ABSTRACT
Rotavirus (RV) causes severe diarrhea in young children and animals worldwide. Several glycans terminating in sialic acids (SAs) and histo-blood group antigens (HBGAs) on intestinal epithelial cell (IEC) surface have been recognized to act as attachment sites for RV. IECs are protected by the double layer of mucus of which O-glycans (including HBGAs and SAs) are a major organic component. Luminal mucins, as well as bacterial glycans, can act as decoy molecules removing RV particles from the gut. The composition of the intestinal mucus is regulated by complex O-glycan-specific interactions among the gut microbiota, RV and the host. In this review, we highlight O-glycan-mediated interactions within the intestinal lumen prior to RV attachment to IECs. A better understanding of the role of mucus is essential for the development of alternative therapeutic tools including the use of pre- and probiotics to control RV infection.
Introduction
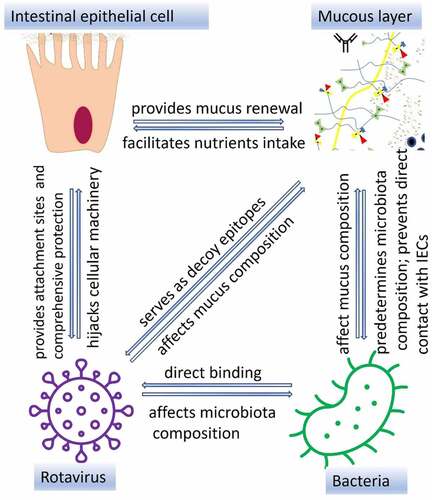
Rotaviruses (RVs) are the major causative agents of acute diarrhea in children and young animals globally.Citation1 RV infects small intestinal epithelial cell (IEC) leading to villus atrophy, increased epithelial cell turnover, enhanced apoptosis, and formation of large vacuoles in enterocytes.Citation2 To infect/enter IECs, RV binds several surface molecules such as sialic acids (SAs)Citation3,Citation4 and histo-blood-group antigens (HBGAs) in genotype-specific manner.Citation5,Citation6 However, presence of RV in the luminal content does not immediately result in its attachment on IECs, since they are protected from external environment by the mucus layer whose major organic component is represented by a variety of mucins, highly glycosylated molecules with complex oligosaccharides, O-glycans, including forementioned HBGAs with or without SA residue.Citation7 Secreted mucins (not attached to IECs) are known to bind RVs thus serving as decoy receptors.Citation8
The mucus layer is also a niche for the gut microbiota whose composition is predetermined by the host factors including the O-glycan profile.Citation9 Growing evidence indicates that microorganisms including beneficial bacteria possess a wide range of factors enabling direct interactions with different components of the host mucus.Citation10 There are several types of interactions between bacteria and mucus in the gut, including selective attachment, mucus degradation, and bacterial regulation of mucus production and composition.Citation8 These bacteria-mucus interactions in the gut also influence the RV pathogenesis and disease outcome. The initial bacterial attachment to O-glycans present in mucus is provided by non-enzymatic glycan-binding proteins referred to as lectins.Citation11 While presence of specific enzymes allows members of the gut microbiota to gradually degrade mucin O-glycansCitation12, some bacteria stimulate O-glycan production by IECs.Citation13 In addition, a wide range of bacteria have also been shown to produce glycans.Citation14 Prior and our recent studies have demonstrated the presence of glycans recognized by human HBGA-specific monoclonal antibodies (Abs) on nonpathogenic phylogenetically diverse Gram-negative and Gram-positive bacteria.Citation15,Citation16 More importantly, bacteria expressing glycans have been shown to bind enteric viruses such as poliovirusesCitation17, norovirusesCitation15 and RVsCitation16 in vitro and in vivo. Thus, these bacterial glycans provide additional attachment sites for RV binding. Taken together, complex interactions within the intestinal mucus result in changes in composition/concentration of decoy receptors for RV thus affecting RV attachment and entry into IECs. This review focuses on the role of O-glycans in the host-microbiota-RV interactions within the GI tract and the implications of these findings on RV disease control strategies.
O-glycans – are major organic constituents of the intestinal mucus
Mucus represents an ancient constituent of the epithelial barrier regulating crucial functions in a wide range of invertebrate and vertebrate species.Citation18 In mammals, mucus is produced by specialized (goblet) epithelial cells scattered in the lining of the gastrointestinal (GI), the respiratory, and the reproductive tracts, as well as the ocular surface.Citation19 Mucus is a complex of proteins, lipids, water, epithelial cells, leukocytes, mucins, and inorganic salts that form a gel-like structure.Citation20 The mucus layer facilitates transport of nutritional components toward the epithelium in the GI tract and the exchange of gases within the respiratory tract. It maintains the viscoelastic properties of the reproductive tract and the preocular tear film. The mucus functions in the gut are mostly determined by mucins, glycoproteins encoded by the family of MUC genes.Citation21
As part of host defense system, mucus within the GI tract serves as a physical barrier that reduces damage to IECs caused by food antigens, commensal microorganisms and the digestive secretions in the gut.Citation20 It also protects IECs from being directly accessed and harmed by various pathogens including parasites, viruses and bacteria.Citation22 Mucus antipathogenic function is achieved in part by the expulsion of pathogen-containing mucus controlled by peristaltic movementsCitation23 and contracting/swaying villi motionsCitation24 contributing to elimination of pathogenic organisms and other particles. Besides these “restrictive” functions, the gut mucus provides attachment sites for certain commensal bacteria promoting GI tract colonization.Citation25 Thus, GI mucus is the primary site where interactions occur between the host and gut microorganisms.
Mucus comprises two layers whose composition and thickness vary throughout the intestine.Citation20 It is the thinnest in the small intestine, while, in the large intestine the mucus layer is up to four times thicker.Citation20 The inner layer is dense and non-penetrable to bacteria under normal physiological conditions. The outer layer, at least two times thicker, is loosely attached, allowing for bacterial binding.Citation26 The permeability of the mucus layer has been found to be age-dependent in swine: it is more penetrable in 2-week old piglets compared to adult pigs.Citation27 Besides, mucus density varies across the intestine,Citation28 which correlates with regional bacterial abundance.Citation20 Taken together, these unique properties of the mucus layers facilitate the corresponding functions of the small and large intestine: nutrient and water absorption (small intestine) and mostly water absorption (large intestine) and protection of IECs against microbial (pathogenic and nonpathogenic) invasion. The latter function is further provided by another important feature of the mucus layer, that of clearing the trapped material by luminal content movement with the average turnover time of the human small intestinal mucus gel and glycocalyx of 6–12 hours.Citation19
Up to 95% of mucus consists of water, while the remaining 5% are the dry matter that contains cell debris, lipids, glycans and various proteins. The major organic part (80% of the total dry matter) of mucus consists of molecules of the mucin family, highly glycosylated proteins. The vast majority of mucin-type glycans are O-linked glycans; however, some N-linked glycans can be found on regions flanking the central protein backbone.Citation29 All mucins are encoded by 22 genes and consist of 80% carbohydrates and 20% proteins.Citation30 There are two structurally and functionally distinct groups of mucins: secreted (gel-forming and non-gel-forming) and membrane-associated. Secreted polymeric gel-forming mucins (MUC2, 5AC, 5B, 6 and 19) that create gel-like structure covering IECs are produced by goblet cells () and secreted monomeric non-gel forming mucins (MUC7 and MUC20) which are water soluble mostly found in bodily fluids such as saliva and tears where they lubricate and protect the eyes and mouth surfaces.Citation31,Citation32 Studies have shown that these structures have region-specific distribution. For example, MUC2 is more abundant in small intestine and colon while MUC5AC and MUC6 predominate the stomach and duodenum.Citation33–35 Secreted mucins (gel forming) lubricate the intestinal mucosa, provide attachment sites for the commensal bacteria, regulate the gut microbiota composition and protect the IECs against pathogen invasion.Citation36 Secreted mucin monomers aggregate via electrostatic and hydrophobic interactions resulting in a net-like polymeric gel structure () that facilitates all the mucus functions.Citation37,Citation38 Both, α-2,3 and α-2,6 N-acetylneuraminic acids (the major SAs) provide a critical role in gel-formation via electrostatic interactions ().Citation37 Mucin core hydrophobic domains further provide gel polymerization ().Citation38 The membrane-associated (transmembrane) mucins (MUC1, 3A, 3B, 4, 12, 13, 15, 16, 17, 18, 20, 21) do not form multimers but bind to IECs via short cytoplasmic domains and form glycocalyx ().Citation39 These mucins play a critical role providing connection between IECs and habitants of the outer mucus layer.Citation40
Figure 1. Schematic representation of secreted and transmembrane mucins. Transmembrane mucins are large and attached to IEC surface via transmembrane and cytoplasmic regions. Cysteine molecules widely present in PTS domains of secreted mucins form intra-disulfide bonds (hydrophobic interactions). In addition, the gel-like structure of secreted mucins is provided by electrostatic interactions within highly glycosylated regions.
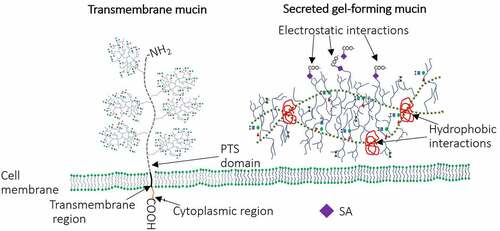
Figure 2. Schematic representation of the mucin family and their structure. Tandem repeat domains – enriched in proline (Pro), threonine (Thr) and/or serine (Ser) (PTS domain) are highly glycosylated [including N-acetylgalactosamine (GalNac), N-acetylglucosamine, fucose, galactose and sialic acid (SA)]. These O-linked glycan domains, represent 50% (w/w) of all mucins.Citation41 Mucin glycosylation occurs within the endoplasmic reticulum and the Golgi apparatus; once glycosylated they are secreted on the apical surface of goblet cells.Citation42 The initial step of mucin-type O-glycosylation in mammals (addition of GalNAc to PTS domain – Tn antigen) is provided by the activity of N-acetylgalactosaminyltransferases (ppGalnac-Ts) - enzymes which are encoded by one of 20 genes. This antigen is further extended by addition of GalNAc, galactose, N-acetylglucosamine and SA (provided by activity of different glycosyltransferases) leading to formation of four different glycan core types that can be included in the structure of MUC1, MUC2, MUC5AC, MUC5B, MUC6–8, MUC 11–13 and MUC 16.Citation39 Activity of FUT2 (active only in secretor individuals) regulates the production of H-type 1 antigen while FUT1 enzyme is responsible for production of H-type 2 antigen (cell-associated antigen). The peripheral terminal region may be presented by l-fucose (Fuc), d-galactose (Gal), N-acetylgalactosamine (GalNac), N-acetylglucosamine (GlcNac) and SA residues, included in the structure of all HBGAs such as A, B, H, Lewis a (Lea), Lewis b (Leb), Lewis x (Lex) and Lewis y (Ley).Citation43.
![Figure 2. Schematic representation of the mucin family and their structure. Tandem repeat domains – enriched in proline (Pro), threonine (Thr) and/or serine (Ser) (PTS domain) are highly glycosylated [including N-acetylgalactosamine (GalNac), N-acetylglucosamine, fucose, galactose and sialic acid (SA)]. These O-linked glycan domains, represent 50% (w/w) of all mucins.Citation41 Mucin glycosylation occurs within the endoplasmic reticulum and the Golgi apparatus; once glycosylated they are secreted on the apical surface of goblet cells.Citation42 The initial step of mucin-type O-glycosylation in mammals (addition of GalNAc to PTS domain – Tn antigen) is provided by the activity of N-acetylgalactosaminyltransferases (ppGalnac-Ts) - enzymes which are encoded by one of 20 genes. This antigen is further extended by addition of GalNAc, galactose, N-acetylglucosamine and SA (provided by activity of different glycosyltransferases) leading to formation of four different glycan core types that can be included in the structure of MUC1, MUC2, MUC5AC, MUC5B, MUC6–8, MUC 11–13 and MUC 16.Citation39 Activity of FUT2 (active only in secretor individuals) regulates the production of H-type 1 antigen while FUT1 enzyme is responsible for production of H-type 2 antigen (cell-associated antigen). The peripheral terminal region may be presented by l-fucose (Fuc), d-galactose (Gal), N-acetylgalactosamine (GalNac), N-acetylglucosamine (GlcNac) and SA residues, included in the structure of all HBGAs such as A, B, H, Lewis a (Lea), Lewis b (Leb), Lewis x (Lex) and Lewis y (Ley).Citation43.](/cms/asset/e6ed8e76-d638-491f-9155-deacdcaa13c1/kgmi_a_2197833_f0002_oc.jpg)
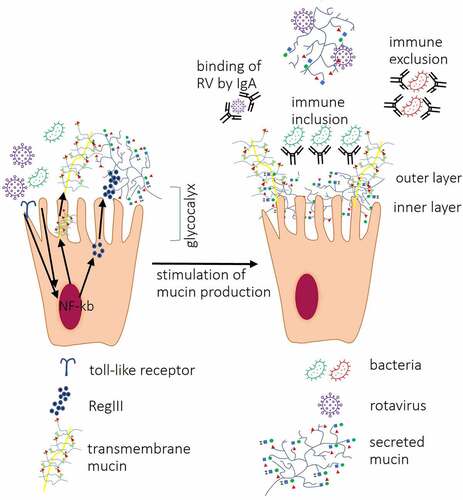
Figure 3. Interactions between IECs and other components of the intestinal mucosa. Interactions between bacterial ligands and Toll-like receptors (TLRs) induce signaling cascades resulting in activation of transcription factor nuclear factor-kappa-chain-enhancer (NF-κb) which lead to increased expression of RegIII proteins, secreted and transmembrane mucins leading to thickening of the mucus layer. Direct contact between RV VP8* and tumor necrosis factor (TNF) receptor associated factor 2 (TRAF2) increases MUC2 transcription. Produced by goblet cells, secreted mucins form two layers: first, dense firmly attached inner layer directly covering IECs surface, non-penetrable for bacteria. Second, loosely attached layer is a habitat for bacteria. Mucin O-glycans within inner and outer layer directly interact with RV and IgA binds RV preventing them to reach IECs. Antibacterial barrier function of mucus layers is further supported by antimicrobial RegIII protein. IgA produced by plasma cells in Peyer’s patches cells block receptors on IEC surface and/or directly bind pathogenic bacteria, immune exclusion, and RV or may facilitate the formation of biofilm (immune inclusion).
The diversity of the GI mucin-type O-glycans is determined by the glycan core structure (cores 1–8, with cores 1–4 being most common), type of HBGA precursor and different terminal modifications that can include fucose, galactose (Gal), N-acetylgalactosamine (GalNAc) and SA ().Citation44 Glycosyltransferases (GTs) provide a critical role in formation of this mucin-type O-glycan biodiversity, while inhibition of these enzymes’ activity results in increased mucus permeability.Citation45 The expression of secreted ABO(H) antigens and the Lewis b (Le b) antigen is regulated by fucosyltransferase 2 (FUT2), encoded by the FUT2 gene, while the expression of membrane-associated H antigen is regulated by FUT1.Citation46,Citation47 Different sialyltransferases are involved in the addition of terminal SAs to O-glycan chains.Citation48 Fucosyltransferase 3 (FUT3) is involved in the biosynthesis of Lewis antigens.Citation49 Three alleles of ABO gene encodes
GTs responsible for converting the H antigen into A and B antigens (alleles A and B), while O allele is an inactive GT that leaves the H antigen unmodified.Citation50 Polymorphisms of fucosyltransferase-encoding genes have been found to determine the fucosyltransferase activity.Citation51 For example, 14 genotypes of FUT3 and 10 genotypes of FUT2 have been recognized only in Chinese population.Citation52 Besides, GTs have been found to interact and compete with each other thus affecting the glycoconjugate profile.Citation53 In addition, O-glycan distribution has been found to vary in different regions of GI tract.Citation47 The biodiversity of mucosal O-glycans may play an evolutionary important role in protection against pathogens known to interact with certain glycans such as HBGAs resulting in lower susceptibility of mammals.Citation54
O-glycans are important ligands for rotavirus attachment and entry
The main targets for RV infection are IECs located at the tips of intestinal villi.Citation55 However, for some strains/genotypes of species A RV (RVA), infection is not limited to IECs, but high-level RVA detection reportedly occurs in extraintestinal tissues including immune cells.Citation56,Citation57 Antigenemia and RNAemia have been reported in children with RVA diarrhea,Citation58,Citation59 suggesting a mechanism of extraintestinal spread of RVAs to highly vascular organs such as liver, cerebrospinal fluid, spleen and lungs. A study by Azevedo and coauthors demonstrated similar observation in pigs inoculated with human RVA.Citation60 However,*Citation61 whether RVA infection is associated with efficient viral replication in immune and/or other blood cells or just passive virus uptake and transport remains to be evaluated. Detection of RVA in extraintestinal tissues has been associated with pathology in some studies. For example, along with the expected changes in the intestine following RVA infection of rats, histopathological changes associated with RVA antigens were observed in the liver and lungs.Citation56 The same study confirmed the ability of G3P5B[3] RVA to infect porcine alveolar macrophages. Recently, a similar observation has been demonstrated for porcine RVA.Citation62 Further, Ciarlet and coauthors observed efficient replication of RVA on cell lines derived from intestine, stomach, breast, bone and lung.Citation63 The wide range of cells permissive for RVA replication may be explained by the presence of common attachment sites in all of these cell types, such as integrins that was reported to enable RVA replication in Chinese hamster ovary cells.Citation63 However, there are no data on the role of O-glycans in extraintestinal replication of RVs.
RV infection requires specific interactions (including virus attachment and entry) between RVs and host cellular attachment sites. RV exposure to the main small intestine proteinase (trypsin) results in the cleavage of the spike protein (VP4) into an N-terminal domain, VP8*, and a C-terminal domain, VP5×.Citation64 The VP8* domain binds to host cellular sialylated glycans.Citation65 However, proteolytic priming of viral particles is not required for the RV binding but is essential for cell membrane penetration and virus entry into the host cellCitation66, suggesting that RV uses different ligands for cell attachment and entry.
There are several surface molecules, such as terminalCitation3 and internalCitation4 (monosialotetrahexosylganglioside, GM1 ganglioside) sialylated glycans, HBGAsCitation6, heat-shock cognate protein (hsc70)Citation67, tight junction proteinsCitation68 and integrinsCitation69 which have been recognized as ligands for RV attachment and entry into IECs. However, the principal receptor for RV attachment/entry remains to be identified. Several approaches have been implemented to dissect the roles of the aforementioned attachment sites in RV replication. For example, cell treatment with sialidase (neuraminidase, NA) cleaving terminal α2,3-, α2,6-, or α2,8-linked SA residues has been found to significantly decrease attachment of some animal RVA strains (simian SA11 G3P[2], RRV G3P[3], bovine NCDV G6P[1] and porcine OSU G5P[7]) to various cells emphasizing the role of SAs as an attachment site for RVA.Citation70 However, most human RVAs (Wa G1P[8], DS-1 G1P[8], ST3 G4P[6] subtype A, K8 G1P[9], S2 G2P[1]) and some animal RVAs (bovine 223 G10P[11], porcine Gottfried G4P[6], equine H2 G3P[12] and FI23 G14P[12]) are not dependent on the presence of sialylated glycans to infect cells.Citation70 These initial observations led to classification of RVAs as NA-sensitive and NA-insensitive (NA-resistant) which later was revisited following evidence that NA treatment only removes terminal SA residues but not the internal ones.Citation71 A study by Haselhorst and colleagues demonstrated an enhanced replication of “sialidase-insensitive” human RVA Wa G1P[8] after SA (α2,3-, α2,6-, or α2,8-linked) removal.Citation72 We have recently demonstrated significantly enhanced replication of porcine RVA RV0084 (G9P[13]) after terminal SA removal with Arthrobacter ureafaciens sialidase (cleaves terminal α2,3-, α2,6-, or α2,8-linked SA residues) treatment.Citation73 Our lab has also demonstrated that human and porcine RVCs utilize sialylated glycans for binding/attachment.Citation74 NA treatment resulted in enhanced porcine RVC Cowden G1P[1] and RVC RV0143 G6P[5] replication, but inhibited the growth of porcine RVC RV0104 G3P[18], further highlighting the role of terminal SA in RV replication.Citation74 Thus, our data suggested that terminal SA residues may mask some other attachment sites recognized by RVs including internal sialylated glycans. For example, internal sialylated glycans have been shown to serve as attachment sites for NA-resistant RVs.Citation4,Citation72,Citation75 Therefore, recently other strategies have been applied to dissect the roles of sialylated glycans in RV replication. Studies have shown that CRISPR/Cas9 knockout of the solute carrier family 35 member A1 gene (SLC35A1, encoding a key GT essential for SA biosynthesis) led to the loss of sialylated glycans on the cell surface.Citation76,Citation77 The latter coincided with loss of NA treatment effects on replication of sialidase sensitive simian RVA.Citation77
The interplay between the RV VP8* domain and sialylated glycans is an example of lectin-glycan interactions characterized by low affinityCitation65,Citation78; while lectins with multiple binding sites have a significantly higher affinity to glycans.Citation79 The ability of lectins to bind sialylated glycans is of importance since neutralizing Abs developed against the SA-binding domain of VP8* inhibit RVA hemagglutination. Thus, VP8* interactions with various glycans are likely to increase the host cell binding capacity of RV.
There are several lectins of different origin (including invertebrates and plants) that bind O-glycans.Citation80 Jolly and colleagues demonstrated ability of galactose-specific plant lectins to inhibit RVA replication in a strain-specific manner.Citation81 The same study showed amino acid sequence similarity (27%) between RV VP5* and the galactose-binding domain of a plant lectin (Ricinus agglutinin) suggestive of similar mechanisms engaged by plant lectins and VP5* while binding host O-glycans. Altogether, these data confirm the significance of highly specific interactions between sugar residues on the IEC surface membranes and within the intestinal mucus with carbohydrate-binding proteins of bacteria (discussed in section 4.1), plants and RVs.
The group of HBGAs play a critical role in RV binding/entry into the IECs. HBGAs are a group of glycans and represent a large family of carbohydrates which consists of more than 300 recognized antigens.Citation82 First detected on the red blood cell surface, these molecules have subsequently been detected in many tissues including epithelial cells lining the respiratory, gastrointestinal, and reproductive tracts, skin and granular secretions.Citation83–87 Along with epithelial cells found to carry high numbers of HBGAs including ABO and Lewis molecules, HBGAs have also been detected in diverse biological fluids such as intestinal content, blood (erythrocytes) and saliva of secretor individuals.Citation86 Thus, HBGAs are present in tissues that are in direct contact with the external environment, and the widespread distribution of sialylated glycans and HBGAs may facilitate RV replication outside of the intestine. The role of HBGAs in RV infection has been demonstrated in our recent study whereby inhibition of HBGA synthesis in porcine ileal enteroids significantly reduced replication of human G1P[8] RVA Wa.Citation73
The glycan-binding specificity of RVs is usually studied in the context of their interactions with HBGA and SA-containing glycan terminal structures. However, specific recognition of mucin cores has also been demonstrated for RVs of different origin ().Citation89,Citation92
Table 1. A summary of glycan cores, SA-containing glycans and HBGA types recognized by different RVA and RVC genotypes in different hosts.
Clinical and in vitro studies have demonstrated that the interactions between RVs and the host O-glycans are both RVA/RVC genotype- and HBGA type-specific (). For example, two RVA genotypes, P[8] (human Wa and RVP) and P[4] (DS1), were shown to recognize both, the Lewis and H-type 1 antigens, while P[6] (ST3) interacted with the H-type 1 antigen only.Citation105 The P[9], P[14] and P[25] genotypes bind to the type A antigens, whereas P[11] interacted with single and repeated N-acetyllactosamine – a precursor of human HBGAs.Citation99,Citation106 However, some studies have demonstrated contradictory results. For example, several epidemiological studies revealed that children with A-type were predominantly infected with a P[8] RVA.Citation105,Citation107,Citation108 This was further corroborated by our results showing that the human RVA Wa strain (G1P[8]) infected and replicated to higher titers in porcine small intestinal enteroids expressing A-antigen.Citation73 However, previously, a crystallography assay failed to confirm that human P[8] RVA Wa binds this antigen.Citation90 Another study by Huang and coauthors in children did not demonstrate any direct binding of type A antigen, however, it generated strong evidence of association.Citation6 The role of HBGA-type A in RV replication has recently been demonstrated in our study whereby porcine RVC Cowden G1P[1] had a higher level of replication in porcine small intestinal enteroids expressing A-antigen while two other genotypes, RV0104 G3[P18] and RV0143 G6[P5] had a preference for H-antigen expressing organoids.Citation74
RV-HBGA interactions have been assessed by X-ray crystallography of a P[14] VP8* in complex with the type A oligosaccharide.Citation91 Based on these findings, human susceptibility to RV infection is determined (at least partially) by their HBGA phenotypes ().
Further, while two α1,2-fucosyltransferases responsible for α(1.2) fucosylation have been recognized (FUT1 and FUT2), in 20% of human population the FUT2 gene is inactive, and such individuals are referred to as non-secretor phenotype ().Citation109 Thus, while in non-secretor individuals O-glycans are expressed only on IECs surface (provided by the activity of FUT1), in secretors individuals (where both FUT1 and FUT2 are active) there are two types of O-glycans: membrane-associated and secreted. The difference in activity of FUT1 and FUT2 enzymes results in different linkages between Gal and GlcNAc (1,3 and 1,4 for type 1 and type 2 precursors, respectively).Citation110 It is of importance to emphasize that expression of HBGA in the small intestine is FUT2-dependent,Citation47 suggesting that non-secretor individuals have a limited glycosylation profile. Clinical studies have not reported RVA infection (P[8] and P[4]) in non-secretor individuals; and even suggested that this phenotype was restricted to P[8] and P[4] RV genotype infections.Citation93,Citation111,Citation112 These data was further supported by a study demonstrating selective recognition of human P[11] to H type 2 antigen (H antigen expressed in non-secretor individuals, ) over H type 1 antigen (expressed in secretor individuals).Citation113 However, recent studies demonstrated P[8] and P[6] but not P[4] infection in non-secretors.Citation97,Citation98 Other studies have shown a strain-specific recognition of precursors type 1 and type 2 for RV P[11].Citation100,Citation101,Citation113 Taken together, RV binding depends on the presence of certain glycan cores, HBGA precursors and terminal sugar residues.
Of interest, FUT2 and Lewis polymorphisms were previously thought to be associated with the low efficacy of RVA vaccines in certain African populations, where the predominant RVA strains as well as FUT2 and Lewis genotype prevalence differ from those in Western populations.Citation93 In contrast to these findings, our recent data dispute this hypothesis by providing evidence that attenuated RVA strains lose their selective affinity for certain HBGAs, but their interactions with SAs remained similar to that of the virulent counterparts.104 The discrepancies described above are likely due to the different models, approaches and assays used in these studies. Thus, experimental data on distinct RV affinity for individual HBGAs are still scarce and/or somewhat inconsistent necessitating additional research.
Interactions between RVAs and intestinal epithelial cells
Mucus layer is the first barrier against RV infection
RV needs to reach the host IEC surface to initiate its replication cycle, which involves complex interactions with various components of the mucosal layer. The ability of viral particles to diffuse through mucus depends on particle size and surface charge.Citation114 While strongly charged particles are trapped, neutral particles diffuse through the mucus.Citation114 Thus, mucus layer acts as a nonspecific defense mechanism of the host against the negatively charged particles, such as outer surface of RVA particles.Citation115 Of importance, MUC2 glycans serve as binding sites for the VP8* domain of the RVA spike protein VP4Citation116 which may be due to the widespread distribution of SA residues and HBGAs as a part of the peripheral carbohydrate structures of this mucin.
Both, extracellular (often extensive) and intracellular domains of transmembrane mucins () play a critical role in protection against pathogenic microorganisms by modulating inflammatory pathways via phosphorylation.Citation40 Cleavage and shedding of the extracellular domain have been suggested to play a role of decoy receptors for pathogenic bacteria.Citation117 In addition, shedding of the extracellular domain of transmembrane mucins is believed to regulate intracellular signal transduction pathways affecting IEC metabolismCitation40 including conformational changes of integrins – another ligand for RV attachment/entry. Shedding of the extracellular domain of transmembrane mucins is regulated by tumor necrosis factor alpha (TNF-α).Citation118 In turn, the production of TNF-α is induced by RV infection.Citation119 Thus, the host response to RV infection increases the cleavage of the highly glycosylated part of membrane associated mucins which leads to increased numbers of decoy epitopes in the mucus layer.
The protective role of purified mucins isolated from human milk against infection caused by several RVAs including SA11 G3P[2], Wa G1P[8], DS-1 G1P[8] and ST3 G4P[6] has been confirmed in vitro and in vivo, demonstrating the beneficial role of non-immunoglobulin factors of breast milk against enteric pathogens.Citation120 The protective role of mucins depends on the origin of the extracted mucins.Citation121 Colonic mucins had a stronger inhibitory effect on RVA replication compared to small intestinal mucins.Citation121 Besides, the same study demonstrated a RV genotype-specific inhibitory effect of crude mucins which was most likely due to the glycan preferences of RVs (). An in vitro study has shown that transmembrane mucin, MUC1, also inhibited RVA infection, whereby MUC1 decreased RVA infection caused by NA-sensitive simian SA11 G3P[2] but not by human NA-insensitive Wa G1P[8].Citation122 The MUC1 is known to be highly sialylatedCitation123 suggesting that the protective role of MUC1 against NA-sensitive RVA is provided by SA residues. Thus, while sialylated O-glycans and HBGAs present on IECs aid in RVA attachment and infection, those secreted in mucus might act as decoy epitopes interfering with the infection. These data emphasize the role of non-secretor status (no HBGA secreted in the mucus layer) in protection against RV infection. However, research to date has not yet determined the role of mucus layer in protection against RV infection in non-secretor individuals.
The key role of mucin sugars in RVA attachment was demonstrated in a study where the carbohydrate removal has been shown to abolish the protective properties of mucins suggesting that the inhibition of RVA infection is O-glycan-mediated.Citation120,Citation121 These results corroborate the findings of in vivo and in vitro studies which demonstrated that the protective role of mucins was associated with the presence of sialylated glycans. Treatment of mucins with NA to remove terminal SA residues led to the loss of its ability to neutralize RVA infection caused by animal SA11 G3P[2], rhesus RV and human Wa G1P[8] RVs in vitro and in vivo.Citation121 However, while the majority of studies have demonstrated protective effects of transmembrane mucins against RV in vitro (as an extract), it is unknown whether the direct interactions between transmembrane mucins and RV facilitate RV attachment to IECs or lead to cleavage of its extracellular domain (with RV bound) and removal from the small intestine via peristaltic and villi contractions. Taken together, secreted and transmembrane mucins regulate O-glycan-mediated interactions between IECs and RV.
Rotavirus infection affects mucus composition
Boshuizen and coauthors demonstrated that RVA infection affects the number of goblet cells along the GI tract in a region-specific manner.Citation124 While no difference in the number of mucin-producing cells has been found in the ileum of RVA infected mice compared to non-infected controls, in the duodenum and jejunum, the numbers of goblet cells were significantly decreased in the RVA infected animals.Citation124 Recently, a study by Engevik and coauthors revealed that RVA depleted the mucin storage in the small but not large intestine and this effect was not due to decreased numbers of goblet cells, further confirming the possibility of direct interactions between RVAs and mucins.Citation8 The same study indicated that mucin depletion was detected within first 48 hours after RVA infection suggesting that the preexisting abundance of mucins in the small intestine and the stimulatory effects of RVA infection on mucin expression do not compensate for high levels of mucins needed at the beginning of infection.Citation8 Thus, these data suggest that mucin-stimulating factors, such as probiotics, may provide an appropriate tool for disease mitigation in the early stages of RVA infection.
More specifically, RV interacts with all types of mucin cores present in the intestine including core 2, 4 and 6.Citation116 For example, increased MUC2 transcription has been shown to be induced via interactions between RV VP8* and cellular TNF-α Receptor Associated Factors (TRAFs) through TRAF2-NF-kB kinase signaling, emphasizing also the role of NF-kB in the pathogenesis of RV infections ().Citation125 Increased production of MUC2 has also been found to be one of defense mechanisms against RVA infection in germ-free (GF) mice.Citation8
The glycosylation profiles of the intestinal mucins vary in the course of RVA infection of mice, whereby at the beginning [1 day post-infection (dpi)] sulfated mucins were predominant, while by the 4 dpi sialomucins became more abundant.Citation124 The ability of extracted mucins to neutralize RVA infection in vitro decreased gradually during the course of infection in mice, further confirming the protective role of early mucins against RVA infection.Citation124 The same study has shown that at 4 dpi the RVA neutralizing activity of mucins extracted from infected animals was higher compared with their noninfected counterparts, suggesting the stimulating role of RVA in mucin glycosylation. However, it is unknown whether the fucosylation pattern in mice is similar to other animals and humans
Overall, these findings suggest that RVA-host interactions induce the production of mucin O-glycans and enhance mucin glycosylation, thereby boosting the protective role of these decoy attachment sites. These studies summarized above confirmed the key role of the mucin concentration/glycosylation at the beginning of the RVA infection. RVA interactions with IECs reduce the amount of mucin decoy attachment sites emphasizing the potential beneficial effects of bacterial mucin-stimulating factors. Besides these factors, mucin concentration and glycosylation status have been found to depend on the diet. High-fat diets were shown to downregulate goblet cell differentiation, glycocalyx formation, and abundance of mucin-stimulating bacteria, while concentration of mucin-degrading bacteria was increased.Citation126,Citation127 However, it is unknown whether a high-fat diet is a confounding factor for RV infection.
Interactions between intestinal epithelial cells and the gut microbiota in the context of RV infection
O-glycan specific host-gut microbiota interactions shape the gut microbiota composition
In nonpathogenic conditions, there is no direct interactions between members of the gut microbiota and IECs. While both mucus layers of the gut mucosa are penetrable to macromolecules and viral particles, the inner mucus layer remains completely impermeable to bacterium-sized particles.Citation28 In addition, there are other components of the innate immune system contributing to the localization and composition of the gut microbiota. For example, host lectins (galectins, C-type lectins and siglecsCitation128 are known to regulate the gut microbiota composition via O-glycan specific interactions. The inner mucus layer in the small intestine is completely penetrable by bacteria in RegIIIγ (C-type lectin)-deficient mice (), whereas in the colon the inner layer completely prevents the contact between bacteria and IECs.Citation129 Similarly, bacterial attachment to components of the intestinal mucus is also O-glycan specific. Bacterial lectins (mostly present on pili, fimbriae and flagella) have been found to directly bind mucin terminal sugar residues.Citation25,Citation130 Selective carbohydrate specificity of bacterial lectins has been demonstrated for several members of the gut microbiota.Citation11,Citation25,Citation131,Citation132 Along with beneficial effect of commensal bacteria lectins on the intestinal homeostasis,Citation133 their O-glycan-specific interactions have also been found to affect RV infection. Selective carbohydrate specificity of plant and bacterial lectins has been shown to block RVA infection in vitro.Citation16,Citation81 The protective effect of bacterial lectins against RV relies on direct interaction between bacterial lectins and membrane-associated host glycans, thus blocking these glycans from directly binding to RV. However, in vivo, the nearly sterile conditions of the inner mucus layerCitation28 restrict contacts between bacterial lectins and membrane-associated O-glycans, thus limiting the beneficial effect of bacterial lectins against RV infection. In addition, bacterial lectins may exacerbate RV infection by blocking the decoy attachment sites (secreted O-glycans) within the outer mucus layer.
Another mechanism that keeps bacteria away from IECs is production of s (secretory) IgA Abs. sIgA Abs bind a broad range of phylogenetically diverse bacteria by recognizing common epitopes such as glycan structure, which further emphasize the role of glycans in host-microbiota interactions.Citation134 sIgA possesses several protective mechanisms, including binding to IECs, Ab independent blocking of pathogens and neutralization of bacterial virulence factors ().Citation135,Citation136 On the other hand, sIgA Abs have been found to promote growth of commensal bacteria, such as B. fragilis, in vitro and in vivo by facilitating the biofilm formation, thus, providing immune inclusion ().Citation137,Citation138 B. fragilis was also shown to upregulate the expression of FUT2 gene.Citation139 Thus, the known association between the increased abundance of B. fragilis in RV-infected individualsCitation140 is a protective mechanism against RV infection. Overall, in the absence of pathological process (steady state), there is no direct bacteria-IECs contact, therefore, the term bacterial colonization should be interpreted as a persistent presence of bacteria within the outer mucus layer. However, the overall effect of the gut microbiota on IECs is mediated by multiple mechanisms that do not require direct contact between the gut bacteria and IECs. First, the metabolism of IECs is regulated by interactions between bacteria and transmembrane mucins.Citation40,Citation117 Second, the gut microbiota promotes the IEC homeostasis through production of microbial soluble factors (discussed below).Citation141
Microbiota regulates mucus production and composition
Studies have shown that mucus composition of GF animals differs from that of conventional counterparts, whereby the inner mucus layer of GF mouse colon was found to be penetrable to bacteria-sized beads.Citation142 However, conventionalizing of GF mice resulted in the impenetrable status of the inner mucus layer.Citation142 Gut microbiota has been found to regulate 10% of the host transcriptome including genes encoding cell proliferation and metabolism.Citation143 The ability of the gut microbiota to influence glycosylation patterns within the intestine has been studied extensively. The presence of microbiota in conventional mice has been shown to increase glycosylation of secreted mucin MUC2 by upregulation of genes encoding GTs compared to GF mice.Citation13 More specifically, MUC2 transcription is induced by the interaction between the gut microbiota and IECs through NF-κB signaling induction ().Citation144 Thus, the presence of microbiota in the gut may be considered as a factor increasing protection against RVA infection.Citation8,Citation145
Interestingly, the effect of the gut microbiota on mucus composition is enzyme and gut region-specific. For example, presence of microbiota was shown to increase expression of St3gal4 sialyltransferase in the small intestine while decreasing the expression of St3gal6 α2,3-sialyltransferase.Citation13 This study also demonstrated that colonization of GF mice led to increased expression of FUT2 in large but not in small intestineCitation13, suggesting that these effects are enzyme- and site-specific.
Gut colonization by microbiota has been found to affect relative ratios between different types of O-glycans. For example, a significant change in sialylated/fucosylated glycan ratio occurs within the gut in the process of colonization. At birth, the expression of sialylated glycans in ‘aseptic’ gut is relatively high compared to the concentration of fucosylated glycans.Citation146 However, the intestinal mucosa of adults is highly fucosylated and characterized by lower expression of SA-containing glycans.Citation146 Similarly, Meng and coauthors have shown that the expression of fucosylated epitopes has been gradually increasing after colonization or during recovery from antibiotic treatment.Citation147 Taken together, these data may provide an additional explanation for the increased susceptibility of younger individuals to RVA infection compared to adults.
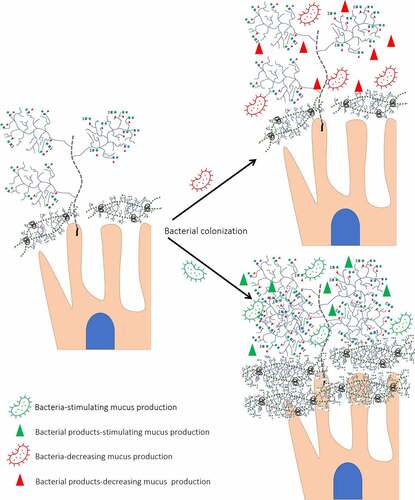
The effect of bacterial colonization is also supported by the activity of members of mitogen-activated protein kinases (MAPK) (extracellular signal-regulated kinases and the c-Jun N-terminal kinase).Citation147 MAPK activation was found to be dependent on the transmembrane mucin cleavage facilitated by bacterial proteases.Citation148 Therefore, while bacterial glycosidases are involved in O-glycans consumption (as discussed below), bacterial proteases are involved in IEC metabolism regulated by interaction with transmembrane mucins ().
Figure 4. Different effects of bacteria on mucin expression/glycosylation. Presence of glycosyl hydrolases allows bacteria to degrade O-glycans decreasing its protective role against RV infection. In addition, bacterial proteases cleave transmembrane mucins. In contrast, bacteria-stimulating bacteria by interaction with transmembrane proteins increases mucin concentration and glycosylation.
Probiotic bacteria have been found to stimulate mucin production in vitro and in vivo, where supplementation of rats with a probiotic mixture (Lactobacilli, Bifidobacteria, and Streptococci) for 7 days led to a 60% increase of basal luminal mucin content.Citation149 This increase coincided with the increased production of gel-forming mucin MUC2, while the expression of genes encoding the membrane-associated MUC1 was only slightly affected. These findings suggest that the probiotics supplementation enhances the production of decoy epitopes which may be helpful in RV binding and elimination. This study also revealed that not only bacterial cells, but also the conditioned media of the probiotic mixture stimulate mucin secretion in vitro. Later, this finding was confirmed in vitro using Bacteroides thetaiotaomicron (B. thetaiotaomicron)-derived conditioned media.Citation150 Of note, incubation of HT-29 and Caco-2 cells with short chain fatty acids (SCFAs) produced by gut microbiota resulted in increased fucosylation (leading to increased amount of HBGAs).Citation151 This emphasized the critical role of the gut microbiota in shaping the intestinal mucus composition. These data suggest that even bacterial metabolites provide beneficial effects on mucin expression further justifying postbiotic use.
A study io mice revealed that while mucin fucosylation protected the host against the invasion by pathogenic bacteria via inhibiting the expression of bacterial virulence genes, increased SA catabolism led to microbial dysbiosis and gut inflammation.Citation152 Thus, sialidase producing bacteria may exacerbate RVA infection in two ways: by degradation of SA residues from mucins leading to reduced numbers of decoy epitopes for RVA binding, and by induction of a pro-inflammatory environment. However, since RVA and RVC have been shown to differently interact with terminal SA-residues (discussed earlier), sialidase-active bacteria are likely to have different effects on replication of different RVs.
A study in mice demonstrated that colonization of the intestine not only led to increased mucin production, but also to increased production of longer O-glycans (i.e. more sialylated, fucosylated), thus protecting core mucins from bacterial proteases.Citation13 Surprisingly, same study reported that some commensal bacteria have an antagonistic effect on mucin secretion.Citation13 While colonization of rats with B. thetaiotaomicron resulted in increased goblet cell proliferation and higher proportion of O-glycans carrying NeuAc or NeuGc residues compared to GF rats, inoculation with another commensal, Faecalibacterium prausnitzii attenuated this effect.Citation153 Caballero-Franco and colleagues demonstrated that bacterial effect on mucus secretion is genus-specific: while conditioned medium of various Lactobacillus species (L. plantarum, L. acidophilus, L. casei, L. debrueckii) increased mucus secretion, less appreciable effect was observed for those obtained from single Bifidobacterium longum culture and combined B. longum + S. salivarius cultures.Citation149
Thus, while the overall effect of bacterial colonization has been found to upregulate mucin glycosylation, the individual features of bacteria should be taken into account when developing optimal strategies to stimulate mucin glycosylation in order to protect IECs from GI tract infection such as RVA infection.
Mucus composition shapes the gut microbiome structure
Similar to glycan-binding specificity of RVs (discussed in section 2), the composition of the gut microbiota has also been found to depend on the presence of secreted O-glycans.Citation9,Citation154,Citation155 Differences in the composition of microbial communities between non-secretors and secretors suggest that more diverse glycosylation profile of secretor individuals might determine gut microbial composition.Citation154 Additionally, lower species richness was demonstrated in non-secretor individuals compared to secretors.Citation155 Another study demonstrated a higher diversity of the two dominant groups of the human intestinal microbiota: Eubacterium rectale-Clostridium coccoides and Clostridium leptum in individuals expressing group B/AB compared to individuals expressing group A and H antigens.Citation9 Whether this difference is due to the ability of bacteria to preferentially utilize certain HBGAs remains to be evaluated. However, Davenport and colleagues did not find an association between the HBGA/secretor status and gut microbiota composition among 1,500 twins suggesting that further studies are required to dissect the role of the host O-glycans in determining gut microbiota composition.Citation156
At the family level, the relative abundance of Prevotellaceae and Paraprevotellaceae was shown to be higher in non-secretor individuals.Citation157 Bacteria belonging to Prevotellaceae family was found to be associated with increased RV shedding and RV-IgA response in individuals vaccinated against RV infection,Citation158 suggesting that this taxa may enhance RV replication. Wacklin and coauthors demonstrated that secretor individuals had a higher richness and diversity of Bifidobacteria (including B. adolescentis) compared to non-secretor counterparts.Citation159 These bacteria has been demonstrated to block RV infection in vitro in a genotype-specific manner,Citation16,Citation160 suggesting that evaluation of anti-RV effect of bacteria requires the use of different RV genotypes/strains/species.Citation9
Mucin sulfation is hypothesized to affect the gut microbiota composition. Of importance, O-linked sulfate may be attached to the 6-hydroxyl of N-acetyl-D-glucosamine (6S-GlcNAc) and terminal D-galactose (Gal) carbohydrates at hydroxyl positions 3-, 4- or 6- (3S-, 4S- and 6S-Gal, respectively).Citation161 Thus, sulfation is likely to affect antigenicity of carbohydrates for bacteria by masking mucin molecules from bacterial glycosidases preventing their degradationCitation162 (discussed in section 4.4). Moreover, RV infection is associated with reduced number of sulfated mucin-containing cells,Citation124 suggesting that sulfation of terminal carbohydrates may increase RV binding.
Mucus as an energy source for microbiota
Taking into account the role of mucin O-glycans serving as decoy receptors for RV binding, the ability of the gut microbiota members to degrade these molecules becomes especially important factor for RV infection. Indeed, O-glycans, as carbohydrates, may serve as a carbon and energy source for the gut microbial community. An earlier study showed that the absence of the gut microbiota in cecum leads to enlarged cecum full of undegraded mucus.Citation163 The ability to degrade mucin molecules has been demonstrated for individual members of all major bacterial phyla of the gut microbiota: Actinobacteria, Bacteroidetes, Firmicutes and Verrucomicrobia Citation164 as well as for several anaerobic and aerotolerant pathogenic bacteria.Citation165,Citation166 Mucin-degrading activity is provided by a group of bacterial enzymes called mucinases which consists of proteases (their role described above), sialidases, glycosidases (glycoside hydrolases: galactosidase, α1–2-fucosidase, α1–3/4-fucosidase) and hexaminidase responsible for the N-acetyl-D-glucosamine, galactose and fucose degradation.Citation167 Wide distribution of Gram-negative Bacteroidetes is associated with its sophisticated enzymatic machinery and the ability to degrade a wide spectrum of complex glycans; while Gram-positive Firmicutes possess a highly selective glycan-degrading activity.Citation168
Several members of the gut microbiota have demonstrated the ability to utilize sialylated glycans within the mucus layer ().Citation169,Citation170 Sialidases have been shown to initiate the sequential degradation of mucins in vitro. These enzymes are encoded by clustered genes called Nan clusters which have been found in E. coli and in members of Clostridia, Bacteroides, and Bifidobacterium. Lack of degradation of SA-containing glycans by Lactobacillus members was consistent with the absence or reduced number of copies of the genes encoding for glycosyl hydrolase enzymes.Citation171 The ability of mucin-degrading bacteria to decrease protective effects of mucus has been demonstrated in vitro, where addition of intestinal murine mucin to MA104 cell line was shown to decrease RVA replication, while pre-treatment of mucin with B. thetaiotaomicron or A. muciniphila led to increased RVA infection.Citation8 In contrast, pre-treatment of mucin with L. acidophilus did not affect the release of mucin oligosaccharides.Citation8 Thus, degradation of sialylated glycans by some bacteria decreases the role of mucus as a decoy epitope for RV. In addition, terminal SAs mask O-glycans from their further degradation by other bacterial enzymes,Citation172 suggesting the different role of sialidase-possessing bacteria for SA-sensitive and insensitive RVs.
Bacterial sialidases are divided into three classes: hydrolytic (cleave α2–3-, α2–6- and α2–8-linked terminal SA residues); trans-sialidases (α2–6- and α2–8- specificity) and recently discovered intramolecular trans-(IT)-sialidases (specificity restricted to α2–3- linkage).Citation173,Citation174 The hydrolitic sialidases with broad spectrum of terminal SA activity were used in a majority of studies describing the role of sialidase treatment in RV infectionCitation3,Citation74, but some bacteria possess sialidase restriction specificity to α2–8-linked terminal SA residues.Citation175 Moreover, other studies also demonstrated sialidase activity against GM-1 ganglioside, suggesting that bacterial sialidases are able to cleave internal SA residues.Citation175,Citation176 Several studies have revealed the significant role of gangliosides in RVA infection,Citation4,Citation94 indicating that sialidase activity against gangliosides may contribute to replication of certain RVs.
However, the ability of bacteria to release free SAs is not always associated with SA consumption by the same bacterial species.Citation177 Some bacteria lacking sialidase activity have been reported to consume Sas. For example, Clostridium difficile has been found to consume free Sas due to the presence of the nan operon (gene encoding catabolic pathways for SA), but this ability is not mediated by sialidase activity.Citation177 These data indicate that presence of sialidases in certain bacteria does not necessarily lead to decreased number of SAs for RV attachment.
Similar to the role of sialylation, mucin sulfation has also been found to protect terminal carbohydrates of O-glycans from bacterial degradation. For example, presence of the wide spectrum of sulfatases has been demonstrated to provide a competitive colonization for B. thetaiotaomicron and A. muciniphila . Citation178,Citation179 These data indicated the role of bacteria with broad spectrum of glycosyl hydrolases (sialidases, sulfatases) necessary for initial degradation of O-glycans in decreased protective effects of intestinal mucins against RVA infection in vitro.Citation8
Mucin degradation has also been reported to be HBGA-specific. While two members of Ruminococcus genus were found to produce HBGA-A and HBGA-H degrading alpha-glycosidase sialidases, two Bifidobacterium strains were also shown to consume some components from the porcine gastric mucin but do not utilize HBGA-A.Citation180 Other human commensal bacteria including E. coli, Enterococcus faecalis, and Bifidobacterium strains have not been found to degrade HBGA-related constituents from porcine gastric mucin.Citation180 Taken together, presence of certain bacteria in mucin glycans might be considered as a factor reducing the number of decoy receptors for RVA infection. However, bacterial fermentation of mucin type O-glycans results in production of SCFAs which are beneficial for IECs integrityCitation181 and immune function.Citation182 Thus, more studies are needed to evaluate the role of mucin-degrading bacteria in replication of different RVs.
RVA/Bacteria interactions with Integrins/Hsc70
Several integrins including α2β1, α4β1, α4β7, αVβ3 and αXβ2 have been shown to be involved in RVA attachment and entry. Graham and coauthors demonstrated that while RVAs of bovine, mice and monkey origin interact with three integrins, α2β1, αXβ2, and αVβ3, none of porcine RVAs (both SA sensitive and insensitive) was integrin-dependent.Citation183 Pathogenic and nonpathogenic bacteria have also been shown to interact with cell host integrins. For example, integrin α4β1 was demonstrated to interact with H. pylori adhesin, an outer membrane protein (OMP).Citation184 Coburn and Cugini demonstrated that αvβ3-integrin-binding protein OMP P66 secreted by B. burgdorferi serves as an adhesin molecule allowing B. burgdorferi to colonize host cells.Citation185 Probiotic extracts bind β3-integrin and Hsc70 (RVA ligands) on MA104 cell surface membranes, limiting RVA attachment and leading to decreased RVA infection.Citation69 Additionally, Hsc70 has been identified as a component of the host cells that is utilized by several pathogens such as E. coli, S. typhimurium and L. monocytogenes for their attachment.Citation186
Studies have shown a direct connection between the expression of integrins and intestinal mucins, where expression level of integrins on cell surface membranes regulated production of mucins. For example, β1-integrin subunit overexpression was found to reduce MUC5AC but not MUC5B levels (secreted mucins).Citation187 Interestingly, α2β1 and β2 integrin expression was shown to be increased after infection caused by human and animal RVAs.Citation188 In turn, mucins were shown to regulate integrin conformation.Citation189 Changes in integrin conformation led to an increase in their affinity for extracellular ligands including pathogens.Citation190 For example, transmembrane mucin MUC1 (its cytoplasmic tail) was demonstrated to affect the integrin-mediated adhesion of Yersinia pseudotuberculosis.Citation191 Therefore, mucins may affect RVA binding to integrins via conformational changes in integrins. Presence of integrin-binding proteins on beneficial bacteria and/or their capability to interact with Hsc70 have not yet been identified, hence warrant further research.
Other host factors affecting microbiota-RV interactions
Besides the intestinal mucus composition, several other factors play significant role in RV-microbiota-host interactions. There is strong evidence of the antimicrobial properties of host bile and to a lesser extent digestive enzymes playing a role in RV-microbiota-host IEC interaction.Citation192 However, some of the gut microbiota members possess bile tolerance, thus this ability is strain specific. For example, within isolated group of Lacticaseibacillus rhamnosus 11 strains were sensitive, 3 – resistant and 8 – tolerant in terms of growth in presence of bile salts.Citation193 This agrees with a study for Bifidobacterium demonstrated the contrasting results for B. infantis and B. longum. Citation194 Bile salt hydrolase activity was shown to play a key role in successful adaptation to growing in the presence of bile salts for members of pathogenic, commensal and probiotic bacteria.Citation195 Of note, the effect of bile salts on the gut microbiota has been shown to be concentration dependent. While low levels of bile salts resulted in increased abundance of Gram-negative bacteria, high levels coincided with increased proliferation of Gram-positive bacteria and reduction of the Gram-negative Bacteroides. Citation196
Members of Lactobacillus, Enteroccocus, Bifidobacterium, Bacteroidetes and Clostridium have been found to possess a variety of enzymes allowing them to transform bile salts.Citation197 Furthermore, GF mice had increased secretion of cholesterol and bile acids compared to their conventional counterparts,Citation198 suggesting that the gut microbiota plays a key role in cholesterol metabolism. Overall, studies have shown that the presence of bile (which is secreted into the duodenum and mainly absorbed in ileum) limits bacterial abundance in the small intestine (with 10Citation4,Citation5 CFU/mL in duodenum compared to distal part of ileum where populations reach up to 10Citation7,Citation8CFU/mL).Citation199
Kim and Chang observed that simian RVA SA11 G3P[2] and human RVA Wa G1P[8] replication was reduced by the bile acid treatment of MA104 cells.Citation200 However, an in vivo study demonstrated the ability of RVA to infect bile duct cells, suggesting that the other components of bile, such as cholesterol, may have an opposite effect on RVA replication in vivo.Citation201 Since RVA replication is cholesterol-dependent,Citation202 the presence of cholesterol in bile but not in bile acid extracts may explain these conflicting results. While little is known about RVC replication, our recent study demonstrated that depletion of cellular cholesterol inhibited replication of porcine RVC Cowden G1P[1], suggesting a similar role of cellular cholesterol in RVC replication.Citation74 These data suggest that along with unique features of the mucus layer in the small intestine reducing bacterial density, the presence of bile further limits the ability of some bacteria to provide their beneficial (mucin-, sIgA- stimulating) effects.
Interactions between microbiota and rotavirus
Direct rotavirus-microbiota interactions
Several studies have demonstrated expression of glycans by bacteria.Citation15,Citation16,Citation203,Citation204 While in mammals, mucins act as a barrier protecting epithelial cells from pathogen attachment, bacterial glycans serve as a defense factor against the host immune system allowing for molecular mimicry and immune evasion, and as virulence factors facilitating host cell invasion.Citation205 Similar to mammals, protein glycosylation in bacteria is catalyzed by GTs.Citation206 Despite the fact that bacterial GTs have a low nucleotide similarity to mammalian GTsCitation207, the enzymatic properties of bacterial GTs are similar to those of human, bovine, mouse and other species.Citation208 More specifically, these similarities have been detected for enzymes responsible for HBGA and SA methabolism.Citation208 Eventually, expression of bacterial glycans and their recognition by RVs and human glycan Abs suggests a critical role of bacteria expressing glycans in RV infection.Citation16
Segmented filamentous bacteria interact directly with RVA affecting its infectivity and disease severity in mice.Citation209 Our lab demonstrated the direct binding of E. coli Nissle 1917 but not L. rhamnosus GG to Wa (G1P[8]) RVA particles or Wa RVA virus-like particles (VP2/4/6) but not to VP2/6 virus-like particles.Citation210 The protective role of E. coli Nissle 1917 against RVA infection has been evaluated and compared with that of L. rhamnosus GG, whereby inoculation of GF piglets with E. coli Nissle 1917 decreased diarrhea severity and virus shedding after RVA challenge to a greater extent than L. rhamnosus GG inoculation.Citation210 Recently, we have shown that E. coli Nissle 1917 but not L. rhamnosus GG bound RVA and RVC and decreased replication of multiple RVA strains in vitro. Citation16 Moreover, members of two genera, Ruminococcus and Oxalobacter that express glycans (A, B, H and Lewis A) on their surface were shown to bind Wa G1P[8] RVA strain.Citation211 Thus, bacterial glycans could provide protection against RVA infection in vivo.
Recent studies have demonstrated that proteins in probiotic extracts binding to Hsc70 and β3 integrin inhibited RVA infection of MA104 cells by blocking viral adhesion rather than entry.Citation69 Studies have also shown that Enterococcus cloacae produces glycans that are capable of binding RVA via interaction with the VP8* domain thereby inhibiting its replication.Citation157 The ability of HBGA-expressing bacteria to directly bind noroviruses (NoVs) has been shown to protect NoVs from acute heat stress and facilitate NoV infection in vitro.Citation212 These studies of RVA and NoV infections emphasize that significant differences occur between in vivo and in vitro infections. In the course of in vivo infection, RVA interacts with O-glycans on the IEC surface as well as secreted O-glycans within the intestinal mucosa and/or glycans present on the bacterial surface and therefore may be removed from the gut by villous movement.Citation213 (). However, conventional continuous cell cultures do not recapitulate in vivo mucus turnover. While gut colonization with commensal microbiota does not result in direct contact between bacteria and the IEC surfaceCitation214, addition of bacteria to continuous cell lines leads to the direct bacterial adhesion and can even cause cell death.Citation215 Thus, in vitro assays have significant limitations for studying the tripartite RVA-bacteria-host interactions.
In addition to using sialylated glycans as an energy source, some bacteria, such as strains of E. coli, P. multocida and B. pseudocatenulatum are known to possess N-acylneuraminate cytidylyltransferases (CMP-Neu5Ac synthetases), enzymes responsible for SA methabolism.Citation216–218 However, production of sialylated glycans in bacteria is not limited to de novo biosynthesis and does not require the presence of all enzymes for SA methabolism.Citation219 Bacterial sialyltransferases allow bacteria to use an external 5´-monophosphate (CMP)-activated SA (e.g., CMP-N-acetyl-neuraminic acid; CMP-Neu5Ac) to synthetize their own SAs.Citation220 Therefore, the sialylated glycans on the bacterial cell surface play a role as anti-recognition molecules, allowing bacteria to remain undetected by the host immune system.Citation221 Thus, bacterial consumption of O-glycans does not necessarily lead to decreased numbers of RVA attachment sites within the intestinal mucosa. However, the role of sialylated glycan-producing bacteria in the context of RVA infection remains poorly understood.
Microbiota upregulate immune responses to RVA infection
Key roles of microbiota include postnatal immune system development, regulation and promotion of protective immunity against pathogens.Citation222,Citation223 sIgA secretion as a part of extrafollicular and T-cell independent Ab responses has been found to be controlled by commensal bacteria.Citation224 The increased transcytosis of IgA through IECs is mediated by the polymeric immunoglobulin receptor (pIgR). Some bacteria upregulate plgR expression by the same MyD88-dependent TLR signalingCitation225 as has been demonstrated for antimicrobial C-type lectins.Citation129 Cash and coauthors observed that the intestine of GF mice had significantly lower concentrations of secreted sIgA, smaller Peyerˈs patches, and reduced RegIIIγ expression.Citation226
Several investigators have evaluated the role of gut microbiota in immune responses to RVA infection.Citation210,Citation227–229 The beneficial effect of probiotics against virus infection has been studied extensively for lactic acid bacteria.Citation227,Citation228 For example, Laino and colleagues showed that L. delbrueckii produces immunomodulatory extracellular polysaccharides (EPSs) that allow for interactions between bacteria and host IECs by interacting with PRRs expressed by nonimmune and immune cells.Citation227 Most recently, Kanmani and colleagues showed that an innate immune response triggered by TLR3 activation in porcine IECs was differentially modulated by EPS from L. delbrueckii. Citation228 Certain probiotic bacteria were demonstrated to upregulate immune responses to RVA vaccine.Citation229 Our lab further demonstrated that colonization of gnotobiotic (Gn) piglets with E. coli Nissle 1917 resulted in increased RVA-specific IgA Ab titers in serum and intestinal contents after vaccination and challenge with human RVA compared with L. rhamnosus GG colonized counterparts.Citation230 Supporting our previous observations,Citation210 this study suggested that the beneficial effect of bacteria on the immune system is strain-specific. Thus, bacteria provide sIgA-stimulating and strain-specific effects in the intestine.
Antibiotic treatment before RVA inoculation increased the concentration of IgA-Ab producing cells in the intestine which correlated with delayed and diminished RVA infection in a mouse model.Citation231 However, in contrast, enhanced RVA infection has been demonstrated in Gn piglets colonized with commensal microbiota and treated with ciprofloxacin compared to the untreated group.Citation145 Others demonstrated that the commensal microbiota supports persistent murine NoV infection, whereas antibiotic treatment resulted in diminished viral shedding and viral loads in intestinal tissues.Citation232 These inconsistencies may reflect the differential effects of the antibiotics used and presence of antimicrobial resistance genes in certain bacteria.
RVA infection affects microbiota composition
RVA infection dramatically alters microbiota composition often decreasing the intestinal microbiota diversity, especially of Proteobacteria and increased number of opportunistic pathogens.Citation233 Our recent study has shown that RVA infection resulted in increased Firmicutes abundance which coincided with reduction in Proteobacteria.Citation234 While the intestinal microbiota in healthy individuals were mainly represented by Bacteroidetes, the dominant phylum of the patients with diarrhea was Firmicutes.Citation235 These changes were not only evident at the phylum level, but variations in microbial composition at the species level associated with RVA infection of children were also noted.Citation140
RVA infection increased the abundance of the mucin-degrading Bacteroides in miceCitation8, humansCitation233 and piglets.Citation236 The abundance of a member of the genus Lactobacillus, lacking mucin-degrading activity, was decreased during the first 3 days of RV infection in mice.Citation8 Remarkably, both, mucin stores and gut microbiota composition were fully restored to the pre-infection levels at day 3 post-infection in a strain-specific manner: RVA infection led to increased abundance of B. fragilis, whereas the abundance of B. vulgatus and B. stercoris was decreased. Thus, RVA infection is complicated by the bacteria-mediated decrease in concentration of protective mucins, further facilitating RV replication. Collectively, these factors may contribute to the increased diarrhea severity and virus shedding during first 2–4 days after infection. However, whether the increased abundance of mucin-degrading bacteria is a mechanism that reduces or increases RVA infection in vivo is unknown. These bacteria may have a prominent effect on sIgA production as shown for B. ovatus Citation237 or carry glycans as decoy epitopes that bind RVA as was demonstrated by our recent study.Citation16 Finally, as was shown for B. thetaiotamicron, bacteria may stimulate O-glycan expression supplying additional decoy epitopes for diverse RVs.Citation150
Conclusions and future perspectives
Despite significant knowledge accumulated in the last 10–15 years regarding RVA pathogenesis, the mechanisms regulating interactions between RVA and host cellular attachment sites remain poorly understood. The discovery of O-glycans such as HBGAs as ligands for RVA attachment/entry has expanded our knowledge about the role of host-related factors that influence RVA infection outcomes. Usually the virus-host interactions at or near the cellular surface membranes are the focus of extensive research. However, the important initial interactions occurring within the intestinal lumen and the mucus layer remain understudied. While many body organ systems, including cardiovascular and nervous, are protected from the external environment by a physical barrier, other body systems such as digestive, respiratory, integumentary, and reproductive, combine both physical barrier and absorptive, transport, and exchange functions to protect themselves from the external environment. These functions are supported by the two mucus layers which are the part of the universal innate immune system of aquatic and terrestrial metazoans. In turn, these functions of mucus are promoted by mucus glycoproteins (mucins, including O-glycans) that are present not only within the intestinal mucosa in secreted and membrane bound forms, but also on the IEC surface where they serve as sites for RVA attachment/entry ().
The gut microbiota regulates mucin production in a strain-specific manner, whereby certain enzymes produce glycans that aid in evasion of host immunity and production of biofilms. On the other hand, mucin-degrading bacteria decrease concentration of O-glycans, reducing their protective effects as decoy attachment sites for various pathogens including RV species. Moreover, these gut microbiota members directly bind host O-glycans sequestering them to restrict viral pathogen binding. All these features of the gut microbiota, including the stimulation of the immune response to RVA are strain-specific, meaning that the effects of the microbiota on the RVA-host interactions is reliant on the microbiota composition. Thus, targeted modulation of gut microbiota composition including pro-, pre- and postbiotics is an appropriate and innovative approach for RV infection control. Since RV – O-glycan interactions are genotype-specific, a similar strategy may be implemented to modulate RV infection outcome. Collectively, RVA infection outcome is a function of multidirectional and complex RV-microbiota-host O-glycans interactions with RV genotype-/bacterial species-dependent characteristics. The findings summarized here suggest that RVA adaptation to the host and genetic diversity are influenced by the host and bacterial glycan variability and the ensuing interactions. This knowledge needs to be further evaluated, expanded and considered for development of effective control measures of RVA and other intestinal pathogens.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Vlasova AN, Amimo JO, Saif LJ. Porcine rotaviruses: epidemiology, immune responses and control strategies. Viruses Internet. 2017 [accessed 2022 Jul 9];9(3):48. doi:10.3390/v9030048.
- Lundgren O, Svensson L. Pathogenesis of Rotavirus diarrhea. Microbes Infect Internet. 2001 [accessed 2022 Apr 4];3(13):1145–31. doi:10.1016/S1286-4579(01)01475-7.
- Ciarlet M, Ludert JE, Iturriza-Gómara M, Liprandi F, Gray JJ, Desselberger U, Estes MK. Initial interaction of rotavirus strains with N-acetylneuraminic (sialic) acid residues on the cell surface correlates with VP4 genotype, not species of origin. J Virol. 2002;76(8):4087–4095. doi:10.1128/jvi.76.8.4087-4095.2002.
- Martínez MA, López S, Arias CF, Isa P. Gangliosides have a functional role during rotavirus cell entry. J Virol. 2013;87(2):1115–1122. doi:10.1128/JVI.01964-12.
- Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK, Prasad BVV. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature Internet. 2012 [accessed 2021 Dec 2];485(7397):256–259. doi:10.1038/nature10996.
- Huang P, Xia M, Tan M, Zhong W, Wei C, Wang L, Morrow A, Jiang X. Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J Virol Internet. 2012 [accessed 2021 Dec 2];86(9):4833–4843. doi:10.1128/JVI.05507-11.
- Zhou J, Zhang W, Liu W, Sheng J, Li M, Chen X, Dong R. Histological study of intestinal goblet cells, IgA, and CD3+ lymphocyte distribution in Huang-huai white goat. Folia Morphol (Warsz). 2020;79(2):303–310. doi:10.5603/FM.a2019.0082.
- Engevik MA, Banks LD, Engevik KA, Chang-Graham AL, Perry JL, Hutchinson DS, Ajami NJ, Petrosino JF, Hyser JM. Rotavirus infection induces glycan availability to promote ileum-specific changes in the microbiome aiding rotavirus virulence. Gut Microbes Internet. 2020 [accessed 2021 Dec 3];11(5):1324–1347. doi:10.1080/19490976.2020.1754714.
- Mäkivuokko H, Lahtinen SJ, Wacklin P, Tuovinen E, Tenkanen H, Nikkilä J, Björklund M, Aranko K, Ouwehand AC, Mättö J. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol. 2012;12:94. doi:10.1186/1471-2180-12-94.
- Sicard J-F, Le Bihan G, Vogeleer P, Jacques M, Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front Cell Infect Microbiol. 2017;7:387. doi:10.3389/fcimb.2017.00387.
- De Oliveira DMP, Hartley-Tassell L, Everest-Dass A, Day CJ, Dabbs RA, Ve T, Kobe B, Nizet V, Packer NH, Walker MJ, et al. Blood group antigen recognition via the group a streptococcal M protein mediates host colonization. mBio Internet. 2017 accessed 2023 Feb 17;8(1):e02237–16. doi:10.1128/mBio.02237-16.
- Fernandez-Julia P, Commane DM, van Sinderen D, Munoz-Munoz J. Cross-feeding interactions between human gut commensals belonging to the Bacteroides and Bifidobacterium genera when grown on dietary glycans. Microbio Res Reports Internet. 2022 [accessed 2023 Feb 13];1(2):12. doi:10.20517/mrr.2021.05.
- Arike L, Holmén-Larsson J, Hansson GC. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology Internet. 2017 [accessed 2022 Apr 12];27(4):318–328. doi:10.1093/glycob/cww134.
- Comstock LE, Kasper DL. Bacterial glycans: key mediators of diverse host immune responses. Cell Internet. 2006 [accessed 2023 Mar 12];126(5):847–850. doi:10.1016/j.cell.2006.08.021.
- Miura T, Sano D, Suenaga A, Yoshimura T, Fuzawa M, Nakagomi T, Nakagomi O, Okabe S. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J Virol Internet. 2013 [accessed 2022 Jan 15;87(17):9441–9451. doi:10.1128/JVI.01060-13.
- Raev SA, Omwando AM, Guo Y, Raque MS, Amimo JO, Saif LJ, Vlasova AN. Glycan-mediated interactions between bacteria, rotavirus and the host cells provide an additional mechanism of antiviral defence. Benef Microbes. 2022;13:1–14. doi:10.3920/BM2022.0026.
- Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host & Microbe Internet. 2014 [accessed 2023 Mar 3];15(1):36–46. doi:10.1016/j.chom.2013.12.004.
- Lang T, Klasson S, Larsson E, Johansson MEV, Hansson GC, Samuelsson T. Searching the evolutionary origin of epithelial mucus protein components-mucins and FCGBP. Mol Biol Evol. 2016;33(8):1921–1936. doi:10.1093/molbev/msw066.
- Johansson MEV. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. Plos One. 2012;7(7):e41009. doi:10.1371/journal.pone.0041009.
- Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol Internet. 2001 [accessed 2021 Dec 2];280(5):G922–929. doi:10.1152/ajpgi.2001.280.5.G922.
- Melhem H, Regan-Komito D, Niess JH. Mucins dynamics in physiological and pathological conditions. Int J Mol Sci Internet. 2021 [accessed 2023 Mar 19];22(24):13642. doi:10.3390/ijms222413642.
- Johansson MEV, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol Internet. 2016 [accessed 2022 Oct 27];16(10):639–649. doi:10.1038/nri.2016.88.
- Patel KS, Thavamani A. Physiology, peristalsis. In: StatPearls Internet. Treasure Island (FL); StatPearls Publishing: 2022 [accessed 2023 Feb 15]. http://www.ncbi.nlm.nih.gov/books/NBK556137/
- Gayer CP, Basson MD. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal Internet. 2009 [accessed 2023 Feb 15];21(8):1237–1244. doi:10.1016/j.cellsig.2009.02.011.
- Uchida H, Kawai Y, Kinoshita H, Kitazawa H, Miura K, Shiiba K, Horii A, Kimura K, Taketomo N, Oda M, et al. Lactic acid bacteria (LAB) bind to human B- orThe permeability of the mucus layer has been fou H-antigens expressed on intestinal mucosa. Biosci Biotechnol Biochem. 2006;70(12):3073–3076. doi:10.1271/bbb.60407.
- Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, Brugiroux S, Keller I, Macpherson JA, Rupp S, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. Internet 2015 [accessed 2023 Feb 17];6(1):8292. doi:10.1038/ncomms9292.
- Krupa L, Bajka B, Staroń R, Dupont D, Singh H, Gutkowski K, Macierzanka A. Comparing the permeability of human and porcine small intestinal mucus for particle transport studies. Sci Rep Internet. 2020 [accessed 2021 Dec 2];10(1):20290. doi:10.1038/s41598-020-77129-4.
- Ermund A, Schütte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol Internet. 2013 [accessed 2022 May 19];305(5):G341–347. doi:10.1152/ajpgi.00046.2013.
- Nason R, Büll C, Konstantinidi A, Sun L, Ye Z, Halim A, Du W, Sørensen DM, Durbesson F, Furukawa S, et al. Display of the human mucinome with defined O-glycans by gene engineered cells. Nat Commun Internet. 2021 [accessed 2023 Feb 17];12(1):4070. doi:10.1038/s41467-021-24366-4.
- Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41(10):1479–1485. doi:10.1177/41.10.8245407.
- Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC2008The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. [accessed 2022 May 19]. 105(39):15064–15069. doi:10.1073/pnas.0803124105
- Hansson GC. Mucins and the microbiome. Annu Rev Biochem Internet. 2020 [accessed 2023 Mar 25];89(1):769–793. doi:10.1146/annurev-biochem-011520-105053.
- Reis CA, David L, Carvalho F, Mandel U, de Bolós C, Mirgorodskaya E, Clausen H, Sobrinho-Simões M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem Internet. 2000 [accessed 2023 Feb 10];48(3):377–388. doi:10.1177/002215540004800307.
- Nordman H, Davies JR, Lindell G, de Bolós, Real F, Carlstedti C, de Bolós F. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J Internet. 2002 [accessed 2023 Feb 10];364(1):191–200. doi:10.1042/bj3640191.
- Magalhães A, Reis CA. Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors. Braz J Med Biol Res Internet. 2010 [accessed 2023 Feb 10]. 43:611–618. 10.1590/S0100-879X2010007500049.
- Bergstrom KSB, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. Plos Pathog. 2010;6(5):e1000902. doi:10.1371/journal.ppat.1000902.
- Baos SC, Phillips DB, Wildling L, McMaster TJ, Berry M. Distribution of sialic acids on mucins and gels: a defense mechanism. Biophys J Internet. 2012 [accessed 2022 Aug 12];102(1):176–184. doi:10.1016/j.bpj.2011.08.058.
- Javitt G, Calvo MLG, Albert L, Reznik N, Ilani T, Diskin R, Fass D. Intestinal gel-forming mucins polymerize by disulfide-mediated dimerization of D3 domains. J Mol Biol Internet. 2019 [accessed 2023 Feb 17];431(19):3740–3752. doi:10.1016/j.jmb.2019.07.018.
- Offner GD, Troxler RF. Heterogeneity of high-molecular-weight human salivary mucins. Adv Dent Res. 2000;14:69–75. doi:10.1177/08959374000140011101.
- Sheng YH, Triyana S, Wang R, Das I, Gerloff K, Florin TH, Sutton P, McGuckin MA. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 2013;6(3):557–568. doi:10.1038/mi.2012.98.
- Coltart DM, Royyuru AK, Williams LJ, Glunz PW, Sames D, Kuduk SD, Schwarz JB, Chen X-T, Danishefsky SJ, Live DH. Principles of mucin architecture: structural studies on synthetic glycopeptides bearing clustered mono-, Di-, Tri-, and hexasaccharide glycodomains. J Am Chem Soc Internet. 2002 [accessed 2023 Feb 17];124(33):9833–9844. doi:10.1021/ja020208f.
- Gill DJ, Chia J, Senewiratne J, Bard F. Regulation of O-glycosylation through golgi-to-ER relocation of initiation enzymes. J Cell Biol Internet. 2010 [accessed 2023 Feb 17];189(5):843–858. doi:10.1083/jcb.201003055.
- Tran DT, Ten Hagen KG. Mucin-type O-glycosylation during development. J Biol Chem. 2013;288(10):6921–6929. doi:10.1074/jbc.R112.418558.
- Brockhausen I, Schachter H, Stanley P 2009. O-Galnac Glycans. In: Essentials of Glycobiology 2nd. Varki A, Cummings R.D, Esko J.D, Freeze H.H, Stanley P, Bertozzi C.R, Hart G.W, Etzler M, editors. Cold Spring Harbor (NY);Cold Spring Harbor Laboratory Press. Internet [accessed 2023 Mar 10]. http://www.ncbi.nlm.nih.gov/books/NBK1896/
- An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204(6):1417–1429. doi:10.1084/jem.20061929.
- Clausen H, Hakomori S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution1. Vox Sang Internet 1989 [accessed 2023 Feb 10];56(1):1–20. doi:10.1111/j.1423-0410.1989.tb03040.x.
- Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS Internet. 2000 [accessed 2022 Mar 27];108(1):1–28. doi:10.1034/j.1600-0463.2000.d01-1.x.
- Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology Internet 2005 [accessed 2023 Feb 17];15(8):805–817. doi:10.1093/glycob/cwi063.
- Kukowska-Latallo JF, Larsen RD, Nair RP, Lowe JB. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes Dev. Internet. 1990 [accessed 2023 Feb 10];4(8):1288–1303. doi:10.1101/gad.4.8.1288.
- Yamamoto F, Clausen H, White T, Marken J, Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature Internet. 1990 [accessed 2023 Feb 10];345(6272):229–233. doi:10.1038/345229a0.
- Ferrer-Admetlla A, Sikora M, Laayouni H, Esteve A, Roubinet F, Blancher A, Calafell F, Bertranpetit J, Casals F. A natural history of FUT2 polymorphism in humans. Mol Biol Evol Internet. 2009 [accessed 2023 Feb 17];26(9):1993–2003. doi:10.1093/molbev/msp108.
- Guo M, Luo G, Lu R, Shi W, Cheng H, Lu Y, Jin K, Yang C, Wang Z, Long J, et al.Distribution of lewis and secretor polymorphisms and corresponding CA19‐9 antigen expression in a Chinese population. FEBS Open Bio Internet. 2017 [accessed 2023 Feb 10]. 7(11):1660–1671. doi: 10.1002/2211-5463.12278.
- Priatel JJ, Chui D, Hiraoka N, Simmons CJ, Richardson KB, Page DM, Fukuda M, Varki NM, Marth JD. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12(3):273–283. doi:10.1016/s1074-7613(00)80180-6.
- Ruvoën-Clouet N, Ganière JP, André-Fontaine G, Blanchard D, Le Pendu J. Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family. J VirolInternet 2000 [accessed 2022 Nov 2];74(24):11950–11954. doi:10.1128/JVI.74.24.11950-11954.2000.
- Bomidi C, Robertson M, Coarfa C, Estes MK, Blutt SE. Single-cell sequencing of rotavirus-infected intestinal epithelium reveals cell-type specific epithelial repair and tuft cell infection. Proc Natl Acad Sci. Internet 2021 [accessed 2023 Mar 11];118(45):e2112814118. doi:10.1073/pnas.2112814118.
- Crawford SE, Patel DG, Cheng E, Berkova Z, Hyser JM, Ciarlet M, Finegold MJ, Conner ME, Estes MK. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J Virol Internet. 2006 [accessed 2022 Aug 8];80(10):4820–4832. doi:10.1128/JVI.80.10.4820-4832.2006.
- Shao L, Fischer DD, Kandasamy S, Rauf A, Langel SN, Wentworth DE, Stucker KM, Halpin RA, Lam HC, Marthaler D, et al. Comparative in vitro and in vivo studies of porcine rotavirus G9P[13] and human rotavirus wa G1P[8]. J Virol Internet 2015 [accessed 2022 Sep 13];90(1):142–151. doi:10.1128/JVI.02401-15.
- Blutt SE, Kirkwood CD, Parreño V, Warfield KL, Ciarlet M, Estes MK, Bok K, Bishop RF, Conner ME. Rotavirus antigenaemia and viraemia: a common event? Lancet. 2003;362(9394):1445–1449. doi:10.1016/S0140-6736(03)14687-9.
- Justino MCA, Campos EA, Mascarenhas JDP, Soares LS, Guerra S, de Fs Furlaneto IP, Pavão MJC Jr, Maciel TS, Farias FP, Bezerra OM, et al. Rotavirus antigenemia as a common event among children hospitalised for severe, acute gastroenteritis in Belém, northern Brazil. BMC Pediatr Internet. 2019 [accessed 2023 Mar 3];19:193. doi:10.1186/s12887-019-1535-2.
- Azevedo MSP, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV, Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. Internet. 2006 [accessed 2023 Mar 3];80(1):372–382. doi:10.1128/JVI.80.1.372-382.2006.
- Gómez-Rial J, Sánchez-Batán S, Rivero-Calle I, Pardo-Seco J, Martinón-Martínez JM, Salas A, Martinón-Torres F. Rotavirus infection beyond the gut. Infect Drug Resist Internet. 2018 [accessed 2022 Aug 8]. 12:55–64. 10.2147/IDR.S186404.
- Nelsen A, Lager KM, Stasko J, Nelson E, Lin C-M, Hause BM. Identification of pulmonary infections with porcine rotavirus a in pigs with respiratory disease. Front Vet Sci. 2022;9:918736. doi:10.3389/fvets.2022.918736.
- Ciarlet M, Crawford SE, Cheng E, Blutt SE, Rice DA, Bergelson JM, Estes MK. VLA-2 (alpha2beta1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J Virol. 2002;76(3):1109–1123. doi:10.1128/jvi.76.3.1109-1123.2002.
- Crawford SE, Mukherjee SK, Estes MK, Lawton JA, Shaw AL, Ramig RF, Prasad BVV. Trypsin cleavage stabilizes the rotavirus VP4 spike. J Virol Internet. 2001 [accessed 2023 Feb 17];75(13):6052–6061. doi:10.1128/JVI.75.13.6052-6061.2001.
- Dormitzer PR, Sun ZY, Wagner G, Harrison SC. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. Embo J Internet. 2002 [accessed 2022 Mar 14];21(5):885. doi:10.1093/emboj/21.5.885.
- Estes MK, Graham DY, Mason BB. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol Internet. 1981 [accessed 2022 Mar 25];39(3):879–888. 10.1128/jvi.39.3.879-888.1981.
- Guerrero CA, Bouyssounade D, Zárate S, Iša P, López T, Espinosa R, Romero P, Méndez E, López S, Arias CF. Heat shock cognate protein 70 is involved in rotavirus cell entry. J Virol Internet. 2002 [accessed 2023 Feb 1];76(8):4096–4102. doi:10.1128/JVI.76.8.4096-4102.2002.
- Torres-Flores JM, Arias CF. Tight Junctions Go Viral! Viruses. Internet. 2015 [accessed 2023 Feb 1];7(9):5145–5154. 10.3390/v7092865.
- Salas-Cárdenas SP, Olaya-Galán NN, Fernández K, Velez F, Guerrero CA, Guitiérrez MF. Decreased rotavirus infection of MA104 cells via probiotic extract binding to Hsc70 and ß3 integrin receptors. Univ Sci Internet. 2018 [accessed 2022 May 19];23(2):219–239. doi:10.11144/Javeriana.SC23-2.drio.
- Fukudome K, Yoshie O, Konno T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989;172(1):196–205. doi:10.1016/0042-6822(89)90121-9.
- Delorme C, Brüssow H, Sidoti J, Roche N, Karlsson K-A, Neeser J-R, Teneberg S. Glycosphingolipid binding specificities of rotavirus: identification of a sialic acid-binding epitope. J Virol Internet. 2001 [accessed 2022 Mar 25];75(5):2276–2287. doi:10.1128/JVI.75.5.2276-2287.2001.
- Haselhorst T, Fleming FE, Dyason JC, Hartnell RD, Yu X, Holloway G, Santegoets K, Kiefel MJ, Blanchard H, Coulson BS, et al. Sialic acid dependence in rotavirus host cell invasion. Nat Chem Biol Internet. 2009 [accessed 2022 Feb 16];5(2):91–93. doi:10.1038/nchembio.134.
- Guo Y, Candelero-Rueda RA, Saif LJ, Vlasova AN. Infection of porcine small intestinal enteroids with human and pig rotavirus a strains reveals contrasting roles for histo-blood group antigens and terminal sialic acids. Plos Pathog. 2021;17(1):e1009237. doi:10.1371/journal.ppat.1009237.
- Guo Y, Raev S, Kick MK, Raque M, Saif LJ, Vlasova AN. Rotavirus C replication in porcine intestinal enteroids reveals roles for cellular cholesterol and Sialic Acids. Viruses Internet. 2022 [accessed 2022 Sep 15];14(8). 10.3390/v14081825.
- Superti F, Donelli G. Gangliosides as binding sites in SA-11 rotavirus infection of LLC-MK2 cells. J Gen Virol Internet 1991 [accessed 2022 Mar 25];72(10):2467–2474. doi:10.1099/0022-1317-72-10-2467.
- Ding S, Diep J, Feng N, Ren L, Li B, Ooi YS, Wang X, Brulois KF, Yasukawa LL, Li X, et al. STAG2 deficiency induces interferon responses via cGAS-STING pathway and restricts virus infection. Nat Commun Internet. 2018 [accessed 2023 Feb 15];9(1):1485. doi:10.1038/s41467-018-03782-z.
- Yamasaki M, Kanai Y, Wakamura Y, Kotaki T, Minami S, Nouda R, Nurdin JA, Kobayashi T. Characterization of sialic acid-independent simian rotavirus mutants in viral infection and pathogenesis. J Virol Internet. 2023 [accessed 2023 Feb 2];97(1):e01397–22. 10.1128/jvi.01397-22.
- Dormitzer PR, Sun ZY, Blixt O, Paulson JC, Wagner G, Harrison SC. Specificity and affinity of sialic acid binding by the rhesus rotavirus VP8* core. J Virol. 2002;76(20):10512–10517. doi:10.1128/jvi.76.20.10512-10517.2002.
- Lee RT, Ichikawa Y, Fay M, Drickamer K, Shao MC, Lee YC. Ligand-binding characteristics of rat serum-type mannose-binding protein (MBP-A). Homology of binding site architecture with mammalian and chicken hepatic lectins. J Biol Chem Internet. 1991 [accessed 2022 Oct 26];266(8):4810–4815. doi:10.1016/S0021-9258(19)67721-5.
- Kletter D, Cao Z, Bern M, Haab B. Determining lectin specificity from glycan array data using motif segregation and GlycoSearch software. Curr Protoc Chem Biol Internet. 2013 [accessed 2023 Feb 17];5(2):157–169. doi:10.1002/9780470559277.ch130028.
- Jolly CL, Beisner BM, Holmes IH. Rotavirus infection of MA104 cells is inhibited by ricinus lectin and separately expressed single binding domains. Virology Internet 2000 [accessed 2022 Jan 15];275(1):89–97. doi:10.1006/viro.2000.0470.
- Storry JR, Clausen FB, Castilho L, Chen Q, Daniels G, Denomme G, Flegel WA, Gassner C, de Haas M, Hyland C, et al. International society of blood transfusion working party on red cell immunogenetics and blood group terminology: report of the Dubai, Copenhagen and Toronto meetings. Vox Sang. 2019;114(1):95–102. doi:10.1111/vox.12717.
- Domino SE, Zhang L, Lowe JB. Molecular cloning, genomic mapping, and expression of two secretor blood group alpha (1,2)fucosyltransferase genes differentially regulated in mouse uterine epithelium and gastrointestinal tract. J Biol Chem. 2001;276(26):23748–23756. doi:10.1074/jbc.M100735200.
- Sarafian VS, Dikov DI, Karaivanov MP. Modulating expression of LAMPs and ABH histo-blood group antigens in normal and neoplastic human skin. Cent Cur J Med Internet. 2006 [accessed 2023 Feb 17];1(2):119–127. doi:10.2478/s11536-006-0012-0.
- Zhang D, Tan M, Zhong W, Xia M, Huang P, Jiang X. Human intestinal organoids express histo-blood group antigens, bind norovirus VLPs, and support limited norovirus replication. Sci Rep Internet. 2017 [accessed 2023 Feb 17]; 7:12621. 10.1038/s41598-017-12736-2.
- de Moraes MTB, Olivares AIO, Fialho AM, Malta FC, da Silva E Mouta Junior S, de Souza Bispo R, Velloso AJ, Alves Leitão GA, Cantelli CP, Nordgren J, et al. Phenotyping of Lewis and secretor HBGA from saliva and detection of new FUT2 gene SNPs from young children from the Amazon presenting acute gastroenteritis and respiratory infection. Infect Genet Evol. 2019;70:61–66. doi:10.1016/j.meegid.2019.02.011.
- Iwamori M, Adachi S, Lin B, Tanaka K, Aoki D, Nomura T. Spermatogenesis-associated changes of fucosylated glycolipids in murine testis. Hum Cell. 2020;33(1):23–28. doi:10.1007/s13577-019-00304-x.
- Liu Y, Ramelot TA, Huang P, Liu Y, Li Z, Feizi T, Zhong W, Wu F-T, Tan M, Kennedy MA, et al. Glycan Specificity of P[19] Rotavirus and Comparison with Those of Related P Genotypes. J Virol Internet. 2016 [accessed 2022 Sep 5];90(21):9983–9996. doi:10.1128/JVI.01494-16.
- Sun X, Dang L, Li D, Qi J, Wang M, Chai W, Zhang Q, Wang H, Bai R, Tan M, et al. Structural Basis of Glycan Recognition in Globally Predominant Human P[8] Rotavirus. Virol Sin Internet. 2020 [accessed 2023 Feb 8];35(2):156–170. doi:10.1007/s12250-019-00164-7.
- Böhm R, Fleming FE, Maggioni A, Dang VT, Holloway G, Coulson BS, von Itzstein M, Haselhorst T. Revisiting the role of histo-blood group antigens in rotavirus host-cell invasion. Nat Commun. 2015;6:5907. doi:10.1038/ncomms6907.
- Hu L, Sankaran B, Laucirica DR, Patil K, Salmen W, Ferreon ACM, Tsoi PS, Lasanajak Y, Smith DF, Ramani S, et al. Glycan recognition in globally dominant human rotaviruses. Nat Commun Internet 2018 [accessed 2021 Dec 28];9(1):2631. doi:10.1038/s41467-018-05098-4.
- Sun X, Li D, Qi J, Chai W, Wang L, Wang L, Peng R, Wang H, Zhang Q, Pang L, et al. Glycan Binding Specificity and Mechanism of Human and Porcine P[6]/P[19] Rotavirus VP8*s. J Virol. 2018;92(14): e00538-18. doi: 10.1128/JVI.00538-18.
- Nordgren J, Sharma S, Bucardo F, Nasir W, Günaydın G, Ouermi D, Nitiema LW, Becker-Dreps S, Simpore J, Hammarström L, et al. Both Lewis and Secretor Status Mediate Susceptibility to Rotavirus Infections in a Rotavirus Genotype–Dependent Manner. Clin Infect Dis Internet 2014 [accessed 2022 Sep 5];59(11):1567–1573. doi:10.1093/cid/ciu633.
- Fleming FE, Böhm R, Dang VT, Holloway G, Haselhorst T, Madge PD, Deveryshetty J, Yu X, Blanchard H, von Itzstein M, et al. Relative roles of GM1 ganglioside, N-acylneuraminic acids, and α2β1 integrin in mediating rotavirus infection. J Virol. 2014;88(8):4558–4571. doi:10.1128/JVI.03431-13.
- Farahmand M, Jalilvand S, Arashkia A, Shahmahmoodi S, Afchangi A, Mollaei-Kandelous Y, Shoja Z. Association between circulating rotavirus genotypes and histo-blood group antigens in the children hospitalized with acute gastroenteritis in Iran. J Med Virol Internet 2021 [accessed 2022 Jun 4];93(8):4817–4823. doi:10.1002/jmv.26808.
- Gozalbo-Rovira R, Ciges-Tomas JR, Vila-Vicent S, Buesa J, Santiso-Bellón C, Monedero V, Yebra MJ, Marina A, Rodríguez-Díaz J. Unraveling the role of the secretor antigen in human rotavirus attachment to histo-blood group antigens. Plos Pathog. Internet. 2019 [accessed 2023 Mar 16];15(6):e1007865. doi:10.1371/journal.ppat.1007865.
- Ayouni S, Sdiri-Loulizi K, de Rougemont A, Estienney M, Ambert-Balay K, Aho S, Hamami S, Aouni M, Neji-Guediche M, Pothier P, et al. Rotavirus P[8] Infections in Persons with Secretor and Nonsecretor Phenotypes, Tunisia. Emerg Infect Dis Internet. 2015 [accessed 2023 Feb 13];21(11):2055–2058. doi:10.3201/eid2111.141901.
- Lee B, Dickson DM, deCamp AC, Ross Colgate E, Diehl SA, Uddin MI, Sharmin S, Islam S, Bhuiyan TR, Alam M, et al. Histo–Blood Group Antigen Phenotype Determines Susceptibility to Genotype-Specific Rotavirus Infections and Impacts Measures of Rotavirus Vaccine Efficacy. J Infect Dis Internet 2018 [accessed 2022 Apr 26];217(9):1399–1407. doi:10.1093/infdis/jiy054.
- Huang P, Jiang B, Tan M, Morrow AL, Jiang X, Jiang X. Poly-LacNAc as an Age-Specific Ligand for Rotavirus P[11] in Neonates and Infants.Gangopadhyay N, editor. Plos One Internet. 2013 [accessed 2021 Dec 2];8(11):e78113. doi:10.1371/journal.pone.0078113.
- Liu Y, Huang P, Jiang B, Tan M, Morrow AL, Jiang X. Poly-LacNAc as an age-specific ligand for rotavirus P[11] in neonates and infants. Plos One. 2013;8(11):e78113. doi:10.1371/journal.pone.0078113.
- Ramani S, Cortes-Penfield NW, Hu L, Crawford SE, Czako R, Smith DF, Kang G, Ramig RF, Le Pendu J, Prasad BVV, et al. The VP8* domain of neonatal rotavirus strain G10P[11] binds to type II precursor glycans. J Virol. 2013;87(13):7255–7264. doi:10.1128/JVI.03518-12.
- Li D, Wang M, Qi J, Zhang Q, Wang H, Pang L, Sun X, Duan Z. Human group A rotavirus P[25] VP8* specifically binds to A-type histo-blood group antigen. Virology. 2021;555:56–63. doi:10.1016/j.virol.2020.12.016.
- Li Z, Gao C, Zhang Y, Palma AS, Childs RA, Silva LM, Liu Y, Jiang X, Liu Y, Chai W, et al. O-Glycome Beam Search Arrays for Carbohydrate Ligand Discovery. Mol Cell Proteomics Internet. 2018 [accessed 2023 Feb 9];17(1):121–133. doi:10.1074/mcp.RA117.000285.
- Ludert JE, Feng N, Yu JH, Broome RL, Hoshino Y, Greenberg HB. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. J Virol Internet. 1996 [accessed 2022 Dec 6];70(1):487–493. 10.1128/jvi.70.1.487-493.1996.
- Pérez-Ortín R, Vila-Vicent S, Carmona-Vicente N, Santiso-Bellón C, Rodríguez-Díaz J, Buesa J. Histo-Blood Group Antigens in Children with Symptomatic Rotavirus Infection. Viruses Internet. 2019 [accessed 2022 Jun 4];11(4):339. doi:10.3390/v11040339.
- Liu Y, Huang P, Tan M, Liu Y, Biesiada J, Meller J, Castello AA, Jiang B, Jiang X. Rotavirus VP8*: Phylogeny, Host Range, and Interaction with Histo-Blood Group Antigens. J Virol Internet. 2012 [accessed 2021 Dec 2];86(18):9899–9910. doi:10.1128/JVI.00979-12.
- Rakau K, Gededzha M, Peenze I, Huang P, Tan M, Steele AD, Seheri LM. The Association between Symptomatic Rotavirus Infection and Histo-Blood Group Antigens in Young Children with Diarrhea in Pretoria, South Africa. Viruses. 2022;14(12):2735. doi:10.3390/v14122735.
- Zhang X-F, Long Y, Tan M, Zhang T, Huang Q, Jiang X, Tan W-F, J-D L, G-F H, Tang S, et al. P[8] and P[4] Rotavirus Infection Associated with Secretor Phenotypes Among Children in South China. Sci Rep Internet. 2016 [accessed 2023 Mar 10];6(1):34591. doi:10.1038/srep34591.
- Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640–4649. doi:10.1074/jbc.270.9.4640.
- Kwon SW, Ahn A, Chung Y. Biological Meaning of the Histo-Blood Group Antigens Composed of Sugar Chains. Korean J of Blood Transfus Internet. 2016 [accessed 2023 Mar 14];26(2):103–122. 10.17945/kjbt.2015.26.2.103.
- Imbert-Marcille B-M, Barbé L, Dupé M, Le Moullac-Vaidye B, Besse B, Peltier C, Ruvoën-Clouet N, Le Pendu J. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J Infect Dis. 2014;209(8):1227–1230. doi:10.1093/infdis/jit655.
- Günaydın G, Nordgren J, Sharma S, Hammarström L. Association of elevated rotavirus-specific antibody titers with HBGA secretor status in Swedish individuals: The FUT2 gene as a putative susceptibility determinant for infection. Virus Res Internet. 2016 [accessed 2022 May 18];211:64–68.10.1016/j.virusres.2015.10.005.
- Hu L, Ramani S, Czako R, Sankaran B, Yu Y, Smith DF, Cummings RD, Estes MK, Venkataram Prasad BV. Structural basis of glycan specificity in neonate-specific bovine-human reassortant rotavirus. Nat Commun. 2015;6:8346. doi:10.1038/ncomms9346.
- Yang X, Forier K, Steukers L, Van Vlierberghe S, Dubruel P, Braeckmans K, Glorieux S, Nauwynck HJ. Immobilization of Pseudorabies Virus in Porcine Tracheal Respiratory Mucus Revealed by Single Particle Tracking. Plos One Internet. 2012 [accessed 2022 Aug 15];7(12):e51054. doi:10.1371/journal.pone.0051054.
- Jiménez-Zaragoza M, Yubero MP, Martín-Forero E, Castón JR, Reguera D, Luque D, de Pablo PJ, Rodríguez JM. Biophysical properties of single rotavirus particles account for the functions of protein shells in a multilayered virus. eLife Internet. [accessed 2023 Feb 9];7:e37295. 10.7554/eLife.37295
- Liu Y, Xu S, Woodruff AL, Xia M, Tan M, Kennedy MA, Jiang X, Zhou ZH. Structural basis of glycan specificity of P[19] VP8*: Implications for rotavirus zoonosis and evolution. Plos Pathog Internet. 2017 [accessed 2022 Aug 30];13(11):e1006707. doi:10.1371/journal.ppat.1006707.
- Vinall LE, King M, Novelli M, Green CA, Daniels G, Hilkens J, Sarner M, Swallow DM. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology. 2002;123(1):41–49. doi:10.1053/gast.2002.34157.
- Thathiah A, Brayman M, Dharmaraj N, Julian JJ, Lagow EL, Carson DD. Tumor necrosis factor alpha stimulates MUC1 synthesis and ectodomain release in a human uterine epithelial cell line. Endocrinology. 2004;145(9):4192–4203. doi:10.1210/en.2004-0399.
- Hakim MS, Ding S, Chen S, Yin Y, Su J, van der Woude CJ, Fuhler GM, Peppelenbosch MP, Pan Q, Wang W. TNF-α exerts potent anti-rotavirus effects via the activation of classical NF-κB pathway. Virus Res Internet. 2018 [accessed 2023 Feb 9];253:28–37.10.1016/j.virusres.2018.05.022.
- Yolken RH, Peterson JA, Vonderfecht SL, Fouts ET, Midthun K, Newburg DS. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest Internet. 1992 [accessed 2022 Apr 26];90(5):1984–1991. 10.1172/JCI116078.
- Chen CC, Baylor M, Bass DM. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105(1):84–92. doi:10.1016/0016-5085(93)90013-3.
- Kvistgaard AS, Pallesen LT, Arias CF, López S, Petersen TE, Heegaard CW, Rasmussen JT. Inhibitory Effects of Human and Bovine Milk Constituents on Rotavirus Infections. J Dairy Sci Internet 2004 [accessed 2022 Apr 12];87(12):4088–4096. doi:10.3168/jds.S0022-0302(04)73551-1.
- Mungul A, Cooper L, Brockhausen I, Ryder K, Mandel U, Clausen H, Rughetti A, Miles DW, Taylor-Papadimitriou J, Burchell JM. Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int J Oncol. 2004;25:937–943.
- Boshuizen JA, Reimerink JHJ, Korteland-van Male AM, van Ham VJJ, Bouma J, Gerwig GJ, Koopmans MPG, Büller HA, Dekker J, Einerhand AWC. Homeostasis and function of goblet cells during rotavirus infection in mice. Virology. 2005;337(2):210–221. doi:10.1016/j.virol.2005.03.039.
- LaMonica R, Kocer SS, Nazarova J, Dowling W, Geimonen E, Shaw RD, Mackow ER. VP4 differentially regulates TRAF2 signaling, disengaging JNK activation while directing NF-kappa B to effect rotavirus-specific cellular responses. J Biol Chem. 2001;276(23):19889–19896. doi:10.1074/jbc.M100499200.
- Lin H, An Y, Hao F, Wang Y, Tang H. Correlations of Fecal Metabonomic and Microbiomic Changes Induced by High-fat Diet in the Pre-Obesity State. Sci Rep Internet. 2016 [accessed 2023 Feb 17]; 6:21618. 10.1038/srep21618.
- Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G, et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci Rep Internet. 2016 [accessed 2022 Sep 5];6(1):28990. doi:10.1038/srep28990.
- Arnold MM, Sen A, Greenberg HB, Patton JT, Hobman TC. The Battle between Rotavirus and Its Host for Control of the Interferon Signaling Pathway. PLoS Pathog Internet. 2013 [accessed 2023 Feb 16];9(1):e1003064. doi:10.1371/journal.ppat.1003064.
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The Antibacterial Lectin RegIIIγ Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science Internet. 2011 [accessed 2022 Mar 30];334(6053):255–258. doi:10.1126/science.1209791.
- Lindén SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin THJ, Sutton P, McGuckin MA. MUC1 Limits Helicobacter pylori Infection both by Steric Hindrance and by Acting as a Releasable Decoy. Plos Pathog Internet. 2009 [accessed 2021 Dec 14;5(10):e1000617. doi:10.1371/journal.ppat.1000617.
- Petrova MI, Imholz NCE, Verhoeven TLA, Balzarini J, Damme EJMV, Schols D, Vanderleyden J, Lebeer S, Biswas I. Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. Plos One Internet. 2016 [accessed 2022 Mar 15];11(8):e0161337. doi:10.1371/journal.pone.0161337.
- Lakhtin VM, Lakhtin MV, Pospelova VV, Shenderov BA. Lectins of lactobacilli and bifidobacteria. II. Probiotic lectins of lactobacilli and bifidobacteria as possible signal molecules regulating inter- and intrapopulation relationships between bacteria and between bacteria and the host. Microb Ecol Health Dis Internet 2007 [[accessed 2022 Mar 15];19(3):153–157. doi:10.1080/08910600701538257.
- Cohen LJ, Han SM, Lau P, Guisado D, Liang Y, Nakashige TG, Ali T, Chiang D, Rahman A, Brady SF. Unraveling function and diversity of bacterial lectins in the human microbiome. Nat Commun Internet. 2022 [accessed 2023 Mar 13];13(1):3101. doi:10.1038/s41467-022-29949-3.
- Pabst O, Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol Internet. 2020 [accessed 2023 Mar 13];13(1):12–21. doi:10.1038/s41385-019-0227-4.
- Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006;74(6):3455–3462. doi:10.1128/IAI.02088-05.
- Martinoli C, Chiavelli A, Rescigno M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity. 2007;27(6):975–984. doi:10.1016/j.immuni.2007.10.011.
- Randal Bollinger R, Everett ML, Palestrant D, Love SD, Lin SS, Parker W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology. Internet 2003 [[accessed 2022 May 21];109(4):580–587. doi:10.1046/j.1365-2567.2003.01700.x.
- Donaldson GP, Ladinsky MS, Yu KB, Sanders JG, Yoo BB, Chou WC, Conner ME, Earl AM, Knight R, Bjorkman PJ, et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science Internet. 2018 [accessed 2023 Mar 13];360(6390):795–800. doi:10.1126/science.aaq0926.
- Nanthakumar NN, Meng D, Newburg DS. Glucocorticoids and microbiota regulate ontogeny of intestinal fucosyltransferase 2 requisite for gut homeostasis. Glycobiology Internet 2013 [accessed 2023 Mar 13];23(10):1131–1141. doi:10.1093/glycob/cwt050.
- Zhang M, Zhang M, Zhang C, Du H, Wei G, Pang X, Zhou H, Liu B, Zhao L. Pattern extraction of structural responses of gut microbiota to rotavirus infection via multivariate statistical analysis of clone library data. FEMS Microbiol Ecol Internet 2009 [accessed 2022 May 21];70(2):177–185. doi:10.1111/j.1574-6941.2009.00694.x.
- Kaur H, Ali SA, Yan F. Interactions between the gut microbiota-derived functional factors and intestinal epithelial cells – implication in the microbiota-host mutualism. Front Immunol Internet. 2022 [accessed 2023 Mar 13];13. 10.3389/fimmu.2022.1006081
- Johansson MEV, Jakobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro AM, Arike L, Wising C, Svensson F, Bäckhed F, et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host & Microbe Internet. 2015 [accessed 2021 Dec 2];18(5):582–592. doi:10.1016/j.chom.2015.10.007.
- Sommer F, Nookaew I, Sommer N, Fogelstrand P, Bäckhed F. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol. 2015;16:62. doi:10.1186/s13059-015-0614-4.
- J-D L, Feng W, Gallup M, Kim J-H, Gum J, Kim Y, Basbaum C. Activation of NF-κB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa -induced mucin overproduction in epithelial cells. Proc Natl Acad Sci U S A Internet. 1998 [accessed 2022 Apr 19];95(10):5718–5723. doi:10.1073/pnas.95.10.5718.
- Paim FC, Langel SN, Fischer DD, Kandasamy S, Shao L, Alhamo MA, Huang H-C, Kumar A, Rajashekara G, Saif LJ, et al. Effects of Escherichia coli Nissle 1917 and Ciprofloxacin on small intestinal epithelial cell mRNA expression in the neonatal piglet model of human rotavirus infection. Gut Pathog Internet. 2016 [accessed 2021 Dec 20];8:66. 10.1186/s13099-016-0148-7.
- Robbe-Masselot C, Maes E, Rousset M, Michalski J-C, Capon C. Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj J. 2009;26(4):397–413. doi:10.1007/s10719-008-9186-9.
- Meng D, Newburg DS, Young C, Baker A, Tonkonogy SL, Sartor RB, Walker WA, Nanthakumar NN. Bacterial symbionts induce a FUT2-dependent fucosylated niche on colonic epithelium via ERK and JNK signaling. Am J Physiol Gastrointest Liver Physiol Internet 2007 [accessed 2021 Dec 14];293(4):G780–787. doi:10.1152/ajpgi.00010.2007.
- Vadaie N, Dionne H, Akajagbor DS, Nickerson SR, Krysan DJ, Cullen PJ. Cleavage of the signaling mucin Msb2 by the aspartyl protease Yps1 is required for MAPK activation in yeast. J Cell Biol Internet. 2008 accessed 2022 Sep 1;181(7):1073–1081. doi:10.1083/jcb.200704079.
- Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol Internet. 2007 [accessed 2022 Jan 8];292(1):G315–322. doi:10.1152/ajpgi.00265.2006.
- Varyukhina S, Freitas M, Bardin S, Robillard E, Tavan E, Sapin C, Grill J-P, Trugnan G. Glycan-modifying bacteria-derived soluble factors from Bacteroides thetaiotaomicron and Lactobacillus casei inhibit rotavirus infection in human intestinal cells. Microb and Infect Internet. 2012 [accessed 2022 Jun 13];14(3):273–278. doi:10.1016/j.micinf.2011.10.007.
- Park D, Xu G, Barboza M, Shah IM, Wong M, Raybould H, Mills DA, Lebrilla CB. Enterocyte glycosylation is responsive to changes in extracellular conditions: implications for membrane functions. Glycobiology Internet. 2017 [accessed 2022 Mar 30];27(9):847–860. doi:10.1093/glycob/cwx041.
- Huang Y-L, Chassard C, Hausmann M, von Itzstein M, Hennet T. Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat Commun. 2015;6:8141. doi:10.1038/ncomms9141.
- Wrzosek L, Miquel S, Noordine M-L, Bouet S, Chevalier-Curt MJ, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, et al.Bacteroides thetaiotaomicron and Faecalibacterium prausnitziiinfluence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol Internet. 2013 [accessed 2022 Jan 8];11(1):61. doi: 10.1186/1741-7007-11-61.
- Rausch P, Rehman A, Kunzel S, Hasler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, Baines JF2011Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotypeInternetaccessed 2021 Dec 21084719030–1903510.1073/pnas.1106408108
- Wacklin P, Tuimala J, Nikkilä J, Tims S, Mäkivuokko H, Alakulppi N, Laine P, Rajilic-Stojanovic M, Paulin L, de Vos WM, et al. Faecal Microbiota Composition in Adults Is Associated with the FUT2 Gene Determining the Secretor Status. Plos One Internet. 2014 [accessed 2021 Dec 11];9(4):e94863. doi:10.1371/journal.pone.0094863.
- Davenport ER, Goodrich JK, Bell JT, Spector TD, Ley RE, Clark AG. ABO antigen and secretor statuses are not associated with gut microbiota composition in 1,500 twins. BMC Genomics Internet. 2016 [accessed 2022 Jun 6];17:941. 10.1186/s12864-016-3290-1.
- Rodríguez-Díaz J, García-Mantrana I, Vila-Vicent S, Gozalbo-Rovira R, Buesa J, Monedero V, Collado MC. Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci Rep Internet. 2017 [accessed 2021 Dec 11];7(1):45559. doi:10.1038/srep45559.
- Harris VC, Haak BW, Handley SA, Jiang B, Velasquez DE, Hykes BL, Droit L, Berbers GAM, Kemper EM, van Leeuwen EMM, et al. Effect of Antibiotic-Mediated Microbiome Modulation on Rotavirus Vaccine Immunogenicity: A Human, Randomized-Control Proof-of-Concept Trial. Cell Host & Microbe Internet. 2018 [accessed 2023 Mar 13];24(2):197–207.e4. doi:10.1016/j.chom.2018.07.005.
- Wacklin P, Mäkivuokko H, Alakulppi N, Nikkilä J, Tenkanen H, Räbinä J, Partanen J, Aranko K, Mättö J. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. Plos One. 2011;6(5):e20113. doi:10.1371/journal.pone.0020113.
- Fernandez-Duarte KP, Olaya-Galán NN, Salas-Cárdenas SP, Lopez-Rozo J, Gutierrez-Fernandez MF. Bifidobacterium adolescentis (DSM 20083) and Lactobacillus casei (Lafti L26-DSL): Probiotics Able to Block the In Vitro Adherence of Rotavirus in MA104 Cells. Probiotics Antimicrob Proteins Internet. 2018 [accessed 2022 Feb 18];10(1):56. doi:10.1007/s12602-017-9277-7.
- Larsson JMH, Karlsson H, Sjövall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19(7):756–766. doi:10.1093/glycob/cwp048.
- Tang PW, Scudder P, Mehmet H, Hounsell EF, Feizi T. Sulphate groups are involved in the antigenicity of keratan sulphate and mask i antigen expression on their poly-N-acetyllactosamine backbones. European J Biochem Internet. 1986 [accessed 2022 May 18];160(3):537–545. doi:10.1111/j.1432-1033.1986.tb10072.x.
- Gustafsson BE, Midtvedt T, Strandberg K. Effects of Microbial Contamination on the Cecum Enlargement of Germfree Rats. Scand J Gastroenterol Internet. 1970 [accessed 2021 Dec 30];5(4):309–314. doi:10.1080/00365521.1970.12096595.
- Raimondi S, Musmeci E, Candeliere F, Amaretti A, Rossi M. Identification of mucin degraders of the human gut microbiota. Sci Rep Internet. 2021 [accessed 2022 May 19];11(1):11094. doi:10.1038/s41598-021-90553-4.
- Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin THJ. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Official Am J Gastroentero Internet. 2010 [accessed 2023 Feb 17];105(11):2420. doi:10.1038/ajg.2010.281.
- Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R, Goodrich-Blair H. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl Environ Microbiol Internet 2015 [accessed 2023 Feb 17];81(11):3655–3662. doi:10.1128/AEM.04050-14.
- Glover JS, Ticer TD, Engevik MA. Characterizing the mucin-degrading capacity of the human gut microbiota. Sci Rep Internet. 2022 [accessed 2022 Sep 2];12(1):8456. doi:10.1038/s41598-022-11819-z.
- Cockburn DW, Koropatkin NM. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J Mol Biol Internet 2016 [accessed 2021 Dec 30];428(16):3230–3252. doi:10.1016/j.jmb.2016.06.021.
- Katoh T, Maeshibu T, Kikkawa K-I, Gotoh A, Tomabechi Y, Nakamura M, Liao W-H, Yamaguchi M, Ashida H, Yamamoto K, et al. Identification and characterization of a sulfoglycosidase from Bifidobacterium bifidum implicated in mucin glycan utilization. Biosci Biotechnol Biochem. 2017;81(10):2018–2027. doi:10.1080/09168451.2017.1361810.
- Meng X, Wang W, Lan T, Yang W, Yu D, Fang X, Wu H. A Purified Aspartic Protease from Akkermansia Muciniphila Plays an Important Role in Degrading Muc2. Int J Mol Sci Internet. 2019 [accessed 2023 Feb 16];21(1):72. doi:10.3390/ijms21010072.
- Zúñiga M, Monedero V, Yebra MJ. Utilization of Host-Derived Glycans by Intestinal Lactobacillus and Bifidobacterium Species. Front Microbiol Internet. 2018 [accessed 2021 Dec 20];9:1917. 10.3389/fmicb.2018.01917.
- György P, Jeanloz RW, von Nicolai H, Zilliken F. Undialyzable growth factors for Lactobacillus bifidus var. pennsylvanicus. Protective effect of sialic acid bound to glycoproteins and oligosaccharides against bacterial degradation. Eur J Biochem. 1974;43(1):29–33. doi:10.1111/j.1432-1033.1974.tb03380.x.
- Schenkman S, Jiang MS, Hart GW, Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991;65(7):1117–1125. doi:10.1016/0092-8674(91)90008-m.
- Tailford LE, Owen CD, Walshaw J, Crost EH, Hardy-Goddard J, Le Gall G, de Vos WM, Taylor GL, Juge N. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat Commun. 2015;6:7624. doi:10.1038/ncomms8624.
- Tanaka H, Ito F, Iwasaki T. Purification and characterization of a sialidase from Bacteroides fragilis SBT3182. Biochem Biophys Res Commun. Internet 1992 [accessed 2023 Feb 13];189(1):524–529. doi:10.1016/0006-291X(92)91589-I.
- Fukano Y, Ito M. Preparation of GM1 ganglioside with sialidase-producing marine bacteria as a microbial biocatalyst. Appl Environ Microbiol Internet. 1997 [accessed 2023 Feb 13];63(5):1861–1865. 10.1128/aem.63.5.1861-1865.1997.
- Almagro-Moreno S, Boyd EF. Insights into the evolution of sialic acid catabolism among bacteria. BMC Evol Biol Internet. 2009 [accessed 2021 Dec 2];9(1):118. doi:10.1186/1471-2148-9-118.
- Luis AS, Jin C, Pereira GV, Glowacki RWP, Gugel SR, Singh S, Byrne DP, Pudlo NA, London JA, Baslé A, et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature. 2021;598(7880):332–337. doi:10.1038/s41586-021-03967-5.
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt 5):1469–1476. doi:10.1099/ijs.0.02873-0.
- Hoskins LC, Agustines M, McKee WB, Boulding ET, Kriaris M, Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985;75(3):944–953. doi:10.1172/JCI111795.
- van der Beek CM, Bloemen JG, van den Broek MA, Lenaerts K, Venema K, Buurman WA, Dejong CH. Hepatic Uptake of Rectally Administered Butyrate Prevents an Increase in Systemic Butyrate Concentrations in Humans. J Nutr. 2015;145(9):2019–2024. doi:10.3945/jn.115.211193.
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes Internet. 2016 [accessed 2023 Mar 13];7(3):189–200. doi:10.1080/19490976.2015.1134082.
- Graham KL, Halasz P, Tan Y, Hewish MJ, Takada Y, Mackow ER, Robinson MK, Coulson BS. Integrin-Using Rotaviruses Bind α2β1 Integrin α2 I Domain via VP4 DGE Sequence and Recognize αXβ2 and αVβ3 by Using VP7 during Cell Entry. J Virol Internet. 2003 [accessed 2022 Jun 16];77(18):9969–9978. doi:10.1128/JVI.77.18.9969-9978.2003.
- Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60(5):861–871. doi:10.1016/0092-8674(90)90099-z.
- Coburn J, Cugini C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin alphavbeta3. Proc Natl Acad Sci U S A. 2003;100(12):7301–7306. doi:10.1073/pnas.1131117100.
- Walker BD, Chua MD, Guttman JA. Hsc70 is a Component of Bacterially Generated Actin-Rich Structures: An Immunolocalization Study. Anat Rec (Hoboken). 2018;301(12):2095–2102. doi:10.1002/ar.23955.
- Iwashita J, Murata J. Integrin β1 subunit regulates cellular and secreted MUC5AC and MUC5B production in NCI–H292 human lung epithelial cells. Biochem and Biophys Reports Internet. 2021 [accessed 2022 Jun 12];28:101124.10.1016/j.bbrep.2021.101124.
- Halasz P, Holloway G, Turner SJ, Coulson BS. Rotavirus Replication in Intestinal Cells Differentially Regulates Integrin Expression by a Phosphatidylinositol 3-Kinase-Dependent Pathway, Resulting in Increased Cell Adhesion and Virus Yield. J Virol Internet. 2008 [accessed 2022 Jun 16];82(1):148–160. doi:10.1128/JVI.01980-07.
- Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, et al.The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature Internet. 2014 [accessed 2022 Aug 29]; 511(7509):319–325. doi: 10.1038/nature13535.
- Iwamoto DV, Calderwood DA. Regulation of integrin-mediated adhesions. Curr Opin Cell Biol. 2015 [accessed 2022 Aug 29]: 36:41–47. 10.1016/j.ceb.2015.06.009.
- Li X, Wubbolts RW, Bleumink-Pluym NMC, van Putten JPM, Strijbis K. The Transmembrane Mucin MUC1 Facilitates β1-Integrin-Mediated Bacterial Invasion. mBio Internet. 2021 accessed 2022 Aug 29;12(2):e03491–20. 10.1128/mBio.03491-20.
- Kastl AJ, Terry NA, Wu GD, Albenberg LG. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell Mol Gastroenterol Hepatol Internet. 2020 [accessed 2022 Mar 30];9(1):33–45. doi:10.1016/j.jcmgh.2019.07.006.
- Chateau N, Deschamps A, Sassi AH. Heterogeneity of bile salts resistance in the Lactobacillus isolates of a probiotic consortium. Lett Appl Microbiol Internet. 1994 [accessed 2022 Mar 30];18(1):42–44. doi:10.1111/j.1472-765X.1994.tb00796.x.
- Ibrahim SA, Bezkorovainy A. Survival of bifidobacteria in the presence of bile salt. J Sci Food Agric Internet. 1993 [accessed 2022 Mar 30];62(4):351–354. doi:10.1002/jsfa.2740620407.
- Begley M, Hill C, Gahan CGM. Bile Salt Hydrolase Activity in Probiotics. Appl Environ Microbiol Internet. 2006 [accessed 2023 Feb 14];72(3):1729–1738. doi:10.1128/AEM.72.3.1729-1738.2006.
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol Internet. 2012 [accessed 2022 Sep 14];303(6):G675–685. doi:10.1152/ajpgi.00152.2012.
- Urdaneta V, Casadesús J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front Med (Lausanne) Internet. 2017 [accessed 2022 Sep 14]. 4:163. 10.3389/fmed.2017.00163.
- Mistry RH, Verkade HJ, Tietge UJF. Reverse Cholesterol Transport Is Increased in Germ-Free Mice—Brief Report. Arteriosclerosis, Thrombosis, and Vascular Biology Internet. 2017 [accessed 2022 Sep 14];37(3):419–422. 10.1161/ATVBAHA.116.308306.
- Hayashi H, Takahashi R, Nishi T, Sakamoto M, Benno Y. Molecular analysis of jejunal, ileal, caecal and rectosigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. J Med Microbiol. 2005;54(11):1093–1101. doi:10.1099/jmm.0.45935-0.
- Kim Y, Chang K-O. Inhibitory Effects of Bile Acids and Synthetic Farnesoid X Receptor Agonists on Rotavirus Replication▿. J Virol Internet. 2011 [accessed 2022 Mar 30];85(23):12570–12577. doi:10.1128/JVI.05839-11.
- Oetzmann von Sochaczewski C, Pintelon I, Brouns I, Dreier A, Klemann C, Timmermans J-P, Petersen C, Kuebler JF. Rotavirus particles in the extrahepatic bile duct in experimental biliary atresia. J Pediatr Surg. 2014;49(4):520–524. doi:10.1016/j.jpedsurg.2013.09.064.
- Sánchez-San Martín C, López T, Arias CF, López S. Characterization of rotavirus cell entry. J Virol. 2004;78(5):2310–2318. doi:10.1128/jvi.78.5.2310-2318.2004.
- Zheng P-Y, Hua J, H-C N, Yeoh K-G, Bow H. Expression of Lewis(b) blood group antigen in Helicobacter pylori does not interfere with bacterial adhesion property. World J Gastroenterol. 2003;9(1):122–124. doi:10.3748/wjg.v9.i1.122.
- Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science. 2005;307(5716):1778–1781. doi:10.1126/science.1106469.
- Moran AP. Relevance of fucosylation and Lewis antigen expression in the bacterial gastroduodenal pathogen Helicobacter pylori. Carbohydr Res. 2008;343(12):1952–1965. doi:10.1016/j.carres.2007.12.012.
- Zhu F, Wu R, Zhang H, Wu H. Structural and biochemical analysis of a bacterial glycosyltransferase. Methods Mol Biol Internet. 2013 [accessed 2023 Feb 17]. 1022:29–39. 10.1007/978-1-62703-465-4_3.
- Martinez-Fleites C, Macauley MS, He Y, Shen DL, Vocadlo DJ, Davies GJ. Structure of an O-GlcNAc transferase homolog provides insight into intracellular glycosylation. Nat Struct Mol Biol Internet. 2008 [accessed 2023 Feb 16];15(7):764–765. doi:10.1038/nsmb.1443.
- Brockhausen I. Crossroads between Bacterial and Mammalian Glycosyltransferases. Front Immunol Internet. 2014 [accessed 2022 Jun 18]. 5:492. 10.3389/fimmu.2014.00492.
- Shi Z, Zou J, Zhang Z, Zhao X, Noriega J, Zhang B, Zhao C, Ingle H, Bittinger K, Mattei LM, et al. Segmented Filamentous Bacteria Prevent and Cure Rotavirus Infection. Cell Internet. 2019 [accessed 2021 Dec 3];179(3):644–658.e13. doi:10.1016/j.cell.2019.09.028.
- Kandasamy S, Vlasova A, Fischer D, Kumar A, Chattha K, Rauf A, Shao L, Neal Langel S, Rajashekara G, Saif L. Differential Effects of Escherichia coli Nissle and Lactobacillus rhamnosus Strain GG on Human Rotavirus Binding, Infection, and B Cell Immunity. J Immun (Balt Md 1950). 2016;196:1780–1789. doi:10.4049/jimmunol.1501705.
- Gozalbo-Rovira R, Rubio-Del-Campo A, Santiso-Bellón C, Vila-Vicent S, Buesa J, Delgado S, Molinero N, Margolles A, Yebra MJ, Collado MC, et al. Interaction of Intestinal Bacteria with Human Rotavirus during Infection in Children. IJMS Internet. 2021 [accessed 2021 Dec 3];22(3):1010. doi:10.3390/ijms22031010.
- Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, et al.Enteric bacteria promote human and mouse norovirus infection of B cells. Science Internet. 2014 [accessed 2022 May 3]. 346(6210):755–759. doi: 10.1126/science.1257147.
- Lim YF, de Loubens C, Love RJ, Lentle RG, Janssen PWM. Flow and mixing by small intestine villi. Food Funct. 2015;6(6):1787–1795. doi:10.1039/c5fo00285k.
- Johansson MEV, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–291. doi:10.1136/gutjnl-2012-303207.
- Letourneau J, Levesque C, Berthiaume F, Jacques M, Mourez M. In Vitro Assay of Bacterial Adhesion onto Mammalian Epithelial Cells. J Vis Exp Internet. 2011 [accessed 2022 Sep 14]. 51:2783. 10.3791/2783.
- Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. A Multifunctional Pasteurella multocida Sialyltransferase: A Powerful Tool for the Synthesis of Sialoside Libraries. J Am Chem Soc Internet. 2005 [accessed 2023 Feb 17];127(50):17618–17619. doi:10.1021/ja0561690.
- Skretas G, Carroll S, DeFrees S, Schwartz MF, Johnson KF, Georgiou G. Expression of active human sialyltransferase ST6GalNAcI in Escherichia coli. Microb Cell Fact Internet. 2009. [accessed 2023 Feb 17]. 8:50. 10.1186/1475-2859-8-50.
- Benítez-Páez A, Moreno FJ, Sanz ML, Sanz Y. Genome Structure of the Symbiont Bifidobacterium pseudocatenulatum CECT 7765 and Gene Expression Profiling in Response to Lactulose-Derived Oligosaccharides. Front Microbiol Internet. 2016 [accessed 2023 Feb 17]. 7:624. 10.3389/fmicb.2016.00624.
- Li Y, Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol Internet. 2012 [accessed 2022 Jun 12];94(4):887–905. doi:10.1007/s00253-012-4040-1.
- Tsukamoto H, Takakura Y, Mine T, Yamamoto T. Photobacterium sp. JT-ISH-224 produces two sialyltransferases, alpha-/beta-galactoside alpha2,3-sialyltransferase and beta-galactoside alpha2,6-sialyltransferase. J Biochem. 2008;143(2):187–197. doi:10.1093/jb/mvm208.
- Yuki N. Current cases in which epitope mimicry is considered a component cause of autoimmune disease: Guillain-Barré syndrome. CMLS, Cell Mol Life Sci Internet. 2000 [accessed 2023 Feb 16];57(4):527–533. doi:10.1007/PL00000714.
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell Internet. 2005 [accessed 2023 Feb 16];122(1):107–118. doi:10.1016/j.cell.2005.05.007.
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor pathway establishes commensal gut colonization. Science Internet. 2011 [accessed 2023 Feb 16];332(6032):974–977. doi:10.1126/science.1206095.
- Hapfelmeier S, Lawson MAE, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al.Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science Internet. 2010 [accessed 2023 Feb 17]. 328(5986):1705–1709. doi: 10.1126/science.1188454.
- Bruno MEC, Rogier EW, Frantz AL, Stefka AT, Thompson SN, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor in intestinal epithelial cells by Enterobacteriaceae: implications for mucosal homeostasis. Immunol Invest. 2010;39(4–5):356–382. doi:10.3109/08820131003622809.
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi:10.1126/science.1127119.
- Laiño J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells. Microorganisms Internet. 2016 [accessed 2022 Dec 2];4(3):27. doi:10.3390/microorganisms4030027.
- Kanmani P, Albarracin L, Kobayashi H, Iida H, Komatsu R, Humayun Kober AKM, Ikeda-Ohtsubo W, Suda Y, Aso H, Makino S, et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol Immunol Internet. 2018 [accessed 2022 Sep 5];93:253–265. doi:10.1016/j.molimm.2017.07.009.
- Chattha KS, Vlasova AN, Kandasamy S, Rajashekara G, Saif LJ. Divergent immunomodulating effects of probiotics on t cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. J Immunol Internet. 2013 accessed 2022 Sep 19;191(5):2446–2456. doi:10.4049/jimmunol.1300678.
- Michael H, Paim FC, Langel SN, Miyazaki A, Fischer DD, Chepngeno J, Amimo J, Deblais L, Rajashekara G, Saif LJ, et al. Escherichia coli nissle 1917 enhances innate and adaptive immune responses in a ciprofloxacin-treated defined-microbiota piglet model of human rotavirus infection. mSphere. 2021;6(2). e00074–21. doi:10.1128/mSphere.00074-21.
- Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis Internet. 2014 [accessed 2021 Dec 3];210(2):171–182. doi:10.1093/infdis/jiu037.
- Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science Internet 2015 [accessed 2021 Dec 3];347(6219):266–269. doi:10.1126/science.1258025.
- Chen S-Y, Tsai C-N, Lee Y-S, Lin C-Y, Huang K-Y, Chao H-C, Lai M-W, Chiu C-H. Intestinal microbiome in children with severe and complicated acute viral gastroenteritis. Sci Rep. 2017;7:46130. doi:10.1038/srep46130.
- Kumar A, Vlasova A, Deblais L, Huang H-C, Wijeratne A, Kandasamy S, Fischer D, Neal Langel S, Paim F, Alhamo M, et al. Impact of nutrition and rotavirus infection on the infant gut microbiota in a humanized pig model. BMC Gastroenterol. 2018;18:93. doi:10.1186/s12876-018-0810-2.
- Jang J-Y, Kim S, Kwon M-S, Lee J, D-H Y, Song R-H, Choi H-J, Park J. Rotavirus-mediated alteration of gut microbiota and its correlation with physiological characteristics in neonatal calves. J Microbiol. 2019;57(2):113–121. doi:10.1007/s12275-019-8549-1.
- Twitchell EL, Tin C, Wen K, Zhang H, Becker-Dreps S, Azcarate-Peril MA, Vilchez S, Li G, Ramesh A, Weiss M, et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog. 2016;8:51. doi:10.1186/s13099-016-0136-y.
- Yang C, Mogno I, Contijoch EJ, Borgerding JN, Aggarwala V, Li Z, Siu S, Grasset EK, Helmus DS, Dubinsky MC, et al. Fecal IgA Levels Are Determined by Strain-Level Differences in Bacteroides ovatus and Are Modifiable by Gut Microbiota Manipulation. Cell Host & Microbe. 2020;27(3):467–475.e6. doi:10.1016/j.chom.2020.01.016.