ABSTRACT
Osteoporosis is a systemic skeletal disease that seriously endangers the health of middle-aged and older adults. Recently, with the continuous deepening of research, an increasing number of studies have revealed gut microbiota as a potential target for osteoporosis, and the research concept of the gut-bone axis has gradually emerged. Additionally, the intake of dietary nutrients and the adoption of dietary patterns may affect the gut microbiota, and alterations in the gut microbiota might also influence the metabolic status of the host, thus adjusting bone metabolism. Based on the gut-bone axis, dietary intake can also participate in the modulation of bone metabolism by altering abundance, diversity, and composition of gut microbiota. Herein, combined with emerging literatures and relevant studies, this review is aimed to summarize the impacts of different dietary components and patterns on osteoporosis by acting on gut microbiota, as well as underlying mechanisms and proper dietary recommendations.
1. Introduction
Osteoporosis is a systemic skeletal disease that seriously endangers the health of middle-aged and elderly individuals, and its severe clinical outcomes mainly include brittle fractures, as well as subsequent disability and death.Citation1–3 With the accelerating process of global population aging, osteoporosis, including chronic diseases such as hypertension, coronary heart disease, and diabetes, has become a pivotal health issue that needs to be faced by whole world.Citation4,Citation5 According to several previous reports, an osteoporotic fracture occurs every 3 s globally, with approximately 50% of the female and 20% of male suffering from a primary osteoporotic fracture after the age of 50 years, and 50% of patients with a primary osteoporotic fracture may experience a secondary osteoporotic fracture in future.Citation6,Citation7 Hence, the prevention and treatment of osteoporosis has gradually become a focus of attention for the health of the middle-aged and elderly individuals, and reducing the prevalence of osteoporosis is of great significance for reducing the medical burden, alleviating the pressure on the social medical system, and improving the quality of life of the population.Citation8
There are approximately 1 × 1014 bacteria in human gut, and the genome carried by it can be 150 times that of the human genome,Citation9 The composition of gut microbiota varies significantly in different individuals, which is universally affected by factors such as the host genotype, age, sex, diet, exercise, and living environment.Citation10,Citation11 Gut microbiota is involved in pivotal physiological processes, such as food digestion, metabolism and absorption of nutrients, energy supplementation, immune modulation, generation of essential vitamins, and the maintenance of gastrointestinal homeostasis,Citation12,Citation13 and the imbalance of gut microbiota is related to irritable bowel syndrome, colon cancer, obesity, diabetes, osteoporosis, gout, and other intestinal and extraintestinal diseases.Citation14 Interactions and connections between gut microbiota and its metabolites in multiple organs and tissues of the host are shown in . Recently, an enhancing number of studies have suggested that the gut microbiota in patients with osteoporosis and associated animal models is somewhat different from that in healthy individuals, and the severity of bone loss is also associated with alterations in gut microbiota,Citation15 which also indicates that gut microbiota could be a potential target for the prevention and treatment of osteoporosis. Meanwhile, with continuous deepening of various studies, the research concept of gut-bone axis has gradually emerged,Citation16 and the cross-linking interactions between multiple organs in host have gradually become a research hotspot. Based on the studies conducted by our research group in the early stages, we showed modulatory influences and implications of gut microbiota on osteoporosis,Citation17 as well as regulatory effects and repercussions of probiotics and prebiotics,Citation18 exercise,Citation19 glucocorticoids,Citation20 and fecal microbiota transplantationCitation21,Citation22 on the gut microbiota for prevention and treatment of osteoporosis.
Figure 1. Interactions and connections between the gut microbiota and its metabolites with multiple organs and tissues of host. Several factors, such as exercise, medication, diets, pollution, age and gender, genotype and so on, might affect the gut microbiota and its metabolites of population, and then cause corresponding changes to the bone, liver, brain, heart, kidney, pancreas, lung, and other organs of the host via a variety of regulation approaches. LPS, lipopolysaccharide; BAs, bile acids; SCFAs, short chain fatty acids; TMAO, trimetlylamine oxide.

Diet is the most direct connection between the gastrointestinal tract and external environment. Strengthening dietary management is a pivotal measure to modulate the gut microbiota, promote the microbial ecological balance, and consolidate the bone health. A growing number of studies have suggested that diets influence the genes and composition of gut microbiota, and the dominant microbiota of different metabolic substrates vary significantly, thereby affecting the structure and function of the gut microbiota.Citation23 Meanwhile, the intake of different dietary nutrients (such as protein, fat, carbohydrates, etc.) and the adoption of different dietary patterns (such as the Western diet, Vegan diet, Mediterranean diet, and so on) might influence the gut microbiota and its metabolites, and the alterations of gut microbiota and its metabolites might also affect the metabolic status of the host, thereby adjusting bone metabolism and modulating the occurrence and development of osteoporosis.Citation24 Hence, based on gut-bone axis, it can be recognized that dietary factors can be involved in the modulation of bone metabolism by altering the abundance, diversity, and composition of the gut microbiota and its metabolites, which further emphasizes the necessity and crucial significance of exploring the close association between the diet, gut microbiota, and osteoporosis. Herein, combined with emerging literatures and relevant researches, this review aims to summarize the effects of different dietary components and patterns on osteoporosis by acting on gut microbiota and its metabolites, as well as the underlying mechanisms and reasonable dietary recommendations, thus renovating the opinions in this research field and providing a reference for future related studies.
2. Inseparable dialogue between gut microbiota and osteoporosis
Gut microbiota is the total collection of microorganisms that colonize the human intestine and feces, which is the largest and most complex ecosystem in the host.Citation25 The gut microbiota of the human body originates from the mother’s uterus and birth canal and forms steadily until the age of 3 years.Citation26 It has a certain self-regulatory ability, assists in maintaining the stability of the intestinal environment, and plays a crucial regulatory role in the nervous, endocrine, immune, and metabolic systems of the human body.Citation27 Under the physiological conditions, the active modulation of gut microbiota helps to repair intestinal mucosal barrier, improve intestinal permeability, reduce inflammatory reactions, promote nutrient absorption, and participate in the multi-level regulation of bone metabolism.Citation28 However, under pathological conditions, the accumulation of inflammation in the intestine might result in insufficient calcium absorption and reduced circulating levels of vitamin D and vitamin K, thus resulting in a decrease in bone mass.Citation29 In addition, as a vital regulator of bone metabolism, the role of gut microbiota in the growth, development, aging, and other pathological alterations of bones should be highlighted.Citation30 A previous study suggested that malnutrition in infants and young children may result in the disruption of the gut microbiota, disrupt the development and maturation of immune system, and influence the normal growth of the skeletal system.Citation31 This study also revealed that normal mice transplanted with the gut microbiota of malnourished children exhibited a significant decline in bone mass after a certain period. In a study, the researchers investigated that germ-free (GF) mice exhibited slower weight gain and lower bone mass than normal mice, while GF mice supplemented with Lactobacillus plantarum maintained the normal growth rate, which suggested the crucial role of gut microbiota in the progression and development of bone.Citation32 Therefore, it is of great significance to protect the gut microbiota to support bone health. The gut microbiota and its metabolites may regulate bone growth and bone strength, as well as bone loss and fracture risk, by influencing activity and function of osteoblasts and osteoclasts.
With the updates and iterations of sequencing and omics technologies in recent years, studies on the gut microbiota and osteoporosis are progressing rapidly. In terms of population-based studies, the researchers investigated the China Multi-Ethnic Cohort study and found that high-altitude exposure may decrease bone mineral density (BMD) in adults, thus enhancing the risk of osteoporosis, and the modulation of gut microbiota might be a potential strategy for alleviating the decrease in BMD.Citation33 The scholars analyzed the gut microbiota of 38 postmenopausal women and indicated that Bacteroides and Rikenellaceae might be involved in the bone metabolism and fracture risk, and further investigations of the underlying microbiota-related pathways in bone metabolism may reveal treatment strategies and facilitate the prevention of osteoporosis.Citation34 Researchers also noticed the serum levels of trimethylamine N-oxide (TMAO), a common metabolite of the gut microbiota, in 286 postmenopausal women with hip fractures and found that elevated serum levels of TMAO were associated with a higher risk of hip fracture, suggesting that elevated serum levels of TMAO might contribute to the risk of osteoporosis and fracture in postmenopausal women.Citation35 In a trial, scholars investigated the additional efficacy of probio-M8, a dairy subspecies of Bifidobacterium, as an adjuvant therapy for postmenopausal osteoporosis and noticed that co-administration of Probio-M8 with conventional drugs/supplements was more efficacious than applying drugs or supplements alone in managing postmenopausal osteoporosis, which emphasized the pivotal significance of supplementing probiotics for the prevention and treatment of postmenopausal osteoporosis.Citation36 In a 1-year randomized, double-blind, and placebo-controlled study, scholars noticed that 100 women with postmenopausal osteoporosis were supplemented with calcium and vitamin D3 tablets and either a hop extract standardized in 8-PN or a placebo for 48 weeks. The results revealed that an 8-PN standardized hop extract can beneficially impacts the bone health of women with postmenopausal osteoporosis.Citation37 Based on gut-bone axis, dietary nutrients absorbed by the gut through metabolic processes of gut microbiota could further act on the bone, inhibit bone resorption, promote bone formation, and reverse bone loss.Citation38 Thus, based on the gut-bone axis, it is acknowledged that an enhancing number of studies have demonstrated an inextricable association between gut microbiota and osteoporosis.
3. Potential mechanisms involved in osteoporosis due to the modulation of gut microbiota
After observing and exploring the corresponding correlation in population cohorts, the researchers further delved into the exploration of deep mechanisms, including the modulation of intestinal epithelial barrier function, inflammation and oxidative stress, neuromodulation, immunomodulation, and endocrine modulation.Citation39 Regarding this, it is worth noting that in the former reviews summarized by our research group in early stages, we have elaborated on potential mechanisms involved in osteoporosis due to modulation of gut microbiota,Citation17–19 which is presented in details in . Specifically, there are multiple mechanisms of modulation of osteoporosis by gut microbiota and its metabolites, including 1) in terms of metabolites, gut microbiota colonized in the intestines of body could produce various metabolites via fermentation, such as short chain fatty acids (SCFAs), indole derivatives, polyamines, adenosine triphosphate, which might have pivotal impacts on bone metabolism.Citation40,Citation41 Therein, SCFAs are the main energy sources of intestinal epithelial cells and have anti-inflammatory impacts on intestinal mucosa, which can enter bloodstream and participate in the biosynthetic pathways, thus altering the activity of osteoclasts and osteoblasts. SCFAs could also promote the bone formation and indirectly influence bone metabolism by promoting the secretion of insulin-like growth factor-1 (IGF-1) and glucagon-like peptide-1 (GLP-1).Citation42 2) Changes of gut microbiota can also result in the disruption of intestinal mucosal barrier function, and enhanced intestinal permeability might cause increased serum lipopolysaccharide (LPS) levels, resulting in metabolic endotoxemia.Citation43 The increased LPS in vivo has been verified to promote bone loss and significantly reduce trabecular volume, lumbar BMD, and vertebral body count in mice models.Citation44 3) Gut microbiota is essential for the immune system function and maturation. Recent studies have also suggested a close interaction between immune system and bone metabolism, known as “osteoimmunology”, which represents the role of immune cells or immune related factors in modulating the bone metabolism.Citation45 Intestinal segmental filamentous bacteria (SFB) were verified to enhance the production of interferon-γ (IFN-γ) and interleukin-17 (IL-17), and after the transplantation of SFB in GF mice, the number of Th17 cells increased and the antibacterial effect of epithelial cells was maintained.Citation46 4) Due to its impacts on synthesis of cortisol, gut hormones, and neurotransmitters, the gut microbiota is also considered a virtual “endocrine organ” with specific effects on bone metabolism. On one hand, gut microbiota affects bone through the disruption of cortisol pathway, as cortisol and exogenous glucocorticoids can not only reduce the calcium absorption, but also promote the apoptosis of osteoblasts and inhibit the proliferation of osteoblasts. On the other hand, excessive glucocorticoids could also reduce the number of osteoblasts and osteoclasts, prolong the lifespan of osteoclasts, and promote apoptosis of osteoblasts.Citation47 5) Gut microbiota can significantly affect the nutrients and heat absorption, thus directly and indirectly affecting bone metabolism. Prebiotic fibers fermented into SCFAs can reduce the pH environment and mineral complexation, such as the formation of calcium phosphate, resulting in more calcium being absorbed to support bone growth or retention.Citation48
Figure 2. Association between gut microbiota and osteoporosis based on the gut-bone axis. Specifically, gut microbiota can regulate metabolic, immune, and inflammatory states of the body. Meanwhile, it can serve as a pivotal regulatory factor in the bone metabolism, regulating the immune system, affecting the generation of osteoclasts, and exerting regulatory effects on the bone. Studies on the regulation of inflammatory factors and hormones in the gut microbiota have shown that anti-inflammatory factors can reduce bone resorption and prevent bone loss. The constantly emerging researches on probiotics regulating bone metabolism has revealed that the increase of beneficial microbiota can promote the enhancement of bone mass and serve as a potential target for preventing and treating osteoporosis. However, the researches on the relationship between gut microbiota and osteoporosis is still in its infancy, and various studies are needed to further clarify its underlying mechanisms of action. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; SCFAs, short chain fatty acids; 5-HT, 5-hydroxytryptamine; NF-kB, nuclear transcription factor-κB; ca, calcium; vit D, vitamin D; SFB, segmented filamentous bacteria; LPS, lipopolysaccharide; GLP-1, glucagon-like peptide-1; IFN-γ, interferon-γ; TGF-β, transforming growth factor-β; GIP, glucose-dependent insulinotropic polypeptide; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-kB ligand; RANK, receptor activator of nuclear factor-kB; ZO-1, zona occludens 1; ALP, alkaline phosphatase; CINP, N.Terminal propeptide of type I collagen; CTX-1, C-terminal telopeptide of type I collagen; IGF-1, insulin-like growth factor-1; TRACP-5b, tartrate-resistant acid phosphatase 5b.

4. The role of probiotics in managing osteoporosis via its effects on gut-bone axis
Probiotics are a type of active microorganisms that are beneficial to host, which are colonized in the human gut and reproductive system, capable of producing precise health benefits, improving the microecological balance, and exerting beneficial effects.Citation49 With continuous alterations in the living structure, more and more probiotics are being developed and utilized, which are inseparable for maintaining stable function of body. In addition to food, the application range of probiotics is becoming increasingly wide, and more and more products, such as toothpaste and beer, are adding probiotics.Citation50 The regulation of probiotics in osteoporosis is an emerging field, and studying how probiotics can improve bone metabolism, prevent, or treat osteoporosis has become a hot research topic, and certain progress has been established.
Current research evidence suggests that supplementing probiotics to restore the balance of gut microbiota and its metabolites is beneficial for bone health. Our former review summarized the contents about the role of probiotics in managing osteoporosis via its effect on gut-bone axisCitation18. Specifically, the scholars noticed that the individuals diagnosed with inflammatory bowel disease or related conditions frequently suffered from osteoporosis due to the changes in intestinal microenvironment and consequent dysbiosis, and investigated that Bifidobacterium lactis BL-99 could be utilized as a beneficial probiotic preparation to prevent the incidence of osteoporosis in mice with ulcerative colitis owing to its abilities to reshape the gut microbiota and suppress the production of pro-inflammatory cytokines.Citation51 Researchers noted that supplementation with Bacteroides vulgatus ATCC 8482 could alleviate the imbalance of gut microbiota in mice with OVX-induced osteoporosis, downregulate the lipopolysaccharide/TLR-4/p-NF-κB pathway, and improve the bone loss and bone microstructure destruction in lumbar spine, which suggested that Bacteroides vulgatus ATCC 8482 might be a probiotic with the potential to ameliorate postmenopausal bone loss.Citation52 Recent studies have also revealed that probiotic therapy could inhibit the activity of osteoclasts in ovariectomy (OVX)-induced mice by inhibiting the secretion of IL-6 and intestinal inflammatory response, thus enhancing the bone mass.Citation53,Citation54
However, current researches on the probiotics for the prevention and treatment of osteoporosis mostly focuses on animal experiments, with few clinical trials reported. A population-based trial revealed that the co-administering Bifidobacterium animalis subsp. lactis Probio-M8 with conventional drugs/supplements was more efficacious than conventional drugs/supplements alone in managing postmenopausal osteoporosis.Citation36 In a double-blind, placebo-controlled study, women from the population who were 75 to 80 years old and had low BMD were randomized to orally receive 1010 colony-forming units of Lactobacillus reuteri ATCC PTA 6475 daily or the placebo, and the results revealed that supplementation with Lactobacillus reuteri ATCC PTA 6475 may be a novel approach to prevent osteoporosis.Citation55 Moreover, in a randomized controlled trial, scholars demonstrated that supplementation of Lactobacillus reuteri ATCC PTA 6475 has potentials to prevent a deterioration of the gut microbiota and inflammatory status in older women with low BMD, which may have beneficial effects on the bone metabolism.Citation56 Thus, it could be acknowledged that an enhancing number of scholars are starting to probe into deep mechanisms of the modulation of gut microbiota and its metabolites from perspectives of probiotic supplementation, and probiotics have also been a potential candidate for the prevention and treatment of osteoporosis.
5. Influences of different dietary nutrients on osteoporosis by acting on the gut microbiota and its metabolites
The composition and contents of nutrients in different kinds of food significantly affect the composition and abundance of the gut microbiota, which in turn influences bone metabolism and regulates osteoporosis.Citation57 Therefore, some microorganisms directly utilize certain nutrients as the source of growth material, and the high intake of this kind of nutrient might result in an enhancement in bacterial abundance and act on bone metabolism.Citation58 For example, microorganisms such as Streptococcus, Bacillus, Propionibacterium, Staphylococcus, Bacteroides, and Clostridium could utilize the protein as a nitrogen source, and higher dietary protein intake could also increase its abundance and diversity.Citation59 In addition, it is also recognized that the alterations in the physiological environment caused by daily dietary intake can influence the selection of microbial communities.Citation60 Taking dietary fat intake as an example, a diet rich in fat may promote bile secretion to facilitate the digestion of lipids, and bile acids have detrimental effects on the cell membrane of bacteria, which enables bile acids to exert a strong selection pressure on microbial groups and cause these gut microbiota-related metabolites to play a negative role in the metabolic modulation of osteoporosis.Citation61 presents and elucidates the effects of dietary nutrients on osteoporosis by acting on gut microbiota and its metabolites.
Figure 3. The effects of dietary nutrients on osteoporosis by acting on gut microbiota and its metabolites. Under the domination of various dietary patterns, gut microbiota is closely related to the synthesis and absorption of essential nutrients, such as protein, vit D, ca, P, and mg, which affect the intestinal mucosal barrier function, intestinal inflammation, metabolites, etc., thereby participating in the modulation of the balance between bone formation represented by osteoblasts and bone absorption represented by osteoclasts via systemic circulation. SCFAs, short chain fatty acids; vit D, vitamin D; ca, calcium; P, phosphorus; mg, magnesium; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; NF-kB, nuclear transcription factor-κB; LPS, lipopolysaccharide; CRP, C-reactive protein; OPG, osteoprotegerin; ALP, alkaline phosphatase; CINP, N.Terminal propeptide of type I collagen; RANKL, receptor activator of nuclear factor-kB ligand; RANK, receptor activator of nuclear factor-kB; CTX-1, C-terminal telopeptide of type I collagen; ZO-1, zona occludens 1; TRACP-5b, tartrate-resistant acid phosphatase 5b.

5.1 Dietary protein and amino acids
Nutritional factors have a crucial impact on osteoporosis, with low calcium intake, vitamin D deficiency, high phosphorus and sodium diets, heavy alcohol consumption, and carbonated beverage consumption being universally considered risk factors for osteoporosis, and adequate protein intake is conducive to bone health.Citation62,Citation63 Dietary protein intake by human body can alter the electron receptors in the respiratory chain through bacterial fermentation, methanation, decarboxylation reactions, and sulfur reduction.Citation64,Citation65 For example, sulfur-containing amino acids, such as methionine and cystinine, can generate hydrogen sulfide under the sulfur reduction of sulfate-reducing bacteria, and amino acids and peptides can produce amines through decarboxylation reactions under the action of Clostridium difficile.Citation66 These metabolic end products alter the growth microenvironment of bacteria and enable bacteria to obtain certain growth advantages, thus altering the composition and abundance of the gut microbiota.Citation67 Meanwhile, studies have reported that the number of Bacteroides, Prevotella, Treponema, Fusobacterium, Clostridium perfringens, Bacillus, and Sarteria in the gut microbiota of individuals on high-protein diets is enhanced compared with that of individuals on conventional diets, whereas the number of Firmicutes decreases.Citation68,Citation69 This alteration has also been verified to be consistent with the reversal of osteoporosis. Researchers revealed that the main metabolites of protein, amino acids, and peptides fermented by gut microbiota are SCFAs, such as acetic acid, propionic acid, and butyric acid, and further participate in the modulation of bone formation and bone resorption by acting on the above SCFAs.Citation70 Scholars revealed that intestinal anaerobic bacteria can synthesize the acetic acid from the glycine, threonine, glutamic acid, lysine, ornithine, aspartic acid, and butyric acid from the threonine, glutamic acid, and lysine, whereas propionic acid is mainly synthesized via threonine metabolism.Citation71 More importantly, the modulation of SCFAs, including the acetic acid, propionic acid, and butyric acid, has been universally reported to be closely associated with the occurrence and development of osteoporosis.Citation72 Meanwhile, Clostridium entericum can utilize the branched chain amino acids (valine, leucine, and isoleucine) to synthesize branched chain fatty acids, and branched chain amino acids and SCFAs are also the potential regulatory factors for metabolic diseases such as osteoporosis, obesity, type 2 diabetes, and so on.Citation73
5.2 Dietary fat
Dietary fat is an effective source of energy for the body. Previous studies have suggested that both the quality and quantity of dietary fat intake might influence the composition and abundance of gut microbiota.Citation74 The unsaturated fatty acids change the composition and abundance of gut microbiota by enhancing ratio of Bacteroidetes to Firmicutes Citation75. N-3 polyunsaturated fatty acids (N-3 PUFA) can result in beneficial alterations in gut microbiota, including the addition of Bifidobacterium, Lactobacillus, Streptococcus, Vibrio desulphuricum, and Akkermania mucophilus.Citation76,Citation77 High intake of saturated fatty acids and trans fatty acids (mainly present in Western diet) enhances risk of cardiovascular and bone metabolism-related diseases, reduces the abundance of Bacteroides, Prevotella, Lactobacillus, and Bifidobacterium, and enhances the abundance of Firmicutes, which may lead to systemic inflammation, bone metabolism disorders, and osteoporosis.Citation78 In addition, current studies on the effects of dietary fat on gut microbiota and osteoporosis have mainly focused on the high-fat diets (HFD). Scholars revealed that long-term HFD could result in decreased bone mass, with microbiota dysbiosis, leaky gut, and systemic inflammation, while the administration of Fructooligosaccharides (FOS) or Galactooligosaccharides (GOS) could significantly enhance the biodiversity and the SCFAs concentrations of gut microbiota in the HFD-fed mice, and reverse high gut permeability and inflammatory cytokines, ultimately protecting against the HFD-induced osteoporosis.Citation79 The researchers analyzed the characteristics of gut microbiota and serum metabolomics in mice with HFD-induced osteoporosis and explored potential correlations. These results suggested that HFD-induced bone loss was accompanied by the expansion of bone marrow adipose tissue and inhibition of bone formation.Citation80 A total of 32 bacterial genera were identified to be significantly correlated with bone loss, and 145 serum metabolites were identified as differential metabolites related to HFD. A former study indicated that probiotic Lactobacillus paracasei HII01, prebiotic xylooligosaccharide, and synbiotics had similarly beneficial effects to improve jawbone microarchitecture in HFD-fed rats by ameliorating osteoclast-related bone resorption and potentiating bone-formation activities.Citation81 Scholars also indicated that exercise can prevent several negative effects of HFD on the bone health of male individuals, and the changes in gut microbiota induced by exercise (reducing the ratio of Firmicutes to Bacteroidetes) might exhibit an emerging mechanism contributing to exercise-induced benefits for the bone health.Citation82 The scholars also summarized that HFD has a crucial effect on bone structure and health, and the imbalance of gut microbiota, deterioration of the intestinal barrier, inflammatory response, oxidative stress, adipokine alterations, and accumulation of bone marrow fat tissue are thought to be potential mechanisms.Citation83 Recently, HFD has been a hot research topic in the academic community, in-depth molecular mechanisms and intervention methods of HFD through the modulation of gut microbiota and its metabolites need to be more identified and explored.
5.3 Dietary carbohydrates
Both digestible and non-digestible carbohydrates may affect the composition and abundance of the gut microbiota, both of which have been verified to enhance the amount of Bifidobacterium.Citation84 The digestible carbohydrates in various fruits (such as glucose, sucrose, and fructose) have been demonstrated to decrease the abundance of Bacteroides and Clostridium.Citation85 The non-digestible carbohydrates (such as resistant starches and sugars) reach the large intestine, where they can be fermented by the gut microbiota to provide energy or produce postbiotics.Citation86,Citation87 Indigestible carbohydrates are also known as dietary fiber. Recently, with the rapid development of research on the gut-bone axis, SCFAs fermented from dietary fiber have become a crucial medium, focusing on the link between gut microbiota and osteoporosis.Citation88 Moreover, dietary fiber alters the gut microbiota by modulating the ratio of Bacteroidetes to Firmicutes, and alters multiple functional pathways (including the amino acid- and lipid-related metabolism), thus participating in the regulation of bone metabolism.Citation89 The researchers analyzed the consequences of insufficient dietary fiber intake in the humanized mice and observed that a low-fiber diet markedly reduced microbial diversity for up to three generations and that microbial diversity cannot be recovered after dietary fiber supplementation.Citation90 On this basis, scholars conducted a research cohort that randomly divided the included population into two groups, where one group was assigned to a high-fat/low-fiber diet for 10 days, while the other group was given a high-fiber/low-fat diet for 10 days.Citation91 Results showed that a HFD slowed down intestinal transit times by as much as 3 days, although specific taxa variations varied between individuals. More importantly, researchers summarized that ingesting more fiber-rich food (including coarse grains, fresh fruits and vegetables), combined with sufficient exercise, proper water intake, and regular bowel movements, might contribute to maintaining human intestinal and bone health, especially in middle-aged and elderly people, to prevent the osteoporosis.Citation92 Therefore, consuming dietary fiber from multiple foods is crucial for maintaining the intestinal health and preventing bone loss.
6. Influences of dietary patterns on osteoporosis by regulating gut microbiota and its metabolites
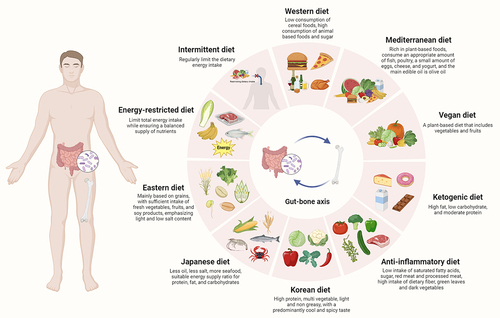
Due to the synergistic influences of multiple nutrients in food, there are certain drawbacks in analyzing the association between specific nutrient elements and bone health, and the association between different kinds of nutrients and osteoporosis is still unclear.Citation93 Dietary patterns refer to relative composition of types and quantities of various food consumed in diets,Citation94 The formation and induction of dietary patterns is a long-term process, affected by various factors, such as the population, agricultural production, food circulation, food processing, consumption level, ingesting habits, cultural traditions, and scientific knowledge of a country or region.Citation95 As a method to evaluate the total consumption of food, the analysis of dietary patterns focuses on analyzing the types of food ingested by research objects, rather than a single food or nutrient element, which has been widely recognized in studies of food nutrition and health.Citation96 Recently, the link between dietary patterns and osteoporosis has attracted increasing attention from researchers. shows the different dietary patterns of osteoporosis by modulating gut microbiota and its metabolites. The effects of different dietary patterns on gut microbiota, metabolites, and bone health are summarized in .
Figure 4. Different dietary patterns on osteoporosis by modulating the gut microbiota and its metabolites. Different dietary nutrients (such as protein, fat, carbohydrates, etc.) and different dietary patterns (such as Western diet, vegan diet, mediterranean diet, etc.) may affect gut microbiota and its metabolites, and the changes of gut microbiota and its metabolites may also influence the metabolic status of host, thereby adjusting the bone metabolism and modulating the occurrence and development of osteoporosis. The effects of different dietary patterns on gut microbiota and bone metabolism are exhibited in this figure.

Table 1. The effects of different dietary patterns on gut microbiota, metabolites, and bone health.
6.1 Western diet
Western diet usually refers to the dietary habits of most people in United States and Europe, with meat and meat products, refined carbohydrates, dairy products, and processed food accounting for a relatively high proportion, whereas fiber accounts for a relatively low proportion.Citation97 Western diet is considered an unhealthy diet with high-fat consumption, especially in low-income and middle-income countries, and the prevalence of hypertension, diabetes, coronary heart disease, and osteoporosis among people on the Western diet is on a significant rise.Citation98 The characteristics of Western diet include the intake of large amounts of refined sugars (mainly candies and desserts, as well as high-sugar soft drinks), animal fats (high intake of saturated and omega-6 fatty acids, low intake of omega-3 fatty acids), processed meat (especially red meat), refined grains, high-fat dairy products, conventionally raised animal products, salt, eggs, potatoes, corn, while fruits, vegetables, whole grains, herbivore products, fish, and nuts are low in intake.Citation119 Taken together, Western diet is low in fiber, vitamins, minerals, and other plant-derived molecules (such as antioxidants). Research evidence from China suggests that adopting a healthy or cautious dietary pattern could prevent hip fractures, while the long-term consumption of a Western diet represented by high-fat food may enhance the risk of hip fracture.Citation120
Western diet promotes alterations in the gut microbiota, leading to the intestinal microecological disorders, intestinal barrier damage, enhanced intestinal permeability, and leakage of toxic bacterial metabolites into the circulatory system, all of which may result in the progression of systemic low-grade inflammation, and then contribute to the occurrence and development of osteoporosis.Citation99,Citation121,Citation122 Long-term consumption of a western diet could result in a decrease in the diversity of gut microbiota, promote the proliferation of Firmicutes and Proteobacteria among the individuals, induce the production of pro-inflammatory cytokines, and facilitate excessive storage of lipids in liver and adipose tissue.Citation100 Meanwhile, HFD can stimulate the proliferation of gram-negative bacteria, leading to endotoxemia, which increases the risk of osteoporosis.Citation123 In summary, the imbalance of gut microbiota driven by Western diet is closely related to intestinal barrier damage, indicating that the Western diet induces adverse changes in gut microbiota and further endangers bone health.
6.2 Mediterranean diet
Mediterranean diet is common in countries or regions bordering Mediterranean Sea, such as Greece, Spain, France, and southern Italy.Citation124 Regarding this kind of dietary pattern, there are more vegetables, fruits, fish, and beans in diets, while there is less red meat.Citation101 As a result, the Mediterranean diet has a relatively high intake of vegetables and fruits, rich in dietary fiber and other complex carbohydrates, and low levels of saturated fatty acids, which contribute to the prevention of diabetes, cardiovascular, and cerebrovascular diseases, and osteoporosis.Citation102 Based on several previous studies, the Mediterranean diet is conducive to the growth and existence of gut microbiota and is related to a lower incidence rate of common chronic diseases, and its regulatory effects on gut microbiota and its metabolites can effectively reduce the risk of chronic diseases.Citation125 Further studies have suggested that some individual components of Mediterranean diet are associated with specific classifications of gut microbiota. For example, grains are linked to Bifidobacterium and Faecalibacterium, olive oil is associated with Tenericutes and Dorea, and the red wine is associated with Faecalibacterium, and so on, the specific classifications of gut microbiota mentioned above have been proven to be related to inhibition of osteoclasts.Citation103,Citation126,Citation127 Meanwhile, scholars suggested that adherence to the Mediterranean diet may enhance the content of SCFAs in the host and increase the proportion of Bacteroidetes to Firmicutes, and thereby be conducive to maintaining the balance of osteoblasts and osteoclasts and promoting the bone health.Citation128 Collectively, Mediterranean diet plays a crucial role in improving the bone health and preventing osteoporosis owing to its unique dietary characteristics.
6.3 Vegan diet
A Vegan diet can promote the abundance and diversity of gut microbiota, while it is related to the duration of dietary pattern adherence, and the rapid alterations in dietary structure can also result in a decline in microbial diversity.Citation104 The long-term vegetarians may experience a decrease in intestinal pH, accompanied by higher levels of SCFAs, lower amounts of Bacteroides, and higher amounts of Prevotella.Citation129 This intestinal environment is conducive to the absorption of calcium in the host, thereby promoting bone formation and inhibiting bone loss.Citation105,Citation106 The scholars indicated in a case-control study that the intake of vegetables and fruits was positively correlated with an increase in BMD in the population, whereas it was negatively correlated with the risk of fractures.Citation130 Researchers also noticed in a population-based study that the serum levels of β-crypto-flavin and β-carotene in 699 postmenopausal women were positively correlated with BMD, indicating that a higher intake of vegetables and fruits may be beneficial to bone health.Citation107 Moreover, compared with the omnivorous population, the contents of fibrous degrading bacteria, such as Spirillum, are higher in vegetarians, and the increase in Akkermania mucophilus and decline in Bacteroides fragilis in body have beneficial effects on obesity, fat accumulation, insulin resistance, and bone metabolism disorders.Citation131 Scholars suggested that Haemophilus, Neisseria, Aggregatibacter, and Veillonella were more prevalent among individuals with a long-term Vegan diet, and these bacteria have been verified to be associated with the bone health in the population.Citation132 In addition, among the population adopting a Vegan diet, the lacto-ovo vegetarians have BMD close to that of omnivores, while the absolute vegetarians have lower BMD and higher risk of fractures, and it is necessary to ensure sufficient intake of calcium, vitamin D, and protein. Collectively, the vegetarians can meet the calcium needs by consuming calcium-rich food such as beans, soy products, nuts, vegetables, and whole grains, but people should seek professional nutritional guidance when choosing Vegan diet based on their own needs and circumstances.
6.4 Anti-inflammatory diet
Diet is crucial to systemic inflammation, and an improper diet (pro-inflammatory food) may facilitate the progression of systemic inflammation, whereas an appropriate diet (anti-inflammatory food) may minimize systemic inflammation, reduce the risk of multiple chronic diseases such as hypertension, diabetes, coronary heart disease, and osteoporosis, optimize the body mass index (BMI), and improve the quality of life.Citation108 The dietary inflammation index (DII) was designed and promoted by scholars to evaluate the overall inflammatory potential of personal diet.Citation133 Scholars revealed that higher DII was associated with an increased risk of osteoporosis in female, while no link was found in male, and a greater pro-inflammatory diet might be related to lower BMD in both female and male.Citation109 Researchers suggested that the consumption of a pro-inflammatory diet enhanced the risk of fracture in Chinese female aged less than 50 years, and high consumption of anti-inflammatory food and low consumption of pro-inflammatory food may be a vital strategy to prevent fractures in female.Citation110 In addition, DII is negatively correlated with the abundance of Faecalibacterium prausnitzii in body, and the decline in the abundance of Faecalibacterium prausnitzii caused by excessive DII might lead to a decrease in the production of SCFAs, thus affecting regulatory effects of gut microbiota-related metabolites on bone formation and inducing the bone loss.Citation111,Citation134 A population-based study also suggested that high inflammatory food intake was associated with a high abundance of Acidaminococcus, and it was positively correlated with circulating plasminogen activator inhibitor-1 (PAI-1), which is an inflammatory marker associated with the risk of obesity, type 2 diabetes, cardiovascular diseases, and osteoporosis.Citation135 As a result, anti-inflammatory diet could provide necessary nutrients for body to maintain intestinal health, and a stable state of gut microbiota can reduce the level of inflammation in intestine, thus reducing the negative impacts of inflammation on bone health.
6.5 Ketogenic diet
The ketogenic diet is an extremely low-carbohydrate diet; although there have not been sufficient and robust experimental designs to definitively recognize the impact of a ketogenic diet on bone health, an increasing number of scholars are focusing on and exploring this direction.Citation136 The scholars noticed that long-term consumption of a ketogenic diet could induce higher levels of ketone and higher fat percentage in the body, resulting in lower body weight and damage to bone mass and mechanical properties.Citation112 Scholars demonstrated that metformin effectively attenuates cancellous bone loss induced by a ketogenic diet and maintains the biomechanical properties of long bones, providing evidence for metformin as a treatment approach for ketogenic diet-induced osteoporosis.Citation113 Moreover, the long-term consumption of a ketogenic diet may decrease the diversity of gut microbiota, reduce the abundance of pathogens such as Escherichia, Salmonella, and Vibrio, enhance the abundance of Lactobacillus and Akkermania mucophilus, promote the generation of SCFAs; and play a role in reducing weight and lowering blood sugar.Citation137 A ketogenic diet could also result in a continuous decrease in the abundance of Bifidobacterium, thereby reducing the level of Th17 cells and improving bone metabolism, and a ketogenic diet containing protein may have stronger effects on reducing the ratio of Firmicutes and enhancing the ratio of Bacteroidetes.Citation114 However, it should be noted that although Ketogenic diet has certain benefits in weight control and other health aspects, its impact on bone health is still controversial and should be treated with caution.
6.6 Korean diet
The characteristics of Korean diet is characterized by high vegetable and whole grain content, low animal-based and saturated fat content, and a high consumption of fermented food.Citation115 A previous study on the Korean population suggested that adding fruits and dairy products as part of the traditional Korean diet might reduce the risk of osteoporosis in postmenopausal Korean women, compared to consuming diets rich in meat, alcohol, and sugar.Citation116 Moreover, subjects with a higher intake of rice, kimchi, and seaweed have a higher risk of osteoporosis.Citation138 After the intervention of traditional Korean diet, abundance of Bacteroidetes in intestinal bacterial community decreased, whereas the abundance of Firmicutes, Weissella, and Coprococcus increased.Citation117,Citation139 In addition, scholars showed in a population-based study that individuals with a long-term intake of Korean diet contained dominant bacteria, including Ruminococcus, Oscillospira, Gemmiger, Blautia, and Coprococcus.Citation140 However, it should be noticed that Korean diet includes partial high-salt food, such as the pickled vegetables and fried chicken. Excessive salt and saturated fat intake may have negative impacts on intestinal and bone health, and the intake of above food needs to be controlled while pursuing the Korean diet.
6.7 Other dietary patterns
Other relatively rare dietary patterns include Japanese diet, Eastern diet, Energy-restricted diet, and Intermittent diet. Therein, 1) Japanese diet is mainly dominated by vegetables, seafood, beans, whole grains, and seaweed. This dietary pattern is rich in nutrients such as fiber, plant protein, healthy fats, and various vitamins and minerals, which contributes to maintaining intestinal and bone health.Citation1412) Eastern diet refers to the dietary patterns of Asian countries, such as China, Japan, South Korea, etc. This dietary pattern is mainly based on vegetables, fruits, beans, whole grains, seafood, etc., rich in fiber, plant protein, healthy fats, vitamins, and minerals. Eastern diet may have positive impacts on osteoporosis by regulating gut microbiota, providing dietary fiber, and probiotics, but more research evidence is needed to verify specific mechanisms.Citation1423) Energy-restricted diet is a dietary pattern that reduces energy intake, typically used to lose weight, or improve metabolic status. Several studies have revealed that a moderate Energy-restricted diet may have positive influences on the bone health by reducing weight, improving metabolic status, and modulating gut microbiota.Citation143,Citation144 Intuitively, reducing body weight may reduce the load on bone and reduce the risk of fractures, while excessive Energy-restricted diet may also lead to insufficient protein intake, which in turn can be harmful to bone health.Citation1454) Intermittent diet refers to the periodic alternating eating and fasting. Existing research evidence suggests that Intermittent diet may have impacts on the composition and function of gut microbiota, enhancing the abundance and diversity of beneficial microbiota, and thus influencing bone health.Citation118 However, current researches on the relationship between Intermittent diet and osteoporosis is still relatively limited, and more research is needed to verify specific mechanisms.Citation146 Moreover, during the process of intermittent diet, individuals should also pay attention to maintaining abundant intake of nutrients, especially the protein and calcium, to ensure the bone health.
7. The reasonable dietary recommendations based on the regulatory influences of gut microbiota and its metabolites on osteoporosis
The gut microbiota plays a crucial role in the modulation of host health, and the composition of the gut microbiota varies significantly among different individuals, which is influenced by various factors such as diet and environment, among which diet is a vital factor that cannot be ignored.Citation147 Different dietary patterns have different effects on the structure, metabolism, and function of gut microbiota and are involved in various physiological activities, such as the food digestion, nutrient metabolic absorption, energy supply, immune modulation, and maintenance of gastrointestinal homeostasis, which are closely associated with the occurrence and development of osteoporosis.Citation148 In addition, diets can affect not only the structure and composition of the gut microbiota but also its genes and function.Citation149 Even bacteria of the same genus have significant differences in functional and core genes under the influence of different dietary patterns.Citation150 The vegan and Mediterranean diets could enhance the diversity of gut microbiota and promote the growth of beneficial bacteria, such as Bifidobacterium, Lactobacillus, and Prevotella, while the Western diet could reduce the diversity of gut microbiota and increase the ratio of Firmicutes to Bacteroidetes.Citation151 Different dietary components may shape the intestinal bacterial community in a time-dependent manner, and the effects of different dietary components on gut microbiota are significantly differentCitation152. Hence, a reasonable dietary composition, proper dietary habits and methods, and appropriate nutrient ratios are the foundation for ensuring human health, whereas unreasonable dietary patterns are the culprit of several chronic diseases, including hypertension, diabetes, and osteoporosis.Citation153 On this basis, it is also essential to propose reasonable dietary recommendations based on the regulatory impacts of gut microbiota and its metabolites on osteoporosis, and shows the different influences on the bone by modulating the gut microbiota and its metabolites under different dietary recommendations.
Figure 5. Different impacts on bone by modulating gut microbiota and its metabolites under different dietary recommendations. The reasonable dietary patterns integrate the characteristics of healthy dietary patterns, such as a Mediterranean diet and an anti-inflammatory diet, mainly including the diversified food, high fiber intake, low sugar intake, low fat intake, and high grains intake, thereby maintaining the bone health and preventing osteoporosis. On the contrary, unhealthy dietary pattern (such as Western diet), represented by high-fat, high sugar, high salt, and high calorie dietary intake, is not conducive to maintaining the intestinal and bone health. SCFAs, short chain fatty acids; TMAO, trimetlylamine oxide; TMA, trimethylamine; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; IL-10, interleukin-10; IL-1β, interleukin-1β.

The reasonable dietary patterns integrate the characteristics of healthy dietary patterns such as a Mediterranean diet and an anti-inflammatory diet, mainly including diversified food, high fiber intake, low sugar intake, low fat intake, and high grains intake.Citation154 The reasonable dietary patterns are mainly based on the principles of food diversification, and it is recommended to consume more vegetables, fruits, beans and their products, fish, and seafood, while consuming less red meat and saturated fatty acids to ensure sufficient intake of plant-based food and a proper amount of animal-based food, thus obtaining a complete variety of dietary nutrients, sufficient quantities, and appropriate proportions, and meeting the physiological needs of middle-aged and older populations to maintain bone health and prevent osteoporosis.Citation155 Reasonable dietary patterns also lay a solid foundation for the shaping of intestinal homeostasis, multiplication of beneficial bacteria, elimination of harmful bacteria, and maintenance of the diversity of gut microbiota.Citation156 Hence, it is essential to encourage middle-aged and elderly individuals to choose the corresponding dietary patterns based on their bone and intestinal conditions and maintain reliable dietary habits.
8. Conclusions and perspectives
The impacts of gut microbiota on the osteoporosis have been widely studied and recognized. As one of the most important symbiotic partners of the human body, the gut microbiota is related to bone health, and there are various factors affecting the gut microbiota and its metabolites, including diet, age, genetics, antibiotics, medication, hormone levels, and emotional stress, which alter and re-shape unique gut microbiota of each person.Citation157 However, in the short term, dietary content and dietary patterns are regarded as the most significant driving factors for shaping gut microbiota and its metabolites. In the long term, diets are also a relatively effective and healthy choice for adjusting and intervening in gut microbiota, which has profound regulatory effects on bone metabolism.Citation158 Hence, based on gut-bone axis, the scientific popularization of dietary regulation on osteoporosis is of great significance, which can be regarded as the most common intervention approach in daily life to participate in the prevention of osteoporosis.
However, from the perspective of the gut-bone axis, it is still essential to identify and recognize the current shortcomings of the effects of diet on gut microbiota and its metabolites in modulating osteoporosis. First, the specific mechanisms by which diets affect the gut microbiota and its metabolites to modulate osteoporosis are not yet fully understood, and there is still a lack of unified conclusions on alterations in the dietary patterns and the characteristics of individuals with osteoporosis.Citation159 Further in-depth researches are needed to determine the effects of different dietary patterns on diversity and composition of the gut microbiota in hosts with osteoporosis. On this basis, large-scale population-based cohorts are still needed to comprehensively assess the effects of demographic characteristics, alterations in dietary patterns, physical activity, daily exercise, and other factors on gut microbiota and its metabolites in individuals with osteoporosis. Second, there is still a lack of a comprehensive layout of dietary patterns to analyze the characteristics of gut microbiota and its metabolites. Current studies tend to focus more on the abundance of characteristic food or nutrients in different dietary patterns to explain their impacts on the gut microbiota, and subsequently on the modulation of osteoporosis.Citation160 It should also be noted that dietary patterns are not static, and some of their components have also been altered over the past few decades.Citation161 Third, current researches have not been able to focus on the metabolic potential and development process of microbial communities. Although the short-term dietary interventions could affect the composition of gut microbiota, long-term interventions could produce relatively stable responses to alterations in the microbiota caused by alterations in diet, and current investigations cannot show alterations in the microbiota over time.Citation162 Nevertheless, with the advancement of sequencing technology, the cost of microbial sequencing may be lower, and the data analysis may be more convenient; thus, studies on microbiomes may become more vigorous. Ultimately, the impacts of different dietary patterns on gut microbiota and its metabolites, and their involvement in the modulation of bone metabolism, have been widely recognized.Citation163 However, further studies are still needed on the interactions between food or nutrients and the gut microbiota and its metabolites, upstream regulatory mechanisms, and downstream effects.Citation164 More scientific popularization of dietary regulation on osteoporosis based on gut-bone axis also needs to be mentioned and expanded. It could be expected that when the above issues are resolved, this approach of improving gut microbiota and its metabolites by adjusting dietary patterns, thus affecting the human gene expression, digestion, absorption, metabolism, and immune processes, and further generating the health effects, might be a more economical, effective, and simple means of reducing the side effects in the future.
Abbreviations
| GF | = | germ-free |
| BMD | = | bone mineral density |
| TMAO | = | trimethylamine N-oxide |
| SCFAs | = | short chain fatty acids |
| IGF-1 | = | insulin-like growth factor-1 |
| GLP-1 | = | glucagon-like peptide-1 |
| LPS | = | lipopolysaccharide |
| SFB | = | segmental filamentous bacteria |
| IFN-γ | = | interferon-γ |
| IL-17 | = | interleukin-17 |
| OVX | = | ovariectomy |
| N-3 PUFA | = | N-3 polyunsaturated fatty acids |
| HFD | = | high-fat diets |
| FOS | = | Fructooligosaccharides |
| GOS | = | Galactooligosaccharides |
| BMI | = | body mass index |
| DII | = | dietary inflammation index |
| PAI-1 | = | plasminogen activator inhibitor-1 |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
There are no research data in this paper.
Additional information
Funding
References
- Ayers C, Kansagara D, Lazur B, Fu R, Kwon A, Harrod C. Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American college of physicians. Ann Intern Med. 2023;176(2):182–25. doi:10.7326/M22-0684.
- Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H, Wang Y, Li Y, Wang L, Fang C, et al. RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep. 2021;22(7):e52481. doi:10.15252/embr.202152481.
- Li X, Wang L, Huang B, Gu Y, Luo Y, Zhi X, Hu Y, Zhang H, Gu Z, Cui J, et al. Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss. Sci Adv. 2020;6(47):6. doi:10.1126/sciadv.abb7135.
- Deardorff WJ, Cenzer I, Nguyen B, Lee SJ. Time to benefit of bisphosphonate therapy for the prevention of fractures among postmenopausal women with osteoporosis: a meta-analysis of randomized clinical trials. JAMA Intern Med. 2022;182(1):33–41. doi:10.1001/jamainternmed.2021.6745.
- Händel MN, Cardoso I, von Bülow C, Rohde JF, Ussing A, Nielsen SM, Christensen R, Body JJ, Brandi ML, Diez-Perez A, et al. Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ. 2023;381:e068033. doi:10.1136/bmj-2021-068033.
- Zhang YW, Lu PP, Li YJ, Dai GC, Cao MM, Xie T, Zhang C, Shi L, Rui Y-F. Low dietary choline intake is associated with the risk of osteoporosis in elderly individuals: a population-based study. Food Funct. 2021;12(14):6442–51. doi:10.1039/D1FO00825K.
- Zhang YW, Lu PP, Li YJ, Dai GC, Chen MH, Zhao YK, Cao MM, Rui YF. Prevalence, characteristics, and associated risk factors of the elderly with hip fractures: a cross-sectional analysis of NHANES 2005–2010. CIA. 2021;16:177–85. doi:10.2147/CIA.S291071.
- Chen X, Zhi X, Wang J, Su J. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2018;6(1):34. doi:10.1038/s41413-018-0035-6.
- Chevalier C, Kieser S, Çolakoğlu M, Hadadi N, Brun J, Rigo D, Suárez-Zamorano N, Spiljar M, Fabbiano S, Busse B, et al. Warmth prevents bone loss through the gut microbiota. Cell Metab. 2020;32(4):575–90.e7. doi:10.1016/j.cmet.2020.08.012.
- Bastings J, Venema K, Blaak EE, Adam TC. Influence of the gut microbiota on satiety signaling. Trends Endocrinol Metab. 2023;34(4):243–255. doi:10.1016/j.tem.2023.02.003.
- Jaswal K, Todd OA, Behnsen J. Neglected gut microbiome: interactions of the non-bacterial gut microbiota with enteric pathogens. Gut Microbes. 2023;15(1):2226916. doi:10.1080/19490976.2023.2226916.
- Zhang T, Cheng JK, Hu YM. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev. 2022;81:101739. doi:10.1016/j.arr.2022.101739.
- Ling Z, Liu X, Cheng Y, Yan X, Wu S. Gut microbiota and aging. Crit Rev Food Sci Nutr. 2022;62(13):3509–3534. doi:10.1080/10408398.2020.1867054.
- Shandilya S, Kumar S, Kumar Jha N, Kumar Kesari K, Ruokolainen J. Interplay of gut microbiota and oxidative stress: perspective on neurodegeneration and neuroprotection. J Adv Res. 2022;38:223–44. doi:10.1016/j.jare.2021.09.005.
- Waldbaum JDH, Xhumari J, Akinsuyi OS, Arjmandi B, Anton S, Roesch LFW. Association between dysbiosis in the gut microbiota of primary osteoporosis patients and bone loss. Aging Dis. 2023;14(6):2081. doi:10.14336/AD.2023.0425.
- He Y, Chen Y. The potential mechanism of the microbiota-gut-bone axis in osteoporosis: a review. Osteoporos Int. 2022;33(12):2495–2506. doi:10.1007/s00198-022-06557-x.
- Zhang YW, Li YJ, Lu PP, Dai GC, Chen XX, Rui YF. The modulatory effect and implication of gut microbiota on osteoporosis: from the perspective of “brain–gut–bone” axis. Food Funct. 2021;12(13):5703–18. doi:10.1039/D0FO03468A.
- Zhang YW, Cao MM, Li YJ, Dai GC, Lu PP, Zhang M, Bai LY, Chen XX, Zhang C, Shi L, et al. The regulative effect and repercussion of probiotics and prebiotics on osteoporosis: involvement of brain-gut-bone axis. Crit Rev Food Sci Nutr. 2023;63(25):7510–7528. doi:10.1080/10408398.2022.2047005.
- Zhang YW, Cao MM, Li YJ, Chen XX, Yu Q, Rui YF. A narrative review of the moderating effects and repercussion of exercise intervention on osteoporosis: ingenious involvement of gut microbiota and its metabolites. J Transl Med. 2022;20(1):490. doi:10.1186/s12967-022-03700-4.
- Zhou RX, Zhang YW, Cao MM, Liu CH, Rui YF, Li YJ. Linking the relation between gut microbiota and glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2023;41(2):145–162. doi:10.1007/s00774-023-01415-0.
- Zhang YW, Cao MM, Li YJ, Zhang RL, Wu MT, Yu Q, Rui YF. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Miner Metab. 2022;40(6):874–89. doi:10.1007/s00774-022-01375-x.
- Zhang YW, Cao MM, Li YJ, Lu PP, Dai GC, Zhang M, Wang H, Rui YF. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Translat. 2022;37:46–60. doi:10.1016/j.jot.2022.08.003.
- Barrea L, Muscogiuri G, Frias-Toral E, Laudisio D, Pugliese G, Castellucci B, Garcia-Velasquez E, Savastano S, Colao A. Nutrition and immune system: from the Mediterranean diet to dietary supplementary through the microbiota. Critic Rev Food Sci Nutr. 2021;61(18):3066–90. doi:10.1080/10408398.2020.1792826.
- Serrano J, Smith KR, Crouch AL, Sharma V, Yi F, Vargova V, LaMoia TE, Dupont LM, Serna V, Tang F, et al. High-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans and mice. Microbiome. 2021;9(1):11. doi:10.1186/s40168-020-00976-w.
- Li S, Hu J, Yao H, Geng F, Nie S. Interaction between four galactans with different structural characteristics and gut microbiota. Crit Rev Food Sci Nutr. 2023;63(19):3653–3663. doi:10.1080/10408398.2021.1992605.
- Mitchell CM, Mazzoni C, Hogstrom L, Bryant A, Bergerat A, Cher A, Pochan S, Herman P, Carrigan M, Sharp K, et al. Delivery mode affects stability of early infant gut microbiota. Cell Rep Med. 2020;1(9):100156. doi:10.1016/j.xcrm.2020.100156.
- Procházková N, Falony G, Dragsted LO, Licht TR, Raes J, Roager HM. Advancing human gut microbiota research by considering gut transit time. Gut. 2023;72(1):180–191. doi:10.1136/gutjnl-2022-328166.
- Jardon KM, Canfora EE, Goossens GH, Blaak EE. Dietary macronutrients and the gut microbiome: a precision nutrition approach to improve cardiometabolic health. Gut. 2022;71(6):1214–1226. doi:10.1136/gutjnl-2020-323715.
- Elmassry MM, Zayed A, Farag MA. Gut homeostasis and microbiota under attack: impact of the different types of food contaminants on gut health. Crit Rev Food Sci Nutr. 2022;62(3):738–763. doi:10.1080/10408398.2020.1828263.
- Radojević D, Bekić M, Gruden-Movsesijan A, Ilić N, Dinić M, Bisenić A, Golić N, Vučević D, Đokić J, Tomić S, et al. Myeloid-derived suppressor cells prevent disruption of the gut barrier, preserve microbiota composition, and potentiate immunoregulatory pathways in a rat model of experimental autoimmune encephalomyelitis. Gut Microbes. 2022;14(1):2127455. doi:10.1080/19490976.2022.2127455.
- Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Sci. 2016;351(6275):351. doi:10.1126/science.aad3311.
- Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Sci. 2016;351(6275):854–857. doi:10.1126/science.aad8588.
- Zuo H, Zheng T, Wu K, Yang T, Wang L, Nima Q, Bai H, Dong K, Fan Z, Huang S, et al. High-altitude exposure decreases bone mineral density and its relationship with gut microbiota: results from the China multi-ethnic cohort (CMEC) study. Environ Res. 2022;215:114206. doi:10.1016/j.envres.2022.114206.
- Ozaki D, Kubota R, Maeno T, Abdelhakim M, Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32(1):145–156. doi:10.1007/s00198-020-05728-y.
- Liu Y, Guo YL, Meng S, Gao H, Sui LJ, Jin S, Li Y, Fan SG. Gut microbiota–dependent trimethylamine N-Oxide are related with hip fracture in postmenopausal women: a matched case-control study. Aging (Albany NY). 2020;12(11):10633–41. doi:10.18632/aging.103283.
- Zhao F, Guo Z, Kwok LY, Zhao Z, Wang K, Li Y, Sun Z, Zhao J, Zhang H. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur J Nutr. 2023;62:965–76. doi:10.1007/s00394-022-03042-3.
- Lecomte M, Tomassi D, Rizzoli R, Tenon M, Berton T, Harney S, Fança-Berthon P. Effect of a hop extract standardized in 8-prenylnaringenin on bone health and gut microbiome in postmenopausal women with osteopenia: a one-year randomized, double-blind, placebo-controlled trial. Nutr. 2023;15(12):15. doi:10.3390/nu15122688.
- Rizzoli R, Biver E, Brennan-Speranza TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9(9):606–21. doi:10.1016/S2213-8587(21)00119-4.
- Tu Y, Yang R, Xu X, Zhou X. The microbiota-gut-bone axis and bone health. J Leukoc Biol. 2021;110(3):525–537. doi:10.1002/JLB.3MR0321-755R.
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. doi:10.1080/19490976.2015.1134082.
- Yan J, Takakura A, Zandi-Nejad K, Charles JF. Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes. 2018;9(1):84–92. doi:10.1080/19490976.2017.1371893.
- Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. 2016;113(47):E7554–e63. doi:10.1073/pnas.1607235113.
- Guan Z, Xuanqi Z, Zhu J, Yuan W, Jia J, Zhang C, Sun T, Leng H, Jiang C, Xu Y, et al. Estrogen deficiency induces bone loss through the gut microbiota. Pharmacol Res. 2023;196:106930. doi:10.1016/j.phrs.2023.106930.
- Wu M, Chen C, Lei H, Cao Z, Zhang C, Du R, Zhang C, Song Y, Qin M, Zhou J, et al. Dietary isoquercetin ameliorates bone loss via restoration of the gut microbiota and lipopolysaccharide-triggered inflammatory status in ovariectomy mice. J Agric Food Chem. 2023;71(43):15981–90. doi:10.1021/acs.jafc.3c00205.
- Guss JD, Horsfield MW, Fontenele FF, Sandoval TN, Luna M, Apoorva F, Lima SF, Bicalho RC, Singh A, Ley RE, et al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32(6):1343–1353. doi:10.1002/jbmr.3114.
- Dar HY, Perrien DS, Pal S, Stoica A, Uppuganti S, Nyman JS, Jones RM, Weitzmann MN, Pacifici R. Callus γδ T cells and microbe-induced intestinal Th17 cells improve fracture healing in mice. J Clin Invest. 2023;133(8). doi:10.1172/JCI166577.
- Ibáñez L, Rouleau M, Wakkach A, Blin-Wakkach C. Gut microbiome and bone. Joint Bone Spine. 2019;86(1):43–47. doi:10.1016/j.jbspin.2018.02.008.
- Whisner CM, Martin BR, Nakatsu CH, Story JA, MacDonald-Clarke CJ, McCabe LD, McCabe GP, Weaver CM. Soluble corn fiber increases calcium absorption associated with shifts in the gut microbiome: a randomized dose-response trial in free-living pubertal females. J Nutr. 2016;146(7):1298–306. doi:10.3945/jn.115.227256.
- Billington EO, Mahajan A, Benham JL, Raman M. Effects of probiotics on bone mineral density and bone turnover: a systematic review. Crit Rev Food Sci Nutr. 2023;63(19):4141–4152. doi:10.1080/10408398.2021.1998760.
- Collins FL, Rios-Arce ND, Schepper JD, Parameswaran N, McCabe LR, Britton RA, Cani PD. The potential of probiotics as a therapy for osteoporosis. Microbiol Spectr. 2017;5(4). doi:10.1128/microbiolspec.BAD-0015-2016.
- Lan H, Liu WH, Zheng H, Feng H, Zhao W, Hung WL, Li H. Bifidobacterium lactis BL-99 protects mice with osteoporosis caused by colitis via gut inflammation and gut microbiota regulation. Food Funct. 2022;13(3):1482–94. doi:10.1039/D1FO02218K.
- Yuan S, Shen J. Bacteroides vulgatus diminishes colonic microbiota dysbiosis ameliorating lumbar bone loss in ovariectomized mice. Bone. 2021;142:115710. doi:10.1016/j.bone.2020.115710.
- Lyu Z, Hu Y, Guo Y, Liu D. Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis. Bone Res. 2023;11(1):31. doi:10.1038/s41413-023-00264-x.
- Lee CS, Kim JY, Kim BK, Lee IO, Park NH, Kim SH. Lactobacillus-fermented milk products attenuate bone loss in an experimental rat model of ovariectomy-induced post-menopausal primary osteoporosis. J Appl Microbiol. 2021;130(6):2041–2062. doi:10.1111/jam.14852.
- Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307–317. doi:10.1111/joim.12805.
- Li P, Ji B, Luo H, Sundh D, Lorentzon M, Nielsen J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbio. 2022;8(1):84. doi:10.1038/s41522-022-00348-2.
- Li J, Li Y, Ivey KL, Wang DD, Wilkinson JE, Franke A, Lee KH, Chan A, Huttenhower C, Hu FB, et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men. Gut. 2022;71(4):724–733. doi:10.1136/gutjnl-2020-322473.
- Chen T, Yang CS. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: implications on health effects. Crit Rev Food Sci Nutr. 2020;60(16):2691–2709. doi:10.1080/10408398.2019.1654430.
- Farsijani S, Cauley JA, Peddada SD, Langsetmo L, Shikany JM, Orwoll ES, Ensrud KE, Cawthon PM, Newman AB. Relation between dietary protein intake and gut microbiome composition in community-dwelling older men: findings from the osteoporotic fractures in men study (MrOS). J Nutr. 2023;152(12):2877–87. doi:10.1093/jn/nxac231.
- Guo Y, Kitamoto S, Kamada N. Microbial adaptation to the healthy and inflamed gut environments. Gut Microbes. 2020;12(1):1857505. doi:10.1080/19490976.2020.1857505.
- Schoeler M, Ellero-Simatos S, Birkner T, Mayneris-Perxachs J, Olsson L, Brolin H, Loeber U, Kraft JD, Polizzi A, Martí-Navas M, et al. The interplay between dietary fatty acids and gut microbiota influences host metabolism and hepatic steatosis. Nat Commun. 2023;14(1):5329. doi:10.1038/s41467-023-41074-3.
- Gasmi A, Bjørklund G, Peana M, Mujawdiya PK, Pivina L, Ongenae A, Piscopo S, Severin B. Phosphocalcic metabolism and the role of vitamin D, vitamin K2, and nattokinase supplementation. Critic Rev Food Sci Nutr. 2022;62(25):7062–71. doi:10.1080/10408398.2021.1910481.
- Zhang YW, Cao MM, Li YJ, Dai GC, Lu PP, Zhang M, Bai LY, Chen XX, Shi L, Zhang C, et al. Dietary protein intake in relation to the risk of osteoporosis in middle-aged and older individuals: a cross-sectional study. J Nutr Health Aging. 2022;26(3):252–258. doi:10.1007/s12603-022-1748-1.
- Zeng X, Xing X, Gupta M, Keber FC, Lopez JG, Lee YCJ, Roichman A, Wang L, Neinast MD, Donia MS, et al. Gut bacterial nutrient preferences quantified in vivo. Cell. 2022;185(18):3441–56.e19. doi:10.1016/j.cell.2022.07.020.
- Humpenöder F, Bodirsky BL, Weindl I, Lotze-Campen H, Linder T, Popp A. Projected environmental benefits of replacing beef with microbial protein. Nature. 2022;605(7908):90–96. doi:10.1038/s41586-022-04629-w.
- Biwer P, Neumann-Schaal M, Henke P, Jahn D, Schulz S. Thiol metabolism and volatile metabolome of clostridioides difficile. Front Microbiol. 2022;13:864587. doi:10.3389/fmicb.2022.864587.
- Nie C, Li Y, Qian H, Ying H, Wang L. Advanced glycation end products in food and their effects on intestinal tract. Crit Rev Food Sci Nutr. 2022;62(11):3103–3115. doi:10.1080/10408398.2020.1863904.
- Tan R, Jin M, Shao Y, Yin J, Li H, Chen T, Shi D, Zhou S, Li J, Yang D, et al. High-sugar, high-fat, and high-protein diets promote antibiotic resistance gene spreading in the mouse intestinal microbiota. Gut Microbes. 2022;14(1):2022442. doi:10.1080/19490976.2021.2022442.
- Huang Z, Boekhorst J, Fogliano V, Capuano E, Wells JM. Impact of high-fiber or high-protein diet on the capacity of human gut microbiota to produce tryptophan catabolites. J Agric Food Chem. 2023;71(18):6956–66. doi:10.1021/acs.jafc.2c08953.
- White BA, Lamed R, Bayer EA, Flint HJ. Biomass utilization by gut microbiomes. Annu Rev Microbiol. 2014;68(1):279–296. doi:10.1146/annurev-micro-092412-155618.
- Neis EP, Dejong CH, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7(4):2930–2946. doi:10.3390/nu7042930.
- El-Saadony MT, Umar M, Hassan FU, Alagawany M, Arif M, Taha AE, Elnesr SS, El-Tarabily KA, Abd El-Hack ME. Applications of butyric acid in poultry production: the dynamics of gut health, performance, nutrient utilization, egg quality, and osteoporosis. Anim Health Res Rev. 2022;23(2):136–46. doi:10.1017/S1466252321000220.
- Carbone L, Bůžková P, Fink HA, Robbins JA, Barzilay JI, Elam RE, Isales C, Connelly MA, Mukamal KJ. Plasma levels of branched chain amino acids, incident hip fractures and bone mineral density of the hip and spine. J Clin Endocrinol Metab. 2023;108(11):e1358–e1364. doi:10.1210/clinem/dgad275.
- Chen H, Ye C, Wu C, Zhang J, Xu L, Wang X, Xu C, Zhang J, Guo Y, Yao Q, et al. Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. Int J Biol Sci. 2023;19(7):2097–2113. doi:10.7150/ijbs.81824.
- Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, Paul Ross R, Stanton C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. doi:10.1016/j.bbi.2016.07.145.
- Wang M, Ma LJ, Yang Y, Xiao Z, Wan JB. N-3 polyunsaturated fatty acids for the management of alcoholic liver disease: a critical review. Crit Rev Food Sci Nutr. 2019;59(sup1):S116–s29. doi:10.1080/10408398.2018.1544542.
- Robertson RC, Kaliannan K, Strain CR, Ross RP, Stanton C, Kang JX. Maternal omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota. Microbiome. 2018;6(1):95. doi:10.1186/s40168-018-0476-6.
- Takeuchi T, Kameyama K, Miyauchi E, Nakanishi Y, Kanaya T, Fujii T, Kato T, Sasaki T, Tachibana N, Negishi H, et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023;35(2):361–75.e9. doi:10.1016/j.cmet.2022.12.013.
- Zhang Z, Lin T, Meng Y, Hu M, Shu L, Jiang H, Gao R, Ma J, Wang C, Zhou X, et al. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism. 2021;119:154767. doi:10.1016/j.metabol.2021.154767.
- Lu L, Tang M, Li J, Xie Y, Li Y, Xie J, Zhou L, Liu Y, Yu X. Gut microbiota and serum metabolic signatures of high-fat-induced bone loss in mice. Front Cell Infect Microbiol. 2021;11:788576. doi:10.3389/fcimb.2021.788576.
- Eaimworawuthikul S, Tunapong W, Chunchai T, Suntornsaratoon P, Charoenphandhu N, Thiennimitr P, Chattipakorn N, Chattipakorn SC. Altered gut microbiota ameliorates bone pathology in the mandible of obese–insulin-resistant rats. Eur J Nutr. 2020;59(4):1453–62. doi:10.1007/s00394-019-02002-8.
- McCabe LR, Irwin R, Tekalur A, Evans C, Schepper JD, Parameswaran N, Ciancio M. Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone. 2019;118:20–31. doi:10.1016/j.bone.2018.03.024.
- Qiao J, Wu Y, Ren Y. The impact of a high fat diet on bones: potential mechanisms. Food Funct. 2021;12(3):963–975. doi:10.1039/D0FO02664F.
- Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Sci. 2021;374(6575):1632–1640. doi:10.1126/science.aaz7015.
- Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host & Microbe. 2018;23(6):705–715. doi:10.1016/j.chom.2018.05.012.
- Rastall RA, Diez-Municio M, Forssten SD, Hamaker B, Meynier A, Moreno FJ, Respondek F, Stah B, Venema K, Wiese M, et al. Structure and function of non-digestible carbohydrates in the gut microbiome. Benef Microbes. 2022;13(2):95–168. doi:10.3920/BM2021.0090.
- Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3(4):289–306. doi:10.4161/gmic.19897.
- Zhou T, Wang M, Ma H, Li X, Heianza Y, Qi L. Dietary fiber, genetic variations of gut microbiota-derived short-chain fatty acids, and bone health in UK biobank. J Clin Endocrinol Metab. 2021;106(1):201–210. doi:10.1210/clinem/dgaa740.
- Martiniakova M, Babikova M, Mondockova V, Blahova J, Kovacova V, Omelka R. The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients. 2022;14(3):14. doi:10.3390/nu14030523.
- Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–215. doi:10.1038/nature16504.
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Sci. 2011;334(6052):105–108. doi:10.1126/science.1208344.
- Wallace TC, Marzorati M, Spence L, Weaver CM, Williamson PS. New frontiers in fibers: innovative and emerging research on the gut microbiome and bone health. J Am Coll Nutr. 2017;36(3):218–222. doi:10.1080/07315724.2016.1257961.
- Ringe JD. Plain vitamin D or active vitamin D in the treatment of osteoporosis: where do we stand today? Arch Osteoporos. 2020;15(1):182. doi:10.1007/s11657-020-00842-0.
- Hu J, Li Y, Wang Z, Li X, Hou T, Ning Z, Huang R, Ma C, Yuan X, Wang D, et al. Association of plant-based dietary patterns with the risk of osteoporosis in community-dwelling adults over 60 years: a cross-sectional study. Osteoporos Int. 2023;34(5):915–923. doi:10.1007/s00198-023-06700-2.
- Shan Z, Wang F, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, Rexrode KM, Rimm EB, Qi L, et al. Healthy eating patterns and risk of total and cause-specific mortality. JAMA Intern Med. 2023;183(2):142–153. doi:10.1001/jamainternmed.2022.6117.
- Wallace TC, Bailey RL, Lappe J, O’Brien KO, Wang DD, Sahni S, Weaver CM. Dairy intake and bone health across the lifespan: a systematic review and expert narrative. Crit Rev Food Sci Nutr. 2021;61(21):3661–707. doi:10.1080/10408398.2020.1810624.
- Yang M, Qi X, Li N, Kaifi JT, Chen S, Wheeler AA, Kimchi ET, Ericsson AC, Rector RS, Staveley-O’Carroll KF, et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat Commun. 2023;14(1):228. doi:10.1038/s41467-023-35861-1.
- Biver E, Herrou J, Larid G, Legrand MA, Gonnelli S, Annweiler C, Chapurlat R, Coxam V, Fardellone P, Thomas T, et al. Dietary recommendations in the prevention and treatment of osteoporosis. Joint Bone Spine. 2023;90(3):105521. doi:10.1016/j.jbspin.2022.105521.
- Lomax TM, Ashraf S, Yilmaz G, Harmancey R. Loss of uncoupling protein 3 attenuates Western diet–induced obesity, systemic inflammation, and insulin resistance in rats. Obesity (Silver Spring). 2020;28(9):1687–97. doi:10.1002/oby.22879.
- Di Giosia P, Stamerra CA, Giorgini P, Jamialahamdi T, Butler AE, Sahebkar A. The role of nutrition in inflammaging. Ageing Res Rev. 2022;77:101596. doi:10.1016/j.arr.2022.101596.
- Kimble R, Gouinguenet P, Ashor A, Stewart C, Deighton K, Matu J, Griffiths A, Malcomson FC, Joel A, Houghton D, et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: a systematic review of randomized controlled trials and observational studies. Crit Rev Food Sci Nutr. 2022;63(27):8698–8719. doi:10.1080/10408398.2022.2057416.
- Wang DD, Nguyen LH, Li Y, Yan Y, Ma W, Rinott E, Ivey KL, Shai I, Willett WC, Hu FB, et al. The gut microbiome modulates the protective association between a mediterranean diet and cardiometabolic disease risk. Nat Med. 2021;27(2):333–343. doi:10.1038/s41591-020-01223-3.
- Rinott E, Meir AY, Tsaban G, Zelicha H, Kaplan A, Knights D, Tuohy K, Scholz MU, Koren O, Stampfer MJ, et al. The effects of the green-mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 2022;14(1):29. doi:10.1186/s13073-022-01015-z.
- Kahleova H, Sutton M, Maracine C, Nichols D, Monsivais P, Holubkov R, Barnard ND. Vegan diet and food costs among adults with overweight: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6(9):e2332106. doi:10.1001/jamanetworkopen.2023.32106.
- Yu Y, Li X, Zheng M, Zhou L, Zhang J, Wang J, Sun B. The potential benefits and mechanisms of protein nutritional intervention on bone health improvement. Crit Rev Food Sci Nutr. 2023:1–15. doi:10.1080/10408398.2023.2168250.
- He W, Xie Z, Thøgersen R, Rasmussen MK, Zachariassen LF, Jørgensen NR, Nørgaard JV, Andersen HJ, Nielsen DS, Hansen AK, et al. Effects of calcium source, inulin, and lactose on gut-bone associations in an ovarierectomized rat model. Mol Nutr Food Res. 2022;66(8):e2100883. doi:10.1002/mnfr.202100883.
- Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Ando F, Yano M. Bone mineral density in post-menopausal female subjects is associated with serum antioxidant carotenoids. Osteoporos Int. 2008;19(2):211–219. doi:10.1007/s00198-007-0457-2.
- Mohammadisima N, Farshbaf-Khalili A, Ostadrahimi A, Pourmoradian S. Positive relation between dietary inflammatory index and osteoporosis in postmenopausal women. Int J Vitam Nutr Res. 2022. doi:10.1024/0300-9831/a000773.
- Zhao S, Gao W, Li J, Sun M, Fang J, Tong L, He Y, Wang Y, Zhang Y, Xu Y, et al. Dietary inflammatory index and osteoporosis: the National health and nutrition examination survey, 2017–2018. Endocrine. 2022;78(3):587–96. doi:10.1007/s12020-022-03178-6.
- Wang L, Ye C, Zhao F, Wu H, Wang R, Zhang Z, Li J. Association between the dietary inflammatory index and the risk of fracture in Chinese adults: longitudinal study. JMIR Public Health Surveill. 2023;9:e43501. doi:10.2196/43501.
- Maldonado-Contreras A, Noel SE, Ward DV, Velez M, Mangano KM. Associations between diet, the gut microbiome, and short-chain fatty acid production among older caribbean latino adults. J Acad Nutr Diet. 2020;120(12):2047–60.e6. doi:10.1016/j.jand.2020.04.018.
- Xu X, Ding J, Wu X, Huang Z, Kong G, Liu Q, Yang, Z., Huang, Z., Zhu, Q. Bone microstructure and metabolism changes under the combined intervention of ketogenic diet with intermittent fasting: an in vivo study of rats. Exp Anim. 2019;68(3):371–380. doi:10.1538/expanim.18-0084.
- Liu Q, Xu X, Yang Z, Liu Y, Wu X, Huang Z, Liu J, Huang Z, Kong G, Ding J, et al. Metformin alleviates the bone loss induced by ketogenic diet: an in vivo study in mice. Calcif Tissue Int. 2019;104(1):59–69. doi:10.1007/s00223-018-0468-3.
- Dahlin M, Singleton SS, David JA, Basuchoudhary A, Wickström R, Mazumder R, Prast-Nielsen S. Higher levels of bifidobacteria and tumor necrosis factor in children with drug-resistant epilepsy are associated with anti-seizure response to the ketogenic diet. EBioMedicine. 2022;80:104061. doi:10.1016/j.ebiom.2022.104061.
- Jang YJ, Kim WK, Han DH, Lee K, Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10(6):696–711. doi:10.1080/19490976.2019.1589281.
- Shin S, Joung H. A dairy and fruit dietary pattern is associated with a reduced likelihood of osteoporosis in Korean postmenopausal women. Br J Nutr. 2013;110(10):1926–1933. doi:10.1017/S0007114513001219.
- Park H, Lee M, Jeong D, Park S, Ji Y, Todorov SD, Holzapfel WH. Safety evaluation and in vivo strain-specific functionality of bacillus strains isolated from Korean traditional fermented foods. Probiot Antimicrob Proteins. 2021;13(1):60–71. doi:10.1007/s12602-020-09672-5.
- Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. 2021;106(1):64–79. doi:10.1210/clinem/dgaa644.
- Matison AP, Mather KA, Flood VM, Reppermund S. Associations between nutrition and the incidence of depression in middle-aged and older adults: a systematic review and meta-analysis of prospective observational population-based studies. Ageing Res Rev. 2021;70:101403. doi:10.1016/j.arr.2021.101403.
- Zeng FF, Wu BH, Fan F, Xie HL, Xue WQ, Zhu HL, Chen YM. Dietary patterns and the risk of hip fractures in elderly Chinese: a matched case-control study. J Clin Endocrinol Metab. 2013;98(6):2347–55. doi:10.1210/jc.2013-1190.
- van den Berg FF, van Dalen D, Hyoju SK, van Santvoort HC, Besselink MG, Wiersinga WJ, Zaborina O, Boermeester MA, Alverdy J. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut. 2021;70(5):915–27. doi:10.1136/gutjnl-2019-320430.
- Norde MM, Collese TS, Giovannucci E, Rogero MM. A posteriori dietary patterns and their association with systemic low-grade inflammation in adults: a systematic review and meta-analysis. Nutr Rev. 2021;79(3):331–350. doi:10.1093/nutrit/nuaa010.
- Las Heras V, Melgar S, MacSharry J, Gahan CGM. The influence of the Western diet on microbiota and gastrointestinal immunity. Annu Rev Food Sci Technol. 2022;13(1):489–512. doi:10.1146/annurev-food-052720-011032.
- Turpin W, Dong M, Sasson G, Raygoza Garay JA, Espin-Garcia O, Lee SH, Neustaeter A, Smith MI, Leibovitzh H, Guttman DS, et al. Mediterranean-like dietary pattern associations with gut microbiome composition and subclinical gastrointestinal inflammation. Gastroenterol. 2022;163(3):685–698. doi:10.1053/j.gastro.2022.05.037.
- Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, Giacco R, Mennella I, Ferracane R, Pons N, et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69(7):1258–1268. doi:10.1136/gutjnl-2019-320438.
- Clark JS, Simpson BS, Murphy KJ. The role of a mediterranean diet and physical activity in decreasing age-related inflammation through modulation of the gut microbiota composition. Br J Nutr. 2022;128(7):1299–1314. doi:10.1017/S0007114521003251.
- Zhu C, Sawrey-Kubicek L, Beals E, Rhodes CH, Houts HE, Sacchi R, Zivkovic AM. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and mediterranean diet in 4 days: a pilot study. Nutr Res. 2020;77:62–72. doi:10.1016/j.nutres.2020.03.005.
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, et al. High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. doi:10.1136/gutjnl-2015-309957.
- Trefflich I, Jabakhanji A, Menzel J, Blaut M, Michalsen A, Lampen A, Abraham K, Weikert C. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit Rev Food Sci Nutr. 2020;60(17):2990–3004. doi:10.1080/10408398.2019.1676697.
- Xie HL, Wu BH, Xue WQ, He MG, Fan F, Ouyang WF, Tu SL, Zhu HL, Chen YM. Greater intake of fruit and vegetables is associated with a lower risk of osteoporotic hip fractures in elderly Chinese: a 1: 1 matched case–control study. Osteoporos Int. 2013;24(11):2827–36. doi:10.1007/s00198-013-2383-9.
- Seel W, Reiners S, Kipp K, Simon MC, Dawczynski C. Role of dietary fiber and energy intake on gut microbiome in vegans, vegetarians, and flexitarians in comparison to omnivores—insights from the nutritional evaluation (NuEva) study. Nutr. 2023;15(8):15. doi:10.3390/nu15081914.
- Zhang C, Björkman A, Cai K, Liu G, Wang C, Li Y, Xia H, Sun L, Kristiansen K, Wang J, et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908. doi:10.3389/fimmu.2018.00908.
- Orchard T, Yildiz V, Steck SE, Hébert JR, Ma Y, Cauley JA, Li W, Mossavar-Rahmani Y, Johnson KC, Sattari M, et al. Dietary inflammatory index, bone mineral density, and risk of fracture in postmenopausal women: results from the women’s health initiative. J Bone Miner Res. 2017;32(5):1136–1146. doi:10.1002/jbmr.3070.
- Ruiz-Saavedra S, Salazar N, Suárez A, de Los Reyes-Gavilán CG, Gueimonde M, González S. Comparison of different dietary indices as predictors of inflammation, oxidative stress and intestinal microbiota in middle-aged and elderly subjects. Nutrients. 2020;12(12):12. doi:10.3390/nu12123828.
- Zheng J, Hoffman KL, Chen JS, Shivappa N, Sood A, Browman GJ, Dirba DD, Hanash S, Wei P, Hebert JR, et al. Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr. 2020;124(9):931–942. doi:10.1017/S0007114520001853.
- Garofalo V, Barbagallo F, Cannarella R, Calogero AE, La Vignera S, Condorelli RA. Effects of the ketogenic diet on bone health: a systematic review. Front Endocrinol (Lausanne). 2023;14:1042744. doi:10.3389/fendo.2023.1042744.
- Jiang Z, Wang X, Zhang H, Yin J, Zhao P, Yin Q, Wang Z. Ketogenic diet protects MPTP-induced mouse model of Parkinson’s disease via altering gut microbiota and metabolites. MedComm. 2023;4(3):e268. doi:10.1002/mco2.268.
- Movassagh EZ, Vatanparast H. Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr. 2017;8(1):1–16. doi:10.3945/an.116.013326.
- Das G, Heredia JB, de Lourdes Pereira M, Coy-Barrera E, Rodrigues Oliveira SM, Gutiérrez-Grijalva EP, Cabanillas-Bojórquez LA, Shin HS, Patra JK. Korean traditional foods as antiviral and respiratory disease prevention and treatments: a detailed review. Trends Food Sci Technol. 2021;116:415–33. doi:10.1016/j.tifs.2021.07.037.
- Wu X, Unno T, Kang S, Park S. A Korean-style balanced diet has a potential connection with ruminococcaceae enterotype and reduction of metabolic syndrome incidence in Korean adults. Nutrients. 2021;13(2):495. doi:10.3390/nu13020495.
- Saji N, Tsuduki T, Murotani K, Hisada T, Sugimoto T, Kimura A, Niida S, Toba K, Sakurai T. Relationship between the Japanese-style diet, gut microbiota, and dementia: a cross-sectional study. Nutrition. 2022;94:111524. doi:10.1016/j.nut.2021.111524.
- Wan MLY, Co VA, El-Nezami H. Dietary polyphenol impact on gut health and microbiota. Crit Rev Food Sci Nutr. 2021;61(4):690–711. doi:10.1080/10408398.2020.1744512.
- Kong CY, Li ZM, Chen HL, Mao YQ, Han B, Guo JJ, Wang LS. An energy-restricted diet including yogurt, fruit, and vegetables alleviates high-fat diet–induced metabolic syndrome in mice by modulating the gut microbiota. J Nutr. 2022;152(11):2429–40. doi:10.1093/jn/nxac181.
- Haji-Ghazi Tehrani L, Mousavi SN, Chiti H, Afshar D. Effect of Atkins versus a low-fat diet on gut microbiota, and cardiometabolic markers in obese women following an energy-restricted diet: randomized, crossover trial. Nutr Metab Cardiovasc Dis. 2022;32(7):1734–1741. doi:10.1016/j.numecd.2022.04.007.
- Netto Cândido TL, da Silva LE, Cândido FG, Valente FX, da Silva JS, Gomes Lopes DR, Do Carmo Gouveia Peluzio M, Mantovani HC, de Cássia Gonçalves Alfenas R. Effect of the ingestion of vegetable oils associated with energy-restricted normofat diet on intestinal microbiota and permeability in overweight women. Food Res Int. 2021;139:109951. doi:10.1016/j.foodres.2020.109951.
- Xie S, Guan C, Huang T, Liu Y, Yuan F, Xu D. Intermittent fasting promotes repair of rotator cuff injury in the early postoperative period by regulating the gut microbiota. J Orthop Translat. 2022;36:216–24. doi:10.1016/j.jot.2022.09.006.
- Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21(4):236–247. doi:10.1038/s41579-022-00805-x.
- Cronin O, Lanham-New SA, Corfe BM, Gregson CL, Darling AL, Ahmadi KR, Gibson PS, Tobias JH, Ward KA, Traka MH, et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis. Calcif Tissue Int. 2022;110(3):273–284. doi:10.1007/s00223-021-00924-2.
- Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13(1):1–24. doi:10.1080/19490976.2021.1897212.
- Perler BK, Friedman ES, Wu GD. The role of the gut microbiota in the relationship between diet and human health. Annu Rev Physiol. 2023;85(1):449–468. doi:10.1146/annurev-physiol-031522-092054.
- Campaniello D, Corbo MR, Sinigaglia M, Speranza B, Racioppo A, Altieri C, Bevilacqua A. How diet and physical activity modulate gut microbiota: evidence, and perspectives. Nutrients. 2022;14(12):14. doi:10.3390/nu14122456.
- Sheflin AM, Melby CL, Carbonero F, Weir TL. Linking dietary patterns with gut microbial composition and function. Gut Microbes. 2017;8(2):113–129. doi:10.1080/19490976.2016.1270809.
- Wang P, Song M, Eliassen AH, Wang M, Fung TT, Clinton SK, Rimm EB, Hu FB, Willett WC, Tabung FK, et al. Optimal dietary patterns for prevention of chronic disease. Nat Med. 2023;29(3):719–728. doi:10.1038/s41591-023-02235-5.
- Malinowska AM, Kok DE, Steegenga WT, Hooiveld G, Chmurzynska A. Human gut microbiota composition and its predicted functional properties in people with western and healthy dietary patterns. Eur J Nutr. 2022;61(8):3887–3903. doi:10.1007/s00394-022-02928-6.
- Fabiani R, Naldini G, Chiavarini M. Dietary patterns in relation to low bone mineral density and fracture risk: a systematic review and meta-analysis. Adv Nutr. 2019;10(2):219–36. doi:10.1093/advances/nmy073.
- Wang L, Wang F, Xiong L, Song H, Ren B, Shen X. A nexus of dietary restriction and gut microbiota: recent insights into metabolic health. Crit Rev Food Sci Nutr. 2023:1–23. doi:10.1080/10408398.2023.2202750.
- Ruigrok R, Weersma RK, Vich Vila A. The emerging role of the small intestinal microbiota in human health and disease. Gut Microbes. 2023;15(1):2201155. doi:10.1080/19490976.2023.2201155.
- Yu L, Gao Y, Ye Z, Duan H, Zhao J, Zhang H, Narbad A, Tian F, Zhai Q, Chen W, et al. Interaction of beta-glucans with gut microbiota: dietary origins, structures, degradation, metabolism, and beneficial function. Crit Rev Food Sci Nutr. 2023:1–26. doi:10.1080/10408398.2023.2217727.
- Chung E, Elmassry MM, Cao JJ, Kaur G, Dufour JM, Hamood AN, Shen CL. Beneficial effect of dietary geranylgeraniol on glucose homeostasis and bone microstructure in obese mice is associated with suppression of proinflammation and modification of gut microbiome. Nutr Res. 2021;93:27–37. doi:10.1016/j.nutres.2021.07.001.
- Zhang J, Yu H, Wang Q, Cai H, Shen F, Ruan S, Wu Y, Liu T, Feng F, Zhao M, et al. Dietary additive octyl and decyl glycerate modulates metabolism and inflammation under different dietary patterns with the contribution of the gut microbiota. Food Funct. 2023;14(1):525–540. doi:10.1039/D2FO03059D.
- Rautava S, Selma-Royo M, Oksanen T, Collado MC, Isolauri E. Shifting pattern of gut microbiota in pregnant women two decades apart – an observational study. Gut Microbes. 2023;15(1):2234656. doi:10.1080/19490976.2023.2234656.
- Bernabeu M, Gharibzahedi SMT, Ganaie AA, Macha MA, Dar BN, Castagnini JM, Garcia-Bonillo C, Meléndez-Martínez AJ, Altintas Z, Barba FJ, et al. The potential modulation of gut microbiota and oxidative stress by dietary carotenoid pigments. Crit Rev Food Sci Nutr. 2023:1–19. doi:10.1080/10408398.2023.2254383.
- Fernández-Murga ML, Olivares M, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reverses the adverse effects of diet-induced obesity through the gut-bone axis. Bone. 2020;141:115580. doi:10.1016/j.bone.2020.115580.
- Shimizu Y. Gut microbiota in common elderly diseases affecting activities of daily living. World J Gastroenterol. 2018;24(42):4750–4758. doi:10.3748/wjg.v24.i42.4750.