superficial and invasive bladder cancer. J Clin Pathol 2004; 57:927-31; PMID:15333651; http://dx.doi.org/10.1136/jcp.2004.016576
- Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. Tenascins in stem cell niches. Matrix Biol: J Int Soc Matrix Biol 2014; 37C:112-23; PMID:24472737; http://dx.doi.org/10.1016/j.matbio.2014.01.007
- Takahashi Y, Sawada G, Kurashige J, Matsumura T, Uchi R, Ueo H, Ishibashi M, Takano Y, Akiyoshi S, Iwaya T, et al. Tumor-derived tenascin-C promotes the epithelial-mesenchymal transition in colorectal cancer cells. Anticancer Res 2013; 33:1927-34; PMID:23645740
- Dandachi N, Hauser-Kronberger C, More E, Wiesener B, Hacker GW, Dietze O, Wirl G. Co-expression of tenascin-C and vimentin in human breast cancer cells indicates phenotypic transdifferentiation during tumour progression: correlation with histopathological parameters, hormone receptors, and oncoproteins. J Pathol 2001; 193:181-9; PMID:11180164; http://dx.doi.org/10.1002/1096-9896(2000)9999:9999%3c::AID-PATH752%3e3.0.CO;2-V
- Beiter K, Hiendlmeyer E, Brabletz T, Hlubek F, Haynl A, Knoll C,, Kirchner T, Jung A. beta-Catenin regulates the expression of tenascin-C in human colorectal tumors. Oncogene 2005; 24:8200-4; PMID:16091738
- Katoh D, Nagaharu K, Shimojo N, Hanamura N, Yamashita M, Kozuka Y, Imanaka-Yoshida K, Yoshida T. Binding of alphavbeta1 and alphavbeta6 integrins to tenascin-C induces epithelial-mesenchymal transition-like change of breast cancer cells. Oncogenesis 2013; 2:e65; PMID:23958855; http://dx.doi.org/10.1038/oncsis.2013.27
- Ramos DM, Dang D, Sadler S. The role of the integrin alpha v beta6 in regulating the epithelial to mesenchymal transition in oral cancer. Anticancer Res 2009; 29:125-30; PMID:19331141
- Brunner A, Tzankov A. The role of structural extracellular matrix proteins in urothelial bladder cancer (review). Biomarker Insights 2007; 2:418-27; PMID:19662222
- Tiitta O, Wahlstrom T, Virtanen I, Gould VE. Tenascin in inflammatory conditions and neoplasms of the urinary bladder. Virchows Archiv B, Cell Pathol Including Mol Pathol 1993; 63:283-7; PMID:7685960; http://dx.doi.org/10.1007/BF02899274
- Deen S, Ball RY. Basement membrane and extracellular interstitial matrix components in bladder neoplasia–evidence of angiogenesis. Histopathology 1994; 25:475-81; PMID:7532615; http://dx.doi.org/10.1111/j.1365-2559.1994.tb00010.x
- Wilson CB, Leopard J, Cheresh DA, Nakamura RM. Extracellular matrix and integrin composition of the normal bladder wall. World J Urol 1996; 14 Suppl 1:S30-7; PMID:8738408; http://dx.doi.org/10.1007/BF00182062
- Champelovier P, El Atifi M, Mantel F, Rostaing B, Simon A, Berger F, Seigneurin D. In vitro tumoral progression of human bladder carcinoma: role for TGFbeta. Eur Urol 2005; 48:846-51; PMID:16046050; http://dx.doi.org/10.1016/j.eururo.2005.06.005
- Booth C, Harnden P, Selby PJ, Southgate J. Towards defining roles and relationships for tenascin-C and TGFbeta-1 in the normal and neoplastic urinary bladder. J Pathol 2002; 198:359-68; PMID:12375269; http://dx.doi.org/10.1002/path.1214
- Ioachim E, Michael M, Stavropoulos NE, Kitsiou E, Salmas M, Malamou-Mitsi V. A clinicopathological study of the expression of extracellular matrix components in urothelial carcinoma. BJU Int 2005; 95:655-9; PMID:15705098; http://dx.doi.org/10.1111/j.1464-410X.2005.05357.x
- Gazzaniga P, Nofroni I, Gandini O, Silvestri I, Frati L, Agliano AM, Gradilone A. Tenascin C and epidermal growth factor receptor as markers of circulating tumoral cells in bladder and colon cancer. Oncol Rep 2005; 14:1199-202; PMID:16211285
- Berndt A, Anger K, Richter P, Borsi L, Brack S, Silacci M, Franz M, Wunderlich H, Gajda M, Zardi L, et al. Differential expression of tenascin-C splicing domains in urothelial carcinomas of the urinary bladder. J Cancer Res Clin Oncol 2006; 132:537-46; PMID:16788848; http://dx.doi.org/10.1007/s00432-006-0106-8
- Matsuda A, Tagawa Y, Yamamoto K, Matsuda H, Kusakabe M. Identification and immunohistochemical localization of annexin II in rat cornea. Curr Eye Res 1999; 19:368-75; PMID:10520233; http://dx.doi.org/10.1076/ceyr.19.4.368.5306
- Zhang Q, Zhao Z, Ma Y, Wang H, Ma J, He X, Zhang D. Combined expression of S100A4 and Annexin A2 predicts disease progression and overall survival in patients with urothelial carcinoma. Urologic Oncol 2014; 32:798-805; PMID:24968947; http://dx.doi.org/10.1016/j.urolonc.2014.02.009
- Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell 1996; 7:883-92; PMID:8816995; http://dx.doi.org/10.1091/mbc.7.6.883
- Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS, Shaw JA, Walker RA, Pringle JH, Jones JL. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res: BCR 2009; 11:R24; PMID:19405959; http://dx.doi.org/10.1186/bcr2251
- Siri A, Knauper V, Veirana N, Caocci F, Murphy G, Zardi L. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem 1995; 270:8650-4; PMID:7536739; http://dx.doi.org/10.1074/jbc.270.15.8650
- Richter P, Tost M, Franz M, Altendorf-Hofmann A, Junker K, Borsi L, Neri D, Kosmehl H, Wunderlich H, Berndt A. B and C domain containing tenascin-C: urinary markers for invasiveness of urothelial carcinoma of the urinary bladder? J Cancer Res Clin Oncol 2009; 135:1351-8; PMID:19326143; http://dx.doi.org/10.1007/s00432-009-0576-6
- Gecks T, Junker K, Franz M, Richter P, Walther M, Voigt A, Neri D, Kosmehl H, Wunderlich H, Kiehntopf M, et al. B domain containing Tenascin-C: a new urine marker for surveillance of patients with urothelial carcinoma of the urinary bladder? Clinica Chimica Acta; Int J Clin Chem 2011; 412:1931-6; PMID:21763295; http://dx.doi.org/10.1016/j.cca.2011.06.038
- Harada T, Shinohara M, Nakamura S, Oka M. An immunohistochemical study of the extracellular matrix in oral squamous cell carcinoma and its association with invasive and metastatic potential. Virchows Archiv: Int J Pathol 1994; 424:257-66; PMID:7514477; http://dx.doi.org/10.1007/BF00194609
- Shrestha P, Sakamoto F, Takagi H, Yamada T, Mori M. Enhanced tenascin immunoreactivity in leukoplakia and squamous cell carcinoma of the oral cavity: an immunohistochemical study. Eur J Cancer Part B, Oral Oncol 1994; 30B:132-7; PMID:7518275; http://dx.doi.org/10.1016/0964-1955(94)90065-5
- Tiitta O, Happonen RP, Virtanen I, Luomanen M. Distribution of tenascin in oral premalignant lesions and squamous cell carcinoma. J Oral Pathol Med: Off Pub Int Assoc Oral Pathol Am Acad Oral Pathol 1994; 23:446-50; PMID:7532219; http://dx.doi.org/10.1111/j.1600-0714.1994.tb00442.x
- Atula T, Hedstrom J, Finne P, Leivo I, Markkanen-Leppanen M, Haglund C. Tenascin-C expression and its prognostic significance in oral and pharyngeal squamous cell carcinoma. Anticancer Res 2003; 23:3051-6; PMID:12926160
- Nagata M, Fujita H, Ida H, Hoshina H, Inoue T, Seki Y, Ohnishi M, Ohyama T, Shingaki S, Kaji M, et al. Identification of potential biomarkers of lymph node metastasis in oral squamous cell carcinoma by cDNA microarray analysis. Int J Cancer J Int Du Cancer 2003; 106:683-9; PMID:12866027; http://dx.doi.org/10.1002/ijc.11283
- Wang Z, Han B, Zhang Z, Pan J, Xia H. Expression of angiopoietin-like 4 and tenascin C but not cathepsin C mRNA predicts prognosis of oral tongue squamous cell carcinoma. Biomar: Biochem Indic Expo, Resp Susceptibility Chem 2010; 15:39-46; PMID:19775228; http://dx.doi.org/10.3109/13547500903261362
- Choi P, Jordan CD, Mendez E, Houck J, Yueh B, Farwell DG, Futran N, Chen C. Examination of oral cancer biomarkers by tissue microarray analysis. Arch Otolaryngol–Head Neck Surg 2008; 134:539-46; PMID:18490578; http://dx.doi.org/10.1001/archotol.134.5.539
- Mori M, Muramatsu Y, Yamada K, Shrestha P, Takai Y. Intracellular localization of tenascin in squamous cell carcinoma of oral cavity: an immunohistochemical study. Anticancer Res 1996; 16:3075-9; PMID:8920770
- Fialka F, Gruber RM, Hitt R, Opitz L, Brunner E, Schliephake H, Kramer FJ. CPA6, FMO2, LGI1, SIAT1 and TNC are differentially expressed in early- and late-stage oral squamous cell carcinoma–a pilot study. Oral Oncol 2008; 44:941-8; PMID:18234543; http://dx.doi.org/10.1016/j.oraloncology.2007.10.011
- Ramos DM, Chen BL, Boylen K, Stern M, Kramer RH, Sheppard D, Nishimura SL, Greenspan D, Zardi L, Pytela R. Stromal fibroblasts influence oral squamous-cell carcinoma cell interactions with tenascin-C. Int J Cancer J Int Du Cancer 1997; 72:369-76; PMID:9219848; http://dx.doi.org/10.1002/(SICI)1097-0215(19970717)72:2%3c369::AID-IJC28%3e3.0.CO;2-9
- Ramos DM, Chen B, Regezi J, Zardi L, Pytela R. Tenascin-C matrix assembly in oral squamous cell carcinoma. Int J Cancer JInt Du Cancer 1998; 75:680-7; PMID:9495234; http://dx.doi.org/10.1002/(SICI)1097-0215(19980302)75:5%3c680::AID-IJC4%3e3.0.CO;2-V
- Berndt A, Borsi L, Hyckel P, Kosmehl H. Fibrillary co-deposition of laminin-5 and large unspliced tenascin-C in the invasive front of oral squamous cell carcinoma in vivo and in vitro. J Cancer Res Clin Oncol 2001; 127:286-92; PMID:11355143; http://dx.doi.org/10.1007/s004320000205
- Franz M, Hansen T, Richter P, Borsi L, Bohmer FD, Hyckel P, Schleier P, Katenkamp D, Zardi L, Kosmehl H, et al. Complex formation of the laminin-5 gamma2 chain and large unspliced tenascin-C in oral squamous cell carcinoma in vitro and in situ: implications for sequential modulation of extracellular matrix in the invasive tumor front. Histochem Cell Biol 2006; 126:125-31; PMID:16344911; http://dx.doi.org/10.1007/s00418-005-0126-5
- Franz M, Hansen T, Borsi L, Geier C, Hyckel P, Schleier P, Richter P, Altendorf-Hofmann A, Kosmehl H, Berndt A. A quantitative co-localization analysis of large unspliced tenascin-C(L) and laminin-5/gamma2-chain in basement membranes of oral squamous cell carcinoma by confocal laser scanning microscopy. J Oral Pathol Med: Off Pub Int Assoc Oral Pathol Am Acad Oral Pathol 2007; 36:6-11; PMID:17181735; http://dx.doi.org/10.1111/j.1600-0714.2006.00492.x
- Thomas GJ, Nystrom ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med: Off Pub Int Assoc Oral Pathol Am Acad Oral Pathol 2006; 35:1-10; PMID:16393247; http://dx.doi.org/10.1111/j.1600-0714.2005.00374.x
- Regezi JA, Ramos DM, Pytela R, Dekker NP, Jordan RC. Tenascin and beta 6 integrin are overexpressed in floor of mouth in situ carcinomas and invasive squamous cell carcinomas. Oral Oncol 2002; 38:332-6; PMID:12076695; http://dx.doi.org/10.1016/S1368-8375(01)00062-8
- Dang D, Yang Y, Li X, Atakilit A, Regezi J, Eisele D, Ellis D, Ramos DM. Matrix metalloproteinases and TGFbeta1 modulate oral tumor cell matrix. Biochem Biophys Res Commun 2004; 316:937-42; PMID:15033492; http://dx.doi.org/10.1016/j.bbrc.2004.02.143
- Ylipalosaari M, Thomas GJ, Nystrom M, Salhimi S, Marshall JF, Huotari V, Tervahartiala T, Sorsa T, Salo T. Alpha v beta 6 integrin down-regulates the MMP-13 expression in oral squamous cell carcinoma cells. Exp Cell Res 2005; 309:273-83; PMID:16024014; http://dx.doi.org/10.1016/j.yexcr.2005.06.008
- Richter P, Umbreit C, Franz M, Berndt A, Grimm S, Uecker A, Böhmer FD, Kosmehl H, Berndt A. EGF/TGFbeta1 co-stimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. J Oral Pathol Med: Off Pub Int Assoc Oral Pathol Am Acad Oral Pathol 2011; 40:46-54; PMID:20819124; http://dx.doi.org/10.1111/j.1600-0714.2010.00936.x
- Qiao B, Johnson NW, Gao J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. Int J Oncol 2010; 37:663-8; PMID:20664935
- Mighell AJ, Thompson J, Hume WJ, Markham AF, Robinson PA. Human tenascin-C: identification of a novel type III repeat in oral cancer and of novel splice variants in normal, malignant and reactive oral mucosae. Int J Cancer J Int Du Cancer 1997; 72:236-40; PMID:9219826; http://dx.doi.org/10.1002/(SICI)1097-0215(19970717)72:2%3c236::AID-IJC6%3e3.0.CO;2-S
- Driemel O, Kosmehl H, Rosenhahn J, Berndt A, Reichert TE, Zardi L, Dahse R. Expression analysis of extracellular matrix components in brush biopsies of oral lesions. Anticancer Res 2007; 27:1565-70; PMID:17595777
- Driemel O, Dahse R, Berndt A, Pistner H, Hakim SG, Zardi L, Reichert TE, Kosmehl H. High-molecular tenascin-C as an indicator of atypical cells in oral brush biopsies. Clin Oral Investigat 2007; 11:93-9; PMID:17111122; http://dx.doi.org/10.1007/s00784-006-0086-8
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 1998; 142:873-81; PMID:9700173; http://dx.doi.org/10.1083/jcb.142.3.873
- Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J: Off Pub Federat Am Soc Exp Biol 2010; 24:4503-12; PMID:20643910; http://dx.doi.org/10.1096/fj.10-154435

