ABSTRACT
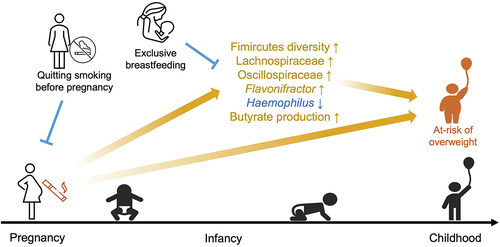
Childhood obesity is linked to maternal smoking during pregnancy. Gut microbiota may partially mediate this association and could be potential targets for intervention; however, its role is understudied. We included 1,592 infants from the Canadian Healthy Infants Longitudinal Development Cohort. Data on environmental exposure and lifestyle factors were collected prenatally and throughout the first three years. Weight outcomes were measured at one and three years of age. Stool samples collected at 3 and 12 months were analyzed by sequencing the V4 region of 16S rRNA to profile microbial compositions and magnetic resonance spectroscopy to quantify the metabolites. We showed that quitting smoking during pregnancy did not lower the risk of offspring being overweight. However, exclusive breastfeeding until the third month of age may alleviate these risks. We also reported that maternal smoking during pregnancy significantly increased Firmicutes abundance and diversity. We further revealed that Firmicutes diversity mediates the elevated risk of childhood overweight and obesity linked to maternal prenatal smoking. This effect possibly occurs through excessive microbial butyrate production. These findings add to the evidence that women should quit smoking before their pregnancies to prevent microbiome-mediated childhood overweight and obesity risk, and indicate the potential obesogenic role of excessive butyrate production in early life.
Introduction
Childhood obesity is a growing concern, affecting over 18% of children and adolescents aged 5–19 globally by 2020. This figure rose rapidly, from just 4% in 1975.Citation1 The increase in prevalence observed from 2010 to 2016 is also worth noting, especially in Southeast Asia, the Western Pacific, and Africa.Citation2,Citation3 Childhood obesity is linked to negative consequences, such as poorer health and self-esteem, being more likely to be bullied, and being more likely to become overweight or obese in adolescence and adulthood.Citation3
Obesity is a multifactorial disease affected by a combination of factors, such as genetic predisposition, cesarean delivery, formula feeding, maternal overweight, dietary habits, reduced physical activity, medications, and secondhand smoke exposure.Citation4,Citation5 Among these factors, early life exposure to tobacco smoke and its relationship with the risk of overweight development is a critical concern, but this has not yet been well studied for obesity risk. A recent study reported an association between childhood exposure to parental smoking and an increased risk of life-course overweight or obesity (OWOB).Citation6 An earlier meta-analysis also suggested that compared to paternal smoking, a direct intrauterine effect on childhood obesity is more prominent through maternal smoking during pregnancy.Citation7 Furthermore, a dose-dependent association between childhood OWOB and maternal smoking during pregnancy has been reported.Citation8
The gut microbiota, the complex and dynamic assembly of microorganisms residing in the gastrointestinal tract (GIT), has been associated with OWOB. Compared to their normal weight counterparts, the GIT of obese adults harbor a higher Firmicutes/Bacteroidetes (F/B) ratio and an enriched abundance of Firmicutes and Proteobacteria.Citation9,Citation10 Increased Bacteroides fragilis and Staphylococcus and depleted Bifidobacterium abundances have been repeatedly reported in association with childhood obesity across multiple cohorts.Citation11 Experimental studies based on fecal microbiota transplantation or supplementation with microbiota-derived molecules have established a causal role of altered microbiota in the development of obesity.Citation9,Citation12 Previous studies have shown that the development of the gut microbiota in early life is influenced by various external and host-related factors.Citation13–18 Being at the interface of external factors and host physiology and correlating with metabolic maturation,Citation13 early-life gut microbiota was unsurprisingly recognized as a mediator in the paths toward childhood OWOB. Our previous study reported that Lachnospiraceae abundance in early infancy (~3 months old) mediated the household disinfectant-related increase in BMI z-scores at ages 1 year and 3 years in the Canadian Healthy Infant Longitudinal Development (CHILD) cohort.Citation14 Similarly, enriched Lachnospiraceae abundance and microbial diversity in early infancy partially explain the increased risk of being overweight in formula-fed infants.Citation15 Other studies within the CHILD cohort also revealed that Lachnospiraceae, Firmicutes richness, Ruminococcaceae, and Bifidobacterium were mediators of the pathway from birth mode to childhood overweight.Citation16,Citation17 Furthermore, a recent study in the same cohort reported that Ruminococcus, Enterobacteriaceae, and Clostridium in late infancy (12 months old) were enriched in children with a rapid BMI growth trajectory.Citation19
The impact of maternal smoking on the infant gut microbiota has recently been discussed.Citation20 Levin et al found that the abundance of Akkermansia and Ruminococcus at one month of age was positively associated with environmental tobacco smoke exposure in a US birth cohort.Citation21 In another study, a German birth cohort study reported an association between maternal smoking during pregnancy and an increased abundance of Blautia and depleted Faecalibacterium abundance in 6-year-old children.Citation22 However, no study has investigated the health consequences of maternal smoking-induced gut microbiota changes in children. Considering the role of infant gut microbiota in the development of childhood OWOB, the potential impact of maternal smoking during pregnancy on early infant gut microbiota and the weight status of children remains to be determined. Although a previous review proposed certain microbiota mediatorsCitation20 in the path between maternal smoking during pregnancy and OWOB in offspring, a study with a large sample size with systematic consideration of potential confounders is necessary. We addressed these important questions in a large subset of the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort study.
Results
This study included a large subsample of 1,592 children with complete data on maternal smoking during pregnancy, BMI z-scores of children aged 1 year, and their gut microbiota data at ~3 months from the CHILD general population birth cohort (, ). Of these children, 90.5% (n = 1,441) had BMI data at the age of 3 years, 87.2% (n = 1,389) had gut microbiota data at 12 months, and 79.6% (n = 1,268) had both.
Figure 1. Schematic diagram for data collection and the association between maternal smoking during pregnancy and BMI z-scores of infants.

Table 1. Characteristics of the study subjects by exposure to maternal smoking during pregnancy.
In years 1 and 3, most children had a normal weight (n = 1,209; 75.9% and n = 963; 66.8%), followed by those who were at risk of being overweight (AROW, n = 277; 17.4% and n = 354; 24.6%), and those who were overweight or obese (OWOB, n = 72; 4.5% and n = 112; 7.8%). Only a small proportion of children were wasted, with a BMI z-score of < −2 (year 1, n = 34; 2.1%; year 3, n = 12; 0.8%). Furthermore, the overall BMI z-score distribution shifted toward higher values at year 3 compared to year 1 (mean ± s.d.:0.62 ± 0.99 vs. 0.18 ± 1.06, p < .001) ().
Maternal smoking during pregnancy increases the risk of being overweight at 1 and 3 years
Over 91% (n = 1,458) of the mothers either never smoked (n = 1,151; 72.3%) or quit smoking prior to pregnancy (n = 307; 19.3%) [median (IQR) years prior to conception:5.6 (2.7, 9.5)]. For the remaining 8.4%, half of them quit smoking (n = 68; 4.3%) and the other half cut (n = 54; 3.4%) or had the same number of cigarettes during pregnancy (n = 12; 0.8%). These self-reported data on smoking prior to and/or during pregnancy were highly correlated with concentrations of infant urinary nicotine metabolites, such as cotinine and trans-3’-hydroxycotinine, measured at the age of 3 months (Methods, Figure S1a-b), regardless of postnatal secondhand smoke exposure (Figure S2). Of note, the earlier mothers quit smoking prior to and during pregnancy, the lower the concentrations of these nicotine metabolites in the children’s urine (years quitting smoking prior to pregnancy: cotinine, Spearman’s Rho = −0.17, p = .006; hydroxycotinine, Spearman’s Rho = −0.28, p < 0.001) (weeks of pregnancy when quitting smoking: cotinine, Spearman’s Rho = 0.29, p = .048; hydroxycotinine, Spearman’s Rho = 0.22, p = .130) (Figure S1c-d). Food intake in 3-year-olds did not differ between children with and without exposure to maternal tobacco smoking during pregnancy, nor between children with normal weight and children who were AROW or OWOB in years 1 and 3 (Table S1).
To quantify the associations between maternal smoking status and childhood weight outcomes, we used generalized linear models with different adjustment levels (Methods, Figure S3, , Table S2). Compared to their non-exposed counterparts, children with exposure to maternal smoking during pregnancy had higher BMI z-scores at 1 year of age [Model 1: β = 0.27, 95% confidence interval (CI) 0.07–0.47], although it was not statistically significant with further adjustments (). This association was more evident for BMI z-scores at 3 years, even in the most adjusted model (Model 3: β = 0.28, 95% CI 0.06–0.49) (). They also had significantly higher odds of becoming AROW or OWOB at 3 years of age (Model 3: OR 1.78, 95% CI 1.11–2.86) but not at 1 year of age (). Interestingly, further analyses revealed that maternal smoking during pregnancy was positively associated with BMI z-score. Specifically, when compared with the children of nonsmoking mothers, quitting smoking prior to pregnancy was not associated with BMI z-scores of offspring at both ages 1 and 3 years (). On the other hand, quitting smoking during pregnancy was associated with increased BMI z-scores at age 3 years of age in the crude model (Model 1: β = 0.32, 95% CI 0.06–0.58); the association became not significant in Models 2 and 3, probably attributed to breastfeeding status being adjusted as a covariate, which may potentially serve as a mediator in the causal pathway. In line with this trend, remaining smoking during pregnancy with reduced or the same number of cigarettes, was associated with the highest BMI z-scores at 3 years of age (Model 3: β = 0.35, 95% CI 0.05–0.66). In addition, infants had a higher odd of becoming at-risk of overweight if they were born to a mother who quit smoking during pregnancy (Model 2: OR = 1.90, 95% CI 1.06–3.42) or smoked cigarettes during pregnancy (Model 2: OR = 2.00, 95% CI 1.05–3.84) ().
We next examined the joint effect of maternal smoking status during pregnancy and breastfeeding status at three months on childhood weight outcomes, given its influence on the above associations. Compared to children of nonsmoking mothers and exclusively breastfed (EBF) at three months, those of nonsmoker mothers but not exclusively breastfed (non-EBF) had higher BMI z-scores at both age 1 year (Model 3: β = 0.21, 95% CI 0.09–0.33) and age 3 years (Model 3: β = 0.13, 95% CI 0.01–0.25), as well as higher odds of being at risk of being overweight at age 1 year (Model 3: OR = 1.46, 95% CI 1.08–1.96) (). Alarmingly, within the non-EBF stratum, those with exposure to maternal smoking during pregnancy had even higher BMI z-scores and odds of becoming at risk of being overweight at both year 1 (BMI z-score Model 3: β = 0.50, 95% CI 0.23–0.76; AROW or OWOB Model 3: OR = 2.01, 95% CI 1.14–3.55) and year 3 (BMI z-score Model 3: β = 0.43, 95% CI 0.17–0.70; AROW or OWOB Model 3: OR = 2.21, 95% CI 1.25–3.90) (). In contrast, those with maternal smoking exposure but exclusively breastfed at three months did not show significantly increased risks as was observed in the non-EBF stratum (p > .05) (). However, given the broad confidence intervals observed in this group, a larger sample size may be required to robustly demonstrate breastfeeding’s effect in attenuating the smoke exposure-associated risks. As revealed by an interaction analysis, maternal smoking during pregnancy was significantly associated for all weight outcomes (except year-1 AROW) only within the stratum of non-EBF infants (Table S3). In addition, there were no multiplicative or additive interactions between these two factors (Table S3).
Since maternal pre-pregnancy weight status is a well-known risk factor for childhood overweight, we also tested the joint effect of maternal pre-pregnancy weight and maternal smoking status during pregnancy. When considering children of normal-weight mothers who did not smoke during pregnancy as the reference group, children of normal-weight mothers who smoked during pregnancy had a higher risk of becoming overweight as opposed to children of nonsmoking OWOB mothers at the age of 3 years (Model 3: OR 2.09, 95% CI 1.15–3.80) (). Additionally, being the highest-risk group, children of smoking OWOB mother had 2.55 (1.26, 5.16) higher odds of being at risk of being overweight at 3 years of age (Model 3) (). In an interaction analysis, maternal smoking during pregnancy was significantly associated weight outcomes at 3 years within the stratum of normal weight mothers; meanwhile, no multiplicative or additive interactions were observed between these two factors (Table S4).
For early life tobacco exposure, it is important to dissect its effect into the direct intrauterine effect due to maternal smoking during pregnancy and the effect of postnatal secondhand smoke exposure. Compared to postnatal secondhand smoke exposure, maternal smoking during pregnancy had a greater impact on the BMI z-scores of the offspring (Figure S4, Table S5). Furthermore, the urine levels of nicotine metabolites in offspring at the age of 3 months were positively correlated with BMI z-scores (Pearson’s correlation tests, p < 0.05), but not with AROW or OWOB in both years 1 and 3 (p > .05, Table S6).
Maternal smoking during pregnancy is associated with altered gut microbiota in offspring
We next investigated the effect of maternal smoking on the infant gut microbiota. According to the self-administered questionnaire, significant differences in the overall gut microbiota composition (measured by Bray-Curtis dissimilarity) were observed in both early infancy (p = .001) and late infancy (p = 0.025) between children who were or were not exposed to tobacco smoke during pregnancy through their mothers (). Alpha diversity indices of the gut microbiota in both early and late infancy were significantly increased in children with a history of exposure to maternal smoking during pregnancy (Table S7), particularly for all diversity indices of the Firmicutes phylum and the phylogenetic diversity index of Actinobacteria (Table S7, , Figure S5a).
Figure 2. Associations between maternal smoking during pregnancy and profiles of gut microbiota in infancy.

Figure 3. Early-infancy microbial mediators in the pathway from maternal smoking during pregnancy to weight outcomes at 1 and 3 years.

As per the LEfSe analysis, several Firmicutes members, particularly those from the Clostridia class, were significantly enriched in children exposed to maternal smoking during pregnancy in early infancy (). These included Lachnospiraceae, Oscillospiraceae, Ruminococcus gnavus, Ruminococcus torques, and Flavonifractor. Megasphaera (a Veillonellaceae member) from the Firmicutes phylum and Sutterella from the Proteobacteria phylum were also higher in this group of children. In contrast, an increased abundance of Blautia, Rothia, and Haemophilus was observed in the non-exposed children (). In late infancy, we observed consistent directions of enrichment for Oscillospiraceae, Ruminococcus torques, Flavonifractor, Sutterella, and Haemophilus as were observed in early infancy. Additionally, increased abundance of Veillonellaceae, mainly Veillonella, was observed in the non-exposed group (). Notably, most of these differential taxa were significantly correlated with maternal smoking exposure levels during pregnancy (Table S8). Besides, in early infancy, their associations with maternal smoking during pregnancy were evident regardless of maternal pre-pregnancy weight status (Figure S5) or breastfeeding status at 3 months (Figure S6), with few exceptions observed for Lachnospiraceae and Haemophilus.
Gut microbial features mediate the effect of maternal prenatal smoking on childhood BMI
We then identified potential gut microbiota mediators in the path from maternal smoking during pregnancy to childhood overweight and obesity. Among microbial features associated with maternal smoking, Chao1 richness and Faith’s phylogenetic diversity (Faith’s PD, a measure of alpha diversity incorporating phylogenetic distance between taxa) within Firmicutes, Lachnospiraceae, Oscillospiraceae, Flavonifractor and Haemophilus were significantly associated with BMI z-scores at both 1 and 3 years of age (p < 0.05, , Figure S7a). All these taxa and indices were related to Firmicutes and were positively associated with BMI z-scores, except Haemophilus, a member of the Proteobacteria phylum, which was inversely correlated with the outcomes.
These microbial features in early infancy significantly mediated the association between maternal smoking during pregnancy and childhood BMI z-scores, especially at the age of 3 years, although with smaller effect sizes (p < 0.05, ). Among these mediators, the two Firmicutes diversity indices (Chao1 richness and Faith’s PD) mediated the highest proportions of the total effects, accounting for 23.3–24.6% of those on year-1 BMI z-scores, and 12.4–15.2% of those on year-3 BMI z-scores. Mediation by late-infancy microbiota was significant only for Firmicutes Chao1 richness (7.2%, p = 0.012) and Faith’s PD (7.1%, p = 0.024) (Figure S7b).
When we looked at mediation effects for the binary outcome (normal weight vs. AROW or OWOB), only the mediation effects of early-infancy Haemophilus abundance (8.9%, p = 0.020) and Firmicutes Chao1 richness (9.9%, p = 0.028) on weight status at age 3 years remained significant (, Figure S7b). Additionally, the early-infancy Firmicutes Faith’s phylogenetic diversity index, and abundances of Oscillospiraceae, Lachnospiraceae and Haemophilus collectively mediated 24.2% (95% CI 8.3% − 54.5%) of the effect on year-3 BMI z-scores and 18.6% (95% CI 3.2% − 41.7%) of the effect on the year-3 binary outcome (Table S9).
Gut microbiota-derived butyrate is a potential metabolic mediator
Since metabolites of the gut microbiota are known to be implicated in overweight and obesity, we further investigated metabolic mediators in these associations. Among early infancy fecal metabolites, we observed elevated butyrate but reduced pyruvate and succinate levels in the gut of children whose mothers had normal weight and smoked during pregnancy (); the concentrations of these metabolites were associated with maternal pre-pregnancy BMI (Table S10). In addition, partial or zero breastfeeding at 3 months was associated with higher butyrate levels across all smoking exposure strata (Figure S8a). Curiously, among exclusively breastfed infants, those whose mothers had quit smoking prior to pregnancy had the highest butyrate levels (Figure S8a). Moreover, butyrate was positively correlated with BMI z-scores at both age 1 (Spearman’s Rho = 0.13, p = .002) and 3 years (Spearman’s Rho = 0.14, p = .001), whereas pyruvate was inversely associated with BMI z-scores at 3 years of age (Spearman’s Rho = −0.16, p < .001) (Table S2). Interestingly, analysis of the PICRUSt2-predicted functional potential of early-infancy microbiota (Methods) showed that pathways linked to butyrate production, such as pathways for succinate fermentation to butyrate, L-lysine fermentation to acetate and butyrate, and acetyl-CoA fermentation to butyrate II, were higher in children with maternal smoke exposure during pregnancy and were significantly correlated with fecal butyrate, pyruvate, and succinate levels (p < .05, ). These pathways were also positively associated with BMI z-scores at 3 years of age (p < .05), among which the pathway for succinate fermentation to butyrate was also significantly positively correlated with BMI z-scores at 1 year of age (p < .05, ). In addition, the pathway for pyruvate fermentation to acetone was also elevated in children exposed to maternal smoke (p < .05; ). These pathways were mainly contributed by Flavonifractor and Lachnoclostridium - two genera associated with exposure to maternal tobacco smoke during pregnancy, and Veillonella, Bifidobacterium, Escherichia/Shigella, Bacteroides, Streptococcus, and Clostridium sensu stricto (Cluster I) (Figure S8b). Among the predicted pathways of the late-infancy microbiota, the pathway for L-lysine fermentation to acetate and butyrate, and the pathway for acetyl-CoA fermentation to butyrate II were also increased in children with maternal smoke exposure (p < .05), but were not correlated with their BMI outcomes (p > .05). Finally, within a limited sample size with both fecal butyrate and weight outcome data available (n = 623), butyrate showed a trend to mediate the association between maternal prenatal smoking and certain childhood weight outcomes (proportion mediated in unadjusted models: year-1 BMI z-scores, 6.7%, p = .066; year-3 AROW or OWOB, 6.4%, p = .054; proportion mediated in adjusted models: year-1 BMI z-scores, 6.4%, p = .182; year-3 AROW or OWOB, 5.1%, p = .198).
Figure 4. Metabolite mediators.

Discussion
In this large cohort study using a multi-omics approach, we are the first to identify several gut microbial mediators, mostly Firmicutes, of the effect of maternal smoking during pregnancy on childhood overweight and obesity, as well as potential metabolic players in these pathways. In general, Firmicutes are highly populated in obese subjects and have been positively associated with smoking in adults.Citation9,Citation10 Here, we build upon these findings and provide evidence of increased Firmicutes diversity in smoking mothers or children with maternal smoke exposure and its obesogenic role.
Being the main producers of short-chain fatty acids (SCFAs) like butyrate,Citation23 higher Firmicutes diversity may result in increased butyrate production in the gut. In agreement with this hypothesis, we observed an elevation in fecal butyrate levels in children with a history of maternal smoking during pregnancy. On the other hand, the decreased concentration of pyruvate, an antagonist of monocarboxylate transporters involved in butyrate transport, may promote butyrate absorption by the host. Despite being generally considered beneficial for weight control,Citation24 several cross-sectional studies have shown that obese subjects have higher fecal SCFA levels than lean ones.Citation25–27 A recent study also showed a positive association between fecal butyrate concentration and child BMI z-scores in toddlers.Citation25 In a clinical trial for weight control, the gut microbial capacity for butyrate production was found to be significantly reduced after a low-energy diet intervention.Citation28 Another recent study based on the CHILD cohort reported the first piece of evidence for a temporal relationship between higher butyrate concentrations measured in early infancy and higher year-1 and year-3 BMI z-scores.Citation29 The current study further illustrates that this relationship is partially attributed to exposure to maternal smoking during pregnancy, an effect that might be alleviated by maintaining exclusive breastfeeding for the first three months.
One possible mechanism of the positive butyrate-childhood obesity correlation is that an overabundance of available butyrate may result in the generation of excessive substrates for the citric acid cycle and lipid biosynthesis,Citation30 which leads to extra weight gain. Additionally, butyrate can enhance growth hormone secretion by anterior pituitary cells in rats.Citation31 This growth hormone increases energy expenditure and reduces fat mass in adults.Citation32 Nonetheless, it also promotes weight gain in normal weight and underweight children.Citation33
Taxa enriched in children with maternal tobacco smoke exposure, such as Sutterella, Ruminococcus, and Lachnospiraceae, were previously reported to be enriched in 12-month or mother’s samples compared to newborn or 4-month samples. Conversely, Haemophilus, which was depleted in children exposed to maternal smoking during pregnancy, was enriched in the newborns.Citation34 Changes in the relative abundances of these age-related taxa, together with elevated Firmicutes diversity, indicate early maturation in the gut microbiota of children with maternal smoke exposure during pregnancy. In the first study quantifying gut microbiota maturity, Subramanian et al. reported persistently immature gut microbiota in malnourished children, which was transiently improved following dietary interventions.Citation35 While the positive association between gut maturity and nutritional status in malnutrition cannot be directly extrapolated to the domain of obesity, our findings indicate that the acceleration of gut microbiota maturity in infancy may induce extra weight gain in childhood. This hypothesis is supported by repeated observations that partially or exclusively formula-fed infants have higher gut microbiota maturity,Citation36 and are more likely to gain weight rapidly.Citation37 These microbiota maturity related taxa, together with those relevant to SCFA production, may serve as potential predictive markers and intervention targets for childhood obesity regardless of smoke exposure during pregnancy.
Among other microbes associated with exposure to maternal smoking and/or childhood weight status, FlavonifractorCitation38 and LachnospiraceaeCitation39 were associated with increased inflammatory cytokines that may facilitate weight gain. Haemophilus was found to be more abundant in the oral microbiome of normal weight subjects and in nonsmokers than in obese and smokers,Citation40 but was reported to be more abundant in obese subjects in another cohort.Citation41 In contrast, Flavonifractor plautti, R. torques, and R. gnavus increased in abundance, whereas Haemophilus parainfluenzae decreased in abundance in adults with active or passive exposure to tobacco smoke in a Dutch cohort.Citation42 H. parainfluenzae was also negatively associated with childhood smoke exposure.Citation42 Flanonifractor and Lachnoclostridium were also enriched in children exposed to maternal smoking during pregnancy and were shown to mediate the associations of the Energy-Adjusted Dietary Inflammatory Index (E-DII score) with visceral adipose tissue and liver fat.Citation43 Although further mechanistic investigation of these mediators is required, suppressing the disease-associated taxa (such as via phage therapy) may reduce the risk of developing obesity and relevant health conditions.
Our study also reports important observational evidence that quitting smoking or reducing the number of cigarettes smoked after conception does not lower the risk of childhood overweight and obesity.Citation44 Smoking may also have a long-term and trans-generational effect through reprogramming gut microbiota and physiology, as higher butyrate levels were observed in the offspring of those who quit smoking prior to pregnancy than in those of nonsmokers. However, quitting smoking before conception can reduce the risk of childhood overweight and obesity. These findings add to the large body of evidence regarding the harmful effects of maternal smoking.Citation7,Citation8 The addition of these findings on obesity risk supplements public health practitioners’ call for potential policy interventions, parents’ decisions to quit maternal smoking prior to pregnancy, and nonsmokers’ decisions to try smoking.
This study was limited by the lack of gut microbiota samples from mothers. Whether maternal smoking during pregnancy affects unnecessary weight gain in children via vertical transmission of obesogenic gut microbiota warrants future longitudinal studies involving the collection of maternal gut microbiota samples. In addition, epigenetic alterations induced by smoke exposure, such as those in the aryl hydrocarbon receptor repressor (AhRR) gene, have been found to be transgenerational.Citation45,Citation46 The target of this repressor, AHR, is implicated in homeostasisCitation47 and can interact bidirectionally with the gut microbiota bidirectionally.Citation48 Therefore, exposure to maternal smoking during pregnancy may affect the assembly of gut microbiota in offspring through epigenetic effects. Furthermore, although we proposed a directed acyclic graph based on biological plausibility, the findings of the current study are still associated. Therefore, experimental studies are required to establish causality. Despite having a large cohort, we were unable to conduct further analyses for certain subgroups with a small sample size, such as testing the mediation role of breastfeeding in the causal pathway. Adjusting for this potential mediator as a covariate might have resulted in bias in the effect estimates. Lastly, a large-scale external cohort is warranted for validation but is currently not available.
In conclusion, this study revealed that the increased diversity of Firmicutes members mediates the effect of maternal smoking during pregnancy on childhood obesity in the offspring. This increase in the diversity of Firmicutes may play an obesogenic role via enhanced butyrate production, despite its general anti-obesity activities in adults. These findings highlight the mediating role of gut microbiota in the effect of prenatal detrimental exposure on childhood outcomes and open up avenues for the future development of microbiome-based weight control in pediatric populations.
Methods
Study design
To understand the influential factors of asthma, allergies, obesity, and other chronic diseases, the CHILD Cohort Study originally recruited 3,264 mothers during the third trimester of their pregnancies from four study sites between 2009 and 2012. Enrollment was confirmed upon singleton live birth at ≥35 weeks of gestation with a birth weight of ≥2,500 g. Major exclusion criteria were 1) in vitro fertilized births to avoid multiple gestation or preterm births, and 2) home births due to missing documentation of maternal intrapartum antibiotic prophylaxis. Mothers were followed up throughout pregnancy, and their children were assessed from birth to 3 years of age. The present study involved a large subset of 1,592 subjects with complete information on maternal smoking status during pregnancy, weight, length/height (at age 1 year and 3 years), and microbiota data (at approximately 3 months and 12 months of age). Samples from the Toronto site were excluded due to the lack of a provincial prescription database for assessing antibiotic medication.
The CHILD Cohort Study received initial approval from the Human Research Ethics Boards at the University of Manitoba, University of Alberta, and University of British Columbia. Written informed consent was obtained from the legal guardians of each participant. The present study was conducted as a sub-study program within the CHILD study, specifically approved by the University of Alberta (SyMBIOTA, Study ID: Pro00010073). Access to the data from the CHILD Cohort Study and the proposal for publication were both granted approval by CHILD (Concept Title C18, Concept Proposal #46, and Publication Proposal #1107).
Exposure and outcome measurements
Maternal smoking status during pregnancy and covariates were collected through standardized questionnaires completed or obtained from hospital records by CHILD study staff (Supplementary Methods). Maternal smoking status during pregnancy was categorized into 1) never smoker, 2) quit smoking before conception, 3) quit smoking during pregnancy, 4) cut the number of cigarettes during pregnancy, and 5) had the same number of cigarettes during pregnancy. In modeling of the effect of detailed maternal smoking statuses during pregnancy, cutting the number of cigarettes during pregnancy and having the same number of cigarettes during pregnancy were combined into one category (“Yes”) due to small sample sizes. These detailed statuses were also dichotomized into 1) “Yes/Smoker”: those who quit smoking during pregnancy, reduced the number of cigarettes, or had the same number of cigarettes (as before conception) during pregnancy; and 2) “No/Non-smoker”: those who quit smoking before conception, or never smoked. Urine levels of two nicotine metabolites, cotinine and trans-3’- hydroxycotinine, measured at 3 months of age by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry (LC-MS), were used to verify the self-reported smoking status during pregnancy.
The weight and length/height of the children were measured by the CHILD study staff at 1 and 3 years of age. From these measures, sex-specific body mass index (BMI, kg/mCitation2-for-age z-scores were computed according to WHO Child Growth reference data using the “igrowup” package for SPSS.Citation49 Sex-specific BMI-for-age z-scores >5 or ≤ −5 were considered outliers and removed from downstream analyses. Weight status was categorized into “Wasted” (BMI z-score < −2), “Normal” (−2 ≤ BMI z-score ≤1), “At-risk overweight” (1 < BMI z-score ≤2), “Overweight” (2 < BMI z-score ≤3) and “Obese” (BMI z-score >3). Weight status was also dichotomized into “Normal” (−2 ≤ BMI z-score ≤1) and “AROW or OWOB” (BMI z-score >1), where those who were “Wasted” (BMI z-score < −2) were excluded.
Taxonomic and metabolomic profiling of fecal microbiota
Fecal samples were collected in early infancy (at age 3.5 ± 1.0 months) during a planned home visit, and in late infancy (at age 12.3 ± 1.4 months) during a clinical study visit using a standard protocol.Citation50 Methods of sample processing, DNA extraction, amplification, and sequencing (the V4 region of 16S ribosomal RNA) were described in our previous study.Citation18 Amplicon sequence variants (ASVs) were generated from sequencing reads and annotated using the QIIME2 pipeline (v2020.6),Citation51 based on which alpha indices (observed ASVs, Chao1 index, Shannon diversity, Simpson’s diversity and Faith’s phylogenetic diversity) and beta diversity (Bray-Curtis dissimilarity) were calculated, and metabolic functions were predicted using PICRUSt2 (Supplementary Methods).Citation52,Citation53
Fecal metabolites were measured in a subset of 3-month samples (n = 623) using magnetic resonance spectroscopy and quantified as micromoles per gram of feces (μmol/g).Citation54 In this study, 33 metabolites detected in > 80% of the samples were analyzed. Nicotine metabolites, namely cotinine and 3-hydroxycotinine, were examined in a subset of samples (n = 1,368) collected at 3 months. Analysis was conducted using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry, and the results were reported as concentrations in ng/mL.Citation55
Statistical analysis
This study was conducted within the CHILD cohort using all available data, and a sample size calculation was not applicable. For bivariate analysis, the Fisher’s exact test was used for categorical data. T-tests/analysis of variance (ANOVA) and Wilcoxon’s tests/Kruskal-Wallis tests were performed for two/four-group comparisons of continuous parameters that were and were not normally distributed. Dunn’s test was used for pairwise comparisons of continuous data between multiple groups, and false discovery rate (FDR) correction was performed using the Benjamini-Hochberg method for multiple comparisons. Spearman’s and Pearson’s tests were performed for correlations between continuous data where appropriate.
Generalized linear models were used to estimate the effect sizes. When modeling the maternal smoking-BMI z-score/weight status relationship, potential confounders and covariates were adjusted for. In Model 1, we adjusted for 1) the minimum set of confounders identified by a direct acyclic graph (DAG) analysis, that is, maternal race/ethnicity and socioeconomic status, and 2) maternal pre-pregnancy BMI, a known factor correlating with childhood weight status (except in the models assessing the joint effects of maternal BMI and smoking status). In Model 2, we further controlled for variables differentially distributed between the exposed and non-exposed groups, including prenatal and postnatal pet exposure, breastfeeding status at three months (except in the models assessing the joint effects of maternal smoking status and breastfeeding status at three months), solid food introduction at three months, and household disinfectant use. Finally, in Model 3, we also controlled for factors that were potentially correlated with the BMI z-scores of children, including prenatal diet calories, infant sex, birth mode, and antibiotic use in the first three months. In addition, the interactionR of R package “interactionR”Citation56 was used to investigate potential interactions between maternal smoking during pregnancy and maternal pre-pregnancy weight status/breast feeding status, with covariates controlled for as in Model 3 above. Maternal education level was adjusted as a proxy for socioeconomic status. Differentially abundant microbial taxa between the two groups were identified using linear discriminant analysis effect size (LEfSe).Citation57
Mediation analysis was conducted using the mediate function of R package “mediation”,Citation58 while confidence intervals and p values were generated using 1,000 bootstraps. To account for confounding effects of exposures and life-style factors on BMI outcomes and microbial abundance or alpha diversity indices, propensity scores were first estimated by the Covariate Balancing Propensity Score (CBPS) method implemented in R package “CBPS”.Citation59 According to the DAG (Figure S3), the fitted factors included maternal race/ethnicity, maternal pre-pregnancy BMI, prenatal and postnatal pet exposure, breastfeeding status at three months, solid food introduction at three months, household disinfectant use, prenatal diet calories, infant sex, birth mode, antibiotic use and the age of sample collection. The propensity score, together with socioeconomic status and maternal race/ethnicity, were then included in the mediation analysis. In addition, the mma function of R package “Multiple Mediation Analysis (mma)”Citation60 was used to explore the combined mediation effects of the potential mediators while adjusting for the covariates mentioned above. In this analysis, Firmicutes Faith’s phylogenetic diversity index, Oscillospiraceae, Lachnospiraceae and Haemophilus were included. Firmicutes Chao1 index was not included because it was highly correlated with the Firmicutes Faith’s phylogenetic diversity index; while Flavonifractor was not included as its family Oscillospiraceae has a larger effect size in the separate model.
In differential abundance analysis, correlation analysis and modeling, microbial taxa (phylum to genus) with a prevalence of < 1% or a mean abundance of < 0.1% at each time point were removed; relative abundance and alpha diversity indices were subjected to rank-based inverse normal transformation.Citation61
Pairwise deletion was performed in bivariate analysis, whereas listwise deletion was performed in modeling missing data. Statistical significance was set at p = 0.05. All statistical analyses were performed using R version 4.1.0.
Author contributions
Study design — Hein Mun Tun, Anita L. Kozyrskyj. Data acquisition — Jaclyn Parks, Tim K. Takaro: environmental exposures, especially smoke exposure; urine metabolite measurement; Catherine J. Field, Piush Mandhane, Theo J. Moraes, Elinor Simons, Stuart E. Turvey, Padmaja Subbarao, Jeffrey R., Brook, James A. Scott: subject recruitment, cohort follow-up, sample collection, biobanking, data registry; James A. Scott: fecal microbiota sequencing; Anita L. Kozyrskyj: measurement of fecal metabolites. Data cleaning — Hogan Kok-Fung Wai, Xi Zhang: cleaning questionnaire data and confirming their accuracy. Data analysis — Ye Peng, Hein Min Tun: Analysis of the microbiota profiles using an updated analytical pipeline, identification of the sub-cohort, associations between smoke exposure and childhood weight outcome, associations between smoke exposure and infant microbiota, mediation analysis. Data interpretation — Ye Peng, Hein Min Tun: all aspects; Hogan Kok-Fung Wai, Jaclyn Parks, Catherine J. Field, Piush Mandhane, Theo J. Moraes, Elinor Simons, Stuart E. Turvey, Padmaja Subbarao, Jeffrey R., Brook, Tim K. Takaro, James A. Scott: epidemiological evidence of the relationship between the exposure, mediator and outcomes; Siew Chien Ng, Xi Zhang, Francis Ka-Leung Chan: potential microbiota-centered mechanisms, clinical implications. Initial drafting — Ye Peng: Introduction, Results, Discussion, Methodologies; Hein Min Tun: Introduction, Results, Discussion. Critical review — Siew Chien Ng, Hogan Kok-Fung Wai, Xi Zhang, Jaclyn Parks, Catherine J. Field, Piush Mandhane, Theo J. Moraes, Elinor Simons, Stuart E. Turvey, Padmaja Subbarao, Jeffrey R., Brook, Tim K. Takaro, James A. Scott, Francis Ka-Leung Chan. All authors approved the final version of the manuscript and agree to be accountable for every aspect in ensuring that questions related to the accuracy of the findings in the manuscript.
Competing interests
Francis Ka-Leung Chan is Board Member of CUHK Medical Centre. He is a co-founder, non-executive Board Chairman and shareholder of GenieBiome Ltd. He receives patent royalties through his affiliated institutions. He has received fees as an advisor and honoraria as a speaker for Eisai Co. Ltd., AstraZeneca, Pfizer Inc., Takeda Pharmaceutical Co., and Takeda (China) Holdings Co. Ltd. Siew Chien Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and Abbvie and received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, Abbvie, and Takeda. Siew Chien Ng has received research grants through her affiliated institutions from Olympus, Ferring, and Abbvie. Siew Chien Ng is a founder member, non-executive director, non-executive scientific advisor, and shareholder of GenieBiome Ltd. Siew Chien Ng receives patent royalties through her affiliated institutions. Francis Ka-Leung Chan, Siew Chien Ng, and Hein Min Tun are named inventors of patent applications held by the CUHK and MagIC that cover the therapeutic and diagnostic use of microbiome. All other coauthors have no conflict of interest.
R2_Supplementary_materials_clean.docx
Download MS Word (2.8 MB)Acknowledgments
The authors thank all the families who participated in this study and the entire CHILD team, which included interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. We also thank Prof. Samuel Yeung-shan Wong, Prof. Kinfai Ho and Miss. Xueqi Wu from the School of Public Health and Primary Care, the Chinese University of Hong Kong for reviewing the manuscript and providing comments and suggestions on the methodologies; Mr. Shilin Zhao from the Department of Medicine & Therapeutics, the Chinese University of Hong Kong for providing advice for the mediation analysis; and Mr. Matthew Wong from the Microbiota I-Center (MagIC), Hong Kong SAR, for reviewing the manuscript.
Data availability statement
De-identified data are available from the corresponding author upon reasonable request. Registration and/or proposal applications may be needed depending on the tier of access requested (https://childstudy.ca/for-researchers/data-access/).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2323234
Additional information
Funding
References
- World Health Organization. Fact sheets on obesity and overweight available from https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=Key%20facts,has%20nearly%20tripled%20since%201975.&text=39%20million%20children%20under%20the,overweight%20or%20obese%20in%202016.
- Ezzati M, Bentham J, Di Cesare M, Bilano V, Bixby H, Zhou B, Adams RJ, Aekplakorn W, Afsana K, Aguilar-Salinas CA. et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–18. doi:10.1016/S0140-6736(17)32129-3.
- World Health Organization. Taking action on childhood obesity (2018). https://apps.who.int/iris/handle/10665/274792.
- Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne). 2021;12:706978. doi:10.3389/fendo.2021.706978.
- Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673–89. doi:10.1007/s40273-014-0243-x.
- Jaakkola JM, Rovio SP, Pahkala K, Viikari J, Ronnemaa T, Jula A, Niinikoski H, Mykkänen J, Juonala M, Hutri-Kähönen N. et al. Childhood exposure to parental smoking and life-course overweight and central obesity. Ann Med. 2021;53(1):208–16. doi:10.1080/07853890.2020.1853215.
- Riedel C, Schonberger K, Yang S, Koshy G, Chen YC, Gopinath B, Ziebarth S, von Kries R. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared with paternal smoking--a meta-analysis. Int J Epidemiol. 2014;43(5):1593–1606. doi:10.1093/ije/dyu150.
- von Kries R, Toschke AM, Koletzko B, Slikker W Jr. Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156(10):954–961. doi:10.1093/aje/kwf128.
- Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11(9):639–47. doi:10.1038/nrmicro3089.
- Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74(9):1251–62. doi:10.1038/s41430-020-0607-6.
- de Cuevillas B, Milagro FI, Tur JA, Gil-Campos M, de Miguel-Etayo P, Martinez JA, de Cuevillas B, de Miguel‐Etayo P, Navas‐Carretero S. Fecal microbiota relationships with childhood obesity: a scoping comprehensive review. Obes Rev. 2022;23(Suppl 1):e13394. doi:10.1111/obr.13394.
- Lee P, Yacyshyn BR, Yacyshyn MB. Gut microbiota and obesity: an opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes Metab. 2019;21(3):479–90. doi:10.1111/dom.13561.
- Jian C, Carpen N, Helve O, de Vos WM, Korpela K, Salonen A. Early-life gut microbiota and its connection to metabolic health in children: perspective on ecological drivers and need for quantitative approach. EBioMedicine. 2021;69:103475. doi:10.1016/j.ebiom.2021.103475.
- Tun MH, Tun HM, Mahoney JJ, Konya TB, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR. et al. Postnatal exposure to household disinfectants, infant gut microbiota and subsequent risk of overweight in children. CMAJ. 2018;190(37):E1097–E1107. doi:10.1503/cmaj.170809.
- Forbes JD, Azad MB, Vehling L, Tun HM, Konya TB, Guttman DS, Field CJ, Lefebvre D, Sears MR, Becker AB. et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018;172(7):172. doi:10.1001/jamapediatrics.2018.1161.
- Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR. et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018;172(4):368–77. doi:10.1001/jamapediatrics.2017.5535.
- Vu K, Lou W, Tun HM, Konya TB, Morales-Lizcano N, Chari RS, Field CJ, Guttman DS, Mandal R, Wishart DS. et al. From birth to overweight and atopic disease: multiple and common pathways of the infant gut microbiome. Gastroenterology. 2021;160(1):128–44 e10. doi:10.1053/j.gastro.2020.08.053.
- Tun HM, Peng Y, Chen B, Konya TB, Morales-Lizcano NP, Chari R, Field CJ, Guttman DS, Becker AB, Mandhane PJ. et al. Ethnicity associations with food sensitization are mediated by gut microbiota development in the first year of life. Gastroenterology. 2021;161(1):94–106. doi:10.1053/j.gastro.2021.03.016.
- Reyna ME, Petersen C, Dai DLY, Dai R, Becker AB, Azad MB, Miliku K, Lefebvre DL, Moraes TJ, Mandhane PJ. et al. Longitudinal body mass index trajectories at preschool age: children with rapid growth have differential composition of the gut microbiota in the first year of life. Int J Obes (Lond). 2022;46(7):1351–1358. doi:10.1038/s41366-022-01117-z.
- McLean C, Jun S, Kozyrskyj A. Impact of maternal smoking on the infant gut microbiota and its association with child overweight: a scoping review. World J Pediatr. 2019;15(4):341–9. doi:10.1007/s12519-019-00278-8.
- Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, Kim H, Zoratti EM, Lukacs NW, Boushey HA. et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep. 2016;6(1):31775. doi:10.1038/srep31775.
- Gschwendtner S, Kang H, Thiering E, Kublik S, Fosel B, Schulz H, Krauss-Etschmann S, Heinrich J, Schöler A, Schloter M. et al. Early life determinants induce sustainable changes in the gut microbiome of six-year-old children. Sci Rep. 2019;9(1):12675. doi:10.1038/s41598-019-49160-7.
- Parada Venegas D, la Fuente MK D, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi:10.3389/fimmu.2019.00277.
- Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–73. doi:10.1038/s41574-019-0156-z.
- Nandy D, Craig SJC, Cai J, Tian Y, Paul IM, Savage JS, Marini ME, Hohman EE, Reimherr ML, Patterson AD. et al. Metabolomic profiling of stool of two-year old children from the INSIGHT study reveals links between butyrate and child weight outcomes. Pediatr Obes. 2022;17(1):e12833. doi:10.1111/ijpo.12833.
- Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18(1):190–195. doi:10.1038/oby.2009.167.
- Teixeira TF, Grzeskowiak L, Franceschini SC, Bressan J, Ferreira CL, Peluzio MC. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br J Nutr. 2013;109(5):914–919. doi:10.1017/S0007114512002723.
- Jian C, Silvestre MP, Middleton D, Korpela K, Jalo E, Broderick D, de Vos WM, Fogelholm M, Taylor MW, Raben A. et al. Gut microbiota predicts body fat change following a low-energy diet: a PREVIEW intervention study. Genome Med. 2022;14(1):54. doi:10.1186/s13073-022-01053-7.
- Bridgman SL, Malmuthuge N, Mandal R, Field CJ, Haqq AM, Mandhane PJ, Moraes TJ, Turvey SE, Simons E, Subbarao P. et al. Childhood body mass index and associations with infant gut metabolites and secretory IgA: findings from a prospective cohort study. Int J Obes (Lond). 2022;46(9):1712–1719. doi:10.1038/s41366-022-01183-3.
- Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: a double-edged sword for health? Adv Nutr. 2018;9(1):21–29. doi:10.1093/advances/nmx009.
- Miletta MC, Petkovic V, Eble A, Ammann RA, Fluck CE, Mullis PE, Blachier F. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PloS One. 2014;9(10):e107388. doi:10.1371/journal.pone.0107388.
- Rasmussen MH. Obesity, growth hormone and weight loss. Mol Cell Endocrinol. 2010;316(2):147–53. doi:10.1016/j.mce.2009.08.017.
- Reinehr T, Lindberg A, Koltowska-Haggstrom M, Ranke M. Is growth hormone treatment in children associated with weight gain?–longitudinal analysis of KIGS data. Clin Endocrinol (Oxf). 2014;81(5):721–6. doi:10.1111/cen.12464.
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703. doi:10.1016/j.chom.2015.04.004.
- Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD. et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–21. doi:10.1038/nature13421.
- Fehr K, Moossavi S, Sbihi H, Boutin RCT, Bode L, Robertson B, Yonemitsu C, Field CJ, Becker AB, Mandhane PJ. et al. Breastmilk feeding practices are associated with the Co-occurrence of bacteria in mothers’ milk and the infant gut: the CHILD cohort study. Cell Host Microbe. 2020;28(2):285–297.e4. doi:10.1016/j.chom.2020.06.009.
- Azad MB, Vehling L, Chan D, Klopp A, Nickel NC, McGavock JM, Becker AB, Mandhane PJ, Turvey SE, Moraes TJ. et al. Infant feeding and weight gain: separating breast milk from breastfeeding and formula from food. Pediatrics. 2018;142(4):142. doi:10.1542/peds.2018-1092.
- Huang S, Mao J, Zhou L, Xiong X, Deng Y. The imbalance of gut microbiota and its correlation with plasma inflammatory cytokines in pemphigus vulgaris patients. Scand J Immunol. 2019;90(3):e12799. doi:10.1111/sji.12799.
- Romani L, Del Chierico F, Chiriaco M, Foligno S, Reddel S, Salvatori G, Cifaldi C, Faraci S, Finocchi A, Rossi P. et al. Gut mucosal and fecal microbiota profiling combined to intestinal immune system in neonates affected by intestinal ischemic injuries. Front Cell Infect Microbiol. 2020;10:59. doi:10.3389/fcimb.2020.00059.
- Kasabreh N. Smoking Thirties: How Tobacco & BMI Shape the Subgingival Microbiome. Dentistry. OhioLINK Electronic Theses and Dissertations Center. Ohio State University, 2019:100.
- Chen X, Sun H, Jiang F, Shen Y, Li X, Hu X, Shen X, Wei P. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ. 2020;8:e8317. doi:10.7717/peerj.8317.
- Gacesa R, Kurilshikov A, Vich Vila A, Sinha T, Klaassen MAY, Bolte LA, Andreu-Sánchez S, Chen L, Collij V, Hu S. et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature. 2022;604(7907):732–9. doi:10.1038/s41586-022-04567-7.
- Lozano CP, Wilkens LR, Shvetsov YB, Maskarinec G, Park SY, Shepherd JA, Boushey CJ, Hebert JR, Wirth MD, Ernst T. et al. Associations of the dietary inflammatory index with total adiposity and ectopic fat through the gut microbiota, LPS, and C-reactive protein in the multiethnic cohort–adiposity phenotype study. Am J Clin Nutr. 2022;115(5):1344–1356. doi:10.1093/ajcn/nqab398.
- Philips EM, Santos S, Trasande L, Aurrekoetxea JJ, Barros H, von Berg A, Bergström A, Bird PK, Brescianini S, Ní Chaoimh C. et al. Changes in parental smoking during pregnancy and risks of adverse birth outcomes and childhood overweight in Europe and North America: an individual participant data meta-analysis of 229,000 singleton births. PLoS Med. 2020;17(8):e1003182. doi:10.1371/journal.pmed.1003182.
- Nakamura A, Francois O, Lepeule J. Epigenetic Alterations of Maternal Tobacco Smoking during Pregnancy: A Narrative Review. Int J Environ Res Public Health. 2021;18(10):18. doi:10.3390/ijerph18105083.
- de Prado-Bert P, Ruiz-Arenas C, Vives-Usano M, Andrusaityte S, Cadiou S, Carracedo Á, de Prado-Bert A, Casas M, Chatzi L, Dadvand P. et al. The early-life exposome and epigenetic age acceleration in children. Environ Int. 2021;155:106683. doi:10.1016/j.envint.2021.106683.
- Stockinger B, Shah K, Wincent E. AHR in the intestinal microenvironment: safeguarding barrier function. Nat Rev Gastroenterol Hepatol. 2021;18(8):559–70. doi:10.1038/s41575-021-00430-8.
- Korecka A, Dona A, Lahiri S, Tett AJ, Al-Asmakh M, Braniste V, D’Arienzo R, Abbaspour A, Reichardt N, Fujii-Kuriyama Y. et al. Bidirectional communication between the aryl hydrocarbon receptor (AhR) and the microbiome tunes host metabolism. Npj Biofilms Microbiomes. 2016;2(1):16014. doi:10.1038/npjbiofilms.2016.14.
- Group WHOMGRS. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi:10.1111/j.1651-2227.2006.tb02378.x.
- Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL. et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185(5):385–94. doi:10.1503/cmaj.121189.
- Bolyen E, Rideout JR, Dillon MR, Bokulich N, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. doi:10.1038/s41587-019-0209-9.
- Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MGI. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38(6):685–688. doi:10.1038/s41587-020-0548-6.
- Caspi R, Billington R, Ferrer L, Foerster H, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, Latendresse M, Mueller LA. et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016;44(D1):D471–80. doi:10.1093/nar/gkv1164.
- Drall KM, Tun HM, Morales-Lizcano NP, Konya TB, Guttman DS, Field CJ, Mandal R, Wishart DS, Becker AB, Azad MB. et al. Clostridioides difficile colonization is differentially associated with gut microbiome profiles by infant feeding modality at 3–4 months of age. Front Immunol. 2019;10:2866. doi:10.3389/fimmu.2019.02866.
- Parks J, McLean KE, McCandless L, de Souza RJ, Brook JR, Scott J, Turvey SE, Mandhane PJ, Becker AB, Azad MB. et al. Assessing secondhand and thirdhand tobacco smoke exposure in Canadian infants using questionnaires, biomarkers, and machine learning. J Expo Sci Environ Epidemiol. 2022;32(1):112–23. doi:10.1038/s41370-021-00350-4.
- Alli BY. InteractionR: an R package for full reporting of effect modification and interaction. Softw Impacts. 2021;10:100147. doi:10.1016/j.simpa.2021.100147.
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):12. doi:10.1186/gb-2011-12-6-r60.
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38. doi:10.18637/jss.v059.i05.
- Fong C, Ratkovic M, Imai K. CBPS: covariate balancing propensity score. R package version 0.23 (2022). Available from https://CRAN.R-project.org/package=CBPS.
- Yu Q, Li B. Mma: multiple mediation analysis. R package version 10.7-1 (2023). Available from https://CRAN.R-project.org/package=mma.
- Brandao Gois MF, Sinha T, Spreckels JE, Vich Vila A, Bolte LA, Weersma RK, Wijmenga C, Fu J, Zhernakova A, Kurilshikov A. et al. Role of the gut microbiome in mediating lactose intolerance symptoms. Gut. 2022;71(1):215–7. doi:10.1136/gutjnl-2020-323911.