ABSTRACT
Development of an effective system for oocyte-cryopreservation is of clinical relevance in reproductive medicine. However, oocyte-preservation is not as effective as embryo preservation. In this study, we used a 37°C pre-equilibrium temperature as part of a modified vitrification method for human oocyte cryopreservation. The effect of the new method on spindle configuration, chromosomal arrangement, and mitochondrial distribution was investigated in in vitro-matured human oocytes. A total of 101 in vitro-matured oocytes were randomly assigned for vitrification at pre-equilibrium temperature of 37°C (37°C Group, n=50) or at room temperature (RT Group, 22-24°C, n=51). The time needed for vitrification in the 37°C group was significantly shorter than that in the RT group. Defective spindles were found in 45.5% and 69.0% oocytes in the 37°C group and RT group, respectively (p < 0.05). Abnormal chromosomes were found in 47.7% and 71.4% oocytes, respectively (p < 0.05). There were no significant differences with respect to oocyte survival rate and mitochondrial distribution pattern between the two groups. These results indicate that vitrification at a pre-equilibrium temperature of 37°C may reduce the incidence of defective spindle configuration and chromosomal abnormalities in in-vitro-matured human oocytes.
Abbreviations: ICSI: intracytoplasmic sperm injection; FSH: follicle-stimulating hormone; MII: metaphase II; EG: ethylene glycol; PROH: 1,2-propanediol
Introduction
Oocyte-cryopreservation is a cutting edge technology for long-term preservation of fertility that, for example, is used to preserve oocytes in cancer patients scheduled to undergo surgery and chemotherapy [Mohsenzadeh et al. Citation2012]. The technology is instrumental in the establishment of human oocyte banks [Cobo et al. Citation2008; Song et al. Citation2011]. Vitrification is an attractive method owing to a higher associated oocyte survival rate as compared to that with slow preservation [Paolo Emanuele et al. Citation2014; Mathias et al. Citation2014]. However, due to factors such as the large size of oocyte cytoplasm, increased damage to the spindle in the meiotic metaphase and use of toxic cryoprotectants, oocyte preservation rates with vitrification have been inferior to those achieved with embryo preservation [Tucker et al. Citation1995]. Several studies have suggested that oocyte preservation may adversely affect embryonic development [Wennerholm Citation2000; Song et al. Citation2011]. Difficulties encountered with oocyte cryopreservation are a major concern, and improvement in oocyte quality after freeze-thawing a key research imperative.
Conventional methods for human oocyte vitrification involve two main steps [Liebermann and Tucker Citation2004]. The first step is the pre-equilibrium process, wherein the oocyte is transferred to a cryoprotectant solution and water in the oocyte is replaced. Subsequently, the oocyte is transferred to a higher concentration of cryoprotectant to concentrate the macromolecules in the oocyte cytoplasm. The entire process is usually carried out at room temperature (RT), of which the first step is more time-consuming. Several constraints are encountered during this process: (i) The oocyte is very sensitive to temperature changes [Coticchio et al. Citation2009]. During the vitrification the oocyte retains some metabolic activity, which may be adversely affected by the sudden change in temperature during the shift from culture temperature (37°C) to pre-equilibrium temperature (RT). (ii) The MII (metaphase II) oocyte is sensitive to osmotic pressure [Mullen et al. Citation2004]. Cryoprotectant solutions, i.e., ethylene glycol (EG) and 1,2-propanediol (PROH) used during the procedure are highly volatile, and may induce a sudden change in osmotic pressure. (iii) The time required for the restoration of oocyte shape during pre-equilibrium exposes the oocyte to toxic cryoprotectants. Longer exposure times are associated with greater damage.
A relatively higher temperature increases the movement of molecules and may shorten the pre-equilibrium time and minimizes the effect of temperature change. Therefore, we chose 37°C (incubation temperature during culture) as the pre-equilibrium temperature. Further, covering the oocyte with oil prevents volatilization of smaller molecules, which may further improve cryopreservation.
Spindle configuration and chromosomal arrangement during meiotic metaphase is known to be sensitive to temperature changes [Coticchio et al. Citation2009]. Mitochondrial distribution plays an important role in the oocyte cytoplasm, and is linked with cytoplasmic maturation and developmental competence since it is maternally inherited [Liu et al. Citation2010]. To explore the impact of the modified method, we examined spindle configuration, chromosomal arrangement, and mitochondrial distribution by immunofluorescent staining. However, the fertilizing potential of the oocyte was not determined in the present study.
Results
Oocyte maturation as reflected by spindle and chromosomal configuration and mitochondria
The mean [± standard deviation (SD)] age of the patients in the 37°C and RT groups was 32.3 (± 3.2) years and 31.7 (± 3.9) years, respectively. The between-group difference was not statistically significant. Further, no significant between-group difference was observed with respect to BMI and basic follicle-stimulating hormone (FSH) between the two groups ().
Table 1. Patients’ characteristics and oocytes survival rates by groups.
Out of 131 GV stage oocytes, 101 oocytes matured to MII (maturation rate: 77.1%). In vitro matured oocytes were vitrified randomly by one of the two methods. Fifty oocytes were vitrified at pre-equilibrium temperature of 37°C, out of which 44 (88.0%) oocytes survived after thawing. The mean (± SD) time required for pre-equilibrium was 2.8 (± 0.90) minutes. Fifty-one oocytes were vitrified at RT pre-equilibrium, out of which 42 (82.4%) oocytes survived after thawing. The time required for pre-equilibrium was 5.2 ± 1.2 minutes. There was no significant between-group difference with respect to oocyte survival rate. However, a significant between-group difference was observed with respect to pre-equilibrium time (p < 0.05) ().
The spindle and chromosomal configuration was then assessed and considered in relation to the mitochondrial distribution. Compared with oocytes vitrified in the 37°C group, a higher number of oocytes in the RT group showed abnormal spindle configuration (20/44 (45.5%) vs. 29/42 (69.0%), respectively; p < 0.05) () and abnormal chromosomes (21/44 (47.7%) vs. 30/42 (71.4%), respectively; p < 0.05) (). The diffuse distribution of mitochondria was considered as normal, while peripheral and semi-peripheral distribution was considered abnormal (). Seventy-five percent (33/44) of the oocytes in the 37°C group showed an evenly diffuse pattern compared to 73.8% (31/42) in the RT group. There was no significant between-group difference in this respect (p > 0.05; ).
Figure 1. Spindle (green) configuration and chromosomal (blue) alignment in human MII oocytes. (A1-3) Representative normal spindle and chromosomal configuration in human MII oocytes in the 37°C group; (B1-3 and C1-3) representative abnormal spindle and chromosomal configuration in the RT group. Arrows indicate the spindle multipoles and displaced chromosomes. Scale bar = 10 µm. MII: metaphase II; RT: room temperature.
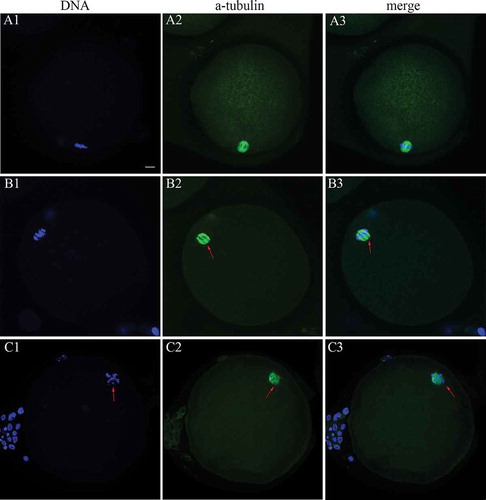
Figure 2. Abnormal spindle, abnormal chromosome, and diffuse mitochondrial distribution rate in the two groups. (A) Representative abnormal spindle rate; (B) representative abnormal chromosome rate; (C) representative diffuse mitochondria rate. The proportion of spindle and abnormal chromosome in the 37°C group was significantly lower than that in the RT group. * indicates p < 0.05; RT: room temperature.
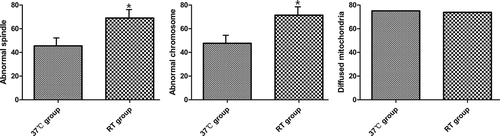
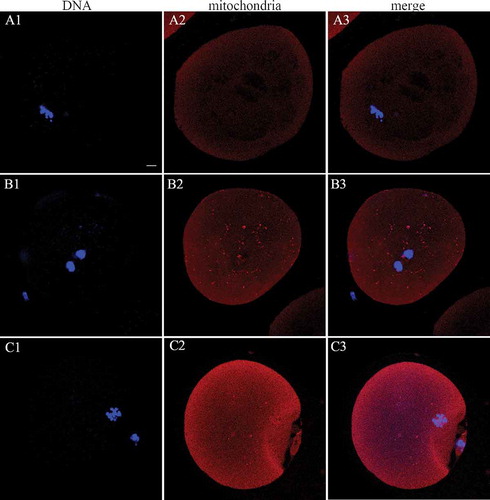
Figure 3. Mitochondrial (red) distribution in human MII oocytes. (A1-3) Peripheral mitochondrial distribution in the MII phase human oocytes in the 37°C group; (B1-3 and C1-3) semiperipheral and diffuse mitochondrial distribution in the RT group, respectively. Scale bar = 10 µm. MII: metaphase II; GV: germinal vesicle; RT: room temperature.
Discussion
The aim of this study was to evaluate a new approach to vitrification of oocytes with respect to vitrification time, spindle configuration, chromosomal arrangement, and mitochondrial distribution. Use of the new method significantly reduced the duration of exposure of the oocyte to the cyroprotectant, which reduced the cytotoxic effect of the cryoprotectant. The spindle and chromosomal abnormalities in the 37°C group were significantly lower than those in the RT group, which indicated a protective effect of the method on oocyte microtubules. No significant between-group differences were observed with respect to oocyte survival rate and mitochondrial distribution.
In our study, GV stage oocytes lacked the surrounding granulosa cells. However, the rate of maturation (77.1%) was comparable to that reported by Abbas et al. (75.33%) [Abbas et al. Citation2013] and Mohamed et al. (70.8%) [Mohamed et al. Citation2011]. The mature oocytes were randomly assigned for vitrification by one of the two methods. The survival rate in the 37°C group (88.0%) was higher than that in the RT group (82.4%). However, the difference was not statistically significant. Vitrification is an efficient method of oocyte cryopreservation. The reported survival rates have varied between 79.9% - 90.4% [Ana et al. Citation2015; Ci et al., Citation2014; Gao et al. Citation2015]. Hence, further modification of the method may have little effect on the survival rate. In the present study, the time needed in the 37°C group for pre-equilibrium was significantly shorter than that in the RT group (p < 0.05). Increased temperature accelerates molecular movement, which may have helped in reducing the pre-equilibration time and thereby, reduced the toxic effects. The oil coating also helped to stabilize the temperature and osmotic pressure during the procedure.
The effect of oocyte cryopreservation and the IVM process on microtubular morphology, chromosomal arrangement, and embryonic development is well-documented [Franciosi et al. Citation2015; Tao et al. Citation2014]. To explore the effects of this new method on the oocyte, we observed temperature sensitive organelles, i.e., spindle and chromosome. In the present study, pre-equilibrium at 37°C was associated with a significant reduction in abnormal spindle and chromosomes (p < 0.05). Pre-equilibrium at 37°C may significantly reduce the adverse effects of temperature on the organelles of human oocyte. Low temperature and shorter exposure to the cryoprotectant agent is known to be associated with a higher rate of abnormal spindle and chromosomes in MII oocytes [Boiso et al. Citation2002]. The following reason may explain the significant difference with respect to the effect on the structure of spindle and chromosome in our study: 1) The 37°C pre-equilibration method avoids a sudden temperature change from culture temperature to vitrification temperature (room temperature previously). 2) Reduced pre-equilibration time decreases the exposure of oocytes to cytotoxic damage. Given that disordered spindle and chromosome(s) are associated with embryonal polyploidy, our results indicate good prospects of this modified method. In our study, there was no significant difference in the distribution of mitochondria between the two groups. A previous study also showed no significant difference in the mitochondrial distribution between in vitro matured oocytes and oocytes matured in vivo [Coticchio et al. Citation2009]. Perhaps the matured oocytes finally develop uniformly mature cytoplasm without the influence of vitrification. However, the mitochondrial distribution cannot be considered representative of their entire functionality. Therefore, further functional investigations are needed. Our findings can be better interpreted if other parameters of cellular metabolism such as ultrastructural evaluation, quantitative analysis, and adenosine triphosphate content, are also analyzed. Hence, further investigation is required to establish the beneficial effect of modified vitrification on oocyte cytoplasmic maturation.
Certain limitations of our study need to be acknowledged. Our study provides only a preliminary explanation of the advantages of pre-equilibrium at 37°C. The research mainly used failed-matured oocytes from ovarian stimulated patients. These oocytes may potentially have developmental problems in vivo. Moreover, the potential for oocyte fertilization and embryo development potential after 37°C pre-equilibrium vitrification method are still unclear, and its clinical value is yet to be fully established.
Vitrification using a pre-equilibrium temperature of 37°C may significantly reduce defective spindle configuration and chromosomal abnormalities of in-vitro matured human oocytes. Our study provides supportive evidence of the benefits of application of oil and use of 37°C temperature in the pre-equilibrium process on oocyte microtubular stability. Stable osmotic pressure and temperature may significantly reduce the harmful effect of freeze-thawing process on spindle and DNA configuration.
Materials and methods
Patients
Immature oocytes were obtained from patients who underwent intracytoplasmic sperm injection (ICSI) for male factors, between December 2014 and July 2015, at the Medical Center for Human Reproduction, Beijing Chaoyang Hospital, Capital Medical University. After denudation, all MII oocytes were used for treatment, while GV stage oocytes were cultured in vitro.
One hundred and thirty one oocytes at GV stage were obtained from sixty-seven patients and cultured in vitro to MII stage. In vitro matured oocytes were randomly assigned to one of the two experimental groups: (i) In the 37°C group, oocytes were vitrified at a pre-equilibrium temperature of 37°C; (ii) in the RT group, oocytes were vitrified at room temperature. In the event that two MII oocytes were obtained from one patient, these were randomly assigned to one of the two groups. If only one MII oocyte was obtained, it was randomly assigned to one of the two groups. The study was approved by the ethics committee at the Beijing Chaoyang Hospital. Written informed consent was obtained from all participants.
Stimulation protocol
Patients prepared for ICSI were administered standard ovarian stimulation using a gonadotropin-releasing hormone agonist (GnRHa; Decapeptyl; Ferring, Kiel, Germany) for down-regulation, followed by ovarian stimulation with 150-300 IU/d recombinant FSH (Gonal-F; Serono Laboratories, Aubonne, Switzerland). Oocyte retrieval was performed 37 h after administration of 6,500 IU recombinant human chorionic gonadotrophin (HCG; Ovidrel; Serono, Rockland, MA, USA), when two or more follicles had acquired a size of at least 18 mm. Corona-cumulus cells were removed with 85 IU/mL hyaluronidase and subjected to mechanical pipetting to assess nuclear maturity. Immature oocytes, which are identified by the absence of polar body and discernible GV nucleus, were collected and cultured in vitro.
In vitro maturation
Immature oocytes were incubated in G-1 medium (Vitrolife, Kungsbacka, Sweden) supplemented with a final concentration of 0.15 IU/mL HCG and 0.075 IU/mL recombinant FSH, at 37°C, in an atmosphere of 5% CO2 and 95% air for in vitro maturation (IVM). The extrusion of the first polar body was used as the criterion for nuclear maturation. The maturity of oocytes was assessed after 20 h of incubation, and then assessed every 4-8 h until 48 h of incubation. When the polar body was extruded, the time and initiated vitrification of the oocytes was recorded.
Vitrification and thawing
In vitro matured oocytes were randomly assigned to one of two groups based on the pre-equilibrium temperature: 37°C group and RT group. In the 37°C group, equilibrium medium (Medicult, Jyllinge, Denmark) was covered with mineral oil (to prevent volatilization) and incubated at 37°C for 30 min. In the RT group, equilibrium medium was covered with mineral oil and incubated at RT for 30 min. All subsequent experiments were carried out in the dark.
Oocytes were transferred into pre-prepared equilibrium medium for several minutes until the oocytes changed from shrunk to a plump state. The time required for the same was recorded. Subsequently, the oocytes were transferred to vitrification medium (Medicult, Jyllinge, Denmark) for 45 s to 60 s at RT and quickly loaded into the Cryoleaf (ORIGIO MediCult, Jyllinge, Denmark) carrier and plunged into liquid nitrogen for storage for at least two w.
The methodology used for thawing is described elsewhere [Munck et al. Citation2015]. Briefly, oocytes were thawed in the thawing medium (Medicult, Jyllinge, Denmark) 1 for a maximum of 3 min at 37°C. Oocytes were then transferred sequentially for 3 min each to the thawing medium 2 and the thawing medium 3 at RT. Oocytes were then washed twice in the thawing medium 4 at 37°C and transferred to G1 medium (Vitrolife, Kungsbacka, Sweden) for 2 h. Thawed oocytes were considered to have ‘morphologically survived’ in the absence of any dark/degenerated or contracted ooplasm and/or cracked zona pellucida.
Fluorescence staining and confocal microscopy
For evaluation of spindle and mitochondria, oocytes were first cultured in 500 nM MitoTracker® Red CMXRos (Molecular Probes, Eugene, OR, USA) for 30 min at 37°C. After 3 washes in PBS, these were fixed and permeabilized as described elsewhere [Yuan et al. Citation2006], and blocked with 5% goat serum at RT for 1 h. Then oocytes were incubated with FITC-conjugated monoclonal antibody against α-tubulin (1:100, Sigma, Saint Louis, MO, USA) for 30 min at 37°C. After washing in 3% BSA, chromosomes were stained with 5 μg/mL DAPI (Sigma-Aldrich, St Louis, MO, USA) for 1 h prior to the final wash. Oocytes were mounted on glass slides and observed under a confocal laser scanning microscope (Leica TCS SP2, Wetzlar, Germany). All operations were performed in dark environment.
Assessment method
Barrel-shaped spindles with chromosomes arranged on a compact equator plate were regarded as having a normal configuration. Spindles with partial or total disorganization of microtubules or those showing irregularly scattered chromosomal arrangement were considered to have abnormal configuration [Shan et al. Citation2010].
The pattern of mitochondrial distribution in human oocytes was defined as peripheral (located largely in the cortical region of the oocytes), diffuse (distributed evenly in the cytoplasm), and semi-peripheral (intermediate pattern between peripheral and diffuse distribution) [Shan et al. Citation2010; Brevini et al. Citation2005]. In most of the mature oocytes, mitochondria were uniformly distributed in the cytoplasm [Sun et al. Citation2001; Liu et al. Citation2010; Brevini et al. Citation2005].
Statistical analyses
All data were pooled from at least three replicates of each experiment. The examinations were performed by three observers who were blind to the source and culture conditions of the oocytes. Between-group differences were assessed by Student t test. P value < 0.05 was considered statistically significant.
Declaration of interest
All authors declare that they have no competing interests.
Acknowledgments
This work was partly supported by the National Natural Science Foundation of China (81370758), and Personnel Training Plan of The Health Care System of Beijing (2013-3-021, 2013-2-009).
Additional information
Notes on contributors
Minghui Liu
Conceived and designed the experiments: YL, SL, WHZ, DPC, MHL. Performed the experiments: WS, MHL. Analyzed the data: YL, SL, WS, MHL. Wrote and revised the paper: All authors. All authors read and approved the final manuscript.
Wenhui Zhou
Conceived and designed the experiments: YL, SL, WHZ, DPC, MHL. Performed the experiments: WS, MHL. Analyzed the data: YL, SL, WS, MHL. Wrote and revised the paper: All authors. All authors read and approved the final manuscript.
Dapeng Chu
Conceived and designed the experiments: YL, SL, WHZ, DPC, MHL. Performed the experiments: WS, MHL. Analyzed the data: YL, SL, WS, MHL. Wrote and revised the paper: All authors. All authors read and approved the final manuscript.
Lei Fu
Conceived and designed the experiments: YL, SL, WHZ, DPC, MHL. Performed the experiments: WS, MHL. Analyzed the data: YL, SL, WS, MHL. Wrote and revised the paper: All authors. All authors read and approved the final manuscript.
Wei Sha
Conceived and designed the experiments: YL, SL, WHZ, DPC, MHL. Performed the experiments: WS, MHL. Analyzed the data: YL, SL, WS, MHL. Wrote and revised the paper: All authors. All authors read and approved the final manuscript.
Shan Liu
Conceived and designed the experiments: YL, SL, WHZ, DPC, MHL. Performed the experiments: WS, MHL. Analyzed the data: YL, SL, WS, MHL. Wrote and revised the paper: All authors. All authors read and approved the final manuscript.
Yuan Li
Conceived and designed the experiments: YL, SL, WHZ, DPC, MHL. Performed the experiments: WS, MHL. Analyzed the data: YL, SL, WS, MHL. Wrote and revised the paper: All authors. All authors read and approved the final manuscript.
References
- Abbas, S., Ahmad, H., Mohammad Ali, K., Mohsen, N., Mohammad, S., Abbas, P., et al. (2013) The effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytes. Eur J Obstet Gynecol Reprod Biol 167: 69–75.
- Ana, C., Nicolás, G., Antonio, P., José, R. (2015) Six years’ experience in ovum donation using vitrified oocytes: report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil Steril 104: 1426–1434.
- Boiso, I., Martí, M., Santaló, J., Ponsá, M., Barri, P.N., Veiga, A. (2002) A confocal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod 17: 1885–1891.
- Brevini, T.A., Vassena, R., Francisci, C., Gandolfi, F. (2005) Role of Adenosine Triphosphate, Active Mitochondria, and Microtubules in the Acquisition of Developmental Competence of Parthenogenetically Activated Pig Oocytes. Biol Reprod 72: 1218–1223.
- Ci, Q., Li, M., Zhang, Y., Ma, S., Gao, Q., Shi, Y. (2014) Confocal microscopic analysis of the microfilament configurations from human vitrification-thawed oocytes matured in vitro. Cryo Letters 35: 544-548(5).
- Cobo, A., Domingo, J., Pérez, S., Crespo, J., Remohí, J., Pellicer, A. (2008) Vitrification: An effective new approach to oocyte banking and preserving fertility in cancer patients. Clin Transl Oncol 10: 268–273.
- Coticchio, G., Bromfield, J.J., Sciajno, R., Gambardella, A., Scaravelli, G., Borini, A., et al. (2009) Vitrification may increase the rate of chromosome misalignment in the metaphase II spindle of human mature oocytes. Reprod Biomed Online 19: 29–34.
- Franciosi, F., Goudet, G., Tessaro, I., Papillier, P., Dalbiestran, R., Reigner, F., et al. ( 2015) In vitro maturation affects chromosome segregation, spindle morphology and acetylation of lysine 16 on histone H4 in horse oocytes. Reprod Fertil Dev 2015 Dec 14. doi: 10.1071/RD15350. [ Epub ahead of print]
- Gao, S., Li, M., Wu, K., Sheng, Y., Tang, R., Chen, Z.J. (2015) Effect of different rehydration temperatures on the survival of human vitrified-warmed oocytes. Assist Reprod Genet 32(8): 1197–1203.
- Liebermann, J., Tucker, M.J. (2004) Vitrifying and warming of human oocytes, embryos, and blastocysts: vitrification procedures as an alternative to conventional cryopreservation. Methods Mol Biol 254: 345–364.
- Liu, S., Li, Y.X., Yan, J.H., Chen, Z.J. (2010) Changes in the distribution of mitochondria before and after in vitro maturation of human oocytes and the effect of in vitro maturation on mitochondria distribution. Fertil Steril 93: 1550–1555.
- Mathias, F.J., D’Souza, F., Uppangala, S., Salian, S.R., Kalthur, G., Adiga, S.K. (2014) Ovarian tissue vitrification is more efficient than slow freezing in protecting oocyte and granulosa cell DNA integrity. Syst Biol Reprod Med 60: 317–322.
- Mohamed, A.K., Astrid, P., Rita, K., Samira, I.R., Thierry, B., Cécile, G., et al. (2011) Vitrification at the germinal vesicle stage does not affect the methylation profile of H19 and KCNQ1OT1 imprinting centers in human oocytes subsequently matured in vitro. Fertil Steril 95: 1955–1960.
- Mohsenzadeh, M., Khalili, M.A., Nazari, S., Jahromi, V.H., Agharahimi, A., Halvaei, I. (2012) Effect of vitrification on morphology and in-vitro maturation outcome of human immature oocytes. Ital J Anat Embryol 117: 190–198.
- Mullen, S.F., Agca, Y., Broermann, D.C., Jenkins, C.L., Johnson, C.A., Critser, J.K. (2004) The effect of osmotic stress on the metaphase II spindle of human oocytes, and the relevance to cryopreservation. Hum Reprod 19: 1148–1154
- Munck, N.D., Petrussa, L., Verheyen, G., Staessen, C., Vandeskelde, Y., Sterckx, J., et al. (2015) Chromosomal meiotic segregation, embryonic developmental kinetics and DNA (hydroxy)methylation analysis consolidate the safety of human oocyte vitrification. Mol Hum Reprod 21: 37–45.
- Paolo Emanuele, L.S., Eleonora, P., Pasquale, P., Vincenzo, V., Roberto, D.L., Paola, D.A., et al. (2014) Human oocyte cryopreservation with slow freezing versus vitrification. Results from the National Italian Registry data, 2007-2011. Fertil Steril 102: 90–95.
- Shan, L., Yuan, L., Feng, H.L., Yan, J.H., Mei, L., Ma, S.Y., et al. (2010) Dynamic modulation of cytoskeleton during in vitro maturation in human oocytes. Am J Obstet Gynecol 203: 1–7.
- Song, W.Y., Sun, Y.P., Jin, H.X., Xin, Z.M., Su, Y.C., Chian, R.C. (2011) Clinical outcome of emergency egg vitrification for women when sperm extraction from the testicular tissues of the male partner is not successful. Syst Biol Reprod Med 57: 210–213.
- Sun, Q.Y., Wu, G.M., Lai, L., Park, K.W., Cabot, R., Cheong, H.T., et al. (2001) Translocation of active mitochondria during pig oocyte maturation, fertilization and early development in vitro. Reproduction 122: 155–163.
- Tao, L., Na, G., Jie-Qiong, L., Mei-Hua, T., Yu-Feng, L. (2014) Vitrification of in vitro matured oocytes: effects on meiotic spindle configuration and mitochondrial function. Int J Clin Exp Pathol 7: 1159–1165.
- Tucker, M.J., Morton, P.C., Sweitzer, C.L., Wright, G., (1995) Cryopreservation of human embryos and oocytes. Curr Opin Obstet Gynecol 7(3): 188–192.
- Wennerholm, W.B. (2000) Cryopreservation of embryos and oocytes: obstetric outcome and health in children. Hum Reprod 15: 18–25.
- Yuan, L., Huai-Liang, F., Yi-Juan, C., Guang-Juan, Z., Yong, Y., Steve, M., et al. (2006) Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril 85: 827–832.

