ABSTRACT
Salmonella Enteritidis is a foodborne enteric pathogen that infects humans and animals, utilizing complex survival strategies. Bacterial small RNA (sRNA) plays an important role in these strategies. However, the virulence regulatory network of S. Enteritidis remains largely incomplete and knowledge of gut virulence mechanisms of sRNAs is limited. Here, we characterized the function of a previously identified Salmonella adhesive-associated sRNA (SaaS) in the intestinal pathogenesis of S. Enteritidis. We found that SaaS promoted bacterial colonization in both cecum and colon of a BALB/c mouse model; it was preferentially expressed in colon. Moreover, our results showed that SaaS enhanced damage to mucosal barrier by affecting expressions of antimicrobial products, decreasing the number of goblet cells, suppressing mucin gene expression, and eventually reducing thickness of mucus layer; it further breached below physical barrier by strengthening invasion into epithelial cells in Caco-2 cell model as well as decreasing tight junction expressions. High throughput 16S rRNA gene sequencing revealed that SaaS also altered gut homeostasis by depleting beneficial gut microbiota while increasing harmful ones. Furthermore, by employing ELISA and western blot analysis, we demonstrated that SaaS regulated intestinal inflammation through sequential activation P38-JNK-ERK MAPK signaling pathway, which enabled immune escape at primary infection stage but strengthened pathogenesis at later stage, respectively. These findings suggest that SaaS plays an essential role in the virulence of S. Enteritidis and reveals its biological role in intestinal pathogenesis.
Introduction
As a food-borne pathogen, Salmonella is responsible for a wide range of enteric diseases from mild, self-limiting gastroenteritis to life-threatening systemic infection. The symptoms of the gastroenteritis caused by Salmonella enterica Serovar Enteritidis (S. Enteritidis) are non-bloody vomiting and nausea, accompanied by abdominal pain, myalgia, headache and arthralgia in people of all ages.Citation1,Citation2 What’s more, for those immunocompromised such as children, older and patients, severe diseases like bacteremia and meningitis can be observed.Citation3 This wide range of clinical pictures makes Salmonella a continuous threat that leads to high disease burden around the world especially in Africa followed by Southeast Asia.Citation4,Citation5 S. Enteritidis, a facultative intracellular Gram-negative pathogen, is one of the most common serotypes of Salmonella. After ingestion it hyper-replicates within its host’s gut tissue causing inflammation, which then reaches mesenteric lymph nodes or further systemic sites such as liver and spleen.Citation6,Citation7 Breaching the intestinal barrier is thought to be the initial step of the S. Enteritidis infection.Citation8 Since animal intestine contains natural microbiota which suppress pathogen growths by competing for nutrients, mucosal barriers preventing pathogens from invading epithelium and physical barriers separating pathogens from internal milieu,Citation9 any damage done to these barriers will lead to increased bacterial dissemination reinstating its virulence.Citation10 Thus, understanding how pathogens break through these barriers is essential for avoiding microbial infections or their progression.
To breach the intestinal barrier, Salmonella employs a variety of virulence factors, among which Salmonella pathogenicity island (SPI)-1 and SPI-2 effectors are predominantly studied. SPI-1 effectors like SopA, SopB and SopE act on actin dynamics, chemokine secretion, and tight junction (TJ) formation during early phase of infection,Citation11 whereas SPI-2 effectors such as SseF and SseG take over post-intracellular growth and survival.Citation12 Other factors include CsgB and PegD important for colonizationCitation13 and AvrA inhibiting JNK MAPK pathway, thus inhibiting inflammatory response and stabilizing tight junctions.Citation14,Citation15 Despite the fact that many of these factors have been well investigated, the regulatory network of S. Enteritidis is still substantially incomplete, and new regulators are constantly being discovered.
Small non-coding RNAs (sRNAs) have been identified in recent years as post-transcriptional regulators that can have a vital effect on diverse cellular processes, such as sRNA4130247 for carbon starvation,Citation16 CyaR for hyperosmotic stress and DsrA for acid stress.Citation17 In vivo conditions are complex and diverse, so sRNAs are thought to play an important role in Salmonella survival and pathogenesis. To date, only a few sRNAs have been found to regulate virulence out of hundreds of candidates in Salmonella; most belong to S. Typhimurium. Research has mostly focused on cell models such as macrophages or epidermic cells to understand possible roles in virulence. To name a few, IsrM, RyhBs, and STnc640 were found involved in bacterial invasion of epithelial cells or intracellular replication and survival in macrophages.Citation18–20 Further in vivo studies are limited, with most stopping with the lethality of animals and bacterial colonization.Citation18 Overall, there is still much more research needed to better understand the actual functions of sRNAs in Salmonella pathogenesis in vivo, particularly within the intestine.
Previous study revealed that Salmonella adhesive-associated sRNA (SaaS) promotes Salmonella virulence by enhancing mortality, systemic inflammation and bacterial dissemination in the intestine.Citation21 However, the role of SaaS in S. Enteritidis intestinal pathogenicity remains unclear. This study aims to investigate how SaaS contributes to the complex interactions among luminal microbes, physical barrier and mucosal barrier for better understanding of its biological role in S. Enteritidis intestinal pathogenicity. Such knowledge is expected to offer insights into development of therapies for bacterial infections and help refine the Salmonella virulence regulatory network.
Results
SaaS is a colon-responsive sRNA that promotes Salmonella colonization of the mouse intestine
To examine the effects of SaaS on Salmonella adherence, we carried out in vivo mouse colonization experiments. The wild strain WT-infected mice and complement strain ΔsaaS/psaaS-infected mice presented similar changes in this assay. As shown in , while the number of CFUs recovered from colon and cecum was significantly higher (P < 0.01) in the WT group than in the ΔsaaS group at 72 and 120 hpi, a dramatic decrease was observed for Salmonella numbers within the latter at 72 hpi compared with 6 hpi. Interestingly, even though the sustaining accumulation makes cecum harbors the most microbiota in the gastrointestinal tract and more Salmonella colonization here, there was no significant difference between WT-infected mice and ΔsaaS-infected mice in terms of the ratio of bacteria colonizing the cecum versus the colon, indicating a possible rule of Salmonella colonization distribution throughout the intestine. For SaaS itself, the results showed that the half-life of SaaS was about 7–8 min, and levels of SaaS were 2.2–2.6-fold greater within colonic tissue than cecal samples (). These results indicate that SaaS, activated by colonic environment, is a positive regulator of Salmonella colonization.
Figure 1. Bacterial dissemination and inflammatory response induced by SaaS in vivo. (a) Bacterial colonization of WT, ΔsaaS and ΔsaaS/psaaS in cecum and colon, and the ratio between bacterial colonization in cecum and bacterial colonization in colon in one mouse; (b) Expression of SaaS in colonized Salmonella in cecum and colon, and half-life of SaaS in the simulated intestinal environment; (c) The concentration of IL-1β, IL-6, TNF-α and iNOS and the expressions of Il18 and Cox2 mRNAs in colon; (d) Representative H&E-stained colonic tissue sections of WT, ΔsaaS and ΔsaaS/psaaS-infected mice (Scale bars, 50 μm). For mRNA expression, the control was set to 1 and indicated by dashed line. Data are represented as means±SD; n = 7–10. Statistical significance was assessed using Student’s t-test. *P < 0.05, **P < 0.01.

SaaS promotes the time-dependent regulation of intestinal inflammatory response
The present study investigated whether SaaS mediated the secretion of cytokines in vivo, with the interleukin (IL)-1β, IL-6, IL-18, tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) levels in colon after infection examined with ELISA or RT-qPCR (). At 6 hpi, there was no significant difference found between all groups for the levels of IL-1β, IL-6, TNF-α and iNOS, while only Il18 and Cox2 mRNA were higher in the ΔsaaS group than that of WT group. At 72 hpi all cytokine levels except Cox2 were significantly higher in the ΔsaaS group compared to those observed from the WT group. Conversely at 120 hpi, except for increased Il18 mRNA expression, lower levels of IL-6, iNOS and Cox2 were detected in the ΔsaaS group than in the WT group, indicating a time-dependent response. In addition, H&E staining showed that although no serious pathological syndromes were observed, the histopathological appearance in the colon exhibited significant differences among groups (). Compared with the WT group, the ΔsaaS group displayed more inflammatory infiltration at 72 hpi. At 120 hpi, an overall enhancement of inflammatory response was evident; however, compared to that in the ΔsaaS group, those infected with WT strain had a more intense infiltration of inflammatory cells. This tendency was consistent with the trends of tested cytokines (). These results indicated that intestinal inflammation is mediated by SaaS in a time-dependent manner.
SaaS contributes to intestinal mechanical and mucosal barrier dysfunction
The integrity of the intestinal barrier is essential for maintaining intestinal immune homeostasis and activating a proper immune response to pathogenic bacteria, with mucosal and physical barriers playing important roles.Citation22 Therefore, we further analyzed the effect of Salmonella mediated by SaaS on the colonic mucosal and physical barrier in vivo. The relative number of goblet cells, mRNA expressions of mucin, thickness of mucus layer and secretory immunoglobulin A (sIgA) levels were all combined to thoroughly evaluate the effects of SaaS on the mucosal barrier (). As shown in , at 72 and 120 hpi, respectively, the relative number of goblet cells was significantly higher (P < 0.01) in the ΔsaaS group than that in the WT group. The virulence factor SopB is the only reported one responsible for regulating the death of goblet cells, which Salmonella uses to inhibit the process of necroptosis in goblet cells.Citation23 Hence we want to know whether SaaS utilizes SopB to lead to the death of goblet cells. Nevertheless, results showed that the level of sopB mRNA in the WT strain was significantly higher than that in the ΔsaaS group (Fig S1), which implies a different mechanism. Meanwhile, gene expression analysis revealed that in the ΔsaaS group, Muc1 (encoding Mucin-1) had higher expression at 6 hpi; Muc2 (encoding Mucin-2) had higher expression throughout infection; while Muc4 (encoding Mucin-4) had higher expression at 72 hpi and 120 hpi (). Furthermore, the expressions of mucin biosynthesis genes B3gnt6 (encoding core 3 1,3-N-acetylglucosaminyltransferase) and St6galnac1 (encoding alpha-N-acetylgalactosaminide alpha-2,6-sialyltransferase 1) were evaluated. There was no significant difference among the groups (), implying that Salmonella has no effect on mucin biosynthesis through SaaS. Hence, it’s the death of goblet cells rather than a decrease in mucin biosynthesis that is responsible for the decreased mucin. As expected, the thickness of mucus layer in the ΔsaaS group was significantly higher than that in the WT group at 120 hpi, indicating an inhibitory role played by SaaS on host’s recovery from damaged mucosal barrier.
Figure 2. Effects of SaaS on the colonic mucus barrier. (a) Representative AB/PAS-stained colonic tissue sections of WT, ΔsaaS and ΔsaaS/psaaS-infected mice (Scale bars, 50 μm); (b) The number of goblet cells (marked with a black arrow), the thickness of mucus layer, sIgA levels and the expressions of Muc1/2/4 mRNA; (c) Expressions of mucin biosynthesis genes including B3gnt6 and St6galnac1. For mRNA expression, the control was set to 1 and indicated by dashed line. Data are represented as means±SD; n = 7–10. Statistical significance was assessed using Student’s t-test. *P < 0.05, **P < 0.01.

Antimicrobial products (AMPs), including alpha defensins (Cryptdins encoded by Cryptdin1/4/5), C-type lectin regenerating islet-derived protein 3 (RegIIIγ encoded by Reg3g and RegIIIβ encoded by Reg3b), secretory phospholipase A2 type IIA (sPLA2 encoded by Pla2g2a), cathelicidins (encoded by Camp), and lysozymes (encoded by Lyz) are released by Paneth cells or goblet cells to provide critical local bactericidal activity protecting the intestinal mucosa.Citation24 Throughout the invasion, there was no significant difference between groups in Cryptdin1/4 and Lyz in this study (). Cryptdin5, Reg3g, and Reg3b mRNA levels were significantly lower (P < 0.01) in the ΔsaaS group compared to the WT group at 6 hpi; however, antimicrobial product expressions showed an opposite trend at 72 hpi, with Reg3g, Reg3b, Camp and Pla2g2a mRNAs being higher in the ΔsaaS group. This trend is maintained, with Cryptdin5 and Camp mRNAs significantly higher (P < 0.01) in the ΔsaaS group, but Reg3g, Reg3b and Pla2g2a mRNAs decreasing.
Figure 3. Effects of SaaS on the colonic antimicrobial products and physical barrier. (a) Expressions of antimicrobial product genes including Cryptdin1/4/5, Reg3g, Reg3b, Pla2g2a, Camp, and Lyz; (b) Invasion and survival of S. Enteritidis to Caco-2 cells, LDH release of Caco-2 cells treated with S. Enteritidis and the expressions of Zo1, Ocln and Cldn mRNA. For mRNA expression, the control was set to 1 and indicated by dashed line. Data are represented as means±SD; n = 7–10. Statistical significance was assessed using Student’s t-test. *P < 0.05, **P < 0.01.

The physical barrier, which is made up of intestinal epithelium and tight junctions (TJs), serves as a secondary protective layer following the mucus layer.Citation25 Dysfunction of TJs or intestinal epithelium often results in increased intestinal permeability, known as leaky gut, which promotes the risk of microbial infection and tissue injury.Citation26,Citation27 In mice infected with ΔsaaS strain, compared to those infected with WT strain, significantly higher gene expression levels of occludin (Ocln) were observed at all times, claudin (Cldn) except at 72 hpi and zonula occludens-1 (Zo1) at 120 hpi from the point of TJs that known as the paracellular route (). To further investigate the effect of SaaS on intestinal permeability, Caco-2 monolayer was used as an in vitro model that mimics the transcellular route. As shown in , CFU recovered from Caco-2 cells was approximately 1.8 fold higher (P < 0.01) in the WT group than in the ΔsaaS group for both invasion and intracellular survival ability. Correspondingly, lactate dehydrogenase (LDH) activities measured in all WT-infected Caco-2 cells were much greater (P < 0.05) than those infected with ΔsaaS. These data suggest that SaaS contributes to increased intestinal permeability and translocation of S. Enteritidis across the intestinal barrier.
SaaS contributes to intestinal biological barrier damage by altering gut microbiota ecology and function
Trillions of bacteria reside on the mucosal and epithelial surfaces of the host intestine, maintaining a relatively stable composition while principally coexisting with the host and interacting with other barriers.Citation28 To access the microbial diversity associated with Salmonella strains in colonic microbiota, we estimated alpha- and beta-diversity.
Richness and diversity of microbiota
The Chao l and ACE indexes are used to positively measure the richness of microbial communities.Citation29,Citation30 The Shannon and Simpson indexes are employed to assess species diversity positively, and evenness negatively, respectively.Citation31 Results showed that values of ACE and Chao1 in the ΔsaaS group were significantly higher than those in other infection groups. The Shannon index for the ΔsaaS group was also higher than in the WT group (). However, no difference was observed for the Simpson index in the colon at 6 hpi. To gain a better understanding of microbial composition, the principal coordinate analysis (PCoA) and clustering analysis were conducted.Citation32 As shown in , gut microbial communities in mice infected with ΔsaaS differed significantly from those in mice infected with WT, ΔsaaS/psaaS or control. Interestingly, changes in gut microbial communities occurred also during infection with ΔsaaS/psaaS at 6 hpi. Overall, these findings reveal that SaaS contributes to the alteration of gut microbial richness and diversity.
Figure 4. Effects of SaaS on the diversity of colon microbiota. (a) Principal coordinate analysis (PCoA) and clustering analysis at 6 hpi; (b) PCoA and clustering analysis at 72 hpi; (c) Composition of colon microbiota at the phyla levels at 6 and 72 hpi; (d) Composition of colon microbiota at the genera levels at 6 and 72 hpi. (e) Differential microbiota at phyla and genera levels and functional prediction of colonic microbial genes at 6 hpi; (f) Differential microbiota at phyla and genera levels and functional prediction of colonic microbial genes at 72 hpi. hpi: hours after infection. Data are represented as means±SD; n = 7–10. Statistical significance was assessed using Student’s t-test. *P < 0.05, **P < 0.01.

Table 1. Alpha diversity characteristics of gut microbiota.
Composition and functional prediction of microbiota
Significant changes induced by SaaS were observed at both phylum and genus levels. At the phylum level (), Firmicutes was found to be the most prevalent in all groups, followed by Bacteroidota and Desulfobacteria at 6 hpi, while at 72 hpi Bacteroidota overtook Firmicutes as the main microbiota in the ΔsaaS group. At the genera level (), Muribaculaceae_norank, Lachnospiraceae NK4A136 group, Ligilactobacillus and Desulfovibrio were among the top four genera. The differential phyla and genera at both infection time points were next analyzed by comparing all groups (). At 6 hpi, the ΔsaaS group had a significantly higher relative abundance of Bacteroidota and lower relative abundance of Proteobacteria compared to the WT group. Alistipes and Parabacteroides belonging to Bacteroidota were more abundant in the ΔsaaS group than in the WT group (). At 72 hpi, similar results including higher abundance of Bacteroidota and lower Proteobacteria at the phyla level as well as higher Parabacteroides at the genera level were observed in the ΔsaaS group (). Furthermore, Firmicutes, Patescibacteria and Actinobacteriota had lower relative abundances in the ΔsaaS group when compared with the WT group. At the genus level, four additional genera were identified in colon contents. In comparison to the WT group, the ΔsaaS group had a greater presence of Muribaculaceae_norank, Lachnospiraceae_uncultured but lower abundances of Ligilactobacillus and Mycobacterium.
We further evaluated the microbial function change based on PICRUSt2 function prediction (). At 6 hpi, compared with WT infection, the ΔsaaS infection decreased membrane transport and lipid metabolism but increased the microbial metabolism of cofactors and vitamins, cellular processes, as well as signaling, immune system and digestive system. This effect was further seen at 72 h with five more functions altered: xenobiotics biodegradation and metabolism were reduced, while nucleotide metabolism, folding/sorting/degradation, transport/catabolism and excretory system were heightened. These findings demonstrate that SaaS promotes the ability of Salmonella to modify gut microbiota composition over time as well as its functionality.
The disruption of Salmonella to intestinal barrier by SaaS was associated with bacterial burden and inflammatory response
To evaluate potential relationships between inflammatory response and bacterial burden with intestinal barriers, Spearman’s correlation analysis was conducted (). In general, IL-18 displayed the most intricate and powerful relationship with intestinal barriers, which was positively correlated with Muc2 and Ocln at 6 and 72 hpi, positively correlated with goblet cells and Muc4 at 72 and 120 hpi, but negatively correlated with R3g3g and Reg3b all through the entire period. Il18 was also positively correlated with Muribaculaceae_norank and Parabacteroides but negatively correlated with Ligilactobacillus and Mycobacterium at 72 hpi. Following IL-18, COX-2 displayed the distinctive relationship with intestinal barriers. Different from those at 6 hpi, COX-2 showed the strongest negative correlation with intestinal barriers like Muc2/4 and Camp in mucosal barrier and all markers in mechanical barrier.
Figure 5. Altered inflammation levels exhibiting correlations with bacterial burden, intestinal barriers. (a) 6 hpi; (b) 72 hpi; (c) 120 hpi. Red color represents significant positive correlation and white color represents significant negative correlation, and the independent right color bars depict correlation coefficients. hpi: hours after infection. Correlation was considered significant when the absolute value of Spearman’s rank correlation coefficient (Spearman’s r) was > 0.5. *P < 0.05; **P < 0.01.

Except IL-18 and COX-2, the correlation between other cytokines and barriers was regular and clear. At 72 hpi, inflammatory response was positively correlated with all mucosal barrier indicators. For biological barrier, inflammatory response was found to be positively associated with Lachnospiraceae_uncultured but negatively associated with Vibrionimonas plus Ligilactobacillus. At 120 hpi, inflammatory response was negatively correlated with goblet cells, thickness of mucus layer, Muc4 and Cryptdin5 but positively correlated with Reg3g and R3g3b, presenting a totally opposite trend with IL-18. Notably, Muc4 showed the strongest correlation with all cytokines throughout the entire period, which suggests it may be a potential target in SaaS-mediated Salmonella invasion.
For bacterial burden, a steady negative correlation with Il18 and goblet cells was observed at both 72 and 120 hpi. At 72 hpi, bacterial burden displayed a positive association with Mycobacterium, but displayed a negative association with Muc2, Ocln, Muribaculaceae_norank and Parabacteroides. At 120 hpi, bacterial burden displayed a positive association with IL-6, iNOS and Reg3 family, but displayed a negative association with thickness of mucus layer, Muc2 and Cryptdin5. These findings confirmed that SaaS helps Salmonella to disrupt intestinal barriers by mediating inflammatory responses.
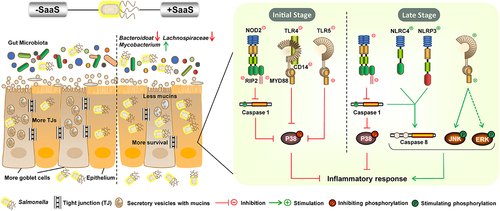
SaaS affects MAPK signaling pathway by mediating the activation pathway
The expression of pro-inflammatory molecules such as iNOS, COX-2, IL-1β, IL-6, IL-18 and TNF-α is known to be regulated by NF-kB (Nuclear factor kappa beta) and MAPK (Mitogen-activated protein kinase) signaling pathways. In general, it is not just the transcriptional activation of target genes but also the total and phosphorylation levels of proteins that are essential for the functioning of MAPK signaling pathway.Citation33 According to mRNA expressions of P38, Erk1/2 (encoding extracellular signal regulated kinase 1 and 2) and Jnk1/2 (encoding c-Jun N-terminal kinase 1 and 2) (Fig S2), their corresponding protein levels were examined in order to evaluate both pathways’ roles in SaaS mediated Salmonella pathogenicity ( and Fig S2). For ERK-MAPK route, there was a continuous increase in phosphorylation levels between all groups (), with a significant difference (P < 0.01) being observed between WT group and ΔsaaS group at 120 hpi (). This suggests an activation process for this particular pathway. On the other hand, P38-MAPK route was earlier shown to be regulated by SaaS due to the higher phosphorylation expression found at 72 hpi in the ΔsaaS group than the WT group, which aligned with the anti-inflammatory phenotype at 72 hpi (). Finally, the JNK-MAPK route was activated by SaaS. As shown in and Fig S2, both the mRNA and phosphorylation levels were significantly higher (P < 0.01) in the WT group than in the ΔsaaS group at 120 hpi. Overall, even though suppression of P38-MAPK route continued, both JNK- and ERK-MAPK routes were subsequently augmented, resulting in a pro-inflammatory phenotype at 120 hpi. In regards to NF-kB pathway, there was no significant difference in both mRNA and protein levels of NF-kB among all groups during entire infection period (Fig S2d). These results revealed that it’s MAPK rather than NF-kB pathway which gets induced by SaaS during Salmonella infection.
Figure 6. Effects of SaaS on protein expressions in MAPK signaling pathways. (a) Phosphorylation level of ERK in western blotting analysis at 6 hpi; (b) Phosphorylation level of ERK and P38 in western blotting analysis at 72 hpi; (c) Phosphorylation level of ERK, P38 and JNK in western blotting analysis at 120 hpi. hpi: hours after infection. Data are represented as means±SD from three independent experiments. Statistical significance was assessed using Student’s t-test. *P < 0.05, **P < 0.01.

We further looked into how SaaS mediated MAPK pathway in vivo. Pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and Nod-like receptors (NLRs), respond to stimuli from local or foreign pathogenic substances.Citation34,Citation35 TLRs and NLRs are two major forms of innate immune sensors, which give immediate responses against invading pathogens or tissue damage. The mRNA levels of TLRs (Tlr4 and Tlr5), NLRs (Nod2 encoding nucleotide-binding oligomerization domain 2, Nlrp3 encoding NLR thermal protein domain associated protein 3 and Nlrc4 encoding NLR family CARD domain-containing protein 4) and their related ligands or downstream factors including Myd88 (encoding myeloid differentiation primary response gene 88), Cd14 (encoding cluster of differentiation 14), Rip2 (encoding receptor-interacting protein 2) and Casp1/3/4/8 (encoding Caspase 1, 3, 4 and 8) were analyzed in mice colon infected with S. Enteritidis (). The results showed that the expression trends for these factors matched the corresponding protein levels at infection time points. At 6 hpi, compared to those in the ΔsaaS group, all factors, except not significant Tlr4, Tlr5, Nlrp3 and Nlrc4, had significantly lower expressions in the WT group. Similarly, lower mRNA levels of Tlr4, Tlr5, Nlrp3 and Casp4 were observed at 72 hpi in WT-infected mice when compared to ΔsaaS-infected mice. On contrary, higher mRNA levels of Tlr5, Nlrp3, Nlrc4 and Casp8 were found at 120 hpi in WT-infected mice compared to those in ΔsaaS-infected ones. These findings suggest that through SaaS, Salmonella activates MAPK signaling pathway sequentially by mediating activation pathways.
Figure 7. Effects of SaaS on the activation pathway of MAPK in colon. (a) Expressions of toll-like receptor systems including Tlr4, Tlr5, Myd88 and Cd14 mRNA; (b) Expressions of nod-like receptor systems including Nod2, Nlrp3, Nlrc4 and Rip2 mRNA; (c) Expressions of caspase family including Casp1, Casp3, Casp4 and Casp8 mRNA. hpi: hours after infection. For mRNA expression, the control was set to 1 and indicated by dashed line. Data are represented as means±SD; n = 7–10. Statistical significance was assessed using Student’s t-test. *P < 0.05, **P < 0.01.

Discussions
Salmonella are important enteric pathogens that invade humans and animals, with S. Enteritidis being one of the most common strains. In order to colonize various tissue sites for further damage, Salmonella needs to quickly adapt to different environments in vivo and break through the complex intestinal barrier. Recent research has found that sRNAs can regulate various genes post-transcriptionally which allows bacteria like Salmonella to adjust accordingly. SaaS, a novel sRNA, was identified as regulating expressions of virulence genes such as invA, prgJ and ssa operon in a simulated intestinal environment.Citation21 Among them, the decrease in expression of invA and prgJ which are part of SPI-1 could weaken the initial invasion and survival of ΔsaaS; this explains why there was a reduction at 72 hpi. When conditions changed from invasion to replication, ssa operon (ssaV, ssaR, ssaT, ssaQ, and ssaU) along with spiA belonging to SPI-2 type three secretion system (T3SS), like a baton, played their roles. The combination of increased expression of spiA, ssaQ, and ssaU as well as decreased levels of ssaV, ssaR and ssaT may have been responsible for the faster rate of replication seen at 120 hpi in this work but subsequently limited and delayed death that reported before.Citation21 Further research into how SaaS interacts with virulence factors will be beneficial in understanding its role in pathogenesis better. At the primary period, Salmonella was observed to have a general anti-inflammatory effect, which was supported by the inhibition of virulence factors like spvC and AvrA.Citation14,Citation36 This enabled it to escape from immune response for better survival. Following this, onset of a potential cytokine storm could be observed based on the high levels of key pro-inflammatory cytokines iNOS, COX-2 and IL-6. Death caused by cytokine storms is the primary cause of mortality in animals infected with Salmonella.Citation37,Citation38 It was observed that iNOS and COX-2 are typically expressed together, indicating an extremely terrible condition.Citation39,Citation40 This suggests that SaaS modulates host immune response by regulating intestinal inflammation over time. However, how exactly increased colonization happens and regulated inflammation contributes toward damage caused by Salmonella remains unclear.
The goblet cells and the secretions they produce, such as MUC1/2/4 and AMPs, act as a first line of defense against Salmonella.Citation26,Citation41,Citation42 Although the mucus layer does not appear to be impaired during initial infection, research suggests that bacteria can still penetrate intestinal regions with an intact mucus barrier,Citation43,Citation44 which was consistent with the death of goblet cells in this work. Though SopB wasn’t involved in the SaaS-mediated death of goblet cells, its roles in helping Salmonella maintain its concrete SCVsCitation45, regulate inflammasome activationCitation46, and promote Salmonella survival in B cellsCitation47 provide additional evidence that SaaS enhances Salmonella virulence. A novel and unknown mechanism of SaaS-mediated death of goblet cells was worth being investigated. The decreased Muc1/2 levels also confirmed that Salmonella can penetrate an intact mucus barrier, and SaaS facilitates this invasion, thus triggering a heightened defensive response such as more AMPs being released. Continuously promoted invasion, in turn, weakened this defensive responseCitation48 and caused more harm to the mucosal barrier at later infection. The damage was further exacerbated by a decrease in IL-18, an interleukin responsible for antiapoptosis and mucosal restitution.Citation49,Citation50 At this point, it is noteworthy that AMPs like RegIII and sPLA2 also have TLRs-mediated pro-inflammatory properties.Citation48 It was speculated that mediated by SaaS, Salmonella affected TLR 4/5 levels and then regulate RegIIIβ/γ secretion, thereby modulating the inflammatory response and compromising the innate defense.Citation51 Decreased expression of Cldn at 72 hpi in this work was also confirmed by studies involving conventional intestine infections with Salmonella.Citation10 In fact, the expression of Cldn can vary according to factors such as the type of injury, time of onset, and duration of the inflammatory response.Citation52 For the slight increase at the beginning of infection, the body’s attempts to seal the intestinal barrier in order to prevent excessive electrolyte loss via diarrhea could be the possible reason.Citation53 Furthermore, increased expression of Cldn has been associated with inflammation or inflammatory bowel diseases according to multiple reports,Citation54 which supported our observation at 120 hpi. Salmonella achieves these diverse regulations usually by controlling TJs distribution or damaging interaction between them using effector proteins.Citation14,Citation55 As the largest component of T3SS, SsaV proteins are promoted by SaaS through direct combination with ssaV mRNA.Citation21 This promotion further intensifies injection of effector proteins, resulting in the increased uptake of Salmonella into the host cells and initiated inflammation which disrupts gut microbiota.Citation56 Some beneficial microbiota, such as Alistipes, Parabacteroides and Lachnospiraceae_uncultured, were shown to have decreased relative abundance here. Alistipes has been associated with the recovery of colitis. Decreased relative abundance of Alistipes was seen alongside inflamed gut environments and decreased Muc1 and Cldn expressions just like this study,Citation57–59 suggesting a synergistic effect between intestinal barriers for successful depletion mediated by SaaS. Also, the depletion of Parabacteroides and Lachnospiraceae_uncultured was confirmed by multiple Salmonella infection models, especially those involving higher virulence Salmonella infection.Citation56,Citation60 While for Ligilactobacillus, a probiotic bacteria used to treat enteric infections,Citation61 the increased abundance could be utilized by Salmonella to suppress inflammatory response for immune escape as reported here,Citation62 confirming the negative correlation between it and pro-inflammatory factors. On the other hand, SaaS increased the relative abundance of deleterious bacteria or making general bacteria harmful such as the Mycobacterium. As an interesting concept “like will to like”,Citation63 the increased Salmonella might be responsible for the increased abundance of Proteobacteria because it includes the similar species such as Enterobacteriaceae family, thus increasing susceptibility to infection.Citation64 The diversity and associated bioactivity of gut microbiota have important effects on the host’s metabolic homeostasis and immune system.Citation65–67 Therefore, a decrease in microbial diversity could be responsible for the malfunctioning metabolism and immune system seen here.
MAPK is critical intersection pathway of cell proliferation, stress responses, inflammation, apoptosis and other signal transduction pathways.Citation32,Citation68 The MAPK pathway has been shown to be time-dependently regulated by SaaS-mediated Salmonella, which is in agreement with the inflammatory response. As the starter of MAPK inflammatory signaling cascades, NLRs and TLRs systems were both suppressed during early infection but enhanced at a later stage, correlating with the decrease of P38 activity and increase in ERK and JNK activities. This suggests a direct regulatory cascade between them. Although NLRs and TLRs usually participate in host defense and tissue repair,Citation69 they have also been utilized by T3SS, such as TcpS mediating TLRCitation70 and PrgJ interacting with NLRC4 inflammasome,Citation71,Citation72 to enhance the virulence of Salmonella in vivo. Inflammasome is innate immune defense mechanism that can become overactivated, leading to the death of host. Both inflammasomes Nlrp3 and Nlrc4 were activated by SaaS, with prgJ being predicted as a target mRNA of SaaS, which was found to be positively regulated by it,Citation21 suggesting activation of NLRC4 through interaction between SaaS and prgJ. Furthermore, an induced NLRC4-Caspase 8 axis based on inhibition of Caspase 1 was observed here, which was confirmed via an apoptosis model built in recent years.Citation73,Citation74 These details raise the possibility that SaaS may interact with prgJ to activate the NLRC4 inflammasome, resulting in NLRC4-Caspase 8-mediated apoptosis of cells. Meanwhile, researches showing that inhibiting JNK signaling can greatly dampen apoptosis in epithelial cells that infected with Salmonella Citation75,Citation76. Correspondingly, the JNK signaling of MAPK was stimulated in this work, which implies an increase in apoptosis mediated by SaaS.
Conclusion
This study is the first to investigate in-depth how Salmonella sRNA affects the intestine, and it revealed a novel regulation of sRNA on MAPK signaling pathway. The present study demonstrates that SaaS promotes the bacterial colonization by increasing itself expression and damaging the physical and mucus barriers. Meanwhile, SaaS damaged intestinal homeostasis and function by decreasing levels of beneficial bacteria and increasing levels of harmful bacteria. Furthermore, SaaS initially alleviates intestinal inflammation by suppressing P38 MAPK pathway and its activation to promote survival and colonization, but it eventually promotes intestinal inflammation by intensifying JNK and ERK MAPK pathways and its activation, raising a cytokine storm. Our research has potential significance as it provides new paradigms for interactions between sRNA and host intestinal immune response during infection by Salmonella or other pathogens.
Materials and methods
Ethics statement
All experiments were carried out in compliance with the relevant guidelines and regulations of the Ethical Committee of Experimental Animal Center of Nanjing Agricultural University.
Construction of SaaS deletion mutant and complemented strain
S. Enteritidis strain NCM61 (Reference genome CP032851.1) isolated from meat-contact surfaces was used as the wild-type (WT).Citation77 The SaaS-deletion mutant ΔsaaS and complemented strain ΔsaaS/psaaS were obtained from the same source as indicated in our previous work.Citation21 Briefly, the ΔsaaS was constructed based on WT according to allelic exchange using the suicide plasmid. First, a bridged target fragment ΔsaaS::Kn (upstream homologous arm-Knr gene-downstream homologous arm) was constructed and subcloned into the suicide plasmid pCVD442, obtaining the pCVD442-ΔsaaS::Kn. Secondly, the donor strain β2155/pCVD442-ΔsaaS::Kn was obtained by transferring pCVD442-ΔsaaS::Kn into Escherichia coli β2155. The conjugation test of above donor strain and the recipient strain WT was performed, and the Salmonella clones obtaining Knr were collected and named as Sen/pCVD442-ΔsaaS::Kn. Finally, deletion mutants (ΔsaaS) were obtained by electroporating plasmid pCP20 into the competent cells of Sen/pCVD442-ΔsaaS::Kn and named as S. Enteritidis strain NCM282.
ΔsaaS/psaaS was then constructed based on ΔsaaS using the low-copy pRK415 expression vector, which was reported in Keen et al., 1988.Citation78 Similarly, the saaS gene and pRK415 expression vector were amplified, and the digested products were ligated for 2 h at 37°C to yield the constructed plasmid pRK415-saaS. pRK415-saaS was subsequently transferred into ΔsaaS and selected on tetracycline (Tc, 10 μg/mL) plates to yield the complemented strain (ΔsaaS/psaaS).
Bacterial cultures and growth conditions
S. Enteritidis strain WT, mutant (ΔsaaS) and complement (ΔsaaS/psaaS) were streaked and grown twice on Luria-Bertani agar (LB; HopeBiotechnology, China) overnight for single colony isolation and then incubated in 6 mL of fresh LB broth at 37°C for 20 h. The cells were harvested by centrifugation at 8000×g for 5 min at 4°C and rinsed three times with 0.85% NaCl solution without activating the cold shock proteins CspE and CspD (Fig S3). Subsequently, the pellets were resuspended in 0.85% NaCl solution to a final concentration of approximately 109 CFU/mL by measuring the OD600 for subsequent experiments.
Animal experiment
Specific-pathogen-free (SPF) female BALB/c mice aged 6 weeks were housed under SPF conditions (humidity, 60 ± 10%; light cycle, 12 h/12 h; temperature, 23.0 ± 0.5°C) (SYXK<Jiangsu>2011–0037). The mice had free access to their diet and water. Following adaptation to the new environment for 1 week, mice were randomly divided into four groups, including PBS (control), WT, ΔsaaS and ΔsaaS/psaaS-infected groups; 1 × 108 CFU S. Enteritidis strains under exponential phase, respectively, in 100 µL of PBS were used for oral gavage to mice that were fasted for 4 h before infection. At 6-, 72- and 120- hour post-infection (hpi), the colon, colonic content and cecum were collected and snap-frozen, separately.
Bacterial burden
The colon and cecum were homogenized and plated on XLD agar. CFU was normalized to the weight of each sample.
ELISA assays
Frozen colon (40–50 mg) was lysed in protein extraction buffer containing protease and phosphatase inhibitors (Beyotime, China). The samples were centrifuged at 13,000 g for 5 min at 4°C, and the protein concentration in the supernatant was determined using a BCA protein assay kit (Thermo Scientific, USA). The IL-1β, IL-6, TNF-α, iNOS and sIgA levels were assessed by ELISA (Angle gene, China) at 6, 72 and 120 hpi according to the kit instructions.
Histological observations
The colon samples were fixed in Carnoy’s fixative solution for alcian blue-periodic acid Schiff (AB-PAS) and 4% paraformaldehyde for hematoxylin and eosin (H&E). The samples were processed with routine histological procedures, dehydration, paraffin embedding, section cutting, deparaffinization and stained according to the previous study.Citation78 The relative number of goblet cells was counted on ten random points per section. The mucus thickness was determined by measuring 10 randomly chosen points per section. These images were obtained from each individual by an operator unaware of sample status using Olympus B×51 (Olympus, Tokyo, Japan).
Quantitative real-time PCR
The colon (10–20 mg) samples were collected, and total RNA was isolated with FastPure Cell/Tissue Total RNA Isolation Kit (Vazyme, China). Reverse transcription of 800 ng of RNA was then performed using a HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme), and 1 µL of cDNA were used for real-time reaction using ChamQ Universal SYBR qPCR Master Mix (Vazyme). A QuantStudio 6 Flex system (Applied Biosystems, United States) was applied with the following protocol: initial denaturation at 95°C/30 s; 40 cycles of denaturation at 95°C/5 s and annealing at 60°C/34 s; and a final melting curve program of 15 s at 95°C, 1 min at 60°C, and 15 s at 95°C. Primers were designed using Primer Premiere (Version 5.0; San Francisco, CA, USA), synthesized (GenScript, China) and listed in Table S1. In parallel, Gapdh and Actb were included as dual internal references for normalization of the gene expression. Fold changes in gene expression were calculated using the 2−ΔΔCT method, where ΔΔCT = ΔCT (WT or ΔsaaS or ΔsaaS/psaaS) - ΔCT (control), ΔCT = CT (target gene) - CT (geometric average of Gapdh and Actb).Citation79
To compare the expression of SaaS in colonized Salmonella in colon and cecum, bacteria were collected and rinsed with PBS three times. Total RNA was extracted with a simple total RNA extraction kit (Tiangen, China) following the manufacturer’s protocol. The process of synthesizing cDNA and determining SaaS expression levels was carried out using the same method as mentioned above. 16S rRNA was used as an internal reference to normalize gene expression data. Fold changes in gene expression were calculated using the 2−ΔΔCT method, where ΔΔCT = ΔCT (Treatment group) - ΔCT (cecum at 6 hpi), ΔCT = CT (SaaS) - CT (16S rRNA). Treatment group means the cecum at 72 hpi or cecum at 120 hpi or colon at 6 hpi or colon at 72 hpi or colon at 120 hpi.
To evaluate the half-life of SaaS, rifampicin stability assays were carried out. Test cultures were cultured overnight at 37°C to exponential phase, adding rifampicin to a final concentration of 250 μg/mL. At 0, 5, 10, 15, and 20 min, the cells were quickly harvested by centrifugation at 12,000×g for 2 min. Pellets were frozen in liquid nitrogen and stored at −80°C. The process of extracting RNA, synthesizing cDNA and determining SaaS expression levels was carried out using the same method as mentioned above. 16S rRNA was used as an internal reference to normalize gene expression data. Fold changes in gene expression were calculated using the 2−ΔΔCT method, where ΔΔCT = ΔCT (Treatment group) - ΔCT (0 min), ΔCT = CT (SaaS) - CT (16S rRNA). Treatment group means the 5 min or 10 min or 15 min or 20 min.
Cell experiments
Bacterial invasion and survival assays
Before infection, Caco-2 cells were washed three times with prewarmed PBS, and the medium was replaced with fresh DMEM without antibiotics and fetal bovine serum. Monolayers of cells were infected with an exponential phase bacterial culture (MOI = 10), then incubated for 3 h at 37°C in a 5% CO2 atmosphere. After incubation, the wells were washed six times with PBS to remove unattached bacteria. Caco-2 cells were then disrupted with 1% Triton X-100 at 37°C for 5 min. Lysate dilutions were plated on XLD agar, and the attachment efficiency was determined by counting the colony-forming units (CFU) per milliliter. The 100 and 10 μg/mL gentamicin were then used to kill surface and extracellular adhered bacteria. The survival levels at 18 h were determined by comparing bacterial recovery from the initial inoculum.
Cell cytotoxicity assays
To verify the viability of Caco-2 cells during infection, the supernatants from above tests were collected, and the release of lactate dehydrogenase (LDH) was determined according to the instruction of lactate dehydrogenase assay kit (Jiancheng, China). The relative LDH release was calculated as LDH release in the supernatant/(LDH release in supernatant + LDH release in cell fraction)×100%.
S rRNA gene sequencing
We detected the composition of intestinal microflora in the colon of mice in the control and S. Enteritidis-infected groups. Total DNA was extracted from colonic contents using the E.Z.N.A.® Soil DNA Kit (Omega Biotek, USA) according to manufacturer’s protocols. The V3-V4 hyper-variable regions of the bacteria 16S rRNA gene were targeted and selected for PCR amplification using a barcode primer 515F (5’-GTGCCAGCMGCCGCGG-3’) and 806 R (5’-GGACTACHVGGGTWTCTAAT-3’) in which barcode is an eight-base sequence unique to each sample. The subsequent detailed PCR protocol and methods for all analyses can be found in Supplementary Material.
Correlation analysis
The Spearman’s correlation coefficients were assessed to determine the relationships between inflammation levels/bacterial burden and intestinal barrier. For each time point, the indicator with no significant difference between treatment groups was excluded. To compare two random indicators, the data from four groups in each indicator was utilized in this comparison method. Correlation was considered significant when the absolute value of Spearman’s rank correlation coefficient was greater than 0.5 and P-value was smaller than 0.05.
Western blotting
Frozen colon (50 mg) was lysed in protein extraction buffer containing protease and phosphatase inhibitors (Beyotime). The samples were centrifuged at 13,000 g for 10 min at 4°C, and the protein concentration in the supernatant was determined using a BCA protein assay kit (Thermo Scientific). Protein (60 μg) was separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF; Millipore, Billerica, USA) membranes. All the primary antibodies were from Cell Signalling Technology (CST, USA) and used with the suitable dilution ratio of 1:1000 for ERK, p-ERK, P38, p-P38, JNK and GAPDH and 1:2000 for p-JNK. Membranes were incubated with appropriate secondary antibodies Goat anti-Mouse IgG (H+L) or Goat anti-Rabbit IgG (H+L) (Thermo Scientific; 1:5000). Quantification of the protein bands was performed with ImageJ (Version 1.53c; NIH, Bethesda, MD, USA) and normalized to GADPH.
Statistical analysis
SAS software (Version 9.2; SAS Institute Inc., USA) was used for statistical analysis. The differences between two groups were analyzed using Student’s t-test. Values of P ≤ 0.05 or 0.01 were considered to be statistically significant or highly significant, respectively. Data were expressed as means ± standard deviation (SD) and figures were constructed using the GraphPad Prism (Version 5.0.3; GraphPad Software Inc., USA). More details about the materials and methods are seen in Supplementary Material.
Supplemental Material
Download MS Word (303.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available in figshare at https://figshare.com/s/cfe1f4b520b47dd62967.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2211184
Additional information
Funding
References
- Eguale T, Gebreyes WA, Asrat D, Alemayehu H, Gunn JS, Engidawork E. Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect Dis. 2015;15:497–21.
- Sanchez-Vargas FM, Abu-El-Haija MA, Gomez-Duarte OG. Salmonella infections: an update on epidemiology, management, and prevention. Travel Med Infect Dis. 2011;9:263–277. doi:10.1016/j.tmaid.2011.11.001.
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis. 2011;17:7–15. doi:10.3201/eid1701.P11101.
- Stilz CR, Cavallo S, Garman K, Dunn JR. Salmonella Enteritidis outbreaks associated with egg-producing farms not regulated by food and drug administration’s egg safety rule. Foodborne Pathog Dis. 2022;19:529–534. doi:10.1089/fpd.2022.0025.
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. Plos Med. 2015;12:e1001921. doi:10.1371/journal.pmed.1001921.
- Knodler LA, Vallance BA, Celli J, Winfree S, Hansen B, Montero M, Steele-Mortimer O. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. null. 2010;107:17733–17741. doi:10.1073/pnas.1006098107.
- Yu HB, Croxen MA, Marchiando AM, Ferreira RBR, Cadwell K, Foster LJ, Finlay BB, Zychlinsky A. Autophagy facilitates Salmonella replication in HeLa cells. mBio. 2014;5:865–879. doi:10.1128/mBio.00865-14.
- Corr SC, Palsson-McDermott EM, Grishina I, Barry SP, Aviello G, Bernard NJ, Casey PG, Ward JBJ, Keely SJ, Dandekar S, et al. MyD88 adaptor-like (Mal) functions in the epithelial barrier and contributes to intestinal integrity via protein kinase C. Mucosal Immunol. 2014;7:57–67. doi:10.1038/mi.2013.24.
- Kim M, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Sasakawa C. Bacterial interactions with the host epithelium. Cell Host Microbe. 2010;5:356–362. doi:10.1016/j.chom.2010.06.006.
- Sun L, Yang S, Deng Q, Dong K, Li Y, Wu S, Huang R. Salmonella effector SpvB disrupts intestinal epithelial barrier integrity for bacterial translocation. Front Cell Infect Microbiol. 2020;17:10–21. doi:10.3389/fcimb.2020.606541.
- Lim JS, Shin M, Kim H, Kim KS, Choy HE, Cho KA. Caveolin-1 mediates Salmonella invasion via the regulation of SopE-dependent Rac1 activation and actin reorganization. J Infect Dis. 2014;210:793–802. doi:10.1093/infdis/jiu152.
- Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi:10.1016/j.immuni.2013.01.009.
- Addwebi TM, Call DR, Shah DH. Contribution of Salmonella Enteritidis virulence factors to intestinal colonization and systemic dissemination in 1-day-old chickens. Poultry Sci. 2014;93:871–881. doi:10.3382/ps.2013-03710.
- Lin Z, Zhang Y, Xia Y, Xu X, Jiao X, Sun J. Salmonella Enteritidis effector AvrA stabilizes intestinal tight junctions via the JNK pathway. J Biol Chem. 2016;291:26837–26849. doi:10.1074/jbc.M116.757393.
- Jiao Y, Zhang Y, Lin Z, Lu R, Xia Y, Meng C, Pan Z, Xu X, Jiao X, Sun J. Salmonella Enteritidis effector AvrA suppresses autophagy by reducing beclin-1 protein. Front Immunol. 2020;11:686–697. doi:10.3389/fimmu.2020.00686.
- Amin SV, Roberts JT, Patterson DG, Coley AB, Allred JA, Denner JM, Johnson JP, Mullen GE, O’Neal TK, Smith JT, et al. Novel small RNA (sRNA) landscape of the starvation-stress response transcriptome of Salmonella enterica serovar typhimurium. Ser Typhi RNA Biol. 2016;13:331–342. doi:10.1080/15476286.2016.1144010.
- Wang HH, Huang MY, Zeng XM, Peng B, Xu XL, Zhou GH. Resistance profiles of Salmonella isolates exposed to stresses and the expression of small non-coding RNAs. null. 2020;11:130–143. doi:10.3389/fmicb.2020.00130.
- Padalon-Brauch G, Hershberg R, Elgrably-Weiss M, Baruch K, Rosenshine I, Margalit H, Altuvia S. Small RNAs encoded within genetic islands of Salmonella Typhimurium show host-induced expression and role in virulence. Nucleic Acids Res. 2008;36:1913–1927. doi:10.1093/nar/gkn050.
- Peñaloza D, Acuña LG, Barros MJ, Núñez P, Montt F, Gil F, Fuentes JA, Calderón IL. The small RNA RyhB homologs from Salmonella Typhimurium restrain the intracellular growth and modulate the SPI-1 gene expression within RAW264.7 macrophages. Microorganisms. 2021;9:635–645. doi:10.3390/microorganisms9030635.
- Meng X, Meng X, Wang J, Wang H, Zhu C, Ni J, Zhu G. Small non-coding RNA STnc640 regulates expression of fimA fimbrial gene and virulence of Salmonella enterica Serovar Enteritidis. BMC Vet Res. 2019;15:319–331. doi:10.1186/s12917-019-2066-7.
- Cai LL, Xie YT, Hu HJ, Xu XL, Wang HH, Zhou GH, Oglesby AG. A small RNA, SaaS, promotes Salmonella pathogenicity by regulating invasion, intracellular growth, and virulence factors. Microbiol Spectr. 2023;11:e0293822. doi:10.1128/spectrum.02938-22.
- Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi:10.1038/nri3608.
- Hu G, Yang Y, Qin X, Qi S, Zhang J, Yu S-X, Du C-T, Chen W. Salmonella outer protein B suppresses colitis development via protecting cell from necroptosis. Front Cell Infect Microbiol. 2019;9:87–99. doi:10.3389/fcimb.2019.00087.
- Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi:10.1038/nrmicro2546.
- Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi:10.1038/nri1499.
- Wang H, Kim JJ, Denou E, Gallagher A, Thornton DJ, Shajib MS, Xia L, Schertzer JD, Grencis RK, Philpott DJ, et al. New role of nod proteins in regulation of intestinal goblet cell response in the context of innate host defense in an enteric parasite infection. Infect Immun. 2016;84:275–285. doi:10.1128/IAI.01187-15.
- Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, Panda SK, Gupta SD, Makharia GK. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch. 2012;460(3):261–270. doi:10.1007/s00428-012-1195-1.
- Ahmer BMM, Gunn JS. Interaction of Salmonella spp. with the intestinal microbiota. null. 2011;2:101–110. doi:10.3389/fmicb.2011.00101.
- Chao A, Yang MCK. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika. 1993;80:193–201. doi:10.1093/biomet/80.1.193.
- Chao A. Nonparametric estimation of the number of classes in a population. Scand Stat Theory Appl. 1984;11:265–270.
- Soetaert K, Heip C. Sample-size dependence of diversity indices and the determination of sufficient sample size in a high-diversity deep-sea environment. Mar Ecol Prog Ser. 1990;59:305–307.
- Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. Isme J. 2011;5:169–172. doi:10.1038/ismej.2010.133.
- Arthur JSC, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi:10.1038/nri3495.
- Bierschenk D, Boucher D, Schroder K. Salmonella-induced inflammasome activation in humans. Mol Immunol. 2017;86:38–43. doi:10.1016/j.molimm.2016.11.009.
- Karki R, Lee E, Place D, Samir P, Mavuluri J, Sharma BR, Balakrishnan A, Malireddi RKS, Geiger R, Zhu Q, et al. IRF8 regulates transcription of NAIPs for NLRC4 inflammasome activation. Cell. 2018;173:920–933. doi:10.1016/j.cell.2018.02.055.
- Zuo L, Zhou L, Wu C, Wang Y, Li Y, Huang R, Wu S. Salmonella spvC gene inhibits pyroptosis and intestinal inflammation to aggravate systemic infection in mice. null. 2020;11:562491. doi:10.3389/fmicb.2020.562491.
- Haschka D, Ymoszuk P, Petzer V, Hilbe R, Heeke S, Dichtl S, Skvortsov S, Demetz E, Berger S, Seifert M, et al. Ferritin H deficiency deteriorates cellular iron handling and worsens Salmonella Typhimurium infection by triggering hyperinflammation. JCI Insight. 2021;6:141760. doi:10.1172/jci.insight.141760.
- Zhan R, Han Q, Zhang C, Tian Z, Zhang J, McCormick BA. Toll-Like receptor 2 (TLR2) and TLR9 play opposing roles in host innate immunity against Salmonella enterica Serovar Typhimurium infection. Infect Immun. 2015;83:1641–1649. doi:10.1128/IAI.02870-14.
- Lin Y, Tang G, Jiao Y, Yuan Y, Zheng Y, Chen Y, Xiao J, Li C, Chen Z, Cao P. Propionibacterium acnes induces intervertebral disc degeneration by promoting iNOS/NO and COX-2/PGE2 activation via the ROS-dependent NF-κB pathway. Oxid Med Cell Longev. 2018;2018:1–12. doi:10.1155/2018/3692752.
- Zachary RS, Naatz A, Corbett JA. CCR5-dependent activation of mTORC1 regulates translation of inducible NO synthase and COX-2 during encephalomyocarditis virus infection. J Immunol. 2015;195:4406–4414. doi:10.4049/jimmunol.1500704.
- Hu G, Song P, Li N, Chen W, Lei Q, Yu S, Zhang X, Du C, Deng X, Han W, et al. AIM2 contributes to the maintenance of intestinal integrity via Akt and protects against Salmonella mucosal infection. Mucosal Immunol. 2016;9:1330–1339. doi:10.1038/mi.2015.142.
- McGuckin MA, Hasnain SZ. Goblet cells as mucosal sentinels for immunity. Mucosal Immunol. 2017;10:1118–1121. doi:10.1038/mi.2016.132.
- Furter M, Sellin ME, Hansson GC, Hardt W. Mucus architecture and near-surface swimming affect distinct Salmonella Typhimurium infection patterns along the murine intestinal tract. Cell Rep. 2019;27:2665–2678. doi:10.1016/j.celrep.2019.04.106.
- Hering NA, Fromm A, Kikhney J, Lee IM, Moter A, Schulzke JD, Bücker R. Yersinia enterocolitica affects intestinal barrier function in the colon. J Infect Dis. 2016;213:1157–1162. doi:10.1093/infdis/jiv571.
- Hu G, Song P, Chen W, Qi S, Yu SX, Du CT, Deng XM, Ouyang HS, Yang YJ. Cirtical role for Salmonella effector SopB in regulating inflammasome activation. Mol Immunol. 2017;90:280–286. doi:10.1016/j.molimm.2017.07.011.
- Luis L, Ana GT, Carlos GT, Abraham GG, Iris EG, Martha ML, Vianney ON. Salmonella promotes its own survival in B cells by inhibiting autophagy. Cells. 2022;11(13):2061–2077. doi:10.3390/cells11132061.
- Zhao SS, Xu QP, Cui YQ, Yao S, Jin S, Zhang Q, Wen Z, Ruan H, Liang X, Chao Y, et al. Salmonella effector SopB reorganizes cytoskeletal vimentin to maintain replication vacuoles for efficient infection. Nat Commun. 2023;14(1):478–496. doi:10.1038/s41467-023-36123-w.
- Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 2012;3:310–321. doi:10.3389/fimmu.2012.00310.
- Zheng XS, Liu L, Meng GX, Zhu S, Zhou RB, Jiang W. IL-18 maintains the homeostasis of mucosal immune system via inflammasome-independent but microbiota-dependent manner. Science Bulletin. 2021;66:2115–2123. doi:10.1016/j.scib.2021.01.025.
- Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation.J. J Immunol. 2008;180:2588–2599. doi:10.4049/jimmunol.180.4.2588.
- Godinez I, Raffatellu M, Chu H, Paixão TA, Haneda T, Santos RL, Bevins CL, Tsolis RM, Bäumler AJ. Interleukin-23 orchestrates mucosal responses to Salmonella enterica Serotype Typhimurium in the intestine. Infect Immun. 2009;77:387–398. doi:10.1128/IAI.00933-08.
- Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397:66–79. doi:10.1111/nyas.13360.
- Splichalova A, Splichalova Z, Karasova D, Rychlik I, Trevisi P, Sinkora M, Splichal I. Impact of the lipopolysaccharide chemotype of Salmonella enterica Serovar Typhimurium on virulence in gnotobiotic piglets. Toxins (Basel). 2019;11:534–543. doi:10.3390/toxins11090534.
- Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, Beauchamp RD, Singh AB, Dhawan P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622–634. doi:10.1136/gutjnl-2012-304241.
- Köhler H, Sakaguchi T, Hurley BP, Kase BJ, Reinecker H, McCormick BA. Salmonella enterica Serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol-Gastr L. 2007;293:178–187. doi:10.1152/ajpgi.00535.2006.
- Borton MA, Sabag-Daigle A, Wu J, Solden LM, O Banion BS, Daly RA, Wolfe RA, Gonzalez JF, Wysocki VH, Ahmer BMM, et al. Chemical and pathogen-induced inflammation disrupt the murine intestinal microbiome. Microbiome. 2017;5:47–53. doi:10.1186/s40168-017-0264-8.
- Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep-Uk. 2015;5:8096–8107. doi:10.1038/srep08096.
- Wu H, Ye L, Lu X, Xie S, Yang Q, Yu Q. Lactobacillus acidophilus alleviated Salmonella-induced goblet cells loss and colitis by Notch pathway. Mol Nutr Food Res. 2018;62:1800552. doi:10.1002/mnfr.201800552.
- Yasuda K, Oh K, Ren B, Tickle TL, Franzosa EA, Wachtman LM, Miller AD, Westmoreland SV, Mansfield KG, Vallender EJ, et al. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. Cell Host & Microbe. 2015;17:385–391. doi:10.1016/j.chom.2015.01.015.
- Li Z, Zhang C, Li B, Zhang S, Haj FG, Zhang G, Lee Y. The modulatory effects of alfalfa polysaccharide on intestinal microbiota and systemic health of Salmonella serotype (ser.) Enteritidis-challenged broilers. Sci Rep. 2021;11:10910. doi:10.1038/s41598-021-90060-6.
- Yuan X, Xue H, Xu X, Jiao X, Pan Z, Zhang Y. Closely related Salmonella Derby strains triggered distinct gut microbiota alteration. Gut Pathog. 2022;14:6–18. doi:10.1186/s13099-022-00480-6.
- Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. null. 2016;7:185–194. doi:10.3389/fmicb.2016.00185.
- Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. Plos Pathog. 2010;6:e1000711. doi:10.1371/journal.ppat.1000711.
- Libertucci J, Young VB. The role of the microbiota in infectious diseases. Nature Microbio. 2019;4:35–45. doi:10.1038/s41564-018-0278-4.
- Zhang LS, Davies SS. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8:46–54. doi:10.1186/s13073-016-0296-x.
- Do M, Lee E, Oh M, Kim Y, Park H. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients. 2018;10:761–777. doi:10.3390/nu10060761.
- Vallianou N, Stratigou T, Christodoulatos GS, Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: current evidence and perspectives. Curr Obes Rep. 2019;8:317–332. doi:10.1007/s13679-019-00352-2.
- Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. WIREs Systems Bio and Medi. 2016;8:227–241. doi:10.1002/wsbm.1331.
- Leemans JC, Kors L, Anders H, Florquin S. Pattern recognition receptors and the inflammasome in kidney disease. Nat Rev Nephrol. 2014;10:398–414. doi:10.1038/nrneph.2014.91.
- Xiong D, Song L, Geng SZ, Jiao Y, Zhou XH, Song HQ, Kang XL, Zhou Y, Xu XL, Sun J, et al. Salmonella coiled-coil- and TIR-containing TcpS evades the innate immune system and subdues inflammation. Cell Rep. 2019;28:804–818. doi:10.1016/j.celrep.2019.06.048.
- Duncan JA, Canna SW. The NLRC4 inflammasome. Immunol Rev. 2018;281:115–123. doi:10.1111/imr.12607.
- Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc Natl Acad Sci. 2013;110:14408–14413. doi:10.1073/pnas.1306376110.
- Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD, Zamboni DS, Seifert HS. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. Plos Pathog. 2017;13:e1006502. doi:10.1371/journal.ppat.1006502.
- Zhang P, Liu Y, Hu L, Huang K, Hong M, Wang Y, Fan X, Ulevitch RJ, Han J. NLRC4 inflammasome–dependent cell death occurs by a complementary series of three death pathways and determines lethality in mice. Sci Adv. 2021;7:9471–9484. doi:10.1126/sciadv.abi9471.
- Wu H, Jones RM, Neish AS. The Salmonella effector AvrA mediates bacterial intracellular survival during infection in vivo. Cell Microbiol. 2012;14(1):28–39. doi:10.1111/j.1462-5822.2011.01694.x.
- M JR, Wu H, Wentworth C, Luo L, Collier-Hyams L, Neish AS. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host & Microbe. 2008;3(4):233–244. doi:10.1016/j.chom.2008.02.016.
- Wang HH, Cai LL, Hu HJ, Xu XL, Zhou GH, Baltrus DA. Complete genome sequence of Salmonella enterica serovar Enteritidis NCM 61, with high potential for biofilm formation, isolated from meat-related sources. Microbio Res Announce. 2019;8:01434–01452. doi:10.1128/MRA.01434-18.
- Keen NT, Tamaki S, Kobayashi D, Troilinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–198. doi:10.1016/0378-1119(88)90117-5.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34–46. doi:10.1186/gb-2002-3-7-research0034.