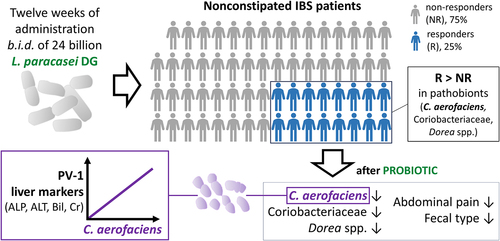
ABSTRACT
Probiotics are exploited for adjuvant treatment in IBS, but reliable guidance for selecting the appropriate probiotic to adopt for different forms of IBS is lacking. We aimed to identify markers for recognizing non-constipated (NC) IBS patients that may show significant clinical improvements upon treatment with the probiotic strain Lacticaseibacillus paracasei DG (LDG). To this purpose, we performed a post-hoc analysis of samples collected during a multicenter, double-blind, parallel-group, placebo-controlled trial in which NC-IBS patients were randomized to receive at least 24 billion CFU LDG or placebo capsules b.i.d. for 12 weeks. The primary clinical endpoint was the composite response based on improved abdominal pain and fecal type. The fecal microbiome and serum markers of intestinal (PV1 and zonulin), liver, and kidney functions were investigated. We found that responders (R) in the probiotic arm (25%) differed from non-responders (NR) based on the abundance of 18 bacterial taxa, including the families Coriobacteriaceae, Dorea spp. and Collinsella aerofaciens, which were overrepresented in R patients. These taxa also distinguished R (but not NR) patients from healthy controls. Probiotic intervention significantly reduced the abundance of these bacteria in R, but not in NR. Analogous results emerged for C. aerofaciens from the analysis of data from a previous trial on IBS with the same probiotic. Finally, C. aerofaciens was positively correlated with the plasmalemmal vesicle associated protein-1 (PV-1) and the markers of liver function. In conclusion, LDG is effective on NC-IBS patients with NC-IBS with a greater abundance of potential pathobionts. Among these, C. aerofaciens has emerged as a potential predictor of probiotic efficacy.
Introduction
Irritable bowel syndrome (IBS) is a common disorder of gut-brain interaction in which recurrent abdominal pain is associated with defecation or a change in bowel habits.Citation1 IBS is a complex and multifactorial condition that may be associated with several potential factors and mechanisms, such as altered intestinal serotonin level and metabolism,Citation2,Citation3 decreased density of peptide YY (PYY) cells in the colon,Citation4,Citation5 high levels of histamine and elevated number of mast cells in intestinal mucosa,Citation6 low-grade mucosal inflammation,Citation7 compromised epithelial barrierCitation8 and altered gut microbiome,Citation9 which can lead to low-grade mucosal inflammation with visceral hypersensitivity.Citation7 However, these aspects can variably and not simultaneously contribute to the symptomatology of IBS patients, which could be hypothetically stratified into a wider number of IBS forms that are currently unknown. According to Rome IV criteria, IBS is subdivided into categories according to bowel habits, including IBS with predominant constipation (IBS-C), IBS with predominant diarrhea (IBS-D), and IBS with mixed bowel habit (IBS-M).Citation10 Ongoing research aims to unravel the complexities of IBS behind this classification and develop targeted therapies to address these underlying mechanisms.
Various therapeutic options for IBS target the underlying pathophysiological aspects of the condition. Unfortunately, no single approach can effectively address this disorder’s diverse manifestations simultaneously. The most common approaches include anti-pain medications (e.g., antispasmodics and neuromodulators), dietary pattern modification (e.g., low-FODMAP diet), psychological intervention, antibiotics (e.g., rifaximin), opioid receptor agonists (e.g., eluxadoline), antagonists of serotonin receptors (e.g., alosetron, ondansetron, ramosetron), secretagogues (e.g., linaclotide), and other medications. In addition, since there is a growing body of evidence indicating the implication of altered intestinal microbiota in IBS,Citation11 supplementation with probiotics and/or prebiotics is often exploited as adjuvant treatment.Citation12,Citation13
Although specific microbial strains have shown promising results,Citation14 meta-analyses have reported inconsistent findings concerning the efficacy of probiotics for various IBS outcome measures, potentially due to wide variability among the probiotic formulations considered [differences in microbial strain(s), dosage, and administration protocol] and pathophysiological differences in IBS subtypesCitation15. Reliable guidelines for selecting appropriate probiotic strains and/or formulations for treating the different forms of IBS are currently lacking.
Lacticaseibacillus paracasei DG is a probiotic bacterium experimentally proven to survive gastrointestinal transit in adultsCitation16 and children,Citation17 to modify the composition of the intestinal microbial ecosystem in healthy adults,Citation18 and to modulate immune responses in different intestinal conditions.Citation19 In addition, a multicenter, randomized, double-blind, crossover, placebo-controlled pilot trial (PROBE-IBS/1; ClinicalTrials.gov Identifier: NCT02371499) showed that strain DG (at least 24 billion CFU/capsule, two capsules per day) induced a significant reduction in Ruminococcus spp., a significant increase in fecal acetate and butyrate, and a significant reduction in the pro-inflammatory cytokine interleukin-15 when administered to IBS patients.Citation20 The results of the PROBE-IBS/1 trial suggested that the clinical efficacy of L. paracasei DG could be greater in patients with IBS-D and IBS-M and encouraged the conduct of a larger study (named PROBE-IBS/2) to assess the effect of EnterolactisⓇ PLUS capsules (a single-strain probiotic formulation containing at least 24 billion CFUs of L. paracasei DG) on abdominal symptoms in non-constipated IBS (NC-IBS) patients without constipation. Here, we present the results of a post-hoc analysis of fecal and serum samples collected from NC-IBS patients in the probiotic arm during the PROBE-IBS/2 study with the aim of identifying potential markers distinguishing the population of patients who achieved the primary clinical endpoint of the trial (responders; R) from non-responders (NR). These results suggest that R patients are characterized by an increased abundance of potential pathobionts, which can be mechanistically linked to the onset of IBS symptoms and can be reduced by intake of the probiotic bacterium L. paracasei DG.
Results
Characteristics of non-constipated IBS patients according to clinical responsiveness
According to the primary endpoint of the PROBE-IBS/2 trial (i.e., composite response over 12 weeks), 16 patients with NC-IBS were considered responders (R: 25.4%). At baseline, R patients were not significantly different from non-responders (NR) patients (n = 47; 74.6%) in terms of age, abdominal pain, fecal type, fecal organic acids, serum markers for intestinal permeability and functioning [i.e., citrulline, plasmalemmal vesicle associated protein-1 (PV-1), and zonulin], and liver and kidney function blood markers (). In contrast, the mean abdominal pain calculated during 12 weeks of treatment was significantly lower in the R group than in the NR group. As expected, abdominal pain and fecal type calculated during the 12-week probiotic intervention period were significantly lower than those during the 2-week run-in (baseline) period only in the R group. In contrast, probiotic treatment did not significantly affect the concentrations of organic acids in feces and serum markers ().
Table 1. Characteristics of the non-constipated IBS patients considered in this study. Mean values ± standard deviation for each parameter are reported.
The fecal abundance of Collinsella aerofaciens and other bacterial taxa may distinguish responder from non-responder NC-IBS patients
R and NR NC-IBS patients were compared regarding fecal bacterial taxonomic structure at baseline (i.e., immediately before the beginning of the 12-week probiotic intake period; V2 in ). Alpha- and beta-diversity analyses did not differentiate between the R and NR groups (not shown). In contrast, we found 18 bacterial taxa that were differentially represented between the two groups (). Specifically, 13 taxa were overrepresented in the R group, including the following Gram-positive taxa belonging to the order Eubacteriales (formerly Clostridiales), genus Dorea, and species Blautia wexlerae, Dorea formicigenerans, Dorea longicatena, and Ruminococcus bromii. In addition, the abundance of the Actinobacteria species Collinsella aerofaciens and a bacterial unit of the Streptococcus thermophilus/salivarius taxonomic group increased in R patients at baseline, together with the Gram-negative class α-Proteobacteria and the Actinobacteria family Coriobacteriaceae. In contrast, only five bacterial taxa were overrepresented in the NR group, including Eggerthella lenta (class Coriobateriia), an undefined Bacteroides species, and Bilophila wadsworthia (class δ-Proteobacteria) (). Subsequently, the fecal abundances of the bacteria found to be significantly different between R and NR patients were used in a PLS discriminant analysis (PLSDA). The resulting score plot significantly separated R patients from NR patients (; Supplementary Figure S1). In addition, the PLSDA loading plot and contribution to component (CC) analysis revealed that 11 taxa contributed significantly to explaining the variability in the PLSDA model reported in : ten taxa for component 1 and two taxa for component 2, including Dorea formicigenerans (cASV0022), which contributed significantly to both components. In particular, the most relevant contributions to component 1 were provided by Collinsella aerofaciens cASV 0011 (CC = 0.95), the whole species C. aerofaciens (CC = 0.81), Ruminococcus bromii cASV 0091 (CC = 0.86), the family Coriobacteriaceae (CC = 0.95), the genus Dorea (CC = 0.79), and undefined Clostridiaceae/Peptostreptococcaceae species (represented mainly by reads ascribed to the Peptostreptococcaceae species Terrisporobacter petrolearius; CC = 0.89) (). These six taxa included the bacterial groups that discriminated R from NR, with the highest abundance in the fecal samples (see heatmap in ), viz., Coriobacteriaceae, Dorea spp., and Collinsella aerofaciens. Notably, the abundance of these bacterial taxa was higher in the R group than in the 100 healthy controls ().
Figure 1. Study scheme (a) and summary of the IBS patients that concluded the PROBE-IBS/2 trial per protocol (PP) in the probiotic arm included in this study (b). NRS, numeric rating scale.

Figure 2. Fecal bacterial taxa distinguishing responder (R) from non-responder (NR) non-constipated IBS patients. P values, derived from Mann-Whitney tests on CLR-transformed bacterial abundances, are color-coded with red indicating taxa increased in the R group. The heatmap displays mean CLR-transformed abundances of reported taxonomic units. Taxonomic lineage is denoted: p, phylum; c, class; o, order; f, family; g, genus; s, species. Corrections to GreenGenes database nomenclature, based on NCBI Taxonomy, are shown in violet. Taxonomic names in blue were determined via manual BLASTN search in GenBank using corresponding read sequences. The histogram on the right illustrates the contribution of each bacterial taxon to the first two components of the PLSDA biplot in , with Roman numerals linking taxa to the PLSDA loading plot. Bold text highlights the 11 bacterial taxa significantly contributing to variability in the PLSDA analysis.

Figure 3. Biplot of PLS discriminant analysis (PLSDA) with prediction background for responder (R) and non-responder (NR) non-constipated IBS patients (panel a) and for R, NR, and healthy controls (HC) (panel b). Roman numerals in panel a refer to bacterial taxa in figure 2. The percentages indicate the explained variation at each axis. The Receiver Operating Characteristic (ROC) curves of the PLSDA model are shown in supplementary Figure S1.

Figure 4. Dot plot of the most abundant bacterial taxa found to better discriminate between responders and non-responder patients in the PROBE-IBS/2 trial. HC, healthy controls (n = 100); R and NR, responder and non-responder NC-IBS patients in the PROBE-IBS/2 trial, respectively; other NC-IBS, other non-constipated IBS patients recruited at baseline during the PROBE-IBS/2 trial (n = 161); statistics is according to Mann-Whitney test; *, P < .05; **, P < .01.

Subsequently, we performed PLSDA with the fecal abundances of the bacteria distinguishing R and NR patients, including the fecal microbiome data of the 100 healthy controls. Interestingly, this analysis revealed that R samples could be distinguished better from the other samples than NR samples from controls (0.91 prediction accuracy for R toward NR + controls; ; Supplementary Figure S1).
The probiotic intervention reduced C. aerofaciens in responder patients
Subsequently, we carried out LEfSe analysis on CLR-transformed taxonomic data using paired statistics to identify the bacterial taxa significantly modified by the probiotic intervention between the time points V2 and V4 in three groups of patients: R, NR, and R+NR. The taxon that increased more after probiotic intake in all three groups of samples was cASV 0254, which was ascribed to the Lacticaseibacillus casei group of species and plausibly corresponds to the probiotic strain administered in this study (L. paracasei DG). The results also revealed that probiotic intake induced a significant reduction in bacterial taxa, mainly in the R group (n = 29) compared to the NR (n = 13) and R+NR (n = 14) groups. In contrast, most of the taxa that significantly increased after probiotic intake were in the R+NR group (n = 25) compared to those in the NR (n = 16) and R (n = 8) groups ( and Supplementary Figure S2). In R patients, a substantial reduction was observed in the majority of altered bacterial taxonomic units after probiotic administration. The affected taxa were predominantly affiliated with the order Eubacteriales, encompassing 24 out of 29 identified taxa. Notable species within this order included Anaerobutyricum hallii, Blautia obeum, Blautia wexlerae, Dorea formicigenerans, and Ruminococcus bromii. Notably, probiotic intervention induced a significant reduction in several taxonomic units reported above to contribute to the distinction between R and NR, viz. Collinsella aerofaciens (cASV 0011), Blautia wexlerae (cASV 0020), Dorea formicigenerans (cASV 0022), the genus Dorea, and the family Coriobacteriaceae (). These taxa were not affected by probiotic intervention in NR patients (Supplementary Figure S2). Furthermore, qPCR experiments conducted on fecal DNA, utilizing species-specific primers, corroborated the heightened abundance of C. aerofaciens in R patients compared to NR counterparts. Importantly, the probiotic intervention reduced C. aerofaciens abundance in R patients (Supplementary Figure S3).
Figure 5. Linear discriminant analysis (LDA) effect size (LEfSe) graphics for responders (R) NC-IBS patients in the probiotic arm. LDA scores indicate taxa significantly (P < 0.05) higher before (V2; negative LDA) or after (V4; positive LDA) probiotic intake. Taxon levels are abbreviated: p, phylum; c, class; o, order; f, family; g, genus; and s, species. Corrections/updates to GreenGenes database nomenclature are marked in violet, while taxonomic names determined through a manual BLASTN search in GenBank using corresponding read sequences are highlighted in blue. Taxon cAVS 254 is identified as the administered probiotic strain (L. paracasei DG; highlighted in green).

The analysis of a previous cohort of patients confirmed that L. paracasei DG intake may reduce C. aerofaciens and abdominal pain in non-constipated IBS
The results described above suggest that the fecal abundance of C. aerofaciens can predict the clinical efficacy of probiotic treatment with L. paracasei DG. To preliminarily confirm this hypothesis, we reconsidered the data of a previous clinical study, which was carried out using the same probiotic formulation (EnterolactisⓇ PLUS) in a population of IBS patients (n = 40; PROBE-IBS/1; multicenter, randomized, double-blind, cross-over, 18-week, placebo-controlled, pilot trial;Citation20 Analysis of the entire population of data (comprehensive IBS-C, IBS-U, IBS-M, and IBS-D) did not reveal any significant changes in C. aerofaciens abundance upon probiotic administration. Nonetheless, when the analysis was performed exclusively in IBS-D and IBS-M patients (n = 17), we observed a significant decrease in the abundance of C. aerofaciens (). Notably, compared to placebo intake, L. paracasei DG administration not only decreased C. aerofaciens abundance but also induced a significant reduction in abdominal pain [P < .01, according to non-parametric repeated measure ANOVA-Type Statistic (RM-ATS) (]. In contrast, abdominal pain did not change significantly in patients with IBS-C and IBM-U (P = 0.34 according to RM-ATS). Finally, when we removed the participants from the analysis to the PROBE-IBS/1 that had an increase in the levels of C. aerofaciens, abdominal pain significantly decreased in the population of patients independent of IBS type (n = 16; P = .027), whereas abdominal pain did not change significantly in the other patients (n = 14; P = .540) (not shown), suggesting that the variation in C. aerofaciens can be inversely associated with the change in abdominal pain in IBS.
Figure 6. Analysis of data collected during the PROBE-IBS/1 cross-over trial. (a) fecal abundance of the bacterial species collinsella aerofaciens in IBS patients before and after the intake of the probiotic product. IBS-CU, constipated and undefined IBS patients; IBS-DM, diarrhea or mixed IBS patients. Statistics is according to Wilcoxon signed-rank test; *, P < .05; n.S., nonsignificant (P > .05). (b) fecal abundance of C. aerofaciens (on the left) and abdominal pain/discomfort (on the right) in IBS-DM patients during the PROBE-IBS/1 trial. NRS, numeric rating scale used to measure abdominal pain/discomfort. Statistics is according to non-parametric repeated measure ANOVA-Type statistic (RM-ATS). The reported P values indicate significant Time×Treatment interaction. **, P < .01; *, P < .05.

The fecal abundance of C. aerofaciens is associated to PV-1 serum levels
In subsequent experiments, we performed a correlation analysis between the fecal abundance of bacterial taxa and serum markers of intestinal function and permeability, viz. citrulline, PV-1, and zonulin. Correlation analysis was first performed with all available data collected from PROBE-IBS/2 participants at baseline (n = 191). For baseline measurements, serum samples were collected at V1, that is, 2 weeks before the collection of fecal samples (V2; see the study scheme in ). Blood and fecal samples were collected on the same day at the end of the probiotic intervention (visit V4). To avoid possible errors due to collection time discrepancy (V1 for blood and V2 for feces), correlation analysis was performed with data from samples collected at V4 (n = 177). The results revealed a significant correlation between serum parameters and numerous bacterial taxa (n = 88 at V1/V2 and n = 123 at V4; Supplementary Figure S4). Nonetheless, only nine taxa displayed the same significant associations at V1/V2 and V4 time points. Specifically, we found significant correlations with serum zonulin levels for Lactobacillus gasseri (cASV 0423), Mediterraneibacter torques (cASV 0191) (positive correlation), and Paratractidigestivibacter faecalis (cASV 349) (negative correlation). In addition, three taxa were found to correlate with citrulline: Comamonas testosteroni (cASV 287), Proteus mirabilis (cASV 287) (negative correlation), and the class Mollicutes (positive correlation) (). Notably, the other three taxa were the same as those reported above among the four bacterial groups that better discriminated between R and NR patients (i.e., C. aerofaciens cASV 0011, R. bromii cASV 0091, and the family Coriobacteriaceae), all of which showed a significant positive correlation with the serum marker PV-1 ().
Figure 7. Correlation analyses between bacterial taxa and serum markers for permeability (panel a) and liver and kidney functionality (panel b). In panel a, correlation analyses were conducted with data from blood samples collected before run-in (visit V1) and fecal samples collected before the probiotic intervention (visit V2), as well as with data from blood and fecal samples collected at the end of the probiotic intervention (visit V4) denoted as V1/V2 and V4, respectively. Only taxa exhibiting consistent significant correlations at both V1/V2 and V4 are displayed. The heatmap represents the τ coefficient of Kendall rank correlation (*, P < .05; **, P < .01; ***, P < .001). Taxonomic lineage is indicated as follows: p, phylum; c, class; o, order; f, family; g, genus; s, species. Corrections/updates to GreenGenes database nomenclature are highlighted in violet, and taxonomic names determined through a manual BLASTN search are in blue. ALT, alanine aminotransferase; AST, aspartate-aminotransferase; Bil, bilirubin; ALP, alkaline phosphatase; BUN, blood urea nitrogen; cr, creatinine.

Finally, we used in the correlation analysis the data referred to serum markers of liver and kidney functioning, which were available for R and NR patients at baseline. The results showed that only for R-associated data, both serum PV-1 and C. aerofaciens were significantly correlated with the serum levels of liver alkaline phosphatase (). In addition, C. aerofaciens was positively correlated with other serum markers, viz. alanine aminotransferase, bilirubin, and creatinine ().
Discussion
IBS is a multifactorial intestinal functional disorder characterized by abdominal pain and altered bowel habits.Citation21 IBS is largely heterogeneous in etiology, pathophysiology, symptom manifestation, and severity, and also within the same subtype.Citation21 As a plausible consequence, attempts to identify effective objective biomarkers for IBS are disappointing.Citation22 The absence of effective biomarkers suggests that IBS patients, in addition to conventional subtyping based on bowel habits, could be further stratified according to other host- or microbiome-associated biomarkers. In addition, person-specific responses to probiotic effects have been reported, supporting the need for personalized probiotic approaches.Citation23 In this context, we searched for potential biomarkers characterizing IBS patients who had significant clinical improvement after taking the L. paracasei DG probiotic within the PROBE-IBS/2 trial.
Several serum markers failed to distinguish clinical responders (R) from non-responders (NR), whereas significant discrimination was obtained according to the abundance of several bacterial taxa. The potential value of the microbiome structure in predicting the response to therapeutic treatment in IBS was recently confirmed by Vervier et al., who found that IBS patients who had significant clinical benefits from a low FODMAP diet regimen were characterized by a distinct microbiome structure, depleted in Bacteroidetes, and enriched in Firmicutes and genes for amino acid and carbohydrate metabolism.Citation24 In particular, the authors defined the distinct microbiota profiles as “pathogenic-like,” in opposition to the microbiota of patients who did not respond to the low FODMAP diet, which was defined as “health-like”.Citation24
Notably, the bacterial taxonomic differences that distinguish R from NR patients also distinguished R patients from healthy controls. In contrast, the bacterial taxonomic differences between NR patients and healthy controls were relatively limited. We can, therefore, hypothesize that the R patients in the PROBE-IBS/2 trial possess an altered (potentially dysbiotic) fecal microbiota. This speculation is supported by the information available in the scientific literature concerning the role of the most abundant discriminative bacterial taxa found in our study: the family Coriobacteriaceae, genus Dorea, and species Collinsella aerofaciens. Several studies have reported that the family Coriobacteriaceae is overrepresented in the intestinal microbiota of IBS patients.Citation25 In addition, Dorea spp. and C. aerofaciens were found to be enhanced in IBS compared to healthy controls, specifically in diarrhea-predominant IBS.Citation26–28 Notably, Dorea spp. and C. aerofaciens produce hydrogen (H2), ethanol, and formate as the main end-products of their glucose metabolism.Citation29,Citation30 Hydrogen is one of the most abundant gases produced by bacteria in the human colon,Citation31 and has been shown to reduce colonic transit.Citation32 Accordingly, intestinal hydrogen production is associated with IBS,Citation33 particularly in NC-IBS patients.Citation34,Citation35
Coriobacteriaceae, Blautia spp., and C. aerofaciens have been associated with various noncommunicable diseases and dysfunctional metabolic conditions. For instance, the abundance of the family Coriobacteriaceae was reported to be increased in patients with type 2 diabetesCitation36 and IBD (both Crohn’s Disease and Ulcerative Colitis)Citation37 compared to healthy controls. Furthermore, the Eubacteriales genus Dorea, which was reported to be increased in multiple sclerosis patients, was demonstrated to include species that promote inflammation by triggering IFN-γ production and to potentially enhance intestinal permeability by degrading mucin and metabolizing sialic acids.Citation38,Citation39 In addition, Coriobacteriaceae, Blautia spp., and C. aerofaciens were all enhanced in overweight/obese subjects.Citation40 In addition, the authors of this study reported a positive association of Dorea spp. and C. aerofaciens with anthropometric parameters (waist circumference) and proposed these bacteria as obesity biomarkers.Citation40
The available literature indicates C. aerofaciens as a pathobiont, which is also overabundant in metabolic syndrome,Citation41 type 2 diabetes,Citation42 autoimmune polyendocrine syndrome type 1,Citation43 and psoriatic arthritis (together with the species Dorea formicigenerans).Citation44 Furthermore, C. aerofaciens exacerbates arthritis in a collagen-induced arthritis model by increasing gut permeability and triggering the expression of IL-17, CXCL1, and CXCL5 in intestinal epithelial cells.Citation45
Interestingly, in our study, probiotic intervention with L. paracasei DG significantly reduced the fecal abundance of Coriobacteriaceae, Dorea spp., and C. aerofaciens. Moreover, a significant decrease in the genus Coriobacteriaceae and species C. aerofaciens after treatment with the probiotic L. paracasei DG was also observed by re-analyzing the data collected during a previous intervention trial involving a different group of NC-IBS patients. Furthermore, in our study, C. aerofaciens and its entire family Coriobacteriaceae were found to positively correlate with plasma vesicle-associated protein-1 (PV-1) and alkaline phosphatase (ALP) serum levels. Simultaneously, PV-1 and ALP positively correlated with each other. PV-1 is a transmembrane glycoprotein expressed on blood vessels and lymphatic endothelial cells, which regulates endothelial permeability.Citation46,Citation47 The increased expression of PV-1 in endothelial cells was found to correlate with the systemic dissemination of enteropathogens and, notably, with higher serum levels of alanine transaminase (ALT),Citation48 indicators of liver damage, similar to ALP. Accordingly, we found that C. aerofaciens, in addition to ALP, was also correlated with the serum levels of the liver function markers ALT and bilirubin. These results suggest that the bacterial community structure of the fecal microbiota in R NC-IBS patients, which is enriched in potentially pro-inflammatory bacteria, may detrimentally influence the gut-liver axis by promoting endothelial leakiness, consequently resulting in the alteration of liver functionality. In support of this hypothesis, recent studies have suggested a potential association between IBS and elevated ALT and metabolic syndrome,Citation49 and the association of a high degree of nonalcoholic fatty liver with an increased risk of IBS.Citation50 In support of this hypothesis, Chen et al. demonstrated that C. aerofaciens may significantly decrease the expression of zonula occludens-1 (ZO-1),Citation45 a tight junction protein also demonstrated to control endothelial adherens junctions.Citation51
This study has several limitations, the most important of which is the limited number of responder NC-IBS patients that we could include in the analyses. In addition, the intestinal microbial ecosystem was exclusively studied through 16S rRNA gene profiling, which does not provide information on strain- or biotype-specific microbial functions. Owing to functional redundancy, the ecosystem services of human-associated microbiomes are more stable than their corresponding taxonomic community structures, which also express geographical/racial variations,Citation52 potentially limiting the generalizability of the results of our study. On the other hand, one strength of this study is that the obtained results concerning C. aerofaciens and the efficacy of the probiotic formulation have also been confirmed with a different dataset generated in a previous independent trial. Furthermore, we found solid scientific literature supporting this hypothesis. Finally, another advantage of this study is that it involved the recruitment of subjects from 20 different centers, allowing the identification of a sufficiently large number of subjects to identify the subgroup of patients with dysbiotic IBS and to increase the generalizability of the obtained results.
Another limitation of this study is the need for more dietary information for the patients during the study. Therefore, it cannot be ruled out that the consumption of certain foods may have influenced the response to probiotic treatment.
Conclusion
In this study, we proposed that a 12-week intake of the probiotic bacterium Lacticaseibacillus paracasei DG can be clinically effective in a subgroup of non-constipated IBS patients characterized by an altered fecal microbiota (dysbiotic NC-IBS patients), which resembles that observed in metabolic syndrome-associated pathologic or pre-pathologic conditions. Among the putative pathobionts increased in patients with dysbiotic NC-IBS, Collinsella aerofaciens, a bacterium reported to contribute to pro-inflammatory immune states, was positively associated with markers of increased endothelial permeability and liver functionality, suggesting the involvement of the gut-liver axis in this subgroup of IBS patients. An analysis of the fecal microbiota focused on these bacteria could permit the identification of NC-IBS patients who can obtain a significant clinical benefit from the probiotic treatment assessed during the PROBE-IBS/2 trial.
Patients and methods
Study design and population
The volunteers and samples in this study were derived from a multicenter, randomized, double-blind, parallel-group, placebo-controlled clinical trial (PSC-DS PROBE2-IBS/17, PROBE-IBS/2), which was carried out as described in ClinicalTrials.gov under the identifier NCT03449628. Eligible patients with symptoms meeting the Rome IV criteria for the diagnosis of IBS without constipation (i.e., patients with IBS-D and IBS-M) were recruited from 20 Italian centers. The inclusion and exclusion criteria are reported in Supplementary Methods (Data supplement file).
This study was primarily based on the analysis of patients that concluded the PROBE-IBS/2 trial per protocol in the probiotic arm (n = 72) (). Nine of them were excluded due to missing samples (n = 1), missing data for the calculation of the composite endpoint (n = 4), and no pain reported during the 2-week run-in period (i.e., mean numeric rating scale [NRS] = 0; n = 4) (). Consequently, 63 patients were included in this study, with a mean age of 35 ± 12 years and sex distribution of 34:29 F/M (). In addition, we also considered samples collected during PROB-IBS/2 at V2 from 172 patients with NC-IBS and 100 healthy controls. The baseline characteristics of the patients are summarized in .
Ethics approval
The protocol was approved by the Ethics Committee of the coordinating center (Hospital S. Orsola Malpighi-Bologna; approval identification no:67/2017/U/Sper on June 13, 2017) and by the Ethics Committee of each participating site. The trial was conducted in compliance with the Declaration of Helsinki and principles of good clinical practice. Written informed consent was obtained from all the participants.
Probiotic product used in the trial
L. casei DGⓇ (Lacticaseibacillus paracasei DG I1572, DSM 34,154) was administered with the formulation EnterolactisⓇ PLUS, consisting of at least 24 billion CFU in a 405 mg hydroxypropyl cellulose capsule (two capsules per day ingested with still water in an empty stomach). Viable counts on a representative number of capsules were performed before and after the trial by spreading serial dilutions on de Man-Rogosa-Sharpe (MRS) agar (Difco Laboratories Inc., Detroit, MI). Each capsule contained an average of 4.1 × 1010 bacterial cells per capsule at the beginning of the study, according to the total bacterial cell count performed by cytofluorimetry (BD Accuri C6; Becton Dickinson Italia, Milan, Italy) upon SYBR green cell labeling. The capsules also contain silicon dioxide and fatty acid magnesium salts as anti-caking agents.
Analysis of the fecal microbial ecosystem
The bacterial community structure of fecal samples was studied through metataxonomics by 16S rRNA gene profiling up to the level of amplicon sequence variants (ASV) clustered at 97% similarity (cASV) as reported in the supplementary methods (Data supplement file). The following organic acids were detected in fecal samples: acetate, butyrate, propionate, valerate, isovalerate, lactate, and succinate. These molecules were detected and quantified in fecal samples by Ultra-Performance Liquid Chromatography – High-Resolution Mass Spectrometry (UPLC-HR-MS) on an Acquity UPLC separation module (Waters, Milford, MA) coupled with an Exactive Orbitrap MS using a HESI-II probe for electrospray ionization (Thermo Scientific, San Jose, CA), as previously described in detailCitation28.
Collinsella aerofaciens quantification through quantitative PCR
The Collinsella aerofaciens species was quantified in fecal samples using a TaqMan real-time quantitative PCR protocol developed in this study as described in detail in the supplementary methods (Data supplement file).
Analysis of serum samples
Serum samples were used for the quantification of citrulline and markers of permeability (zonulin and PV-1), liver [alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin (Bil), alkaline phosphatase (ALP)], and kidney [blood urea nitrogen (BUN) and creatinine (Cr)] functionality, as described in the Supplementary Methods (Data supplement file).
Data analysis, bioinformatics, and statistics
Collected data were analyzed after separating the patients into two groups: responders (R) and non-responders (NR). R and NR were defined according to the primary endpoint of PROBE-IBS/2, which was based on the composite response over 12 weeks. In detail, a patient was considered R when recorded on ≥ 50% of the days, a reduction of ≥ 30% from their average baseline score for their worst abdominal pain, and on the same days, a stool consistency score ≤ 5.Citation53 The standard 11-point numeric rating scale (0 = none to 10 = worst possible pain) was used to measure abdominal pain, and the stool form was measured using the Bristol Stool Form Scale (BSFS).
Statistical calculations, including partial least squares discriminant analysis (PLSDA), were performed using R programming language (version 3.4.2). Read abundances underwent centered log-ratio transformation (CLR). Differently abundant taxa between the groups were identified using linear discriminant analysis (LDA) combined with the effect size (LEfSe) algorithmCitation54 on CLR-transformed taxonomic abundances. A cutoff value of LDA score (log10) above 2.0 was chosen. For paired/unpaired matches, paired/unpaired Student’s t test or Mann-Whitney U and Wilcoxon Signed-Ranks tests were adopted depending on normal distribution assessed through Shapiro-Francia test. A non-parametric repeated measures ANOVA-Type Statistic (RM-ATS) was also used where appropriate. Correlation analyses were performed by calculating Kendall’s τ rank correlation coefficient.
Collaborators
Bruno Annibale, Roma; Guido Basilisco, Milano; Leonilde Bonfrate, Bari; Michele Cicala, Roma; Rocco Cosintino, Roma; Antonio Di Sabatino, Pavia; Raffaella Ferraro, Vercelli; Francesca Galeazzi, Padova; Bastianello Germanà, Belluno; Giovanni Maconi, Milano; Santino Marchi, Pisa; Gerardo Nardone, Napoli; Matteo Neri, Chieti; Fabio Pace, Seriate; Piero Portincasa, Bari; Franco Radaelli, Como; Marcello Rodi, Vercelli; Giovanni Sarnelli, Napoli; Paolo Usai, Cagliari.
Competing interests
SG received research/educational grants and/or speaker/consultation fees from several food and pharmaceutical companies, including Sofar S.p.A. WF, an employee of Sofar S.p.A., which financially supported the study. The probiotic product used in this study was commercialized by a company that financially supported the study.
Data supplement file_Guglielmetti_R1_CLEAN.docx
Download MS Word (709.3 KB)Data availability statement
Metataxonomic raw sequencing data are available as FASTQ files in the European Nucleotide Archive (ENA) of the European Bioinformatics Institute under the accession code PRJEB56302. Metataxonomic raw sequencing data related to PROBE-IBS/120 are under accession code PRJEB18753. Processed data are included in the article or uploaded as supplementary materials. All the other data are available upon request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2298246
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016. doi:10.1053/j.gastro.2016.02.031.
- Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130(1):34–16. doi: 10.1053/j.gastro.2005.09.031.
- Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3(4):349–357. doi: 10.1016/s1542-3565(04)00726-8.
- Simren M, Stotzer PO, Sjovall H, Abrahamsson H, Bjornsson ES. Abnormal levels of neuropeptide Y and peptide YY in the colon in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2003;15:55–62. doi:10.1097/00042737-200301000-00010.
- El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20(9):2383–2391. doi: 10.3748/wjg.v20.i9.2383.
- Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. doi:10.1053/j.gastro.2006.11.039.
- Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The microbiome and irritable bowel syndrome - a review on the pathophysiology, Current research and future therapy. Front Microbiol. 2019;10:1136. doi:10.3389/fmicb.2019.01136.
- Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201. doi:10.1136/gut.2007.140806.
- Shaikh SD, Sun N, Canakis A, Park WY, Weber HC. Irritable bowel syndrome and the gut microbiome: a comprehensive review. J Clin Med. 2023;12(7):2558. doi: 10.3390/jcm12072558.
- Drossman DA, Hasler WL. Rome IV—functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150(6):1257–1261. doi: 10.1053/j.gastro.2016.03.035.
- Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017;312:G52–G62. doi:10.1152/ajpgi.00338.2016.
- Milner E, Stevens B, An M, Lam V, Ainsworth M, Dihle P, Stearns J, Dombrowski A, Rego D, Segars K. Utilizing probiotics for the prevention and treatment of gastrointestinal diseases. Front Microbiol. 2021;12:689958. doi: 10.3389/fmicb.2021.689958.
- Andriulli A, Neri M, Loguercio C, Terreni N, Merla A, Cardarella MP, Federico A, Chilovi F, Milandri GL, De Bona M, et al. Clinical trial on the efficacy of a new symbiotic formulation, flortec, in patients with irritable bowel syndrome: a multicenter, randomized study. J Clin Gastroenterol. 2008;42(Supplement 3):S218–S23. doi:10.1097/MCG.0b013e31817fadd6.
- McFarland LV, Karakan T, Karatas A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;41:101154. doi:10.1016/j.eclinm.2021.101154.
- Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044–1060. doi: 10.1111/apt.15001.
- Arioli S, Koirala R, Taverniti V, Fiore W, Guglielmetti S. Quantitative recovery of viable Lactobacillus paracasei CNCM I-1572 (L. casei DG®) after gastrointestinal passage in healthy adults. Front Microbiol. 2018;9:1720. doi: 10.3389/fmicb.2018.01720.
- Radicioni M, Koirala R, Fiore W, Leuratti C, Guglielmetti S, Arioli S. Survival of L. casei DG® (Lactobacillus paracasei CNCMI1572) in the gastrointestinal tract of a healthy paediatric population. Eur J Nutr. 2019;58(8):3161–3170. doi: 10.1007/s00394-018-1860-5.
- Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, Stuknyte M, Chouaia B, Riso P, Guglielmetti S. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144:1787–1796. doi: 10.3945/jn.114.197723.
- Bretto E, D’Amico F, Fiore W, Tursi A, Danese S. Lactobacillus paracasei CNCM I 1572: a promising Candidate for management of colonic diverticular disease. J Clin Med. 2022;11(7):11. doi: 10.3390/jcm11071916.
- Cremon C, Guglielmetti S, Gargari G, Taverniti V, Castellazzi AM, Valsecchi C, Tagliacarne C, Fiore W, Bellini M, Bertani L, et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: a pilot randomized clinical trial. United Eu Gastroenterol J. 2018;6(4):604–613. doi:10.1177/2050640617736478.
- Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675–1688. doi: 10.1016/S0140-6736(20)31548-8.
- Kim JH, Lin E, Pimentel M. Biomarkers of irritable bowel syndrome. J Neurogastroenterol Motil. 2017;23(1):20–26. doi: 10.5056/jnm16135.
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik R-Z, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–405.e21. doi:10.1016/j.cell.2018.08.041.
- Vervier K, Moss S, Kumar N, Adoum A, Barne M, Browne H, Kaser A, Kiely CJ, Neville BA, Powell N, et al. Two microbiota subtypes identified in irritable bowel syndrome with distinct responses to the low FODMAP diet. Gut. 2022;71(9):1821–1830. doi:10.1136/gutjnl-2021-325177.
- Duan R, Zhu S, Wang B, Duan L. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol. 2019;10(2):e00012. doi: 10.14309/ctg.0000000000000012.
- Maharshak N, Ringel Y, Katibian D, Lundqvist A, Sartor RB, Carroll IM, Ringel-Kulka T. Fecal and mucosa-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Dig Dis Sci. 2018;63(7):1890–1899. doi: 10.1007/s10620-018-5086-4.
- Altomare A, Di Rosa C, Imperia E, Emerenziani S, Cicala M, Guarino MPL. Diarrhea predominant-Irritable Bowel Syndrome (IBS-D): effects of different nutritional patterns on intestinal dysbiosis and symptoms. Nutrients. 2021;13(5):1506. doi: 10.3390/nu13051506.
- Gargari G, Taverniti V, Gardana C, Cremon C, Canducci F, Pagano I, Barbaro MR, Bellacosa L, Castellazzi AM, Valsecchi C, et al. Fecal Clostridiales distribution and short-chain fatty acids reflect bowel habits in irritable bowel syndrome. Environ Microbiol. 2018;20:3201–3213. doi:10.1111/1462-2920.14271.
- Taras D, Simmering R, Collins MD, Lawson PA, Blaut M. Reclassification of Eubacterium formicigenerans Holdeman and Moore 1974 as Dorea formicigenerans gen. nov., comb. nov., and description of Dorea longicatena sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2002;52:423–428. doi: 10.1099/00207713-52-2-423.
- Kageyama A, Benno YC, Omura S. Bergey’s manual of systematics of archaea and bacteria. J Gen Appl Microbiol. 2007;53:1–6. doi: 10.2323/jgam.53.1.
- Scaldaferri F, Nardone O, Lopetuso LR, Petito V, Bibbo S, Laterza L, Gerardi V, Bruno G, Scoleri I, Diroma A, et al. Intestinal gas production and gastrointestinal symptoms: from pathogenesis to clinical implication. Eur Rev Med Pharmacol Sci. 2013;17 Suppl 2(Suppl 2):2–10.
- Jahng J, Jung IS, Choi EJ, Conklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol Motil. 2012;24(2):185–90, e92. doi: 10.1111/j.1365-2982.2011.01819.x.
- Kumar S, Misra A, Ghoshal UC. Patients with irritable bowel syndrome exhale more hydrogen than healthy subjects in fasting state. J Neurogastroenterol Motil. 2010;16(3):299–305. doi: 10.5056/jnm.2010.16.3.299.
- Lee SH, Cho DY, Joo NS, Kim KN. Effect of eradicating hydrogen-forming small intestinal bacterial overgrowth with rifaximin on body weight change. Med. 2019;98(51):e18396. doi: 10.1097/MD.0000000000018396.
- Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16(1):40–46. doi: 10.5056/jnm.2010.16.1.40.
- Afolayan AO, Adebusoye LA, Cadmus EO, Ayeni FA. Insights into the gut microbiota of Nigerian elderly with type 2 diabetes and non-diabetic elderly persons. Heliyon. 2020;6(5):e03971. doi: 10.1016/j.heliyon.2020.e03971.
- Alam MT, Amos GCA, Murphy ARJ, Murch S, Wellington EMH, Arasaradnam RP. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020;12(1):1. doi: 10.1186/s13099-019-0341-6.
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–36 e8. doi:10.1016/j.cell.2016.10.020.
- Crost EH, Tailford LE, Le Gall G, Fons M, Henrissat B, Juge N, de Crécy-Lagard V. Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain-dependent. PLoS ONE. 2013;8(10):e76341. doi: 10.1371/journal.pone.0076341.
- Companys J, Gosalbes MJ, Pla-Paga L, Calderon-Perez L, Llaurado E, Pedret A, Valls RM, Jimenez-Hernandez N, Sandoval-Ramirez BA, Del Bas JM, et al. Gut microbiota profile and its association with clinical variables and dietary intake in overweight/obese and lean subjects: a cross-sectional study. Nutrients. 2021;13(6):2032. doi:10.3390/nu13062032.
- Gallardo-Becerra L, Cornejo-Granados F, Garcia-Lopez R, Valdez-Lara A, Bikel S, Canizales-Quinteros S, Lopez-Contreras BE, Mendoza-Vargas A, Nielsen H, Ochoa-Leyva A. Metatranscriptomic analysis to define the secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb Cell Fact. 2020;19(1):61. doi: 10.1186/s12934-020-01319-y.
- Kulkarni P, Devkumar P, Chattopadhyay I. Could dysbiosis of inflammatory and anti-inflammatory gut bacteria have an implications in the development of type 2 diabetes? A pilot investigation. BMC Res Notes. 2021;14(1):52. doi: 10.1186/s13104-021-05466-2.
- Petersen AO, Jokinen M, Plichta DR, Liebisch G, Gronwald W, Dettmer K, Oefner PJ, Vlamakis H, Chung DC, Ranki A, et al. Cytokine-specific autoantibodies shape the gut microbiome in autoimmune polyendocrine syndrome type 1. J Allergy Clin Immunol. 2021;148(3):876–888. doi:10.1016/j.jaci.2021.03.025.
- Shapiro J, Cohen NA, Shalev V, Uzan A, Koren O, Maharshak N. Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J Dermatol. 2019;46:595–603. doi: 10.1111/1346-8138.14933.
- Chen J, Wright K, Davis JM, Jeraldo P, Marietta EV, Murray J, Nelson H, Matteson EL, Taneja V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8(1):43. doi: 10.1186/s13073-016-0299-7.
- Herrnberger L, Seitz R, Kuespert S, Bosl MR, Fuchshofer R, Tamm ER. Lack of endothelial diaphragms in fenestrae and caveolae of mutant plvap-deficient mice. Histochem Cell Biol. 2012;138(5):709–724. doi: 10.1007/s00418-012-0987-3.
- Rantakari P, Auvinen K, Jappinen N, Kapraali M, Valtonen J, Karikoski M, Gerke H, Iftakhar EKI, Keuschnigg J, Umemoto E, et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat Immunol. 2015;16(4):386–396. doi:10.1038/ni.3101.
- Spadoni I, Pietrelli A, Pesole G, Rescigno M. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier. Gut Microbes. 2016;7(6):540–548. doi: 10.1080/19490976.2016.1239681.
- Lee SH, Kim KN, Kim KM, Joo NS. Irritable bowel syndrome may be associated with elevated alanine aminotransferase and metabolic syndrome. Yonsei Med J. 2016;57(1):146–152. doi: 10.3349/ymj.2016.57.1.146.
- Wu S, Yuan C, Yang Z, Liu S, Zhang Q, Zhang S, Zhu S. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: a prospective cohort study. BMC Med. 2022;20(1):262. doi: 10.1186/s12916-022-02460-8.
- Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208(6):821–838. doi: 10.1083/jcb.201404140.
- Gupta VK, Paul S, Dutta DC. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol. 2017;8:1162. doi: 10.3389/fmicb.2017.01162.
- Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, Davenport JM, McIntyre G, Lopez R, Turner L, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242–253. doi:10.1056/NEJMoa1505180.
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60.