Abstract
Prolonged epididymal sperm storage in vespertilionid and rhinolophid bats, provides an interesting experimental model for the study of spermatozoa epididymal maturation. We examined the presence of the cytoplasmic droplet, and the sequential induction of capacitation and the acrosome reaction in spermatozoa obtained from different epididymal regions (caput, corpus, cauda) throughout the annual reproductive cycle of Corynorhinus mexicanus (C. mexicanus). This is a vespertilionid bat that stores spermatozoa in the epididymis for several months after testes regression. The number of sperm recovered from the different epididymal regions indicate that epididymal transit in C. mexicanus is rapid. The persistence of a high percentage of sperm cells with cytoplasmic droplet in cauda epididymis was observed in addition to a low index of capacitation and acrosome reaction in sperm cells obtained from the corpus epididymis. There was a significant increase in the percentage of capacitated and acrosome reacted spermatozoa during the storage of sperm cells in the cauda epididymis and the percentage of capacitated spermatozoa was consistently, and significantly, higher (p < 0.05) in cauda compared to the corpus epididymis at all studied dates. Tthe process of epididymal maturation in C. mexicanus is completed in the caudal region of this organ encompassing a significant period. Our results also indicate that in C. mexicanus, and in other vespertilionid and rhinolophid bats that show the same temporal asynchrony in the function of male reproductive organs, the final phases of epididymal maturation and storage are, apparently, independent of testicular function.
| Abbreviations | ||
| C. mexicanus | = | Corynorhinus mexicanus (MEXICAN BIG-EARED BAT) |
| BWW | = | Biggers, Whitten, and Whittingham medium |
| CTC | = | chlortetracycline |
| ROS | = | reactive oxygen species |
Introduction
It has been reported that in some vespertilionid and rhinolophid bats, while testes development and spermatogenesis take place mainly in summer, development of accessory sex glands, expression of libido and breeding activity take place in the autumn after the testes have totally regressed [Krutzsch [Citation2000]; León-Galván et al. [Citation2005]]. This significant temporal asynchrony between development and function of male primary and secondary reproductive organs must be accompanied by important modifications in the reproductive physiology of the epididymis. Spermatozoa, produced during active spermatogenesis must be primarily stored in the cauda epididymis for periods which may extend for several months apparently without adequate testicular function [Racey and Entwistle [Citation2000]]. While prolonged storage of spermatozoa may be just a natural consequence of hibernating hypothermia, it is now known that there are several species of non-hibernating tropical bats (vespertilionids) that show epididymal sperm storage [Gopalakrishna and Bhatia [Citation1980]; Karim and Banerjee [Citation1985]; Singh and Krishna [Citation1995]]. In addition, many of these species that store spermatozoa do not hibernate but only show periodic phases of torpor during the hibernating period [Avery [Citation1985]; López-Wilchis Citation[1989]].
The prolonged epididymal sperm storage is interesting if we keep in mind that the anatomic-functional changes known as epididymal maturation develop gradually as the sperm cells progress from the caput down to the cauda regions of the epididymis in a species specific way [Yanagimachi [Citation1994]]. These important physiological properties of mammalian sperm cells necessary for fertilization include: the loss of cytoplasmic droplet, the potential to move progressively [Orgebin-Crist [Citation1987]; Yanagimachi [Citation1994]], capacitation potential [de Lamirande et al. [Citation1997]], the ability to undergo the acrosomal reaction [Brucker and Lipford [Citation1995]], and to interact with the zona pellucida of the egg [Bedford [Citation1998]]. Epididymal maturation in most mammalian species studied to date, including human, depends on androgen secretion by the testes and requires approximately 10–20 days [Holland et al. [Citation1992]; Orgebin-Crist [Citation1996]; Moore and Akhondi [Citation1996]]. Epididymal maturation is necessary to acquire progressive motility and capacitation potential, to recognize the zona pellucida, and finally to undergo the acrosome reaction [Cooper [Citation1986]]. Adequate maturation of spermatozoa will ensure fertilization of the oocyte. Generally epididymal maturation is accomplished during the transport of the spermatozoa through the initial portion of the epididymis, caput and corpus, so that when spermatozoa reach the cauda they are mature, i.e. they have lost the cytoplasmic droplet and must be fully capable of capacitation, and the acrosomal reaction. This can be assessed by submitting mature spermatozoa to well tested in vitro procedures [García-Macedo et al. [Citation2001]; Ávalos-Rodríguez et al. [Citation2004]]. Upon reaching the tail, the epididymis provides an adequate microenvironment in which the spermatozoa are protected during storage. Usually this prolonged stay of the sperm on the tail of the epididymis is dependent on testicular endocrine function.
Occurrence of prolonged epididymal sperm storage has been reported in the Mexican big-eared bat, Corynorhinus mexicanus, Allen 1916, a vespertilionid that belongs to the group that only experiences torpor during the hibernating period. Their annual reproductive cycle involves an important temporal asynchrony between testicular and epididymal development and function [López-Wilchis Citation[1989]; Geiser and Ruf [Citation1995]; León-Galván et al. [Citation2005]]. This makes C. mexicanus a good model to study epididymal sperm maturation in seasonal mammals. They present temporal asynchrony between development and function of the male primary and secondary reproductive organs. For this reason, we assessed the number, viability, presence of cytoplasmic droplet, capacitation potential and the acrosome reaction in the spermatozoa obtained from different epididymal regions (caput, corpus and cauda) throughout the annual reproductive cycle of C. mexicanus.
Results
Testes recrudescence was clearly observed in May, and reached a peak weight in August (). Then the testes underwent a profound involution and were totally regressed in November (). Epididymal organ growth started in June, maximal size was reached in September, and then a gradual involution occurred to its smallest size in February. Clear asynchrony was also observed between epididymal regions and development. The caput reached its peak growth in September, whereas the caudal region showed maximal weight in October (). Epididymis caput involution occurred at least one month before the associated regression of the cauda. Cauda epididymis, flattened in non-breeding bats become swollen and bulbous during autumn when it was engorged with spermatozoa [León-Galván et al. Citation[1999]].
Figure 1 Changes in testicular and epididymal (caput and cauda) mass of adult male Corynorhinus mexicanus during the annual reproductive cycle. Bars indicate the mean±SE and the values in parentheses indicate the number of bats. Different letters indicate statistically significant differences (P < 0.05) between values in the same trace (ANOVA plus Bonferroni post-hoc test applied to the testis and epididymal segment data). The inserts show images of the macroscopical testes regression in Corynorhinus mexicanus: (a) testes of male bats captured on August 18, when maximal size is observed, and (b) testes of male bats captured on October 30, when full regression occurs. Metric units indicate millimeters.
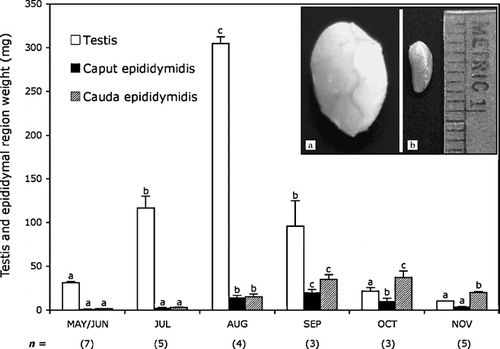
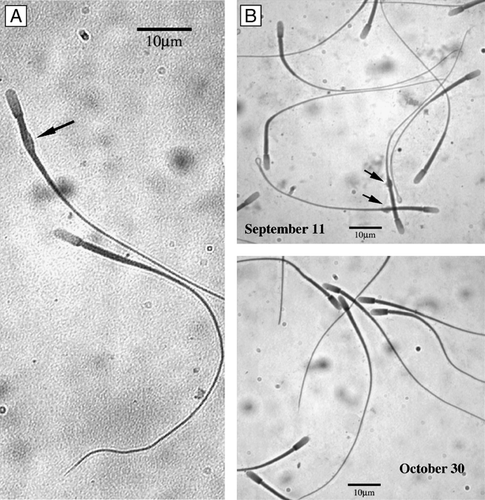
Sperm recovery from the different epididymal regions suggest that spermatozoa transit through the epididymis in C. mexicanus is rapid [Peirce and Breed [Citation2001]]. Sperm number was always low in the caput and in the corpus, never exceeding 30 million (), while in cauda even at the earliest time assayed it was already at 77.2 million, reaching ∼200 million by the end of September. Sperm cells have almost disappeared in the caput epididymis at the beginning of October, and in the corpus by the end of this month while maintaining high concentrations in the cauda (). The percentage of sperm cells with a cytoplasmic droplet was high in cauda epididymis (∼20%) in September then steadily decreased reaching almost nil values by the end of October ( and ).
Determination of the Number of Sperm Cells Recovered from Epididymal Regions at Selected Dates, and Percentage of Spermatozoa with a Cytoplasmic Droplet
Figure 2 C. mexicanus spermatozoa. With cytoplasmic droplet (A, arrow). (B) Sperm cells exhibiting a high percentage with cytoplasmic droplet in the cauda epididymis (∼20%) in September that steadily decrease, reaching almost nil at the end of October.
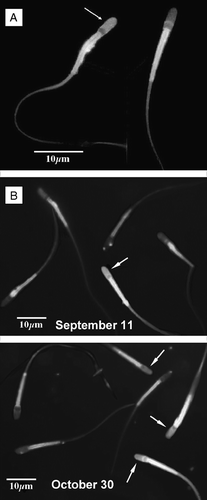
C. mexicanus spermatozoa responded satisfactorily when submitted to capacitation under the conditions utilized for other mammalian sperm cells [García-Macedo et al. [Citation2001]; Ávalos-Rodríguez et al. [Citation2004]], i.e., incubation during 6 h at 39°C under a 5% CO2/95% air atmosphere, in the presence of 2.5 mM Ca2+. After staining with chlortetracycline, capacitated spermatozoa were easily distinguished by the presence of bright fluorescence in the acrosome region ( and , arrows). It is important to note that the neck region of most sperm cells are brilliantly stained by chlortetracycline ().
Figure 3 C. mexicanus spermatozoa capacitation. Spermatozoa were subject to the capacitation reaction during a 6 h incubation at 39°C under a 5% CO2/95% air atmosphere, in the presence of 2.5 mM Ca2+. Evaluation of the percentage of capacitated sperm was done by the chlortetracycline (CTC) fluorescence method. Percentage of capacitated sperm was observed at high magnification, using an epifluorescence Zeiss microscope with a 2Fl filter (405 nm excitation, 460 nm fluorescence and 510 nm barrier filters). (A) Presence of bright fluorescence in the acrosome region (arrow) was considered as providing evidence of capacitation. The neck region was always brilliantly stained by chlortetracycline. (B) Comparison between the epididymal cauda spermatozoa observed on September 11 and October 30, showing the increase in the percentages of capacitated sperm as the spermatozoa reside in the cauda epididymis.
Previously capacitated cauda epididymal spermatozoa showed a linear increase in the percentage of acrosomal reactions induced by either progesterone or A23187 reaching 32±5, and 34±4 (mean±S.D.), respectively, with time during the 60 min incubation [Meizel and Turner [Citation1993]]. No statistical significant differences were found in the percentage of sperm undergoing the acrosomal reaction induced both with A23187 or progesterone. For brevity, only the results obtained using progesterone and after 60 min incubation post-capacitation are shown.
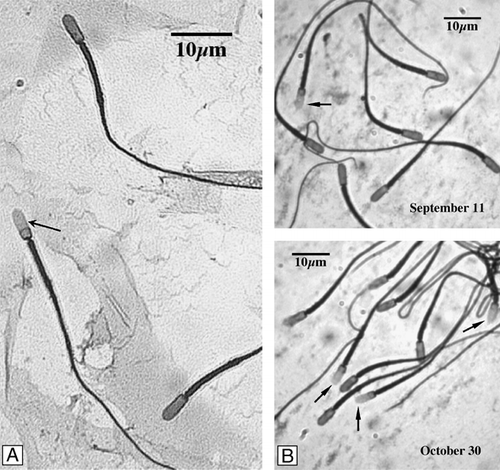
Acrosome-reacted spermatozoa were easily identified by the CBB method (). Blue staining of the acrosomal region forming a distinct apical ridge, indicates intact acrosomes of the unreacted spermatozoa (). In most cases when the acrosome persisted it was accompanied by staining of the entire anterior half, acrosomal region, of the sperm cell [García-Macedo et al. [Citation2001]]. Lack of blue staining was interpreted as characteristic of acrosomally-reacted spermatozoa (, arrow).
Figure 4 Acrosome reaction of C. mexicanus epididymal sperm. The acrosome reaction was assessed during a 60 min incubation, of previously capacitated spermatozoa, in the presence of 3.18 μM progesterone (final concentration). Afterwards aliquots were removed, fixed, and the percentage of acrosomally reacted spermatozoa determined by the coomasie brilliant blue method. Presence and number of stained and unstained spermatozoa was determined at high magnification. (A) Lack of blue staining of the acrosome region (arrow) indicates reacted acrosomes. Blue staining of the acrosome region forming a distinct apical ridge, indicates intact acrosomes of unreacted spermatozoa. (B) Comparison between the epididymal cauda spermatozoa observed in September 11 and October 30, showing the increase in the percentage of acrosome reacted sperm.
When the sperm cells were obtained from the caput epididymis of C. mexicanus the number of capacitated and acrosome reacted spermatozoa was always less than 5%, indicating the presence of a spontaneous process. This percentage was routinely subtracted from the results obtained when the procedures were performed in the spermatozoa obtained from the remaining epididymal regions. The low percentage of capacitation on corpus sperm cells during September (), increased in October reaching its highest value (19.4%) on October 30. In the cauda epididymis, the percentage of capacitated spermatozoa increased significantly (p < 0.05) between September 11 and September 23 and then remained stable around 30%. This is similar to the percentage capacitation obtained from corpus and cauda epididymal spermatozoa in other mammalian species [Yanagimachi [Citation1994]]. Positive progesterone induction of the acrosome reaction followed a similar pattern, increasing in corpus from September 11 to the end of October, but always remaining low, while in the cauda it reached that previously observed by October 30.
Percentage of Capacitated and Acrosome Reacted Spermatozoa Recovered from Different Epididymal Regions of C. mexicanus
Discussion
In passing through the epididymal conduit, spermatozoa must actively interact with their environment [Reyes-Moreno et al. Citation[2007]]. A necessary resultof this active interaction is epididymal maturation, a process that makes the spermatozoa fully capable of undergoing all the necessary processes (progressive motility, capacitation, hyperactivation and acrosomal reaction) to fertilize of the oocyte [Yanagimachi [Citation1994]; de Lamirande et al. [Citation1997]]. The average epididymal transit time in mammals is about 10 days. Each one of the epididymal regions is usually associated with somewhat specialized functions. For example the caput function is mainly reabsorptive, corpus has a net secretory function, and the cauda, predominantly, a storage function. In most mammalian species sperm maturation is achieved during the progress of the spermatozoa through the initial portions of the epididymis, so that when they initiate their storage period in the cauda, sperm have matured [Orgebin-Crist [Citation1987]; Yanagimachi [Citation1994]]. Sperm cells are stored for periods that can extend from days to weeks (depending on the species) until ejaculation. So, in most mammals, the main function of the cauda epididymis seems to be that of sperm storage, ensuring viability and fertility of sperm cells during store.
Epididymal physiology seems to behave differently in C. mexicanus. This is likely echoed in all other vespertilionid and rhinolophid bats that show the same temporal asynchrony in development and function of male reproductive organs. The persistence of a high percentage of sperm cells with a cytoplasmic droplet, low index of capacitation and acrosome reaction in sperm cells obtained from the corpus epididymis is observed. This is followed by a significant increase in the percentage of capacitated and acrosome reacted spermatozoa during the stay of the sperm cells in the cauda epididymis, the percentage of capacitated spermatozoa being consistently, and significantly, higher (p < 0.05) in cauda than in corpus epididymis on all the studied dates, and of the sperm capable of undergoing the acrosome reaction during October. Almost all of our results point to the fact that epididymal maturation in C. mexicanus is completed in the caudal region of this organ involving a significant period that was previously considered as storage.
Our observations suggest that the initial aspects of epididymal maturation, such as nuclear condensation and mitochondrial stabilization [Arenas-Ríos et al. [Citation2007]] may be similar in these bat-species as those observed in many other mammals. However the results presented herein suggest that the final morpho-physiological changes of sperm maturation, including loss of cytoplasmic droplet, capacity for progressive motility, preparation for hyper activation and for sperm–oocyte fusion (capacitation and acrosome reaction), may be just initiated at the time at which spermatozoa initiate their residence in the cauda epididymis. Perhaps the long sperm storage periods observed in these species may be indispensable to sperm maturation.
Endocrine regulation of epididymal physiology appears to also be different in Corynorhinus mexicanus. Although it has been recently shown that estrogens may be functionally indispensable for epididymal function [McCarthy et al. [Citation2006]], it is known that epididymal functions in the majority of mammals, including epididymal sperm transport, sperm maturation and sperm storage, heavily depend on testicular androgen secretion [Holland et al. [Citation1992]; Orgebin-Crist [Citation1996]; Moore and Akhondi [Citation1996]]. When, in otherwise intact animals, the cauda epididymis is physiologically isolated in situ by ligation of both of its extremes, the stored spermatozoa degenerate within several weeks but do not decrease noticeably in number [Lubicz-Nawrocki [Citation1976]; Jones [Citation2004]]. However, when subject to bilateral orchidectomy at the same time that the cauda epididymis is isolated, spermatozoa rapidly die and practically disappear (>90%) in a few days. This effect of bilateral castration can be prevented by the administration of testosterone [Jones [Citation2004]]. In other seasonally breeding males, as the levels of testosterone decline, the number of spermatozoa in the epididymis decreases and finally disappears [Millar [Citation1972]]. Our observations in C. mexicanus [López-Wilchis Citation[1989]; León-Galván et al. [Citation1999], [Citation2005]; Arenas-Ríos et al. [Citation2005], [Citation2007]] permit us assurance that testes are totally regressed during sperm storage yet sperm maturation in the epididymis, is completely functional. The results presented herein indicate that in C. mexicanus, and surely in other vespertilionid and rhinolophid bats which show the same temporal asynchrony in the development and function of male reproductive organs, the final phases of epididymal maturation and storage are, apparently, independent of testicular function. Although different patterns of testosterone secretion have been defined according to the bat species [Martin and Bernard [Citation2000]], in general, while steroidogenic activity of the Leydig cells and high plasma testosterone coincide with spermatogenesis in summer, in the winter-months, when accessory glands are active (or kept in a hypertrophied state), the Leydig cells are inactive and plasma testosterone is low [Krutzsch [Citation2000]].
This apparent physiological independence of epididymal important functions from testes physiology, require a full exploration of secretion and blood levels of steroid and other hormones in these bat species. It has been suggested that high levels of steroid-binding-globulin may play an important role in reproductive function after testes involution. Unfortunately there are not adequate measurements of steroid-binding-globulin in bats [Martin and Bernard [Citation2000]].
In addition to promoting sperm maturation and providing a place for sperm storage, the epididymis plays a role in the transport of spermatozoa along the duct and protects spermatozoa from harmful substances during its transport from the rete testis to the epididymal cauda. Many important tasks related to these processes appear to be under redox control [Leclerc et al. [Citation1997]; de Lamirande et al. [Citation1997]; Lewis and Aitken [Citation2001]; Ford [Citation2004]]. We have recently shown [Arenas-Ríos et al. [Citation2007]] that, in C. mexicanus, redox equilibrium of the micro-environments associated with the milieus by which mammalian spermatozoa must progress during its transit through the epididymis seems to be specifically and differentially controlled with the compartmentalization of epididymal functions. Our data on reactive oxygen species (ROS) related enzyme activities (glutathione peroxidase, catalase and superoxide dismutase) stress the existence of a careful differentially regulated equilibrium between the activities of these enzymes in the cauda and in the caput epididymis that appear to be specifically related to the precise maturation/storage function of the different epididymal regions.
Materials and methods
Chlortetracycline (CTC), coomassie brilliant blue G-250 (CBB), HEPES (N-(2-hydroxy-ethyl) piperazine-N′-(2-ethanesulfonic acid)), sodium pyruvate, Tris (tris-(hydroxyl-methyl) aminomethane), sodium lactate, D-(+) glucose, bovine serum albumin (fatty acids free), and other salts were purchased from Sigma Chemical Co (St. Louis, Missouri, USA); calcium ionophore A23187 was purchased from Boehringer-Mannheim (Germany). All other reagents were at least analytical grade.
Adult male C. mexicanus were captured every two weeks from September 11 to October 30, 2006 in México (19°37′N, 98°02′W). Bats were always captured before or as they left their roost. Individuals were sexed, weighed and their adult status determined as previously described [Kunz and Anthony [Citation1982]; León-Galván et al. [Citation2005]]. In total 16 adult male bats were examined. Mean body weight was 7.5 g (range 7.2–8.0 g) and the mean forearm length was 41.8 mm (range 41.5–43.1 mm).
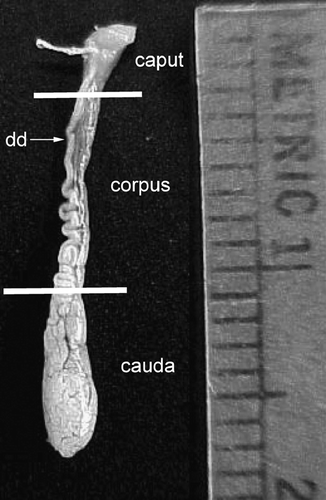
Animals for each determination were captured on the same day, unless two captures, realized on the same week, were necessary. Animals were euthanized and the epididymes isolated, cleared of fat and connective tissue and weighed in a Mettler model AB204 balance (±0.01 mg). Caput, corpus and cauda segments of the epididymes isolated as previously indicated [Arenas-Ríos et al. [Citation2005]]. Briefly, isolation of caput from the corpus was carried out by dissecting at the level at which the walls of the epididymis became clearly parallel (, upper line). The cauda epididymis is flattened in appearance in non-breeding bats and easily differentiated from breeding bats when they become swollen and bulbous. In non-breeding bats the cauda epididymis were isolated by dissecting at the site in which the deferens became clearly separated from the epididymis (, lower line).
Figure 5 Fully developed epididymis of adult male Corynorhinus mexicanus. The caput, corpus and cauda regions are shown. Isolation of caput from the corpus was done by dissecting at the level at which the walls of the epididymis became clearly parallel (upper line). The cauda epididymis was isolated by dissecting at the site in which the deferens became clearly separated from the epididymis (lower line). Metric units indicate millimeters.
Isolated epididymal regions were cleared of fat and connective tissue, rinsed in Biggers, Whitten, and Whittingham medium (BWW) [Biggers et al. [Citation1971]], and weighed. Spermatozoa from the different epididymal regions were obtained as previously described [Ambríz et al. [Citation2003]]. Determinations of sperm number, viability, and presence of cytoplasmic droplet were carried out using standard microscopic procedures.
Evaluation of the percentage of capacitated sperm and assessment of the acrosomal reaction were carried out as previously described [García-Macedo et al. [Citation2001]; Ávalos-Rodríguez et al. [Citation2004]]. Capacitation of bat spermatozoa was accomplished by incubating the sperm suspension for 6 h at 39°C in the presence of 2.5 mM Ca2+ in loosely caped polypropylene conical centrifuge tubes under a 5% CO2/95% atmosphere. After the 6 h capacitating time, 50 μl duplicated aliquots of the sperm cell suspensions were used to determine viability and motility of the incubated spermatozoa and to evaluate the percentage of capacitated spermatozoa. Evaluation of the percentage of capacitated sperm was by the CTC fluorescence method as described by Green et al. [[Citation1994]]. Briefly, the sperm suspension was mixed with 10 μl of 12% paraformaldehyde solution in 0.5 M Tris-HCl 0.5 M, pH 7.4 and 50 μl of a freshly prepared CTC solution (750 mM CTC, 130 mM NaCl, 5 mM cysteine, 20 mM Tris-HCl, pH 7.8). 10 μl of this mixture were smeared in a glass slide previously coated with polylysine [Mazia et al. [Citation1975]], covered with a solution of 0.22 M of the antioxidant 1,4-diazabicyclo-(2,2,2)-octane (DABCO) in 90% glycerol to prevent photo bleaching [Köhn et al. [Citation1997]] and immediately observed by epifluorescence using a Zeiss microscope with a 2Fl filter (405 nm excitation, 460 nm fluorescence and 510 nm barrier filters). At least 200 spermatozoa were counted and classified according to their acrosome fluorescence.
The acrosome reaction was induced in previously capacitated spermatozoa, either by the addition of 100 μl of A23187 diluted stock solution to a final concentration of 10 μM of the ionophore, or by the addition of progesterone to a final concentration of 3.18 μM [Sabeur et al. [Citation1996]] in the capacitating medium and the incubation was continued under the same conditions. After 15, 30, 45 and 60 min at 37°C, in accord with our previous results [García-Macedo et al. [Citation2001]; Ávalos-Rodríguez et al. [Citation2004]], samples were removed, fixed, and the percentage of acrosome reacted spermatozoa was determined by CBB staining [Miller et al. [Citation1993]].
All microscopically counted sperm samples employed double blind procedures and were verified by two independent observers, each counting at least 100 spermatozoa on each slide. In all cases after classification of at least 200 spermatozoa per slide according to the fluorescence or staining patterns of their acrosomes, the percentage of the capacitated or acrosome-reacted spermatozoa was calculated.
Realization of this work, including the use and handling of animals, was reviewed and approved by the Consejo Divisional de Ciencias Biológicas y de la Salud (Biology and Health Sciences Divisional Board) de la Unidad Iztapalapa de la Universidad Autónoma Metropolitana. The animals were cared for in accordance to the Guidelines of the American Society of Mammalogist for the use of wild mammals in research [William et al. [Citation2007]].
Statistical Analysis
Comparisons among groups were made with one-way ANOVA [Sokal and Rohlf [Citation1995]] followed by the Bonferroni post hoc test. Homogeneity of variances was tested by Bartlett and Levene tests [Sokal and Rohlf [Citation1995]]. Statistical analysis was performed using the Stata 7.0 statistical package (Statacorp 2001, Texas). Differences were considered statistically significant when p < 0.05.
Acknowledgments
This work was supported partially by the Consejo Nacional de Ciencia y Tecnología (Beca crédito No. 169578 and convenio No. 400200-5-31743-N).
References
- Ambriz D., Contreras J. L., Hernández O., Mercado E., Cervantes F., Rosado A. Estudio comparativo de los testículos, epidídimos, glándulas sexuales accesorias y espermatozoides de tres especies de lagomorofos (Romerolagus diazi, Lepus californicus y Oryctolagus cuniculus). Acta Zool Mex (n.s.) 2003; 88: 257–269
- Arenas-Ríos E., León-Galván M. A., Mercado P. E., López-Wilchis R., Cervantes D. L. M. I., Rosado A. Superoxide dismutase, catalase, and glutathione peroxidase in the testis of the Mexican big-eared bat (Corynorhinus mexicanus) during its annual reproductive cycle. Comp Biochem Physiol A 2007; 148: 150–158
- Arenas-Ríos E., León-Galván M. A., Mercado P. E., Rosado A. Superoxide dismutase, catalase, and glutathione peroxidase during epididymal maturation and prolonged storage of spermatozoa in the Mexican big-eared bat (Corynorhinus mexicanus). Can J Zool 2005; 83: 1556–1565
- Ávalos-Rodríguez A., Ortíz-Muñíz A. R., Ortega-Camarillo C., Vergara-Onofre M., Rosado-García A., Rosales A. M. Fluorometric study of rabbit sperm head membrana phospholipid asymmetry during capacitation and acrosome reaction using Annexin-V FITC. Arch Androl 2004; 50: 273–285
- Avery M. I. Winter activity of pipistrelle bats. J Animal Ecol 1985; 54(3)721–738
- Bedford J. M. Mammalian fertilization misread? Sperm penetration of the eutherian zona pellucida is unlikely to be a lytic event. Biol Reprod 1998; 59: 1275–1287
- Biggers J. D., Whitten W. K., Whittingham D. G. The culture of mouse embryos in vitro. Methods of Mammalian Embryology, J. C. (Ed.), Daniel. Freeman, San Francisco 1971; 93–116
- Brucker C., Lipford G. B. The human sperm acrosome reaction: Physiology and regulatory mechanisms. An update. Hum Reprod Update 1995; 1: 51–62
- Cooper, T. G. The Epididymis, Sperm Maturation and Fertilisation. Springer-Verlag, Berlín 1986, p. 240.
- de Lamirande E., Jiang H., Zini A., Kodama H., Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod 1997; 2: 48–54
- Ford W. C. L. Regulation of sperm function by reactive oxygen species. Hum Reprod Update 2004; 10(5)387–399
- García-Macedo R., Rosales A. M., Hernández-Pérez O., Chavaría M. E., Reyes A., Rosado A. Effect of Bafilomycin A-1, a specific inhibitor of vacuolar ATPases on the capacitation of rabbit spermatozoa. Andrologia 2001; 33: 113–121
- Geiser F., Ruf T. Hibernation versus daily torpor in mammals and birds: Physiological variables and classification of torpor patterns. Physiol Zool 1995; 68(6)935–966
- Gopalakrishna A., Bhatia D. Storage of spermatozoa in the epididymis of the bat Hipposideros speoris (Schneider). Curr Sci 1980; 49: 951–953
- Green C. M., Cockie S. M., Watson P. F., Fraser L. R. Stimulating effect of pyroglutamylproline amide a prostatic TRH-related tripeptide, on mouse sperm capacitation and fertilizing ability in vitro. Mol Reprod Dev 1994; 38: 215–223
- Holland M. K., Vreeburg J. T. M., Orgebin-Crist M. C. Testicular regulation of epididymal protein secretion. J Androl 1992; 13: 266–273
- Jones R. Sperm survival versus degradation in the mammalian epididymis: A hipotesis. Biol Reprod 2004; 71: 1405–1411
- Karim K. B., Banerjee S. Storage of spermatozoa in the epididymis of the tropical bat, Rhinopoma hardwickei hardwickei (Gray). Anat Rec 1985; 211: 95A
- Köhn F. M., Mack S. R., Schill W. B., Zaneveld L. J. D. Detection of human sperm acrosome reaction: Comparison between methods using double staining, Pisum sativum agglutinin, concanavalin A and transmission electron microscopy. Hum Reprod 1997; 12: 714–721
- Krutzsch P. H. Anatomy, physiology and cyclicity of the male reproductive tract. Reproductive Biology of Bats, E. G. Crichton, P. H. Krutzsch. Academic press, London 2000; 91–155
- Kunz T. H., Anthony E. L. P. Age estimation and post-natal growth in the little brown bat, Myotis lucifugus. J Mammal 1982; 63: 23–32
- Leclerc P., de Lamirande E., Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radical Biol Med 1997; 22(4)643–656
- León-Galván M. A., Fonseca T., López-Wilchis R., Rosado A. Prolonged storage of spermatozoa in the genital tract of female Mexican big-eared bat (Corynorhinus mexicanus): The role of lipid peroxidatin. Can J Zool 1999; 77: 7–12
- León-Galván M. A., López-Wilchis R., Hernández P. O., Arenas R. E., Rosado A. Male reproductive cycle of the mexican big-eared bats, Corynorhinus mexicanus (chiroptera: vespertilionidae). Southwest Nat 2005; 50: 453–460
- Lewis B., Aitken R. J. Impact of epididymal maturation on the tyrosine phosphorylation patterns exhibited by rat spermatozoa. Biol Reprod 2001; 64(5)1545–1556
- López-Wilchis, R. (1989). Biología de Plecotus mexicanus (Chiroptera: Vepertilionidae) en el Estado de Tlaxcala. México. Doctoral thesis, Universidad Nacional Autonoma de México, México.
- Lubicz-Nawrocki C. M. The effect of metabolites of testosterone on the development of fertilizing ability by spermatozoa in the epididymis of castrated hamsters. J Exp Zool 1976; 197: 89–95
- Martin L., Bernard R. T. F. Endocrine regulation of reproduction in bats: The role of circulating gonadal hormones. Reproductive Biology of Bats, G. E. Crichton, P. H. Krutzsch. Academic Press, London 2000; 27–64
- Mazia D., Schatten G., Sale W. Adhesion of cells to surfaces coated with polylysine. J Cell Biol 1975; 66: 198–200
- McCarthy M. J., At-Taras E. E., Pearl C. A., Nitta-Oda B. S., Roser J. F., Conley A. J., Berger T. Suppression of endogenous estrogen during development affects porcine epididymal sperm maturation. Mol Reprod Dev 2006; 73: 1122–1128
- Meizel S., Turner K. O. Initiation of human sperm acrosome reaction by Thapsigargin. J Exp Zool 1993; 267: 350–355
- Millar R. P. Degradation of spermatozoa in the epididymis of a seasonally breeding mammal, the rock hyrax, Procavia capensis. J Reprod Fertil 1972; 30: 447–450
- Miller D. J., Gong X., Shur B. D. Sperm require b-N-acetylglucosaminidase to penetrate through the egg zona pellucida. Dev 1993; 118: 1279–1289
- Moore H. D. M., Akhondi M. A. In vitro maturation of mammalian spermatozoa. J Reprod Fertil 1996; 1(1)54–60
- Orgebin-Crist M. C. Post-testicular development of mammalian spermatozoa. Morphological Basis of Human Reproduction Function, D. M. de Kretser, O. Spera. Acta Medica, Plenum Press, New York 1987; 115–174
- Orgebin-Crist M. C. Androgens and epididymal function. Pharmacology, Biology, and Clinical Applications of Androgens, S. Bhasin, H. L. Gabelnick, J. M. Spieler, R. S. Swerdloff, C. Wang. Wiley-Liss, Inc., New York 1996; 27–38
- Peirce E. J., Breed W. G. A comparative study of sperm production in two species of Australian arid zone rodents (Pseudomys australis, Notomys alexis) with marked differences in testis size. Reproduction 2001; 121: 239–247
- Racey P. A., Entwistle C. A. Life-history and reproductive strategies of bats. Reproductive biology of bats, E. G. Crichton, P. H. Krutzsch. Academic Press, London 2000; 363–414
- Reyes Moreno, C., Laflamme, J., Frenette, G., Sirard, M. A., Sullivan, R. (2007). Spermatozoa modulate epididymal cell proliferation and protein secretion in vitro. Mol. Reprod. Dev. Published Online: 20 Sep 2007.
- Sabeur K., Edwards D. P., Meizel S. Human sperm plasma membrane progesterone receptor(s) and the acrosome reaction. Biol Reprod 1996; 54: 993–1001
- Singh K., Krishna A. Inhibitory effects of melatonin on testosterone but not on androstenedione production during winter in the vespertilionid bat, Scotophilus heathi. J Pineal Res 1995; 19(3)127–132
- Sokal, R. R., Rohlf, F. J. Biometry ed. Freeman H.L. and Co., New York 1995, pp. 887.
- William L., Gannon R., Sikes S. Guidelines of the American Society of Mammalogist for the use of wild mammals in research. J of Mammalogy, 2007; 88(3)809–823
- Yanagimachi R. Mammalian fertilization. The Physiology of Reproduction, E. Knobil, J. D. Neill. Raven Press, New York 1994; 189–317