ABSTRACT
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease, and its prevalence has increased worldwide in recent years. Additionally, there is a close relationship between MASLD and gut microbiota-derived metabolites. However, the mechanisms of MASLD and its metabolites are still unclear. We demonstrated decreased indole-3-propionic acid (IPA) and indole-3-acetic acid (IAA) in the feces of patients with hepatic steatosis compared to healthy controls. Here, IPA and IAA administration ameliorated hepatic steatosis and inflammation in an animal model of WD-induced MASLD by suppressing the NF-κB signaling pathway through a reduction in endotoxin levels and inactivation of macrophages. Bifidobacterium bifidum metabolizes tryptophan to produce IAA, and B. bifidum effectively prevents hepatic steatosis and inflammation through the production of IAA. Our study demonstrates that IPA and IAA derived from the gut microbiota have novel preventive or therapeutic potential for MASLD treatment.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is recognized as the most common chronic liver disease, and the prevalence of MASLD has increased recently in the Asia-Pacific region.Citation1,Citation2 Recently, three international expert panels proposed a new terminology MASLD.Citation3 The definition of MASLD requires the presence of steatotic liver disease and at least one cardiometabolic risk factor, while maintaining the alcohol and concomitant liver disease exclusion criteria of MASLD. MASLD-related cirrhosis can subsequently lead to hepatocellular carcinoma.Citation4
There are trillions of microbes in our intestines, which are collectively referred to as the gut-microbiota.Citation5,Citation6 The composition and function of the gut microbiota can be affected by various factors, including age, sex, genetic factors, lifestyle, medication, or dietary differences.Citation7 The intestinal microbiota consists of a large number of strains that belong to phyla such as Firmicutes, Bacteroides, Actinobacteria, Proteobacteria, and Verrucomicrobia. Approximately 99% of the gut microbiota exists as commensal bacteria, including Lactobacilli and Bifidobacteria. Citation8 Gut microbiota imbalance contributes to the development of obesity-related diseases, including metabolic syndrome and MASLD.Citation9,Citation10
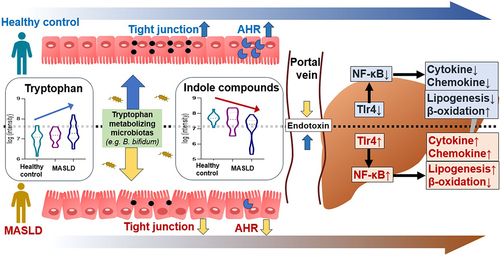
Tryptophan (Trp) is an essential amino acid needed for protein synthesis, that is not produced by the human body and must be consumed through food.Citation11 Trp acts as a precursor for various substances and is metabolized into indole substances including indole, tryptamine, indole ethanol, indole-3-propionic acid (IPA), indole-3-acetic acid (IAA), indole-3-lactic acid, skatole, indole aldehyde, and indole-3-acrylic acid, through various pathways.Citation12,Citation13 According to recent evidence, indole metabolites are known to be effective in hepatic protection.Citation14,Citation15 As such, Trp availability is an important factor in controlling protein biosynthesis. In our previous study, we identified a pattern of decreased IPA and IAA in fecal metabolites of MASLD patients and Trp-related metabolites including IPA and IAA have been shown to have a variety of effects on the liver.Citation16 However, the specific mechanisms of IPA and IAA in the prevention of MASLD are not well understood. It is also necessary to identify specific metabolites according to changes in the microbial community.
Therefore, in this study, we aimed to investigate the underlying mechanisms of IPA and IAA in a Western diet (WD)-induced mouse model for MASLD prevention based on human data showing a decrease in IPA and IAA as MASLD progresses.
Materials and methods
Human data
A prospective cohort study was conducted between April 2017 and March 2020 (ClinicalTrials.gov NCT04339725). This study involved liver disease patients who were followed up by the Department of Liver Diseases, University Hospital. The groups were 19 healthy controls, 16 MASLD patients without hepatitis, and 26 MASLD patients with hepatitis. This project followed the ethics of the 1975 Helsinki Declaration, as reflected by a prior approval by the institutional review board for human research in hospitals (2016–134). Informed consent was obtained from all participants. All authors had access to the study data and reviewed and approved the final manuscript.
MASLD is defined as hepatic steatosis and one or more of the five cardiometabolic risk factors.Citation17,Citation18 MASLD was diagnosed using blood chemistry, clincal parameter, liver biopsy, or imaging study (ultrasound or computed tomography scan). Patients with elevated liver enzymes aminotransferase (AST) or aspartate aminotransferase (ALT) ≥ 50 IU/L or hepatitis on liver pathology were included in the hepatitis group. Enrolled patients with MASLD who did not drink excessive alcohol (male >60 and female >40 g/wk). Patients with autoimmune liver disease, alcohol use disorder, pancreatitis, hemochromatosis, viral liver disease, pregnancy, Wilson’s disease, drug-induced liver injury, and other cancers were excluded. At enrollment, patients taking medications affecting the gut microbiome were excluded. As a control group, normal people visiting the hospital for health examination were recruited. The patient’s disease was treated regardless of the study.
Animals and diets
Animals were under human care, and all procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All procedures were approved by the Animal Experiment Management Committee (2020–40, 2021–53) of Hallym University College of Medicine. Six-week-old specific pathogen-free male C57BL/6J mice were purchased from Dooyeol Biotech (Seoul, Korea). All mice were individually housed in steel microisolator cages at 22 ± 2°C with a 12/12 h light/dark cycle. Mice had ad libitum access to water and food throughout the experiment and were monitored daily. The experiment included an acclimatization period for all groups, during which mice in all groups had an acclimatization period to eating normal diet for 1 week. IPA and IAA were administered at 0.1 mg/ml diluted in water. As an additional animal experiment, Bifidobacterium bifidum was orally administered at a concentration of 10Citation9 CFU/100ul daily for 12 weeks. WD (TD88137, Seoul, Korea) was purchased, and the nutritional composition was 42% fat, 42.7% carbohydrate, and 15% protein.
Liver histological analysis
Specimens were fixed with 10% formalin and routinely embedded in paraffin, and the tissue sections were processed with hematoxylin and eosin. To grade fatty liver, nonalcoholic fatty liver disease activity score (NAS), an objective index consisting of steatosis, lobular inflammation, and ballooning, was evaluated.Citation19 All biopsy specimens were analyzed by a single liver pathologist. Fatty liver was classified as grade 0 to 3 according to the Clinical Research Network scoring system (0: <5%, 1: 5%–33%, 2: 34%–66%, and 3: >66%). of steatosis). Inflammation was graded from 0 to 3 (0: none, 1: 1–2 foci per × 20 field, 2: 2–4 foci per × 20 field, 3: >4 foci per × 20 field). According to NAS-based guidelines, it may be helpful to recognize histological scoring of systems that cover the full spectrum of MASLD.
Stool analysis for 16S rRNA amplicon sequencing
Human feces were stored at −20°C as soon as the patient received 2–3 g of feces using the kit (stool paper and stool box) and moved to −80°C within 1 day. For mice feces, whole intestine was collected during euthanizing and frozen at −80°C and stored. Genomic DNA for metagenomic sequencing was extracted with a QIAamp stool kit (Qiagen, Germany) and library was prepared with a NEBNext Ultra II FS DNA Library Prep Kit for Illumina (New England BioLabs, USA) according to the manufacturer’s directions. The quantification of libraries was checked using a Qubit dsDNA HS assay kit (ThermoFisher Scientific, USA) and confirmed by qPCR with KAPA SYBR FAST qPCR Master Mix kit (Kapa Biosystems, USA). The quality of libraries was assessed on a Bioanalyzer 2100 (Agilent, USA) using a DNA 12,000 chip. All libraries were sequenced on the NovaSeq 6000 platform (Illumina, USA) with a paired end (PE) 150 bp reads. Assays were performed according to previous methods.Citation20 In brief, DNA was extracted with a QIAamp stool kit, and amplification of the V3-V4 region of the bacterial 16S rRNA gene was conducted using barcoded fusion primers. The forward fusion primer contained p5 adapter, i5 index, and gene-specific primer 341F (5′-AATGATACGGCGACCACCG AGATCTACAC-XXXXXXXX-TCGTCGGCAGCGTCAGATGTGTATAAGAGAC AG – CCTACGGGNGGCWGCAG-3′; underlining indicates the target region primer and X indicates the barcode region), and the reverse fusion primer contained p7 adapter, i7 index, and gene-specific primer 805 R (5′-CAAGCAGAAGACGGCATACGAGAT-XXXXXXXXGTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHV GGGTATCTAATCC-3′), in which included sequencing adapters and dual-index barcodes of the Nextera XT kit (Illumina, San Diego, CA, USA). The amplification was performed in the C1000 touch thermal cycler polymerase chain reaction system (Bio-Rad Laboratories, Inc., USA) with the following conditions: initial denaturation of 3 min at 95°C; followed by 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s and final extension at 72°C for of 5 min. Each amplified PCR product was confirmed with 1% agarose gel electrophoresis and visualized on a Gel Doc XR+ imaging system (Bio-Rad laboratories, Inc., USA). The amplified products were purified and size-selected by Agencourt AMPure XP beads (Beckman Coulter, USA). Library was constructed with pooled PCR products and quality of library was assessed on a Bioanalyzer 2100 (Agilent, USA) using a DNA 12,000 chip and quantified by qPCR with KAPA SYBR FAST qPCR Master Mix kit (Kapa Biosystems, USA). Sequencing was carried out according to the manufacturer’s instructions at CJ Bioscience, Inc. (Seoul, Republic of Korea) with the Illumina MiSeq platform using reagent kit V3 in PE 250 bp mode. Microbiome taxonomic profiling was conducted with the EZBioCloud platform (CJ Bioscience Inc., Republic of Korea) using the database version PKSSU4.0. After taxonomic profiling of each sample, comparative MTP analyzer of EZBioCloud was used for the comparative analysis of the samples.Citation21 The number of operational taxonomic units (OTUs) picking was conducted with UCLUST and CDHIT with 97% of similarity cutoff.Citation22 Subsequently, Good’s coverage, rarefaction, and alpha-diversity indices including Simpson, Jackknife, ACE, Chao1, Shannon, and NPShannon were calculated. Beta-diversity was shown by clustering using the and principal coordinate analysis (PCoA) unweighted pair group method with arithmetic mean (UPGMA).
Metabolite analysis of human stool samples
Metabolic profiles in the mouse cecum were obtained using a combination of gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) methods. Cecal samples were thawed at 4°C and mixed with 1.1 ml cold extraction solvent I (acetonitrile/water 1:1, v/v). The mixture was vortexed for 1 min, sonicated for 5 min under ice, and centrifuged at 13,200 rpm for 5 min at 4°C. Each supernatant (500 μl) was transferred to a new 2 ml tube for SCFA analysis. The remaining supernatant was mixed with 600 μl of cold extraction solvent II (acetonitrile/methanol, 1:3, v/v). For the second extraction step, the mixture was vortexed for 1 min and centrifuged at 13,200 rpm for 5 min at 4°C. The supernatant (500 μl) was aliquoted and transferred to new 1.5 ml tubes for gas chromatography time-of-flight mass spectrometry and liquid chromatography Orbitrap mass spectrometry. Aliquots were concentrated to complete dryness using a speed vacuum concentrator (SCANVAC, Korea). Detailed analyzes were performed according to previous research methods.
Strain preparation
Lactobacilli used in the preliminary study were isolated from various sources, including sour milk, cheese, feces of healthy Korean adults, and feces of newborn babies. B. bifidum is a lactobacillus strain isolated from the feces of newborns. B. bifidum was inoculated into flasks containing de Man, Rogosa and Sharpe medium (BD/Difco). Strains were incubated under anaerobic conditions at 37°C for 24 h. Stocks of each strain were prepared by mixing the culture with an equivalent 20% skim milk solution and then storing the mixture at −80°C.
Measurement of IAA production by Salkowski’s reagent
Cultures of each strain were previously grown in liquid culture anaerobic conditions at 35° with or without Trp. After 24 h, sample cultures were separated by centrifugation at 4000 rpm for 10 min at 4°C. Prepared by diluting 50:1 of Salkowski’s reagent, 35% HClO4 (perchloric acid) and 0.5 M FeCl3, reacted 1:1 with the bacterial culture medium and incubated in the dark at 30°C for 30 min. The absorbance is then measured at 530 nm. Calculate the concentration by comparing the optical density of the test sample to a standard IAA curve (10–100 μg ml).
Western blot analysis, LBP ELISA assay, TEER assay, Quant-Seq Microarray, cell related methods, and RT-PCR: supplementary file
Statistical analysis
All values were expressed as mean ± SEM, and analysis was performed between two or more groups by one-way ANOVA. A p-value <.05 was considered to indicate statistical significance. All statistical analyzes were performed using the GraphPad Prism software version. 8 (GraphPad Software Inc., San Diego, CA, USA).
Results
IPA and IAA are decreased in the stool of patients with MASLD
To study how metabolites derived from the gut microbiota are altered as MASLD progresses, we analyzed the microbiota with 16S rRNA gene sequencing and the levels of specific microbiota-derived metabolites in stool samples from 19 healthy controls (HCs) and 42 MASLD patients (, Supplementary Figure S1 and Figure S2, and Supplementary Table S1). In the analysis of composition, phylum composition, and the Firmicutes/Bacteroidetes (F/B) ratio, statistically significant differences were observed among the groups (). The abundance of Bifidobacterium was increased as liver disease progressed (). At the species level, B. bifidum was significantly increased in MASLD. B. longum was increased in MASLD. However, statistically, there was no difference. The levels of B. adolescentis, B. catenulatum, and B. breve were increased as liver disease progressed (Supplementary Figure S1e)
Figure 1. Human data.

We analyzed Trp metabolites including indole derivatives and kynurenine (Kyn), in the stool of patients.Citation23 Trp was significantly increased in MASLD patients. On the other hand, MASLD, IPA significantly decreased in MASLD patients. AA levels decreased in MASLD patients, but this was not statistically significant. (). However, there was a significant decrease in changes according to the disease progression (Supplementary Figure S2a). In the volcano plot, the IPA level was the most significantly decreased compound in MASLD patients (). Other indole derivatives did not reveal the difference between healthy controls and MASLD patients (). The level of Kyn was not significantly different among the groups (data not shown).
Fecal samples from MASLD patients were observed to have lower levels of major short-chain fatty acids (SCFAs) than stool samples from HCs (Supplementary Figure S2b). In contrast, the levels of cholic acid and chenodeoxycholic acid were significantly increased (Supplementary Figure S2c). In addition, the levels of the liver function markers AST and ALT were increased in the blood of MASLD patients (Supplementary Table S1). Overall, these clinical data indicate that IPA and IAA are candidate metabolites of preventive value and suggest a potential role in the pathogenesis of hepatic steatosis.
IPA and IAA attenuate the progression of WD-induced MASLD
We first divided mice into four groups to study the effects of IPA and IAA on hepatic lipid metabolism in MASLD: normal control (NC), WD, and groups supplemented with IPA and IAA under WD conditions (). Treatment with IPA and IAA improved the liver/body ratio (L/B) compared to that in the WD group, and the L/B ratio was significantly improved with IPA treatment (). Hematoxylin and eosin staining upon liver histopathology showed abnormal lipid droplets and inflammation in WD-fed mice, which were reduced by IPA and IAA treatment; the inflammation grade and ballooning grade were greatly reduced. The NAS improved with IPA and IAA treatment, especially IAA treatment, which resulted in a significant improvement ().
Figure 2. IPA and IAA ameliorate WD-induced hepatic steatosis.

In addition, compared to those in the NC group, the levels of the liver function indicators AST and ALT were significantly increased in the mice fed a WD, and liver function was significantly improved through IPA and IAA supplementation. On the other hand, there was no difference between triglyceride (TG) and cholesterol (CHOL) levels (). These data demonstrated improvement in both histological and serologic changes with IPA and IAA administration for 12 weeks, suggesting a protective role for IPA and IAA in WD-induced hepatic steatosis.
Administration of IPA and IAA ameliorates WD-induced hepatic steatosis and inflammation in mice
To evaluate liver inflammation and lipid metabolism in WD-fed mice, we detected changes in the mRNA expression of markers related to inflammation and lipid metabolism in liver tissue. First, the expression of tumor necrosis factor (Tnf)-α, a proinflammatory cytokine, was significantly increased in mice fed a WD, which was significantly improved through IPA and IAA administration. However, there was no significant difference in interleukin 1 beta (Il-1β) and Il-6 expression between the WD group and the IPA and IAA groups (). We also evaluated the mRNA expression of chemokines that activate inflamed tissues and play a pivotal role in the pathogenesis of MASLD.Citation24 The C-X-C motif chemokine ligand 10 (Cxcl10) level was significantly decreased in the IPA and IAA groups compared to that in the WD group (). Next, the gene expression of carnitine palmitoyltransferase-1a (Cpt1a), peroxisome proliferator-activated receptor-α (Ppar-α), and acyl-CoA oxidase 1 (Acox1), which are genes involved in the β-oxidation of fatty acids, was significantly increased by IPA and IAA administration (). Fatty acid synthase (Fasn), acetyl-CoA carboxylase 1 (Acc1), cluster of differentiation 36 (Cd36), and genes involved in adipogenesis and transport were induced in the WD group, whereas they were significantly suppressed in the IPA and IAA groups ().
Figure 3. Administration of IPA and IAA alleviates WD-induced hepatic steatosis and inflammation in mice.

Trp metabolites, indole compounds, are known as ligands of the aryl hydrocarbon receptor (AHR), and IAA is a representative AHR ligand.Citation23 Thus, the levels of cytochrome P450 family 1 subfamily B member 1 (Cyp1b1) and cytochrome P450 family 1 subfamily A member 2 (Cyp1a2), which are genes regulated by AHR in the ileum, were significantly increased by IAA administration, suggesting that AHR was activated in the ileum (). In contrast, the expression of AHR in liver tissue was not activated (Supplementary Figure S3a). Increased proinflammatory cytokine expression were decreased by IPA and IAA (Supplementary Figure S3b). Overall, these results suggest that the administration of IPA and IAA to WD-fed mice improves hepatic steatosis by regulating the inflammatory response and fatty acid synthesis and β-oxidation in the liver.
The treatment of Caco-2 cells with IPA and IAA increased the transepithelial electrical resistance (TEER) values by 1.2-fold and 1.6-fold, respectively, compared with those of untreated controls ().
Treatment with IPA and IAA reduced the endotoxin response, thereby inhibiting the activation of macrophages and NF-κB signaling
We evaluated whether treatment with IPA and IAA improved liver inflammation by reducing intestinal-derived endotoxin, reducing the activity of liver macrophages, and finally inhibiting NF-κB signaling. First, we evaluated the mRNA level of Toll-like receptor 4 (TLR4), which is activated by recognizing lipopolysaccharide (LPS), in the livers of WD-induced mice. As a result, the level of Tlr4 increased in the WD-induced mouse group, while treatment with IPA and IAA showed a decrease its levels to levels similar to those of the NC group, but did not show statistical significance (). In the Western blot analysis, IAA treatment significantly decreased the expression of Tlr4 (). Elevated levels of LPS binding protein (LBP), a protein that binds serum endotoxin induced by a WD, were significantly reduced in the IAA-treated group (). Next, we evaluated the mRNA levels of C-C motif ligand 2 (Ccl2) and C-C motif ligand 5 (Ccl5), which are representative chemokines that attract monocytes and macrophages to the site of inflammation. The levels of Ccl2 and Ccl5 were significantly increased in WD-fed mice, but these levels were significantly decreased with IPA and IAA treatment (). Based on this finding, we investigated the effect of IPA and IAA treatment on the protein level of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a protein complex involved in cytokine production and cell survival in macrophages. Interestingly, the phosphorylated protein levels of p65 and IκBα were increased in WD-fed mice but were restored by treatment with IPA and IAA, and the change with IAA treatment was significant ().
Figure 4. Administration of IPA and IAA suppresses the increase in endotoxin and the inflammatory response caused by WD.

To evaluate the anti-inflammatory effects of IPA and IAA, we measured the levels of cytokines and chemokines in LPS-stimulated macrophages. RAW264.7 cells were pretreated with IPA and IAA in a dose-dependent manner and then treated with LPS. IPA and IAA significantly reduced Tnf-α, and Ccl2 in LPS-stimulated Raw264.7 cells (). mRNA levels of Ahr with IPA and IAA treatment on Raw264.7 cell and CaCo2 cells were increased (). These results suggest that IPA and IAA treatment increase Ahr expression and suppress the expression of endotoxin-stimulated Tlr4, thereby inhibiting macrophage activation and NF-κB signaling and ultimately improving inflammation.
IPA and IAA increased the expression of Acox1 and Cpt1, fatty acid oxidation markers, in AML12 cells (Supplementary Figure S4a and S4b). Inflammation-related cytokines were reduced by IPA and IAA treatment in RAW264.7 cells (Supplementary Figure S4c and S4d). These results suggest that IPA and IAA play a role in controlling fat metabolism and inflammation.
IPA and IAA modulate the alteration of RNA transcription in the liver
To investigate how IPA and IAA contribute to liver protection from reduced inflammation and lipid accumulation in WD-induced mice, we performed RNA-seq of livers harvested from NC, WD, IPA and IAA mice and profiled liver transcripts All significant findings had a fold change > 2, normalized data (log2) >1, p < .05, and WD/NC, IPA/WD, and IAA/WD were compared. p-values were adjusted for FDR (False Discovery Rate). Differentially expressed gene (DEG) clustering heatmap analysis showed significant upregulation and downregulation in IPA and IAA treatment compared to WD-induced mice, and dendrograms indicating expression similarity between samples were relatively similar in the IPA and IAA groups, unlike in the WD group (). Clustering analysis with significant DEGs revealed a unique expression profile for tissues in each group.
Figure 5. IPA and IAA treatment altered transcriptomic in the liver.

We analyzed the WD group vs. IPA and IAA in the Gene Ontology (GO) biological process category. First, in the IPA group, regulation of Il-6 production and leukocyte activation, which contribute to inflammation, were downregulated, and biological oxidation, an energy-generating response in living cells, was upregulated. Next, in the IAA group, the negative regulation of protein phosphorylation was downregulated, and the metabolism of lipids and lipid catabolic processes involved in lipid metabolism were upregulated (). Principal component analysis (PCA) showed a distinct separation between the four groups according to group type (). Next, we performed gene set enrichment analysis (GSEA) to identify pathways regulated by IPA and IAA in WD-induced mice. Not surprisingly, we found significant upregulation of the Trp metabolism pathway in mice administered IPA and IAA, which are metabolites of Trp (). Genes differentially expressed in the liver were identified by comparing the IPA and IAA-administered mouse groups with the WD group.
IPA administration upregulated 34 genes and downregulated 55 genes, and IAA administration resulted in 37 upregulated genes and 48 downregulated genes. Genes with common changes in IPA/WD and IAA/WD were 26 upregulated genes and 27 downregulated genes. Interestingly, consistent with previous studies, the gene expression of Ccl2, a chemokine that attracts monocytes and macrophages to the site of inflammation, was significantly downregulated, and the gene expression of Cyp1a2, a gene associated with AHR, was significantly increased.
The correlation of genes was statistically anal-yzed and visualized (). A total of 53 genes commonly regulated by IPA/IAA (). The comparative analysis revealed 43 regulated genes that crossed WD/NC, IPA/WD, and IAA/WD, satisfying the three conditions of fold change > 2, normalized data (log2) >1, and p < .05 ( and Supplementary Table S3). shows a total of 43 regulated genes statistically analyzed and visualized for correlation based on the protein-protein interaction database. Ccl2, inflammation marker, is decreased in IPA and IAA groups. Mup (major urinary protein), which inhibits insulin resistance and glucose tolerance, and cytochrome P450 relating genes were closely related and increased IPA and IAA groups (). These RNA-seq studies indicate that IPA and IAA administration activate the Trp metabolic pathway and that this activation is attributable to the regulation of transcription related to inflammation and lipid metabolism. Esm1 (endothelial cell-specific molecule 1) and lymphocyte antigens 6 (Ly6d), inflammation markers, were decreased in IPA and IAA groups. In addition, the level of Cyp1a2 was increased in IPA and IAA groups as shown in .
WD-induced intestinal microbiota imbalance is ameliorated by IPA and IAA administration, and B. bifidum is a potent IAA-producing strain
We performed 16S rRNA-based intestinal microbial taxonomic profiling to investigate the potential relevance of IPA and IAA administration to changes in intestinal microbial composition and to specific bacteria that produce indole compounds. Among the ɑ-diversity indicators, Simpson and Shannon indices showed no significant difference across all groups (). On the other hand, β-diversity, which indicates the difference in the composition of the gut microbiota between samples and groups, showed a distinct difference across all groups based on principal coordinate analysis (PCoA) and showed a large change in the microbial composition due to WD supply, and the IPA and IAA groups were partially separated (). Cluster analysis also showed that there were differences between WD group and indole groups that were administered concurrently with WD (). Several studies have shown that the F/B ratio is associated with obesity and various diseases.Citation25 The F/B ratio was decreased in the IPA and IAA groups compared to that in the WD group but was not statistically significant (). The abundance of Bifidobacterium was significantly increased in the IPA and IAA groups (). Each group had a different genus composition (Supplementary Figure S5).
Figure 6. Modulating microbial taxonomic abundance of IPA and IAA on WD-induced changes.

We tested the ability to produce IAA by adding Trp, a precursor of IAA, through a Salkowski assay using 13 strains, which are representative strains that are approved for use as a probiotics in South Korea. The control strains E. coli and B. bifidum showed positive results, suggesting that B. bifidum can metabolize IAA using Trp (). Therefore, measurements were made from 0 h to 72 h to determine the correlation between the growth of B. bifidum and IAA production. B. bifidum growth peaked at 24 h with and without Trp, but the IAA concentration increased up to 24 h only in the medium containing Trp ().
B. bifidum attenuates the progression of WD-induced MASLD
Our previous findings revealed that B. bifidum is a novel bacterium capable of producing IAA. Therefore, we hypothesized that IAA-producing B. bifidum could protect against liver dysfunction in a WD-induced MASLD mouse model. To prove this hypothesis, we divided mice into the NC group and the WD group. To evaluate whether B. bifidum metabolizes Trp, an IAA precursor, under WD conditions, B. bifidum with or without TRP was administered by gavage. In addition, L. plantarum Q180 and Trp were included as controls (). Since Trp administration is known to induce hepatic steatosis, we evaluated whether hepatic steatosis was attenuated through B. bifidum metabolic ability.Citation26 The L/B ratio increased in the WD group and the TRP group, but supplementation with B. bifidum and L. plantarum improved the L/B ratio, and in particular, the group treated with TRP and B. bifidum had a significantly decreased L/B ratio ().
Figure 7. B. bifidum ameliorates WD-induced hepatic steatosis.

Hematoxylin and eosin staining showed abnormal lipid droplets and inflammation in WD-fed mice, which were alleviated by administration of B. bifidum, and showed an effect of relieving hepatic steatosis in the group administered Trp coadministered with B. bifidum. As a result, the inflammation grade and balloon grade were significantly reduced, and the NAS was significantly improved with B. bifidum treatment (). In addition, the levels of AST and ALT, liver function indicators, were significantly increased in the mice of the WD and Trp groups, and liver function was significantly improved through the administration of B. bifidum. On the other hand, there was no significant difference in TG levels, and the CHOL levels were significantly reduced only in the group treated with Trp and B. bifidum (). These data demonstrated improvement in both histological and serological changes with B. bifidum administration for 12 weeks, suggesting that B. bifidum may play a protective role by metabolizing Trp, especially in Trp-induced hepatic steatosis.
Administration of B. bifidum alleviates WD-induced hepatic steatosis and inflammation
To determine whether B. bifidum mediates an inhibitory effect on hepatic steatosis, we evaluated WD-induced mice in groups treated with B. bifidum alone or in combination with Trp and B. bifidum. First, the levels of Tnf-α, a proinflammatory cytokine, were significantly increased in the WD group, but significantly improved in the strain-treated group. However, as in previous studies, there was no difference between Il-6 and Il-1β levels ( and Supplementary Figure S6a). Levels of the chemokine Cxcl10 were decreased in the group treated with B. bifidum alone, and levels of Ccl2 and Ccl5, which recruit macrophages, were greatly reduced in the group treated with B. bifidum and cultured medium (). In the TEER test with L, plantarum and B. bifidum, B. bifidum increased tight junction function (). Next, the expression of genes involved in the β-oxidation of fatty acids, Ppar-α and Acox1, increased in the group treated with the strain, especially in the group administered Trp and B. bifidum together (). The expression of Ppar-γ, which is involved in fat synthesis, was increased in the WD group and greatly improved by B. bifidum treatment. However, unlike previous studies, B. bifidum treatment did not significantly alter the expression of genes related to fat synthesis (Supplementary Figure S6b). In Raw264.7 cell analysis, B. bifidum and culture medium effectively improved the expression of inflammation (Supplementary Figure S7). In the microbiota analysis, each group showed typical findings of beta-diversity, phyrum and genus composition, and proportion of bifidobacterium (Supplementary Figu-re S8).
Figure 8. Administration of B. bifidum alleviates WD-induced hepatic steatosis and inflammation in mice.

We also evaluated genes associated with AHR, which binds IAA. AHR expression was almost absent in the WD group but increased in the Trp and strain groups. In particular, AHR expression was significantly increased in the group administered Trp and B. bifidum together, suggesting that B. bifidum can metabolize Trp to generate IAA to activate AHR in the ileum ( and Supplementary Figure S9a). Conversely, consistent with previous studies, the expression level of AHR in liver tissue was not found to be activated by B. bifidum treatment (Supplementary Figure S6c).
Our results suggest that B. bifidum can reduce translocation and inhibit the activation of macrophages, as in previous studies. Additionally, these results were not improved in the Trp group, suggesting that B. bifidum attenuated inflammation in the liver by metabolizing Trp. Serum IAA levels were increased in the B. bifidum groups (alone and with Trp)(). In the human data alone, decreased cecal IAA and IPA levels in WD were recovered in B. bifidum group (Supplementary Figure S9b and Supplementary Table S2). In liver tissue, changed IAA and IPA levels were recovered in the B. bifidum group (Supplementary Figure S9c).
Next, we evaluated the role of AHR by using AHR antagonist, CH-223191 (Sigma Korea, Seoul)(). level of inflammatory cytokine, NAS score, and fat regulation factors, which were improved in the B. bifidum with Trp group, were worsened (Supplementary Figure S9c, S9d, and S9f). This result suggests that indole producing microbiota increased AHR expression on ileum and inhibited MASLD progression.
Overall, treatment with B. bifidum alone could improve hepatic inflammation and steatosis via AHR. In addition, although the Trp group experienced exacerbation of hepatic inflammation and hepatic steatosis, the administration of B. bifidum had an anti-MASLD effect, which validated our hypothesis.
Discussion
MASLD is currently the most common liver disease in Western countries, and the resulting gut dysbiosis is strongly associated with MASLD and obesity-related diseases, including metabolic syndrome.Citation27 The mechanisms linking obesity and the gut microbiome are being elucidated through a combination of vigorous studies in human and animal models.Citation28 Improving the gut microbiota using pre-, pro-, para-, or postbiotics is a promising approach for preventing or treating MASLD by regulating the intestinal-liver axis.Citation29,Citation30 We aimed to identify the main bacteria that attenuate MASLD by studying the preventive effect of IPA and IAA on MASLD and by investigating bacteria that produce IAA using Trp.
Indole compounds, which are Trp metabolites derived from intestinal microorganisms, play an important role in intestinal homeostasis by liganding with AHR and are related to the pathogenesis of intestinal diseases such as inflammatory bowel disease and colitis. However, liver disease associated with AHR remains largely unexplored.Citation31,Citation32 The specific mechanisms of IPA and IAA in preventing and treating MASLD are not well understood. In a previous report, C. sporogenes, an intestinal microorganism that synthesizes IPA using Trp, is well known, but an intestinal microorganism that produces IAA has not been well reported.Citation33
Trp has three main pathways: Kyn, serotonin, and indole.Citation23 The levels of the indoles IPA and IAA tended to decrease in these pathways as MASLD worsened. Importantly, the level of Trp, a precursor of indole, was increased in patients with hepatic steatosis, suggesting that the metabolic pathway of Trp to IPA and IAA by the gut microbiota was impaired. Therefore, our study suggests a developmental inhibitory role due to supplementation with IPA and IAA in the MASLD phenotype.
To study the inhibitory effects of IPA and IAA supplementation on MASLD, we first characterized a mouse model of MASLD that induces hepatic steatosis. Our results showed that exposure to a WD for 12 weeks induced inflammation and steatosis. Supplementation with IPA and IAA decreased the L/B ratio compared to that in the WD group, consistent with the histology and blood biochemistry results, leading to significant recovery of NAS levels, AST, ALT, TG, and CHOL. Here, we found that IPA and IAA administration significantly reduced the severity of WD-induced liver inflammation, as indicated by the mRNA levels of Tnf-α and Cxcl10.
Moreover, our study demonstrated that Ppar-α upregulates Cpt1a expression, increases mitochondrial β-oxidation, and regulates the uptake and elimination of fatty acids.Citation34,Citation35 Genes regulating adipogenesis in the liver were significantly reduced by IPA and IAA administration. These results provide evidence for the anti-MASLD effect of the administration of IPA and IAA. IAA derived from Trp has been reported as an AHR ligand.Citation36 In addition, genes related to AHR were greatly increased by IAA administration. This finding suggests that IAA administration contributed to the inhibition of MASLD progression by maintaining intestinal homeostasis through the AHR ligand and regulating the gut-liver axis.
A growing body of evidence indicates that bacterial endotoxins, such as LPS, are closely related to MASLD.Citation37 Endotoxins stimulate cells in the liver by releasing inflammatory cytokines and chemokines by a Tlr-4-mediated mechanism.Citation38 We have shown in in vitro studies that IPA and IAA treatment directly affect macrophages by reducing endotoxin signaling. Specifically, the expression level of Tlr4 mRNA, which recognizes and activates LPS, was reduced by IPA and IAA treatment. Protein levels also showed the same results, with ultimately reduced levels of LBP in the serum. Recent studies have shown evidence of important roles for chemokines in the pathogenesis of MASLD, and Ccl2 and Ccl5 are recognized as important mediators in liver damage and inflammatory tissues, as they are associated with macrophages.Citation39 Previous studies have shown evidence that LPS-stimulated macrophages contribute to the inflammatory response by activating members of the NF-ϰB transcription factor family.Citation40 Here, we speculated that LPS is a metabolite that can inhibit macrophage activity and inhibit the NF-kB signaling pathway due to IPA and IAA treatment. Our findings show that IPA and IAA treatment inhibited WD-induced activation of macrophages. Interestingly, Ccl2 RNA transcript expression was also found to be decreased. Additionally, IPA and IAA treatments showed efficacy in relieving inflammation by inactivating NF-ϰB signaling, as speculated. Our findings demonstrated that IPA and IAA treatment contributed to macrophage inactivation by reducing endotoxin levels in MASLD progression. In addition, consistent with their anti-inflammatory effects, NF-ϰB signaling is also important in IPA and IAA treatment. IPA and IAA are therefore potential target metabolites for therapies aimed at inhibiting or preventing the progression of MASLD. We confirmed the anti-inflammatory effects of IPA and IAA on LPS-induced inflammation in the murine macrophage line RAW264.7 in vitro. Treatment with IPA and IAA contributed to anti-inflammatory effects in a dose-dependent manner, as observed in the in vivo experiment.
Mechanistically, we used liver transcriptome analysis to highlight the importance of gene function in IPA and IAA treatment in the liver. IPA treatment downregulated the production of Il-6 and the regulation of leukocyte activation, which contribute to inflammation. Conversely, biological oxidation increased. IAA also downregulated the negative regulation of protein phosphorylation, and the metabolism of lipids was upregulated. These different results suggest that IPA and IAA act on MASLD prevention through different mechanisms.
Recently, lipid metabolism disorder has been shown to be a risk factor for hyperlipidemia, atherosclerosis, and MASLD.Citation41 An imbalance in lipid metabolism can lead to several disorders in the pathways involved in fatty acid synthesis and very low-density lipoprotein secretion and β-oxidative changes.Citation42 Therefore, our study demonstrates that IPA and IAA treatment can attenuate hepatic steatosis by improving lipid metabolism. We also confirmed that IPA and IAA treatments increased Trp metabolism compared to that in the WD group. Additionally, when comparing the WD group with the IPA and IAA groups, Ccl2 was downregulated, and the AHR marker Cyp1a2 was upregulated, which is consistent with previous studies. Therefore, IPA and IAA administration increases Trp metabolism, maintenance of energy homeostasis, and lipid metabolism, suggesting the possibility of mediating transcriptional signal regulation related to metabolism and inflammation.
Recently, increasing evidence has suggested a close link between liver diseases such as MASLD, including impairment of intestinal endothelial barrier function that allows translocation of gut microbiome components and leads to liver inflammation.Citation43 The intestinal microbiome composition is associated with MASLD patients, and as liver disease progresses, Bacteroides decrease and Firmicutes increase.Citation44 This is consistent with the gut microbiota composition of MASLD patients in our study. Our study found similarities in the gut microbiota in the IPA- and IAA-treated groups, different from the WD-induced group. Additionally, various metabolites produced by the gut microbiota reach the liver via gut signaling pathways.Citation45
Mar et al. provided evidence of a few bacterial isolates present in the gut microbiome that synthesize IAA.Citation46 In this study, we screened 12 probiotic cultures with and without Trp and confirmed the relative abundance of IAA in B. bifidum. This suggests that B. bifidum is a strain that produces potent IAA using Trp. Bifidobacterium is the most common bacterium in the infant gut microbiome. Bifidobacterium is also one of the major genera of bacteria that make up the mammalian gastrointestinal microbiome.Citation47 The genus Bifidobacterium has a fructose-6-phosphate phosphoketolase pathway used for carbohydrate fermentation. Therefore, many metabolic studies of Bifidobacterium have focused on oligosaccharide metabolism.Citation48 Among them, B. bifidum has been shown in previous studies to preserve intestinal integrity by reducing mucosal damage and improving hyperglycemia and dyslipidemia in diabetic mouse models.Citation49 However, the role of B. bifidum in MASLD is not clear. Additionally, there are few studies on whether B. bifidum combined with prebiotics has an anti-MASLD effect. Therefore, further investigation into the efficacy of B. bifidum in MASLD is needed. In addition, we investigated whether the progression of MASLD could be inhibited by prebiotics and probiotics.
As expected, the livers of WD-induced mice were dilated and hypertrophied, and as expected, the Trp group was also the same. This dilation and hypertrophy was found to be significantly improved by B. bifidum. In particular, the progression of MASLD was inhibited in the group treated with Trp and B. bifidum together. Additionally, histologically, the WD group and the Trp group were the same, and they were significantly improved by B. bifidum administration. In terms of blood biochemistry, liver enzymes were significantly reduced in response to B. bifidum. Further analysis showed that B. bifidum treatment downregulated proinflammatory cytokines and chemokines. Therefore, administration of probiotic B. bifidum and prebiotic Trp ameliorated hepatic inflammation and steatosis, resulting in a potential preventive effect against MASLD.
In this study, we showed that the community levels of metabolites produced by the gut microbiota differ according to the degree of MASLD progression. SCFAs, such as propionate, acetate, and butyrate, are metabolites that have a variety of physiological functions and immune system regulation.Citation50 Conversely, the levels of BAs, especially cholic acid and chenodeoxycholic acid, were increased. As previously reported, BAs are regulated by farnesoid X receptor (FXR), of which 95% are actively reabsorbed in the terminal ileum and recycled in the liver, and FXR plays an important role in regulating lipid metabolism and suppressing inflammation in the liver.Citation50 As a result, the underlying cause of the increased bile acids in the feces of patients with hepatic steatosis suggests an impairment of FXR function.
In this study, B. bifidum is enriched in MASLD patients and the Bifidobacterium genus was enriched in NASH patients and present at constant levels in MASLD patients. In addition, Bifidobacterium was depleted in WD-fed mice. These conflicting results suggest that the role of Bifidobacterium may differ between human and mouse MASLD.
In conclusion, IPA and IAA levels were found to be reduced in patients with MASLD compared to those in healthy individuals. In our study, using an animal model of WD-induced MASLD, we demonstrated that administration of IPA and IAA ameliorated diet-induced hepatic steatosis and liver inflammation. These IPA and IAA administrations revealed the mechanism of anti-MASLD by inhibiting the NF-κB pathway through reduction of endotoxin levels and inactivation of macrophages. Importantly, we found that the probiotic B. bifidum produced Trp-derived IAA and showed an inhibitory effect on MASLD progression. Our data therefore highlight the potential prognostic value of B. bifidum-derived IAAs and their contribution to controlling hepatic steatosis. Therefore, our data suggest that B. bifidum derived IAAs are likely to be of clinical importance.
Author contributions
K.T.S. and B.H.M. concepted and designed study. B.H.M. wrote the main manuscript text. B.H.M., H.G., G.H.K., J.J.J., S.S., R.G., S.M.W., K.K.O., S.J.Y., H.J.P., J.A.E., M.K.J., J.Y.H., and S.H.H. analyzed and interpreted data. K.T.S., S.D., N.S., and T.P. drafted and revised the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Availability of data and materials
All data are available in manuscript and supplementary file.
Ethical approval statement
The animals received humane care and all procedures were performed in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. This project followed the ethics of the 1975 Helsinki Declaration, as reflected by a prior approval by the institutional review board for human research in hospitals (2016–134). Informed consent was obtained from all participants. All authors had access to the study data and reviewed and approved the final manuscript.
Supplementary_2023.docx
Download MS Word (1.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2307568
Additional information
Funding
References
- Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–22. doi:10.1038/s41591-018-0104-9.
- Kawaguchi T, Tsutsumi T, Nakano D, Eslam M, George J, Torimura T. MAFLD enhances clinical practice for liver disease in the Asia-Pacific region. Clin Mol Hepatol. 2022;28(2):150–163. doi:10.3350/cmh.2021.0310.
- Fukunaga S, Mukasa M, Nakano D, Tsutsumi T, Kawaguchi T. Changing from NAFLD to MASLD: similar cumulative incidence of reflux esophagitis between NAFLD and MASLD. Clin Mol Hepatol. 2024;30(1):121–123. doi:10.3350/cmh.2023.0437.
- Parry SA, Hodson L. Influence of dietary macronutrients on liver fat accumulation and metabolism. J Invest Med. 2017;65(8):1102–15. doi:10.1136/jim-2017-000524.
- Blandino G, Inturri R, Lazzara F, Di Rosa M, Malaguarnera L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016;42(5):303–15. doi:10.1016/j.diabet.2016.04.004.
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–48. doi:10.1016/j.metabol.2015.12.012.
- Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018:361. doi:10.1136/bmj.k2179.
- Koning M, Herrema H, Nieuwdorp M, Meijnikman AS. Targeting nonalcoholic fatty liver disease via gut microbiome-centered therapies. Gut Microbes. 2023;15(1):2226922. doi:10.1080/19490976.2023.2226922.
- Jennison E, Byrne CD. The role of the gut microbiome and diet in the pathogenesis of non-alcoholic fatty liver disease. Clin Mol Hepatol. 2021;27(1):22–43. doi:10.3350/cmh.2020.0129.
- Lee NY, Yoon SJ, Han DH, Gupta H, Youn GS, Shin MJ, Ham YL, Kwak MJ, Kim BY, Yu JS. et al. Lactobacillus and pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes. 2020;11(4):882–99. doi:10.1080/19490976.2020.1712984.
- Modoux M, Rolhion N, Mani S, Sokol H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci. 2021;42(1):60–73. doi:10.1016/j.tips.2020.11.006.
- Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D. Tryptophan metabolism and related pathways in psychoneuroimmunology: the impact of nutrition and lifestyle. Neuropsychobiology. 2020;79(1–2):89–99. doi:10.1159/000496293.
- Yoon SJ, Yu JS, Min BH, Gupta H, Won SM, Park HJ, Han SH, Kim BY, Kim KH, Kim BK. et al. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front Microbiol. 2023;14:1129904. doi:10.3389/fmicb.2023.1129904.
- Ji Y, Yin Y, Li Z, Zhang W. Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD). Nutrients. 2019;11(8):1712. doi:10.3390/nu11081712.
- Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23(4):1099–111. doi:10.1016/j.celrep.2018.03.109.
- Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107(1):228–233. doi:10.1073/pnas.0906112107.
- Cusi K, Younossi Z, Roden M. From NAFLD to MASLD: promise and pitfalls of a new definitiondagger. Hepatology. 2023;19:10–97. doi:10.1016/j.jhep.2023.09.038.
- Kim GA, Moon JH, Kim W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: implication of janus-faced modernity. Clin Mol Hepatol. 2023;29(4):831–43. doi:10.3350/cmh.2023.0277.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp‐Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. doi:10.1002/hep.20701.
- Yu JS, Youn GS, Choi J, Kim CH, Kim BY, Yang SJ, Lee JH, Park TS, Kim BK, Kim YB. Lactobacillus lactis and pediococcus pentosaceus‐driven reprogramming of gut microbiome and metabolome ameliorates the progression of non‐alcoholic fatty liver disease. Clin Transl Med. 2021;11(12):e634. doi:10.1002/ctm2.634.
- Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67(5):1613–7. doi:10.1099/ijsem.0.001755.
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–1. doi:10.1093/bioinformatics/btq461.
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host & Microbe. 2018;23(6):716–24. doi:10.1016/j.chom.2018.05.003.
- Roh YS, Seki E. Chemokines and chemokine receptors in the development of NAFLD. Adv Exp Med Biol. 2018;1061:45–53. doi:10.1007/978-981-10-8684-7_4.
- Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach S. et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. doi:10.1186/s12866-017-1027-1.
- Osawa Y, Kanamori H, Seki E, Hoshi M, Ohtaki H, Yasuda Y, Ito H, Suetsugu A, Nagaki M, Moriwaki H. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011;286(40):34800–8. doi:10.1074/jbc.M111.235473.
- Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13(7):412–25. doi:10.1038/nrgastro.2016.85.
- Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi:10.1038/nature18846.
- Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clement K. Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology. 2020;158(7):1881–98. doi:10.1053/j.gastro.2020.01.049.
- Xue L, He J, Gao N, Lu X, Li M, Wu X, Liu Z, Jin Y, Liu J, Xu J. et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. 2017;7(1):45176. doi:10.1038/srep45176.
- Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11(4):1024–38. doi:10.1038/s41385-018-0019-2.
- Qian M, Liu J, Zhao D, Cai P, Pan C, Jia W, Gao Y, Zhang Y, Zhang N, Zhang Y. Aryl hydrocarbon receptor deficiency in intestinal epithelial cells aggravates alcohol-related liver disease. Cell Mol Gastroenterol Hepatol. 2022;13(1):233–56. doi:10.1016/j.jcmgh.2021.08.014.
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci. 2009;106:3698–3703.
- Reddy JK, Hashimoto T. P Eroxisomal β-oxidation and peroxisome proliferator–activated Receptor α: An Adaptive Metabolic System. Annu Rev Nutr. 2001;21(1):193. doi:10.1146/annurev.nutr.21.1.193.
- Rogue A, Lambert C, Jossé R, Antherieu S, Spire C, Claude N, Guillouzo A, Agoulnik I. Comparative gene expression profiles induced by PPARγ and PPARα/γ agonists in human hepatocytes. PloS ONE. 2011;6(4):e18816. doi:10.1371/journal.pone.0018816.
- Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab Dispos. 2015;43(10):1522–35. doi:10.1124/dmd.115.064246.
- Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16(1):44–54. doi:10.1016/j.cmet.2012.05.012.
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatol. 2009;50(2):638. doi:10.1002/hep.23009.
- Li M, Sun X, Zhao J, Xia L, Li J, Xu M, Wang B, Guo H, Yu C, Gao Y. et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. 2020;17(7):753–64. doi:10.1038/s41423-019-0279-0.
- Hwang D, Jang BC, Yu G, Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-kappaB signaling pathways in macrophages. Biochem Pharmacol. 1997;54(1):87–96. doi:10.1016/s0006-2952(97)00154-8.
- Yeh ML, Yu ML. From nonalcoholic steatohepatitis, metabolic dysfunction-associated fatty liver disease, to steatotic liver disease: updates of nomenclature and impact on clinical trials. Clin Mol Hepatol. 2023;29(4):969–72. doi:10.3350/cmh.2023.0359.
- Chi Y, Wu Z, Du C, Zhang M, Wang X, Xie A, Wang P, Li R. Regulatory effects mediated by ulvan oligosaccharide and its zinc complex on lipid metabolism in high-fat diet-fed mice. Carbohydr Polym. 2023;300:120249. doi:10.1016/j.carbpol.2022.120249.
- Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76(8):1541–58. doi:10.1007/s00018-019-03011-w.
- Gupta H, Min B-H, Ganesan R, Gebru YA, Sharma SP, Park E, Won S-M, Jeong J-J, Lee S-B, Cha M-G. Gut microbiome in non-alcoholic fatty liver disease: from mechanisms to therapeutic role. Biomedicines. 2022;10(3):550. doi:10.3390/biomedicines10030550.
- Dai X, Hou H, Zhang W, Liu T, Li Y, Wang S, Wang B, Cao H. Microbial metabolites: critical regulators in NAFLD. Front Microbiol. 2020;11:567654. doi:10.3389/fmicb.2020.567654.
- Mar JS, Ota N, Pokorzynski ND, Peng Y, Jaochico A, Sangaraju D, Skippington E, Lekkerkerker AN, Rothenberg ME, Tan MW. et al. IL-22 alters gut microbiota composition and function to increase aryl hydrocarbon receptor activity in mice and humans. Microbiome. 2023;11(1):47. doi:10.1186/s40168-023-01486-1.
- Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F. et al. Diversity of bifidobacteria within the infant gut microbiota. PloS ONE. 2012;7(5):e36957. doi:10.1371/journal.pone.0036957.
- da Silva JGV, Vieira AT, Sousa TJ, Viana MVC, Parise D, Sampaio B, da Silva AL, de Jesus LCL, de Castro Oliveira L, Aburjaile FF. Comparative genomics and in silico gene evaluation involved in the probiotic potential of Bifidobacterium longum 51A. Gene. 2021;795:145781. doi:10.1016/j.gene.2021.145781.
- Sharma P, Bhardwaj P, Singh R. Administration of lactobacillus casei and bifidobacterium bifidum ameliorated hyperglycemia, dyslipidemia, and oxidative stress in diabetic rats. Int J Prev Med. 2016;7. doi:10.4103/2008-7802.188870.
- Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70(6):1174–82. doi:10.1136/gutjnl-2020-323071.