?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ziziphus jujuba cv. Yulingdang (YLD) is a jujube cultivar that has been approved as a new plant cultivar by the State Forestry Administration of China. The physicochemical properties and antioxidant activity of YLD fruit were evaluated at seven stages of maturation, ranging from fruit setting to red ripe. The antioxidant activity of YLD fruit was assessed through total phenolics, total flavonoids, and methods including the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging rate, β-carotene-linoleic acid emulsification method, and reducing power. These measures were observed to decrease gradually as maturation progressed. The study also indicated an increase in total sugar and total acid levels, along with a decrease in moisture, soluble protein, and the sugar-acid ratio during maturation. Understanding this information is essential to fully exploit the commercial potential of YLD fruit.
1. Introduction
Jujube (Ziziphus jujuba Mill, Rhamnaceae family) has a cultivation history of over 4,000 years (Stoli & Stănică, Citation2021). It is widely distributed throughout China, now widely cultivated in many parts of the world, including the Middle East, Europe, and North America. With over 700 cultivars are cultivated (Lu et al., Citation2021), including the well-known Winter Jujube, Li Jujube, Golden Silk Jujube, etc. Jujube, as a native economically significant forestry crop in China, has garnered considerable attention from Chinese consumers. In addition to being consumed fresh, jujube is commonly processed into various forms such as dried fruit, candied fruit, fruit tea, and jujube cake, which are regarded as a popular functional food (Choi et al., Citation2011).
Jujube is a highly nutritious and flavorful fruit, abundant in beneficial nutrients present in both its skin and flesh. Traditional Chinese Medicine (TCM) and recent pharmacological studies have demonstrated that fresh jujube contains essential components such as sugars, polysaccharides, vitamins, proteins, phenolics, flavonoids, and other bioactive compounds (Ji et al., Citation2021; Wang et al., Citation2022). These bioactive substances are believed to possess various health-promoting properties, including the ability to promote intestinal motility, alleviate fatigue, and slow down the oxidation process (Chen & Tsim, Citation2020; Wojdyło et al., Citation2016).
Fruit ripening is a vital and complex process, and the appropriate harvesting time has a significant impact on the quality of the fruit and the desired flavor (Patel & Rao, Citation2009). It involves various biochemical reactions like pectin hydrolysis, sugar and acid metabolism, and the synthesis of carotenoids and phenolic compounds (Ji et al., Citation2022). Understanding the changes in physicochemical properties and antioxidant activity of jujubes during their maturation stages has far-reaching implications for the jujube industry. Reche et al. (Citation2021) conducted a physicochemical characterization of jujube at four different maturity stages to investigate their phenolics content and antioxidant activity, aiming to offer guidance for fruit harvesting. Yan et al. (Citation2022) analyzed the physicochemical and antioxidant activity of eight jujube cultivars and identify a suitable maturity stage and optimal harvest time to obtain fruit containing high nutritional and functional characteristics.
YLD is a distinctive jujube cultivar cultivated in Fuyang City, Anhui Province, China, and has received national validation as a new plant cultivar approved by the State Forestry Administration of China in 2013. Due to its desirable size, thin skin, and crunchy texture, YLD is being cultivated on a large scale, generating substantial economic benefits. At present, the research on YLD mainly focuses on the cultivation and yield of the jujube tree (Chen et al., Citation2021). Little research has been conducted on the quality of YLD when consumed fresh. Exploring the physicochemical and antioxidant properties can enrich the research on the quality aspects of YLD. This analysis can provide a more rigorous and scientifically grounded assessment of YLD quality compared to relying solely on empirical data and will enable YLD growers and consumers to gain a deeper understanding and make more informed judgments about the quality of YLD.
In this study, we present a comprehensive analysis of the physicochemical properties and antioxidant activity of YLD during the different stages of maturity. The aim is to provide valuable guidance for the harvesting and processing of YLD and its significance in the development of the YLD jujube fruit market.
2. Materials and methods
2.1. Materials
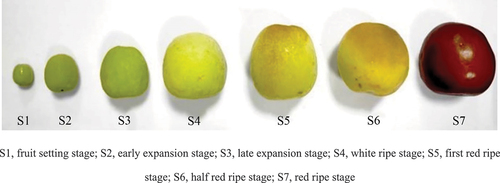
YLD were collected at the Ninglaozhuang Jujube Garden in Fuyang City, China (longitude: 115°44’14.576“E, latitude: 32°59’36.614“N, altitude: 32 m), from 6 July 2020, to 7 September 2020. Jujube fruits of uniform size with stems free from pests and mechanical damage () were randomly collected at seven different maturity stages (S1, fruit setting stage; S2, early expansion stage; S3, late expansion stage; S4, white ripe stage; S5, first red ripe stage; S6, half red ripe stage; S7, red ripe stage) each from three fixed trees, about 150 samples per maturity stage. The samples were promptly placed in an insulated box with crushed ice and ice packs to maintain a low temperature during transportation to the laboratory. Peeled, pitted, and chopped, with a knife, packaged in 1 g packets on tinfoil, and stored at −80°C after flash freezing in liquid nitrogen. All samples were pretreated on the day of collection.
2.2. Moisture content determination
Moisture content was measured by drying in an air oven at 105°C (Obi et al., Citation2016). The moisture content was the mass difference before and after drying, and the results were expressed as percentages by mass (fresh weight).
2.3. Total sugar content determination (TSC)
TSC was determined by the Anthrone method (Zhang et al., Citation2022) with a simple modification. 1 g of sample was homogenized (T18 digital Ultra-Turrax, IKA, Germany) with 10 ml distilled water. The homogenate was tightly sealed and subjected to boiling for a duration of 30 min and filtered. The residue was further boiled for another 10 min with the addition of 5–10 mL of distilled water, filtered, and washed twice with water. Combined the filtrate and adjusted it to 100 mL. 0.5 mL of the sample extract was transferred to a test tube, followed by the addition of 1.5 mL distilled water, 0.5 mL of anthracene-ethyl acetate reagent (1.0 g of analytical grade anthrone dissolved in 50 mL of ethyl acetate), and 5.0 mL of concentrated sulfuric acid. It was then boiled in a water bath for 1 min, and the absorbance at 630 nm (UV-1900i, Shimadzu, Japan) was determined. A standard curve was plotted with a solution of sucrose, and the results were expressed as percentages by mass (fresh weight).
2.4. Total acid content determination (TAC)
TAC was assessed following the method outlined by Tilahun et al. (Citation2020) with a slight modification. Briefly, 10 g of the YLD samples were homogenized with 25 mL of CO2-free water at 80°C. The mixture was then boiled for 30 min, cooled to room temperature, and adjusted to a final volume of 100 mL with CO2-free water. After filtration, the resulting filtrate was collected. 20 mL of the filtrate was taken and mixed with two drops of 1% phenolphthalein indicator. Titration was conducted using a calibrated NaOH solution until the solution turned pink and the color remained stable for 0.5 min. As a blank control, titration was performed using CO2-free water instead of the filtrate. The results were expressed as percentages by mass (fresh weight).
2.5. Sugar-acid ratio
The sugar-acid ratio is calculated as follows:
2.6. Soluble protein content determination (SPC)
SPC was analyzed by the Coomassie brilliant blue method (Zhang et al., Citation2022). Briefly, 2 g of YLD fruit was homogenized with 5 mL of water and centrifuged at 4°C for 20 min at 12,000×g (Allegra 64 R, Beckman Coulter, U.S.A.). The supernatant was collected and kept at a low temperature. 1.0 mL of the extract was added to a test tube, and then 5 mL of Coomassie Brilliant Blue G-250 solution was added. The mixture was thoroughly mixed and incubated for 2 min. The absorbance at 595 nm was measured and compared to a standard curve of bovine serum albumin (BSA). It was expressed as the mass of soluble protein per gram of jujube (fresh weight), i.e. mg/g.
2.7. Ascorbic acid content determination (AAC)
AAC is determined using an ascorbic acid assay kit (Jiancheng Institute of Biological Engineering, Nanjing, China).
2.8. Antioxidant activity determination
2.8.1. Extraction of antioxidant active compounds
The antioxidant active compounds of YLD were extracted using the reported method with a slight modification (Selani et al., Citation2016). Briefly, 10 g of a jujube fruit sample was mixed with 30 mL of 80% methanol, homogenized twice at 4000 rpm for 60 s each time, sonicated in an ice bath (power: 360 W, time: 20 min), and centrifuged at 4°C for 10 min at 3000 rpm. The supernatant was collected, and the residue underwent this procedure three more times. Subsequently, the collected supernatants were combined and concentrated by evaporation at 45°C (RE-52AA rotary evaporator, Yarong, China) until the remaining weight of extract was less than 10% of the original extract weight. All the samples were then stored at −80°C in light-protected conditions.
2.8.2. Total phenolics content determination (TPC)
Determination of TPC in samples by the modified Folin-Ciocalteu method (Shi et al., Citation2018). Take 125 µL of the extract and combine it with 500 µL deionized water and 125 µL Folin-Ciocalteu reagent. The solution was incubated at room temperature for 6 min, and then 1.25 mL of 7% sodium carbonate and 1 mL of deionized water were added. The mixture was stored at room temperature for 1.5 h. The absorbance was measured at 760 nm. The results were quantified using a gallic acid standard curve and expressed as the mass of gallic acid per gram of jujube (fresh weight), i.e. mg/g.
2.8.3. Total flavonoids content determination (TFC)
TFC was determined by the method described in a previous study (Silva & Sirasa, Citation2018) with a slight modification. 100 µL of the sample solution, prepared by diluting at a ratio of 1:2, was mixed with 200 µL of 5% (w/v) sodium nitrite and 200 µL of 10% (w/v) aluminum nitrate solution, incubated for 6 min. 2 mL of 4% (w/v) NaOH solution was added to the mixture, then diluted with deionized water to a final volume of 5 mL and incubated for a further 15 minutes. The absorbance was determined at a wavelength of 510 nm. The results were quantified using a rutin standard curve and expressed as the mass of rutin per gram of jujube (fresh weight), i.e. mg/g.
2.8.4. DPPH radical scavenging rate
The removal rate of DPPH radicals was measured as previously described (Vanti et al., Citation2021) with appropriate modification. 1 mL of sample extract was mixed with 1 mL of 50 µg/mL 2,2-diphenyl-1-picrylhydrazyl (DPPH) methanol solution, and the absorbance was measured at 517 nm after 30 min at room temperature in the dark. Control solutions were prepared similarly, but methanol was used instead of the sample. The formula was calculated as follows:
where A1 and A2 are the absorbances of control and sample tubes, respectively.
2.8.5. β-carotene-linoleic acid emulsification method
The reagents of β-carotene (2 mg), linoleic acid (45 mg), and Tween-40 (350 mg) were well mixed with chloroform (10 mL). The chloroform was completely evaporated at 35°C with a rotary evaporator, and then 100 mL of distilled water was added. The reagent was completely mixed to prepare a mixture of β-carotene-linoleic acid. 4 mL of the mixture was placed in a test tube, and 100 µL of sample extract was added and incubated at 50°C for 1 h. The absorbance was measured at 470 nm (Gao et al., Citation2011).
The antioxidant activity coefficient can be calculated as follows:
where As60 is the absorbance of a sample tube at 60 min; Ac0 is the absorbance of a blank tube at 0 min, and Ac60 is the absorbance of a blank tube at 60 min.
2.8.6. Reducing power
1 mL of the sample extract was combined with 2 mL of 0.2 M phosphate buffer (pH = 6.6) and 2 mL of 1% (w/v) K3Fe(CN)6 and then incubated in a 50°C water bath for 20 min. Subsequently, 2 mL of 10% (w/v) trichloroacetic acid was added. 2 mL of the reaction mixture was combined with 2 mL of deionized water and 0.4 mL of 0.1% (w/v) FeCl3 and incubated for 30 min in the dark. The absorbance was measured at 700 nm (Siddhuraju et al., Citation2002).
2.9. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA method) using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, N.Y., U.S.A.). Origin 2021 (Origin Lab, Northampton, MA, U.S.A.) was used for graphing, and three replicates were performed for each group of experiments; significant differences were indicated as * (p < .05) and ** (p < .01).
3. Results and discussion
3.1. The physicochemical properties of YLD at different stages of maturity
The moisture content plays a crucial role in determining the sensory attributes and tissue structure of the fruit. As shown in , there was a gradual reduction in moisture content as the jujube fruit underwent ripening, leading to a higher concentration of dry matter in the fruit. At the fruit setting stage, early expansion stage, and late expansion stage, water constitutes approximately 85–89% of the fresh weight. This high moisture content is associated with rapid cell division and expansion during this growth phase. The moisture content of YLD decreased from 88.03% at the fruit setting stage to 69.51% at the red ripe stage, and at the first red ripe stage, half red ripe, and red ripe stage were significantly different.
Figure 2. Changes of moisture content in YLD at different stages of maturity. Different lowercase letters between the columns are significantly different at p < .05. Error bars represent the standard deviation.
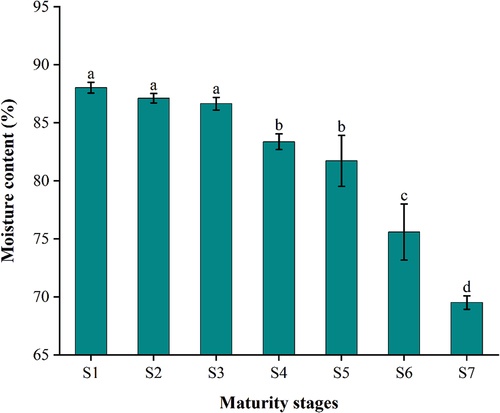
During ripening, the metabolism and the rate of water absorption of YLD gradually decreased. Consequently, the moisture content of the jujube fruit decreased throughout the ripening process, which aligns with the findings reported for Ziziphus jujuba cv. Pear (85.49–74.59%) (Wu et al., Citation2012). Compared to the moisture content of Ziziphus jujuba cv. Dongzao (76.3–58.6%) (Zhang et al., Citation2020) from the white ripe stage to the red stage, we found that the YLD has the highest moisture. This may be due to the differences between cultivars or the effects of irrigation and rainfall (Cui et al., Citation2008).
Ascorbic acid is a very important water soluble vitamin that plays a critical role in maintaining good health and preventing various diseases, and one of the most powerful antioxidants (Zheng et al., Citation2022). The levels of ascorbic acid in the fruit exhibit dynamic changes during different stages of development. As illustrated in , the AAC of YLD at different stages of maturity ranged from 0.10 to 0.14 mg/g, at the first red ripening stage the AAC reached 0.14 mg/g. The overall trend from the white ripe to red ripe stages showed an initial increase, followed by a subsequent decrease. This decline can be attributed to the heightened enzymatic reactions and oxidation processes that occur during maturation. Also, ascorbic acid is involved in cell division and growth, which actively occur in the early stages of fruit development (Smirnoff & Wheeler, Citation2000). Su et al. (Citation2019) compared 13 fresh jujube cultivars and found that the change in ascorbic acid content during jujube fruit ripening differed among cultivars. The trend of AAC in YLD was similar to that of Ziziphus jujuba cv. Fucuimi, Ziziphus jujuba cv. Early Crisp King, Ziziphus jujuba cv. Honey Pot and Ziziphus jujuba cv. Toad.
Figure 3. Changes of ascorbic acid content (a) and soluble protein content (b) in YLD at different stages of maturity. Different lowercase and uppercase letters between the columns are significantly different at p < .05. Error bars represent the standard deviation.
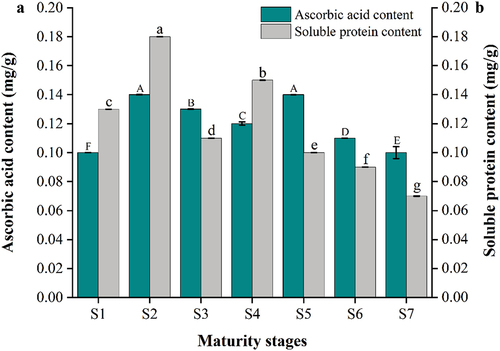
The SPC of fruits and vegetables is one of the important indicators of quality and nutritional value, and it plays an important role as an osmoregulatory substance, as well as a nutrient for the water retention capacity of cells. showed the variations of the SPC in YLD at different stages of maturity, spanning a range of 0.07 to 0.18 mg/g. The SPC content was probably influenced by the type and contents of free amino acids at different stages of maturity, which showed a downward trend.
Sugar and acid contents are among the most important compounds in fruit, as they are important factors in determining pulp and juice quality and influencing consumer purchases (Ma et al., Citation2022). Appropriate sugar and acid levels are necessary for good fruit flavor (Basak et al., Citation2022).
As shown in , the TSC in YLD gradually increased from 15.92% to 61.41% during the maturation from the fruit setting stage to the red ripe stage. The TSC at the red ripe stage exhibited a significant increase of approximately 3.86 times compared to the fruit setting stage. This elevation in TSC is likely attributed to the hydrolysis process, particularly the breakdown of starch within the jujubes during the ripening process (Zheng et al., Citation2012). The TAC of YLD gradually increased with maturity, ranging from 3.86% to 24.87%. The TAC at the red ripe stage is about 6.44 times higher than that at the fruit setting stage. The increasing trend of TAC could be attributed to the accumulation of some organic acid fractions in jujube fruit during the ripening process. Compared to the TSC and TAC of 10 common jujube cultivars in China (Gao et al., Citation2012), we found that YLD exhibited higher levels of total sugar and total acid content.
Table 1. Changes of total sugar content, total acid content, and sugar-acid ratio in YLD at different stages of maturity.
The sugar-acid ratio plays a crucial role in determining the fruit flavor, and there were significant variations in the sugar-acid ratio of jujube fruits at different maturity stages (Tian et al., Citation2019). The sugar-acid ratio of YLD fruit from 4.13 at the fruit setting stage to 2.47 at the red ripe stage with a downward trend, while the sugar-acid ratios of Dongzao jujube (Zhang et al., Citation2020), Pear jujube (Wu et al., Citation2012) and Ziziphus jujuba cv. Lingwulong jujubes (Gou et al., Citation2014) tended to increase at a slow rate. The different pattern variations of YLD could be one of the reasons for its special flavor.
3.2. The antioxidant activity of YLD at different stages of maturity
3.2.1. Total phenolics and total flavonoids content
Phenolics play an important physiological role in fruits, which not only improve fruit quality but have a significant antioxidant activity contributing to the various health benefits and potential. shows the changes in the TPC of YLD at different stages of maturity. As is shown, the TPC decreased as the jujube fruit matured, with a range of 4.78 to 3.95 mg/g. Notably, the highest TPC was observed at the fruit setting stage.
Figure 4. Changes of total phenolics content (a) and total flavonoids content (b) in YLD jujube at different stages of maturity. Different lowercase and uppercase letters between the folded lines are significantly different at p < .05. Error bars represent the standard deviation.
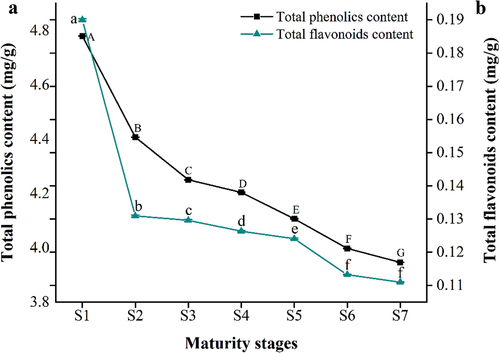
As a secondary metabolite, flavonoids have a wide range of health-promoting properties. Because of their antioxidant, anti-inflammatory, anti-mutagenic, and anti-cancer properties, flavonoids are essential ingredients for various applications, such as nutraceuticals, pharmaceuticals, and medicinal foods (Hossain, Citation2019). As shown in , the TFC of YLD fruits at different stages of maturity also tended to decrease, with the highest TFC in jujube fruits at the fruit setting stage. The TFC of YLD fruit (0.19 mg/g) in the fruit setting stage was 1.73-fold higher than that at the red ripe stage (0.11 mg/g). The TFC of YLD at the half red ripe to red ripe stages was stable.
The TPC and TFC of the jujube fruits were analyzed at various periods. The TPC of the YLD fruits during the same period was much higher than the TFC. The analysis revealed a declining trend in the TPC and TFC of YLD as the jujube fruit reached maturity. The trends of total phenolics and total flavonoids of YLD were consistent with those of Ziziphus jujuba cv. Junzao and Ziziphus jujuba cv. Lizao (Yan et al., Citation2022). This pattern of variation may be caused by the gradual shutdown of various phenolics synthases as the fruit ripens (Li et al., Citation2019). Particularly, the higher phenolics and flavonoids content at the initial stages of fruit development play a pivotal role as defensive compounds, protecting the fruit against environmental stresses and pathogens (Kumar et al., Citation2020).
3.2.2. Antioxidant activity
Antioxidant capacity for different maturity stages was evaluated by DPPH radical scavenging capacity, β-carotene-linoleic acid system, and reducing power assays, the results are illustrated in .
Figure 5. Changes of DPPH radical scavenging rate (a), reducing power (b), and antioxidant activity coefficient (c) in YLD at different stages of maturity. Different lowercase letters, uppercase letters, and Greek letters between the columns are significantly different at p < .05. Error bars represent the standard deviation.
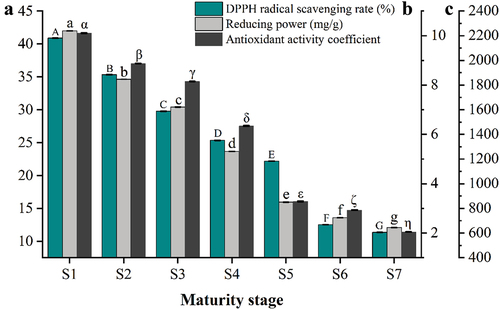
The DPPH free radical is a stable free radical commonly used to determine the free radical scavenging capacity of natural compounds. The absorbance decreases in the presence of antioxidant agents. As shown in , the removal rate of DPPH free radicals showed an overall rapid downward trend as the YLD fruit ripened further. During the maturation of jujube fruit, the lowest removal rate of DPPH free radicals was observed at the red ripe stage (11.35%), whereas the highest removal rate was recorded at the fruit set stage (40.89%). The difference in content could be related to the fact that the TPC and TFC of the fruit were much higher at the fruit set stage than at the red ripe stage.
The reducing power is one of the important indicators used to determine the level of antioxidant capacity. As shown in , the total reducing power of jujube samples at each stage of maturity surpassed 2.0 mg/g, and the total reducing power tended to decrease within the range of 10.20–2.22 mg/g.
The β-carotene-linoleic acid emulsification method operates on the principle of a discoloration reaction between β-carotene and linoleic acid free radicals. In the presence of antioxidant active compounds, the degradation and decomposition of β-carotene is significantly inhibited. The antioxidant activity coefficient is calculated by dividing the absorbance difference between the sample and blank control at 60 minutes by the absorbance difference between the blank control at 0 and 60 minutes (Gao et al., Citation2011). As shown in , the antioxidant activity coefficient of YLD exhibited a range of 2,200.83 to 1,467.94 during the fruit set to white ripe stages, while it ranged from 856.35 to 608.58 during the first red ripe stage to the subsequent red ripe stages. The antioxidant activity coefficient overall tends to decrease.
Three antioxidant capacity assays were used in this study and concluded that the DPPH free radical scavenging rate, antioxidant activity coefficient and total reducing power consistently decreased as YLD matured. This observation may be attributed to the variations in total phenolics and total flavonoids content at different stages of maturity.
3.3. Correlation analysis
illustrates the correlation between each component and the antioxidant activity at different maturity stages of YLD. As shown, the TSC was significantly correlated with the TPC and TFC with an R2 of −0.840 and −0.669, respectively (p < .01). The TSC continued to increase in YLD, while the TPC and TFC continued to decrease, which could be due to the enzymes responsible for breaking down complex carbohydrates into simpler sugars becoming more active, and the process of color change of skin during growth (Gao et al., Citation2012; Shen et al., Citation2015).
Table 2. Correlation of antioxidant activity and components of YLD at different stages of maturity.
Total phenolics and total flavonoids showed a high positive correlation with the antioxidant activity of the extracts. The R2 was 0.944, 0.906, 0.954, 0.815, 0.757, and 0.833, respectively (p < .01). The DPPH free radicals, antioxidant activity coefficient, and total reducing power were significantly positively correlated with each other. At the fruit setting stage, YLD had a higher antioxidant activity due to a faster synthesis of total phenolics and total flavonoids and a higher content of them. As the fruit matures, there was a decrease in the synthesis of total phenolics and total flavonoids, resulting in lower content levels. Accordingly, the antioxidant activity of YLD also tended to decrease. It has been suggested that the polyphenol oxidase contained in jujube fruit will be gradually released as the fruit ripens, which accelerates the oxidation of phenolics to quinones in the fruit, resulting in a decrease in antioxidant activity (Jiang, Citation2009).
4. Conclusions
Understanding these changes during the different stages of maturation is essential for improving the quality of fruits and developing functional foods with added health benefits. YLD fruit has high TSC and TAC. As ripening progresses, there is an observable increase of TSC and TAC, while the moisture content, sugar-acid ratio, and SPC show a decrease. The physicochemical properties of YLD fruit change gradually as the fruit ripens, resulting in fresh jujube fruit with high-quality flavor. This desirable quality makes YLD suitable not only as a premium fresh-eat jujube but also processed as dried jujube, jujube juice with an extended shelf life and distinctive taste. The TPC, TFC, and antioxidant activity of YLD exhibited significant correlations with the developmental stages and maturation of the jujube fruit. YLD can be used as a high-quality raw material for the development of pharmacological and functional foods, as well as a good source of natural antioxidant agents from the daily human diet.
Acknowledgments
Thanks to Anhui Ecological Fermentation Engineering Research Center for Functional Fruit Beverage and Fuyang Academy of Agricultural Sciences for providing the experimental site and materials for this experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Basak, J. K., Madhavi, B. G. K., Paudel, B., Kim, N. E., & Kim, H. T. (2022). Prediction of total soluble solids and pH of strawberry fruits using RGB, HSV and HSL colour spaces and machine learning models. Foods, 11(14), 2086. https://doi.org/10.3390/foods11142086
- Chen, L., Shi, L., Teng, H., & Zhao, W. (2021). Varietal characteristics and high yield cultivation technique of jujube cultivar Yulingdang. Journal of Fruit Resources, 2(3), 90–94. https://doi.org/10.16010/j.cnki.14-1127/s.2021.03.029
- Chen, J., & Tsim, K. W. K. (2020). A review of edible jujube, the Ziziphus jujuba fruit: A health food supplement for anemia prevalence. Frontiers in Pharmacology, 11. https://doi.org/10.3389/fphar.2020.593655
- Choi, S. H., Ahn, J. B., Kozukue, N., Levin, C. E., & Friedman, M. (2011). Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. Journal of Agricultural and Food Chemistry, 59(12), 6594–6604. https://doi.org/10.1021/jf200371r
- Cui, N., Du, T., Kang, S., Li, F., Zhang, J., Wang, M., & Li, Z. (2008). Regulated deficit irrigation improved fruit quality and water use efficiency of pear-jujube trees. Agricultural Water Management, 95(4), 489–497. https://doi.org/10.1016/j.agwat.2007.11.007
- Gao, Q. H., Wu, P. T., Liu, J. R., Wu, C. S., Parry, J. W., & Wang, M. (2011). Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Scientia Horticulturae, 130(1), 67–72. https://doi.org/10.1016/j.scienta.2011.06.005
- Gao, Q. H., Wu, C. S., Yu, J. G., Wang, M., Ma, Y. J., & Li, C. L. (2012). Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. Journal of Food Science, 77(11), C1218–C1225. https://doi.org/10.1111/j.1750-3841.2012.02946.x
- Gou, Q., Wang, M., Ji, X., Shen, J., Wang, Y., & Duan, X. (2014). Dietary and nutritional properties of Lingwu Long jujube at various stages of maturation. Modern Food Science & Technology, 30(11), 98–104. https://doi.org/10.13982/j.mfst.1673-9078.2014.11.019
- Hossain, M. A. (2019). A phytopharmacological review on the Omani medicinal plant: Ziziphus jujube. Journal of King Saud University - Science, 31(4), 1352–1357. https://doi.org/10.1016/j.jksus.2018.12.003
- Jiang, X. (2009). Study on Active Substances and Total Antioxidant Capacity of Lingwu Long Jujube [ Master’s Thesis]. Tianjin University of Science and Technology. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkVR3-_UaYGQCi3Eil_xtLb4LZ-_YhwLFXM1UdjdKkbuuPgiusIragP7Cp_W5JU_9S&uniplatform=NZKPT
- Ji, X., Cheng, Y., Tian, J., Zhang, S., Jing, Y., & Shi, M. (2021). Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chemical and Biological Technologies in Agriculture, 8(1), 54. https://doi.org/10.1186/s40538-021-00255-2
- Ji, X., Guo, J., Ding, D., Gao, J., Hao, L., Guo, X., & Liu, Y. (2022). Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus jujuba cv. Muzao. Journal of Food Measurement and Characterization, 16(3), 2191–2200. https://doi.org/10.1007/s11694-022-01288-3
- Kumar, S., Abedin, M., Singh, A. K., & Das, S. (2020). Role of phenolic compounds in plant-defensive mechanisms. In R. Lone; R. Shuab, and A. N. Kamili (Eds.), Plant phenolics in sustainable Agriculture: volume 1 (pp. 517–532). Springer Singapore.
- Li, X., Jin, L., Pan, X., Yang, L., & Guo, W. (2019). Proteins expression and metabolite profile insight into phenolic biosynthesis during highbush blueberry fruit maturation. Food Chemistry, 290, 216–228. https://doi.org/10.1016/j.foodchem.2019.03.115
- Lu, Y., Bao, T., Mo, J., Ni, J., & Chen, W. (2021). Research advances in bioactive components and health benefits of jujube (Ziziphus jujuba Mill.) fruit. Journal of Zhejiang University-SCIENCE B, 22(6), 431–449. https://doi.org/10.1631/jzus.B2000594
- Ma, W. F., Li, Y. B., Nai, G. J., Liang, G. P., Ma, Z. H., Chen, B. H., & Mao, J. (2022). Changes and response mechanism of sugar and organic acids in fruits under water deficit stress. PeerJ, 10, e13691. https://doi.org/10.7717/peerj.13691
- Obi, O. F., Ezeoha, S. L., & Egwu, C. O. (2016). Evaluation of air oven moisture content determination procedures for pearl millet (Pennisetum glaucum L.). International Journal of Food Properties, 19(2), 454–466. https://doi.org/10.1080/10942912.2015.1038566
- Patel, P. R., & Rao, T. V. R. (2009). Physiological changes in relation to growth and ripening of khirni [Manilkara hexandra (Roxb.) Dubard] fruit. Fruits, 64(3), 139–146. https://doi.org/10.1051/fruits/2009009
- Reche, J., Almansa, M. S., Hernández, F., Amorós, A., & Legua, P. (2021). Physicochemical and antioxidant capacity of jujube (Ziziphus jujuba Mill.) at different maturation stages. Agronomy, 11(1), 132. https://doi.org/10.3390/agronomy11010132
- Selani, M. M., Bianchini, A., Ratnayake, W. S., Flores, R. A., Massarioli, A. P., Alencar, S. M. D., & Brazaca, S. G. C. (2016). Physicochemical, functional and antioxidant properties of tropical fruits co-products. Plant Foods for Human Nutrition, 71(2), 137–144. https://doi.org/10.1007/s11130-016-0531-z
- Shen, J., Wang, M., Gou, Q., Ji, X., Wang, M., & Wang, Y. (2015). Changes in phenolic components and antioxidant activity of jujube fruits (Ziziphus jujuba Mill. Cv. Lingwuchangzao) during different growth stages. Food Science, 36(8), 191–195. https://doi.org/10.7506/spkx1002-6630-201508035
- Shi, Q., Zhang, Z., Su, J., Zhou, J., & Li, X. (2018). Comparative analysis of pigments, phenolics, and antioxidant activity of Chinese jujube (Ziziphus jujuba Mill.) during fruit development. Molecules, 23(8), 1917. https://doi.org/10.3390/molecules23081917
- Siddhuraju, P., Mohan, P. S., & Becker, K. (2002). Studies on the antioxidant activity of Indian laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chemistry, 79(1), 61–67. https://doi.org/10.1016/S0308-8146(02)00179-6
- Silva, K. D. R. R., & Sirasa, M. S. F. (2018). Antioxidant properties of selected fruit cultivars grown in Sri Lanka. Food Chemistry, 238, 203–208. https://doi.org/10.1016/j.foodchem.2016.08.102
- Smirnoff, N., & Wheeler, G. L. (2000). Ascorbic acid in plants: Biosynthesis and function. Critical Reviews in Biochemistry and Molecular Biology, 35(4), 291–314. https://doi.org/10.1080/10409230008984166
- Stoli, I., & Stănică, F. (2021). Review on some features of the Chinese jujube (Ziziphus jujuba Mill.). Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca Horticulture, 78(1), 10. https://doi.org/10.15835/buasvmcn-hort:2020.0033
- Su, J., Kang, C., Shi, Q., Zhang, Z., & Li, X. (2019). Main physical properties and nutritional indexes of fresh jujube fruits during ripening process in Northern Shaanxi. Journal of Northwest A&F University-Natural Science Edition, 47(10), 129–138+145. https://doi.org/10.13207/j.cnki.jnwafu.2019.10.016
- Tian, J., Cao, Y., Chen, S., Fang, Z., Chen, J., Liu, D., & Ye, X. (2019). Juices processing characteristics of Chinese bayberry from different cultivars. Food Science & Nutrition, 7(2), 404–411. https://doi.org/10.1002/fsn3.778
- Tilahun, S., Choi, H. R., Park, D. S., Lee, Y. M., Choi, J. H., Baek, M. W., Hyok, K., Park, S. M., & Jeong, C. S. (2020). Ripening quality of kiwifruit cultivars is affected by harvest time. Scientia Horticulturae, 261, 108936. https://doi.org/10.1016/j.scienta.2019.108936
- Vanti, G. L., Leshem, Y., & Masaphy, S. (2021). Resistance response enhancement and reduction of botrytis cinerea infection in strawberry fruit by morchella conica mycelial extract. Postharvest Biology and Technology, 175, 111470. https://doi.org/10.1016/j.postharvbio.2021.111470
- Wang, C., Wang, R., Fu, C., Jiang, X., Li, X., Han, G., & Zhang, J. (2022). Combining bioactive compounds and antioxidant activity profiling provide insights into assessment of geographical features of Chinese jujube. Food Bioscience, 46, 101573. https://doi.org/10.1016/j.fbio.2022.101573
- Wojdyło, A., Carbonell-Barrachina, Á. A., Legua, P., & Hernández, F. (2016). Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chemistry, 201, 307–314. https://doi.org/10.1016/j.foodchem.2016.01.090
- Wu, C. S., Gao, Q. H., Guo, X. D., Yu, J. G., & Wang, M. (2012). Effect of ripening stage on physicochemical properties and antioxidant profiles of a promising table fruit ‘pear-jujube’ (Zizyphus jujuba Mill.). Scientia Horticulturae, 148, 177–184. https://doi.org/10.1016/j.scienta.2012.09.026
- Yan, M., Wang, Y., Watharkar, R. B., Pu, Y., Wu, C., Lin, M., Lu, D., Liu, M., Bao, J., & Xia, Y. (2022). Physicochemical and antioxidant activity of fruit harvested from eight jujube (Ziziphus jujuba Mill.) cultivars at different development stages. Scientific Reports, 12(1). https://doi.org/10.1038/s41598-022-06313-5
- Zhang, Y., Li, S., Deng, M., Gui, R., Liu, Y., Chen, X., Lin, Y., Li, M., Wang, Y., He, W., Chen, Q., Zhang, Y., Luo, Y., Wang, X., & Tang, H. (2022). Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chemistry: X, 15, 100384. https://doi.org/10.1016/j.fochx.2022.100384
- Zhang, H., Pu, J., Tang, Y., Wang, M., Tian, K., Wang, Y., Luo, X., & Deng, Q. (2022). Changes in phenolic compounds and antioxidant activity during development of ‘Qiangcuili’ and ‘Cuihongli’ fruit. Foods, 11(20), 3198. https://doi.org/10.3390/foods11203198
- Zhang, Q., Wang, L., Wang, Z., Liu, Z., Zhao, Z., Zhou, G., Liu, M., & Liu, P. (2020). Variations of the nutritional composition of jujube fruit (Ziziphus jujuba Mill.) during maturation stages. International Journal of Food Properties, 23(1), 1066–1081. https://doi.org/10.1080/10942912.2020.1770281
- Zheng, X., Gong, M., Zhang, Q., Tan, H., Li, L., Tang, Y., Li, Z., Peng, M., & Deng, W. (2022). Metabolism and regulation of ascorbic acid in fruits. Plants, 11(12), 1602. https://doi.org/10.3390/plants11121602
- Zheng, H. Z., Kim, Y. I., & Chung, S. K. (2012). A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chemistry, 131(1), 106–110. https://doi.org/10.1016/j.foodchem.2011.08.038