ABSTRACT
Human milk oligosaccharides (HMOs) are the third most important solid component in human milk and act in tandem with other bioactive components. Individual HMO levels and distribution vary greatly between mothers by multiple variables, such as secretor status, race, geographic region, environmental conditions, season, maternal diet, and weight, gestational age and mode of delivery. HMOs improve the gastrointestinal barrier and also promote a bifidobacterium-rich gut microbiome, which protects against infection, strengthens the epithelial barrier, and creates immunomodulatory metabolites. HMOs fulfil a variety of physiologic functions including potential support to the immune system, brain development, and cognitive function. Supplementing infant formula with HMOs is safe and promotes a healthy development of the infant revealing benefits for microbiota composition and infection prevention. Because of limited data comparing the effect of non-human oligosaccharides to HMOs, it is not known if HMOs offer an additional clinical benefit over non-human oligosaccharides. Better knowledge of the factors influencing HMO composition and their functions will help to understand their short- and long-term benefits.
Introduction
The World Health Organization (WHO) and pediatric societies recommend breastfeeding within the first hour of life and to breastfeed exclusively for the first six months, continuing for up to two yearsCitation1–3. Human milk is the only recommended source of nutrition for newborns because of its unique composition and the fact that it is naturally occurring and ideally suited to support crucial developmental processes in infancy. In addition, to providing essential nutrients, human milk also contains a plethora of bioactive components that promote healthy growth and development and help to preserve a healthy microbiota and the infant’s immune system.Citation4–6. There are numerous health benefits associated with breastfeeding and human milk, both for mothers (lower risks of breast and ovarian cancer, hypertension, and type 2 diabetes) and their newborns (short- and long term). Short-term benefits include fewer cases of diarrhea, pneumonia, otitis media, atopic dermatitis, and sudden infant death syndrome; long-term benefits include fewer cases of type 2 diabetes, leukemia, autistic spectrum disorders, and obesity; and beneficial effects on IQ and social behaviorCitation5,Citation7–13.
The difference between non-breastfed and breastfed infants in morbidity and mortality was hypothesized to be related to the composition of human milk. The relationship between breastfeeding and infant’s health is based on its nutritional and bioactive components including human milk oligosaccharides (HMOs)Citation4,Citation14,Citation15. In the early 1900s, Moro and Tissier independently found a predominance of bifidobacteria in the stools of breastfed compared to non-breastfed infantsCitation16. It was discovered that the oligosaccharides present in human milk did stimulate the growth of bifidobacteria, and in the 1950s the first clear description of the structure of the most abundant HMOs were unraveledCitation17–19.
HMOs provide a variety of physiologic functions, including the establishment of a balanced infant’s gut microbiota, the strengthening of the gastrointestinal barrier, prevention of infections, and potential support to the immune system, brain, and cognitive developmentCitation4–6,Citation14,Citation15. This review aims to summarize up-to-date information about the functional effects of HMOs, such as supporting the development of a healthy gastro-intestinal microbiome, inhibiting the adhesion of pathogens, promoting the development of a balanced the immune system, and their contribution to brain development and cognitive function.
Method
We searched for relevant studies published in the English language in PubMed, EmBase, Scopus between 2000 and August 2022. We used search terms: “human milk oligosaccharide” AND “breast feeding”, OR “breastfed”, OR “human milk”, OR “formula”, OR “infant formula” and OR “nutrition”. We researched the relevant literature and summarized the most up-to-date information about the functional effects of HMOs, as well as, evaluated preclinical, observational, and randomized controlled clinical trials with HMO-containing infant formulas.
Human Milk Oligosaccharides (HMOs): composition and related factors
Human milk contains numerous structurally different oligosaccharides, indigestible carbohydrates for humans. Human milk contains much more oligosaccharides than the milk of any animal. Human milk oligosaccharides (HMOs) are the third most important solid component in human milk after lactose and lipids, while having a minimal nutritional value for the infantCitation4–6,Citation20,Citation21. Over 200 structurally different HMOs have currently been identifiedCitation20,Citation22. HMOs withstand both heat and cold, and remain therefore unaffected by pasteurization and freeze-dryingCitation23. HMOs are resistant to pancreatic and brush border enzymes, as well as to the low stomach pH. The majority of HMOs are either metabolized by the infant’s gut microbiota or excreted intact. Approximately, 1 to 2% of the ingested HMOs are absorbed, get into the systemic circulation, and are eliminated via urineCitation14.
HMOs are multifunctional, unconjugated, and non-digestible glycans. HMOs are build out of five monosaccharide components: galactose, glucose, fucose, N-acetylglucosamine, and the sialic acid derivative N-acetyl-neuraminic acidCitation14,Citation15,Citation24. Abbreviations of common HMOs were shown in .
Table 1. Abbreviation of HMOs.
Three major HMO categories are present in human milk of secretor mothersCitation6,Citation14,Citation15,Citation25,Citation26
Neutral fucosylated HMOs (35–50%; e.g., 2′-FL and DFL)
Acidic sialylated HMOs (12–14%) e.g., 3′-SL and 6′-SL
Neutral non-fucosylated HMOs (42–55%, e.g., LNnT, LNT).
The levels and distribution of HMOs vary widely from woman to woman but also for a single woman according to the duration of lactation and many other variables (such as regional, seasonal etc.)Citation5,Citation27,Citation28. Conze et alCitation27 performed a weighted analysis of 2′-FL, 3′- FL, LNT, 3′-SL, and 6′-SL concentrations in human milk from previously published reports and reported the following median (± standard deviation) levels: for 2’-FL: 2.56 ± 0.054 (IQR 1.14–3.89 g/L), for 3’-FL a median of 0.32 ± 0.045 (IQR 0.057–1.1 g/L), for LNT 0.82 ± 0.0057 (IQR 0.35–1.5 g/L), for 3’-SL 0.23 ± 0.0018 (IQR 0.10–0.42 g/L) and for 6’-SL 0.33 ± 0.003 (IQR 0.09–0.54 g/L)Citation27.
HMOs range in concentration from 20 to 25 g/L (average 9–22 g/L) in colostrum to 10–15 g/L (average 8–19 g/L) in mature milk, and 4–6 g/L after 6 monthsCitation15,Citation22,Citation29–34. About 10 grams of HMOs are consumed daily by a term infant ingesting 800 milliliters of human milkCitation26.
Individual HMO concentrations vary by secretor status and Lewis blood-type status, race, geographic region, ethnicity, environmental conditions, season, maternal diet, physiological status, parity, gestational age, and mode of deliveryCitation4,Citation5,Citation14,Citation15,Citation28,Citation32,Citation33,Citation35–41. In secretor women (account for 70–80% of all women), 2′-FL is the most prevalent HMO, and persists at around 1 g/L after one yearCitation35,Citation36. Most HMO concentrations decrease over the course of lactation. However, some HMOs, including 3’-SL, 3’-FL, and DSLNT increase in concentration throughout the first months of breastfeeding and even beyond one year of lactationCitation4,Citation30,Citation33,Citation37. Recent research by Plows and colleaguesCitation33 examined HMO levels over two-years and confirmed that the majority of HMO concentrations decrease significantly over the course of lactation among Hispanic mothers in the United States, with the exception of 2’-FL, LSTb, and DSLNT, which showed no change, as well as a 10-fold increase of 3’-FL, and a 2-fold increase of 3’-SL from the first month to the 24th month of lactation. Although it is not known if these variations in HMO-levels have a clinical impact, the stability or growth of certain HMOs during lactation suggests that they may have crucial biological activitiesCitation33.
Maternal secretor and Lewis blood-type status affect HMO fucosylation. Le gene encodes Lewis blood group antigens (FUT3 gene) and generates fucosylated HMOs in mammary glands. Se is another HMOs-related geneCitation5,Citation42. Se and Le genes encode mammary gland enzymes FUT2 and FUT3 involved in fucosylated HMO production. Se and Le genes encode FUT2 and FUT3, which classify lactating mothers into four typesCitation14. Lactating mothers who express active FUT2 are called “secretors,” and their milk is rich in 2′-FL and LNFP I. Non-secretors are lactating mothers who do not express active FUT2. Their milk contains few or no 1–2 fucosylated HMOs, including 2′-FLCitation14. Variations in FUT2 negative genotypes contribute to geographic variances in HMO profilesCitation43. Secretor mothers have greater mean total HMO concentrations than non-secretor mothers, and most HMOs differ by secretor status, but not DSLNTCitation37. Lactating mothers that express FUT3 are Lewis-positive, and their milk contains 3’-FL and LNFP II. Lewis-negative mothers don’t produce FUT3. Non-secretor mothers’ milk has more neutral, non-fucosylated HMOs due to a lack of FUT2Citation40. Cheema et al.Citation28 found that human milk samples were dominated by five HMOs: 2′-FL, 3’-FL, LNT, DFLNT, and LNFP II. The secretor mothers exhibited larger amounts of 2′-FL, DFLac, LNnT, LNFP I, DFLNT, and LSTc, whereas non-secretors had higher concentrations of 3FL, LNFPII, LNT, LSTb, DFLNH, and FDSLNHCitation28. Se and Le gene mutations alter FUT2 and FUT3 enzyme production, modifying the HMO structureCitation14.
The most important variations within HMO distribution are the amount of fucosylated HMOs, which are prominent in secretor individualsCitation44. Although the genetic profile of the mother was found to have a significant effect on the HMO composition in the mother’s breast milk, particularly fucosylated HMOs, the stage of lactation is a major determinant of the HMO quantity, and epigenetics may also have a significant effect on the HMOs’ expressionCitation4,Citation20,Citation30.
HMO concentrations and profiles vary geographically. Among healthy breastfeeding women of 11 different nationalities, McGuire et al.Citation43 found that the concentration of 3’-FL was at least four times higher in milk collected in Sweden than in milk collected in rural Gambia, while the concentration of DSLNT was about four times lower in Sweden than in rural Gambia. Furthermore, in Gambia, lactating mothers produce considerably less HMOs (LNnT) during the wet than during the dry seasonCitation45.
Additional maternal and environmental variables contribute to HMO variability, although their impact may be modestCitation16. It was reported that after a cesarean section, human milk had lower levels of 3′-SL, 2′-FL, and 6′-GL than after vaginal deliveryCitation32. Parity affects as well the concentration of HMOsCitation28. While parity was found to be negatively associated with LNFP III in non-secretor mothers, it was found to be positively associated with LNFP II and FDSLNH in both secretor and non-secretor mothersCitation28. It is likely that parity affects HMO content due to the correlation between maternal body mass index (BMI) and human milk fatty acid composition as well as fat and protein concentration, which increases with each additional deliveryCitation28. Regarding the effects of prematurity on HMOs, higher levels of 3′-SL, 6′-SL, LNT, and LNDFH-I were detected in maternal milk after preterm than after term delivery. At the same time, the proportions of 3′-SL and 6′-SL also differed considerably according to the milk maturation stageCitation46–48. FUT2-dependent HMOs like 2’-FL and LNFP I are slightly lower in early milk of mothers who delivered pretermCitation28. But again, as stated before, it is not known if these variations in HMO concentration do have a major clinical impact.
Maternal adiposity has been reported to be positively, negatively or not related to the amount of individual and/or total HMO concentrations. Maternal body composition was shown to be related to human milk microbiota, HMO composition, and newborn body compositionCitation28. Maternal obesity was associated with lower concentrations of several fucosylated and sialylated HMOs. Infants born to obese mothers had reduced intakes of numerous fucosylated and sialylated HMOs, and obesity in mothers was associated with lower concentrations of these HMOsCitation49. Milk from mothers who were overweight before pregnancy had higher concentrations of LNT and LNnT than milk from mothers who had a normal weightCitation50. Only among secretor mothers has pre-pregnancy BMI been found to have a positive correlation with both 2′-FL and DFLacCitation51. Depending on maternal secretor status, correlations between maternal weight, BMI, and body composition measurements and 2′-FL and LNH concentrations variedCitation28. Adiposity measurements were positively associated with 2′-FL and FLNH concentrations in secretor and non-secretor mothers, and with 3′-SL concentrations in non-secretorsCitation28. McGuire et al.Citation43 also showed a positive correlation between maternal weight and 2′-FL and BMI, but not LNH, FLNH, and FLNH. They also discovered a positive correlation between weight and LNFP III and DFLNT, and a negative correlation between weight and BMI and LNnT and DSLNT. Selma-Royo et al.Citation52 found no connection between maternal BMI and either individual HMO profiles or clusters of HMOs. Secretor mothers have a greater dietary effect on HMO profiles than non-secretor mothers. Dietary fibers, polyphenols, and several insoluble polysaccharides, pectin, and MUFA are associated with the secretor HMO profiles. However, Plows et al.Citation33 found that increases in HMOs over the course of 24 months of lactation were unaffected by maternal age, BMI or socioeconomic level. In Norwegian mothers, no difference in HMO composition was reported between vegan, vegetarian, and non-vegetarian mothersCitation53.
HMO composition is influenced by many variables, including genetic background, environment, dietary intake, and many other factors. However, except for secretor versus non-secretor mothers, there is little evidence that these changes are of clinical impact.
HMOs and anthropometry
There is limited information about how HMOs affect infant body composition. Total HMO intake is not related with growth and adiposity, although some specific HMOs are related with infant growth in the first six months. The difference in weight between breastfed newborns of secretor and non-secretor women may be explained in part by the fact that several HMOs are both positively and adversely linked with baby food responsivenessCitation4,Citation28,Citation48,Citation54. A narrative review reported that several observational studies have investigated if a link could be found between HMOs and infant growth in term-born breastfed infantsCitation4. Only few relationships were consistently reported across studiesCitation4. FLNH, LNnT, and LNFP III were negatively associated with infant anthropometric measurements and body composition, while DFLNH was positively associatedCitation4.
Cheema et alCitation28 demonstrated that anthropometrics, fat-free mass, and adiposity are all strongly linked with HMO intake, with correlations modulated by secretor status. Certain HMOs, such DFLNH and LNnT, appear to serve a protective role by controlling fat formation, perhaps protecting newborns from later-life obesityCitation28. Regardless of maternal secretor status, child body composition was positively associated with 2′-FL, 3-FL, DFLac, DFLNH, DFLNT, and LSTb intakesCitation28.
In infants of non-secretor mothers, DFLNT concentrations were positively- and FLNH, 6′-SL, and FDSLNH were negatively associated with infant anthropometric measurements and body compositionCitation28.
In infants born from secretor mothers, 3′-SL intake was linked to weight, length, fat-free mass, and weight for ageCitation28. 3‘SL was the only HMO linked with greater weight for length increases in the first four months of lactation in a recent European multicenter study of 370 mother-infant dyadsCitation55. Still in secretor mothers, HMO composition at three months after birth was linked to weight and height during the first five years of lifeCitation51,Citation56. An inverse relationship between HMO diversity and LNnT concentration and a direct relationship between 2′-FL concentration and z-scores was reported for children’s height and weight z-scoresCitation51. However, other studies reported different: a negative correlation between LNnT and food responsiveness in the first month of life, but DFLNT and DSLNT showed this correlation solely among secretorsCitation54. Positive associations were seen between DSLNH, FLNH, LNH, LSTc, and food responsiveness at 6 months in both the overall population and in secretors exclusivelyCitation54.
In a Gambian study, researchers found that different HMOs, and 3’-SL in particular, affected infants’ weight-for-age z scores, whereas relative sialylation of HMOs did notCitation45. Infants receiving higher total HMO concentrations had higher percentages of fat-free mass and a lower fat-to-fat-free mass ratio and fat-free mass-to-fat-mass ratiosCitation57. Alderete et al.Citation58 showed that lower infant weight at one and six months, as well as reduced lean and fat mass at six months, were associated with higher levels of LNFP1 and a positive correlation was observed with greater fat mass and LNFP-II and DSLNT.
In 2016, two cohorts of mothers in Malawi, one in healthy 6-month-old, and another in severely stunted infants, HMO compositions were studiedCitation59. Breast milk of undernourished infants has lower levels of HMOs than the milk that healthy babies receivedCitation59. Among secreting mothers, there was no difference in HMO concentrations between those infants who were healthy or stunted. But milk from non-secretor mothers of stunted infants had lower levels of fucosylated and sialylated HMOs than infants with normal growthCitation60. These results suggest that milk of non-secretor mothers would be less conducive to child growth due to an inefficient compensation for a lack of fucosylated HMOsCitation48,Citation60.
According to data from Bangladesh, there is an increased likelihood of severe acute malnutrition for every unit increase in the relative abundance of sialylated HMOsCitation61. Fifty-four percent of the infants with severe acute malnutrition and 58% of the infants who were not malnourished were born to women who were secretors. Fucosylated or undecorated HMOs were not shown to be significantly linked to severe acute malnutrition. This suggests that human milk with a higher relative abundance of sialylated HMOs might have a detrimental effect on the nutritional health of children under the age ofCitation61.
Two hypotheses may be related to the plausibility of HMOs on anthropometric measurements: i) certain HMO-microbiota pairs may affect infant anthropometry, and ii) HMOs affect food-responsiveness and appetite via a microbiome-driven process that affects the entero-endocrine system or the central nervous systemCitation4. The developing gut microbiome is regarded as a crucial determinant determining infant growth, along with the environment, genes, epigenetics, and metabolismCitation62. Sprenger et al.Citation4 hypothesize that differences in maternal nutritional status and in the composition of the mother’s gut microbiota (including epigenetic and genetic changes) may be significant confounding variables. Randomized controlled trials (RCTs) and mechanistic studies are needed to show if the inclusion of specific HMOs could aid to promote growth in specific circumstances of faltering growth or in preterm-born infants.
HMOs and microbiota
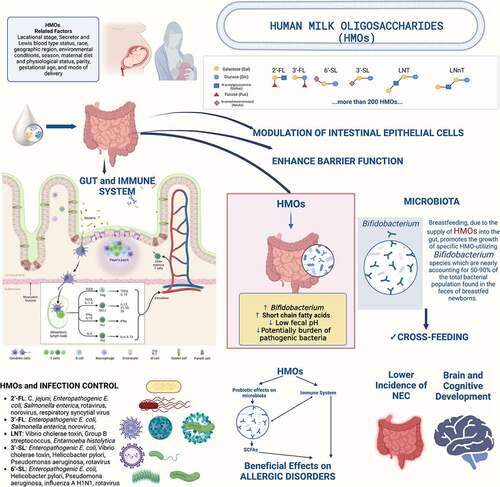
Gut microbiota composition is established in early life and is influenced by many variables, such as delivery mode, gestational age, maternal, and infant/toddler nutrition, antibiotic use, presence of siblings, local environment, geographic location, and host genetics has short- and long-lasting effects on healthCitation63. The content of HMOs in mother’s milk is one of the variables determining the composition of the gut microbiota in the infantCitation63. Infant microbiota is characterized primarily by low diversity and high variability, even more than in adultsCitation64. Breastfed infants have a significantly different microbiota and metabolome compared to formula-fed onesCitation65. Bifidobacteria are among the first colonizers of the infant gut and sustaining this abundance of Bifidobacteria is crucial to preserving the gut microbiota composition. Several studies have shown that HMOs influence the gut microbiota composition via bifidogenic and anti-pathogenic effects and by potentially interacting with the gut epithelium to alter the physical interactions between microbes and their hostsCitation66. Breastfeeding, due to the supply of HMOs into the gut, promotes the growth of specific HMO-utilizing Bifidobacterium species which are nearly accounting for 50–90% of the total bacterial population found in the feces of breastfed newbornsCitation67. In the first 1000 days of life, the gut microbiota of healthy breastfed infants is typically dominated by ‘infant-type’ bifidobacteria, including Bifidobacterium longum subsp. Infantis, B. bifidum, B. breve, and B. longum subsp. longumCitation68. Some members of the Bifidobacterium genus can metabolize HMOs, but not all of them can, and not all HMOs cause the same changes in the composition and/or activity of the gut microbiota and have the same effects on host well-being and health. B. longum subsp. Infantis is the most effective consumer of HMOs, and B. bifidum and B. breve can also partially consume HMOsCitation20. Bifidobacterium bifidum and B. longum subsp. infantis, two avid HMO consumers, dominate through inhibitory effects in which the early arriving species apparently depletes resources for later arriving speciesCitation69. Bifidobacterium longum would be a moderate competitor, as it cannot consume LNnT, but can consume LNT and specific fucosylated sugars such as 2′-FL, 3-FL, LDFT, and LNFP I. Bifidobacterium breve, a species with limited HMO-utilization ability, limited to LNT and LNnT, can benefit from facilitative priority effects and dominates by utilizing fucose, an HMO degradant not utilized by the other bifidobacterial species like B. bifidum and B. infantisCitation69. Several Bacteroides species are known to utilize HMOs as well. Bacteroides have been reported to dominate in the absence of bifidobacteria, and mutual exclusion may be occurring through the depletion of HMOsCitation68. Bacteroides thetaiotamicron, found in a healthy mature gut, provides metabolic and immune support and is an effective HMO degraderCitation70.
The diversity of bifidobacteria in is closely correlated with whether or not the mother is a secretor for the enzyme FUT2Citation71. Observational studies showed that secretor milk status (due to its high levels of 2′-FL and other Fucosyl-HMOs) are associated with bifidobacteria dominated early gut microbiota in breastfed infantsCitation43,Citation72,Citation73. Stool from infants with a microbiome harboring this 2‘FL utilizing capacity has been shown to have a lower pH and provides better protection against specific diarrheal diseasesCitation73. Bifidobacteria isolated from the stool of secretor breast milk-fed infants were able to utilize 2′-FL as the sole carbon source, indicating a more pronounced bifidobacterial metabolic activity targeting fucosylated HMOsCitation4,Citation74. Conversely, the gut microbiota of infants born to non-secretor mothers is depleted of bifidobacteria because to the absence of 2′-FL in human milk, which may result in a diminished level of biological defense against infectionsCitation34. In contrast, bifidobacteria colonization is slowed by non-secretor human milk, while Clostridium and Enterobacteriaceae are encouragedCitation73.
When HMOs are fermented by bacteria, SCFAs are produced, creating a low-pH environment in the colon that encourages the growth of beneficial bacteria and inhibits pathogensCitation20,Citation75. These SCFAs have multiple beneficial physiological effects, such as acting as anti-inflammatory agents, serving as energy substrates for intestinal epithelial cells, and promoting gastrointestinal motilityCitation6,Citation76. Cross-feeding (when one kind of bacterium’s metabolic byproducts are used as a food source by another type of bacterium in the environment) is encouraged by the presence of HMOsCitation77,Citation78. The bifidobacterial population in the infant’s gut is composed of a co-group of multiple Bifidobacterium strains, rather than one strain dominating, and competing to the exclusion of all others. On the one hand, the cross-feeding effect among bifidobacterial species/strains is associated with the ability to thrive in HMOs of multiple Bifidobacterium members in the infant’s gut. Fermentation products of HMO-degrading infant-type Bifidobacterium species may suppress other gut microbes and opportunistic pathogens that do not use HMOs. This competitive advantage in the HMO use of the developing gastrointestinal tract greatly affects the survival and persistence of beneficial Bifidobacterium species and lessens the burden of potentially harmful or pathogenic bacteriaCitation6,Citation68. On the other hand, certain bifidobacterial taxa cooperate with non-bifidobacterial taxa (including HMO consumers and non-HMO consumers) to maximize the nutrient consumption of HMOs, thus contributing to increased bifidobacterial diversity and dominance-gainingCitation68. Schwab et al.Citation79 showed that Eubacterium hallii consumes the fermentation products of HMO by bifidobacteria and generates butyrate and propionate. The cooperation of the bacterial community in the neonatal intestine to maximize the utilization of HMOs, so as to maintain the intestinal immune balance of newborns. Overall, infant-type Bifidobacterium species are well adapted to the infant gut and efficiently consume HMOs, and their presence influences both immediate and long-term health outcomesCitation68,Citation80. Since HMO composition differs between mothers, it’s reasonable to assume that each mother’s milk has a unique effect on her infant’s gut microbiota.
In addition to the widespread indirect effects resulting from microbial fermentation of HMOs, recent research has described the direct benefits of HMOs on gut healthCitation6. 3´-FL stimulated production of mucin and antimicrobial peptides in goblet cells, and 2′-FL may have a similar effect on goblet cell function when inflammatory stressors are also presentCitation81. Natividad et al.Citation82 used in vitro models that replicate the microbial ecology and the intestinal epithelium to evaluate the impact of lactose, 2’-FL, 2’-FL + LNnT, and a mixture of six HMOs (2’-FL, LNnT, DFLac, LNT, 3’-SL, and 6’−SL) on newborn gut microbiota and intestinal barrier integrity. Although the SCFA levels were higher and bifidogenic potential was present in all the products examined, only the fermented medium from the HMOs provided protection against inflammatory gut barrier disruption. The most butyrate-producing bacteria were enriched by the six HMOs formulation, whereas 2’-FL/LNnT and six HMOs promoted the greatest diversity within the Bifidobactericeae familyCitation82.
Since the intestinal epithelial glycocalyx is crucial for microbial colonization, Kong et al.Citation83 conducted the first study to examine the development of this barrier in relation to HMOs. They found that 2′-FL and 3-FL stimulate glycocalyx formation and have a direct effect on the growth of epithelial cell lines. HMOs have been proven to directly modulate goblet cells, causing them to produce more mucus, another important component of the intestinal barrier systemCitation81.
There is a limited information on the complicated relationships between the human milk microbiome and different types of HMOsCitation5,Citation28. Although the potential biological influence on the newborn is still unclear, there is an association between maternal secretor status and HMOs with human milk microbiotaCitation71. Maternal factors including body composition are related to human milk microbiota and HMO composition. Individual HMO concentrations may influence human milk bacterial profiles during the exclusive breastfeeding period. Total HMOs and 2′-FL were positively associated with the relative amount of Staphylococcus, whereas 3′-SL was negatively correlated with the proportions of Ralstonia and Novosphingobium in 16 human milk samplesCitation84. Staphylococcus epidermidis, Streptococcus salivarius, Cutibacterium acnes, Gemella haemolysans, and Veillonella nakazawae all had correlations (positive and negative) with HMO concentrationsCitation28. In colostrum, a higher total HMO concentration is associated with higher counts of Bifidobacteria. Sialylated HMOs were positively correlated with B. breve, and non-fucosylated/non-sialylated HMOs were positively correlated with B. longum. There were also favorable associations found between fucosylated HMOs and Akkermansia muciniphila and between fucosylated/sialylated HMOs and Staphylococcus aureusCitation85. Only in non-secretor mothers, several HMOs were correlated negatively with Streptococcus parasanguis, Gemella haemolysans, and Cutibacterium acnes. Among the secretor mothers, 3′-SL was negatively associated with Staphylococcus epidermidis. Moossavi et al.Citation86 found that 3′-SL, 6′-SL, LSTb, LSTc, DSLNT, and DSLNH all have positive relationships with Staphylococcus spp.
HMOs are the third most important component of human milk and are crucial for the development of a healthy early life gut microbiome. As a result, it is evident that HMOs encourage the growth of a bifidobacteria-rich gut microbiome.
HMOs and necrotizing enterocolitis (NEC)
In preterm newborns, breastfeeding has been linked to a lower incidence of NEC compared to formula feedingCitation4,Citation87,Citation88. In a murine model of NEC, HMOs raise mucin levels and lower bacterial attachmentCitation89. FUT-2 non-secretor and low secretor status in premature newborns is associated with a higher risk for NEC, gram-negative sepsis, and deathCitation90. HMO diversity and specifically DSLNT were shown in observational studies to be associated with NECCitation4,Citation87,Citation91–93. Although explanations for the association between DSLNT and NEC remain elusive, an age-appropriate microbiome progression was suggestedCitation91. DSLNT was shown to increase survival rate and reduce pathology scores in a rat model of NECCitation94. More studies are needed to understand the link between DSLNT and NEC risk.
Protective effects against (severity of) NEC were observed for 6’−SL and 2’-FL in experimental modelsCitation87,Citation94–96. Both 2’−FL and 6’-SL suppress toll like receptor-4 activation, which is linked to the onset of NEC, and hence decrease inflammation in mouse and piglet models of NECCitation95. However, clinical observations could not confirm a relation between 2′-FL or 6’−SL with NEC risk.
HMO and infections
In, the amount of HMOs is associated with a decreased prevalence of diarrhea, overall infections, and morbidityCitation97–100. FUT2 alleles are associated with a higher risk of infant gastrointestinal and respiratory illnessesCitation101. At the age of 2 years, diarrhea due to stable toxin-Escherichia coli infection and of unknown etiology were both reduced in breastfed infants with high levels of alpha 1,2-linked fucosylated-HMOsCitation102. Higher levels of LNFP-II in colostrum were associated with reduced respiratory and gastrointestinal infections by 6 and 12 weeksCitation98. Torres-Roldan et al.Citation110 investigated the HMOs’ composition and infection rates in very-low-birth-weight infants, FDSLNH was found to protect for late-onset neonatal sepsisCitation103.
In breastfed newborns in Mexico, the incidence of Campylobacter diarrhea was decreased in infants whose mothers’ milk had a high percentage of 2′-FLCitation97. The protection offered by HMOs was limited to the duration of breastfeedingCitation105. Furthermore, high levels of LNDFH-I, another 2-linked fucosyloligaosaccharide, protect against calicivirus diarrhea including norovirusCitation97. Population studies show significantly higher levels of LNnT, 2’−FL and 6’-SL in milk of mothers of rotavirus-positive neonates with gastrointestinal symptomsCitation104. However, it is unknown whether high levels of these HMOs are a natural reaction to the rotavirus infection or whether they provide poorer protection against a rotavirus infection than lower levelsCitation104. Secretor-positive human milk inhibits norovirus particles, while secretor-negative milk does not, suggesting that alpha 1,2 linked fucosylated-HMOs may be implicatedCitation105. Both 3-FL and 2’-FL have been found to bind norovirusCitation33.
Higher concentrations of LNF-II in human milk at two weeks postpartum were associated with fewer respiratory problems in infants by 6 and 12 weeks of ageCitation106. Mother’s milk of sick infants contains more of certain HMOs (LNT) than healthy infants, while other HMOs (LNFP1) are less frequent in sick infantsCitation45. However, the levels of HMOs could not be related to physician reported data on infections (otitis media, upper and lower respiratory tract infections)Citation107.
HIV-infected women have larger relative abundances of 3’-SL in their milk than HIV-negative mothersCitation108. HIV-infected women with total HMOs above the median (1.87 g/L) are less likely to transmit HIV via breastfeeding, although there was no difference related to secretor or Lewis statusCitation109. A higher LNnT concentration correlated with reduced transmission. Independent of other known risk factors, higher concentrations of non-3’-SL HMOs were associated with decreased likelihood of postnatal HIV transmission. In Zambian children, breastfeeding was protective against mortality only in uninfected children with high concentrations of fucosylated HMOsCitation110. Higher amounts of 2’-FL and LNFP I, as well as 3-FL and LNFP II/III, were substantially associated with a decreased mortality in children who were not HIV-infectedCitation110. Breastfeeding was found to reduce mortality risk for HIV-infected children, but no consistent relationships were found between HMOs and mortalityCitation110.
Some potential modes of action for HMOs include weakening, preventing, and deviating pathogens from adhering to their cognate cell surface ligandsCitation6. Several viruses and bacteria have been found to bind to HMOsCitation4. Many infectious agents, including viruses (including influenza virus, respiratory syncytial virus, coronaviruses, rotavirus, HIV, and norovirus), bacteria (including Streptococcus pneumoniae, Haemophilus influenza, Group B streptococci (GBS)), and protozoan parasites, require adhesion to the surface of epithelial cells in order to replicate and, in some cases, infiltrate and cause diseaseCitation80,Citation111. HMOs act as soluble decoy receptors that block the attachment of specific viral, bacterial, or protozoan parasite pathogens to the epithelial cell surfaceCitation117. Pathogens that are not bound to the cell surface are washed away harmlessly. Animal models have indicated that increasing acetate, in combination with other metabolites, increases protection from gastrointestinal and respiratory infectionsCitation112,Citation113.
Regarding to anti-infective properties of HMOs, studies showedCitation6,Citation80,Citation114,Citation115
2’-FL: C. jejuni, Enteropathogenic E. coli, Salmonella enterica, rotavirus, norovirus, respiratory syncytial virus
3’-FL: Enteropathogenic E. coli, Salmonella enterica, norovirus,
LNT: Vibrio cholerae toxin, Group B streptococcus, Entamoeba histolytica
3’-SL: Enteropathogenic E. coli, Vibrio cholerae toxin, Helicobacter pylori, Pseudomonas aeruginosa, rotavirus, influenza
6’-SL: Enteropathogenic E. coli, Helicobacter pylori, Pseudomonas aeruginosa, influenza A H1N1, rotavirus
LNnT: pneumococci, influenza
Some HMOs are bacteriostatic against GBS, causing neonatal sepsis, pneumonia, and meningitisCitation87. Non-sialylated HMOs, LNT and LNDFH-I (1–2 mg/L daily), delay the growth of GBS with 96–98%Citation116. HMOs also showed antibacterial action against, Acinetobacter baumannii, and Staphylococcus aureusCitation41. In neonates, HMOs alter the growth and morphogenesis of C. albicans, which then makes it more difficult for the pathogen to attach, invade, and cause diseaseCitation126.
According to basic and animal research, HMOs appear to have a role in the treatment and prevention of bacterial, viral, protozoal, and fungal diseases. It is important to note that the majority of the evidence presented in support of the anti-adhesive effects of HMOs originates from experimental studies. It will need well-designed and powered mother-infant dyad observation studies and, more crucially, intervention studies to demonstrate that a single HMO or a mixture of several HMOs reduces the incidence and/or severity of a diversity of infectious diseases.
HMO and immune development
The immune system develops over the course of gestation and continues to be postnatal in relation to exposure of microorganisms. HMOs can modify host epithelial and immune cell responses and contribute to the development of the gastrointestinal immune systemCitation4–6,Citation20,Citation48,Citation117. It has been hypothesized that HMOs influence the responses of epithelial cells and immune cells by modifying cell proliferation, differentiation, and apoptosis, as well as cell signaling pathways and cell surface glycosylation, so modulating immunological functions. Intestinal epithelial barrier cells can be directly affected by HMOs of varying structures. Direct interactions between HMOs and infant intestinal epithelial cells affect their gene expression, cell cycle, and cell surface glycosylation and regulate their growth, differentiation and apoptosisCitation20. The establishment of the infant gut microbiota and its metabolic activity is thought to be an important mechanism through which HMOs affect immune system developmentCitation4.
In addition, when HMOs reach the colon and are then absorbed intact into the circulation, they may play a systemic immunomodulatory role by mediating cell-cell interactions in the immune system. Intestinal health and intestinal barrier function constitute the first defense line in innate immunityCitation4–6,Citation20,Citation48,Citation118,Citation119. As shown in vitro, HMOs inhibit cell proliferation, promote cell differentiation, death, and maturation, and strengthen the barrier functionCitation7,Citation31,Citation94,Citation120. Modulations in gene expression caused by HMOs have an immediate effect on intestinal epithelial cells, altering their surface glycans and eliciting different cellular responses. The generation of cytokines by lymphocytes is altered by HMOs, which may result in a more balanced TH1/TH2 response. Growing evidence from in vitro research suggests that HMOs directly control immunological responses by altering immune cell populations and cytokine release in infants, in addition to their indirect effects on the immune system via changes in gut microbiotaCitation94.
HMOs may also affect immune system receptors. Galectins, glycan-binding proteins, regulate intracellular signaling, cell – cell communication, proliferation, and survivalCitation121. Galectins may be HMO receptors for the immune system developmentCitation5. HMOs can act locally or systemically on mucosa-associated lymphoid cellsCitation15.
HMOs contain tolerogenic factors influencing human monocyte-derived dendritic cells and elevated Interleukin (IL)-10, IL-27, and IL-6 levels but not IL-12p70 and tumor necrosis factor-alphaCitation122. 2′-FL increases Th1-type interferon-gamma and regulates IL-10 production, suggesting a Th1 responseCitation123. CD11(+) mesenteric lymph node dendritic cells exposed to 3’-SL can produce cytokines that boost Th1 and Th17 immune cellsCitation124. Three weeks of 2’-FL administration to Caco-2Bbe cells, reduced the permeability and upregulated tight junction proteinsCitation125. 2’-FL can boost innate and adaptive immunity in influenza-specific mouse models and reduce respiratory viral infectionsCitation126. In a mouse influenza vaccination model, dietary 2′-FL improved humoral and cellular immune responses, boosting vaccine-specific delayed-type hypersensitivity and immunoglobulin proliferation.Citation127.
HMO and allergy
The prebiotic effects and the immunological programming provided by HMOs also affect individual susceptibility to allergies. A balanced microbiota and microbiome provide immunological benefits by lowering the risk of allergic disorders through the synthesis of SCFAs, such as butyrate and propionate, which have anti-inflammatory and anti-allergic qualities. It is known since more than 20 years that the gastrointestinal microbiota differs in allergic and non-allergic infants before symptoms of allergy developCitation120,Citation128,Citation129. A significant reduction in the probability of acquiring immunoglobulin E (IgE) mediated eczema at the age of two years was observed in C-section-born, allergy-prone breastfed infants whose mothers expressed FUT2, resulting in 2′-FL synthesis in human milkCitation39. C-section infants who were administered human milk containing FUT2-dependent oligosaccharides were shown to have a lower incidence for IgE-associated eczema at the age of 2 yearsCitation39. It was only in infants born via C-section that these associations between IgE-associated eczema and consumption of FUT2-dependent milk oligosaccharides were observedCitation38. The authors did not find an association with HMOs and allergic disorders at 5 years of ageCitation39. When compared to milk with high LNFP III concentrations, infants who received human milk with low LNFP III concentrations were more likely to develop cow’s milk protein allergy (CMPA)Citation38. The mothers’ FUT2 status was associated with a delayed onset of CMPA, and CMPA infants born to non-secretor moms (FUT2 negative) were more likely to develop IgE-mediated CMPA. Lower levels of DSLNT and 6′-SL were associated with atopic dermatitisCitation38. Concentrations of nine neutral HMOs were not associated with the chance of having an allergic disease up to the age of 18 months, according to a case-control study in 20 mother-infant pairs from a larger birth cohortCitation97.
Regarding to relationship between food sensitization, a large clinical observation study (421 mother – infant dyads) demonstrates that HMO composition is associated with the development of food sensitizationCitation130. The HMO profiles associated with lower risk of food sensitization were characterized by higher concentrations of FDSLNH, LNFP II, LNnT, LNFP I, LSTc and FLNH, and lower concentrations of LNH, LNT, 2′-FL, and DSLNHCitation130. In an ovalbumin sensitized mouse model, 2’-FL and 6-FL stabilize mast cells by inducing expression of T regulatory cells and activate the IL-10(+) regulatory cells to reduced symptoms of food allergyCitation131.
By influencing the colonization of the gut microbiota and producing butyrate, microbiota composition of human milk helps the prevention of development of food allergiesCitation50. The development of a microbiome dominated by bifidobacteria was significantly delayed in infants fed secretor-negative human milk compared to those fed secretor-positive breastmilk at three months of ageCitation132. In particular, B. breve has been linked to a decreased incidence of eczemaCitation133. Among infants with a family history of atopy, reduced Bifidobacteriaceae abundance in infancy is related with a higher risk of eczemaCitation133. However, another study found no significant association between the intake of particular HMOs (measured at 6 weeks and 6 months) and the risk of atopic dermatitisCitation134.
Breastfeeding has been shown to reduce the likelihood of developing food allergy, eczema, and asthma, at least during early life, although there is a lack of consistency in reporting of breastfeeding duration, diagnostic criteria for atopic dermatitis, and assessment ageCitation135.
HMO and brain/cognitive development
Sialic acid is considered a key conditioned nutrient during early development. Although the mechanisms are not completely understood, the high levels of sialic acid in human milk, especially in the form of sialylated milk oligosaccharides, are considered an important bioactive component linked to infant brain and cognitive developmentCitation4,Citation6,Citation136. Both 3’−SL and 6’-SL have been shown to enhance learning and memory and play a role in the gut microbiota-brain axisCitation137–139.
Cho et al.Citation149 showed that the association between human milk 3’-SL concentration and cognition, particularly language functions, in typically children who received human milk containing alpha tetrasaccharide (an HMO, which only be detected in the mothers with blood type A. High levels of 6′-SL have been linked to better cognitive and motor development at 18 months of age, as well as better language development at 12 months of ageCitation140,Citation141.
In the brain, fucosylated proteins are found along the neuronal synapses, particularly in the hippocampus, where they play a crucial role in the development of memory and learningCitation142. There is experimental evidence that 2’-FL interferes with cognitive processes, including enhanced cognitive ability, learning, and memoryCitation143. Early exposure to 2′-FL and 6`-SL represents a critical time window for the positive influence on the cognitive development at 2 yearsCitation48,Citation140,Citation141. Although human data are scant, one study found that breastfed infants with greater 2’-FL intake at one month of birth had better cognitive development at 24 months of age and improved motor skillsCitation144,Citation145. A higher concentration of fucosylated HMOs was linked to better linguistic development between the ages of 12 and 18 monthsCitation140.
In summary, studies suggest a role of HMOs in brain and cognitive development, but more data are needed. The mechanisms of action need to be further unraveled.
HMO and diabetes
2’-FL, 3’-SL, 6-SL, and LNnT may have protective effects on the development of type-1 diabetes. In an animal model, early life intake of HMOs delayed and suppressed type-1 diabetes development in non-obese diabetic mice and reduced the development of severe pancreatic insulitis in later lifeCitation126.
HMO and infant formula
Effects of HMOs containing infant formula on anthropometry
Although the WHO recommends exclusive breastfeeding since birth to 6 months of age, some infants will not receive human milk. The energy and nutrition need of a growing infant can be met by infant formula, which typically is cow’s milk based. However, cows and human milk differ substantially in the composition of macro- and micro-nutrients, and in the content of bioactive componentsCitation26. In fact, HMOs are virtually absent in cow’s milk (or any animal milk), and their variety is much lower than in human milkCitation146. Observational studies revealed that many disorders such as NEC, irritable bowel syndrome, obesity, allergies, and eczema, are more common in formula-fed compared to breastfed infantsCitation20. The early microbiota development and effect on immune system development in cow’s milk formula fed infants might be affected by the lack of HMOsCitation147. Nowadays, it is possible to supplement infant formula with mixtures of HMOs. The effects of HMOs in infant formula have been evaluated in several randomized clinical trials ().
Table 2. HMOs in formula in research.
HMO production technologies involve novel processes, which are approved by the regulatory authorities, such as the European Food Safety Agency (EFSA) or the Federal Drug Administration (FDA) in the United States. Both the EFSA in 2015 and the FDA in 2016 approved 2’-FL and LNnT to be added to infant formula, and the first formulas containing HMOs were commercialized in Spain and the USA in 2016. The EFSA indicated that the addition of 2′-FL and LNnT at a ratio of 2:1 to infant formula is safe below 1 -year-old, with a maximum dosage for 2′-FL of 1.2 g/L and for LNnT of 0.6 g/LCitation20. In 2019, the FDA stipulated that the maximum dosage of 2′-FL in infant formula is 2.4 g/L, and for LNnT 0.6 g/LCitation20. HMOs have obtained the Generally Recognized as Safe (GRAS) status. The number of HMOs that can be synthesized on an industrial scale has steadily increased, and nowadays formulas containing seven HMOs (2’-FL, 3´-FL, LDFT, LNnT, LNT, 3’-SL, 6’−SL) are studied. Some oligosaccharides, identical to those in human milk, can be produced by fermentation or other techniques. To be clear, the oligosaccharides added to infant formula do not originate from human milk, even if they have an identical structure. Therefore, HMOs that do not originate from human milk should preferably be called “human identical milk oligosaccharides” (HiMOs)Citation4.
Already in 2005, LNnT was shown to be safe in 228 infants aged 6–24 months during a 16-week follow-up period, with a slight non-significant trend for higher weight and heightCitation148. Marriage et alCitation149 conducted a prospective, randomized, controlled growth and tolerance study, with a formula containing 2′-FL and GOS in healthy full-term infants and showed similar weight, length, and head circumference to breastfed babies from enrollment (0–5 days) to four monthsCitation149. This formula was well-tolerated and comparable for average stool consistency, number of stools per day, and percent of feedings associated with spitting up or vomit with the control group fed GOS supplemented formula. The formula supplemented with 2′-FL resulted in a growth similar to that of breast-fed infantsCitation149. In a multicenter, RCT in Italy and Belgium, Puccio et al.Citation118 reported the first clinical trial with infant formula supplemented with 2′-FL (1.0 g/L) and LNnT (0.5 g/L) up to the age of 6 monthsCitation118. The 2′-FL and LNnT supplemented formula was well-tolerated and supported age-appropriate growth; infant had softer stools and fewer nighttime wake-ups at two months, while cesarean-born babies had a lower incidence of colic at four monthsCitation118. Infants receiving HMO-containing formula had significantly fewer parent-reported lower respiratory tract infections, antipyretic, and antibiotic use up to the age of 12 months (although the supplementation was limited to the age of 6 months)Citation118. Parschat et alCitation160 conducted a multicenter, randomized, controlled, parallel-group clinical study in Germany, Italy, and Spain to evaluate the safety and tolerability of a five HMO blend (5.75 g/L total, comprising 52% 2′-FL, 13% 3’-FL, 26% LNT, 4% 3′-SL, and 5% 6′-SL) and its effect on growth when applied over a 16-week periodCitation150. The primary outcome was the mean daily body weight increment over a 4-month period. The observed mean values for daily weight increase of~28.7 g/day were similar to those reported in studies comparing infant formula with 2′-FL plus GOS, 2′-FL plus LNnT, or 2′-FL plus 3′-GL and GOS/FOSCitation118,Citation149–151. Lasekan et alCitation152 performed a randomized, double-blind, controlled parallel feeding trial with five HMOs (2′-FL, 3-FL, LNT, 3′-SL, and 6′-SL) containing formula in the United States, mostly during the COVID-19 pandemic, while stay-at-home orders were in placeCitation152. The test formula was again found to be safe and well tolerated and weight gain and length did not differ between the groups. Compared to the control group, infants given test formula had more frequent and softer stoolsCitation152. Vandenplas et al.Citation151 studied growth, safety, and tolerance in healthy infants consuming a partly fermented infant formula with postbiotics and the HMOs 3′–GL) and 2′-FL, and a specific prebiotic mixture of short-chain GOS (scGOS) and long-chain fructo-oligosaccharides (lcFOS). Equivalence in weight gain (primary endpoint), length, and head circumference gain of up to 17 weeks was also confirmed with the test formula. There were no statistically significant differences between the formula groups for regurgitation, vomiting, watery, or hard stools at any timepointCitation151. Ramirez-Farias and colleaguesCitation153 examined extensively hydrolyzed formula (eHF) with 2′-FL (0.2 g/L) for growth, tolerance, and compliance in a non-randomized, single-group, multicenter study. Infants (0–60 days old) with suspected food protein allergy, persistent feeding intolerance, or presenting conditions where an eHF was deemed appropriate were enrolled in a 2-month feeding with an experimental formula. This study shows that eHF formula with 2′-FL was well-tolerated and provided a significant improvement of weight for age z-scoresCitation153. An eHF with two HMOs (2′-FL at 1.0 g/L and LNnT at 0.5 g/L) confirmed a non-inferiority of the test formula for weight gain per day at the 4-month visit, and there were no statistically significant differences between the groups on any of the anthropometric parameters measured during the course of the trial. Gold et al.Citation154 showed in an open-label, non-randomized, multicenter study of an amino acid-based formula supplemented with two HMOs (2′-FL and LNnT) for 4 months, with the option to continue feeding it for additional 8 months, and showed that the weight-for-age Z score improved from −0.31 at the start of the trial to+0.28 at the end of the study. Additionally, linear and head growth followed the WHO child growth reference and showed a similar, slight upward trend.
HMOs in infant formula and gastro-intestinal tolerance
Infant formula supplemented with 2′FL alone, 2′FL combined with LNnT, and a blend of five HMOs (2′-FL, 3-FL, LNT, 3′-SL, 6′-SL) in formula with intact and hydrolyzed protein have all been shown to be well tolerated in clinical trialsCitation118,Citation149,Citation150,Citation153,Citation155–157. Stool consistency, flatulence, and the frequency of spitting up/vomiting were similar in infants given formula containing with or without HMOsCitation149,Citation157,Citation158. In an RCT testing, a mix of 5 HMOs in infant formula, the stools in the HMO supplemented formula group were more soft, frequent, and yellow. They were more similar to the breastfed infants’ stools than the stools of the non-supplemented formula groupCitation150,Citation172. A formula including 2′-FL and LNnT showed softer stool consistency in another investigationCitation118. Stool consistency in infants fed 2′-FL and FOS-containing formula was found to be comparable to that of breast-fed infantsCitation157,Citation158.
The effect of HMOs in infant formula on microbiota composition
There is a significant difference in intestinal microbiota composition between breast-fed infants and formula, without supplementation of biotics, fed infantsCitation136. Supplementation with HMOs may therefore potentially increase bifidobacteria and bring microbiota composition closer to that of breastfed infants. In RCT, the microbiota composition in a 2’-FL formula (1 g/L) group was only just significantly different at 2 months and just not at 3 months of age, bringing the microbiota composition somehow closer to that of breastfed infantsCitation159.
The development of the microbiota composition was tested via stool cultures during incubation with 2’-FL of three breast and three formula fed infantsCitation160. The composition of the microbiome at baseline was dependent on the mode of feeding and on the ability to degrade 2’-FL. When looking at the degradation of 2’-FL, the fecal cultures could be divided into slow and fast degraders regardless of mode of feeding. However, since there were only six infants no conclusions can be drawnCitation160. Another multicenter study examined fecal cultures of infants receiving either a formula with a mix of five HMOs at a concentration of 1.5 g/L, a formula with a mix of five HMOs at a concentration of 2.5 g/L, a non-supplemented formula, or breast milkCitation25. The microbiota composition of infants receiving formulas supplemented with HMOs was significantly different to those in the non-supplemented group and were closer to the composition of the breastfed infants. The concentration of B. infantis was statistically higher in the HMO supplemented than in the non-supplemented group, approaching the composition of breastfed infants. Significantly less Clostridium difficile was seen in the HMO supplemented group in comparison to the non-supplemented group suggesting a lesser chance of diarrheal illness. No significant differences were seen between the lower and higher dose HMO supplemented formulasCitation25. In a randomized, double-blind, multicenter clinical experiment, after a three-month intervention, infant formula containing 2′-FL and LNnT enhanced the abundance of Bifidobacterium and Streptococcus and changed the microbiome of cesarean section infants’ group to that observed in vaginal delivery infantsCitation161. This study suggests that the association between formula with 2′-FL and LNnT and lower parent-reported morbidity and medication use may be linked to gut microbiota community typesCitation161. A bifidogenic effect in infants receiving formula with two HMOs (2′-FL and LNnT) which was more pronounced in the cesarean-born infants, however, found no effect on B. infantisCitation161,Citation162. An amino acid-based formula supplemented with 1 g/L 2’FL and 0.5 g/L LNnT confirmed an enrichment in Bifidobacteria and reduction of ProteobacteriaCitation154.
Bifidobacteria abundance and metabolic activity could be associated to decreased respiratory tract infectionsCitation66,Citation72,Citation163. Increased gamma-glutamylation and N-acetylation of amino acids, and decreased inflammatory signaling lipids, are the three most notable molecular pathwaysCitation66.
Bosheva et alCitation25 studied gut maturation effects (microbiota, metabolites, and selected maturation indicators) of an infant formula containing five HMOs (2′-FL, 3-FL, LNT, 3′-SL, 6′-SL). In the first 6 months of life, the HMO supplemented formula shifted the gut microbiome closer to that of breastfed infants with higher bifidobacteria, particularly B. infantis, and lower C. difficile Citation25. Formula with these 5 HMOs suggest that the HMOs may boost infant intestinal immune development and gut barrier function. HMO-supplemented formula helps restore dysbiosis in cesarean-born infantsCitation25.
Estorninos and colleaguesCitation164 evaluated the effects of bovine milk-derived oligosaccharides (primarily composed of GOS with inherent concentrations of sialylated oligosaccharides structurally identical to some in human milk) and reported similar effects on gut microbiota and intestinal immunity in healthy term formula-fed infantsCitation164.
Effects of HMOs containing infant formula on infectious disease prevention
Breastfed children are less likely to suffer from respiratory and gastrointestinal infections than formula fed infantsCitation26,Citation98,Citation118,Citation149. Research with formulas supplemented with HMOs found (as a secondary outcome) a decreased rate of respiratory tract infections and bronchitis, as well as a decreased need for antibiotics and antipyreticsCitation118,Citation161,Citation165. These effects did persist beyond the six-month intervention periodCitation118,Citation161. Further analyses of the same data have linked a microbiome community structure highly dominated by Bifidobacterium species at 3 months of age with a decreased need for antibiotics, lending credence to the observation that 2’-FL and LNnT supplementation reduces the risk of respiratory infections and the need for antibioticsCitation161. Acetate, one of the compounds produced by the HMO-stimulated metabolic activity of Bifidobacterium, may aid in lowering the risk of respiratory tract infections. Another study found that infants who were fed a formula containing 2′-FL (0.2 g/L) and GOS (2.2 g/L) had a lower incidence of illnesses and infestations as reported by the investigatorsCitation149. Supporting the hypothesis that the HMO-containing formula provides immune system benefits, in the study by Lasekan et al.Citation152 fewer infants needed to visit a healthcare professional. However, Parschat et al.Citation150 found no evidence that infant formula containing five HMOs reduced the risk of infection in infants. Leung et al.Citation166 enrolled 461 infants aged 1–2.5 years in China in an RCT testing three young child formulas containing bioactive proteins and/or 2’-FL and/or milk fat for six months and found no difference in the incidence of upper respiratory or gastrointestinal tract infections between all groups.
There is theoretical evidence that HMO supplementation in formula fed infants may have beneficial impacts on microbiota composition, immunological function, and other parameters, hence reducing the prevalence of infections. However, clinical data are not unequivocal and no study was powered to evaluate the effect on infections as a primary outcome.
Effects of HMOs containing infant formula on the immune system
The effects of HMO 2′-FL enriched feeding formulae on immune function biomarkers in term infants were studiedCitation165. At the age of three months, the groups receiving an HMO-supplemented formula had a higher secretory immunoglobulin A and lower alpha-1-antitrypsin in comparison to the non-supplemented group possibly offering immunological benefitsCitation25. A randomized, double-blind, controlled growth and tolerance study was conducted with healthy singleton infants who were enrolled by 5 days of age and fed either formula or human milk exclusively from the time of enrollment to the age of 4 monthsCitation165. GOS was given to the control group, whereas GOS plus either 0.2 or 1.0 g/L 2′-FL was given to the study group and compared to the breastfeeding reference group. Concentrations of plasma inflammatory cytokines were 29–83% lower in infants fed formulas with 2’-FL and GOS than did infants fed the control formula including GOS only. Infants whose formula contained 2′-FL showed innate cytokine profiles more similar to those of breastfed infants. Biomarkers of immune functions such as plasma cytokine concentrations, cytokines released by ex vivo stimulation of peripheral blood mononuclear cells (PBMCs), and percentages of major lymphocyte subsets within the PBMCs population were used in this study to demonstrate the impact of 2′-FL-fortified formulas on the developing immune system. 2′-FL reduced the gap in total T lymphocyte proportions between breastfed infants, which is an indicator of improved adaptive immunity. The discrepancies in apoptotic cell percentages between breastfeeding and control groups were also reduced by 2′-FL, especially in CD8+ T cells and CD8+ T cell subset. These results suggest that compared to GOS alone, supplementing infant formula with 2′-FL promotes immunological development and modulation in a way that is comparable to that of breastfed infantsCitation165.
Effects of HMOs containing infant formula on allergy
Infants diagnosed with CMPA who are not breastfed are treated with a cow’s milk elimination diet, eHF or amino acid formulaCitation167. Preclinical studies have indicated that 2′-FL can reduce allergic responses in a food allergy modelCitation120,Citation131. Laboratory analysis of 2′-FL and LNnT batches showed no evidence of residual milk allergens, despite the fact that HMOs are produced via biofermentation from lactose, which in theory might bring a risk of residual milk allergen contaminationCitation168.
An eHF with two HMOs (2′-FL at 1.0 g/L and LNnT at 0.5 g/L) showed a similar reduction in the supplemented and non-supplemented eHF, with the Cow’s Milk-Related Symptom Score (CoMiSSTM) dropping to the levels seen in presumed healthy infants. Otitis media and upper respiratory tract infections were significantly reduced in the HMO group by 12 months, and lower respiratory tract and gastrointestinal infections were reduced by 30–40%, however without statistical significanceCitation169.
In an open-label study testing an AAF with two HMOs (2´-FL and LNnT) a significant reduction in symptoms was noted between enrollment and Visit 1, as reported by parents, and between Visit 1 and subsequent visits, as assessed by physiciansCitation154. Control of skin symptoms was generally excellent.
Non-human Oligosaccharides
Non-human oligosaccharides were also shown to enhance the development of a bifidobacteria dominated gastrointestinal microbiomeCitation26. RCTs evaluating GOS/FOS as well as only-GOS enriched formulas have demonstrated a stimulating effect on the growth of Bifidobacteria and/or Lactobacilli Citation26. GOS, FOS, and GOS/FOS mixtures (the most studied being a 9:1 mixture of scGOS and lcFOS) are the most researched prebiotics componentsCitation26,Citation170,Citation171. Clinical studies have shown that supplementation of infant formula with a mixture of scGOS and lcFOS (9:1) leads to a more favorable gut microbiota composition and activity, closer to that observed in breastfed infants. There was no statistically significant difference between infants fed GOS/FOS enriched formulas and those receiving regular formulas in terms of weight, height, or head circumferenceCitation172. Moreover, scGOS and lcFOS in infant formula has also been associated with a lower number of infections, fever episodes, and antibiotic prescriptionsCitation170,Citation171. Beneficial effects on Bifidobacteria and Lactobacilli growth in infants given a scGOS/lcFOS supplemented formula were observed to be sustained even after the formula was discontinued, at least for a few monthsCitation172.
Infant formulae with added prebiotics have been linked to a lower fecal pH and a SCFAs pattern closer to that of breastfed infants, without increased frequency of stoolCitation26. Non-human oligosaccharides also promote the growth of a bifidobacteria-dominated gut microbiome, selectively stimulate the growth of Bifidobacteria and/or LactobacilliCitation26. Clinical investigations have demonstrated that adding a mixture of scGOS and lcFOS (9:1) to infant formula results in a more favorable gut microbiota composition and activity, closer to breastfed infants. Beneficial effects on Bifidobacteria and Lactobacilli growth in infants fed with a scGOS/lcFOS supplemented formula were observed to be sustained even after months of discontinuing the formula178. It has been shown that some bifidobacteria only grow in the presence of human milk oligosaccharidesCitation45. However, it is not known if this has any clinical impact for the infant. There are almost no data comparing the effects of HMO and non-human oligosaccharides in infants. Only in the study by Marriage et al.Citation149 there was only-GOS group compared to two GOS group with different levels of 2´-FL. As a consequence, there is no evidence to state that HMOs added to infant formula are more effective than non-human oligosaccharides.
Limitations
Today, there is still a dearth of information on the addition of HMOs to infant formula. No definitive conclusions can be drawn on whether supplemented or non-supplemented formula yields better clinical outcomes because to the limited data from the current research. Due to the differences in design and primary outcomes of the clinical trials, there is inconsistency in the findings. The optimal dosing of HMOs also necessitates fine-tuning. There are substantial variations in the studies in terms of study design, location, lactation sampling, the number of time periods at which development parameters are assessed, the specific HMOs that were analyzed, and the statistical methodologies utilized to predict the correlationsCitation4. The majority of the included studies have a relatively small sample size to quantify disease outcomes, which reduces their precision and statistical ability to find meaningful relationships. Because of these differences, it is not possible to do a meta-analysis. The benefits of sialylated-HMOs are not well recognized, despite the fact that neutral oligosaccharides like 2′-FL and 3′-FL have been the subject of substantial research into their involvement in infant nutrition, growth, and development in both pre-clinical and clinical settings. There is an immediate need for more investigations on the health advantages of HMOs in human milk with varying structural compositionsCitation136. There are almost 200 different oligosaccharides in human milk, but today only five are added to infant formula, while studies with seven are going on. An increase in the number of HMOs used could enhance the outcomes. However, the ideal dosage of HMOs in infant formula is still up for debate, as HMO levels fluctuate in breast milk. Therefore, if the formula is administered at a consistent HMO concentration and ratio, formula-fed infants may consume less of specific HMOs in the early stages of the trial, but more HMOs afterward than breastfed infants. The fact that statistical associations do not imply a causal relation further emphasizes the need for randomized, placebo-controlled interventional trials and supplementary mechanistic studies.
In conclusion, HMOs are a major ingredient of human milk, which is the best source of nutrition for infants. HMOs act in tandem with other bioactive components and also act through many pathways that converge to specific activities, as is predicted from many biological processes. HMOs are known to support a healthy gut microbiome, build the gastrointestinal barrier and promote brain growth and cognitive function, among other important physiological roles. A growing body of research also suggests that particular HMOs contribute to the development of immunological competence, both locally and systemically, in part through influencing the metabolism of particular bacteria, such as particular Bifidobacterium species. The study of milk microbiota and HMOs relies heavily on the strain-specific characterization of beneficial human microbiota organisms and their consumption of specified HMOs. Human milk research is a promising field since more benefits and correlations between components will be uncovered as time goes on. Regarding formula feeding, more clinical trials in children are needed comparing the multiple effects of non-human to human oligosaccharides supplementation.
Author contribution
All authors contributed to evaluation of available article, summarizing the data, drafting, and critical review of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- WHO. Infant and Young Child Feeding. (2021. Available online at: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding.
- Meek JY, Noble L. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022 Jul 1;150(1):e2022057988. doi:10.1542/peds.2022-057988.
- Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, Michaelsen KF, Mihatsch W, Moreno LA, Puntis J, Shamir R, et al. Breast-feeding: a commentary by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2009 Jul;49(1):112–33. doi:10.1097/MPG.0b013e31819f1e05.
- Sprenger N, Tytgat HLP, Binia A, Austin S, Singhal A. Biology of human milk oligosaccharides: from basic science to clinical evidence. J Hum Nutr Diet. 2022 Apr; 35(2): 280–299. 10.1111/jhn.12990
- Moubareck CA. Human milk microbiota and oligosaccharides: a glimpse into benefits, diversity, and correlations. Nutrients. 2021 Mar 29;13(4):1123.
- Hill DR, Chow JM, Buck RH. Multifunctional benefits of prevalent HMOs: implications for infant health. Nutrients. 2021 Sep 25;13(10):3364. doi:10.3390/nu13103364.
- Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, Bahl R. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015 Dec; 104(467): 3–13. 10.1111/apa.13147
- Christensen N, Bruun S, Søndergaard J, Christesen HT, Fisker N, Zachariassen G, Sangild PT, Husby S. Breastfeeding and infections in early childhood: a cohort study. Pediatrics. 2020 Nov; 146(5): e20191892. 10.1542/peds.2019-1892
- Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015 Dec; 104(467): 30–37. 10.1111/apa.13133
- Tschiderer L, Seekircher L, Kunutsor SK, Peters SAE, O’keeffe LM, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: systematic review and meta-analysis involving data from 8 studies and 1 192 700 parous women. J Am Heart Assoc. 2022 Jan 18;11(2):e022746. doi:10.1161/JAHA.121.022746.
- Stordal B. Breastfeeding reduces the risk of breast cancer: a call for action in high-income countries with low rates of breastfeeding. Cancer Med. 2022 Sep 26;12(4):4616–4625. doi:10.1002/cam4.5288.
- Schraw JM, Bailey HD, Bonaventure A, Mora AM, Roman E, Mueller BA, Clavel J, Petridou ET, Karalexi M, Ntzani E, et al. Infant feeding practices and childhood acute leukemia: findings from the childhood cancer & leukemia international consortium. Int J Cancer. 2022 Oct 1;151(7):1013–1023. doi:10.1002/ijc.34062.
- Matsumoto N, Yorifuji T, Nakamura K, Ikeda M, Tsukahara H, Doi H. Breastfeeding and risk of food allergy: a nationwide birth cohort in Japan. Allergol Int. 2020 Jan;69(1):91–97.
- Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015 Nov;91(11):619–622.
- Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012 Sep;22(9):1147–1162.
- Kunz C. Historical aspects of human milk oligosaccharides. Adv Nutr. 2012 May 1;3(3):430S–439S.
- Kuhn R. Les oligosaccharides du lait [Oligosaccharides of milk]. Bull Soc Chim Biol (Paris). 1958;40:297–314.
- Grimmonprez L, Montreuil J. Etude des fractions glycanniques des glycosphingolipides totaux de la membrane des globules lipidiques du lait de femme [The glycan fraction of the total glycosphingolipids of the human milk fat globule membrane]. Biochimie. 1977;59:899–907.
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722.
- Zhang B, Li LQ, Liu F, Wu JY. Human milk oligosaccharides and infant gut microbiota: molecular structures, utilization strategies and immune function. Carbohydr Polym. 2022 Jan 15;276:118738.
- Urashima T, Asakuma S, Leo F, Fukuda K, Messer M, Oftedal OT. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv Nutr. 2012 May 1;3(3):473S–482S.
- Wiciński M, Sawicka E, Gębalski J, Kubiak K, Malinowski B. Human milk oligosaccharides: health benefits, potential applications in infant formulas, and pharmacology. Nutrients. 2020 Jan 20;12(1):266.
- Hahn WH, Kim J, Song S, Park S, Kang NM. The human milk oligosaccharides are not affected by pasteurization and freeze-drying. J Matern Fetal Neonatal Med. 2019 Mar;32(6):985–991.
- Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr. 2014;34:143–169.
- Bosheva M, Tokodi I, Krasnow A, Pedersen HK, Lukjancenko O, Eklund AC, Grathwohl D, Sprenger N, Berger B, Cercamondi CI. HMO study investigator consortium. infant formula with a specific blend of five human milk oligosaccharides drives the gut microbiota development and improves gut maturation markers: a randomized controlled trial. Front Nutr. 2022 Jul 6;9:920362.
- Fabiano V, Indrio F, Verduci E, Calcaterra V, Pop TL, Mari A, Zuccotti GV, Cullu Cokugras F, Pettoello-Mantovani M, Goulet O. Term infant formulas influencing gut microbiota: an overview. Nutrients. 2021 Nov 23;13(12):4200.
- Conze DB, Kruger CL, Symonds JM, Lodder R, Schönknecht YB, Ho M, Derya SM, Parkot J, Parschat K. Weighted analysis of 2’-fucosyllactose, 3-fucosyllactose, lacto-N-tetraose, 3’-sialyllactose, and 6’-sialyllactose concentrations in human milk. Food Chem Toxicol. 2022 May;163:112877.
- Cheema AS, Gridneva Z, Furst AJ, Roman AS, Trevenen ML, Turlach BA, Lai CT, Stinson LF, Bode L, Payne MS, et al. Human milk oligosaccharides and bacterial profile modulate infant body composition during exclusive breastfeeding. Int J Mol Sci. 2022 Mar 5;23(5):2865.
- Zivkovic AM, Barile D. Bovine milk as a source of functional oligosaccharides for improving human health. Adv Nutr. 2011 May;2(3):284–289.
- Thum C, Wall CR, Weiss GA, Wang W, Szeto IM, Day L. Changes in HMO concentrations throughout lactation: influencing factors, health effects and opportunities. Nutrients. 2021 Jun 30;13(7):2272.
- Zhang S, Li T, Xie J, Zhang D, Pi C, Zhou L, Yang W. Gold standard for nutrition: a review of human milk oligosaccharide and its effects on infant gut microbiota. Microb Cell Fact. 2021 May 28;20(1):108.
- Samuel TM, Binia A, de Castro CA, Thakkar SK, Billeaud C, Agosti M, Al-Jashi I, Costeira MJ, Marchini G, Martínez-Costa C, et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci Rep. 2019 Aug 13;9(1):11767.
- Plows JF, Berger PK, Jones RB, Alderete TL, Yonemitsu C, Najera JA, Khwajazada S, Bode L, Goran MI. Longitudinal changes in human milk oligosaccharides (HMOs) over the course of 24 months of lactation. J Nutr. 2021 Apr 8;151(4):876–882.
- Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010 Nov;104(9):1261–1271.
- Liu S, Cai X, Wang J, Mao Y, Zou Y, Tian F, Peng B, Hu J, Zhao Y, Wang S. Six oligosaccharides’ variation in breast milk: a study in south China from 0 to 400 days postpartum. Nutrients. 2021 Nov 11;13(11):4017.
- Zhu Y, Wan L, Li W, Ni D, Zhang W, Yan X, Mu W. Recent advances on 2’-fucosyllactose: physiological properties, applications, and production approaches. Crit Rev Food Sci Nutr. 2022;62:2083–2092.
- Azad MB, Robertson B, Atakora F, Becker AB, Subbarao P, Moraes TJ, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR, et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J Nutr. 2018 Nov 1;148(11):1733–1742.
- Seppo AE, Autran CA, Bode L, Järvinen KM. Human milk oligosaccharides and development of cow’s milk allergy in infants. J Allergy Clin Immunol. 2017 Feb;139(2):708–711.e5.
- Sprenger N, Odenwald H, Kukkonen AK, Kuitunen M, Savilahti E, Kunz C. FUT2-dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr. 2017 Apr;56(3):1293–1301.
- Thurl S, Munzert M, Boehm G, Matthews C, Stahl B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr Rev. 2017 Nov 1;75(11):920–933.
- Ackerman DL, Craft KM, Doster RS, Weitkamp JH, Aronoff DM, Gaddy JA, Townsend SD. Antimicrobial and antibiofilm activity of human milk Oligosaccharides against Streptococcus agalactiae, Staphylococcus aureus, and Acinetobacter baumannii. ACS Infect Dis. 2018 Mar 9;4(3):315–324.
- Kunz C, Meyer C, Collado MC, Geiger L, García-Mantrana I, Bertua-Ríos B, Martínez-Costa C, Borsch C, Rudloff S. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroenterol Nutr. 2017 May;64(5):789–798.
- McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr. 2017 May;105(5):1086–1100.
- Totten SM, Zivkovic AM, Wu S, Ngyuen U, Freeman SL, Ruhaak LR, Darboe MK, German JB, Prentice AM, Lebrilla CB. Comprehensive profiles of human milk oligosaccharides yield highly sensitive and specific markers for determining secretor status in lactating mothers. J Proteome Res. 2012 Dec 7;11(12):6124–6133.
- Davis JC, Lewis ZT, Krishnan S, Bernstein RM, Moore SE, Prentice AM, Mills DA, Lebrilla CB, Zivkovic AM. Growth and morbidity of Gambian infants are influenced by maternal milk Oligosaccharides and Infant Gut Microbiota. Sci Rep. 2017 Jan 12;7:40466.
- Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C, Coppa GV. Preterm milk oligosaccharides during the first month of lactation. Pediatrics. 2011 Dec;128(6):e1520–31.
- Sundekilde UK, Downey E, O’mahony JA, O’shea CA, Ryan CA, Kelly AL, Bertram HC. The effect of gestational and lactational age on the human milk Metabolome. Nutrients. 2016 May 19;8(5):304.
- Corona L, Lussu A, Bosco A, Pintus R, Cesare Marincola F, Fanos V, Dessì A. Human milk oligosaccharides: a comprehensive review towards Metabolomics. Children (Basel). 2021 Sep 14;8(9):804.
- Saben JL, Sims CR, Abraham A, Bode L, Andres A. Human milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients. 2021 Jan 29;13(2):446.
- Wang S, Wei Y, Liu L, Li Z. Association between breastmilk microbiota and food allergy in infants. Front Cell Infect Microbiol. 2022 Jan 12;11:770913.
- Lagström H, Rautava S, Ollila H, Kaljonen A, Turta O, Mäkelä J, Yonemitsu C, Gupta J, Bode L. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am J Clin Nutr. 2020 Apr 1;111(4):769–778.
- Selma-Royo M, Calvo Lerma J, Cortés-Macías E, Collado MC. Human milk microbiome: from actual knowledge to future perspective. Semin Perinatol. 2021 Oct;45(6):151450.
- Neville J, Pawlak R, Chang M, Furst A, Bode L, Perrin MT. A cross-sectional assessment of human milk oligosaccharide composition of vegan, vegetarian, and nonvegetarian mothers. Breastfeed Med. 2022 Mar;17(3):210–217.
- Plows JF, Berger PK, Jones RB, Yonemitsu C, Ryoo JH, Alderete TL, Bode L, Goran MI. Associations between human milk oligosaccharides (HMOs) and eating behaviour in Hispanic infants at 1 and 6 months of age. Pediatr Obes. 2020 Dec;15(12):e12686.
- Binia A, Lavalle L, Chen C, Austin S, Agosti M, Al-Jashi I, Pereira AB, Costeira MJ, Silva MG, Marchini G, et al. Human milk oligosaccharides, infant growth, and adiposity over the first 4 months of lactation. Pediatr Res. 2021 Sep;90(3):684–693.
- Menzel P, Vogel M, Austin S, Sprenger N, Grafe N, Hilbert C, Jurkutat A, Kiess W, Binia A. Concentrations of oligosaccharides in human milk and child growth. BMC Pediatr. 2021 Oct 30;21(1):481.
- Gridneva Z, Rea A, Tie WJ, Lai CT, Kugananthan S, Ward LC, Murray K, Hartmann PE, Geddes DT. Carbohydrates in human milk and body composition of term infants during the first 12 months of Lactation. Nutrients. 2019 Jun 28;11(7):1472.
- Alderete TL, Autran C, Brekke BE, Knight R, Bode L, Goran MI, Fields DA. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. 2015 Dec;102(6):1381–1388.
- Charbonneau MR, O’donnell D, Blanton LV, Totten SM, Davis JC, Barratt MJ, Cheng J, Guruge J, Talcott M, Bain JR, et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 2016 Feb 25;164(5):859–871.
- Spevacek AR, Smilowitz JT, Chin EL, Underwood MA, German JB, Slupsky CM. Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. J Nutr. 2015 Aug;145(8):1698–1708.
- Nuzhat S, Palit P, Mahfuz M, Islam MR, Hasan SMT, Islam MM, Sarker SA, Kyle DJ, Flannery RL, Vinjamuri A, et al. Association of human milk oligosaccharides and nutritional status of young infants among Bangladeshi mother-infant dyads. Sci Rep. 2022 Jun 8;12(1):9456.
- Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth - first 1000 days and beyond. Trends Microbiol. 2019 Feb;27(2):131–147.
- Laursen MF. Gut Microbiota development: influence of diet from infancy to toddlerhood. Ann Nutr Metab. 2021 Aug;30:1–14.
- Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017 Mar;23(3):314–326.
- Stewart CJ, Ajami NJ, O’brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018 Oct;562(7728):583–588.
- Martin FP, Tytgat HLP, Krogh Pedersen H, Moine D, Eklund AC, Berger B, Sprenger N. Host-microbial co-metabolites modulated by human milk oligosaccharides relate to reduced risk of respiratory tract infections. Front Nutr. 2022 Aug 4;9:935711.
- Hao H, Zhu L, Faden HS. The milk-based diet of infancy and the gut microbiome. Gastroenterol Rep (Oxf). 2019 Aug;7(4):246–249.
- Lin C, Lin Y, Zhang H, Wang G, Zhao J, Zhang H, Chen W. Intestinal ‘infant-type’ bifidobacteria mediate immune system development in the first 1000 days of life. Nutrients. 2022 Apr 2;14(7):1498.
- Ojima MN, Jiang L, Arzamasov AA, Yoshida K, Odamaki T, Xiao J, Nakajima A, Kitaoka M, Hirose J, Urashima T, et al. Priority effects shape the structure of infant-type Bifidobacterium communities on human milk oligosaccharides. Isme J. 2022 Sep;16(9):2265–2279.
- Masi AC, Stewart CJ. Untangling human milk oligosaccharides and infant gut microbiome. iScience. 2021 Dec 1;25(1):103542.
- Cabrera-Rubio R, Kunz C, Rudloff S, García-Mantrana I, Crehuá-Gaudiza E, Martínez-Costa C, Collado MC. Association of maternal secretor status and human milk oligosaccharides with milk microbiota: an observational pilot study. J Pediatr Gastroenterol Nutr. 2019 Feb;68(2):256–263.
- Matsuki T, Yahagi K, Mori H, Matsumoto H, Hara T, Tajima S, Ogawa E, Kodama H, Yamamoto K, Yamada T, et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun. 2016 Jun 24;7:11939.
- Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG, Van Tassell ML, Miller MJ, Jin YS, German JB, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. 2015 Apr 10;3:13.
- Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr. 2005 May;135(5):1304–1307.
- Yu ZT, Chen C, Newburg DS. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology. 2013 Nov;23(11):1281–1292.
- Bridgman SL, Azad MB, Field CJ, Haqq AM, Becker AB, Mandhane PJ, Subbarao P, Turvey SE, Sears MR, Scott JA, et al. Fecal short-chain fatty acid variations by breastfeeding status in infants at 4 months: differences in relative versus absolute concentrations. Front Nutr. 2017 Apr 10;4:11.
- D’souza G, Shitut S, Preussger D, Yousif G, Waschina S, Kost C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat Prod Rep. 2018 May 1;35(5):455–488.
- Bode L, Jantscher-Krenn E. Structure-function relationships of human milk oligosaccharides. Adv Nutr. 2012 May 1;3(3):383S–391S.
- Schwab C, Ruscheweyh HJ, Bunesova V, Pham VT, Beerenwinkel N, Lacroix C. Trophic interactions of infant Bifidobacteria and Eubacterium hallii during L-Fucose and Fucosyllactose degradation. Front Microbiol. 2017 Jan 30;8:95.
- Dogra SK, Martin FP, Donnicola D, Julita M, Berger B, Sprenger N. Human milk Oligosaccharide-stimulated Bifidobacterium species contribute to prevent later respiratory tract infections. Microorganisms. 2021 Sep 12;9(9):1939.
- Cheng L, Kong C, Walvoort MTC, Faas MM, de Vos P. Human milk Oligosaccharides differently modulate goblet cells under homeostatic, proinflammatory conditions and ER stress. Mol Nutr Food Res. 2020 Mar;64(5):e1900976.
- Natividad JM, Marsaux B, Rodenas CLG, Rytz A, Vandevijver G, Marzorati M, Van den Abbeele P, Calatayud M, Rochat F. Human milk Oligosaccharides and Lactose differentially affect infant Gut Microbiota and Intestinal barrier in vitro. Nutrients. 2022 Jun 19;14(12):2546.
- Kong C, Elderman M, Cheng L, de Haan Bj, Nauta A, de Vos P. Modulation of intestinal Epithelial Glycocalyx development by human milk Oligosaccharides and non-digestible carbohydrates. Mol Nutr Food Res. 2019 Sep;63(17):e1900303.
- Williams JE, Price WJ, Shafii B, Yahvah KM, Bode L, McGuire MA, McGuire MK. Relationships among microbial communities, maternal cells, oligosaccharides, and macronutrients in human milk. J Hum Lact. 2017 Aug;33(3):540–551.
- Aakko J, Kumar H, Rautava S, Wise A, Autran C, Bode L, Isolauri E, Salminen S. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef Microbes. 2017 Aug 24;8(4):563–567.
- Moossavi S, Atakora F, Miliku K, Sepehri S, Robertson B, Duan QL, Becker AB, Mandhane PJ, Turvey SE, Moraes TJ, et al. Integrated analysis of human milk microbiota with oligosaccharides and fatty acids in the CHILD cohort. Front Nutr. 2019 May 16;6:58.
- Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence ECH, Patel AL, Hou J, Lewis NE, Bode L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut. 2018 Jun;67(6):1064–1070.
- Patel AL, Kim JH. Human milk and necrotizing enterocolitis. Semin Pediatr Surg. 2018 Feb;27(1):34–38.
- Wu RY, Li B, Koike Y, Määttänen P, Miyake H, Cadete M, Johnson-Henry KC, Botts SR, Lee C, Abrahamsson TR, et al. Human milk Oligosaccharides increase mucin expression in experimental Necrotizing Enterocolitis. Mol Nutr Food Res. 2019 Feb;63(3):e1800658.
- Morrow AL, Meinzen-Derr J, Huang P, Schibler KR, Cahill T, Keddache M, Kallapur SG, Newburg DS, Tabangin M, Warner BB, et al. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J Pediatr. 2011 May;158(5):745–751.
- Masi AC, Embleton ND, Lamb CA, Young G, Granger CL, Najera J, Smith DP, Hoffman KL, Petrosino JF, Bode L, et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut. 2021 Dec;70(12):2273–2282.
- Wejryd E, Martí M, Marchini G, Werme A, Jonsson B, Landberg E, Abrahamsson TR. Low diversity of human milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants. Nutrients. 2018 Oct 20;10(10):1556.
- Nolan LS, Rimer JM, Good M. The role of human milk Oligosaccharides and Probiotics on the Neonatal Microbiome and risk of Necrotizing Enterocolitis: a narrative review. Nutrients. 2020 Oct 6;12(10):3052.
- Jantscher-Krenn E, Lauwaet T, Bliss LA, Reed SL, Gillin FD, Bode L. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. Br J Nutr. 2012 Nov 28;108(10):1839–1846.
- Sodhi CP, Wipf P, Yamaguchi Y, Fulton WB, Kovler M, Niño DF, Zhou Q, Banfield E, Werts AD, Ladd MR, et al. The human milk oligosaccharides 2’-fucosyllactose and 6’-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr Res. 2021 Jan;89(1):91–101.
- Good M, Sodhi CP, Yamaguchi Y, Jia H, Lu P, Fulton WB, Martin LY, Prindle T, Nino DF, Zhou Q, et al. The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr. 2016 Oct;116(7):1175–1187.
- Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004 Sep;145(3):297–303.
- Stepans MB, Wilhelm SL, Hertzog M, Rodehorst TK, Blaney S, Clemens B, Polak JJ, Newburg DS. Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med. 2006 Winter;1(4):207–215.
- Hamer DH, Solomon H, Das G, Knabe T, Beard J, Simon J, Nisar YB, MacLeod WB. Importance of breastfeeding and complementary feeding for management and prevention of childhood diarrhoea in low- and middle-income countries. J Glob Health. 2022 Aug 3;12:10011.
- Triantis V, Bode L, van Neerven RJJ. Immunological effects of human milk Oligosaccharides. Front Pediatr. 2018 Jul 2;6:190.
- Barton SJ, Murray R, Lillycrop KA, Inskip HM, Harvey NC, Cooper C, Karnani N, Zolezzi IS, Sprenger N, Godfrey KM, et al. FUT2 genetic variants and reported respiratory and gastrointestinal illnesses during infancy. J Infect Dis. 2019 Feb 15;219(5):836–843.
- Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Guerrero ML, Meinzen-Derr JK, Morrow AL. Human milk alphal,2-linked fucosylated oligosaccharides decrease risk of diarrhea due to stable toxin of E. coli in breastfed infants. Adv Exp Med Biol. 2004;554:457–461.
- Torres Roldan VD, Urtecho SM, Gupta J, Yonemitsu C, Cárcamo CP, Bode L, Ochoa TJ. Human milk oligosaccharides and their association with late-onset neonatal sepsis in Peruvian very-low-birth-weight infants. Am J Clin Nutr. 2020 Jul 1;112(1):106–112.
- Ramani S, Stewart CJ, Laucirica DR, Ajami NJ, Robertson B, Autran CA, Shinge D, Rani S, Anandan S, Hu L, et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat Commun. 2018 Nov 27;9(1):5010.
- Ruvoën-Clouet N, Mas E, Marionneau S, Guillon P, Lombardo D, Le Pendu J. Bile-salt-stimulated lipase and mucins from milk of ‘secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem J. 2006 Feb 1;393(Pt 3):627–634.
- Hanisch FG, Hansman GS, Morozov V, Kunz C, Schroten H. Avidity of α-fucose on human milk oligosaccharides and blood group-unrelated oligo/polyfucoses is essential for potent norovirus-binding targets. J Biol Chem. 2018 Jul 27;293(30):11955–11965.
- Siziba LP, Mank M, Stahl B, Kurz D, Gonsalves J, Blijenberg B, Rothenbacher D, Genuneit J. Associations of human milk Oligosaccharides with otitis media and lower and upper respiratory tract infections up to 2 years: the Ulm SPATZ health study. Front Nutr. 2021 Oct 25;8:761129.
- Van Niekerk E, Autran CA, Nel DG, Kirsten GF, Blaauw R, Bode L. Human milk oligosaccharides differ between HIV-infected and HIV-uninfected mothers and are related to necrotizing enterocolitis incidence in their preterm very-low-birth-weight infants. J Nutr. 2014 Aug;144(8):1227–1233.
- Bode L, Kuhn L, Kim HY, Hsiao L, Nissan C, Sinkala M, Kankasa C, Mwiya M, Thea DM, Aldrovandi GM. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr. 2012 Oct;96(4):831–839.
- Kuhn L, Kim HY, Hsiao L, Nissan C, Kankasa C, Mwiya M, Thea DM, Aldrovandi GM, Bode L. Oligosaccharide composition of breast milk influences survival of uninfected children born to HIV-infected mothers in Lusaka, Zambia. J Nutr. 2015 Jan;145(1):66–72.
- Moore RE, Xu LL, Townsend SD. Prospecting human milk Oligosaccharides as a defense against viral infections. ACS Infect Dis. 2021 Feb 12;7(2):254–263.
- Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011 Jan 27;469(7331):543–547.
- Antunes KH, Fachi JL, de Paula R, da Silva EF, Pral LP, Dos Santos AÁ, Dias GBM, Vargas JE, Puga R, Mayer FQ, et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat Commun. 2019 Jul 22;10(1):3273.
- Yu Y, Mishra S, Song X, Lasanajak Y, Bradley KC, Tappert MM, Air GM, Steinhauer DA, Halder S, Cotmore S, et al. Functional glycomic analysis of human milk glycans reveals the presence of virus receptors and embryonic stem cell biomarkers. J Biol Chem. 2012 Dec 28;287(53):44784–44799.
- Duska-McEwen G, Senft AP, Ruetschilling TL, Barrett EG, Buck RH. Human milk oligosaccharides enhance innate immunity to respiratory syncytial virus and influenza in vitro. Food Nutr Sci. 2014;5:1387–1398.
- Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, Prudden AR, Boons GJ, Lewis AL, Doran KS, et al. Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem. 2017 Jul 7;292(27):11243–11249.
- Donovan SM, Comstock SS. Human milk Oligosaccharides influence Neonatal Mucosal and systemic immunity. Ann Nutr Metab. 2016;69:42–51.
- Puccio G, Alliet P, Cajozzo C, Janssens E, Corsello G, Sprenger N, Wernimont S, Egli D, Gosoniu L, Steenhout P. Effects of infant formula with human milk Oligosaccharides on growth and morbidity: a randomized multicenter trial. J Pediatr Gastroenterol Nutr. 2017 Apr;64(4):624–631.
- Singh RP, Niharika J, Kondepudi KK, Bishnoi M, Tingirikari JMR. Recent understanding of human milk oligosaccharides in establishing infant gut microbiome and roles in immune system. Food Res Int. 2022 Jan;151:110884.
- Walsh C, Lane JA, van Sinderen D, Hickey RM. Human milk oligosaccharides: shaping the infant gut microbiota and supporting health. J Funct Foods. 2020 Sep;72:104074.
- Johannes L, Jacob R, Leffler H. Galectins at a glance. J Cell Sci. 2018 May 1;131(9):jcs208884.
- Xiao L, van De Worp WR, Stassen R, van Maastrigt C, Kettelarij N, Stahl B, Blijenberg B, Overbeek SA, Folkerts G, Garssen J, et al. Human milk oligosaccharides promote immune tolerance via direct interactions with human dendritic cells. Eur J Immunol. 2019 Jul;49(7):1001–1014.
- Ayechu-Muruzabal V, Overbeek SA, Kostadinova AI, Stahl B, Garssen J, Van’t Land B, Willemsen LEM. Exposure of intestinal Epithelial cells to 2’-Fucosyllactose and CpG enhances Galectin release and instructs dendritic cells to drive Th1 and regulatory-type immune development. Biomolecules. 2020 May 19;10(5):784.
- Kurakevich E, Hennet T, Hausmann M, Rogler G, Borsig L. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17444–17449.
- Šuligoj T, Vigsnæs LK, Abbeele PVD, Apostolou A, Karalis K, Savva GM, McConnell B, Juge N. Effects of human milk oligosaccharides on the adult Gut Microbiota and barrier function. Nutrients. 2020 Sep 13;12(9):2808.
- Xiao L, Van’t Land B, Engen PA, Naqib A, Green SJ, Nato A, Leusink-Muis T, Garssen J, Keshavarzian A, Stahl B, et al. Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci Rep. 2018 Mar 1;8(1):3829.
- Xiao L, Leusink-Muis T, Kettelarij N, van Ark I, Blijenberg B, Hesen NA, Stahl B, Overbeek SA, Garssen J, Folkerts G, et al. Human milk Oligosaccharide 2’-Fucosyllactose improves innate and adaptive immunity in an influenza-specific murine vaccination model. Front Immunol. 2018 Mar 9;9:452.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999 Mar;29(3):342–346.
- Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001 Oct;108(4):516–520.
- Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, Forsythe P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy. 2015 Sep;70(9):1091–1102.
- Korpela K, Salonen A, Hickman B, Kunz C, Sprenger N, Kukkonen K, Savilahti E, Kuitunen M, de Vos WM. Fucosylated oligosaccharides in mother’s milk alleviate the effects of caesarean birth on infant gut microbiota. Sci Rep. 2018 Sep 13;8(1):13757.
- Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J Allergy Clin Immunol. 2019 Feb;143(2):467–485.
- Siziba LP, Mank M, Stahl B, Kurz D, Gonsalves J, Blijenberg B, Rothenbacher D, Genuneit J. Human milk oligosaccharide profiles and child atopic dermatitis up to 2 years of age: the Ulm SPATZ health study. Pediatr Allergy Immunol. 2022 Feb;33(2):e13740.
- Han SM, Binia A, Godfrey KM, El-Heis S, Cutfield WS. Do human milk oligosaccharides protect against infant atopic disorders and food allergy? Nutrients. 2020 Oct 21;12(10):3212.
- Hobbs M, Jahan M, Ghorashi SA, Wang B. Current perspective of Sialylated milk Oligosaccharides in mammalian milk: implications for brain and gut health of newborns. Foods. 2021 Feb 21;10(2):473.
- Hauser J, Pisa E, Arias Vásquez A, Tomasi F, Traversa A, Chiodi V, Martin FP, Sprenger N, Lukjancenko O, Zollinger A, et al. Sialylated human milk oligosaccharides program cognitive development through a non-genomic transmission mode. Mol Psychiatry. 2021 Jul;26(7):2854–2871.
- Oliveros E, Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado-García JM, Buck R, Rueda R, Martín MJ. Sialic acid and Sialylated Oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients. 2018 Oct 16;10(10):1519.
- Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. The prebiotics 3‘sialyllactose and 6‘sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun. 2015 Nov;50:166–177.
- Cho S, Zhu Z, Li T, Baluyot K, Howell BR, Hazlett HC, Elison JT, Hauser J, Sprenger N, Wu D, et al. Human milk 3’-Sialyllactose is positively associated with language development during infancy. Am J Clin Nutr. 2021 Aug 2;114(2):588–597.
- Oliveros E, Ramirez M, Vazquez E, Barranco A, Gruart A, Delgado-Garcia JM, Buck R, Rueda R, Martin MJ. Oral supplementation of 2’-fucosyllactose during lactation improves memory and learning in rats. J Nutr Biochem. 2016 May;31:20–27.
- Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B, Hsieh-Wilson LC. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc Natl Acad Sci U S A. 2006 Jan 3;103(1):21–26.
- Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado-García JM, Martínez-Lara E, Blanco S, Martín MJ, Castanys E, Buck R, et al. Effects of a human milk oligosaccharide, 2’-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem. 2015 May;26(5):455–465.
- Oliveros E, Martín MJ, Torres-Espínola FJ, Segura-Moreno MT, Ramírez M, Santos A, Buck R, Rueda R, Escudero M, Catena A, et al. Human milk levels of 2′-fucosyllactose and 6′-sialyllactose are positively associated with infant neurodevelopment and are not impacted by maternal BMI or diabetic status. J Nutr Food Sci. 2021;4:100024.
- Berger PK, Plows JF, Jones RB, Alderete TL, Yonemitsu C, Poulsen M, Ryoo JH, Peterson BS, Bode L, Goran MI. Human milk oligosaccharide 2’-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS One. 2020 Feb 12;15(2):e0228323.
- Urashima T, Taufik E, Fukuda K, Asakuma S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci Biotechnol Biochem. 2013;77:455–466.
- Hegar B, Wibowo Y, Basrowi RW, Ranuh RG, Sudarmo SM, Munasir Z, Atthiyah AF, Widodo AD, Supriatmo KM, Suryawan A, et al. The role of two human milk Oligosaccharides, 2’-Fucosyllactose and Lacto-N-Neotetraose, in infant nutrition. Pediatr Gastroenterol Hepatol Nutr. 2019 Jul;22(4):330–340.
- Prieto PA. In vitro and clinical experiences with a human milk oligosaccharide, Lacto-N-neoTetraose, and Fructooligosaccharides. Foods Food Ingred J Jpn. 2005;210:1018–1030.
- Marriage BJ, Buck RH, Goehring KC, Oliver JS, Williams JA. Infants fed a lower calorie formula with 2‘FL show growth and 2‘FL uptake like breast-fed infants. J Pediatr Gastroenterol Nutr. 2015 Dec;61(6):649–658.
- Parschat K, Melsaether C, Jäpelt KR, Jennewein S. Clinical evaluation of 16-week supplementation with 5HMO-Mix in healthy-term human infants to determine tolerability, safety, and effect on growth. Nutrients. 2021 Aug 20;13(8):2871.
- Vandenplas Y, de Halleux V, Arciszewska M, Lach P, Pokhylko V, Klymenko V, Schoen S, Abrahamse-Berkeveld M, Mulder KA, Porcel Rubio R, et al. A Partly fermented infant formula with postbiotics including 3’-GL, specific Oligosaccharides, 2’-FL, and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants: a double-blind, randomised, controlled, multi-country trial. Nutrients. 2020 Nov 20;12(11):3560.
- Lasekan J, Choe Y, Dvoretskiy S, Devitt A, Zhang S, Mackey A, Wulf K, Buck R, Steele C, Johnson M, et al. Growth and gastrointestinal tolerance in healthy term infants fed milk-based infant formula supplemented with five human milk Oligosaccharides (HMOs): a randomized multicenter trial. Nutrients. 2022 Jun 24;14(13):2625.
- Ramirez-Farias C, Baggs GE, Marriage BJ. Growth, tolerance, and compliance of infants fed an extensively hydrolyzed infant formula with added 2’-FL Fucosyllactose (2’-FL) human milk Oligosaccharide. Nutrients. 2021 Jan 9;13(1):186.
- Gold MS, Quinn PJ, Campbell DE, Peake J, Smart J, Robinson M, O’sullivan M, Vogt JK, Pedersen HK, Liu X, et al. Effects of an amino acid-based formula supplemented with two human milk oligosaccharides on growth, tolerability, safety, and gut microbiome in infants with cow’s milk protein allergy. Nutrients. 2022 May 30;14(11):2297.
- Storm HM, Shepard J, Czerkies LM, Kineman B, Cohen SS, Reichert H, Carvalho R. 2’-Fucosyllactose is well tolerated in a 100% whey, partially hydrolyzed infant formula with Bifidobacterium lactis: a randomized controlled trial. Glob Pediatr Health. 2019 Mar 15;6:2333794X19833995.
- Román E, Moreno Villares JM, Domínguez Ortega F, Carmona Martínez A, Picó Sirvent L, Santana Sandoval L, Casas Rivero J, Alshweki A, Cercamondi C, Dahbane S, et al. Real-world study in infants fed with an infant formula with two human milk oligosaccharides. Nutr Hosp. 2020 Aug 27;37(4):698–706.
- Kajzer J, Oliver J, Marriage B. Gastrointestinal tolerance of formula supplemented with oligosaccharides. Faseb J. 2016;30:671–674.
- Reverri EJ, Devitt AA, Kajzer JA, Baggs GE, Borschel MW. Review of the clinical experiences of feeding infants formula containing the human milk Oligosaccharide 2’-Fucosyllactose. Nutrients. 2018 Sep 21;10(10):1346.
- Alliet P, Vandenplas Y, Roggero P, Jespers SNJ, Peeters S, Stalens JP, Kortman GAM, Amico M, Berger B, Sprenger N, et al. Safety and efficacy of a probiotic-containing infant formula supplemented with 2’-fucosyllactose: a double-blind randomized controlled trial. Nutr J. 2022 Feb 22;21(1):11.
- Nogacka AM, Arboleya S, Nikpoor N, Auger J, Salazar N, Cuesta I, Alvarez-Buylla JR, Mantecón L, Solís G, Gueimonde M, et al. In Vitro probiotic modulation of the intestinal Microbiota and 2‘fucosyllactose consumption in fecal cultures from infants at two months of age. Microorganisms. 2022 Jan 29;10(2):318.
- Berger B, Porta N, Foata F, Grathwohl D, Delley M, Moine D, Charpagne A, Siegwald L, Descombes P, Alliet P, et al. Linking human milk Oligosaccharides, infant fecal community types, and later risk to require antibiotics. mBio. 2020 Mar 17;11(2):e03196–19.
- Vandenplas Y, Berger B, Carnielli VP, Ksiazyk J, Lagström H, Sanchez Luna M, Migacheva N, Mosselmans JM, Picaud JC, Possner M, et al. Human milk Oligosaccharides: 2’-Fucosyllactose (2’-FL) and Lacto-N-Neotetraose (LNnT) in infant formula. Nutrients. 2018 Aug 24;10(9):1161.
- Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010 May 12;58(9):5334–5340.
- Estorninos E, Lawenko RB, Palestroque E, Sprenger N, Benyacoub J, Kortman GAM, Boekhorst J, Bettler J, Cercamondi CI, Berger B. Term infant formula supplemented with milk-derived oligosaccharides shifts the gut microbiota closer to that of human milk-fed infants and improves intestinal immune defense: a randomized controlled trial. Am J Clin Nutr. 2022 Jan 11;115(1):142–153.
- Goehring KC, Marriage BJ, Oliver JS, Wilder JA, Barrett EG, Buck RH. Similar to those who are breastfed, infants fed a formula containing 2’-Fucosyllactose have lower inflammatory Cytokines in a randomized controlled trial. J Nutr. 2016 Dec;146(12):2559–2566.
- Leung TF, Ulfman LH, Chong MKC, Hon KL, Imsl K, Chan PKS, Delsing DJ, Kortman GAM, Bovee-Oudenhoven IMJ. A randomized controlled trial of different young child formulas on upper respiratory and gastrointestinal tract infections in Chinese toddlers. Pediatr Allergy Immunol. 2020 Oct;31(7):745–754.
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Staiano A, et al. European society of pediatric Gastroenterology, Hepatology, and nutrition. diagnostic approach and management of cow’s-milk protein allergy in infants and children: eSPGHAN GI committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012 Aug;55(2):221–229.
- Nowak-Wegrzyn A, Czerkies L, Reyes K, Collins B, Heine RG. Confirmed Hypoallergenicity of a novel whey-based extensively hydrolyzed infant formula containing two human milk Oligosaccharides. Nutrients. 2019 Jun 26;11(7):1447.
- Vandenplas Y, Żołnowska M, Berni Canani R, Ludman S, Tengelyi Z, Moreno-Álvarez A, Goh AEN, Gosoniu ML, Kirwan BA, Tadi M, et al. Effects of an extensively hydrolyzed formula supplemented with two human milk Oligosaccharides on growth, tolerability, safety and infection risk in infants with cow’s milk protein allergy: a randomized, multi-center trial. Nutrients. 2022 Jan 26;14(3):530.
- Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007 Nov;137(11):2420–2424.
- Arslanoglu S, Moro GE, Boehm G, Wienz F, Stahl B, Bertino E. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012 Jul-Sep;26(3 Suppl):49–59.
- Skórka A, Pieścik-Lech M, Kołodziej M, Szajewska H. Infant formulae supplemented with prebiotics: are they better than unsupplemented formulae? An updated systematic review. Br J Nutr. 2018 Apr;119(7):810–825.
- Salvini F, Riva E, Salvatici E, Boehm G, Jelinek J, Banderali G, Giovannini M. A specific prebiotic mixture added to starting infant formula has long-lasting bifidogenic effects. J Nutr. 2011 Jul;141(7):1335–1339.