ABSTRACT
Carbapenem-resistant Klebsiella pneumoniae (CR-Kp) is a significant threat to public health worldwide. The primary reservoir for CR-Kp is the intestinal tract. There, the bacterium is usually present at low density but can bloom following antibiotic treatment, mostly in hospital settings. The impact of disturbances in the intestinal environment on the fitness, survival, expansion, and drug susceptibility of this pathogen is not well-understood, yet it may be relevant to devise strategies to tackle CR-Kp colonization and infection. Here, we adopted an in vivo model to examine the transcriptional adaptation of a CR-Kp clinical isolate to immune activation in the intestine. We report that as early as 6 hours following host treatment with anti-CD3 antibody, CR-Kp underwent rapid transcriptional changes including downregulation of genes involved in sugar utilization and amino acid biosynthesis and upregulation of genes involved in amino acid uptake and catabolism, antibiotic resistance, and stress response. In agreement with these findings, treatment increased the concentration of oxidative species and amino acids in the mouse intestine. Genes encoding for proteins containing the domain of unknown function (DUF) 1471 were strongly upregulated, however their deletion did not impair CR-Kp fitness in vivo upon immune activation. Transcription factor enrichment analysis identified the global regulator cAMP-Receptor Protein, CRP, as a potential orchestrator of the observed transcriptional signature. In keeping with the recognized role of CRP in regulating utilization of alternative carbon sources, crp deletion in CR-Kp resulted in strongly impaired gut colonization, although this effect was not amplified by immune activation. Thus, following intestinal colonization, which occurs in a CRP-dependent manner, CR-Kp can rapidly respond to immune cues by implementing a well-defined and complex transcriptional program whose direct relevance toward bacterial fitness warrants further investigation. Additional analyses utilizing this model may identify key factors to tackle CR-Kp colonization of the intestine.
Introduction
Klebsiella pneumoniae (Kp) is a gram-negative, non-motile rod, endowed with a prominent sugar capsule, which causes human respiratory, urinary tract, and systemic infections.Citation1 Currently, there are no vaccines available against Kp, and antibiotic therapy remains the standard intervention strategy in case of infection. However, due to the large number of antibiotic resistance genes (ARGs) acquired by Kp strains over the last decade, treatments have become largely ineffective. In fact, Kp is currently listed amongst the most dangerous nosocomial multidrug-resistant (MDR) pathogens, grouped under the acronym ‘ESKAPE’,Citation2 and considered a global health priority.Citation3 In particular, a single Kp strain known as ST (Sequence Type) 258 has acquired resistance to carbapenems, last-resort antibiotics for the treatment of MDR infections, and has spread globally, becoming endemic in multiple countries.Citation1,Citation3–5
Carbapenem resistance is primarily achieved through the production of carbapenemases, such as the Klebsiella pneumoniae carbapenemase (KPC), the New Delhi metallo-beta-lactamase (NDM), and the OXA-48-like enzymes.Citation1
The gastrointestinal (GI) tract is a major reservoir of Kp, including carbapenem-resistant (CR)-Kp strains, and overgrowth promoted by antibiotic treatment, can favor infection both in humans and animal models and spread amongst hospitalized patients.Citation6–10 Disseminated infections caused by CR-Kp are extremely difficult to treat due to antibiotic inefficacy.Citation8,Citation11 Understanding the molecular mechanisms that regulate CR-Kp fitness in the gut lumen is therefore of primary importance, and multiple animal models have been established to investigate this issue.Citation6,Citation8,Citation12–15 Several variables can affect fitness and propagation of pathobionts in the intestinal microenvironment. For instance, commensal microbes can directly or indirectly limit the capacity of Kp to colonize the intestinal lumen.Citation6,Citation14,Citation16 In addition, host immunity is a key modulator of Enterobacteriaceae fitness in the gut.Citation17 Landmark studies have uncovered that, by producing reactive oxygen and nitrogen species, inflammation can boost the luminal expansion of bacterial species capable of using those substrates for respiration, such as E. coli and Salmonella spp.Citation18,Citation19 Similarly, the capacity to metabolize amino acids such as L-serine, whose luminal concentrations increase in the inflamed intestine, favors E. coli outgrowth in mouse models.Citation20 In line with these notions, Klebsiella species are significantly more abundant in the intestine of patients with inflammatory bowel disease (IBD) and can even promote colitis in a variety of animal models.Citation21–27
Despite the clinical relevance of CR-Kp in the context of intestinal colonization and inflammation, the molecular programs adopted by this pathobiont in the gut lumen, and their relations with the immune status of the host, have not been investigated. Unraveling the mechanisms through which CR-Kp adapts to inflammatory cues in the intestine may suggest tailored approaches to facilitate clearance of this relevant pathobiont.
Here, we performed transcriptional profiling of intestinal CR-Kp ST-258 (MH-258) in mice undergoing acute immune activation and identified bacterial genes that were subsequently differentially regulated. The immunity-induced transcriptional program of CR-Kp is established rapidly and encompasses switches in carbon source utilization, activation of stress response mechanisms, and modulation of antibiotic resistance. Importantly, a core transcriptional signature was confirmed through experiments carried out in distinct vivaria, implying its robustness. We report that genes encoding for protein containing a domain of unknown function common to multiple paralogs within the Enterobacteriaceae family (DUF1471) are strongly induced by host immunity. Selective deletion of these genes did not reduce the capacity of CR-Kp to colonize the inflamed intestine, suggesting that they may serve other, unknown functions. On the contrary, we describe that the cAMP-Receptor Protein (CRP), a predicted master regulator of the above transcriptional signature, is strictly required to establish intestinal colonization in the first place.
Our work sets the stage for a comprehensive understanding of functional regulation in intestinal CR-Kp on the basis of host immune status. This knowledge may assist the development of novel therapeutic approaches to tackle CR-Kp intestinal carriage and bloom via rational modulation of the gut microenvironment.
Methods
Genome, plasmids, primers, spacers, donor DNA, Kp bacterial strains, and growth conditions
The genome of the Klebsiella strain utilized in this study was annotated using PATRIC, which employs the RAST tool kit.Citation28 NCBI gene identifiers for RAST-predicted peg (protein encoding genes) are provided in Supplementary Table S2.
All the plasmids, bacterial strains, and cell lines used in this study are listed in Supplementary Table S3. All the primers used in this study are listed in Supplementary Table S4. The spacers and DNA donors used in this study are listed in Supplementary Table S5. The primers used in this study were purchased from Microsynth (Balgach, Switzerland) and from LubioScience GmbH (Zürich, Switzerland). K. pneumoniae strains were grown in lysogeny broth (LB) medium (per liter, 5 g of yeast extract, 10 g of tryptone, 10 g of NaCl [pH 7.2 to 7.4]). Antibiotics were added to the following concentrations: 30ug/ml apramycin, 100ug/ml rifampicin, 50 ug/ml neomycin. Unless otherwise noted, we utilized bacteria in the early log phase (OD = 0.1–0.4) from sub-inoculations of overnight cultures. A linear relation between OD values and CFUs is maintained in this range.
The temperature-sensitive pCasKp plasmid and the pSGKp-Rif were provided by Dr. Liang Chen (Hackensack Meridian School of Medicine & Center for Discovery and Innovation) (Wang, Y. et al., 2018).
Mouse husbandry
All experiments using wild-type mice were performed with C57BL/6J female mice that were 6–9 weeks old; mice were purchased from Charles Rivers. All mice were housed in a sterile, autoclaved cages with irradiated food and autoclaved water. Mouse handling and cage changes were performed by investigators wearing gloves, masks, and sterile gowns. All mice were maintained in a specific-pathogen-free facility at the Centre Médical Universitaire (CMU) of the University of Geneva, Switzerland.
All of the animal procedures and experiments were approved and performed in accordance with the guidelines of the animal research committee of Geneva, Switzerland.
Mouse intestinal reconstitution and intranasal inoculation
Kp strains (i.e. WT ST258 and derived mutants) were maintained in glycerol stock at −80°C. To generate the inoculum to be administered to mice, bacteria were inoculated in LB-broth supplemented with carbenicillin (100µg/ml) and neomycin (50ug/ml) and incubated overnight at 37°C 220rpm. O.n. cultures were sub-inoculated 1:500 in fresh LB medium and incubated at 37°C 220rpm. At OD ~0.1–0.4, bacteria were washed once with ice-cold PBS.
For mouse reconstitution, bacteria were resuspended in ice-cold PBS, to prevent further replication, at a concentration of 0.5 × 106CFU/ml. About 200µl of bacterial mixture (0.1×105CFUs) was administered to ampicillin-treated mice by oral gavage.
Depending on the local microbiota flora, mice were administered either ampicillin only (0.5 g/l, MSKCC facility) or AMNV in drinking water (ampicillin, metronidazole, neomycin, vancomycin; 0.5 g/l each, CMU facility) starting 3 days prior to reconstitution with Kp. After oral inoculation of Kp, AMNV in drinking water was replaced with ampicillin (0.5 g/l) for the whole duration of the experiment.
Preparation of competent cells and electroporation
The preparation of Kp competent cells and electroporation of Kp were performed as described previously (Wang, Y. et al., 2018). Briefly, 1 ml of Kp WT overnight culture was diluted into 100 ml of LB broth and incubated at 37°C. When the optical density at 600 nm (OD600) of the cell culture reached ~ 0.5–0.7, the culture was chilled on ice for 20 min and harvested by centrifugation at 7,200 × g for 5 min. The supernatant was discarded, and the cells were resuspended with 5 ml of sterile ice-cold 10% glycerol. The centrifugation and resuspension steps were repeated twice. Finally, the cells were resuspended with 1 ml of ice-cold 10% glycerol. Fifty-microliter aliquots were frozen and stored at −80°C.
For the pCasKP-harboring K. pneumoniae strain, 1 ml of overnight culture from a fresh single colony was diluted into 100 ml of LB broth containing 30ug/ml apramycin and incubated at 30°C. When the cell density reached an OD600 of ~0.2, 1 ml of 20% L-arabinose was added for induction of the lambda Red recombineering operon of pCasKP. After induction at 30°C for 2 h, the culture was prepared as electrocompetent cells in a way similar to that of the WT Kp.
For electroporation, 50 µl of electrocompetent cells were thawed on ice for several minutes. Then, the cells were mixed with no more than 5 µl plasmid or donor template. The mixture was transferred into a 2-mm electroporation cuvette (BTX) and electroporated at 2.5 kV, 200Ω, and 25uF using an ECM 630 electroporation system (BTX). After being pulsed, the cells were recovered in 1 ml antibiotic-free LB broth and incubated at 30°C for 1.5 h before being plated onto LB agar plates supplemented with the required antibiotics. The plates were incubated at 30°C overnight.
Genome editing
The pSGKp plasmid was extracted from Escherichia coli DH5α using the PureLink™ Quick Plasmid Miniprep Kits (Invitrogen). Plasmid was linearized with BsaI (New England Biolab) into FastDigest Buffer (10X) (ThermoFisher) for 20 min at 37°C. Then the plasmid was gel purified using the GeneJET PCR purification kit (Thermoscientific) and quantified by nanodrop. In parallel, phosphorylation and annealing of the spacers were performed as follows: 5 µl of spacer forward and 5µl spacer reverse were incubated for 30 min at 37°C with 5 µl 10× T4DNA Ligase (NEB) and 1µl T4 polynucleotide kinase (NEB) in a 50 µl reaction. The spacers were then ligated for 1 h into the linearized pSGKp plasmid through a 10 µl reaction at room temperature, in presence of 1µl of 10× T4DNA Ligase Buffer (NEB) and 1µl of T4 DNA Ligase (NEB). The ligated product was then transformed in E. coli DH5α ECO112 (ThermoScientific) following manufacturer’s instructions. The bacteria were grown overnight at 37°C on LB-agar supplemented with 100ug/ml Rifampicin. Transformed colonies were identified through colony PCR using DreamTaq Green PCR (ThermoScientific) and confirmed by Sanger sequencing (Microsynth). Transformed clones were stored in glycerol stock at −80°C.
When needed, pSGKp containing the spacers was extracted from E. Coli DH5α overnight cultures using the PureLink™ Quick Plasmid Miniprep Kits (Invitrogen). The extracted plasmid was used for electroporation. After electroporation and overnight culture of electroporated Kp, the mutagenesis was checked through colony PCR and Sanger sequencing as explained before. Then, either only the pSGKp plasmid was cured from Kp or both pCasKp and pSGKp were cured.
For the curing of the pSGKp, bacteria were grown at 30°C overnight in LB broth supplemented with 30ug/ml apramycin and 5% sucrose. Then, bacteria were grown at 30°C overnight on LB-agar supplemented with 30ug/ml apramycin (to check the presence of the pCasKp plasmid) and LB-agar supplemented with 30ug/ml apramycin and 100ug/ml rifampicin (to check the absence of the pSGKp plasmid). The mutant bacteria that had successfully cured the pSGKp plasmid were used for another round of electroporation.
For the curing of both plasmid, bacteria were grown at 37°C overnight in LB broth. Then, they were plated on LB-agar supplemented with 5% sucrose. Finally, clones were picked and streaked on LB-agar plates supplemented with 30 µg/ml apramycin, LB-agar plates supplemented with 100 µg/ml with rifampicin and LB-agar plates (to confirm successful curing).
Immune stimulation
Kp-reconstituted WT mice were injected i.p. with anti-CD3 antibody (clone 145-2C11, BioXCell; 100ug/mouse in 200 µl PBS) and sacrificed at the indicated time points.
Dextran sodium sulfate immune stimulation
Kp-reconstituted WT mice were administered Dextran Sodium Sulfate (DSS) ad-libitum in water (3% DSS in water) and sacrificed at the indicated time points. Fecal pellets were sampled at the indicated time points and were mechanically homogenized (bead beating). Seven serials 1:10 dilutions of the mixture were plated onto LB-agar supplemented with carbenicillin (100ug/ml) and neomycin (50ug/ml). After 24 h incubation at 37°C, CFUs were counted.
Lung infection and organ processing
For intranasal inoculation, bacteria were resuspended in ice-cold PBS at a concentration of 1 × 108CFU/ml. 40 µl of bacteria mixture was administered intranasally to mice anesthetized with isoflurane. Intranasally inoculated mice were sacrificed 24 h after intranasal inoculation. Lungs were mechanically homogenized (bead beating) and 8 serials 1:10 dilutions of the mixture were spot-plated onto LB-agar supplemented with carbenicillin (100 µg/ml) and neomycin (50 µg/ml).
RNA extraction (mouse tissue, murine cell lines)
RNA was isolated from cecum tissue or cell lines, using mechanical homogenization (bead beating) and TRIzol isolation. Briefly, samples were placed into 1 ml of TRIzol (Invitrogen), bead homogenized, and spinned down for 10 min at 12’000 × g 4°C. Then, 0.2 ml of chloroform were added to the tube, followed by 15 sec of vortexing, and incubation at room temperature for 2-3 min. Tubes were centrifuged for 15 min at 12’000 × g 4°C. The RNA was then precipitated transferring the aqueous phase into a new tube and adding 0.5 ml isopropanol. The mixture was incubated at room temperature for 10 min, followed by a centrifugation at 12’000 × g 4°C for 10 min. Finally, the RNA was washed by removing the supernatant and adding 1 ml of 75% ethanol. RNA was pelleted at 7’500 × g 4°C for 5 min, supernatant was removed, and the RNA pellet was air dried for 5 min at room temperature. Then the RNA was resuspended in 100 µl of nuclease-free water and stored at −80°C.
RNA extraction (Kp RNA)
Cecal content was extracted from dissected mice immediately after sacrificed and snap-frozen in dry ice. RNA was isolated from fecal pellets or cecal contents using the RNeasy PowerMicrobiome Kit (Qiagen), following the manufacturer’s instructions. RNA was eluted in 100 µl nuclease-free water and stored at −80°C.
RT qPCR (mouse)
RNA was isolated from cecum tissue or RAW 267.4 macrophages, using mechanical homogenization and TRIzol isolation, as described above. cDNA was obtained using the iScript Reverse Transcription Supermix (Bio-Rad). RT-qPCR was performed on cDNA using the PowerUP SYBR Green Master Mix (Applied Biosystems) and custom primers (Supplementary Table S3). Gene expression was normalized to expression of GAPDH in the control group. Reactions were run on a QuantStudio 6 PRO (Applied Biosystems). The cycling conditions were as follows: 95°C for 20 sec, followed by 40 cycles of 95°C for 1 sec and 60°C for 20 sec. A melt curve has been performed after the 40 cycles as follows: 95°C for 1 sec, 60°C for 20 sec and 95°C for 1 sec.
RT qPCR (Klebsiella)
Kp RNA was extracted from fecal pellets or cecal content as indicated above, cDNA was generated using the QuantiTect reverse transcriptase kit (Qiagen). qPCR was carried out using the PowerUP SYBR Green Master Mix (Applied Biosystems) and dedicated custom primers for the target genes and housekeeping genes selected from our RNA-seq datasets based on low variance in expression despite host immune activation (Supplementary Table S3). Reactions were run on a QuantStudio 6 PRO (Applied Biosystems). The cycling conditions were as follows: 95°C for 20 sec, followed by 40 cycles of 95°C for 1 sec and 60°C for 20 sec. A melt curve was generated after the 40 cycles using the following conditions: 95°C for 1 sec, 60°C for 20 sec and 95°C for 1 sec.
DNA extraction
Kp DNA was extracted from fecal pellets or cecal contents or overnight liquid cultures (12 h LB broth supplemented with carbenicillin (100ug/ml) and neomycin (50ug/ml)) by mechanical disruption (bead beating), followed by extraction using the QIAamp PowerFecal Pro DNA Kits (Qiagen), according to manufacturer’s instructions. After extraction, DNA was eluted in 100 µl nuclease-free water and store at −20°C.
qPCR to measure competitive index
Kp DNA extracted from fecal pellets or cecal content was subjected to quantitative PCR using the following custom Kp member-specific primers (Supplementary Table S3).
868 WT: fwd: 5’-tttagccgccagttcctgac-3’; rev: 5’-ttctctctttcggtgccagc-3’; 868KO: fwd: 5’-cgacgagggtaatctttact-3’; rev: 5’-ttcgacgaggtgaacatcgt-3’ (for WT vs 6KO competition).
CRPWT: fwd: 5’-ggttgcccactttctcagac-3’; rev: 5’-cctgtttgaagagggtcagg-3’; CRPKO: fwd: 5’-acgccgcttagcaacgcgcggtt-3’; rev: 5’-tgctaaaacagtctggatgct-3’ (for WT vs CRPKO competition).
Standard curves were generated by amplification of DNA extracted from pure overnight liquid culture (12 h LB-broth supplemented with carbenicillin (100ug/ml) and neomycin (50ug/ml)) of the WT Kp, the 6KO Kp and the Δcrp Kp (six serials 1:10 dilutions, from 3.3ng/ µl, 3 µl, ~10ug total DNA). Internal controls consisting of equal amount of WT DNA and mutant DNA (50:50, WT:6KO or WT: Δcrp), and different ratios od WT DNA over mutant DNA (75:25, WT:6KO or WT: Δcrp; 25:75, WT:6KO or WT: Δcrp), were always performed to confirm accuracy of the qPCR. PowerUP SYBR Green Master Mix (Applied Biosystems) was used to perform the reactions. Reactions were run on a QuantStudio 6 PRO (Applied Biosystems). The cycling conditions were as follows: 95°C for 20 sec, followed by 40 cycles of 95°C for 1 sec and 60°C for 20 sec. A melt curve was generated after the 40 cycles as follows: 95°C for 1 sec, 60°C for 20 sec, and 95°C for 1 sec.
RNA sequencing and analysis (Kp RNA)
Kp RNA sample libraries were prepared using the Illumina Stranded Total RNA Prep kit with the Ribo-zero Plus and sequenced using the Illumina NovaSeq 6000 with single-end reads 100bp (experiment CMU1), paired-end reads 100 bp (MSK1) or MiSeq with paired-end reads 150 bp (experiment MSK2). Raw sequence reads were filtered using Trimmomatic (v.0.39),Citation29 sequentially aligned to the mouse genome to remove mouse-derived sequences and then to the Kp genome using bowtie2 (v.2.2.5).Citation30 BAM files obtained were analyzed using R (v.4.0.3). Reads were assigned to genes using FeatureCounts from the Rsubread package (v.2.4.2).Citation31 Reads were then analyzed using ‘DeSeq2’ package (v.1.30.0).Citation32 GSEA was performed using the package ‘clusterProfiler’ (v.4.6.0).Citation33
RNA sequencing and analysis (murine RNA)
Murine RNA sample libraries for experiments CMU1 were prepared using the Illumina Stranded Total RNA Prep kit with the Ribo-zero Plus and sequenced using the Illumina NovaSeq 6000 with single read 100bp. Libraries were prepared on a 1:6 mixture of RNA extracted from the mouse tissue and from the respective bacteria, so that each library would produce both mouse and Klebsiella-derived reads. Raw sequence reads were aligned to the reference mouse genome UCSC mm10 using STAR (v.2.7.4.a). Reads were assigned to genes using HTSeq counts (v.0.9.1). Reads were then analyzed in R (v.4.0.3) using ‘DeSeq2’ package (v.1.30.0). GSEA was performed using the package ‘clusterProfiler’ (v.4.6.0) using R (v.4.2.2).
Transcription factors enrichment analysis
A generic database of 201 transcription factors (TF) motifs of prokaryote genomes was downloaded from MEME suite website (https://meme-suite.org/meme/db/motifs, Prodoric database). The DNA sequence of our Klebsiella pneumoniae genome was scanned with these motifs using function scan_sequences() from R Bioconductor package universalmotif. All hits matching the genome with a p-value lower than 1e-6 were collected (n = 71459 hits), and we associate to each TF, the genes that have a hit for its motif in their promoter region (defined as 0-500bp upstream of the gene). This process builds our regulon database on which we conducted enrichment analysis tests, with a hypergeometric test to assert whether differentially expressed genes found by RNA-seq were enriched for some TF.
H2O2 assays
Kp strains were grown overnight in LB broth supplemented with carbenicillin (100 ug/ml) and neomycin (50 ug/ml) at 37°C, and then diluted 1:200 in fresh LB broth supplemented with the very same antibiotics at 37°C. At O.D. ~0.1–0.4, cells were diluted 1:100 in LB broth and challenged with 2.5 mM H2O2 for 1 h at 37°C. Viability of Kp strains was assessed through plating of serial 1:10 dilutions on LB-agar plates.
Stimulations for qPCR were carried out as follows. Kp strains were grown overnight in LB broth supplemented with carbenicillin (100 ug/ml) and neomycin (50 ug/ml) at 37°C and then diluted 1:200 in fresh LB broth supplemented with the very same antibiotics at 37°C. At O.D. ~0.6, 1 ml of Kp culture was mixed with 1 ml of LB-broth supplemented with 2.5 mM (H2O2) (final H2O2 concentration 1.25 mM) and incubated for 15 min at 37°C. Bacteria were spinned down at 3000 rpm for 10 min at 4°C, the supernatant was removed, and the cells were resuspended into TRIzol before proceeding with a RNA extraction as indicated above.
H2O2 measurement in cecal content
WT mice were treated with AMNV for 4 days and then stimulated with anti-CD3 antibody (100ug/200 µl PBS) i.p. injection. 5 h post-injection, mice were sacrificed and cecal contents were collected and resuspended in PBS pH 7.4. Cecal contents were centrifuged at 3’000rpm for 10 min at 4°C. 40 µl of supernatant was mixed with 10 µl of 5 × concentrated mix of AmplexRed (0.125 mM) and horseradish peroxidase (0.5 U/ml). The mixture was incubated for 30 min at RT in the dark. Concentration of H2O2 was then determined by measuring the fluorescence at 590 nm on a SpectraMax Paradigm MultiMode Microplate Reader (Molecular Devices).
Serum killing assay
Kp strains were grown overnight in LB broth supplemented with carbenicillin (100 µg/ml) and neomycin (50ug/ml) at 37°C and then diluted 1:200 in fresh LB broth supplemented with the very same antibiotics at 37°C. At O.D. ~0.1–0.4, cells are diluted 1:100 in LB broth and challenged with activated human serum for 1 h at 37°C. Viability of Kp strains was assessed through plating of serial 1:10 dilutions on LB-agar plates.
Bile acids stress assay
Kp strains were grown overnight in LB broth supplemented with carbenicillin (100 µg/ml) and neomycin (50 µg/ml) at 37°C, and then diluted 1:200 in fresh LB broth supplemented with the very same antibiotics at 37°C. At O.D. ~0.1–0.4, viability to bile acids of Kp strains was assessed through plating of serial 1:10 dilutions on LB-agar plates supplemented with different concentration of bile acids (Cholic acid: 0.05%, 0.1%; Deoxycholic acid: 0.05%, 0.1%, 0.2%). After 24 h incubation at 37°C, CFUs were counted.
Macrophage killing assay
RAW264.7 macrophages were grown in DMEM high glucose + 10% FBS + 1% Pen/Strep. At 90–100% confluence, RAW264.7 cells were seeded at 10Citation6/ml in a 96-well plate and incubated overnight at 37°C. For the Interferon-γ (IFN- γ) stimulation: the culture medium was supplemented with 100 ng/ml IFN- γ for the whole experiment. In parallel, Kp strains were grown overnight at 37°C 220rpm, in LB broth supplemented with carbenicillin (100 µg/ml) and neomycin (50ug/ml). Then, Kp cultures were diluted 1:200 in fresh LB medium. At OD600 ~ 0.1–0.4, bacteria were washed twice with PBS by centrifuging at 3’000rpm 4°C, followed by a resuspension in antibiotic-free complete DMEM. Macrophages were washed once with PBS and antibiotic-free DMEM + 10% FBS was added. Bacteria were added to wells at MOI = 10 in 30 µl, and spin at 200 × g 5 min. Macrophages were incubated with Kp strains for 1 h at 37°C and wash twice with PBS. Then, cells were incubated with DMEM + 10% FBS supplemented with 300 ug/ml gentamycin for 30 min 37°C, to kill extracellular bacteria. Following incubation, cells were washed again twice with PBS and incubated for 3 h at 37°C in DMEM + 10% FBS supplemented with 50 ug/ml gentamycin. To assess recovery of intracellular bacteria, cells were washed twice with PBS and lysed with PBS + 0.1% Triton X-100, then the mixture was serial 1:10 diluted seven times and plated onto LB-agar plates. After 24 h incubation at 37°C, CFUs were counted.
Macrophage activation assay
RAW264.7 macrophages were grown in DMEM high glucose + 10% FBS + 1% Penicillin/Streptomycin (Pen/Strep) (ThermoFisher). At 90–100% confluence, RAW264.7 cells were seeded at 0.5 × 106/ml in a 48 well-plate and incubated overnight at 37°C. In parallel, Kp strains were grown overnight at 37°C 220rpm, in LB broth supplemented with carbenicillin (100ug/ml) and neomycin (50ug/ml). Then, Kp cultures were diluted 1:200 in fresh LB medium. At OD600 ~0.1–0.4, bacteria were washed twice with PBS by centrifuging at 3’000rpm 4°C, followed by a resuspension in antibiotic-free complete DMEM. Macrophages were washed once with PBS and antibiotic-free DMEM + 10% FBS was added. Bacteria were added to wells at MOI = 10 in 30ul and spun at 200 × g 5 min. Macrophages were incubated with Kp strains for 1 h at 37°C and washed twice with PBS. Then cells were incubated with DMEM + 10% FBS supplemented with 100 µg/ml gentamycin for 30 min 37°C, to kill extracellular bacteria. Following incubation, cells were washed again twice with PBS and incubated for 3 h at 37°C in DMEM + 10% FBS supplemented with 50ug/ml gentamycin. To assess the gene expression in macrophages, RNA was extracted with TRIzol and RT-qPCR was performed on the target genes, as explained previously.
Antibiotic susceptibility assay
Kp strains were grown overnight in LB broth supplemented with carbenicillin (100 µg/ml) and neomycin (50 µg/ml) at 37°C, and then diluted 1:200 in fresh LB broth supplemented with the very same antibiotics at 37°C. At O.D. ~0.8–1, cells were diluted 1:1000 in LB broth and challenged for 3 h at 37°C with the following antibiotics: meropenem (100 µg/ml) and colistin (64ug/ml). Viability of Kp strains was assessed through plating of serial 1:10 dilutions on LB-agar plates.
Growth curve in vitro
Kp strains were grown overnight in M9 medium supplemented with 0.5% D-glucose, carbenicillin (100 µg/ml) and neomycin (50ug/ml) at 37°C, and then diluted 1:250 in fresh M9 medium. 100 µl of this dilution were then put ontop of 100 µl M9 medium supplemented with different carbon sources (i.e. 2% D-glucose or 2% D-mannose or 2% L-serine+L-threonine+L-leucine) or ontop of 100 µl LB broth, in a flat-bottom well plate. In-vitro growth of Kp was estimated by measuring every 15 min for 24 h the optical density at 600 nm on a TECAN Infinite 200 PRO (TECAN) in aerobic conditions.
Metabolomics analysis
Metabolite extraction
Fecal material were pre-extracted and homogenized by the addition of 400 µL of MeOH:H2O (4:1), in the Cryolys Precellys 24 sample Homogenizer (2 × 20 seconds at 10,000 rpm, Bertin Technologies, Rockville, MD, US) with ceramic beads. The bead beater was air-cooled down at a flow rate of 110 L/min at 6 bar. Homogenized extracts were centrifuged for 15 min at 4000 x g at 4°C (Hermle, Gosheim, Germany). The resulting supernatant was collected and evaporated to dryness in a vacuum concentrator (LabConco, Missouri, US). Dried sample extracts were resuspended in MeOH:H2O (4:1, v/v) according to the sample weight.
Protein quantification
The protein pellets were evaporated and lysed in 20 mM Tris-HCl (pH 7.5), 4 M guanidine hydrochloride, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin using the Cryolys Precellys 24 sample Homogenizer (2 × 20 secat 10,000 rpm, Bertin Technologies, Rockville, MD, US) with ceramic beads. BCA Protein Assay Kit (Thermo Scientific, Masschusetts, US) was used to measure (A562nm) total protein concentration (Hidex, Turku, Finland).
Data acquisition
Extracted samples were analyzed by Hydrophilic Interaction Liquid Chromatography coupled to tandem mass spectrometry (HILIC – MS/MS)Citation1,Citation2 in both positive and negative ionization modes using a 6495 triple quadrupole system (QqQ) interfaced with 1290 UHPLC system (Agilent Technologies). In positive mode, the chromatographic separation was carried out in an Acquity BEH Amide, 1.7 μm, 100 mm × 2.1 mm I.D. column (Waters, Massachusetts, US). Mobile phase was composed of A = 20 mM ammonium formate and 0.1% FA in water and B = 0.1% FA in ACN. The linear gradient elution from 95% B (0–1.5 min) down to 45% B was applied (1.5 min −17 min) and this conditions were held for 2 min. Then initial chromatographic condition were maintained as a post-run during 5 min for column re-equilibration. The flow rate was 400 μL/min, column temperature 25°C and sample injection volume 2 µl. ESI source conditions were set as follows: dry gas temperature 290°C, nebulizer 35 psi and flow 14 L/min, sheath gas temperature 350°C and flow 12 L/min, nozzle voltage 0 V, and capillary voltage 2000 V. Dynamic Multiple Reaction Monitoring (DMRM) was used as acquisition mode with a total cycle time of 600 ms. Optimized collision energies for each metabolite were applied. In negative mode, a SeQuant ZIC-pHILIC (100 mm, 2.1 mm I.D. and 5 μm particle size, Merck, Damstadt, Germany) column was used. The mobile phase was composed of A = 20 mM ammonium Acetate and 20 mM NH4OH in water at pH 9.7 and B = 100% ACN. The linear gradient elution from 90% (0–1.5 min) to 50% B (8–11 min) down to 45% B (12–15 min). Finally, the initial chromatographic conditions were established as a post-run during 9 min for column re-equilibration. The flow rate was 300 μL/min, column temperature 30°C and sample injection volume 2 µl. ESI source conditions were set as follows: dry gas temperature 290°C and flow 14 L/min, sheath gas temperature 350°C, nebulizer 45 psi, and flow 12 L/min, nozzle voltage 0 V, and capillary voltage −2000 V. Dynamic Multiple Reaction Monitoring (dMRM) was used as acquisition mode with a total cycle time of 600 ms. Optimized collision energies for each metabolite were applied.
Quantification and statistical analysis
Cumulative data are shown as mean±SEM or mean± SD. Statistical tests used include Student’s t-test and Wilcoxon test, Pearson’s correlation. Unless otherwise stated, p values < .05 were considered significant. Detailed information on the n of biological replicates and statistical tests used can be found in figure legends. Statistical analyzes were performed using R (v.4.0.3) or GraphPad Prism (v.9.2.0).
Protein sequence annotation
Protein sequences associated to the coding genes identified by RAST were further annotated with Uniprot database. More specifically, we collected 274,630 unirpot entries associated to the taxonomy of Klebsiella pneumoniae (Taxon ID 573) and align our protein sequences to them using NCBI blastp (v2.13.0). Alignments were processed with an R script to retain at most one uniprot entry per input sequence, with the following criteria: 1) only retain alignments with at least 70% identity; 2) only retains alignment where at least 70% of the query and subject sequences are covered; 3) exclude alignments to unreviewed entries if there exists another alignment to a reviewed SwissProt entry; 4) exclude alignment without a primary gene name if there exist another alignment with one; 5) keep the best alignment for each query sequence prioritizing reviewed alignments and highest bit_scores.
Results
Rapid and robust transcriptional adaptation of intestinal CR-Kp upon immune response
We previously showed that, in a mouse model of i.p. injection with anti-CD3 antibody, immune activation promotes rapid and robust functional reprogramming of commensal microbes via transcriptional modulation.Citation34 Intestinal symbionts adapted to host immune responses within hours, by regulating expression of hundreds of genes, a sizable fraction of which were involved in stress response or utilization of alternative carbon sources. Importantly, we found that transcriptional rewiring of bacteria was associated with dramatic changes in the concentration of luminal metabolites, including decrease in short chain fatty acids (SCFAs) and increase in amino acids and aromatic compounds, all known modulators of host pathophysiology.Citation34
To investigate whether and how immune activation promotes reprogramming of intestinal MDR Klebsiella pneumoniae, we reconstituted antibiotic-treated mice with a previously characterized clinical isolate of Sequence Type-258 carbapenem-resistant (KPC) Kp (MH-258, for Memorial Hospital ST-258, hereby CR-Kp)Citation8,Citation13,Citation35 (). As reported previously,Citation12 CR-Kp reached high densities in the intestine (), without inducing signs of overt inflammation, consistent with the notion that most Kp isolates are avirulent in the gut lumen (data not shown).Citation21 One week following reconstitution, we injected animals i.p. with anti-CD3 antibody, which activates T cells producing a systemic cytokine storm and local activation in the intestinal tissue.Citation36,Citation37 This model has been widely employed to recapitulate GVHD-induced diarrhea and promotes short-lasting responses and self-limited sickness in the animalsCitation36–39. We found that the intestinal content was relatively preserved in the cecum over the first 24 h (), while it was ablated in small intestine and colon (data not shown). In the cecum, CR-Kp loads remained constant over time, suggesting that the anti-CD3 treatment does not promote significant bactericidal activity (). As expected, anti-CD3 injection induced expression in the intestinal tissue of genes encoding for inflammatory mediators including IFN-γ, TNF-α, iNos and lipocalin, which were robustly upregulated already 1.5-3 h post-injection (p.i.), and peaked between 3-9 h p.i., decreasing to various degrees over the following 15 h ().
Figure 1. Anti-CD3 treatment modulates transcriptional activity of Carbapenem-resistant Klebsiella pneumoniae.
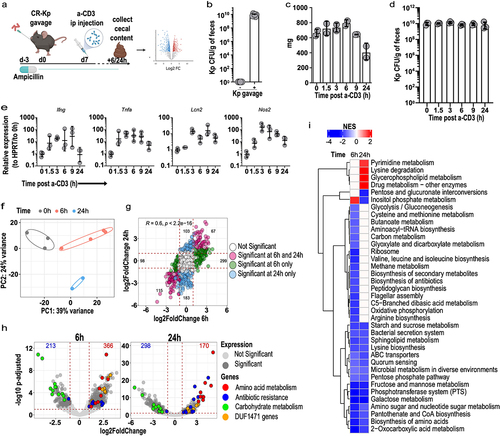
Having confirmed local immune activation in CR-Kp-reconstituted, anti-CD3 treated mice, we conducted RNA-sequencing (RNA-seq) on RNA extracted from their cecal content. Principal component analysis (PCA) revealed a robust shift in CR-Kp transcriptome already 6 h after anti-CD3 treatment, which further evolved at 24 h (). Gene expression profiles were highly concordant at these two time points, possibly indicating persistence of some of the perturbing mediators produced by the treatment (). Gene set enrichment analysis revealed that carbohydrate metabolism (glycolysis/gluconeogenesis, glucose, mannose, starch, sucrose metabolism, as well as phosphotransferase systems, pentose phosphate pathway, amino sugar, and nucleotide sugar metabolism) was strongly and rapidly down-regulated, together with amino acid biosynthesis ().
Analysis at the gene level confirmed that immune activation had produced a very broad and complex rearrangement of CR-Kp transcriptional profile. Hundreds of differentially expressed genes (DEGs) could be identified 6 h (366 up- and 213 down-regulated, respectively) and 24 h post-treatment (170 up- and 298 down-regulated, respectively) (). An in-depth analysis of the top 40 DEGs identified at least four categories of genes, based on function and expression trends, that were over-represented (; for corresponding UniProt annotation of the entries, see Supplementary Table S1). Of note, similar trends of differential expression for analogous categories of genes were previously detected in experiments utilizing a consortium of bona fide commensals, indicating the existence of common strategies across bacterial phyla to cope with the effects of immune activation in the intestinal lumen.Citation34 Down-regulated genes, as highlighted by GSEA, were mostly involved in sugar metabolism or amino acid biosynthesis. The phosphotransferase (PTS) system for mannose (peg.4426–4429), glucitol/sorbitol (peg.1533–5), and glucose (peg.4639) were among the top down-regulated genes. Similarly, expression of the genes encoding the maltoporin A/phage lambda receptor protein and the ABC transporter for maltose/maltodextrins (peg.5545–51) was decreased ().
Three groups of genes were on the contrary robustly up-regulated. The first concerned amino acid metabolism and included: a di-tripeptide/H+ symporter dtpB (peg.630); serine/threonine degradation operons (peg.2562/2566–1427/1428), critical to promote fitness of E. coli in a model of DSS-induced colitis;Citation20,Citation26 a lysine/ornithine antiporter and an inducible lysine decarboxylase (peg.4501–4502), implicated in stress response in Candida albicans;Citation40 an alanine-alanine dipeptide exporter (peg.1568) activated in E. coli as a safety valve in conditions of amino acid excess.Citation41 Second, we detected genes involved in antibiotic-resistance, including: multidrug resistance protein D (emrD) (peg.401), drug resistance transporter emrB/qacA subfamily (peg.450), multiple antibiotic resistance protein marR (peg.3288), marA (peg.3289) and marB (peg.3290), multidrug resistance RND efflux system membrane fusion protein mexE (peg.1603), multidrug resistance protein ermA (peg.1553) and ermB (peg.1552). Of note, such up-regulation occurred either preferentially or exclusively 24 h post injection, perhaps indicating over time accumulation of toxic metabolites in the bacterial cells because of immune-mediated perturbations. The third group included genes involved in stress response or host-microbe interaction, most notably genes encoding for proteins containing the domain of unknown function (DUF) 1471. The DUF1471 is a small domain (~70 AA) found across multiple paralogue proteins encoded by Enterobacteriaceae, where it is present in 1–3 copies. Some of these proteins have been described to enhance resistance to stressors such as CdCl2 or H2O2, or enable interactions between the bacterium and the host.Citation42 The above findings were confirmed in an independent RNAseq experiment and further validated by qPCR (Supplementary Figure S1a-c).
Taken together, our data indicate that immune activation or its consequences on luminal metabolites promotes rapid, complex, and well-defined transcriptional rewiring in intestinal CR-Kp, possibly affecting its metabolism (decrease sugar utilization, increased amino acid catabolism), resistance to stressors and antibiotics, or interaction with host cells.
Identification of an immune-dependent CR-Kp transcriptional signature conserved across vivaria and microbiota configuration
Differences across animal facilities are known to reduce reproducibility of findings in studies involving gut microbes, primarily because of the distinct microbial configurations hosted by the respective animals. We took advantage of the relocation of our laboratory to a different institution to determine a reliable signature of immune-dependent CR-Kp transcriptional rewiring. In the new vivarium, mice harbored a microbiota that resulted largely resistant to ampicillin (data not shown). To deplete the endogenous flora, we therefore employed a broader-spectrum antibiotic regimen, namely a combination of ampicillin, metronidazole, neomycin, and vancomycin (AMNV), followed by gavage with CR-Kp and ad libitum treatment with ampicillin (). In these settings, CR-Kp could colonize the intestine, albeit to lower densities than those reached previously (~108-109 vs ~ 1010 CFUs/g of feces) (, compare with ).
Figure 2. A dual-seq experiment validates mouse and CR-Kp transcriptional shifts following immune activation.
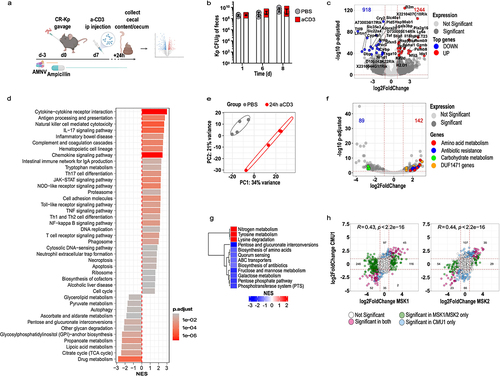
To obtain information about bacterial and host transcriptional rearrangements, we performed sumultanous RNA sequencing (Dual-Seq)Citation43 on RNA extracted from cecal content and tissue of mice reconstituted with CR-Kp and treated with PBS or anti-CD3 24 h prior. Analysis of the mouse transcriptome revealed substantial up-regulation of immune-related genes, including many interferon-regulated genes (Gbp2-6-10, CXCL9, Ly6A, Igtp, MHC), T cell effector molecules (Gzma, Gzmb), cytokines and enzymes involved in inflammatory responses (Tnf, Ido1, Nos2, Lcn2) to a comparable extent to what observed in our initial experiments (). GSEA confirmed up-regulation of multiple immune-related pathways and identified down-regulation of several carbon metabolism pathways, including pyruvate metabolism, pentose and glucuronate interconversion, TCA cycle, and glycan degradation ().
On the bacterial side, we detected hundreds of up- and down-regulated genes in CR-Kp from anti-CD3 treated animals, which belonged to the functional categories identified in our previous experiments (). In agreement with our initial findings, GSEA highlighted down-regulation of PTS, pentose phosphate, fructose and mannose metabolism, and biosynthesis of amino acids, while degradation of amino acids was enhanced (). Furthermore, individual gene expression levels robustly and significantly correlated across different experiments ().
Notably, aggregate PCA revealed that samples clustered primarily on the basis of the experiment, indicating that experiment-dependent variables (likely the nature of the residual microbiota) may produce substantial variability in the baseline transcriptome of CR-Kp (Supplementary Figure S2). However, upon anti-CD3 treatment, a concordant shift along the second component axis occurred across experiments (Supplementary Figure S2). Thus, we integrated the three datasets to identify a common transcriptional signature that may be induced regardless of environmental variables. We found that 40 genes were consistently differentially regulated across experiments (padj <0.05, absolute (log2FC) >1), most of which ranked amongst the top DEG and belonged to the functional categories discussed above (). Furthermore, 127 additional genes were significantly differentially expressed in two out of three experiments, and showed mostly comparable modulation patterns across the three experiments, suggesting that the conserved signature may actually be broader (Supplementary Figure S3).
Figure 3. Identification of a robust transcriptional signature of CR-Kp adaptation to intestinal immunity.
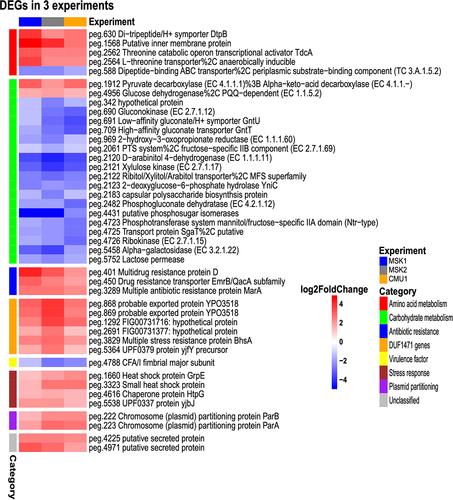
Overall, we identified multiple genes that are rapidly and consistently regulated in intestinal CR-Kp following immune activation and may modulate bacterial fitness or interaction with the host under inflammatory conditions.
Oxidative species drive expression of DUF1471-encoding genes
To begin investigating the functional relevance of the above signature, we decided to focus DUF1471-encoding genes, which were rapidly and strongly induced by immune activation ().
A conserved domain search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) identified 13 proteins encompassing a DUF1471 domain in the employed CR-Kp strain (peg.713, peg.868, peg.869, peg.1292, peg.2691, peg.3406, peg.3504, peg.3829, peg.4153, peg.4155, peg.4504, peg.5364, peg.5376). Six hour post injection, 6–7 of these were consistently found within the top 50 up-regulated genes (significant enrichment as per hypergeometric test, p value~ 9 × 10−11). This observation suggested that expression of these genes may be coordinated and potentially relevant for bacterial fitness in the inflammatory milieu.
These genes were predicted to encode proteins of reduced length (61–91 amino acids) which displayed very little sequence similarity, despite the common presence of a DUF1471 domain (Supplementary Figure S4a). All the predicted proteins contained a signal peptide, suggesting localization either in the periplasm or in the extracellular space (ProtCompB, Softberry, Inc) (Supplementary Table S2). Based on homology (Supplementary Table S1), peg.868 and peg.869 were annotated as YhcN, a stress-induced protein that promotes resistance to CdCl2 or H2O2.Citation44 Peg.3829 corresponds to multiple stress resistance protein BhsA, which promotes survival to acid, heat treatment, hydrogen peroxide, and cadmium and negatively regulate inter-bacterial adhesion and biofilm.Citation45 Peg.1292 was reported to decrease Kp adhesive capacity by negatively regulating type III fimbriaeCitation46 but also to reduce capsule and biofilm formation.Citation47 Peg.4155 encodes Yibj, which can promote biofilm formation and motility.Citation48 Finally, peg.5364 encodes the YjfY protein, which has not been characterized. However, a STRING analysis revealed that Yibj is connected to BhsA (peg.3829), YhcN (peg.868/9), and YbiJ (peg.4155) in E. coli based on co-occurrence and text mining (Supplementary Figure S4b).
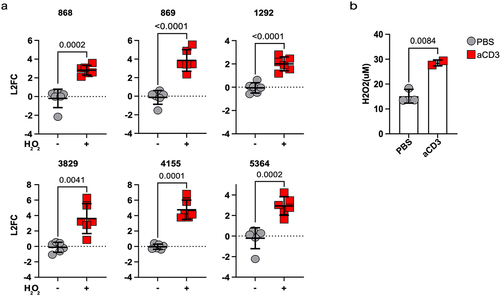
As several of these genes had been previously shown to promote resistance to H2O2, we asked if this molecule could promote their expression. Indeed, exposing CR-Kpn to H2O2 in culture, resulted in rapid up-regulation of these genes (). Notably, i.p. injection of mice with anti-CD3 antibody also resulted in rapid accumulation of H2O2, suggesting that release of oxidizing agents could promote or contribute to up-regulation of DUF1471-encoding genes in vivo ().
Figure 4. DUF1471 protein-encoding genes can be up-regulated by reactive oxygen species.
Cryptic function of DUF1471-engoding genes
To address a potential role for the above genes in regulating bacterial fitness in response to H2O2 or other stressors, we generated individual isogenic deletion mutants and tested their survival through in vitro oxidative stress assays but observed no defect as compared to WT Kp (data not shown). Given the presence of a common domain in the proteins encoded by the above genes, we reasoned that despite the little homology displayed they may possess redundant functions, so that the absence of one member could be functionally compensated by the presence of others.
To account for this possibility, we generated a scarless mutant strain lacking 6 DUF1471-encoding genes of interest (hereby referred to as ‘6KO’) using a recently published CRISPR-based approachCitation49,Citation50 (Supplementary Figure S5). To our surprise, in vitro survival of the 6KO CR-Kp was comparable to that of WT CR-Kp in response to several broadly utilized bacterial stressors, including H2O2, human serum, and bile acids (). Similarly, when testing acidic stress resistance, biofilm formation or surface hydrophobicity, which were other reported roles for individual DUF1471-encoding genes,Citation44–48,Citation51 we detected no difference between 6KO and WT CR-Kp (data not shown).
Figure 5. Absence of 6 DUF1471 protein-encoding genes does not affect the fitness and the virulence of Klebsiella pneumoniae in-vitro and in-vivo.
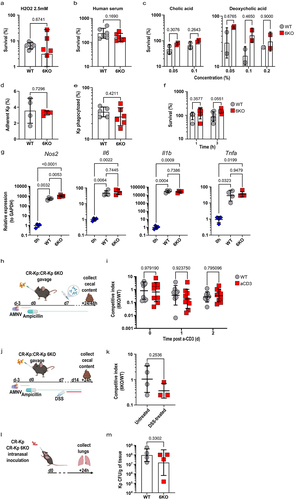
DUF1471-genes can modulate interaction with host cells.Citation42,Citation45,Citation52 However, 6KO CR-Kp mutants showed no defect in cell adhesion and were as resistant as the parental strain to macrophage killing (). Consistent with this observation, we found that the activation profile promoted by WT and 6KO CR-Kp in macrophages was comparable and encompassed strong up-regulation of inflammatory genes such as Il1b, Il6, and Tnfa (). In two independent experiments, we detected a minimal, albeit significant, increase in the induction of Nos2 by 6KO CR-Kp, which we deemed irrelevant ( and data not shown).
Competition assays carried out in vivo, in mice reconstituted with 1:1 ratios of WT and 6KO CR-Kp and treated with anti-CD3 or DSS to promote acute or sustained intestinal inflammation, revealed only non-significant trends toward a loss of fitness in the mutant strain (). Similarly, DUF1471 gene products where not required for CR-Kp to withstand immune insults in a model of lung infection (). The function of the DUF1471-bearing proteins was not masked by the overarching protection provided by the capsule, as capsule deletionCitation13 in WT and 6KO CR-Kp did not uncover any difference between the two strains in the above in vitro and in vivo assays (Supplementary Figure S6a-l).
Thus, while robust up-regulation of DUF1471-encoding genes occurs in the gut lumen of immune-activated mice, as a result of oxidative stress or other inflammation-derived signals, the function of the encoded proteins in the studied CR-Kp strain remains cryptic.
Broad shifts in luminal metabolome following anti-CD3 treatment
The above results suggested that stress-response factors may be redundant in function, making it difficult to address their individual role in the utilized model. Following immune activation, genes involved in carbon metabolism were also strongly modulated. We reasoned that investigating the metabolic rewiring occurring in CR-Kp, which potentially reflects shifts in carbon source availability, may provide alternative strategies to interfere with adaptation of the bacterium to the intestinal milieu.
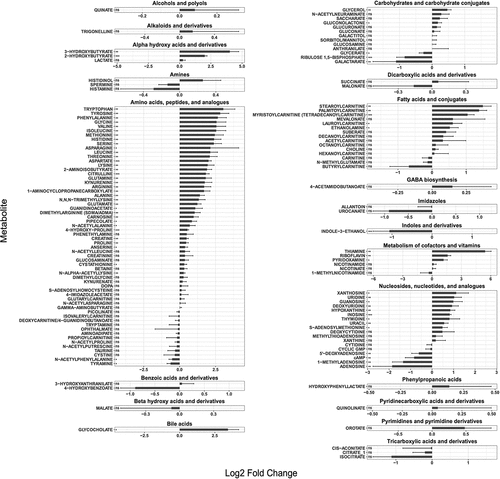
To this aim, we performed a metabolomics analysis of the cecal content from mice 5 hours following PBS or anti-CD3 treated treatment. For this analysis, we utilized mice treated with broad-spectrum antibiotics and confirmed to be microbiota-depleted by plating (Supplementary Figure S7), so to minimize the impact of bacteria-derived metabolites. We detected very significant changes in the metabolic landscape of the cecum, including a strong increase in the concentration of amino acid, which is consistent with our previously published data obtained in SPF mice,Citation34 as well as with the transcriptional signature detected in CR-Kp (up-regulation of amino acid import and catabolism, and down-regulation of amino acid biosynthesis) (). Notable shifts were observed for many different classes of molecules, and included a strong increase in bile acids, nucleotides and nucleosides, vitamins, and cofactor (particularly thiamine), and also fatty acids ( and data not shown). Of note, we were unable to detect monosaccharides such as glucose, fructose and mannose, in the cecum of both PBS and anti-CD3 treated mice. Similar results were obtained via stimulation of animals with distinct immune agonists, indicating that the described metabolic switches likely represent a common outcome of different immune responses (data not shown). Thus, immune activation profoundly remodels the metabolic landscape in the intestinal lumen, possibly changing availability of carbon sources, and triggering rapid adaptation of CR-Kp.
Figure 6. Major metabolic shifts are observed in the gut lumen during inflammation.
CRP may be an important modulator of CR-Kp transcriptional adaptation, and is required for intestinal colonization
The broad transcriptional shifts occurring in CR-Kp following immune activation, and the striking differences detected in concentration of metabolites, including potential carbon sources, prompted us to investigate whether a global regulator may direct the pathobiont’s multifaceted rewiring. We performed a bioinformatic analysis utilizing MEME suite (version 5.5.4) to identify transcription factors potentially responsible for the transcriptional signature depicted in . This analysis predicted several transcription factors that could theoretically regulate a sizable fraction of such core signature (). Of these, only two resulted statistically significant, and the cAMP Receptor Protein (CRP) was the one regulating the highest number of genes. CRP is known to modulate both carbon response (i.e. switch to the utilization of distinct carbon sources, including amino acids, in the absence of glucose) as well as stress response and virulence.Citation53–55 Accordingly, some DUF1471 genes as well as Serine/Threonine hydratase were predicted to be regulated by this transcription factor (). To test the relevance of CRP in the utilized assays, we generated an isogenic mutant lacking this gene. Δcrp CR-Kp could grow similarly to WT CR-Kp in LB and minimal medium M9 supplemented with glucose, displaying slightly delayed kinetics (). However, the strain’s growth was severely impaired and completely abolished when mannose or amino acids were used, respectively, as the sole carbon source (), consistent with the role of CRP in directing metabolic shift to alternative carbon sources.
Figure 7. CRP is an important regulator of CR-Kp intestinal colonization but is dispensable for adaptation to inflammation.
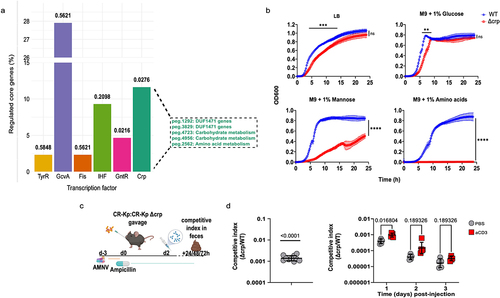
Next, we tested whether CRP might be responsible for adaptation to immune activation in vivo, using a competitive index assay (). Importantly, we found Δcrp CR-Kp to be severely defective in colonization of the gut, both in the context of competitive index, as well as in that of individual reconstitution, suggesting that this transcription factor is crucial for Klebsiella to adapt to the carbon sources available in the intestinal lumen ( left, and data not shown). Anti-CD3 treatment did not further impair the mutant’s fitness, indicating that CRP may not contribute to adaptation to metabolites released upon immune activation or, alternatively, that the inherent defect caused by CRP deficiency may overshadow deficits in the adaptation to the inflamed milieu (, right).
Discussion
MDR bacterial infections are a global health threat, and the WHO has included them in the list of top-10 worldwide healthcare priorities. A recent meta-analysis estimated that roughly 600’000 deaths were caused by antibiotic-resistant Klebsiella pneumoniae in 2019 and that almost half of these were directly attributable to the resistance mechanism, i.e. they would not have occurred had the pathogen responded to treatment.Citation56
The major reservoir of MDR Kp is the intestinal tract. A sizable fraction of individuals carries low amounts of intestinal MDR-Kp, which blooms following antibiotic treatment mostly in healthcare settings, facilitating infection and transmission to other patients.Citation8–10,Citation57 Several mouse models have been established to recapitulate intestinal MDR-Kp colonization and identify the underlying mechanisms of adaptation and transmission;Citation6,Citation8,Citation12,Citation13 however, the interplay between the bacterium and host immunity in this context has not been studied. Dissecting the adaptation of Kp to immune cues in the intestine is particularly relevant, as this bacterium is enriched in the stools of IBD patients, and experiments carried out in animal models have confirmed that some Kp strains can promote enteric inflammation.Citation21–23–Citation27–58
Here, using an established model of acute immune activation,Citation34 we found that carbapenem-resistant (CR)-Kp underwent sustained shifts in gene expression as early as 6 hours and for at least 24 h following the initial immune stimulus. We could confirm modulation of genes and pathways impacted by such rapid adaptation across experiments performed in distinct vivaria and following different antibiotic treatments. The detected transcriptional shifts underlain an overall metabolic rewiring, including strong down-regulation of sugar metabolism (PTS system, metabolism of galactose, fructose, mannose, starch and sucrose, xylose) and amino acid synthesis, and promotion of amino acid import and degradation (threonine catabolic operon transcriptional activator TdcA, threonine and serine transporters and dehydratases). In agreement with these transcriptional data, we detected heightened luminal concentrations of amino acid following anti-CD3 treatment via metabolomics analyzes. Such transcriptional and metabolic shifts are in line with what we previously reported for commensal bacteriaCitation34 and others described for E. coli in a model of DSS-induced colitis.Citation20
Of note, metabolomics analyses were carried out in microbiota-depleted mice, suggesting that amino acid accumulating in the gut should be of host origin, although a definitive proof will require experiments in germ-free mice. Whether the increase in luminal concentrations of amino acids following immune activation is caused by enhanced protein degradation, active release, or reduced absorption, remains to be determined. However, this phenomenon appears to be a hallmark of intestinal inflammation in animal modelsCitation20 and human patients.Citation59,Citation60
In vitro, amino acids that are inexpensive to produce such as serine, glycine, threonine, and aspartate, when present in sufficient amounts, can be taken up by E. coli and catabolized rather than used as building blocks to ensure faster growth even in the presence of glucose. Accumulation of amino acid degradation metabolites such as pyruvate and oxaloacetate can directly inhibit the phosphotransferase system (PTS).Citation61 As we could not detect glucose in the cecal content of antibiotic-treated mice, we speculate that upon inflammation CR-Kp may be taking up amino acids that suddenly become more available, and that the shutdown of PTS/sugar metabolism may be a downstream consequence. Interestingly, PTS gene expression inversely correlates with capsule proficiency in Klebsiella strains, and deletion of an glucose PTS system EIIA gene enhanced transcription of capsular enzymes promoting production of a thicker capsule and augmenting resistance to hydrogen peroxide in vitro.Citation62 Thus, while the above metabolic rewiring may simply reflect changes in carbon sources upon inflammation in the gut, it may also represent an evolutionary strategy to promote fitness in the host in the presence of an immune threat.
We detected consistent and robust up-regulation of several genes encoding the domain of unknown function 1471 (DUF1471) following immune activation. Proteins containing this domain have been described in other Enterobacteriaceae (including E. coli and Salmonella spp.) and can be grouped into several families, whose paralog members have been described to modulate stress responses, adhesion to host or biofilm formation. Expression of some of these genes is regulated by the cyclic AMP receptor protein (CRP).Citation46 These genes were previously shown to enhance bacterial fitness and survival in stress conditions such as exposure to H2O2 across multiple studies, mostly in E. coli.Citation44,Citation45,Citation51 This potential role seems consistent with our findings that H2O2 concentrations increase in the intestinal lumen following anti-CD3 treatment and that H2O2 triggers DUF1471 gene up-regulation in vitro. To our surprise, neither single isogenic Klebsiella mutants nor a mutant strain lacking 6 relevant DUF1471 genes displayed fitness defects in vitro, upon exposure to stressors or cells, or in vivo in models of colonization and infection. It is possible that such discrepancy depends on the bacterial species and strains utilized in different studies. Further investigations will be required to verify the role of this shared domain, which may confer proteins some common functional features. Our results also raise the possibility that a network of effector genes may be involved in promoting adaptation of Klebsiella pneumoniae to the inflamed intestine, and that deletion of individual factors may not be sufficient to impair bacterial fitness in these settings.
Motif recognition analyses identified the cAMP-Receptor Protein (CRP) as a potential regulator of the described adaptation signature. CRP is a known regulator of the carbon response, i.e. switch to alternative carbon source metabolism in the absence of glucose. Further, CRP has been implicated in the regulation of multiple stress and virulence factors, together with antibiotic resistance.Citation53–55, Citation63–65Using an isogenic Δcrp mutant we could not observe, at least in the tested experimental conditions, any role for this gene in modulating fitness in an immune-activated scenario; however, we report that crp is per se crucial to establish colonization of the gut in the first place. This is in keeping with our failure to detect glucose in the mouse intestine via metabolomics approaches. It is still possible that CRP plays a role in adaptation to inflammatory conditions, but that such effect is overshadowed by the stronger fitness defect on colonization, therefore further experiments will be necessary to obtain a definitive answer to this question.
Our data confirm previous findings in other Enterobacteriaceae and greatly expand our understanding of how Klebsiella adapts to the intestinal environment and its perturbations. E. coli, a widely studied member of Enterobacteriaceae which is closely related to Kp, was previously shown to transcriptionally adapt to inflammation in different mouse models of colitis, including DSS treatmentCitation20 and IL-10−/− mono-association.Citation66 In these scenarios, E. coli was found to down-regulate genes involved monosaccharide-utilizationCitation20 and up-regulate genes promoting stress-response, iron-metabolism and amino acid catabolism.Citation20,Citation66 YcfR (Bhsa), corresponding to our peg.3829, was also upregulated in this dataset.
The findings described in our study may have important implications for therapeutic approaches aimed at diminishing the density of Klebsiella pneumoniae in the gut of patients. In fact, while the study of these genes was not the object of the current work, we identified multiple genes of potential therapeutic relevance as to be modulated by immune activation. The phage lambda receptor Maltoporin, was significantly and strongly downregulated following anti-CD3 treatment in our experiments, possibly corroborating the notion that perturbations of the intestinal milieu can affect sensitivity to phage infection by regulating gene expression.Citation67 Our results suggest therefore that the immune status of a host may impair the efficacy of phage therapy, at least in the intestinal lumen. Genes encoding drug efflux pumps were also modulated, particularly at later time points (24 h), which might provide resistance to toxic metabolites accumulating inside the cell as the immune response progresses. For instance, we observed up-
Genes encoding drug efflux pumps were also modulated, particularly at later time points (24 h), which might provide resistance to toxic metabolites accumulating inside the cell as the immune response progresses. For instance, we observed up-regulation of the mexE/mexF efflux system, known to extrude antibiotic molecules of multiple classes in Pseudomonas aeruginosa and E. coli;Citation68,Citation69 multidrug resistance protein D (emrD) which belongs to the major facilitator superfamily (MFS) superfamily,Citation70 and is supposed to extrude uncouplers (e.g. benzalkonium, SDS), but also arabinose;Citation71 multiple antibiotic resistance protein MarA, a transcription factor belonging to the AraC/XylS family of regulators which indirectly promotes expression of the acrAB genes encoding a multidrug efflux pump. Thus, our findings suggest that sensitivity of luminal Kp ST258 to antibiotics may vary depending on immune status, which could be relevant in clinical scenarios in which Klebsiella intestinal carriage represents a risk factor, and occurs either in the presence or absence of intestinal inflammation.
In conclusion, we found that CR-Kp capacity to colonize the intestine requires CRP and that once colonization is established the bacterium can rapidly sense immune activation likely through changes in available carbon sources and accumulation of stressors such as oxidative species and adapt by modulating genes involved in the relative pathways. Activated pathways are likely to be highly redundant, as targeted deletion of key genes involved in the resulting transcriptional signatures did not impair bacterial fitness in inflammatory conditions.
This information may provide guidance to design future strategies to tackle CR-Kp intestinal overgrowth and interaction with immune responses.
Supplemental Material
Download Zip (8.4 MB)Acknowledgments
We thank Diego Andrey, Roberto Sierra-Miranda, Melanie Roch, William Kelley and Wolf-Dietrich Hardt for constructive discussion. Also, I would need to add the following at the end:Sequencing experiments were performed at the iGE3 Genomics Platform of the University of Geneva (https://ige3.genomics.unige.ch). Figures depicting experimental layouts were created with BioRender.com.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings reported in this study are openly available on Yareta [DOI: 10.26037/yareta:qbe6nnbwlbg2ne7ob43cbdkklq].
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2340486
Additional information
Funding
References
- Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80(3):629–26. doi:10.1128/MMBR.00078-15.
- Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–1081. doi:10.1086/533452.
- Brink AJ. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr Opin Infect Dis. 2019;32(6):609–616. doi:10.1097/QCO.0000000000000608.
- Pitout JD, Nordmann P, Poirel L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob Agents Chemother. 2015;59(10):5873–5884. doi:10.1128/AAC.01019-15.
- Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–696. doi:10.1016/j.tim.2014.09.003.
- Sim CK, Kashaf SS, Stacy A, Proctor DM, Almeida A, Bouladoux N, Chen M, Finn RD, Belkaid Y, Conlan S. et al. A mouse model of occult intestinal colonization demonstrating antibiotic-induced outgrowth of carbapenem-resistant Enterobacteriaceae. Microbiome. 2022;10(1):43. doi:10.1186/s40168-021-01207-6.
- Calderon-Gonzalez R, Lee A, Lopez-Campos G, Hancock SJ, Sa-Pessoa J, Dumigan A, McMullan R, Campbell EL, Bengoechea JA. Modelling the gastrointestinal carriage of Klebsiella pneumoniae infections. mBio. 2023;14(1):e03121–03122. doi:10.1128/mbio.03121-22.
- Jung HJ, Littmann ER, Seok R, Leiner IM, Taur Y, Peled J, van den Brink M, Ling L, Chen L, Kreiswirth BN. et al. Genome-wide screening for enteric colonization factors in carbapenem-resistant ST258 Klebsiella pneumoniae. mBio. 2019;10(2):10–128. doi:10.1128/mBio.02663-18.
- David S, Reuter S, Harris SR, Glasner C, Feltwell T, Argimon S, Abudahab K, Goater R, Giani T, Errico G. et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4(11):1919–1929. doi:10.1038/s41564-019-0492-8.
- Gorrie CL, Mirčeta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, Pratt NF, Garlick JS, Watson KM, Pilcher DV. et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis. 2017;65(2):208–215. doi:10.1093/cid/cix270.
- Stoma I, Littmann ER, Peled JU, Giralt S, van den Brink MRM, Pamer EG, Taur Y. Compositional flux within the intestinal microbiota and risk for bloodstream infection with gram-negative bacteria. Clin Infect Dis. 2021;73(11):e4627–e4635. doi:10.1093/cid/ciaa068.
- Caballero S, Carter R, Ke X, Sušac B, Leiner IM, Kim GJ, Miller L, Ling L, Manova K, Pamer EG. et al. Distinct but spatially overlapping intestinal niches for vancomycin-resistant enterococcus faecium and carbapenem-resistant Klebsiella pneumoniae. PLoS Pathog. 2015;11(9):e1005132. doi:10.1371/journal.ppat.1005132.
- Jung HJ, Sorbara MT, Pamer EG, Mecsas J. TAM mediates adaptation of carbapenem-resistant Klebsiella pneumoniae to antimicrobial stress during host colonization and infection. PLoS Pathog. 2021;17(2):e1009309. doi:10.1371/journal.ppat.1009309.
- Sorbara MT, Dubin K, Littmann ER, Moody TU, Fontana E, Seok R, Leiner IM, Taur Y, Peled JU, van den Brink MRM. et al. Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J Exp Med. 2019;216(1):84–98. doi:10.1084/jem.20181639.
- Stacy A, Andrade-Oliveira V, McCulloch JA, Hild B, Oh JH, Perez-Chaparro PJ, Sim CK, Lim AI, Link VM, Enamorado M. et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell. 2021;184(3):615–627.e17. doi:10.1016/j.cell.2020.12.011.
- Sequeira RP, McDonald JAK, Marchesi JR, Clarke TB. Commensal Bacteroidetes protect against Klebsiella pneumoniae colonization and transmission through IL-36 signalling. Nat Microbiol. 2020;5(2):304–313. doi:10.1038/s41564-019-0640-1.
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J. et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5(10):2177–2189. doi:10.1371/journal.pbio.0050244.
- Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–429. doi:10.1038/nature09415.
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD. et al. Host-Derived Nitrate Boosts Growth of E. coli in the Inflamed Gut. Science. 2013;339(6120):708–711. doi:10.1126/science.1232467.
- Kitamoto S, Alteri CJ, Rodrigues M, Nagao-Kitamoto H, Sugihara K, Himpsl SD, Bazzi M, Miyoshi M, Nishioka T, Hayashi A. et al. Dietary L-serine confers a competitive fitness advantage to enterobacteriaceae in the inflamed gut. Nat Microbiol. 2020;5(1):116–125. doi:10.1038/s41564-019-0591-6.
- Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T. et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017;358(6361):359–365. doi:10.1126/science.aan4526.
- Rashid T, Ebringer A, Wilson C. The role of Klebsiella in Crohn’s disease with a potential for the use of antimicrobial measures. Int J Rheumatol. 2013;2013:610393. doi:10.1155/2013/610393.
- Lee IA, Kim DH. Klebsiella pneumoniae increases the risk of inflammation and colitis in a murine model of intestinal bowel disease. Scand J Gastroenterol. 2011;46(6):684–693. doi:10.3109/00365521.2011.560678.
- Tiwana H, Walmsley RS, Wilson C, Yiannakou JY, Ciclitira PJ, Wakefield AJ, Ebringer A. Characterization of the humoral immune response to Klebsiella species in inflammatory bowel disease and ankylosing spondylitis. Rheumatology. 1998;37(5):525–531. doi:10.1093/rheumatology/37.5.525.
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN. et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8(3):292–300. doi:10.1016/j.chom.2010.08.004.
- Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P. et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell. 2020;182(2):447–462.e14. doi:10.1016/j.cell.2020.05.048.
- Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, Silberberg Y, Atarashi K, Furuichi M, Oka A. et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185(16):2879–2898.e24. doi:10.1016/j.cell.2022.07.003.
- Davis JJ, Wattam AR, Aziz RK, Brettin T, Butler R, Butler RM, Chlenski P, Conrad N, Dickerman A, Dietrich EM. et al. The PATRIC Bioinformatics Resource Center: expanding data and analysis capabilities. Nucleic Acids Res. 2020;48(D1):D606–D612. doi:10.1093/nar/gkz943.
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi:10.1093/bioinformatics/btu170.
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. doi:10.1038/nmeth.1923.
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. doi:10.1093/bioinformatics/btt656.
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi:10.1186/s13059-014-0550-8.
- Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS: J Integr Biol. 2012;16(5):284–287. doi:10.1089/omi.2011.0118.
- Becattini S, Sorbara MT, Kim SG, Littmann EL, Dong Q, Walsh G, Wright R, Amoretti L, Fontana E, Hohl TM. et al. Rapid transcriptional and metabolic adaptation of intestinal microbes to host immune activation. Cell Host Microbe. 2021;29(3):378–393.e5. doi:10.1016/j.chom.2021.01.003.
- Xiong H, Carter RA, Leiner IM, Tang Y-W, Chen L, Kreiswirth BN, Pamer EG. Distinct contributions of neutrophils and CCR2+ monocytes to pulmonary clearance of different Klebsiella pneumoniae strains. Infect Immun. 2015;83(9):3418–3427. doi:10.1128/IAI.00678-15.
- Radojevic N, McKay DM, Merger M, Vallance BA, Collins SM, Croitoru K. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol Regul Integr Comp Physiol. 1999;276(3):R715–R723. doi:10.1152/ajpregu.1999.276.3.R715.
- Merger M, Viney JL, Borojevic R, Steele-Norwood D, Zhou P, Clark DA, Riddell R, Maric R, Podack ER, Croitoru K. et al. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor in T cell induced mucosal damage in the mouse intestine. Gut. 2002;51(2):155–163. doi:10.1136/gut.51.2.155.
- Ferran C, Sheehan K, Dy M, Schreiber R, Merite S, Landais P, Noel L-H, Grau G, Bluestone J, Bach J-F. et al. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: Further evidence for transient in vivo T cell activation. Eur J Immunol. 1990;20(3):509–515. doi:10.1002/eji.1830200308.
- Xu H, Cai F, Li P, Wang X, Yao Y, Chang X, Bi Z, Sun H, Zhuang H, Hua Z-C. et al. Characterization and analysis of the temporal and spatial dynamic of several enteritis modeling methodologies. Front Immunol. 2021;12:727664. doi:10.3389/fimmu.2021.727664.
- Olin-Sandoval V, Yu JSL, Miller-Fleming L, Alam MT, Kamrad S, Correia-Melo C, Haas R, Segal J, Peña Navarro DA, Herrera-Dominguez L. et al. Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism. Nature. 2019;572(7768):249–253. doi:10.1038/s41586-019-1442-6.
- Kim S, Ihara K, Katsube S, Hori H, Ando T, Isogai E, Yoneyama H. Characterization of the l-alanine exporter AlaE of Escherichia coli and its potential role in protecting cells from a toxic-level accumulation of l-alanine and its derivatives. Microbiologyopen. 2015;4(4):632–643. doi:10.1002/mbo3.269.
- Eletsky A, Michalska K, Houliston S, Zhang Q, Daily MD, Xu X, Cui H, Yee A, Lemak A, Wu B. et al. Structural and functional characterization of DUF1471 domains of Salmonella proteins SrfN, YdgH/SssB, and YahO. PLoS One. 2014;9(7):e101787. doi:10.1371/journal.pone.0101787.
- Westermann AJ, Förstner KU, Amman F, Barquist L, Chao Y, Schulte LN, Müller L, Reinhardt R, Stadler PF, Vogel J. et al. Dual RNA-seq unveils noncoding RNA functions in host–pathogen interactions. Nature. 2016;529(7587):496–501. doi:10.1038/nature16547.
- Lee J, Hiibel SR, Reardon KF, Wood TK. Identification of stress-related proteins in Escherichia coli using the pollutant cis-dichloroethylene. J Appl Microbiol. 2010;108:2088–2102. doi:10.1111/j.1365-2672.2009.04611.x.
- Zhang XS, Garcia-Contreras R, Wood TK. YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J Bacteriol. 2007;189(8):3051–3062. doi:10.1128/JB.01832-06.
- Luo M, Yang S, Li X, Liu P, Xue J, Zhou X, Su K, Xu X, Qing Y, Qiu J. et al. The KP1_4563 gene is regulated by the cAMP receptor protein and controls type 3 fimbrial function in Klebsiella pneumoniae NTUH-K2044. PLoS One. 2017;12(7):e0180666. doi:10.1371/journal.pone.0180666.
- Horng YT, Dewi Panjaitan NS, Chang H-J, Wei Y-H, Chien C-C, Yang H-C, Chang H-Y, Soo P-C. A protein containing the DUF1471 domain regulates biofilm formation and capsule production in Klebsiella pneumoniae. J Microbiol Immunol Infect. 2022;55(6):1246–1254. doi:10.1016/j.jmii.2021.11.005.
- Hancock V, Vejborg RM, Klemm P. Functional genomics of probiotic Escherichia Coli Nissle 1917 and 83972, and UPEC strain CFT073: comparison of transcriptomes, growth and biofilm formation. Mol Genet Genomics. 2010;284(6):437–454. doi:10.1007/s00438-010-0578-8.
- Wang Y, Wang S, Chen W, Song L, Zhang Y, Shen Z, Yu F, Li M, Ji Q. CRISPR-Cas9 and CRISPR-Assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl Environ Microb. 2018;84(23):e01834–01818. doi:10.1128/aem.01834-18.
- Hao M, He Y, Zhang H, Liao X-P, Liu Y-H, Sun J, Du H, Kreiswirth BN, Chen L. CRISPR-Cas9-Mediated Carbapenemase Gene and Plasmid Curing in Carbapenem-Resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2020;64(9). doi:10.1128/aac.00843-20.
- Elistratova AA, Matrosova LE, Khilyas IV, Shirshikova TV, Danilova IV, Laikov AV, Romanova YD, Sierra-Bakhshi CG, Sharipova MR, Bogomolnaya LM. et al. Serratia marcescens DUF1471-containing protein SrfN is needed for adaptation to acid and oxidative stresses. mSphere. 2022;7(6):e00212–00222. doi:10.1128/msphere.00212-22.
- Salazar JK, Deng K, Tortorello ML, Brandl MT, Wang H, Zhang W. Genes ycfR, sirA and yigG contribute to the surface attachment of Salmonella enterica Typhimurium and Saintpaul to fresh produce. PLoS One. 2013;8(2):e57272. doi:10.1371/journal.pone.0057272.
- Lin CT, Lin T-H, Wu C-C, Wan L, Huang C-F, Peng H-L. CRP-Cyclic AMP regulates the expression of type 3 Fimbriae via cyclic di-GMP in Klebsiella pneumoniae. PLoS One. 2016;11(9):e0162884. doi:10.1371/journal.pone.0162884.
- Lin D, Fan J, Wang J, Liu L, Xu L, Li F, Yang J, Li B. The fructose-specific phosphotransferase system of Klebsiella pneumoniae is regulated by global regulator CRP and linked to virulence and growth. Infect Immun. 2018;86(8):10–128. doi:10.1128/IAI.00340-18.
- Xue J, Tan B, Yang S, Luo M, Xia H, Zhang X, Zhou X, Yang X, Yang R, Li Y. et al. Influence of cAMP receptor protein (CRP) on bacterial virulence and transcriptional regulation of allS by CRP in Klebsiella pneumoniae. Gene. 2016;593(1):28–33. doi:10.1016/j.gene.2016.08.006.
- Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi:10.1016/S0140-6736(21)02724-0.
- Qin X, Wu S, Hao M, Zhu J, Ding B, Yang Y, Xu X, Wang M, Yang F, Hu F. et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: Epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis. 2020;221(Supplement_2):S206–S214. doi:10.1093/infdis/jiz622.
- Tiwana H. et al. Correlation between the immune responses to collagens type I, III, IV and V and Klebsiella pneumoniae in patients with Crohn’s disease and ankylosing spondylitis. Rheumatology. 2001;40:15–23. doi:10.1093/rheumatology/40.1.15.
- Jagt JZ, Verburgt CM, de Vries R, de Boer NKH, Benninga MA, de Jonge WJ, van Limbergen JE, de Meij TGJ. Faecal metabolomics in paediatric inflammatory bowel disease: A systematic review. J Crohns Colitis. 2022;16(11):1777–1790. doi:10.1093/ecco-jcc/jjac079.
- Vich Vila A, Hu S, Andreu-Sánchez S, Collij V, Jansen BH, Augustijn HE, Bolte LA, Ruigrok RAAA, Abu-Ali G, Giallourakis C. et al. Faecal metabolome and its determinants in inflammatory bowel disease. Gut. 2023;72(8):1472–1485. doi:10.1136/gutjnl-2022-328048.
- Zampieri M, Horl M, Hotz F, Muller NF, Sauer U. Regulatory mechanisms underlying coordination of amino acid and glucose catabolism in Escherichia coli. Nat Commun. 2019;10(1):3354. doi:10.1038/s41467-019-11331-5.
- Panjaitan NSD, Horng Y-T, Chien C-C, Yang H-C, You R-I, Soo P-C. The PTS components in Klebsiella pneumoniae affect bacterial capsular polysaccharide production and macrophage phagocytosis resistance. Microorganisms. 2021;9(2):335. doi:10.3390/microorganisms9020335.
- Liu L, Li F, Xu L, Wang J, Li M, Yuan J, Wang H, Yang R, Li B. Cyclic AMP-CRP modulates the cell morphology of Klebsiella pneumoniae in high-glucose environment. Front Microbiol. 2019;10:2984. doi:10.3389/fmicb.2019.02984.
- Panjaitan NSD, Horng YT, Cheng SW, Chung WT, Soo PC. EtcABC, a Putative EII complex, regulates type 3 fimbriae via CRP-cAMP signaling in Klebsiella pneumoniae. Front Microbiol. 2019;10:1558. doi:10.3389/fmicb.2019.01558.
- Zeng J, Hong Y, Zhao N, Liu Q, Zhu W, Xiao L, Wang W, Chen M, Hong S, Wu L. et al. A broadly applicable, stress-mediated bacterial death pathway regulated by the phosphotransferase system (PTS) and the cAMP-Crp cascade. Proc Natl Acad Sci U S A. 2022;119(23):e2118566119. doi:10.1073/pnas.2118566119.
- Patwa LG, Fan T, Tchaptchet S, Liu Y, Lussier YA, Sartor RB, Hansen JJ. Chronic intestinal inflammation induces stress-response genes in commensal Escherichia coli. Gastroenterology. 2011;141(5):1842–1851.e10. doi:10.1053/j.gastro.2011.06.064.
- Lourenco M, Chaffringeon L, Lamy-Besnier Q, Titécat M, Pédron T, Sismeiro O, Legendre R, Varet H, Coppée J-Y, Bérard M. et al. The gut environment regulates bacterial gene expression which modulates susceptibility to bacteriophage infection. Cell Host Microbe. 2022;30(4):556–569.e5. doi:10.1016/j.chom.2022.03.014.
- Alav I, Kobylka J, Kuth MS, Pos KM, Picard M, Blair JMA, Bavro VN. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in gram-negative bacteria. Chem Rev. 2021;121(9):5479–5596. doi:10.1021/acs.chemrev.1c00055.
- Kohler T, Michéa‐Hamzehpour M, Henze U, Gotoh N, Kocjancic Curty L, Pechère J-C. Characterization of MexE–MexF–OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23(2):345–354. doi:10.1046/j.1365-2958.1997.2281594.x.
- Nishino K, Yamaguchi A. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J Bacteriol. 2001;183(20):5803–5812. doi:10.1128/JB.183.20.5803-5812.2001.
- Koita K, Rao CV, Driessen A. Identification and analysis of the putative pentose sugar efflux transporters in Escherichia coli. PLoS One. 2012;7(8):e43700. doi:10.1371/journal.pone.0043700.

