Abstract
Most of the studies investigating the relationship between varicocele and male infertility are mainly focused on the testicles. It is obvious that varicocele would affect the morphology and function of the epididymis which is an intrascrotal organ. In this study, the effects of experimental left varicocele (ELV) on the epididymal morphology were investigated in adult rats. ELV was induced via partial obstruction of the left renal vein in 20 Sprague-Dawley adult rats. An additional twelve rats served as controls, and another twelve served as shams. Half of the rats in the groups were sacrified by the end of the first month of the experiment, and the rest were sacrified by the end of the second month. Epididymides were weighed; tubular diameters of the caput, corpus, and cauda of the epididymis were measured. The TUNEL assay was used to assess apoptosis within the epididymal tubules. The mean weight of each right and left epididymis in the varicocele group was lower than that in the control and sham groups (p < 0.01). In the varicocele group, the left epididymis weighted less than the right by the end of the second month (p < 0.01). The mean tubular diameter in the varicocele group was narrower than that in the control and sham groups (p < 0.001). Tubular diameter was significantly narrower in the caput segments in rats with varicocele by the end of the second month (p < 0.001). Apoptosis was significantly increased in principal cells of the epididymal epithelium in the varicocele groups. The apoptotic cells in the caput epididymis epithelium were more numerous than those in the other segments. In conclusion, ELV significantly decreases epididymal weight and tubular diameters presenting increased apoptosis within the principal cells. There is a positive correlation between the epididymal damage and the duration of varicocele.
| Abbreviations | ||
| ELV | = | Experimental left varicocele |
| TUNEL | = | Terminal deoxynucleotidyl transferase mediated dUTP nick end labeling |
INTRODUCTION
Varicocele is a dilation of the internal spermatic vein (pampiniform plexus) and it is generally recognized as the most common treatable cause of male infertility. Despite extensive investigation of the utility of varicocelectomy, the association between the varicocele and infertility has not been clearly established [Schlesinger et al. [Citation1994]].
Many theories have been proposed to explain the mechanism by which varicocele causes deleterious effects on spermatogenesis and fertility. These include renal and adrenal venous reflux, hormonal dysfunction, hypoxia and stasis, increased testicular blood flow and temperature [Asci et al. [Citation1999]; Marmar [Citation2001]; Schoor et al. [Citation2001]; Fretz and Sandlow [Citation2002]]. Various studies of testicular tissue with varicoceles have demonstrated increased apoptosis among developing germ cells, which may cause oligozoospermia [Turner and Lopez [Citation1990]; Simsek et al. [Citation1998]; Kilinc et al. [Citation2004]; Chen et al. [Citation2004]; Benoff et al. [Citation2004]; Barqawi et al. [Citation2004]; Ku et al. [Citation2005]]. A recent experimental study showed that testicular germ cell apoptosis decreased after varicocelectomy [Fazlioglu et al. [Citation2008]]. The investigations on the relationship between varicocele and male infertility are mostly focused on the testicles. Although varicocelectomy decreased the intratesticular temperature, fertility was achieved only in about one third of the patients [Schlesinger et al. [Citation1994]]. Whereas testicular functions may improve after varicocelectomy, it is not known if the epididymal disturbance is resolved. In two thirds of the patients who are not fertile after varicocelectomy, one of the causative factors may be epididymal disturbance [Schlesinger et al. [Citation1994]].
Testicular spermatozoa are immotile and incapable of fertilizing ova. During the passage through the epididymis, spermatozoa gain motility and the capability to fertilize oocytes [Asci [Citation2004]]. The epididymis, which has a key role in maturation of sperm motility and fertilization capacity, is also responsible for sperm storage and transport. Experimental studies demonstrated that the epididymal structures and functions are influenced for example, by temperature, varicocele, orchidectomy and bisphenol administration [Foldesy and Bedford [Citation1982]; Fan and Robaire [Citation1998]; Jara et al. [Citation2002]; Chitra et al. [Citation2003]]. Abdominal placement of the epididymis causes abnormalities in sperm storage and electrolyte transport functions [Wong et al. [Citation1982]].
In addition, varicocele increases intrascrotal temperature, and high temperature may cause deleterious effects on epididymal morphology and functions. The mechanism responsible for alterations to the epididymal epithelium influenced by varicocele remains unknown. In this study, the effect of experimental left varicocele on the epididymal morphology was studied in the rat model.
RESULTS
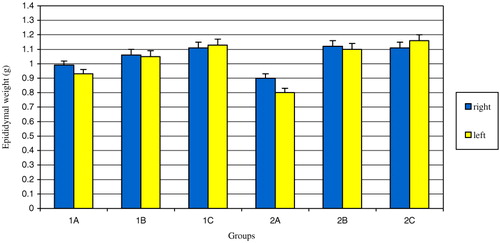
During the experiment, a single rat in group 1A expired. Right and left whole epididymal weights of varicocele groups (1A and 2A) were less than those of the sham (1B and 2B) and control (1C and 2C) groups (p < 0.01) (). Also, the mean left epididymal weight was statistically less than the mean right epididymal weight in group 2A (p < 0.05) (). No statistically significant difference was observed in the mean left and right caput epididymal tubule diameters in the varicocele group compared with sham and control groups by the end of the first month.
FIGURE 1 The comparison of the mean epididymal weights of the groups. The difference between right epididymal weights of groups 1A, 1B and 1C was statistically significant (p=0.007). The difference between left epididymal weights of groups 1A, 1B and 1C was statistically significant (p=0.006). The difference between right epididymal weights of groups 2A, 2B and 2C was statistically significant (p=0.009). The difference between left epididymal weights of groups 2A, 2B and 2C was statistically significant (p=0.003). The difference between right and left epididymal weights of group 2A was statistically significant (p < 0.05).
A statistically significant decrease was noted in the mean corpus epididymal tubular diameters in varicocele group 1A compared with sham group 1B and control group 1C by the end of the first month (p < 0.001). A statistically significant decrease was noted in the mean cauda epididymal tubular diameters in varicocele group 1A compared with sham group 1B and control group 1C by the end of the first month (p < 0.001). In the varicocele group 2A, the mean diameters of all the epididymal segments (caput, corpus, cauda) were found narrower than the sham group 2B and the control group 2C by the end of the second month (p < 0.001).
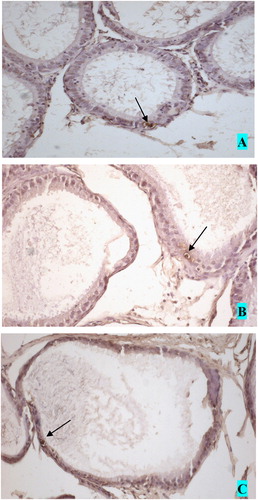
As summarized in , the mean left cauda epididymal tubular diameter was significantly smaller than that of the right side in the varicocele group at the end of second month of the study (p < 0.001). The mean left cauda tubular diameter was smaller in the sham group compared with the control group at the end of the second month of the study. The number of TUNEL-positive cells in the epididymal epithelium in groups 1A and 2A were higher than those in the other groups. An example of the brown stained epididymis epithelial principle cells is shown in and apoptotic cells in the epididymal epithelium are shown in . The rate of apoptosis in the caput epididymis epithelium of the varicocele groups was higher than that in the other epididymal segments of the same group ().
The Comparison of the Mean Tubular Diameters of the Epididymides (μm)
FIGURE 2 Apoptotic epididymal principal cell stained with TUNEL method. Black arrow shows apoptotic principal cell. Magnification (×400).
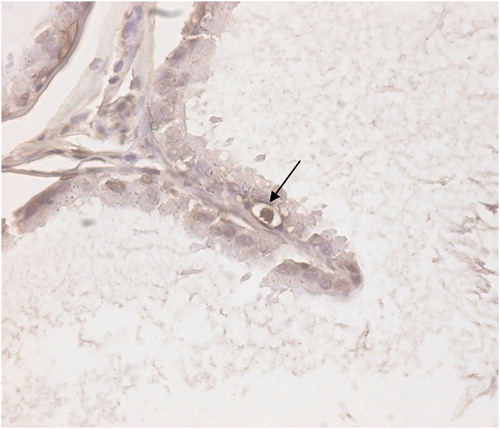
FIGURE 3 Apoptotic cells in the epididymal epithelium. A. caput, B. corpus and C. cauda epididymis. Magnification (×200).
TUNEL-Positive Cell Distribution in the Epididymal Epithelium. [0 is (−), 0.1–0.5 is (+), 0.51–1.0 is (++) and 1.0 < is (+++)]
DISCUSSION
The epididymis plays an important role in male reproduction by providing a microenvironment for sperm maturation and storage. The functions and structure of the epididymis are dependent upon androgens and several testicular factors produced by the testis [Asci [Citation2004]]. Post-testicular spermatozoal maturation requires the specialized epididymal environment which is regulated by selective secretory and absorptive functions [Asci [Citation2004]]. The trials studying the interplay between varicocele and infertility have chiefly focused on the histological structure, and endocrine and exocrine functions of the testicles [Asci et al. [Citation1999]; Marmar [Citation2001]]. With its anatomical, endocrine, and exocrine connections with the testicles within the scrotum, it is not possible for the epididymis to remain uninfluenced by the incriminated pathophysiological factors of varicocele, including hyperthermia. Experimental varicocele has demonstrated that the epididymis suffers bilaterally from hyperthermia [Wang et al. [Citation1991]].
The epididymis which is responsible for sperm fertility maturation, consist of functional tubule and luminal fluid. In our study, the mean epididymal weight of the varicocele group was found to be lower than those of the control and sham groups. Moreover, at the second month, the left epididymides displayed lower weights than those on the other side. This may be attributed to the deleterious effects of varicocele spread over time. Although there are certain technical difficulties, the measurement of epididymal volume as well as testicle volume can give some important information for evaluating an infertile patient with varicocele.
The changes within the epididymis can provide important explanatory findings about varicocele induced infertility. On one hand, Turner et al. [[Citation1987]] reported that the concentration of epidydimidal Na+ and K+ and the blood-testicle barrier remained uneffected within the first month of varicocele. On the other hand, Hurt et al. [[Citation1986]] reported that ELV decreases sperm motility and concentration within the cauda epididymis, and Caflisch [[Citation1992]] reported that the acidity of the epididymal fluid has increased. Using an adult rat experimental varicocele model, Wang et al. [[Citation1991]] have reported damage and scantiness within epididymal epithelial microvili. This epididymal damage can be attributed to the changes in the composition of the seminiferous tubular fluid, as well as the direct injury inflicted by varicocele.
The effect of ELV on epididymal tubular diameter was first studied in the adolescent rat model [Zhang et al. [Citation2003]]. This showed a marked decrease in tubular diameter and a marked increase in epididymal interstitial mass. At the first month, the decrease in the tubular diameter was significant within the bilateral capital and left caudal segments, whereas by the end of the second month, the decrease was evident in all segments throughout both epididymides. The difference between the right and left sides was insignificant [Zhang et al. [Citation2003]]. The results of this study are very similar to the data reported here. As presented above, substantial narrowing of the tubules was limited to the corpus and cauda epididymis, whereas by the second month, the caput epididymis was also affected. These results indicate the extent of epididymal damage over the time in which varicocele persists. The mean tubular diameter of the cauda epidydimis was found to be significantly smaller than the contralateral within the varicocele group at the end of second month. This likely reflects the initial presence of the varicocele on the left side, and the duration of the ailment. Previous studies have demonstrated a more pronounced progressive damage on the varicocele side [Saypol et al. [Citation1981]; Kass et al. [Citation1987]; Turner and Lopez [Citation1990]; Asci et al. [Citation1999]].
It is known that germ cell apoptosis increases during spermatogenesis by 35–40% [Hikim et al. [Citation1998]]. Experimental studies suggest that varicocele and other pathologies increase germ cell apoptosis [Kilinc et al. [Citation2004]; Chen et al. [Citation2004]; Benoff et al. [Citation2004]; Barqawi et al. [Citation2004]]. In contrast, Fujisawa et al. [[Citation1999]] have reported a decrease of germ cell apoptosis within the testicles of infertile men with varicocele utilizing the methods described in the aforementioned study.
The principle cells are the basic cells of the epididymal tubule epithelium, and are androgen dependent [Asci [Citation2004]]. The androgen is supplied by testicular androgens coming into the tubule lumen via efferent canals, and to a lower extent, circulating androgens from the bloodstream. As the aim of this study is to evaluate the morphological changes of the epididymis which depend on varicocele, serum and epididymal androgen levels were not measured, and testicular functions (spermatogenesis) were not evaluated. The principle cells are the mainstay of sperm maturation in the epididymis. The impact of varicocele on the principle cells of the epididymal tubules remains to be resolved. It has been shown that some experimentally induced pathological conditions result in increased apoptosis of the epididymal epithelium [Fan and Robaire [Citation1998]; Turner and Riley [Citation1999]; Jara et al. [Citation2002]]. Orchidectomy and efferent canal ligation have been shown to increase apoptosis within the initial and caputal segments, and caudal segments of the epididymides, respectively [Fan and Robaire [Citation1998]; Qiu et al. [Citation2004]]. More apoptotic cells were detected within the caputal segment than those within the caudal segment in rats exposed to diethylstilbestrol [Qiu et al. [Citation2004]].
In our study, it has been confirmed that varicocele increases apoptosis within the epididymal epithelium. Apoptosis was more pronounced within the caputal segment than those of the other segments of epididymis. The caput epididymis has an increased androgen demand for its function. However, varicocele is not known to produce an abrupt cessation of androgen supply like orchiectomy. This suggests the contribution of other patophysiological factors (especially hyperthermia) in increased apoptosis. Although we found increases of apoptosis within all three epididymal segments, with cauda being the most prominent, it has been reported by others that the increased abdominal temperature only affects the caudal segments of epididymis [Jara et al. [Citation2002]].
Normally, the epididymal epithelium has a slow turnover rate, thus with barely detectable apoptosis, if any [Fan and Robaire [Citation1998]]. While some of the apoptotic principle cell bodies are excreted into the lumen, the rest are removed by the adjacent cells via phagocytosis [Jara et al. [Citation2002]]. A high level of apoptosis encountered within the varicocele group may be suggestive of the deleterious effect of varicocele on the epididymal epithelium. The involvement of the contralateral epidydimis is consistent with previous studies reporting bilateral damage caused by varicocele [Saypol et al. [Citation1981]; Turner and Lopez [Citation1990]].
In conclusion, our study shows that varicocele significantly decreases the epididymal weight and tubule diameter on the involved side. Moreover, it increases apoptosis throughout the epididymal tubule epithelium, especially within the caputal segment. ELV also disrupts the contralateral side that increases in scale with the duration of the ailment. Although further confirmational studies are warranted, we believe that the epididymal involvement also has an important role on the infertility caused by varicocele as well as testicular damage.
MATERIALS AND METHODS
Animals
The study was approved by the Animal Care and Ethics Committee of Ondokuz Mayis University School of Medicine. A total of 44 adult Sprague-Dawley rats weighing 400±55 g were obtained from the Ondokuz Mayis University School of Medicine Surgical Research Laboratories. During the experiment, all rats were maintained on a 12-hour dark-light cycle with room temperature of 22°C. They were fed on standard rat ration and tap water.
Surgical Procedures
All surgical procedures were performed in aseptic conditions under general anaesthesia provided by the intramuscular injection of 10 mg/kg xylazine hydrochloride and 80 mg/kg ketamine hydrochloride combination. First generation cephalosporin was administered to all subjects for prophylactic antibiotherapy.
Experimental Left Varicocele
ELV was created in a total of 20 rats using a technique previously described by Saypol et al. [[Citation1981]]. The abdominal wall was cleaned with a solution of povidone iodine and a midline incision was made to expose the left renal vein. Partial left renal vein ligation was performed with a 4/0 silk ligature placed medial to the insertion of the left adrenal vein. The ligature was placed around the vein and a parallel 0.85 mm metal probe. After tying the ligature, the probe was removed, producing an approximate 50% decrease in renal vein diameter. After ligation, the renal vein immediately dilated in all rats. Abdominal midline incisions were closed with 3/0 silk suture.
Sham Operation
Twelve animals that were submitted to laparotomy and renal vein handling without renal vein ligation served as sham controls.
Control Group
Twelve rats not submitted to any surgical procedures were used as the control group. Half of the rats in each of the varicocele, sham and control groups (group 1A, 1B or 1C, respectively) were sacrificed at 4 weeks following surgery and the other half (group 2A, 2B or 2C) were sacrificed at 8 weeks following surgery. Bilateral epididymides were removed via mid scrotal incision. The epididymides were isolated by excising the efferent ducts and vas deferens. The weights of epididymides were measured by using a sensitive scale (XE220A Precisa). The epididymides were dissected into caput, corpus and cauda segments and were fixed in a 10% neutral buffered formaline solution for 24 hours.
Epididymal Morphological Study
The epididymides were processed for the routine tissue follow-up with Shandon Citadel 2000 apparatus in separate cassettes, and parafin blocks were prepared. Two sections were taken from each parafin block to measure tubule diameter and evaluate the level of apoptosis in the epithelium of epididymis. The sections were stained with Periodic Acid Schift (PAS) to measure the epididymal tubule diameter. The apoptosis in the epithelium of the tubule was investigated with TUNEL method.
Measurement of Epididymal Tubular Diameters
The PAS stained sections were evaluated blindly by two pathologists (MK and IA). Microscopic tubule images were collected under ×100 magnification (Zeiss, Axiophot, Germany) using a digital camera (Insight Diagnostic Instrument, USA). The images were evaluated using Morphometry analysis software (Samba Technologies 2005, Morphometry Analysis, France). Ten of the most round tubules per section were selected and examined. The distances between the parallel walls of the tubules were measured and the mean diameters of the tubules were calculated.
Apoptosis in the Epididymal Epithelium
Apoptosis was assessed by TUNEL (terminal deoxynucleotidyl transferase mediated dUTP nick end labeling) using the in situ cell death detection kit, POD (Roch, Germany, 2004). Tissue sections, which were subjected to 3 (triethoxysilyl) propylamine (APES, Merck) treatments, were taken on adhesive microscope slides. After incubation overnight at 56°C the samples were deparaffinized using xylene and alcohol. Specimens were washed with distilled water and microwaved for 5 min at 350 watt in the antigen retrieval solution (0.1 M pH 6.0 citrate buffers). They were cooled at room temperature then carefully washed with distilled water. To eliminate the endogenous peroxidase activity, they were kept in a solution of 3% hyrogen peroxide in methanol for 5 min. They were then again immersed in distilled water and the TUNEL reaction mixture was dropped onto each microscope slide so that the tissues were completely covered. The slides were then incubated at 37°C for 1 hour and then washed with distilled water. Antibody blocking then proceeded for 5 min, then POD (conjugated with horseradish peroxidase) was dropped on the slides. Next, specimens were incubated at 37°C for 30 min and then washed with distilled water. A 3,3′ diamino benzidine (DAB) including kit (DAKO, Carpinteria, USA) was used as the staining agent. Slides were kept in cromojen for 2 to 5 min. The stained slides were immersed in distilled water and kept in mayers-hemotoksilen for 10–15 seconds. After being dipped into xylene and alcohol, they were closed.
The sections stained by TUNEL were evaluated using an Olympus BX 50 light microscope. Brown stained epididymis epithelial principal cells of 50 tubules in each epididymal segment were counted under ×400 magnification (). Accordingly, the mean TUNEL-positive cell number is expressed as: 0 (−); between 0.1 and 0.5 (+); 0.51–1.0 (++) and; >1.1 (+++).
Statistical Analysis
Epididymal weights and separately calculated diameters of caput, corpus and cauda epididymis of each group were divided blindly, then statistically compared by using variance analysis arranged according to the randomized block appropriate for factorial testing design.
The number of apoptotic cells were assumed to scatter appropriately for Poisson distribution with logaritmic association function. Data were analysed using SAS PROC GENMOD and Lagrance Statistical Test aproach.
Acknowledgments
Authors thank Dr. Serhat Arslan from Veterinary Medicine, Ondokuz Mayis University, for technical support, and statistical analysis. This work was supported by a grant from the Ondokuz Mayis University Research Fund.
References
- Asci R., Sarikaya S., Buyukalpelli R., Yilmaz A. F., Yildiz S. The effects of experimental varicocele on testicular histology and fertility in monorchic adult rats. BJU Int 1999; 83: 493–497
- Asci R. Epididymal physiology. Male Reproductive System Disorders and Treatment, A. Kadioglu, S. Cayan, B. Semerci, I. Orhan, R. Asci, M. O. Yaman, M. F. Usta, M. Kendirci. Turkish Assosiation of Andrology Press, Istanbul 2004; 124–133
- Barqawi A., Caruso A., Meacham R. B. Experimental varicocele induces testicular germ cell apoptosis in the rat. J Urol 2004; 171: 501–503
- Benoff S. H., Millan C., Hurley I. R., Napolitano B., Marmar J. L. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Hum Reprod 2004; 19: 616–627
- Caflisch C. R. Acidification of testicular and epididymal fluids in the rat after surgically-induced varicocele. Int J Androl 1992; 15: 238–245
- Chen C. H., Lee S. S., Chen D. C., Chien H. H., Chen I. C., Chu Y. N., Liu J. Y., Chen W. H., Wu G. J. Apoptosis and kinematics of ejaculated spermatozoa in patients with varicocele. J Androl 2004; 25: 348–353
- Chitra K. C., Rao K. R., Mathur P. P. Effect of experimental varicocele on structure and function of epididymis in adolescent rats: A histological and biochemical study. Asian J Androl 2003; 5: 203–208
- Fan X., Robaire B. Orchidectomy induces a wave of apoptotic cell death in the epididymis. Endocrinology 1998; 139: 2128–2136
- Fazlioglu A., Yilmaz I., Mete O., Kurtulus F., Parlakkilic O., Güctas O., Cek M. The effect of varicocele repair on experimental varicocele-induced testicular germ cell apoptosis. J Androl 2008; 29: 29–34
- Foldesy R. G., Bedford J. M. Biology of the scrotum: I. Temperature and androgen as determinants of the sperm storage capacity of the rat cauda epididymis. Biol Reprod 1982; 26: 673–682
- Fretz P. C., Sandlow J. I. Varicocele: Current concepts in pathophysiology, diagnosis, and treatment. Urol Clin North Am 2002; 29: 921–937
- Fujisawa M., Hiramine C., Tanaka H., Okada H., Arakawa S., Kamidono S. Decrease in apoptosis of germ cells in the testes of infertile men with varicocele. World J Urol 1999; 17: 296–300
- Hikim A. P., Wang C., Lue Y., Johnson L., Wang X. H., Swerdloff R. S. Spontaneous germ cell apoptosis in humans: Evidence for ethnic differences in the susceptibility of germ cells to programmed cell death. J Clin Endocrinol Metab 1998; 83: 152–156
- Hurt G. S., Howards S. S., Turner T. T. Repair of experimental varicoceles in the rat. Long-term effects on testicular blood flow and temperature and cauda epididymidal sperm concentration and motility. J Androl 1986; 7: 271–276
- Jara M., Esponda P., Carballada R. Abdominal temperature induces region-specific p53-independent apoptosis in the cauda epididymidis of the mouse. Biol Reprod 2002; 67: 1189–1196
- Kass E. J., Chandra R. S., Belman A. B. Testicular histology in the adolescent with varicocele. Pediatrics 1987; 79: 996–998
- Kilinc F., Guvel S., Kayaselcuk F., Aygun C., Egilmez T., Ozkardes H. p53 expression and apoptosis in varicocele in the rat testis. J Urol 2004; 172: 2475–2478
- Ku J. H., Shim H. B., Kim S. W., Paick J. S. The role of apoptosis in the pathogenesis of varicocele. BJU Int 2005; 96: 1092–1096
- Marmar J. L. The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update 2001; 7: 461–472
- Qiu C.-H., Ohe M., Koibuchi N., Matsuzaki S. Apoptosis in the epididymal epithelium of adult male golden hamster exposed to diethylstilbestrol. J Histochem Cytochem 2004; 52: 187–192
- Saypol D. C., Howards S. S., Turner T. T., Miller E. D., Jr. Influence of surgically induced varicocele on testicular blood flow, temperature, and histology in adult rats and dogs. J Clin Invest 1981; 68: 39–45
- Schlesinger M. H., Wilets I. F., Nagler H. M. Treatment outcome after varicocelectomy. A critical analysis. Urol Clin N Am 1994; 21: 517–529
- Schoor R. A., Elhanbly S. M., Niederberger C. The pathophysiology of varicocele-associated male infertility. Curr Urol Rep 2001; 2: 432–436
- Simsek F., Turkeri L., Cevik I., Bircan K., Akdas A. Role of apoptosis in testicular tissue damage caused by varicocele. Arch Esp Urol 1998; 51: 947–950
- Turner T. T., Jones C. E., Roddy M. S. Experimental varicocele does not affect the blood-testis barrier, epididymal electrolyte concentrations, or testicular blood gas concentrations. Biol Reprod 1987; 36: 926–932
- Turner T. T., Lopez T. J. Testicular blood flow in peri-pubertal and older rats with unilateral experimental varicocele and investigation into the mechanism of the bilateral response to a unilateral lesion. J Urol 1990; 144: 1018–1021
- Turner T. T., Riley T. A. p53 independent, region-specific epithelial apoptosis is induced in the rat epididymis by deprivation of luminal factors. Mol Reprod Dev 1999; 53: 188–197
- Wang R., Chang J. S., Zhou X. M., Chen D. Y. Varicocele in the rat: A new experimental model. Effect on histology, ultrastructure and temperature of the testis and the epididymis. Urol Res 1991; 19: 319–322
- Wong P. Y., Au C. L., Bedford J. M. Biology of the scrotum: II. Suppression by abdominal temperature of transepithelial ion and water transport in the cauda epididymidis. Biol Reprod 1982; 26: 683–689
- Zhang Q. Y., Qiu S. D., Ma X. N., Yu H. M., Wu Y. W. Effect of experimental varicocele on structure and function of epididymis in adolescent rats. Asian J Androl 2003; 5: 108–112
