Abstract
Mercury induces structural and functional damage in several organs, however the effects of subtoxic doses of the metal on the male reproductive system are not well defined. In order to analyze testicular and epididymal morphological alterations and changes in IL-4 or IFN-γ serum levels, adult male Sprague-Dawley rats received 0.01, 0.05 or 0.1 μg/ml of mercuric chloride (HgCl2) in deionized water for 1 to 7 months by oral route. Controls received deionized water alone. Twenty rats, separated in four groups of five animals each, were used per time of exposure. Progressive degenerative lesions consisting of lack of germ cell cohesion and desquamation, arrest at spermatocyte stage and hypospermatogenesis were observed in seminiferous epithelium by light and electron microscopy. Leydig cells showed cytoplasmic vacuolation and nuclear signs of cell death. Loss of peritubular cell aggregation was evidenced in the epididymis. Mercury accumulation was detected in both organs by mass spectroscopy. Rats showed enhanced IFN-γ serum levels as compared to controls but only reached significance after 7 months of mercury administration. Subtoxic doses of inorganic mercury could lead to reproductive and immunological alterations. The results demonstrate that sublethal concentrations of mercuric chloride are enough to induce morphological and ultrastructural modifications in male reproductive organs. These contribute to functional alterations of spermatogenesis with arrest at spermatocyte stage, hypospermatogenesis and possibly impaired steroidogenesis which together could affect male fertility.
| Abbreviations | ||
| HgCl2 | = | mercuric chloride |
| ROS | = | reactive oxygen species |
| RER | = | rough endoplasmic reticulum |
| GSH | = | glutathione |
| MHC | = | Major Histocompatibility Complex |
| IFN-γ | = | Interferon-gamma |
| IL-4 | = | Interleukin-4 |
Introduction
Mercury is a highly toxic environmental pollutant continuously released from the earth's crust [Berlin et al. Citation2007; Clarkson and Magos Citation2006]. Since this metal is ubiquitous in the environment, it is nearly impossible for most humans to avoid exposure to some form of mercury on a regular basis. Mercury can exist in several physical and chemical forms, including elemental mercury vapor (Hg0), inorganic mercurous (Hg+) and mercuric (Hg2+) compounds, and organic mercuric compounds. Due to industrialization and changes in the environment during the last century, humans and animals are exposed to all mercury forms [Clarkson and Magos Citation2006]. Mercury causes toxic effects in a number of tissues and organs, depending on the chemical form of mercury and the level, time and route of exposure. Differences in the mechanisms involved in the transport and metabolism of inorganic and organic forms of mercury are likely to be responsible for the disparity in their distribution in tissues and organs, pattern of biological effect, and toxicity [Zalups Citation2000]. The kidney is the primary target organ where inorganic mercury is taken up, accumulates, and expresses toxicity. Organic mercuric compounds are less nephrotoxic than inorganic compounds, show a systemic distribution and affect other target organs that include hematopoietic and neural tissues. [Zalups Citation2000; WHO Citation1991; Affelska-Jercha Citation1999]. Once absorbed, mercury has a low excretion rate. A major proportion of absorbed metal accumulates in kidneys, nervous tissue and liver. All forms of mercury cause toxic effects, including neurotoxicity, nephrotoxicity and gastrointestinal toxicity [Ercal et al. Citation2001]. Moreover, mercury alters the endocrine function and, similar to other heavy metals, influences the activity of the immune system [Rowley and Monestier Citation2005]. Metal-induced imbalance in immune regulation can lead to inadequate or excessive production of either inflammatory or anti-inflammatory cytokines resulting in chronic inflammatory processes or autoimmune diseases [Lawrence and McCabe Citation2002]. Mercury induces autoimmune glomerulonephritis in susceptible strains of rodents associated with imbalances in Th1/Th2 cytokines. A skewing towards Th2-type cytokines that include IL-4 and IL-10 dominates [Rowley and Monestier Citation2005]. Although numerous studies have investigated the toxicity of mercury on different organs, there is scarce information about the effects of mercury on the male reproductive system. Male infertility is an important health problem. Industrial and environmental pollutants that include heavy metals, have all been blamed for damaging the reproductive system, since many of them can act as endocrine-disrupters, inducing changes in male reproductive function [Pilliére Citation2005]. The higher incidence of reproductive disorders in humans together with the numerous reports indicating world-wide diminished sperm counts during the last century have caused increased interest in studying the toxicology of the male reproductive system [Creasy Citation2003]. The effects of lead on the male reproductive system causing alteration in spermatogenesis and diminished fertility have been extensively studied; however, the effects of mercury are not as well-defined. The induction of male reproductive toxicity in experimental animals by mercury intoxication suggests that mercury may adversely affect male fertility [Rao and Sharma Citation2001].
Mercury exposure can exert deleterious effects on the male reproductive system as indicated by the progressive histological changes induced in testis by mercury administration to experimental animals, depending on the dose and time of metal exposure [Lee and Dixon Citation1975; Chowdhury et al. Citation1986; Massanyi et al. Citation2007]. Some case reports suggest an association between high levels of mercury and reproductive disorders since hypospermia, teratozoospermia and decreased spermatogenesis have been reported in humans exposed to elemental mercury causing male infertility [Popescu Citation1978]. Nevertheless, different epidemiological studies have reported contradictory results on the effects of either organic or inorganic mercury on male reproductive function. This could be due to disparities of criteria in the methodological approach used in these studies and the presence of other contaminants [Chia et al. Citation1992]. For this reason, experimental models are being used to clarify the pathophysiological mechanisms mediating mercury-induced damage on the male reproductive system [Alcser et al. Citation1989; Ernst et al. Citation1991]. Most investigations using these models examine the effects of high doses of mercury administered by parenteral routes. However, the effects of acute and often very high doses of mercury in animals cannot be extrapolated to humans chronically exposed to relatively low concentrations [Schuurs Citation1999]. Moreover, the greatest effect of endocrine-disrupters can occur at the lowest doses. For many chemicals, increasing the dose will increase the toxic effect. However, for endocrine-disrupters, lower rather than higher doses can have the highest effect [Allsopp et al. Citation1997]. Few studies have investigated the effects of endocrine-disrupters on the male reproductive system at low doses, which are more relevant to current environmental levels [Zahir et al. Citation2005]. Based on the observations mentioned above, we were interested in studying the effects of the oral administration of subtoxic doses of mercuric chloride (HgCl2) on the reproductive system of male Sprague-Dawley rats. In the present work, we have assessed the morphological and ultrastructural modifications in testes and epididymides as a function of mercury ingestion for different periods of times. We have analyzed the levels of IL-4 and IFN-γ in serum in order to determine the cytokine profile induced under experimental conditions. The integrity of different biological systems depends on the balance of pro-inflammatory (IFN-γ) and anti-inflammatory (IL-4) cytokines that control a preferential cellular or humoral immune response. An imbalance in the production of these cytokines could lead to immunologically-mediated alterations in male reproductive organs.
Results
Effects of the Oral Administration of Mercury on Food Ingestion and Water Consumption
No significant differences in mean daily food ingestion were detected between control rats and those that received different concentrations of mercury in the drinking water for 1, 2 or 3 months. Furthermore, mean water consumption was similar in the different groups studied (). When daily ingestion of mercury was calculated on basis of water consumption, significant statistical differences were observed among the groups treated with different concentrations of the metal (). As expected, the rats that received higher concentrations of mercury in the drinking water ingested significantly higher amounts of mercury.
Daily Water Consumption and Hg Ingestion in Rats that Received Sublethal Doses of Mercuric Chloride by Oral Route†
Effects of the Oral Administration of Mercury on Body Weight and on Testis and Epididymis Weights
In general, the administration of mercury did not influence body weight or testis and epididymis weights since no significant differences were observed among the experimental groups (). When the relationships between the male reproductive organs’ weights and body weight were analyzed, only the group of rats that received 0.10 μg/ml of HgCl2 for 1 month, showed a significant lower ratio epididymis/body weight than rats in the control group or rats that received 0.01 μg/ml of HgCl2 for a similar length of exposure.
Body Weight and Relationships between the Male Reproductive Organ Weights and Body Weight in Rats that Received Sublethal Doses of Mercuric Chloride by Oral Route†
Mercury Concentration in Testis, Epididymis and Serum from Rats that Received Mercury by Oral Administration
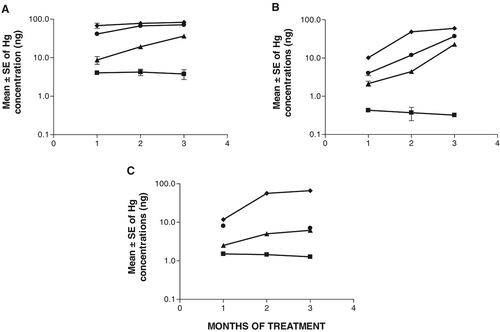
shows the concentrations of mercury detected in testis by means of mass spectroscopy. Since the concentration of mercury was not normally distributed, logarithmic transformation was applied for statistical analysis. Significantly higher mercury concentrations were observed in testes from rats treated with mercury as compared to control rats (P < 0.001), except in rats treated with 0.01 μg/ml of mercury for 1 or 2 months. Furthermore, the concentrations increased with time of exposure (P < 0.01) in groups treated with 0.01 or 0.05 μg/ml of mercury. Similar results were obtained for the epididymis () and for serum ().
Figure 1 Mercury concentrations in testis, epididymis or serum from rats that received sublethal doses of the metal by oral route. ▪ Control rats; ▴ Rats that received 0.01 μg/ml of Hg; • Rats that received 0.05 μg/ml of Hg; ♦ Rats that received 0.1 μg/ml of Hg. (A) Testis; (B) Epididymis; and (C) Serum.
Histopathological Effects of the Oral Administration of Sublethal Doses of Mercury on the Testis and Epididymis
When analyzed by light microscopy, tissue sections of either testis or epididymis from animals that received mercury, showed lesions that progressed with time as compared with tissue sections from control rats (). Seminiferous tubules of controls presented a well defined form and cohesion as well as a high density of spermatozoa. In contrast, seminiferous tubules in testis tissue sections from rats that received mercury for 1 month showed epithelium disorganization, loss of cohesion and germ cell shedding as well as luminal obliteration, independent of the dose used (–). Furthermore, after 2 months of exposure with either 0.05 or 0.1 μg/ml of mercury we observed progressive degeneration with spermatogenic arrest at the spermatocyte stage and significant reduction in sperm density (). Multinucleated giant cells consisting of immature spermatids were observed occasionally either at the germinal epithelium or released into the tubular lumen, after 2 months of exposure (). In rats that received mercury, Sertoli cells showed increased severity in cellular alterations. Vacuolation was observed after 1 month exposure to mercury, however karyolysis and necrosis were observed after 2 months yielding more seriously deteriorated tubules (results not shown). Furthermore, rats that received 0.1 μg/ml of mercury in the drinking water for 3 months showed tubular atrophy (). Leydig cells appeared scattered at the interstitial tissue, from the first month of mercury administration. Furthermore, cytoplasm vacuolation and nuclear signs of necrosis such as karyolysis and karyorrhexis were observed after 2 months of exposure that was accentuated after 3 months (results not shown). No leuko-lymphocytic infiltrate at the testicular interstitium was observed at any dose or time point of mercury administration studied.
Figure 2 Effects of the oral administration of Hg on testis morphology. (A) Microphotography of a tissue section from a control rat receiving deionized water (HE, 160X). (B) Cohesion loss in tubular cells (asterisks) and (C) Luminal obliteration (asterisks) in sections of rats that received 0.05 μg/ml of Hg for 1 month (HE, 250X). (D) Arrest at the spermatocyte stage (asterisks) and (E) Multinucleated giant cells in sections from rats that received 0.05 μg/ml of Hg for 2 months (HE, 100X, 400X). (F) Tubular atrophy in a section from a rat that received 0.1 μg/ml of Hg for 3 months (VG, 400X).
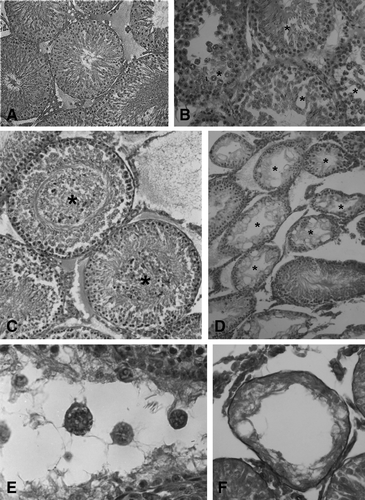
The lesions were not uniformly distributed among all the animals, since some animals showed more severely damaged organs than others from the same experimental group. Furthermore, focal lesions were observed in either testis or epididymis, since damaged tubules alternating with apparently normal tubules were present at the same tissue section. As can be seen in , the severity of lesions was minimal after 1 month of exposure with doses of 0.05 or 0.1 μg/ml of mercury and progressed to moderate after 2 months of exposure.
Severity of Lesions in Seminiferous Tubules from Rats that Received Mercuric Chloride by Oral Route
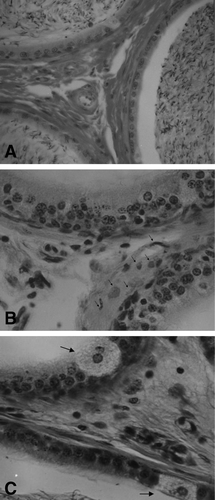
In rats that received mercury, alterations in the epididymis were less severe than those detected in the testis. Some degenerative changes that included peritubular cell dissociation, were observed in the epididymis from rats treated with 0.1 μg/ml of mercury for 1 month, as compared with those of control rats ( and ). Furthermore, we detected hyalinization of the cytoplasm and nuclear degeneration (pyknosis and karyolysis) after 2 months of mercury administration, and multinucleated giant cells with foamy cytoplasm after 3 months, even at the lower dose of mercury tested ().
Figure 3 Effects of the oral administration of Hg on epididymis morphology. (A) Microphotography of a tissue section from a control rat that only received deionized water (VG, 250X). (B) Tissue section from a rat that received 0.01 μg/ml of Hg for 1 month, showing peritubular cell dissociation (arrows) (Iron H, 400X). (C) Tissue section from a rat that received 0.01 μg/ml of Hg for 3 months showing multinucleated giant cells (arrows) (Iron H, 400X).
Effects of the Oral Administration of Sublethal Doses of Mercury on Testis and Epididymis Ultrastructure
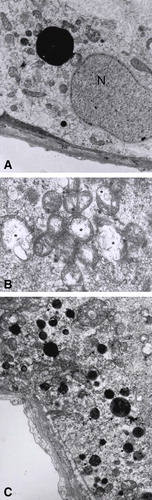
When testis tissue sections from rats treated with mercury were analyzed by electron microscopy we also observed some differences as compared with those obtained from control rats (). Ultrastructural alterations were observed even in tubules that apparently appeared normal by light microscopy. Alterations were primarily observed in Sertoli cells, that presented different degrees of mitochondrial degeneration after 1 month of mercury ingestion, even at the lower concentration of mercury tested. This degeneration ranged from discrete edema to severe expansion of the matrix compartment with loss of cristae structure. These lesions were accompanied by Golgi distension and an increased number of secondary lysosomes mainly at the basal compartment ( and ). Mitochondrial degeneration was also observed in germ cells, mainly in spermatids.
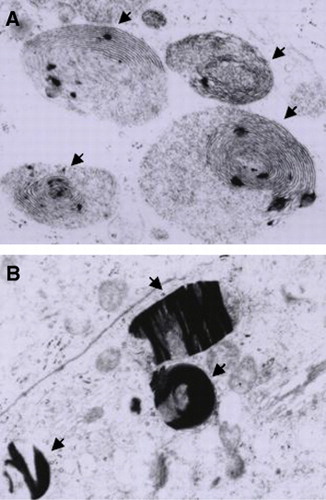
Figure 4 Effects of the oral administration of Hg on the ultrastucture of testis. (A) Microphotography of a tissue section from a control rat that only received deionized water. N: Sertoli cell nucleus, Lys: lysosome, m: mitochondria, tw: tubular wall (6000X). (B) Damaged mitochondria (asterisks) (11500X), (C) Increased number of secondary lysosomes (arrows) and Golgi dilation (asterisks) in sections from rats that received 0.05 μg/ml of Hg for 3 months (5200X).
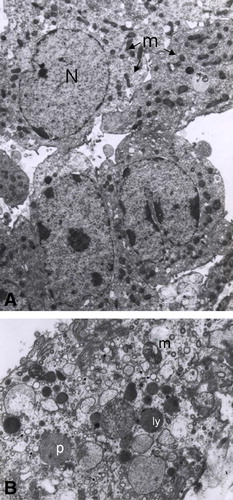
When compared to the control (), some Leydig cells from animals treated for either 2 or 3 months with 0.05 or 0.1 μg/ml of mercury showed mitochondrial degeneration similar to that detected in Sertoli cells. Moreover, the smooth endoplasmic reticulum was dilated ().
Figure 5 Leydig cell alteration in testis from rats that received Hg by oral route. (A) Microphotography of a tissue section from a control rat that only received deionized water. N: nucleus, m: mitochondria (arrows) (2950X). (B) Section from a rat treated with 0.05 μg/ml of Hg for 3 months showing peroxisomes (p), secondary lysosomes (ly) damaged mitochondria (m) and dilated endoplasmic reticulum (arrows) (6600X).
In contrast to the control group, epididymal tissue sections obtained from rats in which mercury was administered orally showed swollen mitochondria, as observed in testis cells. Furthermore, parallel concentric lamella-like myelinic figures in the cytoplasm () as well as an increased number of lysosomes presenting crystal deposits were observed ().
Effects of the Oral Administration of Sublethal Doses of Mercury on IFN-γ and IL-4 Serum Levels
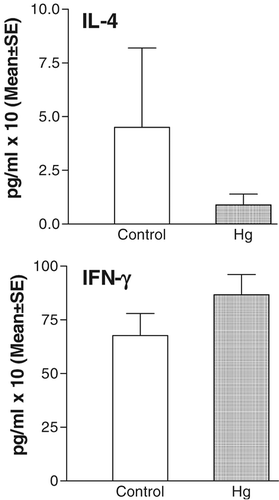
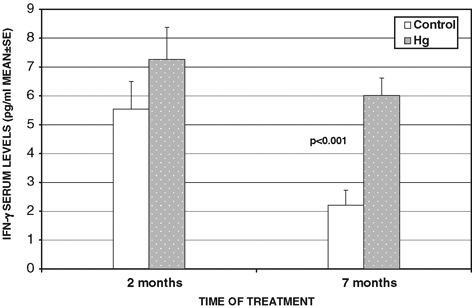
Our first approach was to analyze cytokine serum levels as pooled results from rats that received mercury for different times and their controls that only received deionized water. In control rats, serum levels of IFN-γ were higher than those of IL-4. The oral administration of mercury caused a decrease in the level of IL-4 and an increase in the level of IFN-γ as compared to the control. However, this was not statistically significant (). Consequently, we decided to analyze levels with time of treatment. In contrast when the variation in the level of IFN-γ was analyzed, a marked difference was observed in sera from rats that received mercury for either 2 or 7 months. A significantly higher level of IFN-γ was detected in rats that received mercury orally for 7 months as compared to the corresponding control ().
Discussion
Environmental exposure to chemicals may be implicated in the rise of several male reproductive disorders recorded in humans over the past 5 decades [Allsopp et al. Citation1997; Carlsen et al. Citation1992]. Levels of some metals like mercury, lead and cadmium have become elevated, even in remote areas, as a result of human activities [Allsopp et al. Citation1997]. The resulting disorders include reduced sperm number, decreased percentage of motile sperm in semen and an increased number of physically abnormal sperm. Many chemicals can act as endocrine-disrupters affecting hormone systems that have been implicated in decreasing sperm count, increasing reproductive problems and weakening the immune system [Allsopp et al. Citation1997]. Among others, these include several persistent organochlorine pesticides, dioxins and heavy metals [Longanathan and Kannan Citation1994; Jobling et al. Citation1995].
Occupational exposure and environmental pollution are the major sources of mercury. The general toxic effects of the metal on human and animal systems are well known with the kidney being the primary target organ where HgCl2 accumulates and exerts toxicity [Rowley and Monestier Citation2005]. However, it is also able to induce neurotoxicity, nephrotoxicity and gastrointestinal toxicity [Ercal et al. Citation2001]. In addition, mercury can alter endocrine function and influences the activity of the immune system [Allsopp et al. Citation1997; Rowley and Monestier Citation2005]. For example, mercury can induce a systemic antibody-mediated autoimmune dysregulation, characterized by a T-dependent polyclonal B-cell activation with lesions in multiple organs.
The effects of mercury on the female reproductive system have been extensively studied [Schuurs Citation1999]. Comparatively, there is scarce information about the effects of mercury on the male reproductive system.
Mercury can have deleterious effects on the male reproductive system. Studies in experimental animals and men demonstrate that mercury compounds affect spermatogenic and steroidogenic function. The administration of mercury to experimental animals induces progressive histological changes in testis that are dependent on the dose and duration of exposure. Changes include decreases in the diameter of seminiferous tubules, disorganization of the basal membrane and aspermatogenesis. These alterations were reported in different animal species [Chowdhury and Arora Citation1982], including fish [Friedmann et al. Citation1996; Wester and Canton Citation1992], birds [McNeil and Bhatnagar Citation1985; Maretta et al. Citation1995], mice [Lee and Dixon Citation1975; Orisakwe et al. Citation2001], rats [Massanyi et al. Citation2007; Homma-Takeda et al. Citation2001] and monkeys [Mohamed et al. Citation1987]. It is important to stress that although some data is available on the effects of inorganic mercury on fertility in experimental animals [Lee and Dixon Citation1975; Orisakwe et al. Citation2001; Rao Citation1989] the majority of works have focused on organic mercury. Studies in humans, have suggested an association between high levels of the metal and reproductive disorders. Elevated testicular mercury in an infertile man employed in the chloralkali industry as well as reproductive toxicity among workers occupationally exposed to mercury compounds have been reported in a series of case reports [Popescu Citation1978; Keck et al. Citation1993]. However, epidemiological studies have reported contradictory results on the effects of organic or inorganic mercury on male reproductive function. The inconsistency in findings can be related to disparity in the levels of metal exposure, among other variables.
In the present study we have observed that the oral administration of subtoxic doses of HgCl2 induced morphological alterations in the reproductive organs of adult male Sprague-Dawley rats without significant modifications in testis or epididymis weight. In testis, we detected progressive degenerative lesions in the seminiferous epithelium that were observed independently of the dose used after 1 month of treatment. These lesions included: lack of germ cell cohesion, desquamation to the lumena, arrest at the spermatocyte stage and hypospermatogenesis, presence of multinucleated giant cells and cytoplasmic vacuolation in Sertoli cells. Similarly, Leydig cells showed cytoplasmic vacuolation and nuclear signs of cell death, after 2 months of treatment. In the epididymis, we detected peritubular cell dissociation and giant cells after 1 month of exposure to mercury. The animals that received mercury showed ultrastructural changes in the testis. Changes included: increase in the number of lysosomes and several levels of degeneration in mitochondria, rough endoplasmic reticulum (RER) and Golgi that depended on the dose and time of treatment. In the epididymis, multivesicular bodies and lysosomal electron-dense accumulations were observed. Our results are consistent with previous demonstrations of mercury-induced alterations in mitochondria size and number, vacuolation, rough endoplasmic reticulum (RER) structural changes in cells from rat kidney [Stacchiotti et al. Citation2003], duck liver [Bhatnagar et al. Citation1982] and in human fetal hepatic cell lines [Bucio et al. Citation1995] depending on the metal dose.
We have detected foci of hypospermatogenesis without significant modifications in testis weight at subtoxic doses of mercury. This is in agreement with the work of Orisakwe et al. [Citation2001], which demonstrated that mercury is able to induce remarkable degenerative lesions on mice testes even at the very low dose of 4 ppm. In contrast, other studies using higher doses of metal have reported adverse effects of HgCl2 on rat testicular tissue with marked testicular spermatogenic degeneration at the spermatocyte stage that paralleled a reduction in testis weight [Chowdhury et al. Citation1986; Chowdhury and Arora 1982]. An increased risk of reproductive disorders, including infertility; have been detected in experimental systems at high doses of mercury.
Animals that received HgCl2 in the drinking water accumulated the mercury in the testis and epididymis as well as in serum as detected by mass spectroscopy. Different studies on the effects of chronic oral or parenteral administration of HgCl2 on the male reproductive system describe the doses administered and sometimes qualitatively demonstrate the metal in tissues [Chowdhury et al. Citation1986; Vachhrajani and Chowdhury Citation1990; Ernst et al. Citation1991]. However, few have quantified the levels of metals in the organs or serum of treated animals [Rao and Sharma Citation2001; Eto et al. Citation1997]. The lack of these values restricts the relationships of the levels of mercury in the organs with histopathologic alterations that can be developed. In the present work we have observed that from the first month of exposure, the ingestion of mercury in the drinking water determined metal accumulation in testis, epididymis and serum at the different doses tested. In testis, metal accumulation reached a plateau after two months of exposure to either 0.05 or 0.1 μg/ml of HgCl2 and corresponds to mercury concentrations in a range from 60–80 ng/g of tissue. Interestingly, the severity of the lesions induced by mercury in the seminiferous tubules was more pronounced from the second month of mercury administration in doses of 0.05 or 0.1 μg/ml. These results allow one to establish a relationship between mercury accumulation and the severity of tissue damage.
Our results are in agreement with previous studies that have reported that mercury, in a wide range of doses, has toxic effects on many organs that include testes and epididymides [Lee and Dixon Citation1975; Chowdhury et al. Citation1986]. This has been followed by specific studies that indicate metal accumulation in testis [Rao and Sharma Citation2001; Eto et al. Citation1997] and have indicated that mercury passes through the blood-testis barrier to induce testicular damage [Lee and Dixon Citation1975; Sharma et al. Citation1996].
The oral administration of mercury induced dose-dependent histopathological changes in testes and epididymides, even at doses as low as 0.01 μg/ml. Interestingly, mitochondrial degeneration was a common finding in almost all cellular lesions. The cellular death induced by mercury has been related to an inhibition in mitochondrial oxidative phosphorylation, conducing to anoxia [WHO Citation1991]. We have observed germinal epithelium disorganization and cytoplasmic vacuolation, together with organelle degeneration in Sertoli cells after 1 month of exposure, independent of the dose used. Results suggest that mercury could act directly on immature germinal cells, inhibiting DNA and RNA synthesis and indirectly by the effect produced on Sertoli cells. Mercury inhibits DNA, RNA and protein synthesis in different spermatogenic cell types of mice and rats as well as cell growth with concomitant abnormal mitosis in other cellular types [Lee and Dixon Citation1975; Chowdhury et al. Citation1986].
Testis spermatogenic and steroidogenic functions are intimately related. The beginning and maintenance of spermatogenesis requires normal levels of testosterone at the intratesticular level and in circulation, with Leydig cells producing approximately half of the circulating testosterone in the blood [Vermeulen Citation1986]. The incubation of Leydig cells with 10–100 mM HgCl2 is sufficient to decrease testosterone production [Ng and Liu Citation1990]. The morphological alterations detected in Leydig cells could likely be associated with impaired testosterone production which likely contribute to the alterations reported in this study.
Heavy metals exert an adverse effect on the viability of isolated rat Leydig cells of the testis, with mercury being the most potent [Ng and Liu Citation1990]. Progressive degeneration of Leydig cells and the decrease in their nuclear diameter and population are associated with a gradual increase in deposition of mercury [Vachhrajani amd Chowdhury Citation1990]. Decreased cell viability parallels a reduction in luteinizing hormone-stimulated testosterone production by Leydig cells [Ng and Liu Citation1990]. Sertoli cells which play an essential role in spermatogenesis, are known to be the target of various toxicants [Monsees et al. Citation2000]. Moreover, the toxicity of mercury on isolated Sertoli cells grown in culture is higher than that of cadmium or arsenic [Espevik et al. Citation1982]. Under our experimental conditions we have observed vacuolation in both Leydig and Sertoli cells. Moreover, Leydig cells showed nuclear signs of cell death. Massanyi et al. [Citation2007] indicated that the increased testicular apoptosis induced by mercury may reflect altered steroidogenesis and subsequently decreased spermatogenesis.
Mercury treatment also induces morphological and functional defects in the epididymis [Rao and Sharma Citation2001; Chowdhury et al. Citation1989]. Mercury ions enter the epididymis through the blood-epididymal barrier [Sharma et al. Citation1996]. The disruption of epididymal physiology has been related to alterations in epididymal ATPase and in sialic acid [Rao and Sharma, Citation2001]. Moreover, mercury intoxication reduces secretory epididymal components necessary for sperm maturation [Rao Citation1997]. Rao and Sharma [Citation2001] have indicated that HgCl2 exerts adverse effects on the testis and epididymis by inducing androgen deficiency and perhaps also through direct toxic effects on these tissues. Our results point to degenerative ultrastructural changes due to the accumulation of degraded substrates in lysosomes as described with other chemicals [Soldani et al. Citation1996; Akbarsha and Sivasamy Citation1998].
Mercury toxicity is related to its high affinity for sulphydryl groups since mercury replaces the hydrogen ion. This metal also reacts with amide, carboxyl and phosphoryl groups producing serious alterations in structural and transport functions in enzymes expressed in different tissues [Ferrer Citation2003]. However, the molecular mechanisms for mercury toxicity remain to be resolved. Recent reports suggest that the biochemical mode of action involves oxidative stress by the generation of reactive oxygen species (ROS) and lipid peroxidation through mitochondrial impairment [Ercal et al. Citation2001; InSug et al. Citation1997; Guo et al. Citation1998; Ararargi et al. Citation2003; Larose et al. Citation2008], leading to cell death via necrosis or apoptosis [Castoldi et al. Citation2001; Bragadin et al. Citation2002; Kim and Sharma Citation2004]. Mercury generates ROS in vivo and in vitro via several different cellular processes, potentially causing extensive cellular damage and cell death by oxidizing proteins, lipids and nucleic acids [Ercal et al. Citation2001]. Antioxidant enzyme activities increase, as a compensatory mechanism, to scavenge ROS levels produced as a result of mercury accumulation and enhances the antioxidant potential of the organs to reduce the oxidative stress [Hussain et al. Citation1999].
Biochemical tests from male reproductive organs show alterations after mercuric chloride treatment. In the testis, mercury treatment is associated with a decline in glutathione, glutathione peroxidase, succinate dehydrogenase, alkaline phosphatase, acid phosphatase activities and increased lipid peroxidation [Rao and Sharma Citation2001]. Moreover, in rats the parenteral administration of HgCl2 increases lipid peroxidation in some organs that include kidney, testis, liver as well as lung, correlating with the severity of hepatotoxicity and nephrotoxicity [Ercal et al. Citation2001]. This suggests that lipid peroxidation is one of the molecular mechanisms for cell injury in acute HgCl2 poisoning [Huang et al. Citation1996]. Furthermore, increased apoptosis is detected in testis of mercury-treated rats [Massanyi et al. Citation2007].
In our experimental conditions we found no significant modifications in either body weight or in daily food ingestion. These observations are in agreement with those reported by [Mahboob et al. Citation2001] by the use of low peroral doses of HgCl2 in mice. These authors detected increased lipid peroxidation in all tissues but with significant enhancement only in the kidneys, testes and epididymides suggesting that these organs were more susceptible to Hg toxicity. Elevated concentration of glutathione (GSH) and superoxide dismutase in the testes were also observed. It could be that under our conditions similar biochemical modifications had been induced.
Another mechanism whereby mercury can cause alterations is through the modulation of immune homeostasis [Lawrence and McCabe Citation2002]. Metals act as haptens that combine with circulating or tissue proteins and give rise to new antigens that can influence antigen presentation by modifying the antigen-presenting complex and interfere with antigen recognition [Kusaka Citation1993]. Metal-induced imbalance in immune regulation can lead to inadequate or excessive production of either inflammatory or anti-inflammatory cytokines resulting in chronic inflammatory processes or autoimmune diseases [Kono et al. Citation1998]. Mercury is associated with the induction of autoimmune glomerulonephritis in susceptible strains of rodents. In rats and mice, the susceptibility for mercury-induced autoimmunity has been linked to both Major Histocompatibility Complex (MHC) and non-MHC genes [Rowley and Monestier Citation2005]. The pathology is mediated by autoantibodies directed against tubular membrane components and has been related to dysregulation of cytokine networks by this metal. Mercury-related autoimmune disease in susceptible animals has been directly related to an imbalance in Th1/Th2 immunoregulation with a skewing towards Th2-type immunity. This type of immunity is characterized by the production of IL-4, IL-5, IL-6, IL-10 and IL-13. Recent reports indicate that interferon-gamma (IFN-γ), a Th1-type cytokine, is also associated with kidney pathology as typified by the mouse model [Kono et al. Citation1998; Hu et al. Citation1999].
The results reported in this communication have shown that control Sprague-Dawley rats have higher levels of IFN-γ in serum than those of IL-4, which is in agreement with previous studies that have indicated that IFN-γ levels almost always are higher than IL-4 levels [Lawrence and McCabe Citation2002]. The oral administration of low doses of HgC12 increased the levels of IFN-γ in serum and decreased those of IL-4 as compared to the controls. However, the differences in the levels of IFN-γ between the rats receiving mercury and the controls were statistically significant only after 7 months of mercury ingestion. Lawrence and McCabe [Citation2002] have proposed that an abnormal ratio of IL-4 to IFN-γ may be more relevant than the actual number of IL-4 or IFN-γ producing cells or the amount of each cytokine produced.
The importance of the genetic background of the exposed animal for the immunomodulatory effects induced by heavy metals has been previously demonstrated by the differential ability of mercury to influence humoral versus cell-mediated immunity in Brown Norway or Lewis strain of rats, respectively [Druet and Pelletier Citation1996; Bagenstose et al. Citation1998; Citation1999]. Brown Norway rats are susceptible to mercury-induced autoimmunity, while the Lewis strain is resistant to mercury-induced autoimmune disease but susceptible to mercury-mediated immunosuppression. While mercury is known to induce autoimmune disease in both rats and mice, the features of the disease, mainly autoantibody profiles and kidney pathology, differ between these species. The possibility exists that in Sprague-Dawley rats, mercury induces immunological mechanisms similar to those reported in some mouse models. It is important to stress that studies carried out in mice have presented conflictive results on the effects of mercury on IFN-γ levels that has been associated with differences in mouse strain [Santarelli et al. Citation2006].
The morphological modifications detected in the present work indicate that oral intake of subtoxic doses of HgCl2 induces changes in either seminiferous or epididymal tubules in Sprague-Dawley rats, altering spermatogenesis. Furthermore, mercury induces alterations in Leydig cells suggesting that it could be associated with impaired testicular steroidogenesis and subsequently contributes to spermatogenic arrest. The results show that under our experimental conditions mercury increases Th1-type cytokines in at least this strain of rat.
Materials and Methods
Animals
Two month-old inbred Sprague-Dawley male rats with 250–300 g of body weight were used. These animals were raised at animal facilities from Instituto Venezolano de Investigaciones Científicas (IVIC), Caracas, Venezuela.
Experimental Design
Animals were distributed at random into four different experimental groups each containing five rats that were fed ad libitum with standard laboratory chow. Control rats had free access to deionized water, and animals in Hg-treated groups had free access to deionized water supplemented with either 0.01, 0.05, or 0.1 μg/ml of Hg in the form of HgCl2. Mercury Lethal Dose 50 (LD50) for rat is 1 mg/kg body weight by intravenous route (iv), or 20 mg/kg body weight by intraperitoneal route (ip) [Chowdhury et al. Citation1989]. Body weight as well as food and water ingestion were measured twice a week for each experimental group. Weights were determined using a mechanical balance (Ohaus, Harvard Trip, Fisher Scientific, USA) with a precision of 0.05 g.
Twenty rats from the different experimental groups were sacrificed with diethyl ether either at 1, 2 or 3 months of Hg administration. In some experiments animals were sacrificed after 7 months of mercury administration. Treatment and anesthesia were administered in accordance with the guidelines of “Principles in the Use of Animals in Toxicology” [American Society of Toxicology Citation1999]. Each type of experiment was repeated three times.
Testes as well as epididymides were bilaterally dissected by aseptic manipulation for histological processing. Serum was isolated from blood samples obtained by cardiac puncture in order to quantify Hg as well as Interferon-gamma (IFN-γ) and Interleukin-4 (IL-4) levels.
Mercury Quantification in Male Reproductive Tissues and Serum
Mass Spectroscopy was used to determine Hg in testes, epididymides and serum samples from rats of the different experimental groups [Jarvis and Gray Citation1992]. Briefly, 1 ml of serum or 0.5 g from each organ was digested with 3 ml of 26% HNO3 by incubation for 1 h at 90°C in teflon containers with hermetic seals. Samples were cooled at room temperature and diluted to 10 ml with distilled water. A calibration curve was obtained by using Hg standards; blanks consisted of 3 ml of HNO3 diluted to 10 ml with distilled water. Mercury concentration was quantified in a Perkin-Elmer ELAN 6000 Mass Spectrometer. Once the blank was subtracted, the values of Hg were corrected for the dilution and weight for each sample.
Processing of Tissue Samples for Light Microscopy Analysis
Testes or epididymides were weighed after dissection. Organs were fixed for 6 to 8 h in Bouin, dehydrated and rinsed. After that, the organs were embedded in Paraplast® and cut in 3–5 μm sections with a microtome. Tissue sections were adhered to microscope slides and stained with Hematoxylin-Eosin. Additionally, to evidence details in nuclei and connective tissue, samples were stained with Iron Hematoxylin (IH) and Van Gieson's Trichromic Stain (VG), respectively [Culling et al. Citation1985].
Evaluation of Lesion Severity in Testis
Severity of the lesions evidenced in seminiferous tubules, presented in tissue sections of Hg-treated animals, was evaluated following the criteria of Chapin et al. [Citation1984]. Severity was considered as “mild”, if less than 10% of the tubules presented in tissue sections from one half or less of the total number of analyzed Hg-treated rats were affected. “Moderate”, if 20–50% of the tubules presented in tissue sections from more than one half of the total number of treated rats were affected. “Severe”, if more than 50% of the tubules, in more than one half of the total number of treated rats were affected.
Processing of Tissue Samples for Electron Microscopy
Fragments of testis or epididymis were removed and processed for electron microscopy. Briefly, tissue samples from rats of the different experimental groups were fixed with glutaraldehyde/cacodylate buffer, dehydrated, included in Epon and cut in 100 nm sections with an ultramicrotome. Tissue sections were conventionally treated for transmission electron microscopy, fixed in a solution of 1% osmium tetraoxide and stained with lead citrate and uranyl acetate for ultrastructure evaluation. Tissue sections were observed in a Phillips CM-100 transmission electron microscope.
Cytokine Determination
Serum levels of Interferon-gamma (IFN-γ) or Interleukin-4 (IL-4) were determined using a commercial ELISA assay following the manufacturer's protocol (Biosource®; Invitrogen Corporation, California, USA). Briefly, 96-well microtiterplates coated with a monoclonal antibody directed to the corresponding cytokine, were incubated for 2 h at room temperature with serum samples or cytokine standard. Plates were washed three times and incubated for an additional 2 h with a monoclonal antibody directed to the corresponding cytokine and conjugated to biotin. A conjugate of Streptavidin-Horseradish Peroxidase was added to the plates after three washes and further incubated for 30 min. The reaction was developed with TBM substrate, and after the addition of Stop Solution, plates were read at 450 nm on an ELISA microplate reader Anthos 2010.
Statistical Analysis
Statistical differences between the experimental groups were evaluated using non-parametric Mann-Whitney “U” test. The level of significance considered was P ≤ 0.05.
Mercury levels in testis, epididymis and serum were analyzed by a mixed general linear model ANOVA following logarithmic transformation. Means were compared using Tukey's studentized multiple range test.
Acknowledgments
The authors want to express their gratitude to Dr. Gloria Lacort and Mrs. Elinor Pelfort from “Laboratorio de Química de los Servicios Científicos y Técnicos de la Universidad de Barcelona”, Barcelona, España Cleusa Gómez (BSc) and to Mr. Danny Pino from “Grupo de Investigaciones en Reproducción Humana and from Departamento de Ciencias Morfológicas, Sección de Histología, Escuela de Ciencias de la Salud, Universidad de Oriente (UDO), Núcleo Bolívar, Ciudad Bolívar, Venezuela. This work was supported by FUNDACITE Guayana, Project No 000703.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Affelska-Jercha A. The toxic effect of mercury in occupational exposure. Med Pr 1999; 50: 305–314
- Akbarsha M. A., Sivasamy P. Male reproductive toxicity of phosphamidon: Histopathological changes in epididymis. Indian J Exp Biol 1998; 36: 34–38
- Alcser K. H., Brix K. A., Fine L. J., Kallenbach L. R., Wolfe R. A. Occupational mercury exposure and male reproductive health. Am J Ind Med 1989; 15: 517–529
- Allsopp, M., Santillo, D. and Johnston, P. (1997) Poisoning the Future: Impacts of Endocrine-Disrupting Chemicals on Wildlife and Human Health. Website: http://archive.greenpeace.org/toxics/reports/ptf/ptfrep.html, May 2007.
- American Society of Toxicology (1999) Guiding principles in the use of animals in toxicology. Revised by the Society of Toxicology. Website: www.toxicology.org/ai/air/air6.asp 15 march 2006.
- Ararargi S., Kondoh M., Kawase M., Saito S., Higashimoto M., Sato M. Mercuric chloride induces apoptosis via a mitochondrial dependent pathway in human leukemia cells. Toxicology 2003; 184: 1–9
- Bagenstose L. M., Salgame P., Monestier M. Mercury-induced autoimmunity in the absence of IL-4. Clin Exp Immunol 1998; 114: 9–12
- Bagenstose L. M., Salgame P., Monestier M. Cytokine regulation of a rodent model of mercuric chloride-induced autoimmunity. Environ Health Perspect 1999; 107(Suppl. 5)807–810
- Berlin M., Zalups R. K., Fowler B. A. Mercury. Handbook on the Toxicology of Metals3rd edition, G. Nordberg, B. A. Fowler, M. Nordberg, L. T. Friberg. Elsevier, Amsterdam 2007; 675–729
- Bhatnagar M. K., Vrablic O. E., Yamashiro S. Ultrastructural alterations of the liver of Pekin ducks fed methyl mercury-containing diets. J Toxicol Environ Health 1982; 10: 981–1003
- Bragadin M., Marton D., Manente D., Grasso M., Toninello T. Methylmercury induces the opening of the permeability transition pore in rat liver mitochondria. J Inorg Biochem 2002; 89: 159–162
- Bucio L., Souza V., Albores A., Sierra A., Chávez E., Cárabez A., Gutiérrez-Ruiz M. C. Cadmium and mercury toxicity in a human fetal hepatic cell line (WRL-68 cells). Toxicology 1995; 102: 285–299
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during the past 50 years. Br Med J 1992; 305: 609–613
- Castoldi A. F., Coccini T., Ceccatelli S., Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Res Bull 2001; 55: 197–203
- Chapin R., White R., Morgan K., Bus J. Studies of lesions induced in the testis and epididymis of F-344 rats by inhaled methyl-chloride. Toxicol Appl Pharmacol 1984; 76: 328–343
- Chia S. E., Ong C. N., Lee S. T., Tsakok F. H. Blood concentrations of lead, cadium, mercury, zinc, and copper and human semen parameters. Arch Androl 1992; 29(2)177–183
- Chowdhury A., Makhija S., Vachhrajani K., Gautam A. Methylmercury and mercuric chloride induced alterations in rat epididymal sperm. Toxicol Lett 1989; 47: 125–134
- Chowdhury A. R., Arora U. Toxic effect of mercury on testes in different animal species. Indian J Physiol Pharmacol 1982; 26: 246–249
- Chowdhury A. R., Vachhrajani K. D., Makhija S., Kashyap S. K. Histomorphometric and biochemical changes in the testicular tissues of rat treated with mercuric chloride. Biomed Biochim Acta 1986; 45: 949–956
- Clarkson T. W., Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 2006; 36: 609–662
- Creasy D. M. Evaluation of testicular toxicology: A synopsis and discussion of the recommendations proposed by the Society of Toxicologic Pathology. Birth Defects Res B Dev Reprod Toxicol 2003; 68: 408–415
- Culling, C. F. A., Allison, R. T., Barr, W. T. Cellular Pathology Technique, London, Butlerworths. London 1985
- Druet P., Pelletier L. Th2 and Th1 autoreactive anti-class II cell lines in the rat suppress or induce autoimmunity. J Autoimmun 1996; 9: 221–226
- Ercal N., Gurer-Orhan H., Aykin-Burns N. Toxic metals and oxidative stress part I: Mechanisms involved in metal induced oxidative damage. Curr Top Med Chem 2001; 1: 529–539
- Ernst E., Møller-Madsen B., Danscher G. Ultrastructural demonstration of mercury in Sertoli and Leydig cells of the rat following methyl mercuric chloride or mercuric chloride treatment. Reprod Toxicol 1991; 5: 205–209
- Espevik T., Lamvik M. K., Sunde A., Eik-Nes K. B. Effects of cadmium on survival and morphology of cultured rat Sertoli cells. J Reprod Fertil 1982; 65: 489–495
- Eto K., Yasutake A., Miyamoto K., Tokunaga H., Otsuka Y. Chronic effects of methylmercury in rats. II. Pathological aspects. Tohoku J Exp Med 1997; 182: 197–205
- Ferrer A. Intoxicación por metales. Metal poisoning. An Sist Sanit Navar 2003; 26: 141–153, Website: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1137-66272003000200008&lng=es&nrm=iso> 15 march 2005
- Friedmann A. S., Watzinb M. C., Brinck-Johnsenc T., Leitera J. C. Low levels of dietary methylmercury inhibit growth and gonadal development in juvenile walleye (Stizostedion vitreum). Aquat Toxicol 1996; 35: 265–278
- Guo L., Miller M. A., Shapiro I. M., Shenker B. J. Mercuric chloride induces apoptosis in human T lymphocytes: Evidence of mitochondrial dysfunction. Toxicol Appl Pharmacol 1998; 153: 250–257
- Homma-Takeda S., Kugenuma Y., Iwamuro T., Kumagai Y., Shimojo N. Impairment of spermatogenesis in rats by methylmercury: Involvement of stage and cell- specific germ cell apoptosis. Toxicology 2001; 169: 25–35
- Hu H., Moller G., Abedi-Valugerdi M. Mechanism of mercury-induced autoimmunity: Both T helper 1-type and T helper 2-type responses are involved. Immunology 1999; 96: 348–357
- Huang Y. L., Cheng S. L., Lin T. H. Lipid peroxidation in rats administrated with mercuric chloride. Biol Trace Elem Res 1996; 52: 193–206
- Hussain S., Atkinson A., Thompson S. J., Khan A. T. Accumulation of mercury and its effect on antioxidant enzymes in brain, liver, and kidneys of mice. J Environ Sci Health B 1999; 34: 645–660
- InSug O., Datar S., Koch C. J., Shapiro I. M., Shenker B. J. Mercuric compounds inhibit human monocyte function by inducing apoptosis: Evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transition and loss of reductive reserve. Toxicology 1997; 124: 211–224
- Jarvis, K., Gray, L. Handbook of Inductively Coupled Plasma Mass Spectrometry. Blackie, London 1992
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some pthalate plasticizers, are weakly oestrogenic. Environ Health Perspect 1995; 103: 582–587
- Keck C., Bergman M., Ernst E., Mooler C., Klinsch S., Nieschlag E. Autometallographic detection of mercury in testicular tissue of an infertile men exposed to mercury vapour. Reprod Toxicol 1993; 7: 469–475
- Kim H., Sharma R. P. Mercury-induced apoptosis and necrosis in murine macrophages: Role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol 2004; 196: 47–57
- Kono D. H., Balomenos D., Pearson D. L., Park M. S., Hildebrandt B., Hultman P., Pollard K. M. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J Immunol 1998; 161: 234–240
- Kusaka Y. Occupational diseases caused by exposure to sensitizing metals. Sangyo Igaku 1993; 35: 75–87
- Larose C., Canuel R., Lucotte M., Di Giulio R. T. Toxicological effects of methylmercury on walleye (Sander vitreus) and perch (Perca flavescens) from lakes of the boreal forest. Comp Biochem Physiol C Toxicol Pharmacol 2008; 147: 139–149
- Lawrence D. A., McCabe M. J. Immunomodulation by metals. Int Immunopharmacol 2002; 2: 293–302
- Lee I. P., Dixon R. L. Effects of mercury on spermatogenesis studied by velocity sedimentation cell separation and serial mating. J Pharmacol Exp Ther 1975; 194: 171–181
- Longanathan B., Kannan K. Global organochlorine contamination trends: An overview. Ambio 1994; 23: 187–189
- Mahboob M., Shireen K. F., Atkinson A., Khan A. T. Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury. J Environ Sci Health B 2001; 36: 687–697
- Maretta M., Marettova E., Skrobanek P., Ledec M. Effect of mercury on the seminiferous epithelium of the fowl testis. Acta Vet Hung 1995; 43: 53–61
- Massanyi P., Lukac N., Slivkova J., Kovacik J., Makarevich A. V., Chrenek P., Toman R., Forgacs Z., Somosy Z., Stawarz R., et al. Mercury-induced alterations in rat kidneys and testes in vivo. J Environ Sci Health A Environ Sci Eng Toxic Hazard Subst Control 2007; 42: 865–870
- McNeil S. I., Bhatnagar M. K. Ultrastructure of the testis of Pekin ducks fed methyl mercury chloride: Seminiferous epithelium. Am J Vet Res 1985; 46: 2019–2025
- Mohamed M. K., Burbacher T. M., Mottet N. K. Effects of methyl mercury on testicular functions in Macaca fascicularis monkeys. Pharmacol Toxicol 1987; 60: 29–36
- Monsees T. K., Franz M., Gebhard S., Winterstein U., Schill W. B., Hayatpour J. Sertoli cells as a target for reproductive hazards. Andrologia 2000; 32: 239–246
- Ng T. B., Liu W. K. Toxic effect of heavy metals on cells isolated from the rat adrenal and testis. In Vitro Cell Dev Biol 1990; 26: 24–28
- Orisakwe O. E., Afonne O. J., Nwobodo E., Asomugha L., Dioka C. E. Low-dose mercury induces testicular damage protected by zinc in mice. Eur J Obstet Gynecol Reprod Biol 2001; 95: 92–96
- Pilliére F. Perturbateurs endocriniens et risques professionnels. Endocrine disruptors and occupational risks. EMC Toxicologie Pathologie 2005; 2: 43–53
- Popescu H. I. Poisoning with alkyl mercuric compounds. Br Med J 1978; 1: 2347
- Rao M. V. Effect of methyl mercury on mouse epididymis and spermatozoa. Biomed Biochim Acta 1989; 8: 577–582
- Rao M. V. Mercury and its effects on mammalian systems—A critical review. Indian J Environ Toxicol 1997; 7: 3–11
- Rao M. V., Sharma P. S. N. Protective effect of vitamin E against mercuric chloride reproductive toxicity in male mice. Reprod Toxicol 2001; 15: 705–712
- Rowley B., Monestier M. Mechanisms of heavy-metal autoimmunity. Mol Immunol 2005; 42: 833–838
- Santarelli L., Bracci M., Mocchegiani E. In vitro and in vivo effects of mercuric chloride on thymic endocrine activity, NK and NKT cell cytotoxicity, cytokine profiles (IL-2, IFN-γ, IL-6): Role of the nitric oxide-l-arginine pathway. Int Immunopharmacol 2006; 6: 376–389
- Schuurs A. H. Reproductive toxicity of occupational mercury. A review of the literature. J Dent 1999; 27: 249–256
- Sharma A. K., Kapadia A. G., Fransis P., Rao M. V. Reversible effects of mercuric chloride on reproductive organs of the male mice. Reprod Toxicol 1996; 10: 153–159
- Soldani P., Pellegrini A., Gesi M., Lenzi P., Paparelli A. Suramin-induced ultrastructural changes in the testis of albino rats. Exp Toxicol Pathol 1996; 48: 299–305
- Stacchiotti A., Borsani E., Rodella L., Rezzani R., Bianchi R., Lavazza A. Dose-dependent mercuric chloride tubular injury in rat kidney. Ultrastruct Pathol 2003; 27: 253–259
- Vachhrajani K. D., Chowdhury A. R. Distribution of mercury and evaluation of testicular steroidogenesis in mercuric chloride and methylmercury administered rats. Indian J Exp Biol 1990; 28: 746–751
- Vermeulen A. Chapter III Leydig cell Physiology. Male reproductive disfunction, R. Santen, R. Swerldorf. Dekker, New York 1986; 49–76
- Wester P. W., Canton H. H. Histopathological effects in Poecilia reticulata (guppy) exposed to methyl mercury chloride. Toxicol Pathol 1992; 20: 81–92
- WHO, 1991. Environmental Health Criteria. Mercury, No 362; World Health Organization; Washington.
- Zahir F., Rizwi S. J., Haq S. K., Khan R. H. Low dose mercury, toxicity and human health. Environ Toxicol Pharmacol 2005; 20: 351–360
- Zalups R. K. Molecular interactions with mercury in the kidney. Pharmacol Rev 2000; 52: 113–144