ABSTRACT
A growing number of studies support that the bidirectional interactions between the gut microbiota, the immune system and the CNS are relevant for the pathophysiology of MS. Several studies have reported alterations in the gut microbiome of MS patients. In addition, a variety of studies in animal models of MS have suggested that specific members of the gut commensal microbiota can exacerbate or ameliorate neuroinflammation. Probiotics represent oral nontoxic immunomodulatory agents that would exert benefits when using in combination with current MS therapy. Here we investigate the effect of Vivomixx on the gut microbiome and central and peripheral immune responses in a murine model of primary progressive MS. Vivomixx administration was associated with increased abundance of many taxa such as Bacteroidetes, Actinobacteria, Tenericutes and TM7. This was accompanied by a clear improvement of the motor disability of Theiler’s virus infected mice; in the CNS Vivomixx reduced microgliosis, astrogliosis and leukocyte infiltration. Notably, the presence of Breg cells (CD19+CD5+CD1dhigh) in the CNS was enhanced by Vivomixx, and while spinal cord gene expression of IL-1β and IL-6 was diminished, the probiotic promoted IL-10 gene expression. One of the most significant findings was the increased plasma levels of butyrate and acetate levels in TMEV-mice that received Vivomixx. Peripheral immunological changes were subtle but interestingly, the probiotic restricted IL-17 production by Th17-polarized CD4+ T-cells purified from the mesenteric lymph nodes of Theiler’s virus infected mice. Our data reinforce the beneficial effects of oral probiotics that would be coadjuvant treatments to current MS therapies.
Introduction
Although the etiology of MS remains unknown, environmental and genetic risk factors are involved in the pathogenesis of the disease.Citation1 Accumulating evidence indicate that microbiome gut dysbiosis may be implicated in the increased incidence of MS in developed countries.Citation2 Specifically, bi-directional interactions between the gut microbiota, the intestinal barrier and the immune system appear to be relevant to the pathophysiology of MS.Citation3–Citation5 The different stages and subtypes of MS are influenced by environmental factors,Citation1 particularly in the relapsing remitting MS forms (RRMS) with current successful therapies. Studies using the encephalomyelitis autoimmune experimental (EAE) model of MS described lower incidence in germ free (GF) or antibiotic treated mice than in specific pathogen free (SPF) mice,Citation6–8 while recolonization restored the susceptibility to develop EAE.Citation3 However, other studies reported a therapeutic role of probiotic administration in a variety of EAE models. On this matter, orally administration of Bacteroides fragilis,Citation9,Citation10 Prevotella histicola,Citation11 different species of Lactobacillus (L. paracasei plus L. plantarum;Citation12 L. Reuteri;Citation13 L. helveticusCitation14), or multi-strain probioticsCitation15 have demonstrated to improve EAE. In addition, probiotics supplementation to RRMS patients has been shown to modulate immunity by exerting anti-inflammatory effects.Citation16,Citation17
Right now, there is little information regarding the association between gut microbes and CNS inflammation/neurodegeneration in the primary progressive MS. Notably, MRI studies in progressive MS show less brain permeability, probably reflecting CNS compartmentalized inflammation. As a result, we have studied the effect of administering the oral multi-strain probiotic, Vivomixx, on the gut microbiome and plasma short chain fatty acids (SCFAs) in the late chronic phase of a murine model of PPMS, the Theiler’s murine encephalomyelitis virus induced demyelinating disease (TMEV-IDD).
Results
Vivomixx treatment on the symptomatic chronic phase of TMEV-IDD improves motor function
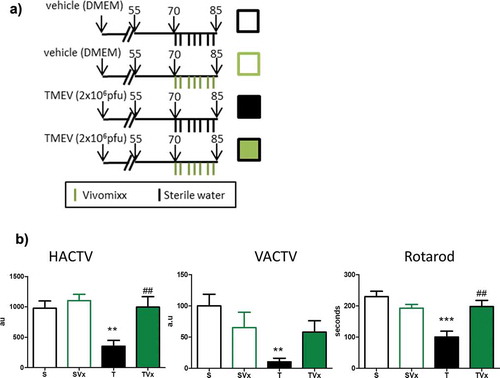
We explored the effect of manipulating the gut microbiota by oral administration of Vivomixx during the chronic phase of disease ()). Consumption of this probiotic improved motor function ()), witnessed through the increase in the horizontal and vertical activity displayed by these mice (HACTV p < .01 vs TMEV-mice), as opposed to the more limited activity of the vehicle treated TMEV-mice (TMEV p < .01 vs sham mice). The reduced latency to fall observed in TMEV-mice on the rotarod performance confirmed the motor improvement induced by Vivomixx supplementation (p < .01 vs TMEV-mice).
Figure 1. Vivomixx-treatment improves the motor disability of TMEV-mice. (a) Scheme of the experimental groups. Mice were administered 3 × 108 cfu Vivomixx by oral gavage three times a week from day 70 pi to 85 dpi. Control mice were administered autoclaved water alone. b) Horizontal (HACTV) and vertical activity (VACTV), as well as motor coordination were evaluated. The data are presented as the means ± SEM: ***p < .001 vs. S; ##p < .01 vs T; n = 10 mice/group studied in two independent experiments.
Effects of oral Vivomixx on gut microbiota and blood content of SCFAs
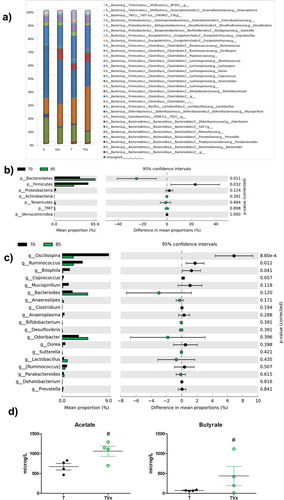
Miseq Illumina analysis of the bacterial population revealed that TMEV-mice showed a decreased relative abundance of Bacteroides, Parabacteroides, Prevotella, Sutterella or Desulfovibrio while an increase of Anaerostipes, Dorea, Erysipelotrichaceae or Coprobacillus were observed ()). Sham mice subjected to oral Vivomixx treatment experiment subtle changes in gut microbiota composition. In the case of Vivomixx-TMEV mice the relative abundance Anaerostipes, Dorea, Oscillospira, Enterobacteraceae or Ruminococcus were decreased while Bacteroides, Odoribacter, Lactobacillus, or Sutterella were increased ()). To give robustness to our data we compare the gut microbiota composition of TMEV-mice before and after Vivomixx treatment, we found an increased relative abundance of Bacteroidetes and a significant decrease in Firmicutes. In addition, the percentage of bacteria belonging to Actinobacteria, Tenericutes and TM7 phyla tend to increase, but not significantly ()). At genus level ()), Vivomixx treatment tend to rebalance microbiota changes increasing the relative abundance of Bacteroides, Odoribacter or Lactobacillus, while Oscillospira, Ruminococcus or Bilophila were significantly reduced. Gut microbiota changes would be related to the increased levels of acetate and butyrate observed in the plasma of TMEV-mice after 15 days of probiotic treatment ()).
Figure 2. Changes in gut microbiota population after Vivomixx treatment. Fecal pellets were collected freshly from each mouse before and after probiotic treatment. A) Stacked bar charts of the relative abundance of main bacterial communities, at genus level, present in the different experimental groups. Extended error bar plots, at phylum (b) and genus level (c), shows the differences in the mean proportions of bacterial taxa in TMEV mice before (black) and after treatment (green). Corrected p-values are shown on the right. D) Quantification of the plasma levels of acetate and butyrate. The data are presented as the means ± SEM: ***p < .001 vs. S; ##p < .01 vs T; n = 5 mice/group.
Oral Vivomixx alters CNS infiltrates in the TMEV-IDD augmenting the frequency of the regulatory B220+CD19+CD5+CD1high B lymphocytes
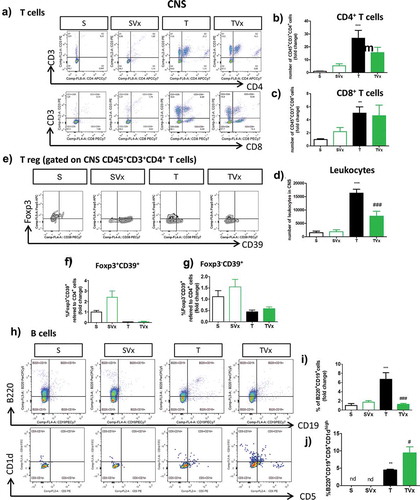
We assessed whether administering Vivomixx altered the T cell subpopulation in the CNS of TMEV-mice ()). A tendency toward a reduction in the proportion of CD4+ T cells was observed ()), with no change in the frequency of CD8+ T cells ()). However, the increase in the number of leukocytes in the CNS of TMEV-infected mice was significantly dampened by probiotic supplementation ()). In addition, probiotic gavage did not significantly modify the proportions of Foxp3+CD39+CD4+ or Foxp3−CD39+CD4+ T cells in the CNS of TMEV-infected mice ()). Conversely, the presence of B cells ()) was strongly influenced by oral Vivomixx administration, which significantly reduced the proportion of B cells (); p < .001 TMEV vs sham; p < .001, TMEV plus Vivomixx vs TMEV). More interestingly, Vivomixx enhanced the presence of the Breg subpopulation B220+CD19+CD5+CD1dhigh cells (); p < .05 vs TMEV).
Figure 3. Effect of Vivomixx treatment on the T and B cell populations in the CNS. A single cell leukocyte suspension was obtained from the brain and spinal cord. A, H) Representative flow cytometry plots of CD45+CD3+CD4+ T cells, CD45+CD3+CD8+ T cells (a), and B220+CD19+ B cells and CD5+CD1dhigh cells gated on B220+CD19+ B cells (h). B-D) Quantification of the change in the number of CD45+CD3+CD4+, CD45+CD3+CD8+ T cells and leukocytes relative to S mice E) Representative flow cytometry plots of CD39Foxp3 cells gated on CD45+CD3+CD4+ T cells. F-G) Percentage of Foxp3+CD39+ and Foxp3−CD39+ T cells gated on CD45+CD3+CD4+ T cells, respectively. The data represent the change relative to the S mice. I) Quantification of the change in the percentage of B220+CD19+ B cells relative to the S mice. J) Percentage of CD5+CD1dhigh cells gated on B220+CD19+ B cells in the CNS: Groups S, T (n = 10); groups SVx, TVx (n = 5); **p < .01 vs S; ***p < .001 vs. S; #p < .05 vs. T; ###p < .001 vs T.
Oral Vivomixx modifies microglial morphology and reduces astrogliosis promoting an anti-inflammatory profile in the spinal cord
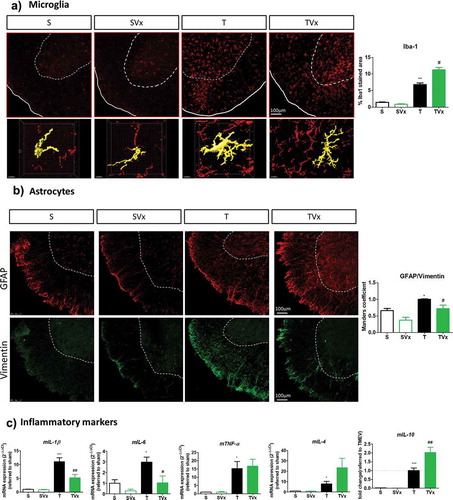
Microglial cells maintain the CNS homeostasis, being activated in MS and its experimental models. As expected, the area of Iba-1 staining is larger in TMEV-mice (p < .01 vs sham mice) and Vivomixx enhanced this effect, further increasing the area of Iba-1 staining (p < .05 vs TMEV: )). Nevertheless, a detailed examination of these cells indicated subtle morphological changes in the spinal cord of mice that received Vivomixx reflecting that microglia exhibited anti-inflammatory activation or attenuated proinflammatory activity ()). In addition, probiotic supplementation was associated with weaker Vimentin-GFAP co-localization in the spinal cord of TMEV-infected mice, whereas TMEV-mice had a higher MoC than sham mice (), p < .05 vs sham mice and p < .05 vs TMEV-mice). Indeed, when we analyzed the expression of pro-inflammatory cytokine genes ()), we found that the elevated expression of IL-1β and IL-6 in TMEV-mice (p < .001 and p < .05 vs sham, respectively) was significantly reduced by Vivomixx (p < .01 and p < .05 vs TMEV-mice, respectively). Conversely, expression of the IL-10 gene was promoted by Vivomixx (p < .01 vs TMEV-mice), while IL-4 expression displayed a tendency toward enhancement. Intriguingly, the elevated TNF-α gene expression observed in TMEV-mice remained unchanged by the probiotic treatment ()).
Figure 4. Vivomixx administration in the chronic phase of TMEV-IDD promotes anti-inflammatory responses in the spinal cord. A) Representative images of transversal spinal cord sections immunostained with Iba-1 to label microglia. Scale bar 100 μm. Below are higher magnification images (63x) of Iba-1 stained cells that indicate the changes in morphology. Scale bar 10 μm. Quantification of the area occupied by Iba-1 staining is also shown on the right. B) Representative images of GFAP (red) and Vimentin (green) staining in the spinal cord together the quantification of Vimentin-GFAP overlap through the Manders coefficient measure. C) qRT-PCR analysis of inflammatory markers (mIL-1β, mIL-6, mTNF-α, mIL-4 or mIL-10) in the spinal cord as assessed using the 2−ΔΔCt method. *p < .05 vs. S; **p < .01 vs. S; ***p < .001 vs. S; #p < .05 vs. T; ##p < .01 vs. T.
Peripheral immunological changes in the symptomatic chronic phase of TMEV-IDD associated with Vivomixx supplementation
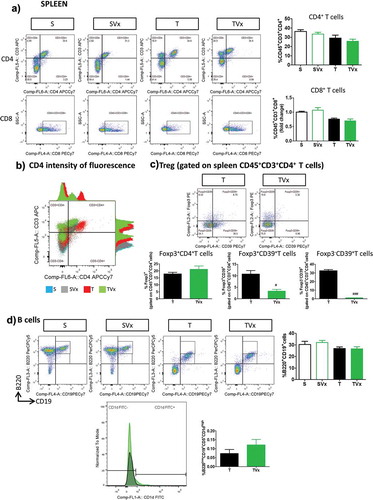
The proportions of CD4+ and CD8+ T cells in the spleen of TMEV-mice were not significantly affected by Vivomixx administration ()), although more intense CD4+ fluorescence was evident in T cells from TMEV-mice and this was dampened when they received the probiotic ()). Although the frequency of Treg cells was similar in TMEV-mice irrespective of their treatment with Vivomixx, the percentage of Foxp3+CD39+ and Foxp3−CD39+ T cells was dramatically reduced in the spleen of TMEV-mice that received Vivomixx (p < .05 and p < .001 vs TMEV, respectively) ()). By contrast, there were no significant changes in the proportion of B cells ()) or in the abundance of Breg cells in the spleen of TMEV-mice receiving Vivomixx.
Figure 5. Subtle changes in the peripheral immunological response after Vivomixx administration to TMEV-mice. A spleen cell suspension was stained to analyze the CD4, CD8, CD39+ Treg and Breg cell populations. a) Representative flow cytometry plots of CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells, along with their corresponding quantification. b) Histogram of fluorescence emitted by CD3+CD4+ spleen cells from representative mice in each experimental group. c) Flow cytometry plots of Foxp3+CD39+ Treg cells gated on CD45+CD3+CD4+ T cells, together with a quantification of the percentage of Foxp3+ T cells (gated on CD4+ T cells), Foxp3−CD39+ and Foxp3+CD39+ Treg cells. d) Representative flow cytometry images and quantification of the B220+CD19+ cells in the spleen, together with a normalized histogram and quantification of CD1d expression in B220+CD19+CD5+ gated cells. Groups. S, T (n = 10); groups SVx, TVx (n = 5); **p < .01 vs S; ***p < .001 vs. S; #p < .05 vs. T; ###p < .001 vs T.
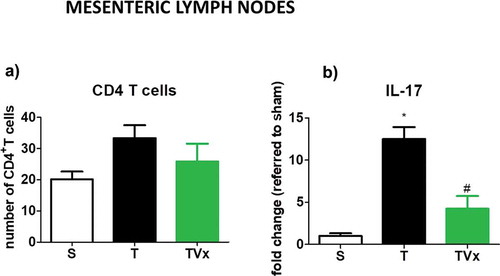
No significant variation was detected in the number of CD4+ T cells in the MLNs under the different conditions studied ()). However, the release of IL-17 by Th17 polarized lymphocytes derived from TMEV-mice was stronger respect to sham mice (p < .05) ()). By contrast, significantly less IL-17 was produced by CD4+ T cells obtained from the MLNs of TMEV-mice that received Vivomixx (p < .05 vs TMEV-mice).
Figure 6. Vivomixx affects the Th17 responses in the MLNs. CD4 T cells isolated from MLN were polarized to Th17 in culture. The expression of IL-17 was measured by ELISA, and the data represent the mean ± SEM of the change relative to the S or T mice from two experiments performed in triplicate: *p < .001 vs S; #p < .05 vs T.
Discussion
In the last decade, evidence has emerged of a relationship between gut microbiota and MS, both in patients and in animal models.Citation4,Citation5,Citation18,Citation19 In addition, there is significant support for the gut microbiota playing a critical role in shaping the immune system.Citation20–22 Several reports described, in EAE models, a therapeutic effect of multi-strain probioticsCitation15 and specific bacterial strains like Bacteroides,Citation9,Citation10 PrevotellaCitation11 or Lactobacillus. However, the involved mechanisms for their benefits seem to be different. For example, while L. paracasei DSM13434 and L. plantarum DSM 15312 increased TGF-β1 in the blood, L. plantarum DSM 15313 enhanced blood levels of IL-27.Citation12 Until date the only probiotic considered a kind of medical food is VivomixxCitation23.Here, we proved the efficacy of Vivomixx treatment in a model of progressive MS whereby oral administration of this probiotic, during the chronic phase improved the motor disability likely associated to changes in gut microbiota.
Differences in the composition of the fecal microbiota at the genus level have been reported in a variety of studies on MS patients.Citation4,Citation5,Citation18,Citation19 In our study TMEV-mice showed changes in gut microbiota population compatible with MS disease like decreases of Prevotella, Bacteroides, Parabacteroides or Coprobacillus relative abundance or an increase of Anaerostipes.Citation18,Citation24,Citation25 Vivomixx treatment tends to decrease Anaerostipes, Dorea, Oscillospira, Enterobacteraceae or Ruminococcus while Bacteroides, Odoribacter, Lactobacillus, or Sutterella were increased. These changes would be associated with the beneficial effects of probiotic treatment. For example, Dorea have been considered to be part of the healthy gut microbiota or patients on disease-modifying treatment show increased abundance of Sutterella and Prevotella.Citation5
In the TMEV-IDD model we found that mice receiving oral Vivomixx had fewer CNS infiltrated leukocytes, particularly B cells, without significant changes in the proportion of CD4+ and CD8+ T cells, Treg cells, or Foxp3+CD39+ T cells. One of the pathways by which the commensal microbiome affects host immunity at distal sites is by regulating the activity of Treg cells through the ectonucleotidase CD39.Citation10 Particularly, CD39+ cells have been associated with the suppression of neuroinflammation by Bacteroides fragilis in the EAE modelCitation26 and its polysacharide A was not able to protect against EAE in CD39-deficient mice.Citation10 Here, TMEV infection decreased CD39 Treg cells population in the CNS, in accordance with our previous resultsCitation27 and previous data from the peripheral blood of MS patients.Citation28 Unlike what we might expect, probiotic treatment did not reverse the decreased CD39+ Tregs suggesting that CD39 activity is not the main pathway involved in the anti-inflammatory effects of Vivomixx. Indeed, our results are consistent with data from MS patients who did not show changes in the frequencies of CD4, CD8 or CD39+T cells following Vivomixx.Citation17
It is known that in EAE, encephalitogenic T cells upregulate their CD4 cell surface expression following antigen recognition and that this was associated with EAE development, for example after transfer to naïve animals.Citation29 Therefore, although oral Vivomixx did not affect the frequency of CD4+ T cells, the fact that this probiotic restricted the surface expression of CD4 on T cells suggest its possible contribution to its beneficial effects in TMEV mice.
Recent reports show that gut microbiota and microbial products can directly regulate B-cell development, activation, and differentiation.Citation30 In addition, the induction of Breg cells in an autoimmune context was seen to depend on a healthy microbiota.Citation31 Notably, in our model of progressive MS, Vivomixx increased the frequency of Breg cells, unveiling a different mechanism of action to that exerted by other probiotics in EAE models where Treg cells have been specifically implicated.Citation26,Citation32 Nonetheless, Bregs would be involved in the beneficial effect of Vivomixx but functional studies are needed to be sure that these cells are working in an effective way. There are many crucial differences between EAE and the TMEV model. First, the TMEV ability to maintain the inflammation into the CNS compartment, with minor effects in the periphery unlike that observed in EAE. On this way, most of studies noticed the induction of IL-10 producing Tregs in the periphery as the main mechanism for EAE protection when mice received many probiotics as LactobacilliCitation12 or Prevotella Histicola.Citation11 The second possibility comes from the different strain of mice used in the models of MS. In EAE models that use SJL/L mice, like we used in the present study, the protective effects against EAE following Lactobacillus helveticus seems to involve decreased IL-6 and reduced Th-17 cells in the spinal cord, without changes in Tregs.Citation14
Preclinical studies suggest that the microbiome plays a critical role in regulating microglial maturation and function.Citation33 Indeed, microglia from TMEV-infected mice administered Vivomixx undergo morphological changes, which could reflect changes in their functional state, in accordance with the emergent hypothesis that microglia may adopt distinct CNS region-specific phenotypes.Citation34 In the spinal cord, the pro-inflammatory environment of TMEV-mice shifted to an anti-inflammatory profile following Vivomixx supplementation.
In an attempt to find a mechanism that may explain the changes in the CNS of TMEV-mice that receive oral Vivomixx we assessed plasma acetate and butyrate. On the whole, it seems that the reduction in bacteria producing SCFAs may be a common characteristic of MS patients.Citation18 A high fiber diet or SCFA supplementation are reported to ameliorate EAECitation35 and, there is growing evidence supporting that circulating metabolites of the microbiota can influence CNS and immune cell activity,Citation33 including microglial function.Citation27,Citation36 One of the most significant findings of the present study was the increased butyrate and acetate levels in the plasma of TMEV-mice that received Vivomixx which may contribute to the altered immune CNS responses and the improved motor activity. Indeed, several reports indicate the beneficial effects of SCFA suppressing inflammation in the EAE model.Citation35,Citation37,Citation38 Furthermore, a recent finding shows that specific SCFAs exert an epigenetic modulation of Breg responses.Citation39,Citation40 Particularly, EAE mice that were subjected to the transference of Breg cells, expanded in vitro in presence of pentanoate, did not develop the classical symptoms. Furthermore, butyrate increased the levels of 5-Hydroxyindole-3-acetic acid, a metabolite of serotonine neurotransmitter which activates the aryl-hydrocarbon receptor (AhR), a newly discovered transcriptional marker for Breg function.Citation41 Here, we speculate that Breg response as well as the reduction of IL17 production by Th17 mesenteric polarized cells may be associated with acetate and butyrate increments following Vivomixx administration to TMEV-mice.
Although, there is no clear evidence of a relationship between SCFA and Bregs at CNS level, astrocytes express AhR that, when activated limit CNS inflammation. In the EAE model the results by Quintana’s groupCitation42 suggest a link between metabolites derived from dietary tryptophan by the commensal gut bacteria and the activation of AhR signaling in astrocytes. It is worth noting that our understanding of the molecular pathways involved in the effects of probiotics and SCFAs on CNS inflammation, in MS and its animal models, is still very limited. This statement also includes microglial cells which are thought to be targeted by SCFAs,Citation36 although their underlying mechanisms remain incomplete too. On this line, more recently, a new pathway in which microbial metabolites may limit the pathogenic activity of microglia and astrocytes has been identified.Citation43 Then, we would like to highlight the reduction of astrogliosis and microgliosis we found in Vivomixx treated-TMEV mice.
An important issue to consider is that MS disease modifying therapies may alter the microbiome directly. As an interesting example, the abundance of Prevotella was increased in MS patients treated with glatiramer acetate taking into account that MS reduced the frequency of these bacteria.Citation44 Furthermore, in a humanized transgenic EAE preclinic model of MS, the combination of human Prevotella histicola and glatiramer acetate did not have a synergistic effect, but Prevotella was as effective as glatiramer in suppressing the disease.Citation11
While dogma dictates that Firmicutes are responsible for butyrate and Bacteroidetes are responsible for acetate and propionate production, there is evidence for alternative pathways.Citation45 There are non-canonical butyrate-producing pathways that use amino acids as primary substrates, including the lysine, 4-aminiobutyrate, pathways. Considering metagenomic analysis of samples from healthy human patients from the Human Microbiome Project a significant overlap between the genes involved in the lysine and 4-aminobutyrate pathways and those expressed by members of the phylum Bacteroidetes was found for butyrate production. Then, it was predicted that these both pathways can contribute to the butyrogenic potential of Bacteroidetes.Citation46 Because members of the Odoribacter genus dominated the lysine and 4-aminobutyrate pathways, our finding about increased Odoribacter abundance would be associated with the increased levels of butyrate in TMEV-mice treated with Vivomixx.
The subtle changes promoted by Vivomixx at spleen level are in accordance with the compartmentalization of inflammation that occurs in progressive MS, characterized by intrinsic CNS processes driven by resident cells like microglia and astrocytes. As such, the main immunological changes in the chronic phase of TMEV-IDD linked to the manipulation of the gut microbiota by oral Vivomixx happen within the CNS. Although probiotics do not modify the host microbiome in a robust and persistent manner, they can influence gut immunity and homeostasis.Citation47 Such effects were evident in the TMEV model through the CNS immune responses, microgliosis and astrogliosis, IL-17 production by ex vivo mesenteric CD4+ T cells, and more importantly, the severity of motor dysfunction.
Regarding probiotics as therapeutic agents, VSL#3 administered to a small number of RRMS patients leads to an anti-inflammatory peripheral immune response.Citation17 It seems clear that both, disease promotion and amelioration may be induced by specific components of the gut microbiota that interact bi-directionally with the host immune system. This concept implies that selective experimental strategies manipulating specific subsets of bacteria or balancing specific gut microbiota may be useful as a therapeutic approach in MS. Our findings may provide a starting point for further mechanistic studies that help to develop adjuvant treatments complementary to the available therapeutic options for progressive MS patients.
Materials and Methods
Animals and TMEV infection
TMEV-infected susceptible female SJL/J mice (Charles River) were maintained under conventional controlled conditions. The right brain hemisphere of 6–8 weeks-old mice was inoculated with 2 × 106 plaque forming units (pfu) of the Daniel (DA) strain of TMEV). Sham control mice received the vehicle alone. Each experimental group was housed in different cages and in different isolated racks in the same room. Each cage contained 5 mice subjected to the same treatment, receiving the same food and sterile water, and manipulated by the same person. All experiments were performed in strict accordance with EU (Directive 2010/63/EU) and Spanish regulations (Royal Decree 53/2013 BOE n° 34 and “Comunidad de Madrid” decree: ES 280790000184). The Ethics Committee on Animal Experimentation at the Instituto Cajal approved all the procedures employed in this study (protocol number: 2013/03 CEEA-IC).
Treatments
TMEV-mice were orally gavaged with 3 × 108 cfu (100 μl) of Vivomixx for 70 to 85 days post infection (dpi) (TVx group; n = 10). Vivomixx, a multi-species probiotic is composed of Lactobacillus paracasei DSM 24734, Lactobacillus plantarum DSM 24730, Lactobacillus acidophilus DSM 24735, Lactobacillus delbruckeii subspecies bulgaricus DSM 24734, Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, Bifidobacterium breve 24732 and Streptococcus thermophilus DSM 24731. Sham or TMEV-mice administered the vehicle (S group, n = 10 or T group, n = 10, respectively) and Sham mice administered Vivomixx (SVx group, n = 5).
Evaluation of motor function
Mice were followed for the progression of demyelinating disease by evaluating spontaneous motor activity on day 70 and 85 pi using an activity cage (Activity Monitor System Omnitech Electronics, Inc.; Colombus, OH, USA) and motor coordination by rotarod test as we previously described.Citation48
Sample collection
Fecal samples freshly obtained from each mouse on day 70 and 85 pi were stored at −80°C. On day 85 pi, spleen and MLN were removed from anesthetized mice (Dolethal; 50 mg/kg body weight) for flow cytometry and ex vivo CD4-T cell studies. Then, anesthetized mice were perfused transcardially with saline to obtain the brain and spinal cord for further FACS analysis.
Metabolite analysis
Blood samples in EDTA-treated BD vacutainer were centrifuged for 10 min at 1500 g in a refrigerated centrifuge within two hours of extraction and aliquots were stored at −20°C. Acetate and butyrate levels were assessed as described previously.Citation27 Validation of the method was carried out usingCitation13C-acetate andCitation13C-butyrate standards. For both analytes the recoveries were above 90% and the precision, in terms of relative standard deviation, was below 10%.
Ex vivo experiments on CD4 T cells
CD4+ T cells were isolated from the MLN using a commercial cell isolation kit and following the manufacturer’s instructions (Miltenyi, Biotec Inc). Cells from each animal were cultured and polarized to Th17 cells in RPMI supplemented with: 2-β Mercaptoethanol (50 μM); immobilized anti-mouse CD3ε; anti-mouse CD28; anti-mouse IFN-γ; anti-mouse IL-4 (all from BD biosciences); hTGF-β and recombinant murine IL-6 (from Peprotech); and recombinant murine IL-23 (BioLegend). The cells were stimulated 3 days later for 5 hours with PMA (100 ng/ml) and ionomycin (500 ng/ml, both from Tocris Bioscience). The supernatants were collected and stored at −20°C for IL-17 measurement by solid phase sandwich ELISA using Quantikine kits (M1700; R&D systems Inc), according to the manufacturer’s instructions. The assay’s sensitivity was 5 pg/ml.
Tissue processing and immunohistochemistry
Anesthetized mice were perfused transcardially with saline and the spinal cord processed as previously described.Citation48 Microglia were labeled with a primary antibody against Iba-1 (ionized calcium binding adaptor molecule 1, 1:1,000: Wako Chemical Pure Industry), while reactive astrogliosis was assessed by GFAP (1:2000: Sigma) and Vimentin (1:1000: Sigma) labeling. Five spinal cord sections were examined per animal and at least three mice per group were used to quantify the immunostained area using ImageJ software (NIH, Bethesda). The white matter area in the ventral and lateral horns was analyzed by immunofluorescence on a Leica TC SP5 confocal microscope. Representative images of the surface of a single microglial cell were obtained with Imaris software. Astroglial activation defined as the amount of Vimentin relative to the GFAP staining (Vimentin-GFAP overlapping) was analyzed using the Manders overlap coefficient (MoC) that quantifies the degree of co-localization of the two fluorophores.
RNA extraction, reverse transcription and RT-PCR
Total RNA from the cervical spinal cord was extracted and then transcribed into cDNA as we previously described.Citation49 Real-time PCR (RT-PCR) was performed with SYBR® and the following oligonucleotide primers mIL-1β, forward 5ʹ-TGG TGT GTG ACG TTC CCA TT-3ʹ, reverse 5ʹ-TCC ATT GAG GTG GAG AGC TTT C-3ʹ; mTNF-α, forward 5ʹ-AGA GGC ACT CCC CCA AAA GA-3ʹ, reverse 5ʹ-CGA TCA CCC CGA AGT TCA GT-3ʹ; mIL-4, forward, 5ʹ-CCACGGATGCGACAAAAATC-3ʹ, reverse 5ʹ-GACGTTTGGCACATCCATCTC-3ʹ; mIL-10, forward 5ʹ-TGA ATT CCC TGG GTG AGA AGC TGA-3ʹ, reverse 5ʹ-TGG CCT TGT AGA CAC CTT GGT CTT-3ʹ; mIL-6, forward 5ʹ-TCC AGA AAC CGC TAT GAA GTT C-3ʹ, reverse 5ʹ-CAC CAG CAT CAG TCC CAA GA-3ʹ; and mRps29 forward 5ʹ-GCC GCG TCT GCT CCA A-3ʹ, reverse 5ʹ-ACA TGT TCA GCC CGT ATT TGC-3ʹ. PCR amplification were initiated incubating at 50°C for 2 min and 95°C for 10 min, and amplification was performed over 40 cycles at 95°C for 15 s and 60°C for 1 min. The cDNA samples were analyzed in triplicate on an Applied Biosystems PRISM 7500 Sequence detection system, normalizing the mRNA expression to the Rps29 gene in each sample and quantifying gene expression by the 2−ΔΔCt method. The results are expressed relative to the Sham mice for each time point.
Flow cytometry antibodies
A CNS or spleen leukocyte suspension was obtained as described previously.Citation49 Freshly isolated cells (106) were incubated with an anti-mouse CD16/CD32 FC (eBioscience) and labeled with anti-mouse antibodies: PE anti-CD25; PE anti-CD3; PerCP-Cy5.5-conjugated anti-CD45; PerCP-Cy5.5-conjugated anti-B220; PE-Cy7-conjugated anti-CD39; PE-Cy7-conjugated anti-CD8a; PE-Cy7-conjugated anti-CD19; APC anti-CD3; APC-Cy7-conjugated anti-CD4; APC-Cy7-conjugated anti-CD11b (all from BD Pharmingen); APC anti-CD3; and PE-Cy7-conjugated anti-CD39 (both from eBioscience). To detect Foxp3, the cells were suspended in the fixation/permeabilization buffer and stained with a PE anti-Foxp3 antibody (BD Pharmingen). Cell viability was assessed with the LIVE/DEAD Fixable Green Dead Cell Stain Kit or LIVE/DEAD Fixable Red Dead Cell Stain Kit (Life Technologies Corporation). At least 10,000 events were acquired in each experiment on a FACSaria flow cytometer (BD Biosciences) and the data were analyzed using FlowJo software (v.10; Tree Star).
16S rRNA analysis of the microbial community
Fecal DNA was isolated using the QIAamp DNA Stool Mini Kit (Qiagen; cat. no. 51504) and following the stool pathogen detection protocol. DNA was extracted following the manufacturer’s instructions with the modifications described previously,Citation27 and the quality of the reads was analyzed with FastQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The microbiome analysis of the paired-end reads was performed using the QIIME2 v2018.8 software (Qualitative Insights into Microbial Ecology)Citation50 and the sequences were grouped as “sequence variants” (SVs), equivalent to the 100% Operational Taxonomic Units (OTUs). Representative sequences from each cluster were queried against the GreenGenes database (version 13_8) to obtain taxonomic assignments at a 97% sequence identity using BLAST. These representative sequences were then used for phylogenetic analysis and for diversity calculations.
Statistical analysis
The data were expressed as the mean ± standard error or the mean (SEM) and they were analyzed using GraphPath Prism5 Software. One-Way ANOVA followed by the Bonferroni post hoc test were used to determine the statistical significance. For non-parametric analyzes, a Kruskall-Wallis and Mann-Whitney U test were applied.
Author contributions
LM and FJC conducted the research, performed the experiments and evaluate the results. AF and MM collaborate in FACs assay; GA realized immunohistochemistry studies; JLLG and HE performed the metabolite analysis; LMV, CE and LCB reviewed the manuscript. LM and CG designed and supervised the study, discussed the experimental setting and wrote the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Laura Ramos and Nieves López de Andradas for their excellent technical support. We are grateful to Dr. Moses Rodríguez (Department of Immunology and Neurology, Mayo Clinic/Foundation, Rochester, MN, USA) for the generous gift of the Theiler’s virus DA strain. We also thank the CBMSO-Genomics & Massive Sequencing service, CBMSO-Cytometry service, the Fundación Parque Científico de Madrid and the Instituto Cajal-Microscopy Unit for their brilliant technical assistance.
Additional information
Funding
References
- Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7:268–15. doi:10.1016/S1474-4422(08)70042-5.
- Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13:25–36. doi:10.1038/nrneurol.2016.187.
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi:10.1038/nature10554.
- Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. doi:10.1038/srep28484.
- Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. doi:10.1038/ncomms12015.
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183:6041–6050. doi:10.4049/jimmunol.0900747.
- Yokote H, Miyake S, Croxford JL, Oki S, Mizusawa H, Yamamura T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol. 2008;173:1714–1723. doi:10.2353/ajpath.2008.080622.
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi:10.1073/pnas.1000082107.
- Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal bacteroides fragilis depends on polysaccharide A expression. J Immunol. 2010;185:4101–4108. doi:10.4049/jimmunol.1001443.
- Wang Y, Begum-Haque S, Telesford KM, Ochoa-Reparaz J, Christy M, Kasper EJ, Kasper DL, Robson SC, Kasper LH. A commensal bacterial product elicits and modulates migratory capacity of CD39+CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes. 2014;5:552–561. doi:10.4161/gmic.29797.
- Shahi SK, Freedman SN, Murra AC, Zarei K, Sompallae R, Gibson-Corley KN, Karandikar NJ, Murray JA, Mangalam AK. Prevotella histicola, a human gut commensal, is as potent as COPAXONE(R) in an animal model of multiple sclerosis. Front Immunol. 2019;10:462. doi:10.3389/fimmu.2019.00462.
- Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Weström B, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010;5(2):e9009. doi:10.1371/journal.pone.0009009.
- He B, Hoang TK, Tian X, Taylor CM, Blanchard E, Luo M, Bhattacharjee MB, Freeborn J, Park S, Couturier J, et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front Immunol. 2019;10:385. doi:10.3389/fimmu.2019.00385.
- Yamashita M, Ukibe K, Matsubara Y, Hosoya T, Sakai F, Kon S, Arima Y, Murakami M, Nakagawa H, Miyazaki T, et al. Lactobacillus helveticus SBT2171 attenuates experimental autoimmune encephalomyelitis in Mice. Front Microbiol. 2017;8:2596. doi:10.3389/fmicb.2017.02596.
- Kwon HK, Kim GC, Kim Y, Hwang W, Jash A, Sahoo A, Kim J-E, Nam JH, Im S-H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin Immunol. 2013;146:217–227. doi:10.1016/j.clim.2013.01.001.
- Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36(5):1245–1249. doi:10.1016/j.clnu.2016.08.015.
- Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, Kivisäkk P, Pierre IV, Hrishikesh L, Gandhi R, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83(6):1147–1161. doi:10.1002/ana.25244.
- Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim S-W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One. 2015;10(9):e0137429. doi:10.1371/journal.pone.0137429.
- Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Curr Treat Options Neurol. 2015;17:344. doi:10.1007/s11940-015-0344-7.
- Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. doi:10.4161/gmic.19320.
- Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi:10.1038/nature18848.
- Colpitts SL, Kasper LH. Influence of the gut microbiome on autoimmunity in the central nervous system. J Immunol. 2017;198:596–604. doi:10.4049/jimmunol.1601438.
- Cheng FS, Pan D, Chang B, Jiang M, Sang LX. Probiotic mixture VSL#3: an overview of basic and clinical studies in chronic diseases. World J Clin Cases. 2020;8:1361–1384. doi:10.12998/wjcc.v8.i8.1361.
- Camara-Lemarroy CR, Metz LM, Yong VW. Focus on the gut-brain axis: multiple sclerosis, the intestinal barrier and the microbiome. World J Gastroenterol. 2018;24:4217–4223. doi:10.3748/wjg.v24.i37.4217.
- Schepici G, Silvestro S, Bramanti P, Mazzon E. The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant. 2019;28:1507–1527. doi:10.1177/0963689719873890.
- Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, Begum-Haque S, Kasper DL, Kasper LH. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39 + Foxp3 + T cells and T reg function. Gut Microbes. 2015;6:234–242. doi:10.1080/19490976.2015.1056973.
- Mestre L, Carrillo-Salinas FJ, Mecha M, Feliu A, Espejo C, Alvarez-Cermeno JC, Villar LM, Guaza C. Manipulation of gut microbiota influences immune responses, axon preservation, and motor disability in a model of progressive multiple sclerosis. Front Immunol. 2019;10:1374. doi:10.3389/fimmu.2019.01374.
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, Mills KHG. CD39 + Foxp3 + regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi:10.4049/jimmunol.0901881.
- Li J, Ridgway W, Fathman CG, Tse HY, Shaw MK. High cell surface expression of CD4 allows distinction of CD4(+)CD25(+) antigen-specific effector T cells from CD4(+)CD25(+) regulatory T cells in murine experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;192:57–67. doi:10.1016/j.jneuroim.2007.09.004.
- Kim M, Kim CH. Regulation of humoral immunity by gut microbial products. Gut Microbes. 2017;8:392–399. doi:10.1080/19490976.2017.1299311.
- Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, Mauri C, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat Med. 2014;20:1334–1339. doi:10.1038/nm.3680.
- Ochoa-Reparaz J, Kasper LH. The influence of gut-derived CD39 regulatory T cells in CNS demyelinating disease. Transl Res. 2017;179:126–138. doi:10.1016/j.trsl.2016.07.016.
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi:10.1038/nn.4476.
- De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, Zhang H-Y, Liu Q-R, Shen H, Xi Z-X, et al. Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron. 2017;95(2):341–56 e6. doi:10.1016/j.neuron.2017.06.020.
- Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee D-H, May C, Wilck N, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43:817–829. doi:10.1016/j.immuni.2015.09.007.
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi:10.1038/nn.4030.
- Mizuno M, Noto D, Kaga N, Chiba A, Miyake S, Ashour HM. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. doi:10.1371/journal.pone.0173032.
- Chitrala KN, Guan H, Singh NP, Busbee B, Gandy A, Mehrpouya-Bahrami P, Ganewatta MS, Tang C, Chatterjee S, Nagarkatti P, et al. CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. Eur J Immunol. 2017;47(7):1188–1199. doi:10.1002/eji.201646792.
- Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10:760. doi:10.1038/s41467-019-08711-2.
- Sanchez HN, Moroney JB, Gan H, Shen T, Im JL, Li T, Taylor JR, Zan H, Casali P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun. 2020;11:60. doi:10.1038/s41467-019-13603-6.
- Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, Alber DG, Krausgruber T, Catalan D, Klein N, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31(4):837–51 e10. doi:10.1016/j.cmet.2020.03.003.
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao -C-C, Patel B, Yan R, Blain M, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–597. doi:10.1038/nm.4106.
- Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi:10.1038/s41586-018-0119-x.
- Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, Choung RS, Ju J, Sompallae R, Gibson-Corley K, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20:1269–1277. doi:10.1016/j.celrep.2017.07.031.
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi:10.1079/PNS2002207.
- Vital M, Howe AC, Tiedje JM, Moran MA. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5(2):e00889. doi:10.1128/mBio.00889-14.
- van Baarlen P, Wells JM, Kleerebezem M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013;34:208–215. doi:10.1016/j.it.2013.01.005.
- Mestre L, Correa F, Arevalo-Martin A, Molina-Holgado E, Valenti M, Ortar G, Marzo V, Guaza C. Pharmacological modulation of the endocannabinoid system in a viral model of multiple sclerosis. J Neurochem. 2005;92:1327–1339. doi:10.1111/j.1471-4159.2004.02979.x.
- Carrillo-Salinas FJ, Mestre L, Mecha M, Feliu A, Del Campo R, Villarrubia N, Espejo C, Montalbán X, Álvarez-Cermeño JC, Villar LM. Gut dysbiosis and neuroimmune responses to brain infection with Theiler’s murine encephalomyelitis virus. Sci Rep. 2017;7:44377. doi:10.1038/srep44377.
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi:10.1038/nmeth.f.303.

