ABSTRACT
Background: The relationship between cutaneous and extracutaneous complications in pediatric patients with type 1 diabetes is unclear. Objective: The objective of the current study is to investigate the relationship between skin disorders and diabetic microangiopathic changes in pediatric and adolescent patients with type 1 diabetes. Patients and methods: Eighty patients with type 1 diabetes and 50 healthy controls were enrolled in the study. All recruited patients were followed up monthly for a total period of 12 month. Monthly visit included thorough clinical examination with system review, as well as whole-body cutaneous examination. HbA1c was assessed every 3 month. Twenty-four hours urine was collected for measurement of urinary albumin. Results: Fifty percent of the screened diabetic cohort had diabetic nephropathy (DN). The overall prevalence of cutaneous lesion among the studied diabetic cohort was high (72.5%), with cutaneous infections (40%) and xerosis (30%) being the most prevalent. The frequency of cutaneous infections, xerosis and rubeosis faciei was higher in patients with nephropathy than in those without nephropathy. Conclusion: cutaneous affection in patients with diabetes may be a clue to the presence of associated microangioapthic complications. The significant association between diabetic nephropathy and cutaneous lesions support the concept that cutaneous lesion in diabetes is a reflection of diabetic angiopathy, highlighting the importance of identifying patients at risk of other microvascular complications.
1. Introduction
Dermatological disorders, usually neglected and frequently underdiagnosed among patients with diabetes, are common complications and encounter a broad spectrum of disorders in both type 1 and type 2 diabetes.Citation1 The frequency of dermatological disorders during the course of diabetes has been reported to range from 30.0 to 91.2%.Citation2-Citation4
Although the mechanism for many diabetes associated skin problems remains unknown, the pathogenesis of others is multifactorial, possibly linked to abnormal carbohydrate metabolism, other altered metabolic pathways, atherosclerosis, microangiopathy, neuron degeneration, and impaired host mechanisms.Citation5
Several studied explored the spectrum of cutaneous affection among diabetic cohort, but to the best of our knowledge, studies investigating the relationship between microvascular complications and cutaneous affection in pediatric patients with type1 diabetes are limited in number.
Early identification of patients who are prone to develop microvascular complication would be an important step for their better management during the clinical course of the disease process. So, the primary objective of the current study is to investigate the relationship of cutaneous and extracutaneous complications in patients with type 1 DM.
2. Patients and method
A cross-sectional study conducted on 80 patients with type 1 diabetes diagnosed according to recommendations of American Diabetes Association.Citation6 Patients were recruited from Diabetes Clinic, of Pediatric Hospital, Ain Shams University. The study was in agreement with the guidelines of the ethics committee at our hospital and an informed consent was obtained from all patients. The research was conducted in accordance with the 1964 Helsinki Declaration or comparable standards. The studied diabetic cohorts were 48 males and 32 female patients, their age ranged from 2–18 years with a mean of 11.69 ± 4.34 years. During the same period, 50 healthy children (32 males and 18 females, mean age 12.2 ± 2.12 years) were selected as controls.
Clinical evaluation of the patients was based on clinical history from the parents with special emphasis on medical history about dermatological diseases, reviewing follow- up sheets, and clinical examination. All patients were subjected to: Detailed history with description of patients' onset of disease, duration of diabetes, daily insulin requirement and presence of complications as well as family history. All recruited patients were followed up monthly for a total period of 12 month. Monthly visit included thorough clinical examination with system review, as well as whole-body cutaneous examination, including hair, nails and visible mucosal surfaces. Anthropometric assessment including: weight, height and body mass index (BMI).
Glycemic control was evaluated every 3 month by assessing HbA1c which was measured by cation exchange high performance liquid chromatography (CE-HPLC). Patients were considered under optimal glycemic control when their HbA1c was < 7% (53 mmol/mol IFCC).Citation7 Twenty-four hours urine was collected for measurement of urinary albumin. Urine albumin (UAE) was measured using an immunonephelometric method, on an Olympus AU640 Analyzer. Diabetic nephropathy was diagnosed if the UAE was ≥ 30 mg/24 h and categorized as either microalbuminuria (30 - 300 mg/24 h) or macroalbuminuria (> 300 mg/24 h). The albumin detection of 24-h urine was done 3 times within 6 months to make sure of the diagnosis for nephropathy.Citation8
Assessment of retinopathy was performed once by a retinal specialist, using direct and indirect opthalmoscopy. None of the recruited patients had either diabetic retinopathy or neuropathy.
Bedside laboratory procedures like the Tzanck smear, KOH mount, and Gram's stain were carried out. To confirm the diagnosis, a skin biopsy was done in a few cases. All the subjects in this study were evaluated during the summer to eliminate the effect of seasonal variation.
Statistical analyses were carried out using SPSS 17.0 software package program. Results for continuous variables were presented in mean ± (SD), for binary variables in percent. The comparison between two groups with qualitative data was done using Chi-square test or Fisher exact test. Independent t-test was used for two independent groups with quantitative data. Paired t-test was used for two paired groups with quantitative data. Pearson correlation coefficients were used to assess the association between normally distributed variables, spearman test was used when variables are not normally distributed. Stepwise logistic regression was used to assess significant associations of cutaneous lesions with clinical and metabolic parameters of the patients with type 1 diabetes. All statistical tests were two-sided with a level of significance being <0.05.
3. Results
The clinical characteristics and biochemical variables of healthy controls and patients with type 1 diabetes are illustrated in . A total of 58 patients (72.5%) had at least one cutaneous disorder. The most common dermatologic diseases among studied diabetic cohort were cutaneous infections (fungal infections) (40%) and xerosis (30%). Fifty percent of the studied diabetic cohort had nephropathy; none had either retinopathy or neuropathy.
Table 1. Demographic and laboratory characteristic of studied patients and healthy controls.
When evaluating the relation between cutaneous and extracutaneous affection in patients with type 1 diabetes, the overall incidence of any cutaneous infection was common in diabetic cohort with microalbuminuria. Additionally xerosis and keratosis pilaris were more prevelant in cohort with microalbumuria, all patients with rubeosis had diabetic nephropathy. The two patients with necrobiosis had diabetic nephropathy ().
Table 2. Distribution of cutaneous disorders in relation to microalbuminuria.
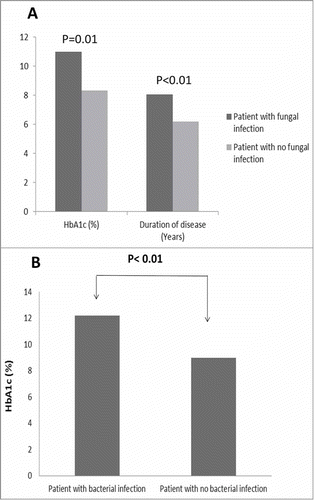
Among the current diabetic cohort, the most common cutaneous manifestation was cutaneous infection, where 40% showed fungal infection and bacterial infection was reported in 23.75%. Patients who experienced fungal infection had significantly higher HbA1c with a mean value of 10.98 ± 1.6% compared to 8.33 ± 2.6%, additionally these patients had longer disease duration with mean of 8.07 ± 3.8 years compared to 6.19 ± 2.9 years (). Bacterial infections in diabetic cohort was associated with a significantly higher HbA1c (P<0.05), with a mean value of 12.2 ± 1.4% compared to 7.45 ± 3.9% in patients with no bacterial infection ().
Figure 1. Effect of glycemic control and disease duration among patients with fungal and bacterial infection. showed that patients with fungal infection had significantly higher HbA1c with a mean value of 10.98 ± 1.6% compared to 8.33± 2.6%(P = 0.01), additionally these patients had longer disease duration with mean of 8.07± 3.8 years compared to 6.19 ±2.9 years(P<0.01). illustrated highly significantly values of HbA1C in patients with bacterial infections with a mean value of 12.2 ± 1.4% compared to 7.45± 3.9% (P < 0.01) in patients with no bacterial infection ().
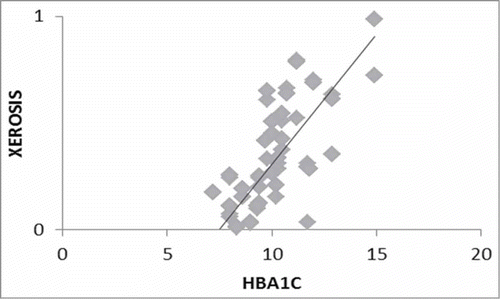
Twenty-four patients (30%) had skin lesions potentially associated with diabetes. Xerosis was found in 30% of patients with type 1 diabetes compared to 8% in healthy controls (P<0.05). Twenty-one patients (87.5%) of the patients with xerosis, had micoalbuminuria. Stepwise logistic regression, to assess the influence of diabetes risk factors and late complications on xerosis, showed that xerosis best correlated with patients' glycemic control (R = 0.66, p<0.01, β-coefficient = 0.103) ().
Figure 2. Correlation between xerosis and HbA1C. Stepwise logistic regression showed that xerosis best correlated with patients' glycemic control (R = 0.66, p < 0.01, β-coefficient = 0.103).
The prevalence of rubeosis in the current type 1 diabetic population was 8.75%; all patients had diabetic nephropathy.
Among the studied diabetic cohort, only two patients showed necrobiosis, both had long duration of disease (11.7 ± 5.4 years), poor glycemic control with diabetic nephropathy. None of the screened age and sex matched healthy controls, showed either rubeosis, necrobiosis, or diabetic hand.
Keratosis pilaris, though not considered a diabetes-related cutaneous manifestation, was significantly more common (17.5%) in patients with type 1 diabetes than in control subjects (4%) (P< 0.05). In the current study, keratosis pilaris was best correlated with xerosis (R = 0.6, p<0.01, β-coefficient = 0.71). Pruritus, localized or generalized without any skin lesions were present in 20% of cases compared to 12% in healthy controls (P<0.05). Among the diabetic cohort, the difference in prevelance of pruritis was non-significant in both patients with and without microalbuminuria (P > 0.05). Other cutaneous affection registered in the current study (acne, eczema, atopic dermatitis and pigmentary lesions) showed no difference between both patients and healthy controls. One patient showed acanthosis nigricans, the patient was male with 10 years duration of diabetes and poor glycemic control (HbA1c = 10.6%).
4. Discussion
The current study highlighted the high prevalence of cutaneous affection among the studied diabetic cohort, where 72.5% had at least one cutaneous disorder. Similarly, Pavlovi'c et al. reported a high prevalence (68%) of cutaneous manifestation in their studied diabetic cohort.Citation2
In a single center study conducted in Egypt, the overall prevalence of dermatologic manifestations was 67.56%.Citation9 A cross-sectional study conducted on a large south Asian cohort with type 1 diabetes, reported that the prevalence of cutaneous manifestation was 67.8%.Citation10
Overall, cutaneous infection and xerosis showed to be highly prevalent and important skin disorders in several studies, regardless DM type. Among cutaneous infections, fungal etiology appears to be the most common and those with bacterial origin are less frequent.Citation11-Citation14
When exploring the relationship between cutaneous and extracutaneous lesions among the current diabetic cohort, higher prevalence of cutaneous affection was observed among patients with microalbuminuria. Similar to our findings, Demirseren et al. found higher prevalence of cutaneous affection among patients with diabetic nephropathy and concluded that long disease duration and poor glycemic control may increase the prevalence of the cutaneous diseases associated with microangiopathy.Citation15
Xerosis was found in 30% of patients and it was more frequent in patients with nephropathy. In agreement with our data, Demirseren et al. reported xerosis in 26.4% of their diabetic cohort and found that xerosis was more frequent in patients with either nephropathy or retinopathy.Citation15 Earlier Pavlovi'c et al. identified xerosis in 22% of studied diabetic cohort.Citation2 Stepwise logistic regression highlighted the strong influence of glycemic control on xerosis. Similarly, Zakharov et al. demonstrated that well-controlled patients with type 1 DM did not present with alteration on the epidermal thickness.Citation16 This data reinforces that skin disorders in DM patients are strongly related to glycemic control.
Consistent with previous findings by Pavlovi'c et al., Keratosis pilaris proved to be significantly more prevalent among patients with type 1 diabetes and was best correlated with xerosis. There they postulated that xerosis plays a significant role in the development of keratosis pilaris.Citation2
Despite possible definition discrepancies across the studies, it is clear that the skin dryness is one of the earliest and most common manifestations of type 1 diabetes.Citation2 DM affects the skin through several mechanisms. Reaching pathological high levels of glucose strongly affects skin homeostasis by inhibiting keratinocyte proliferation and migration, protein biosynthesis, inducing endothelial cell apoptosis, decreasing nitric oxide synthesis and impairing phagocytosis and chemotaxis from several cells.Citation17,Citation18 Besides hyperglycemia induce direct damage, high glucose levels also induce advanced glycation end products (AGE) formation. AGEs are formed from glycation of proteins, lipids and nucleic acids that act in several pathways, inducing reactive oxygen species (ROS) formation, impairing ROS clearance, as well as intra and extracellular proteins function, and inducing pro inflammatory cytokine through nuclear factor κβ (NF-κβ) pathway.Citation17-Citation19
The prevalence of rubeosis faciei in our patients with type 1 diabetes was rather high (8.75%), a similar high prevalence was noticed by Pavlovi'c et al..Citation2 It is presumed that venular dilation in the cheeks of patients with diabetes underlies rubeosis faciei and is caused by hyperglycemia-induced sluggish microcirculation.Citation20
Vascular endothelial dysfunction is a central event in the pathogenesis of diabetic microangioapthic changes. In the current study, xerosis and rubeosis facei were significantly associated with diabetic nephropathy. Microangiopathy is proposed to be involved in the etiology of these disorders. It has been demonstrated that diffuse thickening of the basement membrane with a progressive increase in permeability are induced by microvascular occlusion.Citation20
Demirseren et al., evaluated the relation between cutaneous and extracutaneous manifestations in a large diabetic cohort with both type 1 and type 2 diabetes, they recruited 52 patients with type 1 diabetes with median age of 35.5 years. They concluded that cutaneous diseases in which microangiopathy accounts for the etiopathogenesis, are significantly associated with diabetic nephropathy, neuropathy, and retinopathy. Additionally they concluded that skin disorders may be clues to the presence of associated microvascular complications of DM.Citation15
In this study pruritus was highly observed among patients with type 1 diabetes (20%), yet the distribution among the diabetic cohort was similar (P>0.05). This is in agreement with findings reported by Youssef et al. and Timshina et al. where they reported prevalence of 15.1% and 15.2% respectively.Citation9,Citation21 Several cutaneous mediators have been suggested to cause pruritus and seemed to be linked to metabolic changes observed in diabetes. Diffuse persistent pruritus without an identifiable clinical cause (pruritus sine material) as well as forms of prurigo, especially prurigo simplex subacuta are commonly observed in patients with diabetes.Citation22
The main limitations of the present study include the cross-sectional nature of the study and the small size of the studied cohort.
5. Conclusion
Cutaneous disorders, especially disorders in which microangiopathy accounts for the etiopathogenesis, are significantly associated with diabetic nephropathy. Diabetes-associated cutaneous lesions among diabetic cohort are begin conditions yet may signify an underlying process of microvascular affection among these patients requiring further assessment. Therefore, detailed and careful dermatological examinations may provide a simple early tool for diagnosis and treatment of extracutaneous complications.
Conflict of interest
Nothing to declare.
References
- Wang YR, Margolis D. The prevalence of diagnosed cutaneous manifestations during ambulatory diabetes visits in the United States, 1998–2002. Dermatology. 2006;212(3):229–34. doi:10.1159/000091249. PMID:16549918.
- Pavlovic MD, Milenkovic T, Dinic M, Misovic M, Daković D, Todorović S, Daković Z, Zecevi RD, Doder R. The prevalence of cutaneous manifestations in young patients with type 1 diabetes. Diabetes Care. 2007;30(8):1964–7. doi:10.2337/dc07-0267. PMID:17519431.
- Shahzad M, Al Robaee A, Al Shobaili HA, Alzolibani AA, Al Marshood AA, Al Moteri B. Skin manifestations in diabetic patients attending a diabetic clinic in the Qassim region, Saudi Arabia. Med Princ Pract. 2011;20(2):137–41. doi:10.1159/000321219. PMID:21252568.
- Hosseini MS, Ehsani AH, Panah FH, Azizi F. The correlation between skin lesions, microabluminuria and other microvascular complications in type 2 diabetic patients. Int J Nephrol Urol. 2010;2:553–60.
- Bhat YJ, Gupta V, Kudyar RP. Cutaneous manifestations of diabetes mellitus. Int J Diabetes Dev Countries. 2006;26:152–5. doi:10.4103/0973-3930.33180.
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40(1):S11–24. PMID:27979889.
- American Diabetes Association. Glycemic Targets. Diabetes Care. 2017;40(1):S48–56.
- Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic Nephropathy: Diagnosis, Prevention, and Treatment. Diabetes Care. 2005;28(1):164–76. doi:10.2337/diacare.28.1.164. PMID:15616252.
- Youssef RM, Ibrahim A, Amin IM, Soliman HM, Ali A. Cutaneous manifestations among Egyptian children and adolescents with type 1 diabetes. Egyptian Pediatric Association Gazette. 2016;64:44–49. doi:10.1016/j.epag.2015.10.001.
- Sawatkar GU, Kanwar AJ, Dogra S, Bhadada SK, Dayal D. Spectrum of cutaneous manifestations of type 1 diabetes mellitus in 500 South Asian patients. Br J Dermatol. 2014;171:1402–6. doi:10.1111/bjd.13077. PMID:24773124.
- Romano G, Moretti G, Di Benedetto A, Giofre C, Di Cesare E, Russo G, Califano L, Cucinotta D. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39(2):101–6. doi:10.1016/S0168-8227(97)00119-8. PMID:9597379.
- Sanad EM, ElFangary MM, Sorour NE, ElNemisy NM. Skin manifestations in Egyptian diabetic patients: a case series study. Egypt J Dermatol Venereol. 2013;33:56–62. doi:10.4103/1110-6530.123941.
- Goyal A, Raina S, Kaushal SS, Mahajan V, Sharma NL. Pattern of cutaneous manifestations in diabetes mellitus. Indian J Dermatol. 2010;55:39–41. doi:10.4103/0019-5154.60349. PMID:20418975.
- Farshchian M, Fereydoonnejad M, Yazdanfar A, Kimyai-Asadi A. Cutaneous manifestations of diabetes mellitus: a case series. Cutis. 2010;86:31–5. PMID:21049764.
- Demirseren DD, Emre S, Akoglu G, Arpacı D, Arman A, Metin A, Cakır B. Relationship between skin diseases and extracutaneous complications of diabetes mellitus: clinical analysis of 750 patients. Am J Clin Dermatol. 2014;15(1):65–70. doi:10.1007/s40257-013-0048-2. PMID:24135944.
- Zakharov P, Talary MS, Kolm I, Caduff A. Full-field optical coherence tomography for the rapid estimation of epidermal thickness: study of patients with diabetes mellitus type 1. Physiol Meas. 2010;31:193–205. doi:10.1088/0967-3334/31/2/006. PMID:20016116.
- Blakytny R, Jude EB. Altered molecular mechanisms of diabetic foot ulcers. Int J Low Extrem Wounds. 2009;8:95–104. doi:10.1177/1534734609337151. PMID:19443898.
- Behm B, Schreml S, Landthaler M, Babilas P. Skin signs in diabetes mellitus. J Eur Acad Dermatol Venereol. 2012;26:1203–11. doi:10.1111/j.1468-3083.2012.04475.x. PMID:22348239.
- Gkogkolou P, Bohm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinology. 2012;4:259–70. doi:10.4161/derm.22028.
- Ngo BT, Hayes KD, DiMiao DJ, Srinivasan SK, Huerter CJ, Rendell MS. Manifestations of cutaneous diabetic microangiopathy. Am J Clin Dermatol. 2005;6(4):225–37. doi:10.2165/00128071-200506040-00003. PMID:16060710.
- Timshina DK, Thappa DM, Agrawal A. A clinical study of dermatoses in diabetes to establish its markers. Indian J Dermatol. 2012;57(1):20–5. doi:10.4103/0019-5154.92671. PMID:22470203.
- Meurer M, Stumvoll M, Szeimies RM. Hautvera¨ nderungen bei. Diabetes mellitus. Skin changes in diabetes mellitus. Hautarzt. 2004;55:428–35. (in German) PMID:15083279.

