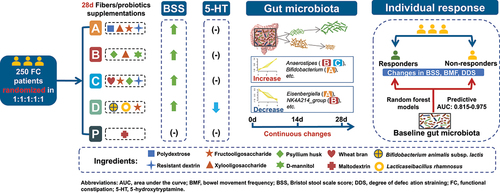
ABSTRACT
Dietary fibers/probiotics may relieve constipation via optimizing gut microbiome, yet with limited trial-based evidences. We aimed to evaluate the effects of formulas with dietary fibers or probiotics on functional constipation symptoms, and to identify modulations of gut microbiota of relevance. We conducted a 4-week double-blinded randomized placebo-controlled trial in 250 adults with functional constipation. Intervention: A: polydextrose; B: psyllium husk; C: wheat bran + psyllium husk; D: Bifidobacterium animalis subsp. lactis HN019 + Lacticaseibacillus rhamnosus HN001; Placebo: maltodextrin. Oligosaccharides were also included in group A to D. 16S rRNA sequencing was used to assess the gut microbiota at weeks 0, 2, and 4. A total of 242 participants completed the study. No time-by-group effect was observed for bowel movement frequency (BMF), Bristol stool scale score (BSS), and degree of defecation straining (DDS), while BSS showed mean increases of 0.95–1.05 in group A to D (all P < 0.05), but not significantly changed in placebo (P = 0.170), and 4-week change of BSS showed similarly superior effects of the interventions as compared placebo. Group D showed a marginal reduction in plasma 5-hydroxytryptamine. Group A resulted in a higher Bifidobacterium abundance than placebo at week 2 and 4. Fourteen genera showed intervention-specific increasing or decreasing trends continuously, among which Anaerostipes showed increasing trends in groups B and C, associated with BMF increase. Random forest models identified specific baseline microbial genera panels predicting intervention responders. In conclusion, we found that the dietary fibers or probiotics may relieve hard stool, with intervention-specific changes in gut microbiota relevant to constipation relief. Baseline gut microbiota may predispose the intervention responsiveness. ClincialTrials.gov number, NCT04667884.
Plain Language Summary
What is the context?
Supplementation of dietary fibers, such as psyllium husk or wheat bran (10 ~ 15 g/day) may relieve constipation symptoms, but bloating and flatulence are major concerns on a high fiber intake.
Functional constipation patients had alternated gut microbiota profiles, while meta-analysis suggested that multispecies probiotics may increase bowel movement frequency and relieve hard stool in functional constipation.
Dietary fibers or probiotics may lead to before-after changes of gut microbiota in patients with functional constipation, but time-series continued changes of gut microbiota during the intervention are unknown.
Elevation of 5-hydroxytryptamine synthesis in enterochromaffin cells may affect bowel movement. And the elevated plasma 5-hydroxytryptamine was observed in functional constipation patients.
What is new?
Daily supplement of three prebiotic formulas with dietary fibers (polydextrose, psyllium husk, wheat bran, together with oligosaccharides), or a probiotic formula with Bifidobacterium animalis subsp. lactis HN019 + Lacticaseibacillus rhamnosus HN001 effectively relieved hard stool in functional constipation patients after 4 weeks intervention.
We identified continued increasing or decreasing gut microbial genera over the intervention. Dietary fiber – gut microbiota (Anaerostipes)—constipation relieve (bowel movement frequency) evidence axis was identified in this human trial.
Probiotic supplementation marginally reduced plasma 5-hydroxytryptamine, possibly associated with changes in BMF-related gut microbial genera.
Intervention-specific baseline gut microbiota well predicted the responsiveness of constipation symptom relief.
What is the impact?
We provided references for the dosage and duration of dietary fiber/probiotics recommendations for adults with functional constipation, and advanced the microbial genera evidences of the fibers/probiotics-microbiota-laxation theory in humans.
Introduction
Characterized by continuously difficult, incomplete, or infrequent defecation, without an organic originCitation1, functional constipation affects approximately 10.1% (Rome IV criteria) of the worldwide population in adultsCitation2, resulted in considerable reductions in quality of life and increase in medical costCitation3. Effective intervention strategies are urgent to relieve the functional constipation difficulties, particularly in rapidly aging populations, such as ChineseCitation4.
The current knowledge in the etiology of functional constipation involves intestinal motility disorders, intestinal secretion disorders, changes in visceral sensitivity, pelvic floor muscle dysfunction, enteric nervous system dysfunction, etc.Citation1. From the perspective of primary care practice, lacking physical activity and insufficient intakes of water and dietary fibers are commonly recognized risk factorsCitation5. Animal models suggested laxative effects of extracted fibers involving water binding or colonic muscular contractionCitation5; yet human observational studies showed mixed associations between dietary fibers and constipation symptomsCitation6,Citation7. Moreover, the effects of supplementation of extracted fibers on constipation syndromes varied, partially related to the differences in fiber category, dosage, and intervention durationCitation5. With outstanding solubility, viscosity, or fermentabilityCitation8, psyllium husk, and wheat bran are widely used as fiber ingredients in the food industryCitation9,Citation10, and a few clinical trials recently suggested their effectiveness in improving bowel movement, stool consistency, and strainingCitation11–13, however, it was not clear whether a combination of psyllium husk and wheat bran with relatively lower dosage would be effective.
Growing evidence has pointed to the pathological role of gut microbiota disturbance in functional constipation, for the observations that functional constipation was associated with decreased beneficial species, increased pathogen species, and reduced species richness in human, and the capability that gut microbiota modulate gut functions via metabolite production, such as short-chain fatty acids (SCFA) and bile acid, and indirect enteric neuroendocrine regulation in animalsCitation14. Dietary fibers, such as psyllium huskCitation11, polydextroseCitation15, xylooligosaccharideCitation16, or probiotic supplementationsCitation17, were reported to be effective in modulating gut microbiota, yet their effects in functional constipation and the mode of gut microbiota–fiber interactions in optimizing intervention benefits are largely unknown in human.
In the current study, we aimed to evaluate the effectiveness of three dietary fiber formulas (polydextrose, psyllium husk, and wheat bran + psyllium husk) and one probiotic supplement (Bifidobacterium animalis subsp. lactis HN019 + Lacticaseibacillus rhamnosus HN001) on the improvement of constipation symptoms among Chinese adults with functional constipation, to identify gut microbiota changes upon intervention, and to explore the potential roles of gut microbiota in optimal intervention responses; hopefully to advance the dietary therapies for functional constipation.
Results
Participants and characteristics
During the intervention, 8 participants dropped out, and 242 (96.8%) participants eventually finished all the intervention procedures and provided biological samples (Figure S1). The overall adherence score was 0.86 ± 0.09 with no group difference. The mean age of the participants was 44.5 ± 16.7 years with 77.2% females (). The baseline variables were comparable among the five groups except that Bristol stool scale score (BSS) were slightly lower in Group C and D (overall P = 0.025). The overall mean bowel movement frequency (BMF) and degree of defecation straining (DDS) were 3.19 ± 1.65 and 2.45 ± 0.78, respectively.
Table 1. Baseline characteristics of the participants.
Effects on constipation symptoms
After 4 weeks of intervention, all the groups presented a significant within-group increase in BMF, and significant within-group decreases in DDS () (all P < 0.05). Overall, no significant time by group effect was observed for all the symptoms () (all P for interaction>0.05); however, BSS significantly increased in the four intervention groups (all P < 0.001), but not significantly changed in the placebo group (P = 0.170). By directly comparing the 4-week BSS change of each intervention group and the value of the placebo group, we observed similar superior effects of the four intervention groups (P = 0.056, P = 0.037, P = 0.058, and P = 0.042, respectively) ( and Table S1).
Figure 1. The changes of constipation symptoms from baseline to week 2 and week 4 by group.

Table 2. Constipation symptoms over the intervention by group a.
Biochemical variables and adverse events
No between-group or within-group change difference was observed in the plasma levels of glucose, and lipid profiles (Table S2). The plasma 5-hydroxytryptamine (5-HT) level tended to reduce only in the group D (median change = −10.75 ng/ml, P = 0.061), which was confirmed by a sensitive analysis among those with adherence score≥0.8 (median change = −13.33 ng/ml, P = 0.020) (), but this reduction did not sustain after FDR adjustment. No direct correlation of the 5-HT change with constipation symptom changes was captured in group D (data not shown), while 5-HT change was correlated with the change of several gut microbes, such as NK4A214_group and Eisenbergiella, etc. (). Yet, this correlation did not sustain with FDR adjustment, similarly to the correlations between 5-HT and gut microbes at different time points. No severe adverse events were reported over the intervention, whereas occasional mild events were reported (Table S3).
Figure 2. Changes in 5-HT and correlations with gut microbiota.

Baseline correlations between gut microbiota and constipation symptoms
At baseline, significant correlations were identified between the relative abundance of gut microbiota genera and BMF after adjusting for age and sex (). The relative abundance of 30 genera (such as UCG.002, Eisenbergiella, UCG.005, Alistipes, Christensenellaceae_R.7_group, and NK4A214_group) were inversely correlated with the BMF, while Bacteroides was positively correlated with BMF (all FDR adjusted P < 0.05). No significant correlation could be captured between gut microbiota and BSS or DDS.
Figure 3. The baseline correlations between gut microbial abundance and constipation symptoms, and the difference in relative abundance of the top 10 genera between intervention groups and placebo group at different stages.

Effects on gut microbiota diversity and composition
At each intervention stage (week 0, 2, 4), the genera community richness (Chao1 estimators) and diversity (Shannon index, ACE estimators) were comparable among groups, similarly for PCoA score (P > 0.05, Figure S2). No single genus showed significant difference between an intervention group and the placebo group at each stage, with full FDR correction (Table S4). For the top 10 genera (), the abundance of Bifidobacterium in group A was significantly higher than in the placebo group at both week 2 (difference of mean abundance = 1.780%, FDR adjusted P = 0.027) and week 4 (2.930%, FDR adjusted P = 0.049); while the abundance of Alistipes in group B was significantly lower than the placebo group at week 2 (1.967%, FDR adjusted P = 0.020). No direct association was detected between the change in Bifidobacterium and changes in constipation symptoms (Table S5)
Constant microbiota changes over the intervention
The group-specific microbial changing patterns were identified, and 14 genera showed continuous increase or decrease trends (). Several genera that inversely associated with baseline BMF showed decreasing trends over the intervention, such as UCG.002, Eisenbergiella, and NK4A214_group. Notably, the Anaerostipes fit the continuously increasing trend both in Group B and Group C. Meanwhile, we observed a positive correlation between Anaerostipes change and BMF change (, Table S6), and an inverse correlation between UCG-002 change and BSS change, but the correlations did not sustain with FDR correction (Table S6). The KEGG annotations of those genera in the constantly changing trends shared common modules of biological processes such as glycolysis, (reductive) citrate cycle, and gluconeogenesis (Table S7).
Figure 4. Constant genera abundance changes over the intervention and selected relevance with constipation.

Baseline microbiota predispose the responsiveness to interventions
With baseline genera abundance profiles as candidate predictors, the mean area under the receiver operating characteristics curves (AUCs) of the random forest models for predicting responders of 3 scenarios in groups A-D exceeded 0.8 (), outstandingly, with an AUC (95%CI) of 0.975 (0.916–1.000) predicting at least 1-unit BMF increase in group B, and an AUC (95%CI) of 0.976 (0.910–1.000) predicting at least 1-unit BSS increase in group C. The differential genera between responders and non-responders contributing to the random forest modeling were presented (), while their KEGG annotations reflected common modules of biological process such as glycolysis, (reductive) citrate cycle, and gluconeogenesis (Table S8)
Figure 5. Baseline gut microbiota predicting the improvement of constipation symptoms upon interventions.

Discussion
In this double-blinded randomized placebo-controlled trial among adults with functional constipation, hard stool was relieved in the three dietary fiber formulas with moderate dosage of polydextrose, psyllium husk, wheat bran plus psyllium husk, or the probiotic formula, accompanied with oligosaccharides. To our knowledge, this is the first human trial that recognized intervention-specific continued changes in gut microbiota, directly linked with constipation relief. Furthermore, our results suggested the capacity of gut microbial genera in shaping the intervention responsiveness in the improvement of BMF, BSS, and DDS.
Recently, several intervention studies suggested that psyllium husk (10 g/day for 8 weeksCitation12, or 10 g/day for 4 weeksCitation18 or wheat bran (15 g/day for 12 weeksCitation13 supplementation with relative high dose was effective in relieving hard stool in constipation patients; however, on the other hand, bloating and flatulence are of substantial concerns on a daily supplementation with a high-doseCitation5. In our results, a daily intake of 5 g psyllium husk (group B), or 2 g psyllium husk plus 5 g wheat bran(group C), along with oligosaccharide for 4 weeks was sufficient to relieve hard stool. Moreover, we observed that daily supplementation of Bifidobacterium animalis subsp.lactis + Lacticaseibacillus rhamnosus also relieved hard stool. Similarly, an earlier meta-analysis suggested that Bifidobacterium animalis subsp.lactis increased the BSS with a mean difference of 0.46, yet with other strainsCitation19. Taken together, our study provided direct evidences of the effectiveness of four new prebiotic or probiotic formulas on relieving hard stool symptoms among constipation patients, with a mean increase in BSS of 0.95–1.05. The dose and durations of our trial may be referred in future clinical practice for functional constipation.
5-HT, a brain neurotransmitter, also has important regulatory effects in gastrointestinal tract, as over 90% of the body 5-HT was synthesized in the enterochromaffin cells of gastrointestinal epitheliumCitation14. Animal models suggested that elevation of 5-HT synthesis may contribute to functional constipation by regulating gut motility triggered by metabolites of indigenous spore-forming microbesCitation20. Supportively, an earlier case-control study showed that platelet-depleted plasma concentrations of 5-HT tended to be elevated in patients of functional constipation, and inversely correlated with BMFCitation21. Supplementation of Bifidobacterium pseudolongum in mice reduced the 5-HT level in the colonic mucosa via diminishing enterochromaffin cellsCitation22. Our study, in human subjects, identified that plasma 5-HT was marginally reduced by 4-week intervention of Bifidobacterium animalis subsp.lactis HN019 + Lacticaseibacillus rhamnosus HN001. Although no direct link between 5-HT change and constipation symptom change could be captured, we showed potential correlations between 5-HT change and alterations in gut microbes, some of which, such as NK4A214_group and Eisenbergiella, were inversely correlated with BMF at baseline. Our results suggested the potential roles of enteric neurohormone, 5-HT, in the probiotic-derived optimizations in gut microbial genera, related to bowel movement in human.
Few was known regarding the gut microbiota alterations promoted by pre- or probiotic supplementation among constipation patients, particularly with repeated measurements over the intervention periods. In our study, we compared group differences in microbe genera level at the three intervention stages. Interestingly, Bifidobacterium was persistently higher in group A than in placebo at weeks 2 and 4, when only assessing the top ten abundant genera. Previous studies have suggested both polydextroseCitation23 and oligosaccharidesCitation16 may effectively increase Bifidobacterium in healthy volunteers, while we observed in functional constipation patients, that intervention of polydextrose and oligosaccharides for 2 to 4 weeks may also enrich Bifidobacterium, which may have multiple health benefits, including inhibiting pathogens, increasing production of SCFA, thus optimizing colonic functionCitation24.
We also applied soft clustering analysis to identify the potential constant microbe changes over the three intervention stages. Fourteen genera showed either increasing or decreasing trends continuously from week 0 to week 2 and 4, with functional annotation highlighting carbohydrate metabolism. Notably, UCG.002 and Eisenbergiella, both inversely associated with BMF at baseline, presented decreasing trends with the supplementation of polydextrose and oligosaccharides (Group A), while both of the two genera were linked with inflammationCitation25,Citation26. Moreover, Anaerostipes showed constant increasing trends in both group B and group C (both including psyllium husk and oligosaccharides), while this increasing trend tended to correlate with the increase in BMF. In an bacteria experiment, commensal Anaerostipes was able to convert dietary inositol into propionate and acetateCitation27, SCFAs which may reduce intestine pH, support colonic motility, inhibit pathogens, and optimize BMFCitation5. Those results may shed light on the potential mechanism underling the effects on relieving constipation by dietary fiber supplementation through optimizing gut microbes, including Anaerostipes, in functional constipation patients.
Host gut microbiota may interact with dietary fibers, or probiotic supplementations, thus affecting microbiome metabolism and inhibiting the growth of pathogensCitation5. Although overall constipation symptoms improved after the intervention, the level of responsiveness showed large individual differences. We hypothesis that the baseline gut microbiota features of a person may substantially predispose the potential responsiveness to a certain intervention strategy. By applying a random forest selection approach, we found that specific baseline microbial profiles well predicted the intervention responsiveness of BMF, BSS, or DDS improvement with outstanding AUCs. Our results from a human trial, may provide comprehensive information regarding the potential interactions between gut microbes and polydextrose, oligosaccharides, psyllium husk, wheat bran, or Bifidobacterium animalis subsp. lactis + Lacticaseibacillus rhamnosus. For instance, proinflammatory genus, Alistipes, was associated with worse constipation symptoms in previous observation studyCitation28,Citation29 and our baseline data. Interestingly, a lower abundance of Alistipes here was identified as a major contributor in predicting BSS increase upon group C intervention (psyllium husk plus wheat bran). Meanwhile, Alistipes showed a decreasing potential when supplemented with psyllium husk for 2 weeks (). In addition, lower baseline abundance of NK4A214_group was identified as a major contributor in predicting BMF increase in group B, while it was also inversely correlated with BMF at baseline, and continuously reduced in group B. The three results collectively suggested NK4A214_group as a possible contributor of constipation, but a sensitive target to psyllium husk and xylooligosaccharide intervention. Collectively, dietary fiber supplementation, may substantially relieve constipation, by interactions with host gut microbes, and modulations of microbe abundance in functional constipation patients; host gut microbiota may predispose the ways of their interactions with fiber intake, resulting in the individual differences in constipation symptom improvement.
Our study has several limitations. Firstly, without sub-categorization for constipation patients, we were unable to detect the intervention effects for different constipation types, such as those with dyssynergic defecation, who might not directly respond to the fiber or probiotics. Secondly, without microbial metabolites data, we were unable to explore the metabolic interactions between host and gut microbiomes, and their associations with clinical improvement. Moreover, the wide age range of the participants may increase the individual variability of gut microbiota, thereafter limited the ability of detecting intervention effects. In addition, the KEGG annotations based on 16S rRNA gene sequences may have a limited resolution.
Conclusion
In conclusion, we demonstrated the effects of dietary fiber or probiotic formulas in relieving hard stool in functional constipation patients. Over the intervention periods, we identified continuously changed microbiota features and baseline microbe profiles predicting intervention responsiveness, all intervention-specific. Together with the correlations between microbiota profile and constipation symptoms, our study provided novel evidences that the pre or probiotic interventions may modulate gut microbiota, associated with intestinal health.
Methods
Study design and participants
This study was a double-blind, randomized, placebo-controlled trial conducted from August 2020 to May 2021. Participants were recruited by advertisements and screened using an online questionnaire posted by the Epidemiology Division, School of Public Health, Xi’an Jiaotong University Health Science Center or the Nutrition Department, Xi’an Daxing Hospital. The inclusion criteria were: age between 18 and 70 years, and diagnosis with functional constipation based on Rome Ⅳ criteria: 1) met two or more of the following conditions in at least 25% of defecations: a, straining, b, lumpy or hard stools with Bristol stool scale score (BSS) rating 1–2), c, sensation of incomplete evacuation, d, sensation of anorectal obstruction/blockage, e, manual maneuvers to facilitate defecation, f, fewer than three spontaneous bowel movement per week; 2) loose stools are rarely present without the use of laxatives; 3) insufficient to be diagnosed with irritable bowel syndrome per Rome Ⅳ criteria. Those symptoms should have appeared for at least 6 months before diagnosis, and met the above diagnostic criteria in the past 3 months. Exclusion criteria included: 1) pregnancy or lactation; 2) unable to take the intervention products or complete the examination as required for any reason; 3) difficult in verbal expression or mental disorders; 4) abnormal liver and kidney function indicated by physical examination; 5) acute gastrointestinal diseases in the recent 4 weeks; 6) constipation caused by surgery in the recent 4 weeks; 7) take products of prebiotics or probiotics, antibiotics, laxatives, anticholinergics, or antidiarrheal drugs more than 1 time/week in the recent 4 weeks; 8) a history of cancer, severe enteritis, intestinal obstruction, inflammatory bowel disease, hypothyroidism, mental illness, stroke, heart disease, liver cirrhosis, renal failure, hematopoietic system diseases, or organic intestinal diseases indicated by colonoscopy or imaging display; 9) participated in other clinical studies within 3 months before enrollment; 10) other health issues unsuitable to participate.
From August 2020 to May 2021, 477 individuals who completed the online screening questionnaire attended the onsite screening session, of which 227 were excluded for not meeting the inclusion criteria, too busy to participate, or other reasons. Finally, 250 participants were recruited and completed the baseline examination (Figure S1). The study was approved by the Ethical Committee of Xi’an Jiaotong University Health Science Center and the Ethical Committee of Xi’an Daxing Hospital. Each participant provided written informed consent before the intervention. The present trial was registered at ClinicalTrials.gov as NCT04667884.
Randomization and blinding
Block randomization (block length = 5) was performed by an independent statistician, who was blinded to treatment allocation. On the first visit (day 0) to the research center, an intervention group was assigned to each participant according to the sealed allocation sequence. The intervention sachets were prepared by BYHEALTH Co., LTD (Guangdong, China) with identical appearance, taste, and smell. Participants and researchers were blinded to treatment assignments until the entire study was completed.
Sample size
The sample size was determined by assuming a difference in bowel movement frequency (BMF) change of 0.98 between any intervention group and the placeboCitation30, with an assumed standard deviation (SD) of BMF as 1.6 in candidate population, if α error and power were set as 0.05 and 80%, respectively. A total of 40 participants were needed in each group. Considering about 20% lost follow-up, we determined 50 participants in each group (250 for 5 groups).
Intervention
All eligible subjects were randomly assigned to one of the five groups: Group A: polydextrose (1.96 g), oligosaccharides (xylooligosaccharide and fructooligosaccharide, 1.95 g) and resistant dextrin (0.75 g) for each sachet; Group B: psyllium husk (2.5 g), oligosaccharides (xylooligosaccharide, 0.74 g) and D-mannitol (1.45 g) for each sachet; Group C: wheat bran (2.5 g), psyllium husk (1 g), oligosaccharides (fructooligosaccharide, 1.9 g) and resistant dextrin (0.5 g) for each sachet; Group D: 7.7 × 10Citation9 CFU Bifidobacterium animalis subsp. lactis (previously named as Bifidobacterium lactis) HN019 + 1.9 × 10Citation9 CFU Lacticaseibacillus rhamnosus (previously named as Lactobacillus rhamnosus) HN001, oligosaccharides (fructooligosaccharide, 0.48 g) for each sachet; Group P (Placebo): maltodextrin (1.5 g) for each sachet. During the intervention phase, participants were required to take 2 sachets with warm water (≤37°C) every day.
At week 0, all the participants received reading materials of lifestyle counseling developed based on Chinese Dietary Guidelines 2016Citation31 and Expert Consensus on Chronic Constipation in ChinaCitation32 (Table S9). Participants were required to visit the research centers at Week 0, 2, and 4 to collect intervention products and finish all measurements, sample collection, and questionnaires. Participants were required to return all remaining intervention products at each follow-up visit, and to report any adverse events, medication use, and hospitalizations. The adherence score was defined as the number of intervention sachets consumed divided by the number of intervention sachets provided.
Data and sample collection
The primary outcome was the change of BMF recorded as the number of bowel movements per week. The secondary outcomes include the changes in BSS, DDS, fasting glucose, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, total cholesterol, triglycerides, and gut microbiome profiles over the 4-week intervention.
A Redcap-based online system was applied to collect questionnaire informationCitation33. Data on demography, lifestyle, and medical history were collected at baseline. Participants were required to report their weekly BMF on days 0 (baseline), 7, 14, 21 and 28 (endpoint). Other constipation outcomes were collected at days 0, 14, and 28, including BSS (type 1: separate hard lumps (hard to pass); type 2: lumpy, hard, sausage-shaped; type 3: sausage-shaped with cracks on the surface; type 4: sausage-shaped or snake-like; smooth and soft; type 5: soft blobs with clear-cut edges (easy to pass); type 6: fluffy pieces with ragged edges; mushy; type 7: entirely liquid, watery, no solid pieces)Citation34, DDS (from 1 as normal to 4 as frequent abdominal pain or burning sensation of the anus that affects defecation)Citation35. Weight was measured to the nearest 0.1 kg, using an electronic scale (Tanita BC-567), while height was measured using a portable stadiometer, to the nearest 0.1 cm, with participants wearing light clothing and shoes off. Blood samples were collected by trained health workers following an overnight fast (≥10 hours) at days 0, 14 and 28. Fasting blood samples were centrifuged, allocated as plasma, buffy coat, and red blood cells, and then immediately stored at −80°C until assay. Plasma levels of glucose, triglycerides, total cholesterol, and HDL/LDL cholesterol were measured by an automatic biochemical analyzer (Beckman Coulter AU680). Plasma 5-HT was determined using an ELISA kit (Elabscience E-EL-0033c).
Fecal sample collection and assessment of gut microbiota
Fecal samples were collected by participants themselves at baseline (3 days before the intervention), or within 3 days before or after visits on days 14 and 28. A collection tube for fecal samples (Real-Bio Technology, Shanghai) with preservation solution was used to enable room temperature preservation of the feces before their visits to the research center. The solution allows the preservation of DNA in the stool at room temperature for 10–14 days. All fecal samples were transferred to −80°C freezer on the day of the visit. A printed version and a video version of sample collection instructions were developed and provided to each participant to ensure the samples meet the requirement (online video: https://figshare.com with the doi: 10.6084/m9.figshare.21542994). DNA extraction and 16S rRNA sequencing were conducted according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China) (detailed in Table S10)
Statistical analyses
The intention-to-treat principle was applied in the data analyses unless otherwise indicated. Descriptive data were presented as mean ± SD for continuous variable or as numbers and percentages for qualitative variables. Baseline characteristics were compared using ANOVA, Kruskal-Wallis test, or Fisher’s exact test per distributions when appropriate. With adjustment for age and sex, a generalized estimating equation (GEE) was also used to assess time effects within-group, group effects, and group × time effects, respectively for the outcomes. Wilcoxon rank-sum test was used for comparing before-after changes in constipation symptoms between each treated group and the placebo group. Partial Spearman’s correlation coefficient was used to assess the correlations between changes in constipation symptoms and changes in other variables over the intervention.
16S rRNA gene sequences were rarefied to the lowest number of sequences per sample (n = 50,331) to complete downstream diversity and composition analyses. The genus-level α-diversity indices of gut microbial community richness (the Ace and Chao1 estimators) and community diversity (the Shannon index) were calculated and compared among all groups at week 0, 2, and 4 using the Kruskal-Wallis test. Permutational multivariate analysis of variance (PERANOVA) based on Bray-Curtis distance was performed to assess global microbiota composition among the five groups at the genus level for each stage. After excluding those bacterial genera that only presented in less than 10% of the total samples, 120 bacterial genera abundance from 16s rRNA sequencing were used in the subsequent genera level analyses. We compared the abundance between an intervention group and the placebo group using the Wilcoxon signed-rank test at weeks 0, 2, and 4. For the top 10 abundant genera, the differences of the mean relative abundance of an intervention group (A-D) relative to the placebo group were presented in heatmap to illustrate the comparison results.
Soft clustering analysis was used to identify changing trends of gut microbiota in each intervention group over three stages by applying the R package “Mfuzz”Citation36,Citation37. The log2 fold changes of continuously increasing or decreasing genera were presented. PICRUSt2 (version v2.2.0-b) was used to predict the Kyoto Encyclopedia of Genes and Genomes (KEGG) module and pathways to infer microbial functional content (Detailed in Table S7). Random forests analysis was used to determine genus-level gut microbiota patterns predicting responders (≥1 increase in BMF; ≥1 increase in BSS score; ≥1 decrease in DDS) using the R package “caret” with the AUC calculated using the R package “pROC”. Related KEGG module and pathways were explored (Detailed in Table S8).
All the analyses were performed using the R (version 4.0.2). FDR-adjusted P value (Q value) was applied in post-hoc analyses (comparisons for bacteria genera and 5-HT, correlation analysis between variables). All the authors had access to the study data and had reviewed and approved the final manuscript.
Author contributions
XLiu, TT, and XZ were involved in the conception and design of the study. HL, YL, YH, BM, JY, XLiao, YY, JL, YS, SD, QW, and LZeng were involved in the acquisition of data. JL, JX, and LK were involved in the lab experiment. HL, YL, YH, FC, KX, and LS were involved in the analysis and interpretation of data. HL, YL, YH, GM, and XLiu were involved in the drafting of the manuscript. XLiu, XW, ZL, LS, LZhang, TT, and XZ were involved in the critical revision of the manuscript for important intellectual content. All the authors revised and approved the final manuscript.
Data availability
The data that support the findings of this study are available as deidentified individual patient data (IPD) in the Figshare public repository (https://doi.org/10.6084/m9.figshare.21561870)
Supplemental Material
Download PDF (2.4 MB)Acknowledgments
We thank all the participants, on-site field workers, investigators in Xi’an Jiaotong University and Xi’an Daxing Hospital, and all the members in Xin Liu Laboratory in assisting the intervention procedure, and discussion of the manuscript.
Disclosure statement
BYHEALTH Co., LTD provided the dietary fiber and probiotic supplements in this study.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2197837
Additional information
Funding
References
- Vriesman MH, Koppen IJN, Camilleri M, Di Lorenzo C, Benninga MA. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol. 2020;17(1):21–15. doi:10.1038/s41575-019-0222-y.
- Barberio B, Judge C, Savarino EV, Ford AC. Global prevalence of functional constipation according to the Rome criteria: a systematic review and meta-analysis. The Lancet Gastroenterol Hepatol. 2021;6(8):638–648. doi:10.1016/S2468-1253(21)00111-4.
- Nag A, Martin SA, Mladsi D, Olayinka-Amao O, Purser M, Vekaria RM. The humanistic and economic burden of chronic idiopathic constipation in the USA: a systematic literature review. Clin Exp Gastroenterol. 2020;13:255–265. doi:10.2147/CEG.S239205.
- Huang L, Jiang H, Zhu M, Wang B, Tong M, Li H, Lin MB. Prevalence and risk factors of chronic constipation among women aged 50 years and older in Shanghai, China. Med Sci Monit. 2017;23:2660–2667. doi:10.12659/MSM.904040.
- Gill SK, Rossi M, Bajka B, Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. 2021;18(2):101–116. doi:10.1038/s41575-020-00375-4.
- Shen L, Huang C, Lu X, Xu X, Jiang Z, Zhu C. Lower dietary fibre intake, but not total water consumption, is associated with constipation: a population-based analysis. J Hum Nutr Diet. 2019;32(4):422–431. doi:10.1111/jhn.12589.
- Markland AD, Palsson O, Goode PS, Burgio KL, Busby-Whitehead J, Whitehead WE. Association of low dietary intake of fiber and liquids with constipation: evidence from the national health and nutrition examination survey. Am J Gastroenterol. 2013;108(5):796–803. doi:10.1038/ajg.2013.73.
- McRorie JW, Fahey GC, Gibb RD, Chey WD. Laxative effects of wheat bran and psyllium: resolving enduring misconceptions about fiber in treatment guidelines for chronic idiopathic constipation. J Am Assoc Nurse Pract. 2020;32(1):15–23. doi:10.1097/JXX.0000000000000346.
- Belorio M, Gomez M. Psyllium: a useful functional ingredient in food systems. Crit Rev Food Sci Nutr. 2022;62(2):527–538. doi:10.1080/10408398.2020.1822276.
- Stevenson L, Phillips F, O’sullivan K, Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int J Food Sci Nutr. 2012;63(8):1001–1013. doi:10.3109/09637486.2012.687366.
- Yang C, Liu S, Li H, Bai X, Shan S, Gao P, Dong X. The effects of psyllium husk on gut microbiota composition and function in chronically constipated women of reproductive age using 16S rRNA gene sequencing analysis. Aging (Albany NY). 2021;13(11):15366–15383. doi:10.18632/aging.203095.
- Noureddin S, Mohsen J, Payman A. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: a randomized trial in patients with type 2 diabetes and chronic constipation. Complement Ther Med. 2018;40:1–7. doi:10.1016/j.ctim.2018.07.004.
- Muller M, Hermes GDA, Emanuel EC, Holst JJ, Zoetendal EG, Smidt H, Troost F, Schaap FG, Damink SO, Jocken JWE, et al. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: a randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes. 2020;12(1):1704141. doi:10.1080/19490976.2019.1704141.
- Wang JK, Yao SK, Ağagündüz D. Roles of gut microbiota and metabolites in pathogenesis of functional constipation. Evid Based Complement Alternat Med. 2021;2021:5560310. doi:10.1155/2021/5560310.
- Ibarra A, Pelipyagina T, Rueffer M, Evans M, Ouwehand AC. Efficacy of polydextrose supplementation on colonic transit time, bowel movements, and gastrointestinal symptoms in adults: a double-blind, randomized, placebo-controlled trial. Nutr. 2019;11(2):11. doi:10.3390/nu11020439.
- Finegold SM, Li Z, Summanen PH, Downes J, Thames G, Corbett K, Dowd S, Krak M, Heber D. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct. 2014;5(3):436–445. doi:10.1039/c3fo60348b.
- Wang L, Tian P, Wang B, Cui S, Zhao J, Zhang H, Qian L, Wang Q, Chen W, Wang G, et al. A randomised, double-blind, placebo-controlled trial of bifidobacterium bifidum CCFM16 for manipulation of the gut microbiota and relief from chronic constipation. Food Funct. 2022;13(3):1628–1640.
- Erdogan A, Rao SSC, Thiruvaiyaru D, Lee YY, Coss Adame E, Valestin J, O’Banion M. Randomised clinical trial: mixed soluble/insoluble fibre vs. psyllium for chronic constipation. Alimentary Pharmacol Therapeutics. 2016;44(1):35–44. doi:10.1111/apt.13647.
- Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100(4):1075–1084. doi:10.3945/ajcn.114.089151.
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler C, Ismagilov R, Mazmanian S, Hsiao E. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi:10.1016/j.cell.2015.02.047.
- Shekhar C, Monaghan PJ, Morris J, Issa B, Whorwell PJ, Keevil B, Houghton LA. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterol. 2013;145(749–57 quiz):e13–4. doi:10.1053/j.gastro.2013.07.014.
- Tatsuoka M, Osaki Y, Ohsaka F, Tsuruta T, Kadota Y, Tochio T, Hino S, Morita T, Sonoyama K. Consumption of indigestible saccharides and administration of bifidobacterium pseudolongum reduce mucosal serotonin in murine colonic mucosa. Br J Nutr. 2022;127(4):513–525. doi:10.1017/S0007114521001306.
- Lamichhane S, Yde CC, Forssten S, Ouwehand AC, Saarinen M, Jensen HM, Gibson GR, Rastall R, Fava F, Bertram HC. Impact of dietary polydextrose fiber on the human gut metabolome. J Agric Food Chem. 2014;62(40):9944–9951. doi:10.1021/jf5031218.
- Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22(6):495–512. doi:10.1111/j.1365-2036.2005.02615.x.
- Li N, Wang J, Liu P, Li J, Xu C. Multi-omics reveals that bifidobacterium breve M-16V may alleviate the immune dysregulation caused by nanopolystyrene. Environ Int. 2022;163:107191. doi:10.1016/j.envint.2022.107191.
- Terzo S, Mule F, Caldara GF, Baldassano S, Puleio R, Vitale M, Cassata G, Ferrantelli V, Amato A. Pistachio consumption alleviates inflammation and improves gut microbiota composition in mice fed a high-fat diet. Int J Mol Sci. 2020;21(1):21. doi:10.3390/ijms21010365.
- Bui TPN, Manneras-Holm L, Puschmann R, Wu H, Troise AD, Nijsse B, Boeren S, Bäckhed F, Fiedler D, deVos WM. Conversion of dietary inositol into propionate and acetate by commensal anaerostipes associates with host health. Nat Commun. 2021;12(1):4798. doi:10.1038/s41467-021-25081-w.
- Sugitani Y, Inoue R, Inatomi O, Nishida A, Morishima S, Imai T, Kawahara M, Naito Y, Andoh A. Mucosa-associated gut microbiome in Japanese patients with functional constipation. J Clin Biochem Nutr. 2021;68(2):187–192. doi:10.3164/jcbn.20-93.
- Tian H, Ye C, Yang B, Cui J, Zheng Z, Wu C, Zhou S, Lv X, Qin N, Qin H, et al. Gut metagenome as a potential diagnostic and predictive biomarker in slow transit constipation. Frontiers in Medicine. 2022;8. doi:10.3389/fmed.2021.777961.
- Zhang C, Jiang J, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Meta-analysis of randomized controlled trials of the effects of probiotics on functional constipation in adults. Clin Nutrition. 2020;39(10):2960–2969. doi:10.1016/j.clnu.2020.01.005.
- Chinese Nutrition Society. Dietary guidelines for Chinese residents. Beijing: Renmin Wei Sheng Chu Ban She; 2016.
- Gastrointestinal Dynamics Group of Gastroenterology Branch of Chinese Medical Association, Collaborative Group of Functional Gastrointestinal Diseases. Expert consensus on chronic constipation in China (2019, Guangzhou). Zhong Hua Xiao Hua Bing Za Zhi. 2019;39:577–598.
- Zhang B, Baibing M, Wentao W, Guoshuai S, Suhang S, Yezhou L, Xiangyu GA, Shaonong DA, Xiangyu GA, Hong YA, et al. Application of research electronic data capture in an interactive online survey system for monitoring hypertension risk factors during pregnancy. Xi’an Jiao Tong da Xue Xue Bao Yi Xue Ban. 2020;41(4):612-616.
- Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Tech Coloproctol. 2001;5(3):163–164. doi:10.1007/s101510100019.
- Ibarra A, Latreille-Barbier M, Donazzolo Y, Pelletier X, Ouwehand AC. Effects of 28-day bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: a double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes. 2018;9(3):236–251. doi:10.1080/19490976.2017.1412908.
- Futschik ME, Carlisle B. Noise-robust soft clustering of gene expression time-course data. J Bioinform Comput Biol. 2005;3(04):965–988. doi:10.1142/S0219720005001375.
- Kumar L, Futschik M. Mfuzz: a software package for soft clustering of microarray data. Bioinformat. 2007;2(1):5–7. doi:10.6026/97320630002005.