ABSTRACT
G-protein coupled receptor 120 (GPR 120) has been implicated in anti-inflammatory functions. However, how GPR120 regulates the neutrophil function remains unknown. This study investigated the role of GPR120 in the regulation of neutrophil function against enteric bacteria. 16S rRNA sequencing was used for measuring the gut microbiota of wild-type (WT) mice and Gpr120−/− mice. Citrobacter rodentium infection and dextran sulfate sodium (DSS)-induced colitis models were performed in WT and Gpr120−/− mice. Mouse peritoneal-derived primary neutrophils were used to determine the neutrophil functions. Gpr120−/− mice showed altered microbiota composition. Gpr120−/− mice exhibited less capacity to clear intestinal Citrobacter rodentium and more severe intestinal inflammation upon infection or DSS insults. Depletion of neutrophils decreased the intestinal clearance of Citrobacter rodentium. GPR120 agonist, CpdA, enhanced WT neutrophil production of reactive oxygen species (ROS) and extracellular traps (NETs), and GPR120-deficient neutrophils demonstrated a lower level of ROS and NETs. CpdA-treated neutrophils showed an enhanced capacity to inhibit the growth of Citrobacter rodentium, which was abrogated by the inhibition of either NETs or ROS. CpdA promoted neutrophil inhibition of the growth of commensal bacteria Escherichia coli O9:H4 and pathobiont Escherichia coli O83:H1 isolated from a Crohn’s disease patient. Mechanically, mTOR activation and glycolysis mediated GPR120 induction of ROS and NETs in neutrophils. Additionally, CpdA promoted the neutrophil production of IL-17 and IL-22, and treatment with a conditioned medium of GPR120-activated neutrophils increased intestinal epithelial cell barrier functions. Our study demonstrated the critical role of GPR120 in neutrophils in protection against enteric bacterial invasion.
Introduction
The intestinal mucosa consists of a single layer of epithelial cells covered by a mucus layer and a number of immune cells underneath. Under normal steady conditions, the intestine is separated from a large quantity of the microbiome, dietary substance, and ingested toxins by the mucus layer and epithelial cells. Meanwhile, there are a lot of immune cells that accumulate into injured mucosa, where commensal microbiota and pathogens invade when intestinal inflammation occursCitation1. It has been shown that innate immune cells, especially neutrophils, are crucial to protecting against bacterial invasion in the intestineCitation2,Citation3.
Neutrophils have long been viewed as the effector cells in acute and chronic inflammationCitation4. Large numbers of neutrophils accumulate in the intestinal mucosa and phagocytose pathogenic microbes upon intestinal inflammation, which damages the intestinal barrierCitation5. However, the protective role of neutrophils has also been recognized in regulating intestinal inflammationCitation3. It has been reported that neutrophils can eliminate bacteria through the production of reactive oxygen species (ROS), formation of extracellular traps (NETs), and secretion of several cytokinesCitation6–8. However, how the neutrophil function is regulated is still not completely understood.
Dietary ω-3 polyunsaturated fatty acids (PUFA) have been implicated in regulating intestinal diseasesCitation9. As a receptor for ω-3 PUFA, G-protein coupled receptor 120 (GPR120) plays a critical role in various physiologic homeostasis mechanismsCitation10. It has also been shown that GPR120 agonist inhibits proinflammatory cytokine production by macrophages and promotes IL-10 production in CD4+ T cellsCitation11–13. Although its role in adipocytes, obesity, and diabetes is well establishedCitation11,Citation14,Citation15, the effect of GPR120 in regulation of intestinal microbiota homeostasis is still unknown. In this report, we demonstrated that GPR120 enhances neutrophil function in controlling gut bacteria, which contributes to inhibiting intestinal inflammation and infection. GPR120 agonist promotes neutrophils to inhibit bacterial growth through upregulation of ROS production and NETs formation, which is mediated by mTOR and glycolysis. In addition, GPR120 increases the neutrophil production of IL-17A and IL-22 and intestinal epithelial cell barrier function.
Results
GPR 120 regulates the gut microbiota.
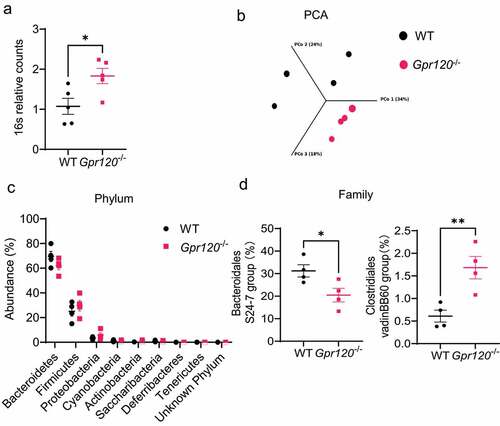
To determine the role of GPR 120 in regulating gut microbiota, we assessed the total gut microbiota between WT and Gpr 120−/− mice by determining 16S DNA counts in feces. We found that there were more bacteria in Gpr 120−/− mice compared with WT mice (). We then determined the intestinal microbiota composition using 16S rRNA sequencing analysis. The principal coordinates based on a Bray-Curtis comparison clearly separated the samples from the WT and Gpr 120−/− mice (). Taxonomically, although there were no significant differences in relative bacteria abundance at the phylum level (), Gpr120−/− mice exhibited a decreased tendency to harbor Bacteroidetes, which has been shown to be decreased in patients with inflammatory bowel disease (IBD)Citation16. Furthermore, we found that Bacteroidales S24–7 group, which belongs to Bacteroidetes phylum, was significantly decreased, and Clostridiales vadinBB60 group, which belongs to Firmicutes phylum, was increased (). Taken together, these results indicate that GPR120 regulates the growth of certain gut microbiota.
Figure 1. Gpr120−/− mice demonstrate altered gut microbiota composition.
Deficiency of GPR120 promotes intestinal inflammation with decreasing clearance of intestinal pathogen
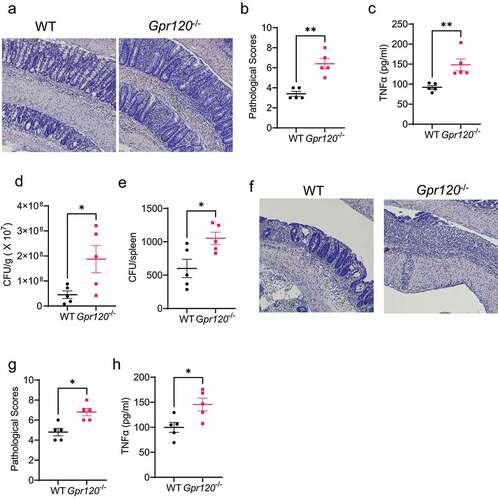
To investigate whether GPR120 regulates pathogen clearance and intestinal inflammation, we first performed Citrobacter rodentium, which is similar to human enteropathogenic Escherichia coli associated with IBD [16], infection in WT and Gpr120−/− mice. After 10 days, we found that Gpr120−/− mice demonstrated more severe colitis compared to WT mice, as demonstrated by higher pathological scores and elevated intestinal TNF-α expression (). In addition, Gpr120−/− mice exhibited increased intestinal Citrobacter rodentium counts in feces and spleens (), indicating that deficiency of GPR120 decreases intestinal pathogen clearance and promotes bacteria translocation to the spleen. To confirm the role of GPR120 in regulating intestinal inflammation, we performed DSS-induced colitis in WT and Gpr120−/− mice. Consistently, deficiency of GPR120 exacerbated intestinal inflammation induced by DSS (). These data suggested the importance of GPR120 in the clearance of intestinal pathogens and control of intestinal inflammation.
Figure 2. Gpr120−/− mice are impaired in the clearance of intestinal Citrobacter rodentium and are susceptible to intestinal inflammation.
Neutrophils protect the intestine against enteric infection of Citrobacter rodentium
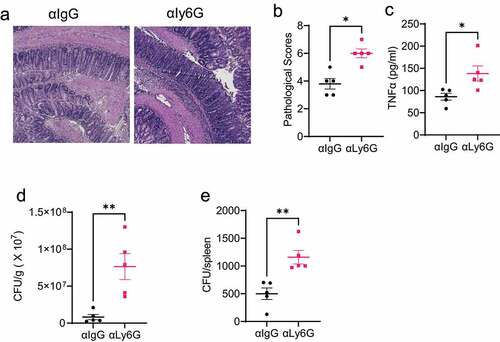
As the first line of defense against gut infection, massive neutrophils accumulate at the inflammation site. We next investigated the role of neutrophils in protecting the host against enteric infection. The WT mice were orally infected with Citrobacter rodentium, and then treated with the control IgG antibody or anti-Ly-6 G neutralizing antibody to deplete neutrophils. Mouse weights were monitored daily, and mice were sacrificed on day 10. The efficiency of the depletion was shown in Supplementary Figure S1A-C. Neutrophil-depleted mice demonstrated more severe intestinal inflammation () than the IgG-treated mice. Additionally, intestinal TNFα secretion was increased in the neutrophil-depleted mice (). Furthermore, the depletion of neutrophils increased Citrobacter rodentium counts in feces () and spleens (), indicating that neutrophils enhance the clearance of Citrobacter rodentium in the intestine and decrease bacteria translocation to other organs.
Figure 3. Depletion of neutrophils decreases the intestinal clearance of Citrobacter rodentium.
GPR120 enhances neutrophil functions related to bacterial killing
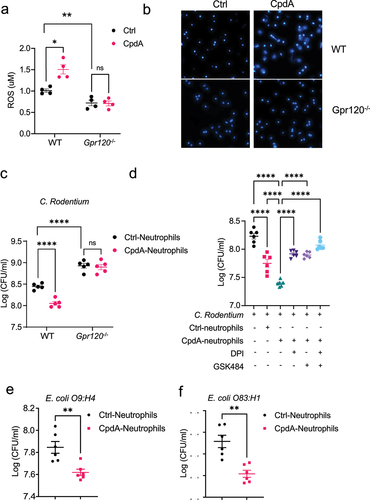
Given that GPR120 regulates intestinal inflammation and intestinal bacteria clearance (), and neutrophils are crucial for protection from enteric pathogen infection (), we next asked whether GPR120 modulates neutrophil functions to clear bacteria. Neutrophils are involved in killing bacteria and pathogens, which is mainly dependent on the production of ROS and the formation of extracellular traps (NETs)Citation17–20. Because of the short life of freshly prepared intestinal neutrophils and the easy preparation of peritoneal neutrophils in large quantitiesCitation21, we used peritoneal neutrophils for in vitro experiments in this study. The purity of prepared peritoneal neutrophils was regularly >95% (Supplementary Figure S2A). We treated WT and GPR120-deficient neutrophils with or without CpdA, a GPR120-selective agonistCitation22. We first determined the toxicity of CpdA on neutrophils and found that CpdA did not affect neutrophils’ viability with doses less than 10 µM (Supplementary Figures S2B-D). Next, we determined whether GPR120 affects ROS production in neutrophils. CpdA-treated WT, but not GPR120-deficient, neutrophils produced a higher level of ROS than control neutrophils (), indicating that CpdA specifically affects on GPR120. In addition, the ROS level was decreased in GPR120-deficient neutrophils compared with WT neutrophils. Then, we investigated the role of GPR120 in regulating the formation of NETs. Neutrophils were treated with or without CpdA in the presence of Hoechst 33,342, a dye that stains the primary component of NETs and nucleic acid. We found that CpdA promoted NETs formation in WT neutrophils, and the level of NETs was decreased in GPR120-deficient neutrophils compared with WT neutrophils (). Consistently, treatment with DHA, a ω-3 PUFA, a natural GPR 120 ligand, promoted ROS production and NETs formation (Supplementary Figures S3A-B).
Figure 4. GPR120 agonist promotes neutrophil inhibition of enteric bacterial growth through the upregulation of ROS and NETs.
CpdA promotes the neutrophil killing of bacteria
To investigate whether CpdA affects neutrophils to kill bacteria, we conducted an anti-bacterial experiment by culturing Citrobacter rodentium with CpdA-treated or control WT and GPR120-deficient neutrophils. As shown in , CpdA-treated WT, but not GPR120-deficient, neutrophils significantly reduced Citrobacter rodentium counts compared with control neutrophils, while the counts were higher when cultured with GPR120-deficient neutrophils compared with WT neutrophils. In addition, DHA-pre-treated neutrophils showed higher capacity to inhibit the growth of Citrobacter rodentium (Supplementary Figure S3C). To determine whether GPR120 promotes neutrophils to kill bacteria through the induction of ROS or/and NETs formation, we cultured Citrobacter rodentium with CpdA-treated or control neutrophils in the presence of the ROS inhibitor, Diphenyleneiodonium (DPI)Citation23, or the NETs inhibitor, GSK484Citation19. Both control and CpdA-treated neutrophils suppressed the growth of Citrobacter rodentium (). Inhibition of ROS or NETs abrogated the CpdA-neutrophil inhibition of bacterial growth (), which was enhanced by the combination of these two inhibitors. In addition, DPI and GSK484 themselves had no effect on growth of Citrobacter rodentium (Supplementary Figure S4). These data indicated that CpdA enhances neutrophil killing of bacteria at least partially through induction of ROS and NETs.
Next, we investigated whether GPR120 enhancement of neutrophil killing is bacterial strain-specific, we cultured Escherichia coli O9:H4, the intestinal commensal bacteria, with CpdA-treated or control neutrophils. CpdA promoted the neutrophil killing of the commensal bacteria (). To determine whether GPR120 also enhances neutrophil killing of pathogenic bacteria in IBD, we cultured Escherichia coli O83:H1, a pathobiont isolated from a patient with Crohn’s diseaseCitation24, with CpdA-treated or control neutrophils. We found that CpdA-treated neutrophils inhibited the growth of Escherichia coli O83:H1 (). Taken all together, these data demonstrated that GPR120 enhances neutrophil killing of both gut commensal bacteria and pathobionts.
GPR120 promotes the neutrophil killing of bacteria through the activation of the mTOR pathway
To investigate the mechanisms underlying GPR120 regulation of neutrophil functions, we determined whether GPR120 enhances mTOR activation, which has been reported to mediate several functions regulated by GPR120 in other cell typesCitation25. We found that CpdA promoted mTOR activation in neutrophils (). To investigate whether GPR120 regulates neutrophil function through enhanced activation of mTOR, we added rapamycin, an mTOR inhibitor, to the neutrophil cultures with CpdA. Blockade of mTOR suppressed ROS production and NETs formation induced by CpdA ().
Figure 5. mTOR mediates GPR120 induction of neutrophil production of ROS and formation of NETs.
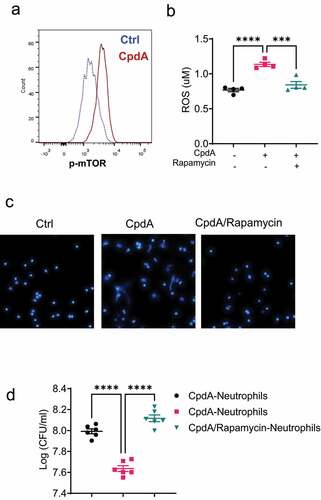
We then investigated whether GPR120 promotes the neutrophil killing of bacteria through the activation of mTOR. We pre-treated neutrophils with CpdA in the presence or absence of rapamycin and then collected the neutrophils to co-culture with Citrobacter rodentium. As shown in , CpdA-treated neutrophils suppressed Citrobacter rodentium growth compared with control neutrophils. However, this effect was abrogated in neutrophils treated with CpdA and mTOR inhibitor. Taken together, these data suggest that GPR120 promotes the neutrophil killing of bacteria by neutrophils through the activation of the mTOR pathway.
Glycolysis mediates GPR120 induction of bacteria-killing by neutrophils
It has been shown that metabolism is crucial in regulating various functions in different types of cellsCitation26,Citation27. Next, we investigated whether GPR120 affects glycolysis and mitochondrial oxidation, the two major metabolic events in neutrophils. We treated neutrophils with or without CpdA for 1 h and then measured their energy phenotype using Seahorse XF Cell Energy Phenotype Test Kit. This assay measures the two major energy-producing pathways, mitochondrial respiration and glycolysis, under baseline and stressed conditions, which are stimulated by the ATP synthase inhibitor oligomycin and the mitochondrial uncoupling agent FCCP. The energy phenotype was shown in . Specifically, there was no difference in Oxygen Consumption Rate (OCR), which represents the mitochondrial respiration level, both in baseline and stressed conditions (). However, CpdA-treated neutrophils showed an increased level of Extracellular Acidification Rate (ECAR), which represents the glycolysis levels, compared with control neutrophils under baseline and stressed conditions (). Taken together, GPR120 promotes glycolysis but not mitochondrial respiration under both baseline and stressed conditions.
Figure 6. GPR120 regulates NETs formation in neutrophils through the upregulation of glycolysis.
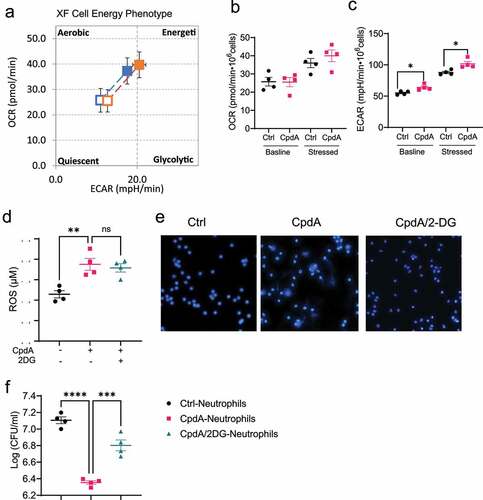
Next, we determined whether glycolysis is involved in the GPR120 regulation of neutrophil functions. We treated neutrophils with or without CpdA in the presence or absence of 2-Deoxy-D-glucose (2DG), an inhibitor of glycolysis. Treatment with 2DG did not affect ROS production induced by CpdA () but suppressed GPR120 induction of NETs formation (). Furthermore, the capacity to inhibit Citrobacter rodentium growth was decreased in neutrophils pre-treated with CpdA and 2DG compared with neutrophils treated with CpdA alone (). These results indicated that the GPR120 promotes neutrophils killing of bacteria at least partially through upregulation of glycolysis.
GPR120-activated neutrophils produce higher levels of IL-17A and IL-22 and promote intestinal epithelial cell barrier function
It has been shown that neutrophils produce IL-17A and IL-22, which promote intestinal production of antimicrobial peptides to inhibit bacterial growth and suppress intestinal inflammationCitation28,Citation29, as well as TGF-β, which induces intestinal epithelial cell production of amphiregulin to promote intestinal epithelial barrier functionCitation30. We next investigated whether GPR120 also regulates neutrophil production of IL-17A, IL-22 and TGF-β. We treated neutrophils with or without CpdA in the absence or presence of IL-23, which stimulates IL-17A and IL-22 in neutrophilsCitation31. As shown in , IL-23 promoted IL-17 and IL-22 production, which was further enhanced by CpdA treatment. In addition, GPR120-deficient neutrophils produced significantly lower levels of IL-17A and IL-22 in the presence of IL-23 (). However, CpdA did not affect TGF-β production (Supplementary Figure S5), indicating that GPR120 specifically regulates IL-22 and IL-17 production in neutrophils. In addition, DHA did not affect IL-17A and IL-22 production (Supplementary Figure S3D).
Figure 7. GPR120 regulates IL-17A and IL-22 production and IEC barrier function in neutrophils.
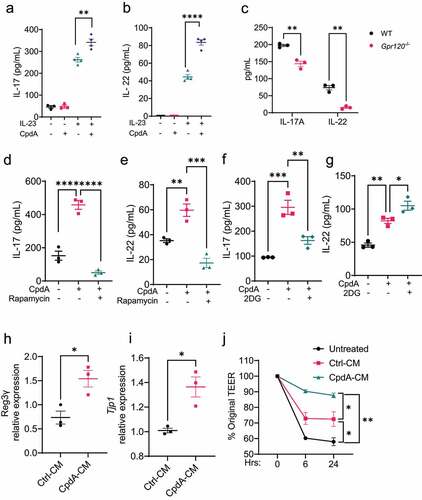
To determine whether mTOR and glycolysis also mediate GPR120 induction of neutrophil production of IL-17A and IL-22, we treated neutrophils with CpdA in the presence or absence of mTOR inhibitor rapamycin or glycolysis inhibitor 2-DG, and measured IL-17A and IL-22 24 h later. As shown in , the addition of rapamycin inhibited GPR120 induction of IL-17A and IL-22. However, inhibition of glycolysis decreased IL-17A production but increased IL-22 production induced by CpdA (), indicating that glycolysis differentially regulated GPR120 induction of IL-17A and IL-22 in neutrophils.
Considering that IL-17A and IL-22 do not affect bacteria directly, we did not investigate whether CpdA induction of IL-17A and IL-22 contributes to inhibiting bacterial growth in this study. IL-17A and IL-22 have been reported to participate in the anti-bacterial activity by promoting intestinal barrier functionsCitation32,Citation33. We collected the culture medium from control and CpdA-treated neutrophils and treated the murine IEC cell line, Mode-K, with the conditioned medium. We found that the conditioned medium of CpdA-treated neutrophils induced higher levels of Reg3γ, an antimicrobial peptide, and tight junction protein– (TJP) expression (). In addition, the conditioned medium from GPR120-treated neutrophils promoted intestinal integrity upon proinflammatory cytokine insults, as demonstrated by a higher level of transepithelial/transendothelial electrical resistance (TEER) ().
Discussion
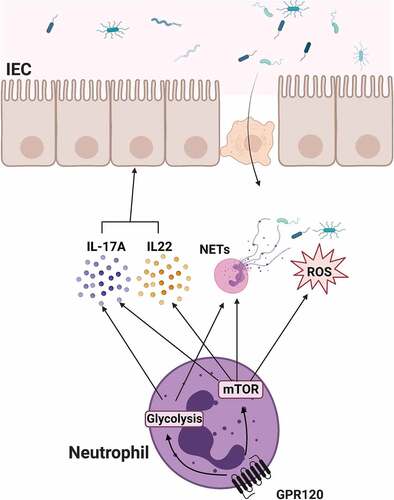
Accumulating evidence demonstrates that neutrophils are critical in regulating homeostasis in several tissues and systems, including the intestine. Besides, dietary components also participate in modulating intestinal homeostasis. ω-3 PUFA is commonly consumed from daily diet and has been implicated in the regulation of intestinal microbiota and intestinal disorders. In the current study, we demonstrated that GPR120, a recently recognized receptor for ω-3 PUFA, promotes neutrophil production of ROS and formation of NETs as well as expression of IL-17A and IL-22 via activation of mTOR and upregulation of glycolysis, which contributes to host against bacteria invade (). Therefore, our study provides novel insights into how GPR120 contributes to the maintenance of intestinal microbiota homeostasis through the regulation of neutrophil functions.
Figure 8. The schematic diagram of GPR120 regulation of neutrophil anti-bacterial function.
The gut microbiota has been well recognized in the regulation of intestinal health and diseases, including IBD. Various mechanisms that control the homeostasis of gut microbiota, and different gut bacteria differentially regulate host responses to gut microbiota and pathogens to promote or inhibit intestinal inflammation. It has been reported that Bacteroidetes is decreased in IBD patientsCitation16. Bacteroidales S24–7 group, which belongs to Bacteroidetes phylum, has been demonstrated to be greatly decreased after the onset of colitisCitation34, indicating its role in modulating intestinal inflammation. In this study, we found that the intestinal bacterial load was increased in Gpr120−/− mice, and Gpr120−/− mice showed different intestinal microbiota profiles, in which the Bacteroidales S24–7 group was significantly decreased, suggesting that GPR120 regulates the amounts and composition of gut microbiota, which may favor the maintenance of a healthy gut microbiome.
Neutrophils are recruited to the infection site and then kill bacteria and pathogens mainly by producing ROS and forming of NETs directlyCitation17–20. Although the effects of GPR120 on other innate cells, including macrophages and dendritic cells, have been investigatedCitation35, the role of GPR120 in regulating functions in neutrophils is still unknown. In this study, we found that the GPR120 agonist promoted both ROS production and NETs formation in neutrophils. Interestingly, GPR120 agonist enhanced neutrophils to inhibit the growth of enteric bacteria, not only the enteric pathogen Citrobacter rodentium, but also gut commensal bacteria Escherichia coli O9:H4 and pathobiont Escherichia coli O83:H1, which might explain the higher intestinal bacterial load in Gpr120−/− mice, indicating that GPR120 contributes to the maintenance of gut microbiota homeostasis probably through regulation of neutrophil controlling of gut bacteria. Furthermore, inhibition of ROS or NETs suppressed neutrophils to kill bacteria induced by GPR120 agonist, indicating that GPR120 promotes neutrophils to inhibit bacteria through upregulation of ROS and formation of NETs. Under healthy conditions, IECs and mucus layer separate commensal bacteria and immune cells, including neutrophils, underneath IECs. Upon injury or colitis or pathogen invasion, commensal bacteria and pathogens could reach IEC and lamina propria. In this case, activation of GPR120 in neutrophils promotes the killing of both commensal bacteria and pathogens when they invade intestinal lamina propria. Although GPR120 promotes neutrophils to kill bacteria, we cannot exclude the possibility of other cells that express GPR120 in changing bacterial composition in Gpr120−/− mice. GPR120 affects neutrophil anti-bacterial function, which might contribute, at least partially, to the altered composition of gut microbiota in Gpr120−/− mice. Therefore, neutrophil-specific GPR120KO mice is important in exploring the role of neutrophil-expressed GPR120 in vivo.
Glycolysis is considered the major metabolism pathway in neutrophils; however, other metabolic pathways, including the pentose phosphate pathway (PPP), the citric acid cycle, and oxidation pathways, have been described recentlyCitation36. In this study, we demonstrated that neutrophils were more glycolytic after the treatment with the GPR120 agonist, whereas the oxidation levels were not altered. Consistent with previous studies showing that glycolysis is involved in NETs formationCitation37,Citation38, inhibition of glycolysis suppressed NETs formation induced by GPR120 agonist, which further inhibited neutrophils from killing bacteria. However, the blockade of glycolysis did not affect ROS production, which mainly relies on PPP in neutrophilsCitation12. Whether PPP is also involved in GPR120 regulation of neutrophils’ anti-bacterial functions was not investigated in this study but should be further studied in the future.
In summary, our study demonstrates that GPR120 promotes neutrophil control of gut bacterial growth. Our study thus provides evidence for GPR120 as a new potential therapeutic target for suppressing intestinal infection and inflammation.
Materials and methods
Mice
Wild-type (WT) C57BL/6 mice were obtained from the Jackson Laboratory, and C57BL/6 Gpr120−/− mice were obtained from Bristol-Myers Squibb. All the mice were bred and maintained in the animal facilities at the University of Texas Medical Branch. Both male and female mice were used. All experiments were reviewed and approved by the Institutional Animal Care and Use Committees of the University of Texas Medical Branch.
Reagents
Neutralizing antibody against Ly-6 G (1A8) was purchased from Bio X Cell. Flow cytometry antibodies, FITC- CD11b, PE/Cy7-Ly6G, and Percp/cy5.5-pmTOR, were purchased from Biolegend. Elisa kits were purchased from BioLegend. Mouse recombinant IL-23 was purchased from BioLegend. Culture medium RPMI 1640, DMEM, and HBSS buffer were purchased from Corning. Thioglycolate broth, CpdA, rapamycin, and 2-Deoxy-D-glucose (2DG) were purchased from Sigma-Aldrich. Diphenyleneiodonium (DPI) and hydrochloride (GSK484) were obtained from Cayman. Amplex® red hydrogen peroxide/peroxidase assay kit and Live/Dead Fixable Dead Cell Stain Kit were purchased from Thermo Fisher Scientific. Citrobacter rodentium strain DBS100 (ATCC) were obtained from ATCC. Escherichia coli O9:H4Citation39 and Escherichia coli O83:H1Citation24 were kindly provided by Dr. Alfredo Torres of UTMB.
Citrobacter rodentium infection mouse model
WT and Gpr120-/- mice were orally administrated with Citrobacter rodentium (5 × 10Citation8/mouse) on day 0. Mice were sacrificed on day 10 post-infection.
For antibody treatment, WT mice were intraperitoneally injected with anti-IgG (4 mg/kg) or anti-Ly6G (4 mg/kg) every day from day 0.
DSS-induced colitis mouse model
WT and Gpr120-/- mice were orally treated with 2% DSS (w/v) in drinking water for 7 days, and the water was changed to normal drinking water for another 3 days. Mice were sacrificed on day 10.
Fecal and splenic Citrobacter rodentium culture
Fresh feces were collected and suspended in cold PBS. After a series of 10-fold dilution, the fecal suspension was seeded onto MacConkey’s agar culture plates. Bacteria CFU counts were normalized to fecal weights. Spleens were immediately collected when mice were sacrificed, homogenized in cold PBS, and seeded onto MacConkey’s agar culture plates. Bacteria counts were normalized to fecal weights. The total bacteria CFU counts in every spleen were determined.
Ex vivo organ culture
The colons were removed and longitudinally opened. After washing the cold RPMI medium three times, two 3-mm circular full-thickness pieces of the colons were obtained using a 3-mm dermal punch and placed in 1 ml complete RPMI media for 24 h at 37 ̊C with 5% CO2. Culture supernatants from the culture were collected for analysis of cytokine content.
ELISA
The cytokine production was measured using ELISA kits (IL-17A, IL-22, TGF-β, and TNF-α) according to the manufacturer’s instructions. Ninety-six-well plates were coated with the indicated cytokine capture antibody overnight at 4°C. After blocking using 1% BSA, samples were added to wells and incubated at room temperature for 2 h, followed by incubation with a detection antibody for 1 h. HRP-labeled streptavidin was then incubated for 30 min. After adding tetramethylbenzidine substrate, the absorbance of each well was measured at 450 nm.
H&E staining and pathological scoring
Colonic tissues were Swiss-rolled and fixed in 10% buffered formalin for 24 h. After dehydration, tissues were embedded, and 5-µm sections were cut. H&E staining was performed after a series of hydrationCitation40. Images were captured by a Leica microscope. Pathological scores were determined by different key parameters based on different mouse modelsCitation41.
Quantitative PCR
Bacterial 16S rDNA: Fecal pellets were collected, and fecal DNA was extracted using phenol chloroform. The same amount of DNA was used for measuring 16S counts by quantitative PCR. Primers are the following: 16S forward: 5’-TCCTACGGGAGGCAGCAGT-3’; 16S reverse: 5’-GGACTAC- CAGGGTATCTAATCCTGTT-3’Citation42. The data were normalized to eukaryotic β-actin.
Gene expression in Mode-K cells: After treatment, Mode-K cells were collected, and total RNA was extracted by TRizol. 200 ng of RNA was used for reverse transcription, and Reg3g and Tjp1 levels were determined by real-time PCR. Primers are following: Reg3g forward: 5’-TCCCAGGCTTATGGCTCCTA-3’; Reg3g reverse: 5’-GCAGGCCAGTTCTGCATCA-3’; Tjp1 forward: 5’- GTTGGTACGGTGCCCTGAAAGA-3’; Tjp1 reverse: 5’- GCTGACAGGTAGGACAGACGAT-3’. The relative expression was normalized to Gapdh.
16S rRNA Sequencing
Fecal bacterial DNA was isolated using a QIAAMP PowerFecal DNA kit (Cat# 12830–50, Lot# 160044775, Qiagen) according to the manufacturer’s instructions. The microbiome samples were analyzed using barcoded high-throughput amplicon sequencing of the bacterial 16S rRNA gene. Quality control and taxonomical assignment of the resulted reads were performed using CLC Genomics Workbench 21.0. Microbial Genomics Module (http://www.clcbio.com). Low-quality reads containing nucleotides with a quality threshold below 0.05 (using the modified Richard Mott algorithm), as well as reads with two or more unknown nucleotides, were removed from the analysis. Reference-based OTU picking was performed using the SILVA SSU v132 97% databaseCitation43. Sequences present in more than one copy but not clustered to the database were then placed into de novo OTUs (97% similarity) and aligned against the reference database with 80% similarity threshold to assign the “closest” taxonomical name where possible. Chimeras were removed from the dataset if the absolute crossover cost was 3 using a k-mer size of 6. The beta diversity was estimated using the Bray-Curtis method based on PCoA axes representing the top three highest variances.
Neutrophil isolation
Mice were injected with 1 ml of 3% thioglycolate broth into the peritoneal cavity. After 4 h, mice were sacrificed, and 10 ml cold PBS buffer containing 5% FBS was injected subsequently intraperitoneally subsequently. After a gentle massage of the abdomen, peritoneal fluid was transferred into centrifuge tubes. Neutrophils were purified using 50% Percoll at 1200 rpm for 20 min.
Neutrophil culture
Neutrophils were cultured in the RPMI 1640 medium containing penicillin-streptomycin and fetal bovine serum at 37°C and 5% CO2 in the presence or absence of CpdA (3 µM) or DHA (5 µM), as well as other inhibitors indicated in the Figure legends. For collecting medium for determining IL-17A and IL-22 production, neutrophils were treated with or without IL-23 (20 ng/mL).
Viability assay
Resazurin (44 µM) was added to the neutrophil culture medium. Cell viability was calculated by subtracting the absorbance at 595 nm from the absorbance at 570 nm at the time points indicated.
Reactive oxygen substrate assay
Amplex® red hydrogen peroxide/peroxidase assay kit was used for measuring ROS production secreted by neutrophils. The reaction mixture, which contains 50 µM Amplex® Red reagent and 0.1 U/mL HRP in HBSS, was added into the 96-well plate and pre-warmed at 37°C for 10 min. WT or GPR120-deficient neutrophils were treated with or without CpdA (3 µM), DHA (5 µM), rapamycin (2 µM), or 2DG (250 µM), and then added to the plate. The ROS was determined by the fluorescence for excitation at 560 nm and emission detection at ~590 nm.
NETs staining
Peritoneal neutrophils were treated with or without CpdA (3 µM), DHA (5 µM), rapamycin (2 µM), or 2DG (250 µM), and then seeded on the poly-lysin-coated coverslips. After 1 h, neutrophils were fixed with 4% paraformaldehyde and stained with Hoechst 33,342 (1 µg/ml) at room temperature for 5 min. The NETs were visualized on a Cytation 5 microscope.
In vitro bacterial killing by neutrophils
Peritoneal neutrophils were cultured with or without CpdA (3 µM) in the presence of rapamycin (2 µM) or 2DG (250 µM) for 1 h. Neutrophils were collected and co-cultured with appropriate aliquots of Citrobacter rodentium strain DBS100 (ATCC), Escherichia coli O9:H4, or Escherichia coli O83:H1, with an initial OD600 value of 0.1–0.2, in the presence or absence of DPI (10 µM) or GSK484 (25uM). The bacterial suspensions were incubated at 37°C for 12 h under aerobic conditions and then transferred to solid MacConkey’s agar culture plates (Citrobacter rodentium) or Luria Broth’s agar culture plates (Escherichia coli O9:H4 and Escherichia coli O83:H1) overnight. Finally, the colony-forming units (CFU) were counted.
Cell metabolism measurement
Neutrophils were pre-treated with or without CpdA (3 µM). After 30 min of treatment, cells (5 × 10Citation5 cells per well) were suspended in Seahorse XF media and seeded into a 96-well Seahorse plate, which was pre-coated with poly-lysin, and subjected to the Seahorse XF Cell Energy Phenotype Assay to determine oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) under baseline and stressed conditions.
Mode-K cell culture and treatment
Neutrophils were cultured with or without CpdA (3 µM) in the RPMI 1640 medium containing penicillin-streptomycin and fetal bovine serum at 37°C and 5% CO2, and the medium was collected after 24 h. Mode-K cells were cultured in 80% DMEM medium containing penicillin-streptomycin, fetal bovine serum, and non-essential amino acid and 20% culture medium of neutrophils at 37°C and 5% CO2. Mode-K cells were collected after 24 h for gene expression.
Transepithelial electrical resistance (TEER) assay
Mode-K cells (300 K) were suspended in 200 µL of DMEM culture medium and then seeded in the insets (0.4 µm polyester membrane). The insets were carefully inserted into the lower chamber (600 µL of DMEM culture medium) of the 24-well Transwell plates. After 24 h, cells were attached to the insert, and the medium in the inserts was changed to a conditioned medium of neutrophils in the presence of proinflammatory cytokines (10 ng/mL of LPS, 40 ng/mL of TNF-α, and 20 ng/mL of IL-1β). TEER levels were determined by Epithelial Volt-Ohm Meter (Millicell ESR-2) at time points indicated.
Statistical analysis
All the data were analyzed using Prism 9.0 (GraphPad Software, San Diego, CA) and presented as mean ± SEM. Analyses were based on whether the data were normally distributed and the number of tested groups for comparison. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Grant support
This work was supported by NIH grants DK112436, DK125011, AI150210, DK124132.
Abbreviations
| CFU | = | colony-forming units |
| DPI | = | Diphenyleneiodonium |
| ECAR | = | Extracellular Acidification Rate |
| GPR120 | = | G-protein coupled receptor 120 |
| NETs | = | neutrophil extracellular traps |
| OCR | = | Oxygen Consumption Rate |
| PPP | = | pentose phosphate pathway |
| PUFA | = | polyunsaturated fatty acids |
| ROS | = | reactive oxygen species |
| WT | = | wild-type |
| 2DG | = | 2-Deoxy-D-glucose. |
Author contributions
Conceptualization: W.Y., and Y.C.; Methodology: Z.Z., W.Y., G.G., and Y.C.; Investigation: Z.Z., W.Y., T.Y., Y.Y. (Yu Yu), X.Z., Y.Y. (Yanbo Yu), C.G., A.J.B., S.Y., G.G., and Y.C.; Resources: Q.Z., M.L.; Writing – original draft preparation: Z.Z., W.Y., and Y.C.; Writing – review and editing: W.Y., and Y.C. with input from all other authors; Supervision: Y.C.; Funding acquisition: Y.C.
Supplemental Material
Download PDF (528.7 KB)Acknowledgments
We appreciate Dr. Sherry Haller of the University of Texas Medical Branch for proofreading the manuscript. was created with Biorender.com.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2190311.
Data availability statement
16S rRNA sequencing data have been deposited in SRA under the BioProject number PRJNA716350 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA716350).
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/19490976.2024.2329383)
Additional information
Funding
References
- Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16:457–17. doi:10.1038/s41579-018-0036-x.
- Davies JM, Abreu MT. The innate immune system and inflammatory bowel disease. Scand J Gastroenterol. 2015;50:24–33. doi:10.3109/00365521.2014.966321.
- Zhou GX, Liu ZJ. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J Dig Dis. 2017;18:495–503. doi:10.1111/1751-2980.12540.
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi:10.1038/nri3024.
- Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi:10.1038/mi.2012.24.
- Zhou G, Yu L, Fang L, Yang W, Yu T, Miao Y, Chen M, Wu K, Chen F, Cong Y, et al. CD177 + neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67(6):1052–1063. doi:10.1136/gutjnl-2016-313535.
- Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017;7:373. doi:10.3389/fcimb.2017.00373.
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science (New York, NY). 2004;303(5663):1532–1535. doi:10.1126/science.1092385.
- Tu M, Wang W, Zhang G, Hammock BD. ω-3 Polyunsaturated fatty acids on Colonic Inflammation and colon cancer: roles of lipid-metabolizing Enzymes Involved. Nutrients. 2020;12(11):3301. doi:10.3390/nu12113301.
- Karakuła-Juchnowicz H, Róg J, Juchnowicz D, Morylowska-Topolska J. GPR120: mechanism of action, role and potential for medical applications. Postepy higieny i medycyny doswiadczalnej (Online). 2017;71:942–953. doi:10.5604/01.3001.0010.5809.
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi:10.1016/j.cell.2010.07.041.
- Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64:362–369. doi:10.1002/iub.1017.
- Yang W, Liu H, Xu L, Yu T, Zhao X, Yao S, Zhao Q, Barnes S, Cohn SM, Dann SM, et al. GPR120 inhibits colitis through regulation of CD4(+) T cell interleukin 10 production. Gastroenterology. 2022;162(1):150–165. doi:10.1053/j.gastro.2021.09.018.
- Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483(7389):350–354. doi:10.1038/nature10798.
- Paschoal VA, Walenta E, Talukdar S, Pessentheiner AR, Osborn O, Hah N, Chi TJ, Tye GL, Armando AM, Evans RM, et al. Positive reinforcing mechanisms between GPR120 and PPARγ modulate insulin sensitivity. Cell Metab. 2020;31(6):1173–88.e5. doi:10.1016/j.cmet.2020.04.020.
- Zhou Y, Zhi F. Lower Level of bacteroides in the gut microbiota is associated with inflammatory bowel disease: a meta-analysis. 2016;2016:5828959. doi:10.1155/2016/5828959.
- El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PMC. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273(1):180–193. doi:10.1111/imr.12447.
- Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016;85:765–792. doi:10.1146/annurev-biochem-060815-014442.
- Twaddell SH, Baines KJ, Grainge C, Gibson PG. The emerging role of neutrophil extracellular traps in respiratory disease. Chest. 2019;156(4):774–782. doi:10.1016/j.chest.2019.06.012.
- Lazzaretto B, Fadeel B. Intra- and extracellular degradation of neutrophil extracellular traps by macrophages and dendritic cells. Journal of Immunology. 2019;203:2276–2290. doi:10.4049/jimmunol.1800159.
- Carlsen ED, Liang Y, Shelite TR, Walker DH, Melby PC, Soong L. Permissive and protective roles for neutrophils in leishmaniasis. Clin Exp Immunol. 2015;182:109–118. doi:10.1111/cei.12674.
- Oh DY, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, Sasik R, Hah N, Chi TJ, Cox JM, et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med. 2014;20(8):942–947. doi:10.1038/nm.3614.
- Kraaij T, Tengström FC, Kamerling SWA, Pusey CD, Scherer HU, Toes REM, Rabelink TJ, van Kooten C, Teng YKO. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun Rev. 2016;15(6):577–584. doi:10.1016/j.autrev.2016.02.018.
- Nash JH, Villegas A, Kropinski AM, Aguilar-Valenzuela R, Konczy P, Mascarenhas M, Ziebell K, Torres AG, Karmali MA, Coombes BK. Genome sequence of adherent-invasive Escherichia coli and comparative genomic analysis with other E. coli pathotypes. BMC Genomics. 2010;11(1):667. doi:10.1186/1471-2164-11-667.
- Gao B, Han YH, Wang L, Lin YJ, Sun Z, Lu WG, Hu Y-Q, Li J-Q, Lin X-S, Liu B-H, et al. Eicosapentaenoic acid attenuates dexamethasone-induced apoptosis by inducing adaptive autophagy via GPR120 in murine bone marrow-derived mesenchymal stem cells. Cell Death & Disease. 2016;7(5):e2235. doi:10.1038/cddis.2016.144.
- Kumar S, Dikshit M. Metabolic Iinsight of neutrophils in health and disease. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.02099.
- Yang W, Yu T, Cong Y. CD4(+) T cell metabolism, gut microbiota, and autoimmune diseases: implication in precision medicine of autoimmune diseases. Precis Clin Med. 2022;5(3):bac018. doi:10.1093/pcmedi/pbac018.
- Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16:185–196. doi:10.1038/s41575-018-0084-8.
- Valeri M, Raffatellu M, Napier B. Cytokines IL-17 and IL-22 in the host response to infection. Pathog Dis. 2016;74(9):111. doi:10.1093/femspd/ftw111.
- Chen F, Yang W, Huang X, Cao AT, Bilotta AJ, Xiao Y, Sun M, Chen L, Ma C, Liu X, et al. Neutrophils promote Amphiregulin production in intestinal Epithelial cells through TGF-β and contribute to intestinal homeostasis. Journal of Immunology. 2018;201(8):2492–2501. doi:10.4049/jimmunol.1800003.
- Chen F, Cao A, Yao S, Evans-Marin HL, Liu H, Wu W, Carlsen ED, Dann SM, Soong L, Sun J, et al. mTOR mediates IL-23 induction of Neutrophil IL-17 and IL-22 production. Journal of Immunology. 2016;196(10):4390–4399. doi:10.4049/jimmunol.1501541.
- Hebert KD, McLaughlin N, Galeas-Pena M, Zhang Z, Eddens T, Govero A, Pilewski JM, Kolls JK, Pociask DA. Targeting the IL-22/IL-22BP axis enhances tight junctions and reduces inflammation during influenza infection. Mucosal Immunol. 2020;13(1):64–74. doi:10.1038/s41385-019-0206-9.
- Xiao Y, Huang X, Zhao Y, Chen F, Sun M, Yang W, Chen L, Yao S, Peniche A, Dann SM, et al. Interleukin-33 promotes REG3γ expression in intestinal Epithelial cells and Regulates gut microbiota. Cell Mol Gastroenterol Hepatol. 2019;8(1):21–36. doi:10.1016/j.jcmgh.2019.02.006.
- Osaka T, Moriyama E, Arai S, Date Y, Yagi J, Kikuchi J, Tsuneda S. Meta-analysis of fecal microbiota and Metabolites in experimental Colitic mice during the inflammatory and healing phases. Nutrients. 2017;9(12):1329. doi:10.3390/nu9121329.
- Zhao C, Zhou J, Meng Y, Shi N, Wang X, Zhou M, Li G, Yang Y. DHA sensor GPR120 in host defense exhibits the dual characteristics of regulating Dendritic cell function and Skewing the balance of Th17/Tregs. Int J Biol Sci. 2020;16(3):374–387. doi:10.7150/ijbs.39551.
- Injarabian L, Devin A, Ransac S, Marteyn BS. Neutrophil metabolic shift during their lifecycle: impact on their survival and activation. Int J Mol Sci. 2019;21(1):21. doi:10.3390/ijms21010287.
- Rodríguez-Espinosa O, Rojas-Espinosa O, Moreno-Altamirano MM, López-Villegas EO, Sánchez-García FJ. Metabolic requirements for neutrophil extracellular traps formation. Immunology. 2015;145:213–224. doi:10.1111/imm.12437.
- Amini P, Stojkov D, Felser A, Jackson CB, Courage C, Schaller A, Gelman L, Soriano ME, Nuoffer J-M, Scorrano L, et al. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat Commun. 2018;9(1):2958. doi:10.1038/s41467-018-05387-y.
- Rasko DA, Rosovitz MJ, Myers GS, Mongodin EF, Fricke WF, Gajer P, Crabtree J, Sebaihia M, Thomson NR, Chaudhuri R, et al. The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. J Bacteriol. 2008;190(20):6881–6893. doi:10.1128/JB.00619-08.
- Yang W, Yu T, Cong Y. Induction of Intestinal Inflammation by adoptive transfer of CBir1 TCR transgenic CD4+ T cells to immunodeficient Mice. J Vis Exp. 2021;178. doi:10.3791/63293-v.
- Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):4457. doi:10.1038/s41467-020-18262-6.
- Yu T, Yang W, Yao S, Yu Y, Wakamiya M, Golovko G, Cong Y. STING promotes intestinal IgA production by regulating Acetate-producing bacteria to maintain host-microbiota Mutualism. Inflamm Bowel Dis. 2023. doi:10.1093/ibd/izac268.
- Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glöckner FO. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014;42(D1):D643–8. doi:10.1093/nar/gkt1209.
