ABSTRACT
The occurrence and progression of tumors are often accompanied by disruptions in the gut microbiota. Inversely, the impact of the gut microbiota on the initiation and progression of cancer is becoming increasingly evident, influencing the tumor microenvironment (TME) for both local and distant tumors. Moreover, it is even suggested to play a significant role in the process of tumor immunotherapy, contributing to high specificity in therapeutic outcomes and long-term effectiveness across various cancer types. Probiotics, with their generally positive influence on the gut microbiota, may serve as effective agents in synergizing cancer immunotherapy. They play a crucial role in activating the immune system to inhibit tumor growth. In summary, this comprehensive review aims to provide valuable insights into the dynamic interactions between probiotics, gut microbiota, and cancer. Furthermore, we highlight recent advances and mechanisms in using probiotics to improve the effectiveness of cancer immunotherapy. By understanding these complex relationships, we may unlock innovative approaches for cancer diagnosis and treatment while optimizing the effects of immunotherapy.
1. Introduction
The complex interplay between the gut microbiota and human well-being has recently emerged as a captivating area of research. Tumors arise and progress in individuals with compromised immune function, where the immune system fails to recognize and eliminate abnormal cells. This process is also accompanied by disturbances in the gut microbiota.Citation1 Numerous studies have shown notable variations in gut microbiota between healthy individuals and cancer patients, with unique microbial signatures observed among various cancer groups. Inversely, the impact of the gut microbiota on the onset and growth of cancer is increasingly clear,Citation2,Citation3 affecting the tumor microenvironment (TME) and impacting both local and distant tumors (). Tumor immunotherapy is an approach to treatment that stimulates the body’s tumor-specific immune responses to harness their inhibitory and cytotoxic functions against cancer cells, which has been rapidly developed and garnered attention due to its high specificity and potential for long-term effectiveness.Citation4 However, the major limitation of tumor immunotherapy is that it does not work for all cancer patients. Substantial evidence has demonstrated that among various types of cancer, some individuals may not show significant treatment responses to ICIs and have side effects due to intestinal microorganisms.Citation5
Within this context, as beneficial microorganisms, probiotics have drawn attention for their ability to modulate the gut microbiota and hold promise in potentially alleviating various health conditions and diseases.Citation6,Citation7 Probiotics also modulate intestinal immune function by interacting with various cell types, including IECs, dendritic cells (DCs), T cells, and B cells, influencing their differentiation, activation, proliferation, and secretion.Citation8–11 In addition, they also play a crucial role in enhancing cancer immunotherapy and providing better inhibition of tumor growth. On the one hand, probiotics can regulate the gut microbiota and immunity, influencing the body’s response to immune inhibitors. On the other hand, probiotics can also translocate to tumor sites and exert their inhibitory effects.Citation12–15
Therefore, this manuscript explores the interplay between the gut microbiota and the TME. We summarize the disturbance of the intestinal microbiota in cancer patients and potential underlying causes, as well as the microbial biomarkers that can be used for the diagnosis and prognostic assessment of cancers.Citation16–18 We also investigated how the gut microbiota affects the tumor microenvironment, including the composition of the tumor microbiota and its influence on cancer development. Furthermore, we highlight recent advances in using probiotics to improve the effectiveness of cancer immunotherapy. This comprehensive review aims to provide valuable insights into the dynamic interactions between probiotics, gut microbiota, and cancer. By understanding these complex relationships, we may unlock innovative approaches for cancer diagnosis and treatment while optimizing the effect of immunotherapy.
2. Gut microbiome and probiotics
The gut microbiota, consisting of a large number of microorganisms, has garnered widespread attention for its multifaceted role in human health. These microorganisms, predominantly anaerobic bacteria, are roughly equivalent in number to human cells,Citation19 with their unique genes exceeding the human genome by approximately 100-fold.Citation20 The gut microbiota contributes to food digestion and nutrient absorption, engages in diverse metabolic processes, and profoundly impacts host immunity maintenance and regulation.Citation21 Consequently, overall health needs to maintain the balance of the gut microbiota. Diet, geography, and health status have the most remarkable effects on gut microbiota composition. In general, it remains in dynamic equilibrium; once the balance of the gut microbiota is disturbed, it can cause the host to lose various functions, including barrier function, immunological function, and inflammation, which makes it harder to fight pathogens,Citation22 thereby inducing various diseases, such as inflammatory bowel diseases;Citation23,Citation24 metabolic disorders, such as obesity and type 2 diabetes;Citation25–27 autoimmune diseases,Citation28,Citation29 such as food allergies,Citation30 eczema,Citation31 and asthma;Citation32 and even the growth and incidence of malignancies ().Citation33,Citation34 In addition, growing interest in the significance of the gut microbiota in disease states has garnered increasing attention, as it can extend beyond the intestinal barrier and impact primary lymphoid organs and the tumor microenvironment.Citation35–38 In tumor immunotherapy research, only a subset of participants demonstrates a significant response, implying that the intestinal microbiota is crucial in tumor immunotherapy.
By intervening in the gut microbiota, probiotics can potentially enhance the host’s well-being and improve the host’s health. Probiotics exert their pleiotropic effects to reduce potential disease risks by employing several key mechanisms by competitively excluding harmful microbes, producing beneficial metabolites, including short-chain fatty acids,Citation39,Citation40 hydrogen peroxide,Citation41 and bacteriocin,Citation42 regulating immunity, and maintaining the integrity of the mucosa.Citation43 Numerous studies have revealed that probiotics can help prevent and treat gastrointestinal diseases,Citation44,Citation45 chronic metabolic diseases,Citation46,Citation47 and cardiovascular and cerebrovascular diseases.Citation48–50 In recent years, some studies have applied probiotics in tumor immunotherapy and found that probiotics can cooperate with tumor immunotherapy to achieve better tumor suppression effects. This also suggests a brighter prospect for using probiotics in treating and preventing diseases.
3. The gut microbiome and cancer
Multiple studies have shed light on the gut microbiota’s function in promoting or inhibiting tumor development.Citation51 The interaction between microorganisms and cancer is multifaceted. Microbes can contact cancerous tissues directly, influencing their behavior.Citation52,Citation53 Additionally, microbes could indirectly affect cancer, including the modulation of host physiology or the induction of systemic inflammation by the gut microbiota, affecting tumors’ spread to distant places.Citation51,Citation54 When dietary or host-released metabolites enter the gut, they undergo biotransformation through microbial catalysis.Citation55 These metabolites can reach remote tissues upon absorption and circulation within the host and influence tumor progression.Citation56 Both individual microbial species and the overall microbial community contribute to tumor initiation and development. For instance, Helicobacter pylori, initially identified as the causative agent of gastritis, has a significant correlation with gastric cancer epidemiologically.Citation57 Gastric cancer risk was considerably decreased following the elimination of H. pylori. Meanwhile, tumors arise and progress in individuals with compromised immune function, where the immune system fails to identify and eliminate abnormal cells;Citation1 these effects occur alongside disturbances in the gut microbiota.
3.1. Characteristics of the gut microbiome in patients with various types of cancer
Numerous studies have shown notable variations in the gut microbiota composition between healthy subjects and cancer patients. Moreover, within different groups of cancer patients, there may be unique microbial signatures ().
Table 1. Characteristics of the Gut Microbiota in Patients with Various Types of Cancer.
With a troubling rise in occurrence among young adults over the past 20 years, colorectal cancer (CRC) has risen to the third most common cancer among all cancers worldwide.Citation115 Numerous studies have illuminated the complex interaction between the gut microbiota and the emergence of CRC. Moreover, certain members of the microbial population have been linked to colorectal cancer. Animal models used in functional investigations have identified several microorganisms, including Escherichia coli, Enterococcus faecalis, Fusobacterium nucleatum, and Bacteroides fragilis, and demonstrated their potential carcinogenic roles in colorectal cancer development.Citation116 B. fragilis can produce B. fragilis toxin (BFT), which triggers inflammatory responses and the abnormal proliferation of intestinal cells.Citation66 Enterotoxigenic B. fragilis causes colonic signal transduction and activates transcription-3 (Stat3) activation and T helper 17 (Th17) cell responses, thereby promoting colon tumorigenesis.Citation117 Patients with colorectal cancer have been found to have more B. fragilis than healthy controls. Furthermore, F. nucleatum, which is more prevalent in the feces of people with CRC, is known to adhere to colorectal cancer cells. This bacterium might aid cancer growth by promoting inflammation and inhibiting the immune response.Citation118,Citation119 In particular, the F. nucleatum can stimulate TLR4 signaling to encourage the development of tumors.Citation67,Citation120 Extracellular superoxide and hydrogen peroxide produced by E. faecalis have been shown to cause DNA damage in colonic epithelial cells.Citation67 Additionally, E. faecalis can produce cytotoxic necrotizing factor 1 (CNF1), which results in the growth and spread of cancer cells.Citation120 Additionally, E. coli and Streptococcus gallolyticus both promote CRC development.Citation68,Citation69 Polyketide synthase-positive (pks+) E. coli-derived colibactin, a small-molecule genotoxin, can induce double-strand DNA breaks (DSBs), causing DNA damage in colonic epithelial cells to facilitate CRC progression.Citation121,Citation122 In addition to these bacterial species that promote colorectal cancer, the intestinal microbial community structure of colorectal cancer patients also has unique characteristics that are significantly different from those of healthy people. Human studies have revealed that CRC patients have increased species richness in the gut microbiota, with decreases in Roseburia and increases in the Bacteroides, Escherichia, Fusobacterium, and Porphyromonas genera.Citation64,Citation65 Another study demonstrated that the genera Prevotella, Peptostreptococcus, Parvimonas and Porphyromonas were increased in the fecal samples of CRC patients.Citation71–74 Among them, Peptostreptococcus anaerobius, which belongs to the genus Peptostreptococcus, is a CRC-enriched bacterium that promoted carcinogenesis by activating the TLR2 and/or TLR4 pathways in mouse models.Citation123 Moreover, Clostridium difficile was also enriched in CRC patients.Citation75
These unique characteristics can be used as biomarkers for early diagnosis. Yu and her team employed seven bacterial species, including B. fragilis, F. nucleatum, Parvimonas micra, Porphyromonas asaccharolytica, Prevotella intermedia, Thermanaerovibrio acidaminovorans, and Alistipes finegoldii, as biomarkers for early-stage colorectal cancer diagnosis. They achieved an area under the receiver-operating characteristics curve (AUC) of 0.80; when combined with clinical information, the accuracy was further enhanced to an AUC of 0.88.Citation124 Additionally, Ma et al. developed an innovative prediction model that utilized single nucleotide variant (SNV) sites within the gut microbiota to diagnose early CRC. This prediction model contained 22 single-nucleotide variant sites (SNVs), primarily derived from Eubacterium rectale and F. nucleatum, and the model demonstrated high accuracy in distinguishing between disease and healthy cohorts (AUC = 73.08%~88.02%). Furthermore, when tested via a meta-analysis that included patients with other metabolic disorders, the model exhibited excellent specificity.Citation23,Citation125
Esophageal cancer has long been regarded as one of the most prevalent cancers in the world, with common symptoms including difficulty swallowing, which can lead to indigestion.Citation126 Park et al. shed light on a potential link between gram-negative bacteria expressing lipopolysaccharides and enhanced inducible nitric oxide synthase expression (iNOS). This may lead to a reduction in esophageal sphincter relaxation, which ultimately contributes to gastroesophageal reflux disease (GERD) and is linked to an increased risk of esophageal cancer.Citation127 In the context of esophageal cancer, distinct alterations in the gut microbiota have been observed. Compared to healthy individuals, patients with esophageal cancer often have lower levels of Bacteroidetes and higher amounts of Firmicutes and Actinobacteria.Citation77 Other research has also shown that individuals with esophageal cancer have lower Bacteroidetes, Fusobacteria, and Spirochaetes in their feces.Citation78
Amino acids, bile acids, short-chain fatty acids, and choline are essential signaling molecules and metabolic substrates that influence liver function.Citation128,Citation129 Intestinal barrier dysfunction allows intestinal-derived bacteria and microbiota metabolites, particularly lipopolysaccharides, to induce CXCL1 expression in hepatocytes in a TLR4-dependent manner and increase CXCR2+ PMN-MDSCs. The increase in PMN-MDSCs could induce liver cells to form an immunosuppressive environment and lead to the occurrence of liver cancer.Citation130 In fecal samples of hepatocellular carcinoma (HCC) patients, the genera Streptococcus, Lactobacillus Prevotella_9, Faecalibacterium, and Bacteroides were enriched, whereas Akkermansia, Subdoligranulum, Prevotella_2, and Faecalibacterium were reduced. These bacteria are all involved in BA synthesis.Citation79–82,Citation131 Meanwhile, in fecal samples of liver cancer patients, lipopolysaccharide (LPS)-producing genera, such as Klebsiella, increased, whereas butyrate-producing genera, such as Ruminococcus, decreased.Citation84 Research has shown that the presence of GelE-positive E. faecalis can promote the progression of liver cancer. This bacterium expressed metallopeptidase GLE, which increased intestinal permeability, led to an increase in plasma lipopolysaccharides and activation of hepatocyte TLR4-MYD88 proliferation signaling, and finally promoted the development of liver cancer.Citation85
Based on these microbes, eight genera, including Faecalibacterium, Lactobacillus, Klebsiella, Citrobacter, Dorea, Ruminococcus Gnavus group, Veillonella, and Burkholderia Caballeronia Paraburkholderia, were proposed to be used in the gut microbiota-based model, and high accuracy (AUC >0.90) was obtained when distinguishing patients with liver cancer from healthy controls.Citation132 Zhang et al. employed only the three genera mentioned above, Faecalibacterium, Burkholderia Caballeronia Paraburkholderia, and Ruminococcus-1, as biomarkers to differentiate between cholangiocarcinoma (CCA) patients and healthy subjects.Citation88 Han et al. employed Enterococcus, Lactobacillus, and Escherichia as biomarkers to distinguish between groups with and without lung cancer and achieved an accuracy of 0.81.Citation103
Chronic inflammation is intimately related to cancer development, and the gut microbiome may impact pancreatic tissue through the inflammatory process, thereby promoting the growth and spread of pancreatic cancer. In patients with pancreatic ductal adenocarcinoma (PDA), comparative investigations of the gut microbiota have revealed that at the phylum level, Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia are more prevalent in these patients’ guts.Citation90 During the inflammatory process, changes in gut permeability occur, which enable the translocation of intestinal microorganisms, particularly those belonging to the Proteobacteria phylum, which migrate to the pancreas and accumulate there. In turn, Toll-like receptor 2 and Toll-like receptor 5 are consequently activated in the pancreas and trigger downstream immune suppression. This combined effect and genetic risk factors promote the development of PDA.Citation90,Citation133 Additionally, another study indicated that pancreatic adenocarcinoma patient feces contained higher concentrations of the bacteria Fusobacterium hwasookii, Veillonella atypica, F. nucleatum, and Alloscardovia omnicolens. At the same time, Bacteroides coprocola, Faecalibacterium prausnitzii, Romboutsia timonensis, and Bifidobacterium bifidum were deficient.Citation91 Similarly, another pancreatic adenocarcinoma patient study confirmed a decrease in F. prausnitzii. Concurrently, Veillonella parvula, V. atypica, and Streptococcus species were more abundant in the intestines.Citation92
Breast cancer predominantly affects females, with a significant increase in breast cancer risk observed during menopause. Hormone levels undergo substantial fluctuations in women before and after menopause, accompanied by significant alterations in the gut microbiota. Consequently, compared to healthy individuals, there are distinct characteristics in the gut microbiota of premenopausal and postmenopausal breast cancer patients. In premenopausal breast cancer patients, more Enterobacteriaceae, Lactobacilli, and aerobic Streptococci were present compared to healthy individuals.Citation94 Additionally, there was an observable increase in the presence of Bacteroides, Clostridia, and aerobic Lactobacilli.Citation95 However, postmenopausal breast cancer patients had a decrease in Bacteroides abundance and an increase in Clostridiales abundance.Citation98 At the species level, postmenopausal breast cancer patients exhibited a decreased abundance of Roseburia inulinivorans and Eubacterium eligens, while Acinetobacter radioresistens, E. coli, Salmonella enterica, Erwinia amylovora, Citrobacter koseri, Shewanella putrefaciens, Enterococcus gallinarum, Actinomyces spp. HPA0247 and F. nucleatum had an increased abundance.Citation96 The reason behind these differences is widely believed to be linked to fluctuations in estrogen levels, which have been linked to the development of breast cancer. Possible mechanisms include, on the one hand, the hypothesis that estrogen metabolism can be influenced by gut bacteria. For postmenopausal women in particular, elevated estrogen levels are a significant risk factor for breast cancer.Citation134 Certain gut microbiota can also induce 16α-hydroxylation of estrogen, which raises the risk of developing breast cancer.Citation135 On the other hand, a high-fat diet may cause the gut microbiota of patients to create steroidal chemicals that are linked to cancers. These steroids may influence estrogen levels or affect breast tissue, which could promote the growth of tumors.Citation136 Furthermore, breast cancer incidence and inflammation are tightly related. The gut microbiota regulates mucosal and systemic immune responses, impacting cancer cells and the surrounding environment.Citation137 This influences obesity status, breast cancer risk, and endogenous and exogenous metabolism.Citation138
These findings highlight the intricate interplay between specific gut microbiomes and the development of colorectal cancer, thereby offering potential avenues for further research and therapeutic interventions.
3.2. The gut microbiome and the tumor microenvironment (TME)
Although the gut microbiota has been identified as a pivotal biomarker and modulator in cancer development and treatment response, recent research has provided evidence that the gut microbiota has a crucial impact on the TME and impacts both local and distant tumors (). One notable example is pancreatic adenocarcinoma, a cancer typically associated with poor survival rates. Research conducted in 2019 on pancreatic adenocarcinoma patients revealed that long-term responders, specifically those surviving for over five years, exhibited a more diverse tumor microbiome than short-term survivors. Notably, some of the microbes within the tumors originated from the gut, which may communicate through the pancreatic duct and may be impacted by the regulation of gut microbial communities.Citation139 This underscores the role of gut microbiota in the tumor microenvironment, a role further supported by experiments involving fecal microbiota transplantation (FMT). FMT from long-term survivors effectively slowed tumor development in pancreatic adenocarcinoma mice and bolstered their immune cell activity.Citation140 Many studies have also reinforced the importance of local microbial communities as essential constituents of the TME. This significance is particularly evident in cancers developing at mucosal locations such as the lungs, skin, and gastrointestinal tract.Citation141–144 Notably, the bacterial populations residing in tumors are unique to certain tumors.Citation145,Citation146
3.2.1. Characteristics of the tumor microbiome in various types of tumors
Microbiomes are present in various tumor tissues and play a vital role in tumor development, metastasis, and treatment. Within the context of cancer development, microbes within the tumor microenvironment can be a double-edged sword. On the one hand, they can facilitate tumor development and spread through mechanisms such as inducing DNA mutations, activating oncogenic pathways, fostering chronic inflammation, and promoting complement system activity and metastasis.Citation147 On the other hand, these tumor-associated microbes may also contribute to inhibiting tumor growth or enhancing the immune system’s response. They can either strengthen or weaken the antitumor immune response, induce different immunotherapeutic effects and outcomes, and impact long-term survival rates.Citation147,Citation148 Notably, recent research by Nejman et al. analyzed seven different cancer types and revealed distinct microbial compositions within tumors and cells across various cancer types.Citation148 Numerous studies have also found that different tumor types exhibit unique microbial community structures in tumors such as those associated with colorectal, lung, and breast ().Citation148–150
The phyla Firmicutes and Bacteroidetes were the most predominant in colorectal cancer tissues.Citation148 Yachida et al. found that the species F. nucleatum and Solobacterium moorei showed a progressive rise from the early to late stages of carcinogenesis in colorectal tumors.Citation72,Citation151 In addition, enterotoxigenic B. fragilis and E. coli both had a high abundance of tumors, and the colonization of tumors by E. coli expressing the genomic island polyketide synthase (pks+ E. coli) leads to an increase in tumor multiplicity and invasion.Citation68,Citation152–154
Lung cancer is the most prevalent cancer, with the highest occurrence and fatality rates. Research has shown that both healthy and diseased lungs are not sterile environments. Tumor tissues harbor unique microbial communities. For instance, Laroumagne et al. detected E. coli, Haemophilus influenzae, Enterobacter, and Staphylococcus enrichment in lung cancer tissues.Citation155 Lee et al. identified phyla such as Firmicutes and TM7 and genera including Veillonella and Megasphaera that were notably elevated in lung cancer tissues.Citation156 Another study revealed specific microbial compositions in lung cancer tumor tissues, including Streptococcus, Acinetobacter, Thermus, Megasphaera, Granulicatella adiacens, Enterococcus, Prevotella, Rothia, Brevundimonas, Propionibacterium, Enterobacter, E. coli and Legionella.Citation157–159 Research has indicated that the Actinobacteria and Firmicutes phyla showed a higher abundance in lung cancer tissues than in healthy individuals.Citation160 The genera Staphylococcus, Escherichia, and Bacillus were the characteristic markers of the lung cancer group.Citation161 Discrepancies in detection results may be attributed to tumor subtypes, sampling locations, different stages of the disease, and various detection methods. Yu et al. found that the abundance of the Thermus genus was increased and related to advanced-stage cancer.Citation143 Yan et al., using quantitative PCR methods, confirmed the significant alterations in Neisseria, Capnocytophaga, and Veillonella among lung cancer patients.Citation156,Citation162,Citation163 In addition, in small-cell carcinoma (SCC), the genera Klebsiella, Comamonas, Rhodoferax, Acidovorax, and Polaromonas are commonly reported.Citation164
In pancreatic ductal adenocarcinoma, at the phylum level, the bacterial phyla Proteobacteria (45%), Bacteroidetes (31%), Firmicutes (22%), and Actinobacteria (1%) were most prevalent in human pancreatic ductal adenocarcinoma (PDA) samples. Proteobacteria dominate the microbiome of pancreatic adenocarcinoma,Citation90 and these bacteria can induce T-cell anergy in a Toll-like receptor-dependent manner, thereby hastening the advancement of tumors. Among them, Gammaproteobacteria were detected in 76% of PDAC patients.Citation165 At the genus level, the genera Pseudomonas and Elizabethkingia were predominant in human PDA samples.Citation90 Bifidobacterium pseudolongum was also detected in the pancreas.Citation90 Additionally, another study indicated that F. nucleatum was enriched in PDAC cohorts.Citation148 Riquelme et al. found that Pseudoxanthomonas, Streptomyces spp., and Saccharopolyspora were enriched in patients with longer survival rates. In contrast, Bacillus clausii was increased in pancreatic adenocarcinoma (PDAC) patients with shorter survival.Citation140
In cholangiocarcinoma tumors, a total of 266 species, including those of Clostridiales, Sphingomonadales, Pseudomonadales, Burkholderiales, Bacillales, and Xanthomonadales, were detected in intrahepatic cholangiocarcinoma.Citation166 There was also an increase in H. pylori, Helicobacter hepaticus, and Helicobacter bilis.Citation167,Citation168 The potential mechanism underlying these changes is that gram-negative bacteria affect hepatocytes to create a microenvironment that is immunosuppressive by inducing CXCR2+ PMN-MDSC accumulation through TLR4-dependent CXCL1 production, thereby promoting the development of liver tumors.Citation169
Breast cancer (BC) is one of the most prevalent malignancies in women. Numerous studies have identified microorganisms important for breast tumor development, progression, and prognosis. Actinobacteria, Proteobacteria, Bacteroidetes, Firmicutes, and Verrucomicrobia were enriched in breast tumor tissues.Citation170 Thompson et al. also confirmed that Firmicutes, Actinobacteria, and Proteobacteria were increased, and the species Mycobacterium phlei and Mycobacterium fortuitum were abundant in breast tissues as well.Citation171 In the four BC subtypes, Proteobacteria and Actinomyces were also confirmed to be significantly enriched.Citation172 According to Xuan et al., Methylobacterium radiotolerans was enriched, while Sphingomonas yanoikuyae was decreased in breast tumor tissue at the genus level.Citation170 Many studies have found that F. nucleatum is enriched and accelerates the development and metastasis of breast tumors.Citation148,Citation173,Citation174 In malignant breast tissue samples, the genus Fusobacterium was found to have a high accumulation.Citation174 F. nucleatum can promote aggressive tumor behaviors through Treg lymphocytes in a chemokine-dependent manner, particularly involving CCL20; this bacterium is thereby associated with an unfavorable prognosis.Citation175 B. fragilis was also detected in cancerous breast tissues.Citation176 Urbaniak et al. indicated that Comamondaceae, Bacteroidetes, Enterobacteriaceae, Bacillus, and Staphylococcus were enriched in BC cancer patients.Citation177 Meng et al. showed that the genus Propionicimonas and families Methylobacteriaceae, Caulobacteraceae, Nocardioidaceae, Rhodobacteraceae, and Micrococcaceae were enriched in BC tissues.Citation178 Additionally, the genus Agrococcus, which is connected to malignancy, and the relative abundance of the family Bacteroidaceae were reduced.Citation178
Table 2. Characteristics of the microbiomes in various types of Tumors.
3.2.2. The tumor microbiome and tumor development
The tumor microbiome, which accounts for approximately 25% of all cells in the tumor environment (including immune cells, cancer-associated fibroblasts, endothelial cells, pericytes, etc.), influences tumor development and treatment through various mechanisms.Citation200 These mechanisms include inducing DNA damage and mutations, thereby directly promoting tumorigenesis by increasing mutagenesis,Citation145,Citation201,Citation202 activating oncogenic signaling pathways, and enhancing cell proliferation and transformation.Citation203,Citation204 Several studies have pointed to members of the Enterobacteriaceae family, which produce colibactin, a DNA-damaging toxin that induces senescence in epithelial cells. Senescent cells activate signaling pathways that ultimately promote the proliferation of neighboring cells unaffected by colibactin damage, thus inducing tumor formation.Citation205 The pks+ E. coli, a bacterium known to cause DNA damage, leads to distinct mutational features in organoids.Citation206 Another group of bacteria capable of inflicting DNA damage includes enterotoxigenic B. fragilis, which produces a toxin called fragilysin. This zinc-dependent metalloproteinase promotes the cleavage of epithelial cell E-cadherin. This cleavage releases β-catenin, activating cell proliferation-regulating transcription factors and facilitating the proliferation of colon epithelial cells.Citation207 Furthermore, F. nucleatum, with its antigen adhesin A (FadA), encourages the development of CRC through the E-cadherin-calcium-catenin-Wnt-β-catenin signaling pathway.Citation208 H. pylori contributes to CRC through the induction of inflammation and by regulating mucosal cell growth and proliferation via critical intracellular signaling pathways.Citation209
Additionally, the tumor microbiome can also break down and metabolize antitumor drugs, reducing their effectiveness and leading to drug resistance.Citation210 For instance, Ravid Straussman’s research has demonstrated that microbes within tumors can directly metabolize gemcitabine, leading to chemotherapy resistance in pancreatic cancer. Gemcitabine resistance was mediated by intratumoral Gammaproteobacteria and Bifidobacterium pseudolongum in mouse models.Citation165,Citation211 Moreover, certain bacteria, including intratumoral Mycoplasma hyorhinis and species of Proteobacteria, can also metabolize gemcitabine into 2,2-difluoro deoxyuridine, rendering the drug inactive and causing resistance.Citation165 Using ciprofloxacin to eliminate bacteria could overcome this resistance in mouse models.
The tumor microbiome can also modulate the host immune system, thereby influencing immune cell activation and polarization and altering the immune milieu within the tumor microenvironment.Citation212 For instance, intratumoral bacteria can activate the functionality of antitumor T cells by producing immunogenic molecules or stimulating interferon signaling.Citation213 Certain intratumoral microbes can affect the synthesis and secretion of cytokines such as IL-6 and TNF-α.Citation214 Numerous studies indicate that the intratumoral microbiota may impact cytokine production, thereby inducing proinflammatory responses that subsequently activate the NF-κB or STAT3 pathway to promote tumor development.Citation215,Citation216 Triner and colleagues showed that intratumoral bacteria induce the production of IL-17, which promotes B-cell infiltration and tumor development.Citation217 Additionally, Toll-like receptors, including TLR4 and TLR5, are closely connected to the interactions between tumors and microorganisms (including the gut and intratumoral microbiota).Citation218
4. The gut microbiome and tumor immunotherapy
4.1. Cancer immunotherapy strategies
Tumor immunotherapy is an approach to treatment that stimulates the body’s tumor-specific immune responses, either actively or passively, to harness their inhibitory and cytotoxic functions against cancer cells.Citation4 It offers advantages such as specificity, efficiency, and minimal harm to healthy tissues. Unlike conventional treatments such as surgery, targeted therapy, and chemotherapy, immunotherapy does not directly kill cancer cells.Citation219 Instead, it mobilizes immune cells within the body capable of recognizing tumors, enhances the body’s immune system’s capabilities, and relies on them to indirectly eliminate and control cancer.Citation220 This method has fewer side effects, making it a safe and effective alternative. Immunotherapy encompasses various approaches, including immunotherapy checkpoint inhibitors, adoptive cellular therapy (CAR-T, TIL, NK, and CIK/DC-CIK), cancer vaccines, molecular targeted therapies, and immunomodulators such as cytokine therapy ().Citation4
Figure 3. Cancer Immunotherapy Strategies included immunotherapy checkpoint inhibitors, adoptive cellular therapy, cancer vaccines, and cytokine therapy.

Immunotherapy checkpoint inhibitors (ICIs) act based on the following principles: Immune checkpoint molecules are crucial for regulating the onset and magnitude of immune responses, preserving self-tolerance and autoimmune responses, and reducing tissue damage.Citation221 These checkpoints are expressed on immune cells and inhibit their functions, thereby preventing an effective antitumor immune response and allowing tumors to evade the immune system.Citation222 Key immune checkpoint molecules associated with cancer are PD-1, Tim-3, CTLA-4, and LAG-3, with the most extensively researched checkpoint inhibitors being PD-1/PD-L1 and CTLA-4 inhibitors.Citation4 Their primary function is to impede the interaction between tumor cells expressing immune checkpoints and immune cells, thus thwarting the inhibitory impact of tumor cells on immune cells.
Adoptive cellular immunotherapy (ACI) acts via the following mechanisms: Adoptive cellular immunotherapy transfers immune cells possessing antitumor activity, encompassing specific and nonspecific responses, into individuals with cancer. Immune cells can directly eliminate or trigger the body’s immune response to eradicate tumor cells.Citation223 Cell-based immunotherapy has always been one of the most active areas in cancer biotherapy. This therapy is particularly suitable for patients with compromised immune function, leading to reduced immune cell numbers and impaired function, especially for patients with hematological or immunological malignancies. (1) Chimeric antigen receptor T-cell (CAR-T) therapy involves extracting immune lymphocytes from the patient’s blood and genetically engineering them. This modification allows T cells to recognize tumor cell antigens while enhancing their antitumor activity. Then, the modified T cells are reintroduced into the host, specifically targeting and killing cancer cells.Citation224 (2) Tumor-infiltrating lymphocyte (TIL) therapy involves isolating lymphocytes infiltrating tumor tissue and selecting those with specific anticancer properties.Citation225 After activation and expansion, these selected lymphocytes are reintroduced into the patient’s body to recognize and target tumor cells specifically. TILs consist of T cells that directly infiltrate tumor tissue, which makes them suitable for solid tumors. (3) Natural killer (NK) cell therapy is based on the following principles: NK cells function as the body’s initial line of defense against cancer and infections and have stronger and more efficient capabilities in killing tumor and virus-infected cells than other immune cells.Citation226 By isolating and expanding from tumor tissue and then reintroducing them into the patient’s body, tumor cells can be killed. (4) Cytokine-induced killer (CIK) cell therapy is based on the following principles: CIK cells are novel, highly proliferative immune cells with cytotoxic properties and some immunological characteristics. These cells express CD3 and CD56 membrane proteins, resembling NK cells with powerful antitumor activity typical of T lymphocytes.Citation227
Tumor vaccines are a therapeutic strategy for boosting the immune system’s capacity to combat cancer. These vaccines may contain tumor-associated antigens to assist the immune system in recognizing and targeting cancer cells. Notably, Provenge (Sipuleucel-T) is currently the world’s first and only tumor vaccine approved by the U.S. Food and Drug Administration (FDA).Citation228 Dendritic cell (DC) vaccines have also made significant breakthroughs in many clinical trials.Citation229
Molecular targeted therapy focuses on reversing malignant biological behaviors at the molecular level, targeting processes that can lead to cellular transformation, such as cell signaling pathways, oncogenes, tumor suppressor genes, cytokine receptors, antitumor angiogenesis, and suicide genes.Citation230 This approach aims to control tumor cell growth and achieve complete tumor regression. This approach offers high selectivity at the molecular and cellular levels by efficiently and selectively targeting tumor cells while minimizing damage to normal tissues. Molecular targeted therapy drugs can be broadly categorized into the following two types: monoclonal antibodies and small molecules. Monoclonal antibodies are a key component of biological targeted therapy.
Immunoadjuvants are adjunctive therapies that enhance the activity of the immune system to combat cancer better. These adjuvants can be used in conjunction with other immunotherapy methods.Citation231
Cytokine therapy takes advantage of immune modulators that can help strengthen the immune system’s response against certain tumors. Commonly used cytokines in antitumor immunotherapy include IL-2, IL-12, INF-γ, and TNF.Citation232
4.2. The gut microbiome and tumor immunotherapy
In recent years, tumor immunotherapy has developed rapidly and garnered attention due to its high specificity and possible long-term effectiveness.Citation4 The most extensively researched immunotherapy strategies are PD-1/PD-L1 and CTLA-4 immune checkpoint inhibitors. Immunotherapy can precisely identify and target tumor cells without causing significant harm to healthy cells. This treatment has shown potential efficacy in various cancer types, including melanoma,Citation233,Citation234 lung cancer,Citation235 colorectal cancer,Citation236 and lymphoma.Citation237 Some patients achieve long-term clinical responses after immunotherapy and maintain resistance to tumors even after the treatment process.Citation238 However, the major limitation of tumor immunotherapy is that it does not work for all cancer patients. Some individuals may not exhibit a significant response to treatment. A study conducted in Israel involved 10 individuals with advanced-stage melanoma whose cancer had continued to progress despite prior treatment with a checkpoint inhibitor.Citation239 Substantial evidence demonstrates that intestinal microorganisms have the potential to impact clinical responses to ICIs and their side effects in a variety of cancer types.Citation5
There is clear evidence indicating that the gut microbiota plays a crucial role in shaping the immune system, is closely related to innate and adaptive immunity, and can impact the clinical responses and adverse effects of immune checkpoint inhibitors in various cancer types.Citation240,Citation241 Components within the gut microbiota can modulate the host’s antitumor immune response.Citation184,Citation242 Several studies have indicated the role of the gut microbiota in regulating the effectiveness of immune checkpoint blockade therapy.Citation185 Recent research by a Canadian team explored the potential of FMT from cancer-free donors to prevent immunotherapy resistance in individuals who had not previously received such treatment. Twenty patients with advanced melanoma were employed. Three individuals responded completely after FMT and immunotherapy, 13 partially responded, and three had persistent disease.Citation243 Additionally, FMT was utilized to evaluate the impact of gut microbiota by transferring fecal microbial communities obtained from responders and nonresponders into a germ-free melanoma model mouse. Intriguingly, researchers found that even without using anti-PD-L1 drugs, transplanting fecal microbiota from responders could lead to tumor shrinkage. A synergistic effect was observed when fecal microbiota transplantation was combined with anti-PD-L1 treatment. However, the combination had no antitumor response in nonresponders.Citation243 In another study, seven melanoma donors who had achieved a durable response to immunotherapy were employed. Another 16 patients with metastatic melanoma, whose cancer had progressed following prior immunotherapy, were enrolled for FMT with the donors’ feces. Among the 16 recipients, six exhibited a positive response, with three achieving a complete response.Citation200 In melanoma patients who were resistant to anti-PD-1 therapy, FMT from individuals who had responded effectively managed to reinstate the tumor’s sensitivity to PD-1 blockade. This led to beneficial alterations in immune and microbial profiles within both the gut and the tumor microenvironment.Citation244,Citation245
Distinct features were observed in the responsive and nonresponsive melanoma patients’ gut microbes. Patients who responded to treatment exhibited elevated levels of unidentified species from the Ruminococcaceae and Faecalibacterium families, along with Ruminococcus bicirculans and Barnesiella intestinihominis. Conversely, nonresponders displayed enrichment in Adlercreutzia equolifaciens, Bacteroides thetaiotaomicron, Bifidobacterium dentium, and unidentified species from the Mogibacterium genus.Citation246 However, Matson et al. discovered a strong connection between the clinical response and commensal microbial composition. Furthermore, they identified that E. faecium, Collinsella aerofaciens, and Bifidobacterium longum were common in fecal samples from melanoma patients who positively responded to immunotherapy therapy. Transplanting fecal samples from responding patients into germ-free mice enhanced T-cell responses and increased the effectiveness of anti-PD-L1 therapy.Citation184 A parallel study delved into the gut microbial populations of melanoma patients and discovered that the abundance of Ruminococcaceae species was linked to clinical responses to checkpoint inhibition.Citation183 McCulloch et al. proposed that an undesirable gut microbiota promotes systemic inflammation and resistance factors that affect immunity and the response to immunotherapy. They also noted that the high abundance of Streptococcus species was associated with treatment-related adverse events and a shorter progression-free survival.Citation247 In other research studies, the antitumor effects of CTLA-4 blockade depended on specific Bacteroides species, such as B. thetaiotaomicron and B. fragilis.Citation242 In contrast, the presence of the Bifidobacterium genus was positively linked to the effectiveness of PD-L1 ligand PD-1 blockade.Citation248 Additionally, the presence of A. muciniphila in stool samples positively correlated with clinical responses to ICIs in both patients and mouse models.Citation185
5. Probiotics regulate the immune system by mediating the gut microbiome
Probiotics are a category of beneficial, active microorganisms that can regulate the gut microbiota and the immune system through multiple mechanisms. (ⅰ) Probiotics can inhibit the colonization and proliferation of pathogenic bacteria in the gut through competitive exclusion, the production of antimicrobial substances, including peptides, bacteriocins, and butyrate,Citation249,Citation250 and modification of the gut environment. Probiotics can increase the abundance of microorganisms in the gut that produce short-chain fatty acids (SCFAs).Citation251 These SCFAs, essential metabolic byproducts of gut microbes, influence immune cells’ metabolism and signal transduction. (ⅱ) Probiotics play a role in maintaining gut permeability and enhancing intestinal barrier function. They achieve this by promoting mucus secretion, increasing tight junction proteins, or reducing intestinal permeability. Research has demonstrated that Lactobacillus rhamnosus GG (LGG) can directly interact with intestinal epithelial cells (IECs) and preserve the integrity of the epithelial barrier.Citation252 Studies have shown that probiotics induce goblet cells to secrete mucins and inhibit pathogen adhesion.Citation253 Lactobacillus plantarum BMCM12 can secrete extracellular proteins that weaken pathogen adhesion, safeguarding the intestinal barrier.Citation254 (ⅲ) Probiotics also modulate intestinal immune function by interacting with various cell types, including IECs, DCs, T cells, and B cells, by influencing their differentiation, activation, proliferation, and secretion. Probiotics engage with IECs or immune cells associated with the lamina propria through Toll-like receptors. This interaction leads to the release of different cytokines and chemokines, activating both innate immune responses and cytokine release by T cells and stimulating mucosal immune cells.Citation255,Citation256 Research has indicated that probiotics entering the gut can stimulate the production of IgA antibodies.Citation257 Oral intake of probiotics effectively increases the number of IgA+ cells in the intestinal lamina propria, thereby reinforcing and sustaining immune surveillance in mucosal areas distant from the gut and promoting the maturation of humoral immune mechanisms.Citation255,Citation258 Probiotics can also augment the number of macrophages and DCs in the lamina propria and enhance their functionality over a specific timeframe. One study demonstrated that specific Lactobacillus probiotic strains activated an in vitro inflammatory response in macrophages by synthesizing proinflammatory mediators, including cytokines and reactive oxygen species (ROS), and were involved in signaling pathways such as nuclear factor-kappa B (NF-kB) and Toll-like receptor 2 (TLR2).Citation257
Probiotics can also impact the host’s immune response by altering the composition and diversity of the gut microbiota. Probiotics can increase the abundance of beneficial bacteria. Treatment with a mixture of Bifidobacterium can modify the gut microbial community, significantly increasing the population of other probiotic species, such as Lactobacillus, Kosakonia, and Cronobacter. These beneficial bacteria can produce metabolites, including SCFAs, which favor immune regulation. Furthermore, the altered symbiotic community enhances the adaptability of mitochondria and intestinal Tregs’ metabolic and inhibitory functions mediated by IL-10. This contributes to maintaining regional immune homeostasis in patients under conditions of CTLA-4 blockade.Citation259 Treg cells are a key mechanism through which probiotics exert their beneficial effects. The mechanism by which probiotics regulate and alleviate inflammatory diseases and atopic dermatitis in neonates and infants was associated with the induction or expansion of Tregs and the manipulation of mucosal DCs to promote regulatory function.Citation260,Citation261 Furthermore, another study has indicated that bacterial strains from Clostridia clusters IV, XIVa, and XVIII play a pivotal role in Treg development in healthy mice, protecting against pathogens. Additionally, species within the Clostridia group, including F. prausnitzii, may promote Treg differentiation by producing SCFAs.
Beyond this, the next-generation probiotics (NGPs), such as the human commensal bacteria F. prausnitzii or A. muciniphila, have emerged as promising candidates. These strains may offer better adaptability to the gut, are being utilized to alleviate obesity, inflammatory bowel disease (IBD), and cancer, and investigate disease mechanisms.Citation262 Research has shown that the polysaccharide A (PSA) produced by F. prausnitzii can modulate the host’s immune system in terms of both health and disease. PSA also plays a critical role in developing the mammalian immune system and activating CD4+ T cells.Citation263 F. prausnitzii can enhance the effects of tumor immunotherapy and reduce its side effects.Citation242 Additionally, the presence of A. muciniphila can improve the clinical efficacy of PD-1 inhibitor treatment in patients with NSCLC.Citation264
6. Probiotics enhance tumor immunotherapy via mediating the gut microbiome
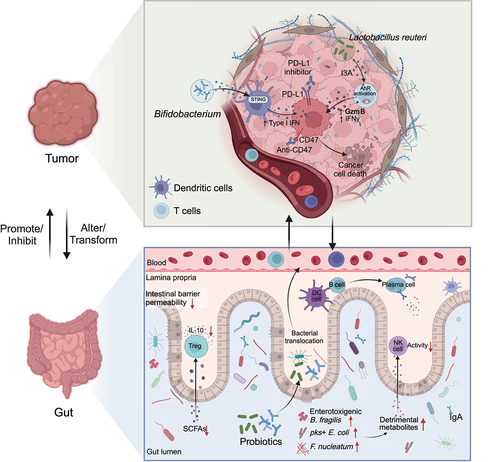
The gut microbiota plays a pivotal role in developing and maturing the host’s innate and adaptive immune systems.Citation265 Given the adaptable characteristics of the microbiome, adjusting the microbiota has recently become an appealing approach with the potential to address resistance to tumor immunotherapy in patients. On the one hand, the FMT strategy can be used to transplant the fecal microbiota of responders. On the other hand, increasing research focuses on the regulatory effects of probiotics on the gut microbiota and their promoting effect with tumor immunotherapy ().
Routy et al. pinpointed that A. muciniphila could mediate the connection between immunotherapy and treatment response. They found that orally administering A. muciniphila after fecal microbiota transplantation with feces from nonresponders reinstated the effectiveness of PD-1 blockade through an interleukin-12-dependent mechanism. This process facilitated the recruitment of CCR9+CXCR3+CD4+ T lymphocytes into epithelial tumors.Citation185 Preclinical oral probiotics in mice showed promising results in melanoma and bladder cancer studies. Administration of Bifidobacterium enhanced tumor control significantly. This approach nearly eradicated the tumors when combined with CD274 (PD-L1) blockade. The main contributing factors to success were the improved function of DCs and the recruitment of CD8+ T cells to the tumor microenvironment.Citation248 Le Noci et al. demonstrated that Lactobacillus rhamnosus could counteract immunosuppression and hinder the implantation of lung tumors. Moreover, tumor metastases were reduced when both antibiotics and probiotics were employed. These collective results suggest that the microbiota in the local environment plays a crucial role in the development of lung cancer by influencing the local immune response.Citation266 Supplementation with Lactobacillus pentosus Probio-M9 probiotics promoted the production of beneficial metabolites, such as butyric acid, in the gut by increasing beneficial microbes such as Lactobacillus and Bifidobacterium. In particular, this bacterium accumulated beneficial metabolites, including α-ketoglutarate, N-acetylglutamine, and pyridoxol derived from blood sources, enhancing the anti-PD-1 immune therapy response.Citation14
In the context of colon cancer, a different study examined the impact of intratumor microbiota on CD47-based cancer immunotherapy. Shi et al. orally administered Bifidobacterium and discovered that Bifidobacterium from the colon accumulates within tumor sites and enhances local anti-CD47 treatment through the STING pathway.Citation267 Additionally, Iida et al. demonstrated that administering Alistipes shahii through oral gavage restored the immunotherapeutic response against colon tumors in mice previously treated with antibiotics.Citation268
In melanoma, C57 mice were orally administered a combination of commensal Bifidobacterium, including B. breve and B. longum, through gavage. This treatment promoted antitumor immunity and enhanced the effectiveness of anti-PD-L1 therapy.Citation248 A recent study published in Cell introduced the only known probiotic capable of homing to tumors and exerting synergistic effects with immunotherapy. Lactobacillus reuteri (Lr) was found to migrate to and colonize melanoma tumors readily and released the dietary tryptophan-derived metabolite indole-3-carbaldehyde (I3A), which stimulated the production of interferon-gamma by CD8+ T cells, leading to the killing of cancer cells and thereby enhanced the efficacy of ICIs. The antitumor immune response induced by I3A secreted by Lr relies on the AhR receptor found on CD8+ T cells.Citation15
7. Conclusion and outlook
This article explores the relationship between gut microbiota and cancer from the perspectives of gut microbial communities and tumors. We summarized the disruption of gut microbiota, particularly in cancer patients, delving into the underlying mechanisms behind this imbalance. Additionally, the microbial biomarkers that appear in cancer diagnosis and prognosis were addressed. Furthermore, we delved into how the gut microbiota affected the tumor microenvironment, including the composition of tumor-associated microbial communities and their influence on cancer development. By untangling the intricate relationships among microbes, the tumor microenvironment, and cancer cells, valuable insights can be gained for potential cancer treatments, including targeted and personalized therapies, to maximize the efficacy of anticancer treatments. In addition, we highlighted recent advancements in using probiotics to enhance the effectiveness of cancer immunotherapy.
Probiotics are a type of beneficial live microorganism that benefits human health. They can regulate intestinal function, enhance immune system function, and inhibit the growth and spread of tumor cells. In recent years, numerous studies have shown that probiotics can synergize with immune checkpoint inhibitors (such as PD-1, PD-L1, or CTLA-4 antibodies) in various ways to modulate the immune responses of cancer patients, enhancing the effectiveness and tolerance of immunotherapy. On the one hand, probiotics can regulate cancer patients’ gut microbiota and immune microenvironment by promoting the secretion of immune factors at the tumor site, thus influencing the body’s response to immune inhibitors and alleviating the treatment side effects caused by chemotherapy. On the other hand, probiotics can also migrate to tumor sites and exert their inhibitory effects, including stimulating the secretion of immune cell factors and producing antitumor substances such as I3A. This comprehensive review offers valuable insights into the dynamic interactions between gut microbial communities, probiotics, and tumors.
Despite promising research results, the relationship between cancer immunotherapy and probiotics still requires further investigation to clarify their efficacy and safety. Research on the promoting effects of probiotics on cancer immunotherapy continued to be a hot topic in human health. Probiotics could emerge as a novel adjuvant for cancer treatment and be combined with other drugs or therapeutic approaches to improve cancer patient’s prognosis and quality of life. Furthermore, by maintaining a healthy gut microbiome, probiotics might have contributed to the prevention of tumor development or the slowing of tumor progression, providing new avenues for early tumor intervention. Additionally, different types of tumors and patients may respond differently to probiotics, emphasizing the importance of personalized treatment and optimizing the potential of immunotherapy strategies in the future.
Abberivation
| TME | = | Tumor microenvironment |
| ICIS | = | Immune checkpoint inhibitors |
| I3A | = | Indole-3-carbaldehyde |
| CRC | = | Colorectal cancer |
| BFT | = | B. Fragilis toxin |
| CNF1 | = | Cytotoxic necrotizing factor 1 |
| pks+ | = | Polyketide synthase-positive |
| DSBS | = | Double-strand DNA breaks |
| AUC | = | Receiver-operating characteristics curve |
| SNV | = | Single nucleotide variant |
| INOS | = | Inducible nitric oxide synthase expression |
| GERD | = | Gastroesophageal reflux disease |
| HCC | = | Hepatocellular carcinoma |
| LPS | = | Lipopolysaccharide |
| ICC | = | Intrahepatic cholangiocarcinoma |
| CCA | = | Cholangiocarcinoma |
| PDA | = | Pancreatic ductal adenocarcinoma |
| PDAC | = | Pancreatic adenocarcinoma |
| ICC | = | Intrahepatic cholangiocarcinoma |
| BC | = | Breast Cancer |
| EOC | = | Epithelial ovarian cancer |
| FMT | = | Fecal microbiota transplantation |
| SCC | = | Small-cell carcinoma |
| OSCC | = | Oral squamous cell carcinoma |
| FADA | = | Antigen adhesin A |
| ACI | = | Adoptive cellular immunotherapy |
| CAR-T | = | Chimeric antigen receptor T-cell |
| TIL | = | Tumor-infiltrating lymphocyte |
| NK | = | Natural killer |
| CIK | = | Cytokine-induced killer |
| DC | = | Dendritic cell |
| FDA | = | U.S. Food and Drug Administration |
| SCFAS | = | Short-chain fatty acids |
| LGG | = | Lactobacillus rhamnosus GG |
| IECS | = | Intestinal epithelial cells |
| ROS | = | Reactive oxygen species |
| NF-kb | = | Nuclear factor-kappa B |
| TLR2 | = | Toll-like receptor 2 |
| Lr | = | Lactobacillus reuteri |
Credit authorship contribution statement
Shuaiming Jiang: Conceptualization, Writing – original & editing, Visualization. Wenyao Ma: Writing – original & editing, Visualization. Chenchen Ma: Data curation, Investigation, Visualization. Zeng Zhang: Investigation, Visualization. Wanli Zhang: Conceptualization, Writing – review & editing. Jiachao Zhang: Conceptualization, Writing – review & editing, Funding acquisition.
Acknowledgments
This research was supported by the National Natural Science Foundation of China [32222066] and the Hainan Province Science and Technology Special Fund under Grant [GHYF2023001].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Finn O. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23:6–31. doi:10.1093/annonc/mds256.
- Roderburg C, Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes. 2014; 5(4):441–445. doi:10.4161/gmic.29599.
- Mima K, Ogino S, Nakagawa S, Sawayama H, Kinoshita K, Krashima R, Ishimoto T, Imai K, Iwatsuki M, Hashimoto D. The role of intestinal bacteria in the development and progression of gastrointestinal tract neoplasms. Surg Oncol. 2017;26:368–376. doi:10.1016/j.suronc.2017.07.011.
- Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020; 17(8):807–821. doi:10.1038/s41423-020-0488-6.
- Bhutiani N, Wargo JA. Gut microbes as biomarkers of ICI response—sharpening the focus. Nat Rev Clin Oncol. 2022; 19(8):495–496. doi:10.1038/s41571-022-00634-0.
- Salminen SJ, Gueimonde M, Isolauri E. Probiotics that modify disease risk. J Nutr. 2005;135:1294–1298. doi:10.1093/jn/135.5.1294.
- Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr. 2000;71:1682S–1687S. doi:10.1093/ajcn/71.6.1682S.
- Ma W, Mao Q, Xia W, Dong G, Yu C, Jiang F. Gut microbiota shapes the efficiency of cancer therapy. Front Microbiol. 2019;10:1050. doi:10.3389/fmicb.2019.01050.
- Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019; 16(10):605–616. doi:10.1038/s41575-019-0173-3.
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016; 167(4):915–932. doi:10.1016/j.cell.2016.10.027.
- Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. Gut microbiome and aging: Physiological and mechanistic insights. J Nutr Health Aging. 2018; 4(4):267–285. doi:10.3233/NHA-170030.
- Dey N, Ciorba MA. Probiotic gut bacteria enhance cancer immunotherapy in a mouse model of melanoma. Gastroenterology. 2016; 151(1):206–207. doi:10.1053/j.gastro.2016.05.015.
- Patil A. Probiotics in cancer treatment: from the laboratory to clinical practice. Preprints. 2023. doi:10.20944/preprints202307.0553.v1.
- Gao G, Shen S, Zhang T, Zhang J, Huang S, Sun Z, Zhang H. Lacticaseibacillus rhamnosus Probio-M9 enhanced the antitumor response to anti-PD-1 therapy by modulating intestinal metabolites. EBioMedicine. 2023;91:91. doi:10.1016/j.ebiom.2023.104533.
- Bender MJ, AC M, Phelps CM, Pandey SP, Laughlin CR, Shapira JH, Sanchez LM, Rana M, Richie TG, Mims TS. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023; 186(9):1846–1862. e26. doi:10.1016/j.cell.2023.03.011.
- Liu F, Li J, Guan Y, Lou Y, Chen H, Xu M, Deng D, Chen J, Ni B, Zhao L. Dysbiosis of the gut microbiome is associated with tumor biomarkers in lung cancer. Int J Biol Sci. 2019; 15(11):2381–2392. doi:10.7150/ijbs.35980.
- Olovo CV, Huang X, Zheng X, Xu M. Faecal microbial biomarkers in early diagnosis of colorectal cancer. J Cell Mol Med. 2021;25:10783–10797. doi:10.1111/jcmm.17010.
- Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Curr Oncol Rep. 2014; 16(10):1–9. doi:10.1007/S11912-014-0406-0.
- Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 2016; 164(3):337–340. doi:10.1016/j.cell.2016.01.013.
- Cani PD, Moens de Hase E, Van Hul M. Gut microbiota and host metabolism: from proof of concept to therapeutic intervention. Microorganisms. 2021; 9(6):1302. doi:10.3390/microorganisms9061302.
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021; 19(1):55–71. doi:10.1038/s41579-020-0433-9.
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014; 157(1):121–141. doi:10.1016/j.cell.2014.03.011.
- Jiang S, Chen D, Ma C, Liu H, Huang S, Zhang J. Establishing a novel inflammatory bowel disease prediction model based on gene markers identified from single nucleotide variants of the intestinal microbiota. iMeta. 2022; 1(3):e40. doi:10.1002/imt2.40.
- Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020; 17(4):223–237. doi:10.1038/s41575-019-0258-z.
- Van Hul M, Cani PD. The gut microbiota in obesity and weight management: microbes as friends or foe? Nat Rev Endocrinol. 2023; 19(5):258–271. doi:10.1038/s41574-022-00794-0.
- Das T, Jayasudha R, Chakravarthy S, Prashanthi GS, Bhargava A, Tyagi M, Rani PK, Pappuru RR, Sharma S, Shivaji S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci Rep. 2021; 11(1):2738. doi:10.1038/s41598-021-82538-0.
- Jiang S, Liu A, Ma W, Liu X, Luo P, Zhan M, Zhou X, Chen L, Zhang J. Lactobacillus gasseri CKCC1913 mediated modulation of the gut–liver axis alleviated insulin resistance and liver damage induced by type 2 diabetes. Food Funct. 2023; 14(18):8504–8520. doi:10.1039/d3fo01701j.
- Miyauchi E, Shimokawa C, Steimle A, Desai MS, Ohno H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat Rev Immunol. 2023; 23(1):9–23. doi:10.1038/s41577-022-00727-y.
- Li B, Selmi C, Tang R, Gershwin ME, Ma X. The microbiome and autoimmunity: a paradigm from the gut–liver axis. Cell Mol Immunol. 2018; 15(6):595–609. doi:10.1038/cmi.2018.7.
- Iweala OI, Nagler CR. The microbiome and food allergy. Annu Rev Immunol. 2019; 37(1):377–403. doi:10.1146/annurev-immunol-042718-041621.
- Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, Martricardi PM, Aberg N, Perkin MR, Tripodi S. et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121(1):129–134. doi:10.1016/j.jaci.2007.09.011.
- Verhulst SL, Vael C, Beunckens C, Nelen V, Goossens H, Desager K. A longitudinal analysis on the association between antibiotic use, intestinal microflora, and wheezing during the first year of life. J Asthma. 2008; 45(9):828–832. doi:10.1080/02770900802339734.
- Jia W, Rajani C, Kaddurah-Daouk R, Li H. Expert insights: The potential role of the gut microbiome-bile acid-brain axis in the development and progression of Alzheimer’s disease and hepatic encephalopathy. Medicinal Research Reviews. 2020; 40(4):1496–1507. doi:10.1002/med.21653.
- Cani PD, Jordan BF. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018; 15(11):671–682. doi:10.1038/s41575-018-0025-6.
- Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, Weber D, Hashimoto D, Slingerland AE, Slingerland JB. et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020;382(9):822–834. doi:10.1056/NEJMoa1900623.
- Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, Niehus R, Staffas A, Dai A, Fontana E. et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;588(7837):303–307. doi:10.1038/s41586-020-2971-8.
- Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. doi:10.1126/science.abc3421.
- Li Y, Tinoco R, Elmén L, Segota I, Xian Y, Fujita Y, Sahu A, Zarecki R, Marie K, Feng Y. et al. Gut microbiota dependent anti-tumor immunity restricts melanoma growth in Rnf5−/− mice. Nat Commun. 2019;10(1):1492. doi:10.1038/s41467-019-09525-y.
- Cheng Y, Liu J, Ling Z. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit Rev Food Sci Nutr. 2022;62:7929–7959. doi:10.1080/10408398.2021.1920884.
- Nagpal R, Wang S, Ahmadi S, Hayes J, Gagliano J, Subashchandrabose S, Kitzman DW, Becton T, Read R, Yadav H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep. 2018; 8(1):12649. doi:10.1038/s41598-018-30114-4.
- Seth A, Yan F, Polk DB, Rao R. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC-and MAP kinase-dependent mechanism. Am J Physiol. 2008; 294(4):1060–1069. doi:10.1152/ajpgi.00202.2007.
- Tiwari SK, Tiwari SK. Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front Cell Infect Microbiol. 2022;12:851140. doi:10.3389/fcimb.2022.851140/full.
- Li H-Y, Zhou D-D, Gan R-Y, Huang S-Y, Zhao C-N, Shang A, Xu X-Y, Li H-B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients. 2021; 13(9):3211. doi:10.3390/nu13093211.
- Madsen KL. The use of probiotics in gastrointestinal disease. Can J Gastroenterol Hepatol. 2001;15:817–822. doi:10.1155/2001/690741.
- Sullivan Å, Nord C. Probiotics and gastrointestinal diseases. J Intern Med. 2005; 257(1):78–92. doi:10.1111/j.1365-2796.2004.01410.x.
- Aggarwal J, Swami G, Kumar M. Probiotics and their effects on metabolic diseases: an update. J Clin Diagn Res. 2013; 7(1):173–177. doi:10.7860/JCDR/2012/5004.2701.
- Huo D, Cen C, Chang H, Ou Q, Jiang S, Pan Y, Chen K, Zhang J. Probiotic Bifidobacterium longum supplied with methimazole improved the thyroid function of Graves’ disease patients through the gut-thyroid axis. Commun Biol. 2021; 4(1):1046. doi:10.1038/s42003-021-02587-z.
- Zhang Z, Li J, Jiang S, Xu M, Ma T, Sun Z, Zhang J. Lactobacillus fermentum HNU312 alleviated oxidative damage and behavioural abnormalities during brain development in early life induced by chronic lead exposure. Ecotox Environ Safe. 2023;251:114543. doi:10.1016/j.ecoenv.2023.114543.
- Ebel B, Lemetais G, Beney L, Cachon R, Sokol H, Langella P, Gervais P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit Rev Food Sci Nutr. 2014; 54(2):175–189. doi:10.1080/10408398.2011.579361.
- Ascher S, Reinhardt C. The gut microbiota: an emerging risk factor for cardiovascular and cerebrovascular disease. Eur J Immunol. 2018; 48(4):564–575. doi:10.1002/eji.201646879.
- Queen J, Shaikh F, Sears CL. Understanding the mechanisms and translational implications of the microbiome for cancer therapy innovation. Nat Cancer. 2023;1–12. doi:10.1038/s43018-023-00602-2.
- Tlaskalová-Hogenová H, Štěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011; 8(2):110–120. doi:10.1038/cmi.2010.67.
- Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017; 67(4):326–344. doi:10.3322/caac.21398.
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016; 8(1):1–12. doi:10.1186/S13073-016-0303-2.
- Zhang J, Lacroix C, Wortmann E, Ruscheweyh H-J, Sunagawa S, Sturla SJ, Schwab C. Gut microbial beta-glucuronidase and glycerol/diol dehydratase activity contribute to dietary heterocyclic amine biotransformation. BMC Microbiol. 2019; 19(1):1–14. doi:10.1186/s12866-019-1483-x.
- Lefort ÉC, Blay J. Apigenin and its impact on gastrointestinal cancers. Mol Nutr Food Res. 2013; 57(1):126–144. doi:10.1002/mnfr.201200424.
- Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010; 10(6):403–414. doi:10.1038/nrc2857.
- Talarico S, Leverich CK, Wei B, Ma J, Cao X, Guo Y, Han G, Yao L, Self S, Zhao Y. et al. Increased H. pylori stool shedding and EPIYA-D cagA alleles are associated with gastric cancer in an East Asian hospital. PloS One. 2018;13(9):e0202925. doi:10.1371/journal.pone.0202925.
- Liang W, Yang Y, Wang H, Wang H, Yu X, Lu Y, Shen S, Teng L. Gut microbiota shifts in patients with gastric cancer in perioperative period. Medicine (Baltimore). 2019; 98(35):e16626. doi:10.1097/MD.0000000000016626.
- Y-F Q, J-N S, L-F R, X-L C, J-H D, Tao K, X-M G, Y-N C, Su W. Intestinal microbiota is altered in patients with gastric cancer from Shanxi Province, China. Dig Dis Sci. 2019; 64(5):1193–1203. doi:10.1007/s10620-018-5411-y.
- Wu J, Zhang C, Xu S, Xiang C, Wang R, Yang D, Lu B, Shi L, Tong R, Teng Y. et al. Fecal microbiome alteration may be a potential marker for gastric cancer. Dis Markers. 2020;2020:3461315. doi:10.1155/2020/3461315.
- Zheng C, Chen T, Wang Y, Gao Y, Kong Y, Liu Z, Deng X. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J Cancer. 2019; 10(3):568. doi:10.7150/jca.29072.
- Sarhadi V, Mathew B, Kokkola A, Karla T, Tikkanen M, Rautelin H, Lahti L, Puolakkainen P, Knuutila S. Gut microbiota of patients with different subtypes of gastric cancer and gastrointestinal stromal tumors. Gut Pathog. 2021; 13(1):11. doi:10.1186/s13099-021-00403-x.
- Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, Zhang D, Xia H, Xu X, Jie Z. et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat Commun. 2015;6(1):6528. doi:10.1038/ncomms7528.
- Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y. et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66(1):70–78. doi:10.1136/gutjnl-2015-309800.
- Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015; 60(2):208–215. doi:10.1093/cid/ciu787.
- Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. 2002; 23(3):529–536. doi:10.1093/carcin/23.3.529.
- Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012; 338(6103):120–123. doi:10.1126/science.1224820.
- Boleij A, Tjalsma H. The itinerary of Streptococcus gallolyticus infection in patients with colonic malignant disease. Lancet Infect Dis. 2013; 13(8):719–724. doi:10.1016/S1473-3099(13)70107-5.
- Keenan JI, Aitchison A, Purcell RV, Greenlees R, Pearson JF, Frizelle FA. Screening for enterotoxigenic Bacteroides fragilis in stool samples. Anaerobe. 2016;40:50–53. doi:10.1016/j.anaerobe.2016.05.004.
- Wirbel J, Pyl PT, Kartal E, Zych K, Kashani A, Milanese A, Fleck JS, Voigt AY, Palleja A, Ponnudurai R. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25(4):679–689. doi:10.1038/s41591-019-0406-6.
- Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Watanabe H, Masuda K, Nishimoto Y, Kubo M. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. 2019;25(6):968–976. doi:10.1038/s41591-019-0458-7.
- Thomas AM, Manghi P, Asnicar F, Pasolli E, Armanini F, Zolfo M, Beghini F, Manara S, Karcher N, Pozzi C. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. 2019;25(4):667–678. doi:10.1038/s41591-019-0405-7.
- Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019; 16(11):690–704. doi:10.1038/s41575-019-0209-8.
- Fang W-J, Jing D-Z, Luo Y, Fu C-Y, Zhao P, Qian J, Tian B-R, Chen X-G, Zheng Y-L, Zheng Y. et al. Clostridium difficile carriage in hospitalized cancer patients: a prospective investigation in eastern China. BMC Infect Dis. 2014;14(1):523. doi:10.1186/1471-2334-14-523.
- Cheng Y, Ling Z, Li L. The intestinal microbiota and colorectal cancer. Front Immunol. 2020;11:615056. doi:10.3389/fimmu.2020.615056.
- Deng Y, Tang D, Hou P, Shen W, Li H, Wang T, Liu R. Dysbiosis of gut microbiota in patients with esophageal cancer. Microb Pathog. 2021;150:104709. doi:10.1016/j.micpath.2020.104709.
- Yang W, Chen C-H, Jia M, Xing X, Gao L, Tsai H-T, Zhang Z, Liu Z, Zeng B, Yeung S-C. Tumor-associated microbiota in esophageal squamous cell carcinoma. Front Cell Dev Biol. 2021;9:641270. doi:10.3389/fcell.2021.641270.
- Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M. Gut dysbiosis associated with hepatitis C virus infection. Clin Infect Dis. 2018; 67(6):869–877. doi:10.1093/cid/ciy205.
- Zhang X, Coker OO, Chu ES, Fu K, Lau HC, Wang Y-X, Chan AW, Wei H, Yang X, Sung JJ. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021; 70(4):761–774. doi:10.1136/gutjnl-2019-319664.
- Zhang Z, Wang D, Qiao S, Wu X, Cao S, Wang L, Su X, Li L. Metabolic and microbial signatures in rat hepatocellular carcinoma treated with caffeic acid and chlorogenic acid. Sci Rep. 2017; 7(1):4508. doi:10.1038/s41598-017-04888-y.
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021; 7(1):6. doi:10.1038/s41572-020-00240-3.
- Ma J, Li J, Jin C, Yang J, Zheng C, Chen K, Xie Y, Yang Y, Bo Z, Wang J. et al. Association of gut microbiome and primary liver cancer: A two‐sample Mendelian randomization and case–control study. Liver Int. 2023; 43(1):221–233. doi:10.1111/liv.15466.
- Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019; 68(6):1014–1023. doi:10.1136/gutjnl-2017-315084.
- Iida N, Mizukoshi E, Yamashita T, Yutani M, Seishima J, Wang Z, Arai K, Okada H, Yamashita T, Sakai Y. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat Cancer. 2021; 2(10):1039–1054. doi:10.1038/s43018-021-00251-3.
- Jia X, Lu S, Zeng Z, Liu Q, Dong Z, Chen Y, Zhu Z, Hong Z, Zhang T, Du G. et al. Characterization of gut microbiota, bile acid metabolism, and cytokines in intrahepatic cholangiocarcinoma. Hepatology. 2020;71(3):893–906. doi:10.1002/hep.30852.
- Ma J, Li J, Jin C, Yang J, Zheng C, Chen K, Xie Y, Yang Y, Bo Z, Wang J. et al. Association of gut microbiome and primary liver cancer: A two-sample Mendelian randomization and case–control study. Liver Int. 2023;43(1):221–233. doi:10.1111/liv.15466.
- Zhang T, Zhang S, Jin C, Lin Z, Deng T, Xie X, Deng L, Li X, Ma J, Ding X. A predictive model based on the gut microbiota improves the diagnostic effect in patients with cholangiocarcinoma. Front Cell Infect Microbiol. 2021;2021:1157. doi:10.3389/fcimb.2021.751795/full.
- Saab M, Mestivier D, Sohrabi M, Rodriguez C, Khonsari MR, Faraji A, Sobhani I, Dudeja P. Characterization of biliary microbiota dysbiosis in extrahepatic cholangiocarcinoma. PloS One. 2021; 16(3):e0247798. doi:10.1371/journal.pone.0247798.
- Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018; 8(4):403–416. doi:10.1158/2159-8290.CD-17-1134.
- Kartal E, Schmidt TS, Molina-Montes E, Rodríguez-Perales S, Wirbel J, Maistrenko OM, Akanni WA, Alhamwe BA, Alves RJ, Carrato A. A faecal microbiota signature with high specificity for pancreatic cancer. Gut. 2022; 71(7):1359–1372. doi:10.1136/gutjnl-2021-324755.
- Nagata N, Nishijima S, Kojima Y, Hisada Y, Imbe K, Miyoshi-Akiyama T, Suda W, Kimura M, Aoki R, Sekine K. Metagenomic identification of microbial signatures predicting pancreatic cancer from a multinational study. Gastroenterology. 2022; 163(1):222–238. doi:10.1053/j.gastro.2022.03.054.
- Bard JM, Luu HT, Dravet F, Michel C, Moyon T, Pagniez A, Nazih H, Bobin-Dubigeon C. Relationship between intestinal microbiota and clinical characteristics of patients with early stage breast cancer. Faseb J. 2015;29:914. doi:10.1096/fasebj.29.1_supplement.914.2.
- Thu MS, Chotirosniramit K, Nopsopon T, Hirankarn N, Pongpirul K. Human gut, breast, and oral microbiome in breast cancer: A systematic review and meta-analysis. Front Oncol. 2023;13:1144021. doi:10.3389/fonc.2023.1144021.
- Minelli EB, Beghini A, Vesentini S, Marchiori L, Nardo G, Cerutti R, Mortani E. Intestinal microflora as an alternative metabolic source of estrogens in women with uterine leiomyoma and breast cancer. Ann N Y Acad Sci. 1990; 595(1):473–479. doi:10.1111/j.1749-6632.1990.tb34337.x.
- Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, Yang H, Ji Y, Wei W, Tan A. et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6(1):136. doi:10.1186/s40168-018-0515-3.
- Goedert JJ, Jones G, Hua X, Xu X, Yu G, Flores R, Falk RT, Gail MH, Shi J, Ravel J. et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: a population-based case-control pilot study. J Natl Cancer Inst. 2015;107:147. doi:10.1093/jnci/djv147.
- Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, Goedert JJ. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99:4632–4640. doi:10.1210/jc.2014-2222.
- Yamamoto ML, Maier I, Dang AT, Berry D, Liu J, Ruegger PM, Yang JI, Soto PA, Presley LL, Reliene R. et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res. 2013;73:4222–4232. doi:10.1158/0008-5472.CAN-13-0022.
- Zhuang H, Cheng L, Wang Y, Zhang Y, Zhao M, Liang G, Zhang M, Li Y, Zhao J, Gao Y. Dysbiosis of the gut microbiome in lung cancer. Front Cell Infect Microbiol. 2019;9:112. doi:10.3389/fcimb.2019.00112.
- Zhang W-Q, Zhao S-K, Luo J-W, Dong X-P, Hao Y-T, Li H, Shan L, Zhou Y, Shi H-B, Zhang Z-Y. Alterations of fecal bacterial communities in patients with lung cancer. Am J Transl Res. 2018; 10(10):3171. doi: AJTR0077798.
- Zheng Y, Fang Z, Xue Y, Zhang J, Zhu J, Gao R, Yao S, Ye Y, Wang S, Lin C. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes. 2020; 11(4):1030–1042. doi:10.1080/19490976.2020.1737487.
- Han W, Wang N, Han M, Liu X, Sun T, Xu J. Identification of microbial markers associated with lung cancer based on multi‐cohort 16 s rRNA analyses: A systematic review and meta‐analysis. Cancer Med. 2023; 12(18):19301–19319. doi:10.1002/cam4.6503.
- Zhang WQ, Zhao SK, Luo JW, Dong XP, Hao YT, Li H, Shan L, Zhou Y, Shi HB, Zhang ZY. et al. Alterations of fecal bacterial communities in patients with lung cancer. Am J Transl Res. 2018;10(10):3171–3185.
- Botticelli A, Putignani L, Zizzari I, Del Chierico F, Reddel S, Di Pietro F, Quagliarello A, Onesti CE, Raffaele G, Mazzuca F. Changes of microbiome profile during nivolumab treatment in NSCLC patients. J Clin Oncol. 2018;36:e15020. doi:10.1200/JCO.2018.36.15_suppl.e15020.
- Gui Q, Li H, Wang A, Zhao X, Tan Z, Chen L, Xu K, Xiao C. The association between gut butyrate‐producing bacteria and non‐small‐cell lung cancer. J Clin Lab Anal. 2020;34:e23318. doi:10.1002/jcla.23318.
- Golombos DM, Ayangbesan A, O’Malley P, Lewicki P, Barlow L, Barbieri CE, Chan C, DuLong C, Abu-Ali G, Huttenhower C. et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Urology. 2018;111:122–128. doi:10.1016/j.urology.2017.08.039.
- Popović V B, M Š, Chow C-E, Chan LS, Roje B, Terzić J. The urinary microbiome associated with bladder cancer. Sci Rep. 2018; 8(1):12157. doi:10.1038/s41598-018-29054-w.
- Wu P, Zhang G, Zhao J, Chen J, Chen Y, Huang W, Zhong J, Zeng J. Profiling the urinary microbiota in male patients with bladder cancer in China. Front Cell Infect Microbiol. 2018;8:167. doi:10.3389/fcimb.2018.00167.
- He C, Li B, Huang L, Teng C, Bao Y, Ren M, Shan Y. Gut microbial composition changes in bladder cancer patients: A case-control study in Harbin, China. Asia Pac J Clin Nutr. 2020; 29(2):395–403. doi:10.6133/apjcn.202007_29(2).0022.
- Hu X, Xu X, Zeng X, Jin R, Wang S, Jiang H, Tang Y, Chen G, Wei J, Chen T. Gut microbiota dysbiosis promotes the development of epithelial ovarian cancer via regulating Hedgehog signaling pathway. Gut Microbes. 2023; 15(1):2221093. doi:10.1080/19490976.2023.2221093.
- Wang Z, Qin X, Hu D, Huang J, Guo E, Xiao R, Li W, Sun C, Chen G. Akkermansia supplementation reverses the tumor-promoting effect of the fecal microbiota transplantation in ovarian cancer. Cell Rep. 2022; 41(13):41. doi:10.1016/j.celrep.2022.111890.
- Kang G-U, Jung D-R, Lee YH, Jeon SY, Han HS, Chong GO, Shin J-H. Dynamics of Fecal microbiota with and without invasive cervical cancer and its application in early diagnosis. Cancers. 2020; 12(12):3800. doi:10.3390/cancers12123800.
- Wang Z, Wang Q, Zhao J, Gong L, Zhang Y, Wang X, Yuan Z. Altered diversity and composition of the gut microbiome in patients with cervical cancer. AMB Express. 2019; 9(1):1–9. doi:10.1186/s13568-019-0763-z.
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023; 73(3):233–254. doi:10.3322/caac.21772.
- Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022; 28(30):4053–4060. doi:10.3748/wjg.v28.i30.4053.
- Wu S, Rhee K-J, Albesiano E, Rabizadeh S, Wu X, Yen H-R, Huso DL, Brancati FL, Wick E, McAllister F. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–1022. doi:10.1038/nm.2015.
- Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016; 70(1):395–411. doi:10.1146/annurev-micro-102215-095513.
- Li S, Konstantinov SR, Smits R, Peppelenbosch MP. Bacterial biofilms in colorectal cancer initiation and progression. Trends Mol Med. 2017; 23(1):18–30. doi:10.1016/j.molmed.2016.11.004.
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015; 13(5):269–284. doi:10.1038/nrmicro3432.
- Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, Brennan CA, Chun E, Ngo L, Samson LD. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019; 363(6428):eaar7785. doi:10.1126/science.aar7785.
- Foegeding NJ, Jones ZS, Byndloss MX. Western lifestyle as a driver of dysbiosis in colorectal cancer. Dis Model Mech. 2021; 14(5):14. doi:10.1242/dmm.049051.
- Tsoi H, Chu ES, Zhang X, Sheng J, Nakatsu G, Ng SC, Chan AW, Chan FK, Sung JJ, Yu J. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology. 2017; 152(6):1419–1433.e5. doi:10.1053/j.gastro.2017.01.009.
- Dai Z, Coker OO, Nakatsu G, WKK W, Zhao L, Chen Z, Chan FKL, Kristiansen K, Sung JJY, Wong SH. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018; 6(1):70. doi:10.1186/s40168-018-0451-2.
- Ma C, Chen K, Wang Y, Cen C, Zhai Q, Zhang J. Establishing a novel colorectal cancer predictive model based on unique gut microbial single nucleotide variant markers. Gut Microbes. 2020; 13(1):1–6. doi:10.1080/19490976.2020.1869505.
- Dixon WG, Solomon DH. Bisphosphonates and esophageal cancer—a pathway through the confusion. Nat Rev Rheumatol. 2011; 7(6):369–372. doi:10.1038/nrrheum.2011.60.
- Park H, Clark E, Cullen JJ, Koland JG, Kim MS, Conklin JL. Expression of inducible nitric oxide synthase in the lower esophageal sphincter of the endotoxemic opossum. J Gastroenterolo. 2002; 37(12):1000–1004. doi:10.1007/s005350200169.
- Petrick JL, Florio AA, Koshiol J, Pfeiffer RM, Yang B, Yu K, Chen CJ, Yang HI, Lee MH, McGlynn KA. Prediagnostic concentrations of circulating bile acids and hepatocellular carcinoma risk: REVEAL-HBV and HCV studies. Int J Cancer. 2020; 147(10):2743–2753. doi:10.1002/ijc.33051.
- McBrearty N, Arzumanyan A, Bichenkov E, Merali S, Merali C, Feitelson M. Short chain fatty acids delay the development of hepatocellular carcinoma in HBx transgenic mice. Neoplasia. 2021; 23(5):529–538. doi:10.1016/j.neo.2021.04.004.
- Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Ma L, Roy S, Fu Q, Brown ZJ. et al. Gut microbiome directs hepatocytes to recruit MDSC and promote cholangiocarcinoma. Cancer Discov. 2020;11(5):1248–1267. doi:10.1158/2159-8290.CD-20-0304.
- Ponziani FR, De Luca A, Picca A, Marzetti E, Petito V, Del Chierico F, Reddel S, Paroni Sterbini F, Sanguinetti M, Putignani L. Gut dysbiosis and fecal calprotectin predict response to immune checkpoint inhibitors in patients with hepatocellular carcinoma. Hepatol Commun. 2022; 6(6):1492–1501. doi:10.1002/hep4.1905.
- Deng T, Li J, He B, Chen B, Liu F, Chen Z, Zheng J, Shi Z, Zhang T, Deng L. Gut microbiome alteration as a diagnostic tool and associated with inflammatory response marker in primary liver cancer. Hepatol Int. 2022; 16(1):99–111. doi:10.1007/s12072-021-10279-3.
- Sethi V, Vitiello GA, Saxena D, Miller G, Dudeja V. The role of the microbiome in immunologic development and its implication for pancreatic cancer immunotherapy. Gastroenterology. 2019; 156(7):2097–2115. doi:10.1053/j.gastro.2018.12.045.
- Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi:10.1093/jnci/djh336.
- Muti P, Bradlow HL, Micheli A, Krogh V, Freudenheim JL, Schunemann HJ, Stanulla M, Yang J, Sepkovic DW, Trevisan M. et al. Estrogen Metabolism and Risk of Breast Cancer: A Prospective Study of the 2: 16α-Hydroxyestrone Ratio in Premenopausal and Postmenopausal Women. Epidemiology. 2000;11(6):635–640. doi:10.1097/00001648-200011000-00004.
- Hill MJ, Goddard P, Williams REO. GUT BACTERIA and ÆTIOLOGY of CANCER of the BREAST. Lancet. 1971; 298(7722):472–473. doi:10.1016/S0140-6736(71)92634-1.
- Laborda-Illanes A, Sanchez-Alcoholado L, Dominguez-Recio ME, Jimenez-Rodriguez B, Lavado R, Comino-Mendez I, Alba E, Queipo-Ortuno MI. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers. 2020; 12(9):2465. doi:10.3390/cancers12092465.
- Wu AH, Tseng C, Vigen C, Yu Y, Cozen W, Garcia AA, Spicer D. Gut microbiome associations with breast cancer risk factors and tumor characteristics: a pilot study. Breast Cancer Res Treat. 2020; 182(2):451–463. doi:10.1007/s10549-020-05702-6.
- Halbrook CJ, Lyssiotis CA, Pasca di Magliano, di Magliano MP A, Pasca di Magliano M. Pancreatic cancer: Advances and challenges. Cell. 2023; 186(8):1729–1754. doi:10.1016/j.cell.2023.02.014.
- Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019; 178(4):795–806. e12. doi:10.1016/j.cell.2019.07.008.
- Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384:691–702. doi:10.1016/S0140-6736(14)61136-3.
- Sommariva M, Le Noci V, Bianchi F, Camelliti S, Balsari A, Tagliabue E, Sfondrini L. The lung microbiota: role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cell Mol Life Sci. 2020; 77(14):2739–2749. doi:10.1007/s00018-020-03452-8.
- Yu G, Gail MH, Consonni D, Carugno M, Humphrys M, Pesatori AC, Caporaso NE, Goedert JJ, Ravel J, Landi MT. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016; 17(1):1–12. doi:10.1186/s13059-016-1021-1.
- Hoste E, Arwert EN, Lal R, South AP, Salas-Alanis JC, Murrell DF, Donati G, Watt FM. Innate sensing of microbial products promotes wound-induced skin cancer. Nat Commun. 2015; 6(1):5932. doi:10.1038/ncomms6932.
- Wong-Rolle A, Wei HK, Zhao C, Jin C. Unexpected guests in the tumor microenvironment: microbiome in cancer. Protein Cell. 2021; 12(5):426–435. doi:10.1007/s13238-020-00813-8.
- Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S. Commensal microbiota promote lung cancer development via γδ T cells. Cell. 2019; 176(5):998–1013. doi:10.1016/j.cell.2018.12.040.
- Yang L, Li A, Wang Y, Zhang Y. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther. 2023; 8(1):35. doi:10.1038/s41392-022-01304-4.
- Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science. 2020; 368(6494):973–980. doi:10.1126/science.aay9189.
- Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020; 579(7800):567–574. doi:10.1038/s41586-020-2095-1.
- Greathouse KL, Stone JK, Harris CC. Cancer-type-specific bacteria: freeloaders or partners? Cancer Cell. 2020; 38(2):158–160. doi:10.1016/j.ccell.2020.06.017.
- Luan C, Xie L, Yang X, Miao H, Lv N, Zhang R, Xiao X, Hu Y, Liu Y, Wu N. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci Rep. 2015; 5(1):7980. doi:10.1038/srep07980.
- Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, Shields CE D, Hechenbleikner EM, Huso DL. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018; 359(6375):592–597. doi:10.1126/science.aah3648.
- Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, Bonnet R, Battista JR. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PloS One. 2013; 8(2):e56964. doi:10.1371/journal.pone.0056964.
- Arthur JC. Microbiota and colorectal cancer: colibactin makes its mark. Nat Rev Gastroenterol Hepatol. 2020; 17(6):317–318. doi:10.1038/s41575-020-0303-y.
- Laroumagne S, Lepage B, Hermant C, Plat G, Phelippeau M, Bigay-Game L, Lozano S, Guibert N, Segonds C, Mallard V. Bronchial colonisation in patients with lung cancer: a prospective study. Eur Respir J. 2013; 42(1):220–229. doi:10.1183/09031936.00062212.
- Lee SH, Sung JY, Yong D, Chun J, Kim SY, Song JH, Chung KS, Kim EY, Jung JY, Kang YA. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer. 2016;102:89–95. doi:10.1016/j.lungcan.2016.10.016.
- Cameron SJ, Lewis KE, Huws SA, Hegarty MJ, Lewis PD, Pachebat JA, Mur LA, Guo NL. A pilot study using metagenomic sequencing of the sputum microbiome suggests potential bacterial biomarkers for lung cancer. PloS One. 2017; 12(5):e0177062. doi:10.1371/journal.pone.0177062.
- Tsay J-C, Wu BG, Badri MH, Clemente JC, Shen N, Meyn P, Li Y, Yie T-A, Lhakhang T, Olsen E. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am J Respir Crit Care Med. 2018; 198(9):1188–1198. doi:10.1164/rccm.201710-2118OC.
- Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019; 37(18):1558. doi:10.1200/JCO.19.00201.
- Zheng X, Sun X, Liu Q, Huang Y, Yuan Y. The composition alteration of respiratory microbiota in lung cancer. Cancer Invest. 2020;38:158–168. doi:10.1080/07357907.2020.1732405.
- Han W, Wang N, Han M, Liu X, Sun T, Xu J. Identification of microbial markers associated with lung cancer based on multi-cohort 16 s rRNA analyses: A systematic review and meta-analysis. Cancer Med. 2023; 12(18):19301–19319. doi:10.1002/cam4.6503.
- Yan X, Yang M, Liu J, Gao R, Hu J, Li J, Zhang L, Shi Y, Guo H, Cheng J. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5:3111. doi: ajcr0009363.
- Cheng C, Wang Z, Wang J, Ding C, Sun C, Liu P, Xu X, Liu Y, Chen B, Gu B. Characterization of the lung microbiome and exploration of potential bacterial biomarkers for lung cancer. Transl Lung Cancer Res. 2020; 9(3):693. doi:10.21037/tlcr-19-590.
- Greathouse KL, White JR, Vargas AJ, Bliskovsky BJ VV, von Muhlinen N, Polley EC, Bowman ED, Khan MA, Robles AI, Robles AI. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018; 19(1):1–16. doi:10.1186/s13059-018-1501-6.
- Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017; 357(6356):1156–1160. doi:10.1126/science.aah5043.
- Chai X, Wang J, Li H, Gao C, Li S, Wei C, Huang J, Tian Y, Yuan J, Lu J. Intratumor microbiome features reveal antitumor potentials of intrahepatic cholangiocarcinoma. Gut Microbes. 2023; 15(1):2156255. doi:10.1080/19490976.2022.2156255.
- Segura-López FK, Güitrón-Cantú A, Torres J. Association between Helicobacter spp. infections and hepatobiliary malignancies: a review. World J Gastroenterol. 2015; 21(5):1414–1423. doi:10.3748/wjg.v21.i5.1414.
- Boonyanugomol W, Chomvarin C, Sripa B, Bhudhisawasdi V, Khuntikeo N, Hahnvajanawong C, Chamsuwan A. Helicobacter pylori in Thai patients with cholangiocarcinoma and its association with biliary inflammation and proliferation. HPB. 2012; 14(3):177–184. doi:10.1111/j.1477-2574.2011.00423.x.
- Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Ma L, Roy S, Fu Q, Brown ZJ. et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021; 11(5):1248–1267. doi:10.1158/2159-8290.CD-17-1134.
- Xuan C, Shamonki JM, Chung A, ML D, Chung M, Sieling PA, Lee DJ, Takabe K. Microbial dysbiosis is associated with human breast cancer. PloS One. 2014; 9(1):e83744. doi:10.1371/journal.pone.0083744.
- Thompson KJ, Ingle JN, Tang X, Chia N, Jeraldo PR, Walther-Antonio MR, Kandimalla KK, Johnson S, Yao JZ, Harrington SC. et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PloS One. 2017; 12(11):e0188873. doi:10.1371/journal.pone.0188873.
- Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Peck KN, AM D, Alwine JC, Robertson ES. Distinct microbial signatures associated with different breast cancer types. Front Microbiol. 2018;9:951. doi:10.3389/fmicb.2018.00951.
- Parhi L, Alon-Maimon T, Sol A, Nejman D, Shhadeh A, Fainsod-Levi T, Yajuk O, Isaacson B, Abed J, Maalouf N. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020; 11(1):3259. doi:10.1038/s41467-020-16967-2.
- Hieken TJ, Chen J, Hoskin TL, Walther-Antonio M, Johnson S, Ramaker S, Xiao J, Radisky DC, Knutson KL, Kalari KR. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. 2016; 6(1):30751. doi:10.1038/srep30751.
- Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, Sawayama H, Kinoshita K, Ishimoto T, Iwatsuki M. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res. 2016;22:5574–5581. doi:10.1158/1078-0432.CCR-16-1786.
- Parida S, Wu S, Siddharth S, Wang G, Muniraj N, Nagalingam A, Hum C, Mistriotis P, Hao H, Talbot CC Jr. A procarcinogenic colon microbe promotes breast tumorigenesis and metastatic progression and concomitantly activates notch and β-catenin axes. Cancer Discov. 2021;11:1138–1157. doi:10.1158/2159-8290.CD-20-0537.
- Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G, Goodrich-Blair H. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016; 82(16):5039–5048. doi:10.1128/aem.01235-16.
- Meng S, Chen B, Yang J, Wang J, Zhu D, Meng Q, Zhang L. Study of microbiomes in aseptically collected samples of human breast tissue using needle biopsy and the potential role of in situ tissue microbiomes for promoting malignancy. Front Oncol. 2018;8:318. doi:10.3389/fonc.2018.00318.
- Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P, Allen-Vercoe E, Moore RA. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012; 22(2):299–306. doi:10.1101/gr.126516.111.
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J. et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012; 22(2):292–298. doi:10.1101/gr.126573.111.
- Replication Study RJ, Iorns E, Denis A, Williams SR, Perfito N, Errington TM. Replication Study: Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Elife. 2018;7:e25801. doi:10.7554/eLife.25801.
- Mrázek J, Mekadim C, Kučerová P, Švejstil R, Salmonová H, Vlasáková J, Tarasová R, Čížková J, Červinková M. Melanoma-related changes in skin microbiome. Folia Microbiol. 2019; 64(3):435–442. doi:10.1007/s12223-018-00670-3.
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews M, Karpinets T, Prieto P, Vicente D, Hoffman K, Wei SC. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018; 359(6371):97–103. doi:10.1126/science.aan4236.
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018; 359(6371):104–108. doi:10.1126/science.aao3290.
- Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018; 359(6371):91–97. doi:10.1126/science.aan3706.
- Yang J, He Q, Lu F, Chen K, Ni Z, Wang H, Zhou C, Zhang Y, Chen B, Bo Z. A distinct microbiota signature precedes the clinical diagnosis of hepatocellular carcinoma. Gut Microbes. 2023; 15(1):2201159. doi:10.1080/19490976.2023.2201159.
- Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019; 11(1):1–13. doi:10.1186/s13099-018-0281-6.
- Sun L, Ke X, Guan A, Jin B, Qu J, Wang Y, Xu X, Li C, Sun H, Xu H. Intratumoural microbiome can predict the prognosis of hepatocellular carcinoma after surgery. Clin Transl Med. 2023; 13(7):e1331. doi:10.1002/ctm2.1331.
- Qu D, Wang Y, Xia Q, Chang J, Jiang X, Zhang H. Intratumoral microbiome of human primary liver cancer. Hepatol Commun. 2022; 6(7):1741–1752. doi:10.1002/hep4.1908.
- Liou J-M, Malfertheiner P, Lee Y-C, Sheu B-S, Sugano K, Cheng H-C, Yeoh K-G, Hsu P-I, Goh K-L, Mahachai V. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020; 69(12):2093–2112. doi:10.1136/gutjnl-2020-322368.
- Baba Y, Hara Y, Toihata T, Kosumi K, Iwatsuki M, Iwagami S, Miyamoto Y, Yoshida N, Komohara Y, Baba H. Relationship between gut microbiome Fusobacterium nucleatum and LINE-1 methylation level in esophageal cancer. Esophagus. 2023; 20(4):704–712. doi:10.1007/s10388-023-01009-9.
- Salachan PV, Rasmussen M, Fredsøe J, Ulhøi B, Borre M, Sørensen KD. Microbiota of the prostate tumor environment investigated by whole-transcriptome profiling. Genome Med. 2022; 14(1):1–18. doi:10.1186/s13073-022-01011-3.
- Cohen RJ, Shannon BA, JE M, Shannon T, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol. 2005; 173(6):1969–1974. doi:10.1097/01.ju.0000158161.15277.78.
- Yow MA, Tabrizi SN, Severi G, Bolton DM, Pedersen J, Giles GG, Southey MC. Characterisation of microbial communities within aggressive prostate cancer tissues. Infect Agent Cancer. 2017; 12(1):1–10. doi:10.1186/s13027-016-0112-7.
- Cavarretta I, Ferrarese R, Cazzaniga W, Saita D, Lucianò R, Ceresola ER, Locatelli I, Visconti L, Lavorgna G, Briganti A. The microbiome of the prostate tumor microenvironment. Eur Urol. 2017;72:625–631. doi:10.1016/j.eururo.2017.03.029.
- Feng Y, Ramnarine VR, Bell R, Volik S, Davicioni E, Hayes VM, Ren S, Collins CC. Metagenomic and metatranscriptomic analysis of human prostate microbiota from patients with prostate cancer. Bmc Genom. 2019; 20(1):1–8. doi:10.1186/s12864-019-5457-z.
- Banerjee S, Alwine JC, Wei Z, Tian T, Shih N, Sperling C, Guzzo T, Feldman MD, Robertson ES. Microbiome signatures in prostate cancer. Carcinogenesis. 2019; 40(6):749–764. doi:10.1093/carcin/bgz008.
- Zheng D-W, Deng W-W, Song W-F, Wu C-C, Liu J, Hong S, Zhuang Z-N, Cheng H, Sun Z-J, Zhang X-Z. Biomaterial-mediated modulation of oral microbiota synergizes with PD-1 blockade in mice with oral squamous cell carcinoma. Nat Biomed Eng. 2022; 6(1):32–43. doi:10.1038/s41551-021-00807-9.
- Galeano Niño JL, Wu H, KD L, Kempchinsky AG, Baryiames A, Barber B, Futran N, Houlton J, Sather C, Sicinska E. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature. 2022; 611(7937):810–817. doi:10.1038/s41586-022-05435-0.
- Erdmann J. How gut bacteria could boost cancer treatments. Nature. 2022; 607(7919):436–439. doi:10.1038/d41586-022-01959-7.
- Clay SL, Fonseca-Pereira D, Garrett WS. Colorectal cancer: the facts in the case of the microbiota. J Clin Investig. 2022; 132(4):132. doi:10.1172/JCI155101.
- Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015; 148(6):1244–1260. doi:10.1053/j.gastro.2014.12.035.
- Vilenchik M, Solit D, Basso A, Huezo H, Lucas B, He H, Rosen N, Spampinato C, Modrich P, Chiosis G. Targeting wide-range oncogenic transformation via PU24FCl, a specific inhibitor of tumor Hsp90. Chem Biol. 2004;11:787–797. doi:10.1016/j.chembiol.2004.04.008.
- Sell S. Alpha-fetoprotein, stem cells and cancer: how study of the production of alpha-fetoprotein during chemical hepatocarcinogenesis led to reaffirmation of the stem cell theory of cancer. Tumor Biol. 2008;29:161–180. doi:10.1159/000143402.
- Secher T, Samba-Louaka A, Oswald E, Nougayrède J-P, Sherman M. Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PloS One. 2013; 8(10):e77157. doi:10.1371/journal.pone.0077157.
- Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, Gurjao C, Manders F, Dalmasso G, Stege PB. Mutational signature in colorectal cancer caused by genotoxic pks+. E coli Nat. 2020;580:269–273. doi:10.1038/s41586-020-2080-8.
- Farge E. Mechanotransduction in development. Curr Top Dev Biol. 2011;95:243–265. doi:10.1016/B978-0-12-385065-2.00008-6.
- Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host & Microbe. 2013; 14(2):195–206. doi:10.1016/j.chom.2013.07.012.
- Peng C, Ouyang Y, Lu N, Li N. The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Front Immunol. 2020;11:1387. doi:10.3389/fimmu.2020.01387.
- Garajová I, Balsano R, Wang H, Leonardi F, Giovannetti E, Deng D, Peters GJ. The role of the microbiome in drug resistance in gastrointestinal cancers. Expert Rev Anticancer Ther. 2021;21:165–176. doi:10.1080/14737140.2021.1844007.
- Wei M-Y, Shi S, Liang C, Meng Q-C, Hua J, Zhang Y-Y, Liu J, Zhang B, Xu J, Yu X-J. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol Cancer. 2019; 18(1):1–15. doi:10.1186/s12943-019-1008-0.
- Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host & Microbe. 2014; 15(3):295–305. doi:10.1016/j.chom.2014.02.003.
- Dai E, Zhu Z, Wahed S, Qu Z, Storkus WJ, Guo ZS. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer. 2021; 20(1):1–27. doi:10.1186/s12943-021-01464-x.
- Ray AL, Berggren KL, Restrepo Cruz S, Gan GN, Beswick EJ. Inhibition of MK2 suppresses IL‐1β, IL‐6, and TNF‐α‐dependent colorectal cancer growth. Int J Cancer. 2018; 142(8):1702–1711. doi:10.1002/ijc.31191.
- Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010; 138(6):2101–2114. doi:10.1053/j.gastro.2010.01.058.
- Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021; 21(10):653–667. doi:10.1038/s41577-021-00534-x.
- Triner D, Devenport SN, Ramakrishnan SK, Ma X, Frieler RA, Greenson JK, Inohara N, Nunez G, Colacino JA, Mortensen RM. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology. 2019; 156(5):1467–1482. doi:10.1053/j.gastro.2018.12.003.
- Testro AG, Visvanathan K. Toll‐like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol. 2009; 24(6):943–954. doi:10.1111/j.1440-1746.2009.05854.x.
- Duan X, Chan C, Lin W. Nanoparticle‐mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed. 2019; 58(3):670–680. doi:10.1002/anie.201804882.
- Wu Z, Li S, Zhu X. The mechanism of stimulating and mobilizing the immune system enhancing the anti-tumor immunity. Front Immunol. 2021;12:682435. doi:10.3389/fimmu.2021.682435.
- Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015;106:945–950. doi:10.1111/cas.12695.
- Cao Y, Wang X, Jin T, Tian Y, Dai C, Widarma C, Song R, Xu F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct Target Ther. 2020; 5(1):250. doi:10.1038/s41392-020-00348-8.
- Li Y, Dong K, Fan X, Xie J, Wang M, Fu S, Li Q. DNT cell-based immunotherapy: progress and applications. J Cancer. 2020; 11(13):3717. doi:10.7150/jca.39717.
- Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T‐cell immunotherapy for human cancer. Am J Hematol. 2019; 94(S1):3–9. doi:10.1002/ajh.25418.
- Zhao Y, Deng J, Rao S, Guo S, Shen J, Du F, Wu X, Chen Y, Li M, Chen M. Tumor infiltrating lymphocyte (TIL) therapy for solid tumor treatment: progressions and challenges. Cancers. 2022; 14(17):4160. doi:10.3390/cancers14174160.
- Oh S, Lee J-H, Kwack K, Choi S-W. Natural killer cell therapy: a new treatment paradigm for solid tumors. Cancers. 2019; 11(10):1534. doi:10.3390/cancers11101534.
- Ma Y, Y-C X, Tang L, Zhang Z, Wang J, Wang H-X. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012; 1(1):1–10. doi:10.1186/2162-3619-1-11.
- Jaroslawski S, Caban A, Toumi M. Sipuleucel-T (Provenge®): Autopsy of an Innovative Change of Paradigm in Cancer Treatment. Value in Health. 2015; 18(7):A479. doi:10.1016/j.jval.2015.09.1294.
- Constantino J, Gomes C, Falcão A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res. 2017; 65(4):798–810. doi:10.1007/s12026-017-8931-1.
- Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. 2018;834:188–196. doi:10.1016/j.ejphar.2018.07.034.
- Banstola A, Jeong J-H, Yook S. Immunoadjuvants for cancer immunotherapy: A review of recent developments. Acta Biomater. 2020;114:16–30. doi:10.1016/j.actbio.2020.07.063.
- Schmidt FM, Lichtblau N, Minkwitz J, Chittka T, Thormann J, Kirkby KC, Sander C, Mergl R, M F, Stumvoll M. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J Psychiatr Res. 2014;55:29–34. doi:10.1016/j.jpsychires.2014.04.021.
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi:10.1200/JCO.2014.58.3377.
- Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994;91:6458–6462. doi:10.1073/pnas.91.14.6458.
- Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, Kiialainen A, Hanhart J, Schill C, Hess C. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018; 24(7):994–1004. doi:10.1038/s41591-018-0057-z.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372(26):2509–2520. doi:10.1056/NEJMoa1500596.
- Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, Fuseri N, Bonnafous C, Czerwinski D, Rajapaksa A. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014; 123(5):678–686. doi:10.1182/blood-2013-08-519199.
- Loo K, Smithy JW, Postow MA, Betof Warner A. Factors determining long-term antitumor responses to immune checkpoint blockade therapy in melanoma. Front Immunol. 2022;12:810388. doi:10.3389/fimmu.2021.810388.
- Levy Z. Can gut bacteria boost cancer treatments? Nature. 2022; 607(7919):436–439. doi:10.1038/d41586-022-01959-7.
- Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018; 33(4):570–580. doi:10.1016/j.ccell.2018.03.015.
- Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125:S33–S40. doi:10.1016/j.jaci.2009.09.017.
- Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015; 350(6264):1079–1084. doi:10.1126/science.aad1329.
- Routy B, Lenehan JG, Miller WH Jr, Jamal R, Messaoudene M, Daisley BA, Hes C, Al KF, Martinez-Gili L, Punčochář M. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat Med. 2023; 29(8):2121–2132. doi:10.1038/s41591-023-02453-x.
- Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021; 371(6529):602–609. doi:10.1126/science.abb5920.
- Davar D, Dzutsev AK, JA M, Rodrigues RR, Chauvin J-M, Morrison RM, Deblasio RN, Menna C, Ding Q, Pagliano O. et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021; 371(6529):595–602. doi:10.1126/science.abb5920.
- Li H, van der Merwe PA, Sivakumar S, van der Merwe PA. Biomarkers of response to PD-1 pathway blockade. Br J Cancer. 2022; 126(12):1663–1675. doi:10.1038/s41416-022-01743-4.
- McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, Balaji AK, Vetizou M, Prescott SM, Fernandes MR. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022; 28(3):545–556. doi:10.1038/s41591-021-01655-5.
- Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Man Lei Y, Jabri B, Alegre M-L. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015; 350(6264):1084–1089. doi:10.1126/science.aac4255.
- Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. 2019;10:S49–S66. doi:10.1093/advances/nmy063.
- Solanki SS, Singh P, Kashyap P, Sansi MS, Ali SA. Promising role of defensins peptides as therapeutics to combat against viral infection. Microb Pathog. 2021;155:104930. doi:10.1016/j.micpath.2021.104930.
- Li J, Sung CYJ, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:1306–1315. doi:10.1073/pnas.1518189113.
- Van Zyl WF, Deane SM, Dicks LM. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes. 2020; 12(1):1831339. doi:10.1080/19490976.2020.1831339.
- Monteagudo-Mera A, Rastall RA, Gibson GR, Charalampopoulos D, Chatzifragkou A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol. 2019; 103(16):6463–6472. doi:10.1007/s00253-019-09978-7.
- Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Fact. 2020; 19(1):1–11. doi:10.1186/s12934-020-1289-4.
- Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Vélez E, Perdigón G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019; 74(2):115–124. doi:10.1159/000496426.
- Kang H-J, Im S-H. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61:S103–S105. doi:10.3177/jnsv.61.S103.
- Rocha-Ramírez L, Pérez-Solano R, Castañón-Alonso S, Moreno Guerrero S, Ramírez Pacheco A, García Garibay M, Eslava C. Probiotic Lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res. 2017;2017:1–14. doi:10.1155/2017/4607491.
- Rinaldi E, Consonni A, Guidesi E, Elli M, Mantegazza R, Baggi F. Gut microbiota and probiotics: novel immune system modulators in myasthenia gravis? Ann N Y Acad Sci. 2018; 1413(1):49–58. doi:10.1111/nyas.13567.
- Sun S, Luo L, Liang W, Yin Q, Guo J, Rush AM, Lv Z, Liang Q, Fischbach MA, Sonnenburg JL. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci U S A. 2020;117:27509–27515. doi:10.1073/pnas.1921223117.
- Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg D, Wahn U, Hamelmann E. Probiotic‐induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory‐dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007; 37(4):498–505. doi:10.1111/j.1365-2222.2006.02629.x.
- Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007; 13(2):236–243. doi:10.3748/wjg.v13.i2.236.
- Singh TP, Natraj BH. Next-generation probiotics: a promising approach towards designing personalized medicine. Crit Rev Microbiol. 2021;47:479–498. doi:10.1080/1040841X.2021.1902940.
- O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microb. 2017; 2(5):1–6. doi:10.1038/nmicrobiol.2017.57.
- Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, Mazieres J, Audigier-Valette C, Moro-Sibilot D, Goldwasser F. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022; 28(2):315–324. doi:10.1038/s41591-021-01655-5.
- Bullman S, Eggermont A, Johnston CD, Zitvogel L. Harnessing the microbiome to restore immunotherapy response. Nat Cancer. 2021; 2(12):1301–1304. doi:10.1038/s43018-021-00300-x.
- Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M, Storti C, Triulzi T, Castelli C, Balsari A. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep. 2018;24:3528–3538. doi:10.1016/j.celrep.2018.08.090.
- Shi Y, Zheng W, Yang K, Harris KG, Ni K, Xue L, Lin W, Chang EB, Weichselbaum RR, Fu Y-X. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020; 217(5):e20192282. doi:10.1084/jem.20192282.
- Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013; 342(6161):967–970. doi:10.1126/science.1240527.