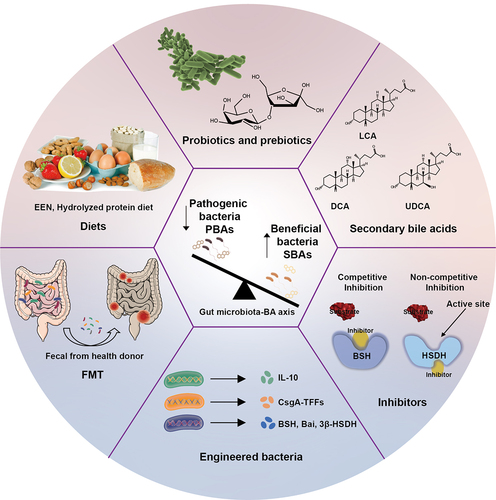
ABSTRACT
Inflammatory bowel disease (IBD) is a chronic and recurrent condition affecting the gastrointestinal tract. Disturbed gut microbiota and abnormal bile acid (BA) metabolism are notable in IBD, suggesting a bidirectional relationship. Specifically, the diversity of the gut microbiota influences BA composition, whereas altered BA profiles can disrupt the microbiota. IBD patients often exhibit increased primary bile acid and reduced secondary bile acid concentrations due to a diminished bacteria population essential for BA metabolism. This imbalance activates BA receptors, undermining intestinal integrity and immune function. Consequently, targeting the microbiota-BA axis may rectify these disturbances, offering symptomatic relief in IBD. Here, the interplay between gut microbiota and bile acids (BAs) is reviewed, with a particular focus on the role of gut microbiota in mediating bile acid biotransformation, and contributions of the gut microbiota-BA axis to IBD pathology to unveil potential novel therapeutic avenues for IBD.
1. Introduction
The human gut microbiome is essential for the digestion and breakdown of complex compounds, such as dietary fibers, and the catabolism of peptides and proteins, serving as a defensive barrier against pathogens and playing a pivotal role in immune regulation.Citation1 The interaction between gut microbiota and their hosts yield metabolites, such as short-chain fatty acids (SCFAs), bile acids (BAs), lipopolysaccharides (LPS), and branched-chain amino acids, which are crucial for the maintenance of intestinal integrity, peripheral tissue function, and modulation of metabolism.Citation2 BAs are complex enterohepatic-derived hormones with profound effects on systemic metabolism; however, their roles are not yet fully understood. Studies in humans and mice have demonstrated that BAs are involved in regulating intestinal inflammation, tumorigenesis, and immune function.Citation3 The dynamic interplay between gut microbiota, their BA metabolites, and the host critically affects our metabolic phenotype, immune function, and the onset of diseases such as cancer, inflammatory bowel disease (IBD), and metabolic diseases, including diabetes, nonalcoholic fatty liver disease (NAFLD), and obesity.Citation4–6
IBD is a chronic, relapsing disorder of the gastrointestinal tract, encompassing conditions such as Crohn’s disease (CD) and ulcerative colitis (UC). Factors influencing IBD include compromised intestinal mucosal barrier function, dysregulation of the intestinal microecology, genetics, and the response of the host immune system. However, the precise causes and mechanisms underlying IBD remain elusive.Citation7 Research has consistently underscored the pivotal role of gut microbiota in IBD, leading to the application of various microbial therapies such as fecal microbiota transplantation (FMT), probiotics, and engineered bacteria. However, despite their clinical use, the mechanisms by which these microbial therapies exert their effects remain insufficiently understood.Citation8 Recent advancements in histological techniques have provided new insights into the relationship between gut microbiota and BAs, leading to the emergence of the gut microbiota-bile acid (BA) axis as a novel target for IBD treatment.Citation9 This review aims to delineate interactions between gut microbiota and BAs, elucidate the mechanisms by which gut microbiota influence BA metabolism, and explore the changes in gut microbiota and BAs observed in IBD patients. Additionally, it provides an overview of emerging IBD therapies based on the gut microbiota-BA axis, offering new insights for clinical prevention, treatment, and diagnosis of IBD.
2. Gut microbiota-BA axis
The diverse microorganisms in the human gut facilitate the biotransformation of BAs, affecting both the composition and size of the BA pool. Conversely, changes in BAs can also alter the composition and abundance of the gut microbiota.
2.1. Gut microbiota mediates BA metabolism
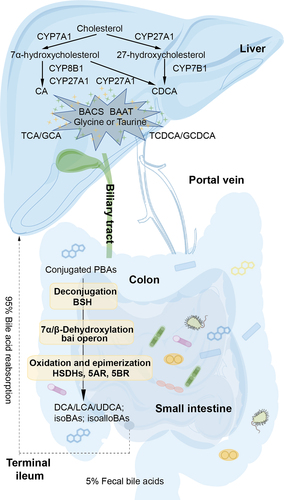
Bile acid synthesis is classified into classical and alternative pathways. In humans, the classical pathway predominates, accounting for over 75% of BA synthesis. Primary bile acids (PBAs), such as cholic acid (CA) and chenodeoxycholic acid (CDCA), are produced from cholesterol with the aid of microsomal cytochrome P450 (CYPs) enzymes, including cholesterol 7α-hydroxylase (CYP7A1) and mitochondrial sterol 27-hydroxylase (CYP27A1).Citation10 Bile acid-CoA synthase (BACS) then converts CA and CDCA into their CoA derivatives, which are subsequently conjugated with glycine or taurine to form glycine-conjugated (predominant in humans) or taurine-conjugated (predominant in mice) BAs through formation of amide bonds catalyzed by bile acid-CoA-amino acid N-acyltransferase (BAAT).Citation10 The conjugated BAs are secreted from the liver into the bile ducts by the bile salt export pump (BSEP), traverse the duct walls into the gallbladder, where they are concentrated, and finally enter the duodenum.Citation11 In the ileum, 95% of BAs bind to plasma proteins for hepatic recirculation, while the remaining 5% are excreted in the feces as part of the enterohepatic cycle that repeats 4 to 12 times daily in humans .Citation12,Citation13 Gut microbiota modifies the BA pool composition through several enzymatic actions, including bile salt hydrolase (BSH)-mediated deconjugation to produce free BAs in the liver, bai gene-mediated 7α/7β-dehydroxylation to produce lithocholic acid (LCA) and deoxycholic acid (DCA) in the colon, and HSDHs-mediated oxidation and epimerization to produce isocholic acids (β-hydroxy) as well as hydroxylation at C6, such as α-muricholic acid (3α, 6β, 7α-trihydroxy-5β-cholan-24-oic acid, αMCA) and β-muricholic acid (3α, 6β, 7β-trihydroxy-5β-cholan-24-oic acid, βMCA).Citation14 Muricholic acids (MCAs) primarily found in mice and also detected in infant urine and feces, are among these secondary bile acids (SBAs).Citation14,Citation15 Furthermore, BAs possess a cyclopentanophenanthrene steroid nucleus, with hydrophilic α-hydroxyl groups above the nucleus plane, and a hydrophobic surface below, contributing to their amphiphilic natureCitation16 and surface tension reduction between the oil and water phases.Citation16 Most of the BAs produced by the liver are taurine or glycine conjugates, which not only ensure solubility by lowering the pKa,Citation17 but also render them impermeable to cell membranes, reducing their critical micellar concentration. These properties of BAs are critical for solubilization of dietary lipids and lipid-soluble vitamins,Citation17 with the enterohepatic cycle described above enabling efficient use of BAs to maintain homeostasis.
Figure 1. Gut microbiota regulates BA metabolism in the hepatic-intestinal circulation. BAs are synthesized from cholesterol catalyzed by CYPs in two ways in the liver, then conjugated with glycine or taurine catalyzed by BACS and BAAT. Conjugated PBAs then undergo a series of reactions including deconjugation, 7α/7β-dehydroxylation, oxidation and epimerization in the colon. Approximately 95% of the BAs reaching the terminal ileum are reabsorbed and thus recycled by the liver.
2.2. BAs reshape the gut microbiota composition
BAs serve as potent antimicrobial agents that are essential to the innate immunity of the intestine.Citation18,Citation19 Their bactericidal action is based on damaging the cell membrane, which subsequently results in leakage of intracellular contents.Citation20 At pH7, unconjugated BAs, such as CDCA and DCA, remain less dissociated, facilitating their passage across hydrophobic cell membranes, unlike their conjugated counterparts, such as glycocholic acid (GCA) and taurocholic acid (TCA).Citation20 As a result, unconjugated BAs demonstrate a more potent antibacterial effect on Staphylococcus aureus. Citation20 Dobson et al. isolated six cholic acid derivatives with significant antimicrobial activity from Bacillus amyloliquefaciens UWI-W23 cultures.Citation21 Furthermore, LCA and its derivatives have been found to exert antibacterial effects on Escherichia coli, Staphylococcus aureus, Bacillus cereus, and Pseudomonas aeruginosa. Citation22 Another BA, ursodeoxycholic acid (UDCA) has also been shown to possess antibacterial and anti-inflammatory properties in Helicobacter pylori-induced gastritis.Citation23 Therefore, BAs and their derivatives offer potential for the development of novel antibiotics against major bacterial pathogens. Additionally, BAs may indirectly modulate the composition of the intestinal microbiota via the farnesoid X receptor (FXR), Takeda G-protein receptor 5 (TGR5), pregnane X receptor (PXR), and vitamin D receptor (VDR). For instance, the FXR agonist obeticholic acid (OCA) has been found to inhibit endogenous BA synthesis and increase the abundance of gram-positive strains, such as Streptococcus thermophilus, Lactobacillus paracasei, Bifidobacterium shortum, and Lactococcus lactis. Citation24 FXR activation has also been observed to induce changes in the composition of the small intestinal microbiota in response to alterations in endogenous BA concentrations.Citation24 A recent study revealed that the gut microbiota of centenarians (average age, 107 years old; n = 160) was enriched in Bacteroides and Alistipes, while the abundance of Streptococcus was reduced compare with older (85–89 years old; n = 112) and young (21–55 years old; n = 47).Citation25 Centenarians exhibited a unique bile acid profile, in particular, had significantly higher levels of isoLCA, 3-oxoLCA, alloLCA, 3-oxoalloLCA and isoalloLCA. Among them, just 2.0 μM isoalloLCA was able to potently inhibit C. difficile 630 production, and the researchers also tested isoalloLCA against other strains. When isoalloLCA was administered to a culture from feces obtained from young healthy volunteers, the abundance of gram-positive bacteria species (e.g., Faecalibacterium, Bifidobacterium, Streptococcus) was significantly reduced, while that of gram-negative species (e.g., Bacteroides, Alistipes) was increased, consistent with the enrichment of Bacteroides and Alistipes and depletion of Streptococcus species seen in the microbiomes of centenarians. This suggests that isoalloLCA has a direct influence on the composition of the gut microbiota, and may help protect the intestine from adverse effects of pathogens.Citation26 In summary, the metabolism of specific BAs plays a crucial role in maintaining intestinal homeostasis, and BA supplementation may affect the composition of the intestinal microbiota.Citation25
3. Mechanisms of bile acid biotransformation by gut microbiota
The gut microbiota metabolizes BAs entering the colon through various processes, including deconjugation, 7α/β-dehydroxylation, oxidation/epimerization, esterification, desulfation, and reconjugation, all of which are mediated by enzymatic catalysis.
3.1. Deconjugation
BSH plays a crucial role in BA-mediated signaling pathways, which regulate lipid uptake, glucose metabolism, and energy homeostasis.Citation27 BSH catalyzes the cleavage of the amide bond in BAs, resulting in release of free BAs such as CA and CDCA, along with glycine or taurine . Glycine and taurine residues may serve as nutrients for gut microbiota.Citation33 Lactobacillus johnsonii (L. johnsonii) PF01 harbors three BSH isoforms, whereas Lactobacillus salivarius LMG14476 contains two, named BSH1 and BSH2.Citation34 The BSH isoforms in L. johnsonii PF01 demonstrate a higher deconjugation rate for glycine-conjugated bile salts than for taurine-conjugated forms.Citation34 Song et al. identified BSH activity in 591 bacterial strains across 117 genera within the human gut, marking a first in this research area. The highest BSH-t3 enzyme activity was detected in Lactobacillus. Citation35 Crystal structures of BSH from various species have been resolved, including Bacteroides thetaiotaomicron (6UFY, blue),Citation28 Ligilactobacillus salivarius (5HKE, yellow),Citation29 Bifidobacterium longum (2HF0, green),Citation30 Clostridium perfringens (2BJF, violet),Citation31 and Enterococcus faecalis (4WL3, pink) , .Citation32 The three-dimensional structures of proteins are crucial for their catalytic functions. BSHs can exist in monomeric, dimeric, and tetrameric forms. Structural analysis reveals that BSH proteins share a highly conserved αββα motif and two sandwiched antiparallel β-sheets, which conform to the structural characteristics of the N-terminal nucleophile (Ntn)-hydrolase family.Citation35 Rossocha et al. pinpointed critical residues in catalysis using C. perfringens BSH (2BJF) as a model .Citation31 The active site of BSHs consists of conserved and functionally important residues, including Cys2, Arg18, Met20, Asn82, Asn175, and Arg228, which interact with reaction products like taurine and deoxycholate. shows hydrogen bonds that are formed between Cys2, Arg18 of BSH and deoxycholate. Phe61 and Ile137 flank ring A and Met20, Ala68, and Phe26 are adjacent to the isovaleric acid side. Moreover, hydrophobic interactions are observed between Ile133 and ring B, and Leu142 and ring D. Interestingly, taurine is observed in a “reversed” orientation with its sulfur group pointing toward Cys2 and leaving the active site with its amino group ahead. Taurine forms only a single hydrogen bond to a protein side chain.Citation31 The catalytic mechanism of BSH of the Ntn-hydrolase superfamily features an autocatalytic cleavage that reveals a nucleophilic residue at the N-terminus .Citation33 This cleavage results in the stabilization of the tetrahedral intermediate by oxygen anion vacancies, with Arg18 potentially enhancing the nucleophilic capability of the Cys2-SH group to participate in autocatalytic processing.Citation33
Figure 2. The deconjugation reaction and the structural features of BSH. (a) Hydrolysis of conjugated BAs by BSH to unconjugated BAs and glycine or taurine. (b) Structural homology between subunits of BSHs from Bacteroides thetaiotaomicron (PDB ID: 6UFY, blue),Citation28 Ligilactobacillus salivarius (PDB ID: 5HKE, yellow),Citation29 Bifidobacterium longum (PDB ID: 2HF0, green),Citation30 Clostridium perfringens (PDB ID: 2BJF, violet),Citation31 and Enterococcus faecalis (PDB ID: 4WL3, pink).Citation32 (c) Overall structural features of BSH, taurine and deoxycholate complex from C. perfringens (PDB ID: 2BJF). The reaction products taurine and deoxycholate are shown in pink. (d) The interactions between BSH and the substrate taurine, deoxycholate, mapped by Discovery Studio Visualizer software. (e) The catalytic mechanism of BSH.
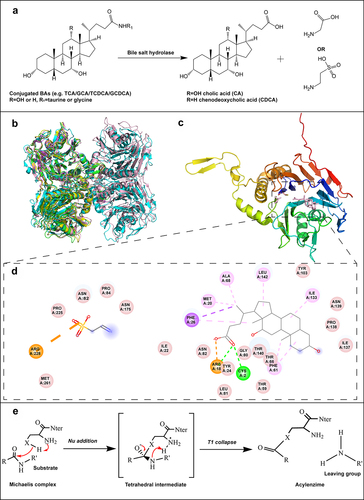
Table 1. Summary of BA biotransformation by microorganisms.
3.2. 7α/7β-dehydroxylation
The C-7 dehydroxylation of PBAs (CA, CDCA) to SBAs (DCA, LCA) is performed via a multistep biochemical pathway. This pathway involves the BA-inducible (bai) operon, encoding proteins predominantly from Clostridium,Citation43,Citation44,Citation73 and Eubacterium. Citation45 Despite its relatively low abundance in the human intestine, the bai operon of Faecalicatena contorta S122 is actively transcribed under host colonization conditions, playing a pivotal role in regulation of the 7α dehydroxylation activity.Citation74 The Bai gene has also been identified in Eggerthella lenta, yet its role in this bacterium awaits functional characterization.Citation75 Studies have demonstrated that E. lenta does not produce DCA or LCA.Citation76 These SBAs are implicated in health conditions such as obesity, gallstones, liver, and colon carcinogenesis,Citation77 highlighting the significance of the 7α-dehydrogenation pathway as a critical bacterial BA biotransformation process in the human gut.
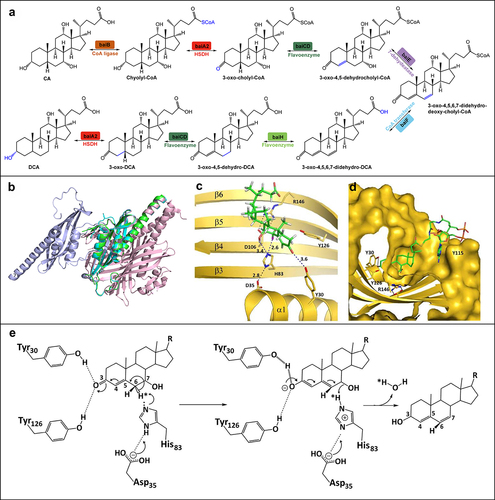
The bai operon, encompassing baiB/CD/E/A2/F/G/H/I, is integral to the 7α-dehydroxylation metabolic pathway. Funabashi et al. isolated and characterized the enzymes involved in this pathway, achieving an in vitro reconstitution.Citation73 The cloning of six enzymes (baiB/CD/A2/E/F/H) from the bai operon of C. sporogenes ATCC 15,579 enabled a complete eight-step conversion of CA to DCA ().Citation73 This conversion commences with the uptake of CA by H+-dependent active transporter (baiG), followed by thioesterification to CoA via a CoA ligase (baiB).Citation83,Citation84 The resulting Cholyl-CoA is then oxidized by HSDH (baiA2) to form 3-oxo-cholyl-CoA.Citation85,Citation86 This intermediate is further oxidized to form 3-oxo-4,5-dehydrocholyl-CoA by a flavoenzyme (baiCD).Citation87 7α-dehydrogenase (baiE) catalyzes the critical 7α-dehydroxylation step, resulting in the formation of 3-oxo-4,5–6,7-didehydro-deoxy-cholyl-CoA.Citation84 CoA is then subsequently removed by CoA transferase (baiF) to form the intermediate, and further reduced by flavoenzyme (baiH, baiCD) and HSDH (baiA2) to produce DCA.Citation85,Citation87–89 BaiI appears to be non-essential in this 7α-dehydroxylation pathway.Citation73 The same pathway is used for the 7α-dehydroxylation of CDCA.
Figure 3. The dehydroxylation pathway for CA and the structural feature of 7α dehydratases. (a) Pathway of intermediate steps in 7α-dehydroxylation of CA by enzymes coded by bai operon genes. (b) The structure homology between BA 7α-dehydratases from Clostridium scindens (PDB ID: 4LEH, light pink),Citation78 Clostridium hylemonae (PDB ID: 4L8O, green),Citation79 Clostridium hiranonis (PDB: 4N3V, violet),Citation80 and Peptacetobacter hiranonis (formerly Clostridium hiranonis, PDB ID: 4L8P, blue).Citation81 (c) Predicted binding mode of 3-oxo-△4-chenodeoxylcholyl-CoA with BA 7α-dehydratases. Blue dashed lines predicted interactions of His83 and Tyr30 with substrate 3-oxo-△4-chenodeoxylcholyl-CoA.Citation82 Adapted with permission from ref. 55, copyright 2016, John Wiley and Sons. (d) Predicted stacking interaction between 3-oxo-△4-chenodeoxycholyl CoA and Tyr115. Carbon atoms of protein residues and product molecules are colored gold and green, respectively. H, O, N, P, and S atoms are colored in gray, red, blue, orange, and olive, respectively.Citation82 Adapted with permission from ref. 55, copyright 2016, John Wiley and Sons. (E) Catalytic mechanism of BA 7α-dehydratases.Citation82 Adapted with permission from ref. 55, copyright 2016, John Wiley and Sons.
The crystal structures of the enzymes encoded by the baiA2/baiB/baiE genes have been resolved.Citation85 BA 7α-dehydrogenase (baiE) is the rate-limiting enzyme in this pathway. Crystal structures of BaiE from Clostridium scinden (PDB ID: 4LEH),Citation78 Clostridium hylemonae (PDB ID: 4L8O),Citation79 Clostridium hiranonis (PDB ID: 4N3V),Citation80 and Peptacetobacter hiranonis (formerly Clostridium hiranonis, PDB ID: 4L8P)Citation81 exhibit a common twisted (α+ β barrel) fold, characteristic of the nuclear transport factor 2 (NTF2) family , . Particularly, BaiE structure from Clostridium scindens (PDB ID: 4LEH) demonstrates trimer formations with a twisted barrel fold .
Docking of the substrate, 3-oxo-ΔCitation4-chenodeoxycholate, into BaiE’s binding pocket highlighted an active center composed of three conserved residues: Tyr30 (α1), Asp35 (α1), and His83 (β3). The side chains of Asp35 and His83 are within proximity of each other at a distance of 2.8 Å, whereas no interaction between Tyr30 and Asp35 or His83 was observed. Asp106 and Arg146 are essential for substrate binding and turnover. A unique ring arrangement formed by the residues above the active site is observed in all apo structures. The conformational flexibility of this ring may be critical for substrate binding . Similarly, the interaction between the adenine group of the CoA moiety of 3-oxo-Δ4-chenodeoxycholyl CoA and baiE was also postulated, where CoA adopts a twisted conformation due to a π–π stacking interaction with Tyr115, allowing pantetheine and AMP moieties of CoA to be exposed to the solvent without any direct interaction with baiE . An analysis of the catalytic mechanism revealed that Tyr30 acts as a general acid (assisted by Tyr126) to facilitate the delocalization of the π-electrons across atoms C3, C4, C5, and C6 . The terminal hydroxyl group of Tyr30 could potentially protonate the oxyanion generated on the C3-oxo group to support negative charge stabilization. This leads to an electron shift that destabilizes the bond between C6 and 6αH. His83 is then strategically positioned to remove the 6αH atom, and protonate the departing C7α-hydroxy group, while Asp35 modulates the pKa of the His83-imidazole ring, aiding the deprotonation and subsequent re-protonation processes that conclude with water molecule release.Citation82
3.3. Oxidation and epimerization
Gut microbiota-mediated oxidation and epimerization occur through the action of HSDHs, which catalyze the reversible oxidation and reduction of the 3α-, 7α-, or 12α-hydroxyl groups of BAs.Citation90,Citation91 Microorganisms such as Clostridium scindens,Citation60 Eubacterium,Citation86 Clostridium hiranonis,Citation60 Eggerthella lenta,Citation76 Escherichia coli,Citation55 and Bacteroides fragilis Citation92 are capable of oxidizing and reducing BAs as they possess at least one HSDH. For instance, the NAD(P)H-dependent 3α-, 3β-, and 12α-HSDHs encoding gene clusters are found in Eggerthella sp. CAG:29815. A 12α-HSDH encoding gene is found in Eggerthella sp. CAG:298,Citation76 Clostridium scindens, Clostridium hylemonae, and Peptacetobacter hiranonis. Citation60 Epimerization leads to the production of more hydrophilic and less toxic isobile acids (isoBAs), which enhance bacterial resistance in the competitive and hostile intestinal environment.Citation93 CDCA, a hydrophobic BA, is converted to its more hydrophilic and less toxic 7β-isomer UDCA by gut microbiota.Citation93,Citation94 It is worth noting that isoBAs also appear to regulate gut microbiota composition and host metabolism. E. lenta and Ruminococcus gnavus contribute to this regulatory action by producing 3-oxoDCA and isodeoxycholic acid (isoDCA), respectively, each through a specific 3α-hydroxysteroid dehydrogenase (3α-HSDH) and 3β-hydroxysteroid dehydrogenase (3β-HSDH) enzyme. IsoDCA, for instance, promotes the growth of Mycobacterium spp. Citation76
Recent studies revealed the involvement of two types of bacterial steroid dehydrogenases, namely 5α-reductase (3-oxo-5α-steroid 4-dehydrogenase, 5AR) and 5β-reductase (3-oxo-5β-steroid 4-dehydrogenase, 5BR), in the production of homologous (5α and 5β) BAs.Citation62 The levels of isolithocholic acid (isoLCA), 3-oxolithocholic acid (3-oxoLCA), allolithocholic acid (alloLCA), 3-oxoallolithocholic acid (3-oxoalloLCA), and isoalloLCA were also found to be significantly higher in the centenarian group (average age, 107 years old) due to the unique gut microbiota in centenarians.Citation25 Analysis of centenarians’ gut microbiota revealed the presence of many strains with strong 3α-HSDH, 3β-HSDH, 5AR, and/or 5BR activity.Citation25 It is plausible that 3-oxoalloLCA is generated from 3-oxo-Δ4-LCA through a 5AR homolog, and the conversion of 3-oxoalloLCA to alloLCA or isoalloLCA involves 3α-HSDH or 3β-HSDH, respectively, which is consistent with the previously-described conversion of 3-oxoDCA to DCA or isoDCA .Citation26,Citation95,Citation96 The crystal structures of several enzymes involved in oxidation and epimerization have been resolved, including 3α-HSDH, 7α-hydroxysteroid dehydrogenase (7α-HSDH), 7β-hydroxysteroid dehydrogenase (7β-HSDH), 5AR, and 5BR . shows that both 5AR (PDB ID: 7C83) and 5BR (PDB ID: CAQ)Citation64 possess alpha-helices and loops, forming a barrel structure, while 5BR wrapping β-sheets in the middle. 3α-HSDH (PDB ID: 1FJH), 7α-HSDH (PDB ID: 5EPO), and 7β-HSDH (PDB ID: 5GT9)Citation58 share a common structural fold. Hence, human steroid 5BR (AKR1D1) could be converted into 3β-HSDH by a single point mutation, Glu120His (PDB ID: 3UZW).Citation54 The wild-type AKR1D1 specifically recognizes D4–3-ketosteroids, whereas the mutant Glu120His does not have 5β-reductase activity, but shows 3β-HSDH activity instead.Citation54
Figure 4. The oxidation and epimerization pathways of CA and CDCA and the structure features of related enzymes. (a) The pathways of CA and CDCA oxidation and epimerization. (b) Three-dimensional structures of different oxidoreductases: 3α-HSDH (PDB ID: 1FJH), 7β-HSDH (PDB ID: 5GT9), 5AR (PDB ID: 7C83), 5BR (PDB ID: 3CAQ). (c) Overall structural features of 7α-HSDH, TCDCA, and NADP+ complex from Clostridium absonum (PDB ID: 5EPO). TCDCA and NADP+ are shown in pink. (d) The interactions between CA 7α-HSDH and TCDCA, NADP+, mapped by Discovery Studio Visualizer software. (e) The catalytic mechanism of HSDHs.
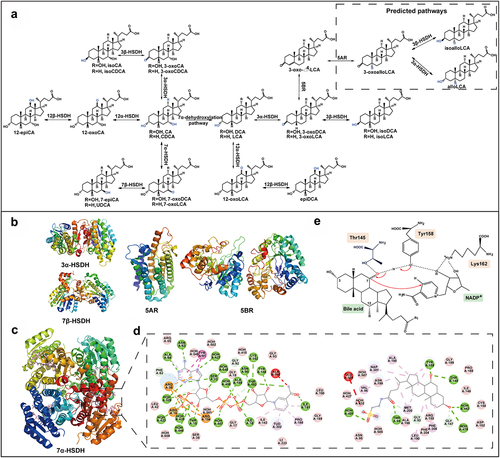
We have previously identified multiple 3α-HSDHs, 7α-HSDHs, and 7β-HSDHs from the black bear gut microbial macrogenome,Citation97 and resolved the crystal structure of 7α-HSDH from Clostridium absonum (CA 7α, PDB ID: 5EPO).Citation56 CA 7α structure (5EPO) was chosen as the basis to investigate the catalytic mechanism of HSDHs . CA 7α is composed of four subunits, each containing seven parallel β-strands and three α-helices on each side. The central β-sheet constitutes the active center. The interactions of CA 7α-HSDH with the substrate and the coenzyme NADP(H) are depicted in . The SDR superfamily features a conserved active center (Asn-Ser(Thr)-Tyr-Lys) and coenzyme binding sites (Thr-Gly-XCitation3-Gly-X-Gly), which are primarily involved in the hydrogen bonding between the enzyme and the substrate. The orientation of NADP(H) is determined by residues including Ser13, Thr15, Arg16, Ile18, Arg38, Asn63, Asn90, Tyr158, Lys162, Ile191, Thr193, Arg194, and Ala195. Thr15, Arg16, Arg38, and Arg194 create a positively charged environment through complex hydrogen bonding interactions with the phosphate group.Citation98 The orientation of the substrate TCDCA in the substrate-binding site is determined by residues including Gly93, Thr94, Thr145, Gly147, Tyr158, Asn199, Phe204, and Phe208. The catalytic mechanism of HSDHs was analyzed as well . The catalytic triad (Thr145-Tyr158-Lys162) was identified based on the crystal structure of CA 7α. Here, Thr145 was found to stabilize substrates, intermediates, and products by forming hydrogen bonds, whereas Tyr158 acts as a catalytic acid/base, and Lys162 serves as part of the proton relay and interacts with the nicotinamide cofactor.Citation56
3.4. Esterification, desulfation, and reconjugation
Bacteria can also esterify BAs with alcohols, SCFAs, and long-chain fatty acids. Bacterial species from genera Bacteroides, Eubacterium, Lactobacillus, Citrobacter, and Peptostreptococcus harbor esterases that convert BAs into their C-24 ethyl esters with ethanol addition.Citation68,Citation99 Methyl esters of DCA are formed by human fecal isolates; however, the mechanism is unknown. It has been hypothesized that the reaction is dependent on C1 transfer (methyl or methoxyl group) to the C24 carboxyl group rather than on methanol as a substrate.Citation68 Bacteria can generate BA fatty acid esters, in which C16 and C18 long-chain fatty acids, as well as SCFAs (acetate), are lined to C3 of isoDCA and isoLCA (FA-conjugated isoBAs, FA-isoBAs).Citation100 Esterification is a detoxification strategy to precipitate BA esters, thereby reducing the concentration of hydrophobic SBAs and toxic fatty acids and alcohols in fecal water.Citation101 Despite progress in liquid chromatography-mass spectrometry approaches to BA profiling, BA esters are rarely detected in fecal samples.Citation100 Esters of BAs account for 10% to 30% of the total BA content in human feces.Citation102 Unfortunately, little is known about the role of gut bacteria in BA esterification. The contribution of FA-isoBAs to intestinal physiology and their role in pathophysiologic conditions such as IBD is currently under investigated.
Sulfation increases the solubility, reduces intestinal absorption, and promotes excretion of BAs in feces and urine. BA-sulfates are also less toxic compared to their unsulfated counterparts. Therefore, sulfation is an important detoxification pathway of BAs.Citation103 BA sulfatase activity has been identified in Clostridium, Peptococcus, Fusobacterium, and Pseudomonas species.Citation69 Sulfatase activity requires a 3α/3β-sulfate group and a free C24 or C26 carboxyl group. Only the BA sulfatase from Comamonas testosterone has been obtained in purified form.Citation69
In the presence of gut microbiota, BAs combine with amino acids to form bile acid-amino acid conjugates. The enzymes responsible for binding amino acids are currently unknown, although previous studies implied the involvement of microbial BSHs in glycine binding. Actinobacteria, Firmicutes, and Bacteroidetes species were shown to have glycine re-conjugation and BSH activities, with the conjugation activities being the highest among all microbial species.Citation60 Recently, Guzior et al. also found that BSH had acyltransferase activity (bile salt hydrolase/transferase, BSH/T). Clostridium perfringens BSH/T rapidly performed acyl transfer when provided with various amino acids and taurocholate, glycocholate or cholate, with an optimum at pH 5.3. These microbially conjugated bile acids (MCBAs) had antimicrobial properties due to have greater hydrophobicity.Citation104 These MCBAs can activate PXR receptor and the aryl hydrocarbon receptor.Citation105 The intrinsic acyltransferase activity of BSH/T greatly diversifies BA chemistry. The physiological effects of amino acid-conjugated BAs, other than those conjugated to glycine or taurine, are not yet fully understood. However, recent studies have indicated that CA conjugated to phenylalanine, leucine, and tyrosine may act as FXR agonists.Citation70 These conjugated BAs are also detected in IBD patients.Citation70 Lucas et al. also found that CDCA, DCA or CA may be conjugated with one or more glutamate, glutamine, aspartate, asparagine, methionine, histidine, lysine, serine, tryptophan, valine, alanine, arginine, phenylalanine, leucine/isoleucine, and tyrosine.Citation71 However, it remains unclear whether BAs conjugated to amino acids enhance intestinal absorption and enterohepatic circulation.
4. Microbiota-derived BAs in IBD and gut immunity
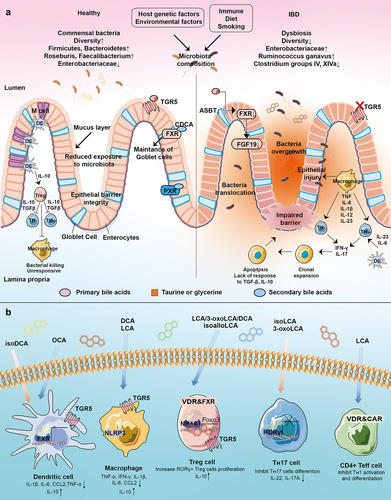
IBD can be influenced by various factors, including genetics, environmental influences, gut microbiota dysbiosis, immune system abnormalities, diet, and smoking. The association between gut microbiota and the development of IBD has long been recognized. Patients with IBD exhibit dysbiosis in their gut microbiota, which triggers an exaggerated immune response toward pro-inflammatory microbes, leading to chronic inflammation and tissue damage in the intestine. This dysbiosis compromises the integrity of the intestinal mucosal barrier, resulting in a reduction in mucus layer thickness and increased intestinal permeability. As a result, microbial components translocate into the intestinal tissue.Citation7 However, the exact role of BAs in IBD pathogenesis remains unknown.Citation97 Here, we summarize the changes in BA and gut microbiota composition in IBD patients as reported in recent experimental studies, along with the effects of major BA receptors on immune responses in the gut .
Figure 5. Interaction of gut microbiota, microbial bile acids, and host immunity. (a) Interactions between the gut microbiota and the host immune response in healthy and IBD states. Gut microbes play an important role in the maintenance of intestinal mucosal immune homeostasis, including the maintenance of an intact intestinal mucosal barrier and a modest immune response to antigens. Disturbances in the abundance and diversity of the gut microbiota can lead to impaired functioning of the host’s intestinal immune system, which can lead to IBD. (b) Bile acids can activate these receptors including LCA, 3-oxoLCA, isoLCA, isoalloLCA, DCA, isoDCA, UDCA, and OCA. IsoDCA inhibits FXR activity in DCs, resulting in the expansion of peripheral Treg cells, while OCA activates FXR to decrease the secretion of TNF-α, IL-1β, IL-6, CCL2 and to increase the level of IL-10. DCA and LCA activate TGR5 on macrophage to block NLRP3 inflammatory vesicle activity, thereby suppressing intestinal inflammation. The activation of TGR5 reduces the level of TNF-α, IFN-γ, IL-1β, IL-6, and CCL2. IsoalloLCA binds to the nuclear hormone receptor NR4A1 at the Foxp3 locus and increases Treg cell differentiation. The combination of LCA/3-oxoLCA increased the RORγt Treg cell population and proliferation via VDR. 3-oxoLCA blocks TH17 differentiation through RORγt and reduces IL-17A and IL-22 significantly. Besides, 3-oxoLCA and isoalloLCA regulate TH17 and Treg cell homeostasis. LCA also inhibits TH1 activation and differentiation via VDR.
4.1. Alteration of gut microbiota and microbiota-derived BAs in IBD
The diversity of beneficial gut microbiota in IBD patients is decreased, whereas that of potentially pathogenic bacteria is increased. The level of PBAs of UC patients increased, which is positively correlated with potentially-pathogenic bacteria, including Proteobacteria, Escherichia coli, and Actinobacteria. Citation106 These pathogenic bacteria cause intestinal infections and inflammation, affecting enterohepatic recycling reabsorption and BA receptor homeostasis.Citation107 Bile acid malabsorption (BAM), a common symptom in patients with IBD, occurs due to decreased activity of the apical sodium-dependent bile acid transporter (ASBT) in the ileum, which subsequently leads to a decrease in intestinal absorption and an increase excretion of BAs in the feces . ASBT is downregulated in active CD, active UC, and CD in remission. The ileum is often the main site of ASBT expression. Therefore, the degree of BAM may be more serious for CD with ileal resection.Citation108 Moreover, IBD patients who undergo ileal resection have elevated levels of PBAs, along with a decrease in microbial diversity.Citation109 FXR also regulates BA production via feedback inhibition of CYP7A1 by the FXR/FGF19/hepatic fibroblast growth factor receptor 4 signaling pathway.Citation110 Thus, BAM induced by ASBT dysfunction in IBD leads to a decrease in the BA pool, a decrease in FXR inhibition, and a subsequent increase in PBA production in intestinal epithelial cells.
Additionally, the gut microbiota in IBD patients show impaired deconjugation and biotransformation, leading to higher levels of conjugated BAs, lower levels of SBAs, and higher levels of PBAs.Citation111–113 These effects have been attributed to a decrease in the abundance of BSH in the gut microbiome.Citation112 Meanwhile, IBD patients exhibited decreased levels of SBAs, such as DCA, LCA, and taurochenodeoxycholic acid (TLCA), indicating a depletion of SBA-producing bacteria like Firmicutes, Clostridium IV and XIVa, Butyricicoccus, Clostridium, Faecalibacterium, Ruminococcaceae, and Roseburia,Citation114–116 which are known to possess BSH and HSDHs enzymes responsible for the deconjugation and biotransformation of BAs.Citation114,Citation117,Citation118 Furthermore, the enzymatic activity of 7α-dehydroxylation (BSH) is inhibited at a pH below 6.5. The stool pH in IBD is acidic (pH 5.2), which therefore inhibits the catalytic action of the enzyme, and thereby prevents the conversion of PBAs to SBAs.Citation119 Connors et al. also found that PBAs were associated with decrease in the activities of enzymes catalyzing BA 7α-dehydroxylation.Citation120 The levels of LCA, DCA, and gene expression required for the conversion of PBAs to SBAs. Moreover, Ruminococcaceae abundance was reduced in UC compared to familial adenomatous polyposis (FAP) .Citation115
Table 2. Gut microbes and BAs changes in IBD.
Changes in ratios of certain BAs may also serve as markers of changes in the gut microbiota. For instance, the ratio DCA/(DCA+CA), which indicates 7α-dehydroxylation, is positively correlated with the presence of Clostridium subcluster XIVa.Citation122 Serum DCA/(DCA+CA) levels are significantly lower in individuals in the remission state of CD and the remission and exacerbation states of UC compared to healthy controls (HCs).Citation122 Furthermore, other correlations between microbiota and BAs were also observed in primary sclerosing cholangitis-IBD (PSC-IBD), where 12% of the operational taxonomic units strongly correlated with fecal BAs. In particular, significant shifts were observed within the Firmicutes (73%) and Bacteroidetes phyla (17%) at the operational taxonomic unit level.Citation125 Overall, these findings highlight the alterations in gut microbiota and its derived BAs in IBD, leading to potential implications for disease pathogenesis. Further research is need to fully understand these complex interaction.
4.2. Gut microbiota-derived BAs regulate intestinal inflammation and immunity
BA receptors are also involved in the regulation of BA-mediated intestinal inflammation.Citation127,Citation128 Immune cells on the intestinal mucosal surface are responsible for the rapid detection and elimination of pathogenic microorganisms, while maintaining immune homeostasis.Citation129 Dysfunction of intestinal immune response can lead to chronic intestinal inflammation, which is a characteristic of IBD.
Healthy human intestinal epithelial cells exhibit high expression of FXR, whereas reduced intestinal FXR signaling is seen in IBD. Impaired FXR signaling can be detrimental in IBD .Citation130–132 Immune disturbances such as elevated macrophage infiltration were observed in FXR−/− mice.Citation133 Specifically, macrophages isolated from (trinitrobenzene sulfonic acid) TNBS-treated FXR−/−mice were found to release more inflammatory cytokines than wild-type (WT) mice.Citation133 FXR exerts anti-inflammatory effects through various mechanisms, such as in the ileum, where high concentrations of conjugated PBAs have direct antimicrobial effects and mediate the antimicrobial program of FXR to prevent bacterial overgrowth.Citation134 In contrast, immune cells in the colon regulate immune homeostasis during microbial colonization. Gut microbiota-derived SBA isoDCA inhibits FXR activity in DCs, thus weakening the immunostimulatory abilities of DCs.Citation95 This anti-inflammatory phenotype acquired by DCs allows peripheral Treg cells to differentiate, and help suppress the immune response during bacterial colonization.Citation95,Citation135 BAs and their analogues can be used to activate FXR to treat IBD. The FXR agonist INT-747 (OCA) significantly decreases TNF-α secretion in activated human peripheral blood mononuclear cells, CD14 monocytes, DCs, and mononuclear cells in lamina propria and decrease severity of colitis in DSS-induced and TNBS- induced colitis model.Citation132 OCA also reduces the expression of proinflammatory cytokines (e.g., IL-1β, IL-6) and chemokines (e.g., CCL2) by activating FXR.Citation132 OCA-activated FXR was also shown to exert anti-inflammatory effects, including increased plasma IL-10 level and inhibition of the decrease in splenic DCs and the increase in Treg cells associated with dextran sulfate sodium salt (DSS)-induced colitis .Citation136 Phenylalanocholic acid, tyrosocholic acid, and leucocholic acid have been identified as novel FXR agonists enriched in the dysbiotic state associated with CD patients.Citation70 These molecules have been found to promote the expression of mouse intestinal FXR target genes Fgf15 and Shp. Nonetheless, further studies are required to determine whether they induce intestinal inflammation and to elucidate FXR-related mechanisms of action.Citation70 Moreover, it is also crucial to carefully consider the FXR-regulating activities of human- and rodent-specific BAs, as they significantly differ from each other. This requires that attention be paid to the circulating BA pool in both experimental animal models and human patients, and how these parameters change in response to diet, inflammation, and dysbiosis is understood. Such detailed consideration is essential to fully comprehend the BA-dependent FXR functions in mucosal immune regulation.
GPBAR1 (TGR5) is a G protein-coupled receptor highly expressed in monocytes/macrophages.Citation137 TGR5 regulates the M1/M2 phenotype of intestinal macrophages, and inhibits the expression of inflammatory genes (TNF-α, IFN-γ, IL-1β, IL-6, and CCL2).Citation138 Activation of TGR5 in intestinal stem cells (ISCs) triggers the SRC/YAP signaling pathway, leading to ISC renewal and proliferation.Citation139 In macrophages, TGR5 activation suppresses intestinal inflammation by blocking NLRP3 inflammatory vesicle phosphorylation and ubiquitination.Citation140,Citation141 DCA/LCA supplementation can reduce intestinal inflammation in DSS murine colitis model by activating TGR5.Citation115 Supplementation with DCA/LCA may also promote ISC stemness, inhibit NLRP3 inflammatory vesicle activity, and reduce the development of colitis by activating TGR5. In addition, PBAs are converted to SBAs (LCA, DCA) through a series of gut microbiota-mediated reactions. LCA and DCA undergo further transformations into isoLCA, alloLCA, 3-oxoLCA, 3-oxoalloLCA, isoalloLCA, isoDCA, and 3-oxoDCA. These metabolites modulate TH17 and Treg cell differentiation, and affect intestinal inflammation.Citation142 IsoalloLCA enhances Treg cell differentiation by activating TGR5 via binding to the nuclear hormone receptor NR4A1 at the Foxp3 locus, whereas 3-oxoLCA blocks TH17 differentiation through inhibiting retinol-associated orphan receptor-γt (RORγt) while expression levels of IL-17A, IL-22 are decreased significantly .Citation142 The dysregulation of TH17/Treg homeostasis in IBD patients is also modulated by 3-oxoLCA and isoalloLCA, suggesting that BA metabolites may directly regulate host immunity by modulating TH17 and Treg cell homeostasis.Citation143 These findings indicate that the gut microbiota-BA axis can impact the onset and progression of inflammation by altering the composition of the intestinal BA pool.
RORγt is a nuclear receptor that plays a role in various inflammatory and autoimmune diseases.Citation144 RORγt Treg cells constitute a unique population of Treg cells that contribute to the response to commensal microbes within the colon.Citation135 Song et al. discovered that microbial BAs, including the combination of LCA/3-oxoLCA, specifically increased the population of RORγt Treg cells.Citation135 The proliferative effect of RORγt Treg cells depends on the VDR activation state.Citation135 VDR activation leads to the formation of a heterodimer with the retinoid X receptor (RXR) which acts as a transcription factor. PXR acts synergistically with CAR to control BA clearance and bilirubin detoxification. LCA and its metabolites 3-oxo-LCA, glycol-LCA, and 6-oxo-LCA activate VDR.Citation145 Reduced VDR expression is commonly observed in mucosal biopsies of IBD patients.Citation146 VDR deficiency in the intestine exacerbates LCA-induced hepatotoxicity, yet intestinal transgenic expression of CYP3A4 protects mice deficient in VDR in the intestine from LCA-induced hepatotoxicity.Citation147 VDR is also expressed in immune cells. LCA inhibits TH1 activation and differentiation via VDR by reducing ERK1/2 phosphorylation in primary human and mouse CD4 TH1 cells .Citation148 In a TNBS-induced colitis model, mice lacking VDR in the colonic epithelium exhibited increased severity of colitis, TH1 and TH17 responses, apoptosis of epithelial cells, and impaired intestinal permeability.Citation149 Thus, while the anti-inflammatory effect of VDR signaling has been demonstrated clearly, the direct effect of BAs on VDR in the context of inflammation has not been shown as clearly, prompting future investigations in this regard.
PXR is expressed across the intestine and in macrophages. PXR is activated by LCA and 3-oxoLCA, resulting in a reduction of intestinal inflammation.Citation150 PXR expression level (gene NR1H4) is an indicator of intracellular BA concentration in the liver and intestine. PXR plays a key role in regulating enterohepatic circulation. Inhibition of PXR leads to decreased expression of transforming growth factor (TGF)-β and IL-10, and increased expression of TNF-α and IL-8.Citation151 PXR agonists induce anti-inflammatory effects in DSS-induced colitis mice by inhibiting NF-κB and reducing cytokine and chemokine expression.Citation152 In a mouse model of necrotizing small intestinal colitis, administration of low-dose LCA was found to reduce intestinal IL-6 expression in a PXR-dependent manner.Citation150,Citation152 Deficiency in both PXR and CAR was found to increase the relative abundance of Lactobacillus, which possesses BSH activity, and corresponding decreased taurine-conjugated PBAs in feces, which may lead to a higher internal burden of taurine and unconjugated BAs.Citation153 Taken together, these findings suggest that dysbiosis of gut microbiota and alterations in BAs may be involved in regulating inflammatory responses through BA receptors. Conversely, BA receptors can also affect the abundance of gut microbiota. Nonetheless, further investigation is needed to understand details of mechanisms of action and quantitative relationships between gut microbiota, microbiota-derived BAs, and BA receptors.
5. IBD treatments targeting the gut microbiota-BA axis
Current medical treatments for IBD typically involve the use of 5-aminosalicylates or sulfasalazine, antibiotics, corticosteroids, immunomodulators, and biologics therapies.Citation154,Citation155 Considering the insights from this review on the effect of the gut microbiota-BA axis on IBD, modulating this axis may represent a promising new treatment strategy .
5.1. Diets
The importance of diet in improving health is widely recognized.Citation156 Diets such as the ‘Western’ diet, which is high in fat and protein but low in fruits and vegetables, were found to be associated with IBD development.Citation157 A diet may trigger intestinal inflammation by altering the composition of gut microbiota and BAs, activating immune cells, and increasing intestinal permeability in IBD patients.Citation158 To this end, dietary therapy has shown promise in improving the health condition of IBD patients. For example, enteral nutrition (EEN) is a first-line treatment for CD in children.Citation159,Citation160 EEN therapy is based on direct anti-inflammatory effects, which improve the composition of gut microbiota, reduce barrier permeability, and modulate the immune system.Citation161,Citation162 Previous studies have also demonstrated that EEN has a significant and lasting impact on the composition of major intestinal bacterial groups.Citation163 Moreover, EEN has been found to partially restore the BA composition in patients, particularly by increasing levels of hydrophobic BA (LCA), which activates TGR5 receptors and reduces inflammation.Citation121 In mice with inflammation, supplementation with fucose was found to increase Lactobacillus abundance and a decrease in T-β-MCA levels in the ileum. Lactobacillus exhibited the highest enzyme activity for the deconjugation of BAs. T-β-MCA acts as an FXR antagonist, and its reduction leads to the reactivation of the FXR-FGF15-CYP7A1 pathway, thereby reducing the BA pool and restoring the dysregulated ecology associated with colitis.Citation164
Dietary fibers are essential for preserving microbial diversity, and the gut microbiota plays a pivotal role in modulating host physiology by metabolizing these fibers.Citation165,Citation166 This interaction can significantly influence BA metabolism as well. For instance, soluble dietary fibers such as oligofructose can promote the conversion of PBAs to SBAs by gut microbiota.Citation166 Additionally, dietary resistant starch supplementation can increase gut luminal DCA abundance in mice.Citation167 These findings imply that dietary fibers could potentially be utilized to regulate gut microbiota and bile acid metabolism. Despite these insights, the impacts of fibers on IBD still present certain ambiguities. While epidemiological studies have indicated a link between a fiber-rich diet and a reduced incidence of IBD, numerous confounding factors complicate this relationship.Citation168 Individuals with IBD often exhibit intolerance to fermentable fiber-rich foods, with certain dietary items acting as triggers for gut symptoms.Citation169 For instance, fermentable carbohydrates such as fructans have been shown to worsen functional gastrointestinal symptoms in IBD patients.Citation170 Parallel complexity can be found in animal studies. For instance, feeding mice a low-fiber diet rather than a naturally fiber-rich diet predisposes mice to severe DSS-induced colitis, which is further exacerbated by the addition of highly soluble fiber inulin to the low-fiber diet. In contrast, the addition of the moderately soluble fiber psyllium to this diet provided robust protection in DSS-induced colitis and a model of T-cell transfer colitis, and psyllium fiber against colitis via activation of FXR.Citation171,Citation172 Thus, the effect of fiber may be related to fiber and environment. Moreover, the influence of fiber appears to be intricately tied to an individual’s microbiota composition. Specifically, Armstrong et al. found that dysregulated inulin fermentation can trigger a pro-inflammatory response through activation of the NLRP3 and TLR2 pathways in patients with IBD who lack the activity of specific fermenting microorganisms.Citation173 Bonazzi et al. also found that individualized microbiota determines the effect of dietary fiber on colitis sensitivity.Citation174 Therefore, individuals with IBD possessing a microbiota resistant to fiber may not necessarily need to eliminate all forms of soluble fiber. Conversely, those with a microbiota sensitive to fiber should meticulously assess their fiber consumption and sources as integral components of disease management. Hence, a comprehensive comprehension of how soluble fiber interacts with a specific gut microbiota is imperative for the development of individualized interventions based on fiber and microbiota, for patients with IBD as well as for healthy individuals.
Sophora alopecuroides L. is a traditional medicine known for its anti-inflammatory properties. Following treatment with Sophora alopecuroides L., the abundance of Firmicutes species was found to increase, whereas that of Bacteroidetes species decreased. Additionally, levels of αMCA, βMCA, ωMCA, and CA were found to increase significantly.Citation175 The presence of Bacteroidetes, Proteobacteria, and Deferribacteres was found to be positively correlated with αMCA, βMCA, ωMCA, and CA, whereas those of Actinobacteria, Tenericutes, Firmicutes, and TM7 were negatively correlated.Citation175 Furthermore, the content of DCA in the intestine of DSS-induced mice was significantly reduced by the presence of Sophora alopecuroides L., indicating its potential to alleviate UC by reducing the cytotoxicity of DCA.Citation175 Another study found that dihydromyricetin (DHM), a nutraceutical supplement, was found to lead to an enrichment of beneficial genera such as Lactobacillus and Akkermansia, and increased levels of unconjugated BAs (CDCA and LCA), thereby activating FXR and TGR5 and maintaining intestinal integrity.Citation176 This suggests that DHM treatment in IBD targets the gut microbiota-BAs-FXR/TGR5 signaling pathway.Citation176 Herba Origani can also promote BA absorption and regulate BA metabolism in DSS-induced UC mice to maintain intestinal mucosal immune homeostasis. It can also enhance the abundance of the Bacteroidota community to restore the gut microbiota dysbiosis.Citation177 In summary, dietary therapy improves the disease symptoms by reducing pathogenic bacteria and increasing the abundance of beneficial commensal bacteria. These bacteria also play a role in modulating BA composition through enzymatic catalytic activity. While dietary therapy is a relatively safe option, changing long-term lifestyle habits may be challenging.Citation178 Moreover, achieving effectiveness of specific diets in different populations also requires further investigation through randomized controllable trials.Citation179
5.2. Probiotics and prebiotics
Probiotics such as Bifidobacterium, Lactobacillus genera, Lactococcus spp., E. coli Nissle 1917, and Streptococcus thermophilus are living microorganisms that are consumed as food supplements to improve gut microbiota and enhance intestinal barrier function.Citation180,Citation181 Probiotics have been approved for alleviating intestinal disorders in IBD.Citation97 For instance, a combination supplement of Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum ZDY2013 has been shown to enrich unidentified Firmicutes and decrease the abundance of Bacteroidetes, while regulating oxidative stress and inflammatory mediators to alleviate UC.Citation182 Similarly, Bifidobacterium longum (B. longum) CECT 7894 has been found to increase the relative abundances of beneficial bacteria such as Bifidobacterium, Blautia, Butyricicoccus, Clostridium, Coprococcus, Gemmiger, and Parabacteroides, while reducing the relative abundances of harmful bacteria like Enterococcus and Pseudomonas. Citation183 Furthermore, B. longum CECT 7894 has been shown to increase the abundance of SBAs such as α-MCA, β-MCA, LCA, CDCA, UDCA, HCA, isoLCA, and isoalloLCA, which were found to be positively associated with the increased abundance of BSH and 7α-dehydroxylases.Citation183 B. longum CECT 7894 was also found to improve the efficacy of infliximab (IFX) in DSS-induced colitis by modulating gut microbiota composition and BA metabolism.Citation183 Another study demonstrated that Lactobacillus casei strain Shirota (LcS) could inhibit the clinical manifestation of DSS-induced mice by increasing the relative abundance of beneficial bacterial species and reducing the relative abundance of pathobionts.Citation184 Additionally, the levels of taurine-conjugated PBAs (TCA, TCDCA) and taurine-conjugated SBAs (TDCA, TUDCA) were found to increase, while those of α-MCA and β-MCA decreased. These changes in the BA profile further suppressed the expression of IFN-γ and nitric oxide (NO), and increased the expression of IL-10 in colonic tissue.Citation184 E. coli Nissle 1917 was also shown to have the same efficacy and safety profile as the gold standard mesalazine in patients with UC.Citation185 Moreover, a mixture of multiple strains tends to be more effective than a single strain. For instance, Tursi et al. studied the effects of supplementation with VSL#3 in patients affected by relapsing UC who were already under treatment with 5-ASA and/or immunosuppressants at stable dose. Compare with placebo group, VSL#3 was found to improve rectal bleeding and decreased ulcerative colitis disease activity index (UCDAI) scores by 50% in UC.Citation186 VSL#3 was also shown to be efficient and safe in children with UC.Citation187 In contrast to traditional single probiotics or simple combinations, members of a designed bacterial consortium (GUT-103 and GUT-108) work synergistically with each other to restore microbial composition and function in IBD patients, providing DCA and LCA, SCFAs, indoles, and antimicrobial agents that are missing in IBD patients.Citation188 7-α-dehydratase (7-α-DH) or 7α-HSDH activity are induced in GUT-103 and GUT-108 strains. GUT-103, which is composed of 17 strains, synergistically provides protective and sustained engraftment in the IBD inflammatory environment, preventing and treating chronic immune-mediated colitis. The therapeutic application of GUT-108 reduces the levels of colitogenic Enterobacteriaceae and increases beneficial resident Clostridium (Clusters IV and XIVa) species, especially Lachnospiraceae including Dorea species and Lachnoclostridium species that are not GUT-108 constituents.Citation188 In addition, GUT-108 strains provide additional redundancy for the synthesis of the protective LCA and DCA.Citation188
Prebiotics are typically carbohydrates with various molecular structures, including polyphenols and polyunsaturated fatty acids.Citation189 These substances may enhance the composition and quantity of beneficial species in the gut microbiota, such as Bifidobacteria and Lactobacilli. These beneficial species utilize the nutrients from prebiotics to support their growth and proliferation.Citation190 In overweight dogs, inulin-type prebiotics have been found to increase the abundance of Firmicutes and decrease the relative abundance of Proteobacteria. In addition, the concentration of fecal total BAs was observed to increase in dogs fed with prebiotics.Citation191 In clinical remission, fermentation of Xylo-Oligosaccharide (XOS) was found to promote the growth of beneficial bacteria such as Roseburia, Bifidobacterium, and Lactobacillus, and thereby alleviate dysbiosis in the feces of UC patients.Citation192 Similarly, stachyose was found to increase the abundance of beneficial microbiota species (Akkermansia and Lactobacillus) and bacterial diversity, thereby mitigating acute colitis in mice.Citation193 Further investigation has demonstrated that prebiotics change the metabolites of gut microbiota to treat UC. High-dose fructans have been shown to increase colonic butyrate production, significantly reduce colitis, and lead to an increased abundance of Bifidobacteriaceae and Lachnospiraceae. However, these shifts were not correlated with improved disease scores.Citation194
These findings highlight the potential of probiotics and prebiotics in targeting the gut microbiota-BA axis for IBD treatment. Nevertheless, it is still challenging to arrive at definitive conclusions regarding the effectiveness of probiotic and prebiotic products due to the high heterogeneity and risk of bias in clinical studies. Large-scale and well-designed trials are necessary before probiotics and prebiotics can be adopted as definitive treatment strategies for IBD. Moreover, the selection of probiotic and prebiotic supplements should be carefully considered in individualized medicine, considering not only the disease itself but also the specific BA metabolic profile of each patient.
5.3. Fecal microbiota transplantation
FMT from a healthy donor to a patient is a therapeutic approach to restore the composition of the gut microbiota.Citation8 Previous studies have shown that FMT resolves 80–90% of antibiotic-resistant Clostridium difficile infections (CDI).Citation195,Citation196 After FMT treatment of CDI, patients were found to show increased levels of SBAs, and decreased levels of PBAs, which is essential for the success of FMT treatment since PBAs (e.g., TCA) can cause the spore formation of C.difficile. Citation197 FMT also yielded positive results for IBD treatment, especially in UC patients. A clinical trial (ClinicalTrials.gov Number: NCT01545908) demonstrated nine patients with active UC without infectious diarrhea, who received fecal transplants from a single donor, and showed remission of inflammation.Citation198 FMT induces remission in UC by increasing microbial diversity. Patients in remission after FMT showed enrichment of Eubacterium hallii, Roseburia inulivorans, and SBAs, while patients without remission showed enrichment of Fusobacterium gonidiaformans, Sutterella wadsworthensis, and Escherichia species (ClinicalTrials.gov, Number: NCT01896635).Citation199 FMT with high-dose and multi-donor was also found to be more effective in active UC.Citation199 FMT is typically delivered via colonoscopic infusion and enemas, but orally administered FMT has recently been assessed as effective in UC as well.Citation200 Taken together, FMT offers potential as a therapy to achieve remission in IBD. However, FMT transplants may also fail. A recent randomized controlled clinical trial in adults with colonic or ileo-colonic CD (ClinicalTrials.gov Number: NCT02097797) showed that the enrichment of Gammaproteo bacteria (Proteobacteria phylum) such as Klebsiella, Actinobacillus, and Haemophilus may affect the success of donor microbiota colonization.Citation201 Other factors still require further consideration, including the mode of delivery, aerobic versus anaerobic stool preparation, number of donors, and the need for single or repeated FMT. To this end, larger trials are necessary to determine the feasibility of FMT as an option for IBD treatment.
5.4. Secondary bile acids
SBAs such as UDCA, DCA, and LCA may offer alternative treatments for IBD.Citation202,Citation203 Oral administration of UDCA, TUDCA, or GUDCA has been shown to reduce intestinal ecological disorders, and increase the ratio of Firmicutes/Bacteroidetes in the intestine of DSS-induced mice.Citation204 UDCA therapy can also increase the abundance of BSH-rich bacteria, such as Akkermansia muciniphila, and prevent the loss of Clostridium cluster XIVa, bacterial species known to be particularly decreased in IBD patients.Citation204 Moreover, UDCA-mediated alleviation of DSS-induced colitis was dependent on the microbiota. UDCA facilitated colonization of Akkermansia, which was associated with the enhancement of the mucus layer upon UDCA treatment and activation of FXR receptor in macrophages.Citation205 These results indicate that UDCA could be a promising treatment option for reducing dysbiosis and ameliorating inflammation in human IBD. The interaction between gut microbiota and fecal BAs is also a risk factor in colorectal cancer neoplasia. In male patients undergoing UDCA treatment, an overrepresentation of Faecalibacterium prausnitzii and an underrepresentation of Ruminococcus gnavus were more prominent in male patients without adenoma recurrence compared to those with recurrence. However, this relationship was not observed in female patients.Citation206 Additionally, UDCA and LCA are protective against DSS-induced increases in epithelial permeability and colonic inflammation. Specifically, UDCA administration was found to increase colonic LCA levels, while LCA administration did not alter UDCA levels.Citation203 Bossche et al. showed that TUDCA attenuated ileitis by alleviating the downregulation of nuclear receptors and BA transporters in TNFΔARE/WT mice.Citation207 Different doses of UDCA may have different effects on patients. For example, a high-dose UDCA (50 mg/kg/day) was found to ameliorate experimental colonic inflammation, while lower doses (10, 25 mg/kg/day) showed no significant effect.Citation208 However, long-term use of high-dose UDCA (28–30 mg/kg/day) was also found to increase the risk of colorectal cancer in patients with UC and primary sclerosing cholangitis.Citation209 Therefore, maintaining the dynamic balance of BAs is crucial for intestinal homeostasis.
An important limitation of oral BAs is their rapid absorption into the hepatic-intestinal circulation of the small intestine and delivery to the liver, resulting in low concentrations within the colon. Therefore, alternative delivery methods need to be explored to achieve therapeutic concentrations in the colon. Recently, rectal administration of DCA and LCA was shown to reduce inflammation in three models of colitis in mice.Citation115 LCA and DCA suppress in vitro proinflammatory cytokine production from human peripheral blood-derived macrophages, which are key mediators of intestinal inflammation in IBD, through activation of the TGR5 receptor. In conclusion, SBAs are promising candidates for IBD treatment. However, BAs may also affect the structure of the intestinal microbial community, which plays a significant role in the development of inflammatory disorders. Therefore, the effect of BA therapy on the fecal microbiota during colitis should also be taken into account for the development of a therapy strategy.
5.5. Inhibitors
The utilization of SBA biotransformation-associated microbial elements such as BSH, HSDH, and bai genes, as targets for the gut-microbiota BA axis, has not been fully explored. Metagenomic analysis of the human microbiome revealed that the abundance and activity of gut BSHs are indeed correlated with IBD.Citation210 Parasar et al. developed chemo proteomic tools to study BSH activity in gut microbiota, and identified altered BSH activities in a DSS-induced mouse model, leading to changes in BA metabolism, and subsequently impacting host metabolism and immunity.Citation211 Similar to biologics that demonstrated significant improvements in inducing and maintaining remission in IBD, BSH inhibitors have also been discovered. Smith et al. discovered BSH inhibitors such as riboflavin, phenethyl caffeate, tetracycline antibiotics, and roxarsone from 2,240 biologically active and structurally diverse compounds using a high-throughput screening system.Citation212 Subsequently, Adhikari et al. identified a covalent pan-inhibitor compound 7 from a rational design candidate library of BSHs, leading to a decrease in the level of conjugated BAs.Citation28 Recently, Adhikari developed a highly potent gut-restricted inhibitor (AAA-10) with low off-target effects and durable efficacy in vivo. After 5 days of oral administration of AAA-10, the abundance of DCA and LCA in the gastrointestinal tract of wild-type mice was found to decrease, suggesting that AAA-10 is highly potent in inhibiting the activity of BSH, and modulating the composition of the BA pool in vivo.Citation213 Taxifolin can inhibit neurosteroid synthesis by inhibiting steroid biosynthesis steroid 5α-reductase 1 (SRD5A1) and 3α-hydroxysteroid dehydrogenase (3α-HSD, AKR1C9).Citation214 Similarly, a competitive inhibitor of 3β-hydroxysteroid dehydrogenase (3β-HSD) may terminate pregnancy by inhibiting the synthesis of progesterone (P4).Citation215 To the best of our knowledge, no inhibitor of HSDH for IBD treatment has been discovered to date. Our knowledge regarding the role of the bai gene is also limited. Funabashi et al. constructed a 7α-dehydroxylation pathway including bai operon in C. sporogenes, and realized a complete eight-step conversion of CA to DCA in vitro. Citation73 SPF mice colonized with the baiH mutant S122 exhibit reduced intestinal inflammation compared to those colonized with the WT Faecalicatena contorta S122, indicating the potential of baiH as a therapeutic drug target for colitis.Citation74 These studies provide novel insights into manipulating the gut microbiota through targeting bai genes. However, no inhibitors of the bai operon have been discovered to date. In section 3, we have provided an extensive discussion of the structural features and catalytic mechanisms of the enzymes involved in the BA metabolic pathway. Structure-function studies of enzymes are vital to evaluate the molecular basis of the interaction between these enzymes and their inhibitors, in addition to providing a theoretical basis for structure-based inhibitor design. While these inhibitors are not yet ready for clinical application as new drug candidates, they can still be utilized as chemical probes to gain a better understanding of the effects of gut microbiota and BAs on host metabolism.
5.6. Engineered bacteria
Orally engineered bacteria can survive in the complex environment of the gastrointestinal tract and express desired target molecules. Engineered bacteria may then target the intestinal mucosa, synthesize and deliver therapeutic molecules on the surface of the intestinal mucosa, and thereby enhance the effectiveness of treatment compared to classical systemic therapies.Citation216 By using synthetic biology techniques such as the CRISPR-Cas system, researchers have developed recombinant bacterial strains that produce active therapeutic agents, such as IL-10 at mucosal sites to inhibit inflammationCitation217 and secrete neutralizing anti-TNF-α nanobodies to block the proinflammatory effects of TNF-α.Citation218 Most therapeutic approaches for IBD are aimed at modulating intestinal inflammation; however, restoring intestinal barrier function and promoting mucosal healing, which has not been studied extensively as a therapeutic strategy, may also be possible. To this end, Lactobacillus lactis was engineered to produce trefoil factor (TFF), then secreted TFF was absorbed in the colon to promote intestinal barrier function and epithelial repairing.Citation218
Recently, E. coli Nissle 1917 was engineered to secrete curli-fused TFFs (CsgA-TFFs) both in vitro and in vivo. This nanofiber-composed matrix was then shown to promote mucosal healing and immunomodulation to prevent DSS-induced colitis in mice.Citation219 Thus, engineered bacteria may be a viable approach, although the treatments are based on animal models. The intestinal ecological dysregulation in IBD leads to reduced levels of microbial BA biotransformation and SBAs while inducing higher levels of conjugated PBAs. In vivo, BAs were found to be modified by the gut microbiota through a mixture of deconjugation, 7α/β-dehydroxylation, and oxidation/epimerization. In this process, the first step involves the catalysis of conjugated BAs to release free BAs by BSH. A deficiency of BSH-active bacteria plays a role in intestinal inflammation and impairs BA metabolism. Therefore, BSH-active bacteria in probiotic preparations are being actively investigated for IBD treatment.Citation220 The second step involves the conversion of PBAs (CA, CDCA) into SBAs (DCA, LCA) through a multistep biochemical pathway carried out by bacteria containing bai genes. DCA and LCA are strong TGR5 agonists with anti-inflammatory properties. Therefore, stimulating the microbial 7α-dehydroxylation (7α-DH) pathway may also promote the production of DCA and LCA, and thereby dampen intestinal inflammation.Citation115 Engineered 7α-DH overexpressing strains and minimal gut microbiota with 7α-DH activity are potential strategies in this regard.Citation221,Citation222 Recently, Funabashi et al. constructed a 7α-dehydroxylation pathway in C. sporogenes based on the characteristics of the enzyme encoded by the bai operon and realized a complete eight-step conversion of CA to DCA in vitro. Citation73 This engineered C. sporogenes can be used to produce DCA and thereby regulate intestinal immunity. In addition, SPF mice colonized with the baiH mutant S122 exhibit reduced intestinal inflammation compared to those colonized with WT Faecalicatena contorta S122.Citation74 The third step involves the oxidation and epimerization of BAs via HSDHs. An engineered Bacillus mimicus strain with 3β-HSDH was developed to produce isoDCA, which promotes the production of colonic RORgt+ Treg cells in a cns1-dependent manner. In DCs, isoDCA interacts with FXR to enhance Treg induction. Recently, Ruminococcus gnavus with 5BR and 3β-HSDH enzyme activity was shown to epimerize DCA to 3-oxoDCA, and subsequently to 3-isoDC, which are less toxic.Citation75 Besides, microbiome-mediated BA metabolism can be disrupted by antibiotic-induced dysbiosis, leading to an overaccumulation of taurocholate in the colon.Citation223 Taurocholate can induce the germination of Clostridioides difficile endospores, thereby causing C.difficile infection (CDI).Citation224 As a result, E. coli Nissle 1917 containing recombinant bile salt hydrolase (Cbh) of Clostridium perfringens (EcN-Cbh) have been engineered to inhibit CDI by deconjugating taurocholate to cholate and reducing the abundance of C.difficile in the murine model. Additionally, EcN-Cbh can also elevate the levels of other bile salts including chenodeoxycholate, lithocholate, and deoxycholate. Therefore, EcN-Cbh can be utilized to regulate BA pool and gut microbiota, presenting promising implications for the use of such engineered microbes to treat IBD.Citation225 These studies suggest that targeting the gut microbiota-BA axis can alleviate intestinal inflammation, reduce BA toxicity, modulate immunity, and thus provide a treatment mechanism for IBD. Engineering bacteria to treat IBD based on the mechanism of gut microbiota catalyzing BA metabolism may also be a feasible mechanism. However, the manipulation of microbial elements associated with SBA production, including BSH, bai, and HSDH genes, through targeting the gut microbiota-BA axis, has not been extensively studied.Citation210 The treatment based on BAs also needs to be validated in humans through high-quality, randomized controlled trials before it can be implemented in clinical practice. Currently, phase II clinical trials are underway to verify whether UDCA reduces inflammation and improves the quality of life in UC pouch patients (ClinicalTrials.gov, Number: NCT03724175).Citation115
6. Conclusion and perspective
Numerous studies to date have investigated the relationship between gut microbiota, microbiota-derived BAs, and IBD. While many reviews have focused on the role of BAs and their receptors in driving immune and microbial changes within the intestines, the interplay between alterations in abundance and function of gut microbiota, BA concentrations, recently discovered metabolic pathways in vivo, the diverse biological roles of BAs, and the specific mechanisms of enzymatic conversion in metabolic pathways need to be elucidated. In this review, we discussed the role of gut microbiota in the metabolism of BAs, with a particular emphasis on providing insights into the structure and catalytic mechanism of key enzymes found in the gut microbiota. Advancements in synthetic biology and understanding of the related catalytic mechanisms allow for the design of engineered bacteria. These bacteria, characterized by high activity and robust thermostability, can be tailored for various pathological and physiological contexts. However, manipulation of SBA transformation-related microbial elements, including BSH and bai genes, remains an underexplored avenue in the gut-microbiota BA axis. We have also discussed changes in the gut microbiota and BAs in IBD, and interactions between the gut microbiota and its metabolized BAs through different BA receptors and immune cells. Thus, targeting the gut microbiota-BA axis holds significant potential for IBD treatment. To this end, further research is necessary to deepen our understanding of the underlying mechanisms and validate their efficacy in clinical practice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kuziel GA, Rakoff-Nahoum S. The gut microbiome. Curr Biol. 2022;32(6):R257–33. doi:10.1016/j.cub.2022.02.023.
- Zheng L, Wen XL, Duan SL. Role of metabolites derived from gut microbiota in inflammatory bowel diseasea. World J Clin Cases. 2022;10(9):2660–2677. doi:10.12998/wjcc.v10.i9.2660.
- Kriaa A, Mariaule V, Jablaoui A, Rhimi S, Mkaouar H, Hernandez J, Korkmaz B, Lesner A, Maguin E, Aghdassi A. et al. Bile acids: key players in inflammatory bowel diseases? Cells. 2022;11(5):901. doi:10.3390/cells11050901.
- Fernandes MR, Aggarwal P, Costa RGF, Cole AM, Trinchieri G. Targeting the gut microbiota for cancer therapy. Nat Rev Cancer. 2022;22(12):703–722. doi:10.1038/s41568-022-00513-x.
- Chen B, Bai Y, Tong F, Yan J, Zhang R, Zhong Y, Tan H, Ma X. Glycoursodeoxycholic acid regulates bile acids level and alters gut microbiota and glycolipid metabolism to attenuate diabetes. Gut Microbes. 2023;15(1):2192155. doi:10.1080/19490976.2023.2192155.
- Wahlström A, Sayin Sama I, Marschall H-U, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24(1):41–50. doi:10.1016/j.cmet.2016.05.005.
- Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, Wilson DC, Cameron F, Henderson P, Kotze PG. et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. 2022;162(4):1147–1159.e1144. doi:10.1053/j.gastro.2021.12.282.
- Varga A, Kocsis B, Sipos D, Kasa P, Vigvari S, Pal S, Dembrovszky F, Farkas K, Peterfi Z. How to apply FMT more effectively, conveniently and flexible - a comparison of FMT methods. Front Cell Infect Microbiol. 2021;11:657320. doi:10.3389/fcimb.2021.657320.
- Cai J, Sun L, Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe. 2022;30(3):289–300. doi:10.1016/j.chom.2022.02.004.
- Axelson M, Ellis E, Mork B, Garmark K, Abrahamsson A, Bjorkhem I, Ericzon BG, Einarsson C. Bile acid synthesis in cultured human hepatocytes: support for an alternative biosynthetic pathway to cholic acid. Hepatology. 2000;31(6):1305–1312. doi:10.1053/jhep.2000.7877.
- Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S, Clerici C, Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47(18):4559–4569. doi:10.1021/jm049904b.
- Wang LX, Frey MR, Kohli R. The role of FGF19 and MALRD1 in enterohepatic bile acid signaling. Front Endocrinol. 2022;12:799648. doi:10.3389/fendo.2021.799648.
- Stellaard F, Lütjohann D. Dynamics of the enterohepatic circulation of bile acids in healthy humans. Am J Physiol-Gastr L. 2021;321(1):G55–G66. doi:10.1152/ajpgi.00476.2020.
- Honda A, Miyazaki T, Iwamoto J, Hirayama T, Morishita Y, Monma T, Ueda H, Mizuno S, Sugiyama F, Takahashi S. et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J Lipid Res. 2020;61(1):54–69. doi:10.1194/jlr.RA119000395.
- Garcia-Canaveras JC, Donato MT, Castell JV, Lahoz A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J Lipid Res. 2012;53(10):2231–2241. doi:10.1194/jlr.D028803.
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. doi:10.1194/jlr.R500013-JLR200.
- Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15(7):804?816. doi:10.3748/wjg.15.804.
- Keitel V, Stindt J, Haussinger D. Bile acid-activated receptors: GPBAR1 (TGR5) and other G protein-coupled receptors. Handb Exp Pharmacol. 2019;256:19–49. doi:10.1007/164_2019_230.
- Percyrobb IW, Collee JG. Bile acids: a pH dependent antibacterial system in the gut? Br Med J. 1972;3(5830):813–815. doi:10.1136/bmj.3.5830.813.
- Sannasiddappa TH, Lund PA, Clarke SR. In vitro antibacterial activity of unconjugated and conjugated bile salts on staphylococcus aureus. Front Microbiol. 2017;8:1581. doi:10.3389/fmicb.2017.01581.
- Dobson TE, Maxwell AR, Ramsubhag A. Antimicrobial cholic acid derivatives from the Pitch Lake bacterium Bacillus amyloliquefaciens UWI-W23. Steroids. 2018;135:50–53. doi:10.1016/j.steroids.2018.04.008.
- Do Nascimento PG, Lemos TL, Almeida MC, de Souza JM, Bizerra AM, Santiago GM, da Costa JG, Coutinho HD. Lithocholic acid and derivatives: Antibacterial activity. Steroids. 2015;104:8–15. doi:10.1016/j.steroids.2015.07.007.
- Thao TDH, Ryu HC, Yoo SH, Rhee DK. Antibacterial and anti-atrophic effects of a highly soluble, acid stable UDCA formula in Helicobacter pylori-induced gastritis. Biochem Pharmacol. 2008;75(11):2135–2146. doi:10.1016/j.bcp.2008.03.008.
- Friedman ES, Li Y, Shen TCD, Jiang J, Chau L, Adorini L, Babakhani F, Edwards J, Shapiro D, Zhao CY. et al. FXR-Dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology. 2018;155(6):1741–1752.e1745. doi:10.1053/j.gastro.2018.08.022.
- Larabi AB, Masson HLP, Bäumler AJ. Bile acids as modulators of gut microbiota composition and function. Gut Microbes. 2023;15(1):2172671. doi:10.1080/19490976.2023.2172671.
- Sato Y, Atarashi K, Plichta DR, Arai Y, Sasajima S, Kearney SM, Suda W, Takeshita K, Sasaki T, Okamoto S. et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature. 2021;599(7885):458–464. doi:10.1038/s41586-021-03832-5.
- Kastl A, Zong WJ, Gershuni VM, Friedman ES, Tanes C, Boateng A, Mitchell WJ, O’Connor K, Bittinger K, Terry NA. et al. Dietary fiber-based regulation of bile salt hydrolase activity in the gut microbiota and its relevance to human disease. Gut Microbes. 2022;14(1):2083417. doi:10.1080/19490976.2022.2083417.
- Adhikari AA, Seegar TCM, Ficarro SB, McCurry MD, Ramachandran D, Yao LN, Chaudhari SN, Ndousse-Fetter S, Banks AS, Marto JA. et al. Development of a covalent inhibitor of gut bacterial bile salt hydrolases. Nat Chem Biol. 2020;16(3):318–326. doi:10.1038/s41589-020-0467-3.
- Xu FZ, Guo FF, Hu XJ, Lin J. Crystal structure of bile salt hydrolase from Lactobacillus salivarius. Acta Crystallogr Sec F, Struc Biol Commun. 2016;72:376–381. doi:10.1107/S2053230X16005707.
- Kumar RS, Brannigan JA, Prabhune AA, Pundle AV, Dodson GG, Dodson EJ, Suresh CG. Structural and functional analysis of a conjugated bile salt hydrolase from Bifidobacterium longum reveals an evolutionary relationship with penicillin V acylase. J Biol Chem. 2006;281(43):32516–32525. doi:10.1074/jbc.M604172200.
- Rossocha M, Schultz-Heienbrok R, von Moeller H, Coleman JP, Saenger W. Conjugated bile acid hydrolase is a tetrameric N-terminal thiol hydrolase with specific recognition of its cholyl but not of its tauryl product. Biochemistry. 2005;44(15):5739–5748. doi:10.1021/bi0473206.
- Madej T, Lanczycki CJ, Zhang D, Thiessen PA, Geer RC, Marchler-Bauer A, Bryant SH. MMDB and VAST+: tracking structural similarities between macromolecular complexes. Nucleic Acids Res. 2014;42(D1):D297–D303. doi:10.1093/nar/gkt1208.
- Jones BV, Begley M, Hill C, Gahan CGM, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA. 2008;105(36):13580–13585. doi:10.1073/pnas.0804437105.
- Bi J, Fang F, Lu S, Du G, Chen J. New insight into the catalytic properties of bile salt hydrolase. J Mol Catal B Enzym. 2013;96:46–51. doi:10.1016/j.molcatb.2013.06.010.
- Song ZW, Cai YY, Lao XZ, Wang X, Lin XX, Cui YY, Kalavagunta PK, Liao J, Jin L, Shang J. et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome. 2019;7(1):9. doi:10.1186/s40168-019-0628-3.
- Coleman JP, Hudson LL. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens. Appl Environ Microb. 1995;61(7):2514–2520. doi:10.1128/AEM.61.7.2514-2520.1995.
- Elkins CA, Moser SA, Savage DC. Genes encoding bile salt hydrolases and conjugated bile salt transporters in Lactobacillus johnsonii 100-100 and other Lactobacillus species. Microbiology. 2001;147:3403–3412. doi:10.1099/00221287-147-12-3403.
- Dussurget O, Cabanes D, Dehoux P, Lecuit M, Buchrieser C, Glaser P, Cossart P. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol Microbiol. 2002;45(4):1095–1106. doi:10.1046/j.1365-2958.2002.03080.x.
- Delpino MV, Marchesini MI, Estein SM, Comerci DJ, Cassataro J, Fossati CA, Baldi PC. A bile salt hydrolase of Brucella abortus contributes to the establishment of a successful infection through the oral route in mice. Infect Immun. 2007;75(1):299–305. doi:10.1128/IAI.00952-06.
- Kawamoto K, Horibe I, Uchida K. Purification and characterization of a new hydrolase for conjugated bile acids, chenodeoxycholyltaurine hydrolase, from bacteroides vulgatus. J Biochem. 1989;106(6):1049–1053. doi:10.1093/oxfordjournals.jbchem.a122962.
- Dean M, Cervellati C, Casanova E, Squerzanti M, Lanzara V, Medici A, de Laureto PP, Bergamini CM. Characterization of cholylglycine hydrolase from a bile-adapted strain of Xanthomonas maltophilia and its application for quantitative hydrolysis of conjugated bile salts. Appl Environ Microbiol. 2002;68(6):3126–3128. doi:10.1128/AEM.68.6.3126-3128.2002.
- Kim GB, Yi SH, Lee BH. Purification and characterization of three different types of bile salt hydrolases from Bifidobacterium strains. J Dairy Sci. 2004;87(2):258–266. doi:10.3168/jds.S0022-0302(04)73164-1.
- Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microb. 2000;66(3):1107–1113. doi:10.1128/aem.66.3.1107-1113.2000.
- Ridlon JM, Kang D-J, Hylemon PB. Isolation and characterization of a bile acid inducible 7alpha-dehydroxylating operon in Clostridium hylemonae TN271. Anaerobe. 2010;16(2):137–146. doi:10.1016/j.anaerobe.2009.05.004.
- Kitahara M, Takamine F, Imamura T, Benno Y. Assignment of Eubacterium sp. VPI 12708 and related strains with high bile acid 7alpha-dehydroxylating activity to Clostridium scindens and proposal of Clostridium hylemonae sp. nov. isolated from human faeces. Int J Syst Evol Microbiol. 2000;50(3):971–978. doi:10.1099/00207713-50-3-971.
- Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7(1):22–39. doi:10.1080/19490976.2015.1127483.
- Grimm C, Maser E, Möbus E, Klebe G, Reuter K, Ficner R. The crystal structure of 3alpha -hydroxysteroid dehydrogenase/carbonyl reductase from Comamonas testosteroni shows a novel oligomerization pattern within the short chain dehydrogenase/reductase family. J Biol Chem. 2000;275(52):41333–41339. doi:10.1074/jbc.M007559200.
- Couture J-F, Pereira De Jésus-Tran K, Roy A-M, Cantin L, Côté P-L, Legrand P, Luu-The V, Labrie F, Breton R. Comparison of crystal structures of human type 3 3α-hydroxysteroid dehydrogenase reveals an “induced-fit” mechanism and a conserved basic motif involved in the binding of androgen. Protein Sci. 2005;14(6):1485–1497. doi:10.1110/ps.051353205.
- Nahoum V, Gangloff A, Legrand P, Zhu D-W, Cantin L, Zhorov BS, Luu-The V, Labrie F, Breton R, Lin S-X. Structure of the human 3α-hydroxysteroid dehydrogenase type 3 in complex with testosterone and NADP at 1.25-Å resolution. J Biol Chem. 2001;276(45):42091–42098. doi:10.1074/jbc.M105610200.
- Nakamura S, Oda M, Kataoka S, Ueda S, Uchiyama S, Yoshida T, Kobayashi Y, Ohkubo T. Apo- and holo-structures of 3alpha-hydroxysteroid dehydrogenase from Pseudomonas sp. B-0831. Loop-helix transition induced by coenzyme binding. J Biol Chem. 2006;281(42):31876–31884. doi:10.1016/S0021-9258(19)84102-9.
- Hoog SS, Pawlowski JE, Alzari PM, Penning TM, Lewis M. Three-dimensional structure of rat liver 3 alpha-hydroxysteroid/dihydrodiol dehydrogenase: a member of the aldo-keto reductase superfamily. Proc Natl Acad Sci USA. 1994;91(7):2517–2521. doi:10.1073/pnas.91.7.2517.
- Zhang B, Hu X-J, Wang X-Q, Thériault J-F, Zhu D-W, Shang P, Labrie F, Lin S-X. Human 3α-hydroxysteroid dehydrogenase type 3: structural clues of 5α-DHT reverse binding and enzyme down-regulation decreasing MCF7 cell growth. Biochem J. 2016;473(8):1037–1046. doi:10.1042/BCJ20160083.
- Jin Y, Stayrook SE, Albert RH, Palackal NT, Penning TM, Lewis M. Crystal structure of human type III 3alpha-hydroxysteroid dehydrogenase/bile acid binding protein complexed with NADP(+) and ursodeoxycholate. Biochemistry. 2001;40(34):10161–10168. doi:10.1021/bi010919a.
- Chen M, Drury JE, Christianson DW, Penning TM. Conversion of human steroid 5β-reductase (AKR1D1) into 3β-hydroxysteroid dehydrogenase by single point mutation E120H: example of perfect enzyme engineering. J Biol Chem. 2012;287(20):16609–16622. doi:10.1074/jbc.M111.338780.
- Tanaka N, Nonaka T, Tanabe T, Yoshimoto T, Tsuru D, Mitsui Y. Crystal structures of the binary and ternary complexes of 7 alpha-hydroxysteroid dehydrogenase from Escherichia coli. Biochemistry. 1996;35(24):7715–7730. doi:10.1021/bi951904d.
- Lou D, Wang B, Tan J, Zhu L, Cen X, Ji Q, Wang Y. The three-dimensional structure of Clostridium absonum 7α-hydroxysteroid dehydrogenase: new insights into the conserved arginines for NADP(H) recognition. Sci Rep. 2016;6(1):22885. doi:10.1038/srep22885.
- Kim K-H, Lee CW, Pardhe BD, Hwang J, Do H, Lee YM, Lee JH, Oh T-J. Crystal structure of an apo 7α-hydroxysteroid dehydrogenase reveals key structural changes induced by substrate and co-factor binding. J Steroid Biochem Mol Biol. 2021;212:105945. doi:10.1016/j.jsbmb.2021.105945.
- Wang R, Wu J, Jin DK, Chen Y, Lv Z, Chen Q, Miao Q, Huo X, Wang F. Structure of NADP+-bound 7β-hydroxysteroid dehydrogenase reveals two cofactor-binding modes. Acta Crystallogr Sec F. 2017;73(5):246–252. doi:10.1107/S2053230X17004460.
- Savino S, Ferrandi EE, Forneris F, Rovida S, Riva S, Monti D, Mattevi A. Structural and biochemical insights into 7β-hydroxysteroid dehydrogenase stereoselectivity. Proteins Struct Funct Bioinf. 2016;84(6):859–865. doi:10.1002/prot.25036.
- Doden H, Sallam Lina A, Devendran S, Ly L, Doden G, Daniel Steven L, Alves João MP, Ridlon Jason M, Müller V. Metabolism of oxo-bile acids and characterization of recombinant 12α-hydroxysteroid dehydrogenases from bile acid 7α-Dehydroxylating human gut bacteria. Appl Environ Microb. 2018;84(10):e00235?00218. doi:10.1128/AEM.00235-18.
- Doden HL, Wolf PG, Gaskins HR, Anantharaman K, Alves JMP, Ridlon JM. Completion of the gut microbial epi-bile acid pathway. Gut Microbes. 2021;13(1):1907271. doi:10.1080/19490976.2021.1907271.
- Lee JW, Cowley ES, Wolf PG, Doden HL, Murai T, Caicedo KYO, Ly LK, Sun F, Takei H, Nittono H. et al. Formation of secondary allo-bile acids by novel enzymes from gut Firmicutes. Gut Microbes. 2022;14(1):2132903. doi:10.1080/19490976.2022.2132903.
- Han Y, Zhuang Q, Sun B, Lv W, Wang S, Xiao Q, Pang B, Zhou Y, Wang F, Chi P. et al. Crystal structure of steroid reductase SRD5A reveals conserved steroid reduction mechanism. Nat Commun. 2021;12(1):449. doi:10.1038/s41467-020-20675-2.
- Faucher F, Cantin L, Luu-The V, Labrie F, Breton R. Crystal structures of human Δ4-3-ketosteroid 5β-reductase (AKR1D1) reveal the presence of an alternative binding site responsible for substrate inhibition. Biochemistry. 2008;47(51):13537–13546. doi:10.1021/bi801276h.
- Drury JE, Di Costanzo L, Penning TM, Christianson DW. Inhibition of human steroid 5β-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex. J Biol Chem. 2009;284(30):19786–19790. doi:10.1074/jbc.C109.016931.
- Di Costanzo L, Drury JE, Christianson DW, Penning TM. Structure and catalytic mechanism of human steroid 5beta-reductase (AKR1D1). Mol Cell Endocrinol. 2009;301(1–2):191–198. doi:10.1016/j.mce.2008.09.013.
- Di Costanzo L, Drury JE, Penning TM, Christianson DW. Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J Biol Chem. 2008;283(24):16830–16839. doi:10.1074/jbc.M801778200.
- Edenharder R, Hammann R. Deoxycholic acid methyl ester — a novel bacterial metabolite of cholic acid. Syst Appl Microbiol. 1985;6(1):18–22. doi:10.1016/S0723-2020(85)80005-9.
- Tazuke Y, Matsuda K, Adachi K, Tsukada Y. Purification and properties of a novel sulfatase from Pseudomonas testosteroni that hydrolyzed 3 beta-hydroxy-5-cholenoic acid 3-sulfate. Biosci Biotechnol Biochem. 1998;62(9):1739–1744. doi:10.1271/bbb.62.1739.
- Quinn RA, Melnik AV, Vrbanac A, Fu T, Patras KA, Christy MP, Bodai Z, Belda-Ferre P, Tripathi A, Chung LK. et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature. 2020;579(7797):123–129. doi:10.1038/s41586-020-2047-9.
- Lucas LN, Barrett K, Kerby RL, Zhang Q, Cattaneo LE, Stevenson D, Rey FE, Amador-Noguez D, Manichanh C. Dominant bacterial phyla from the human gut show widespread ability to transform and conjugate bile acids. mSystems. 2021;6(4):e0080521. doi:10.1128/mSystems.00805-21.
- Huijghebaert SM, Hofmann AF. Influence of the amino acid moiety on deconjugation of bile acid amidates by cholylglycine hydrolase or human fecal cultures. J Lipid Res. 1988;27(7):742–752. doi:10.1016/S0022-2275(20)38791-5.
- Funabashi M, Grove TL, Wang M, Varma Y, McFadden ME, Brown LC, Guo C, Higginbottom S, Almo SC, Fischbach MA. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582(7813):566–570. doi:10.1038/s41586-020-2396-4.
- Jin WB, Li TT, Huo D, Qu SP, Li XV, Arifuzzaman M, Lima SF, Shi HQ, Wang AL, Putzel GG. et al. Genetic manipulation of gut microbes enables single-gene interrogation in a complex microbiome. Cell. 2022;185(3):547–562.e22. doi:10.1016/j.cell.2021.12.035.
- Devlin AS, Fischbach MA. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat Chem Biol. 2015;11(9):685–690. doi:10.1038/NCHEMBIO.1864.
- Harris SC, Devendran S, Méndez-García C, Mythen SM, Wright CL, Fields CJ, Hernandez AG, Cann I, Hylemon PB, Ridlon JM. Bile acid oxidation by Eggerthella lenta strains C592 and DSM 2243T. Gut Microbes. 2018;9(6):523–539. doi:10.1080/19490976.2018.1458180.
- Berr F, Kullak-Ublick GA, Paumgartner G, Munzing W, Hylemon PB. 7 alpha-dehydroxylating bacteria enhance deoxycholic acid input and cholesterol saturation of bile in patients with gallstones. Gastroenterology. 1996;111(6):1611–1620. doi:10.1016/S0016-5085(96)70024-0.
- Joint Center for Structural Genomics. RCSB PDB - 4LEH: crystal structure of a bile-acid 7-alpha dehydratase (CLOSCI_03134) from Clostridium scindens ATCC 35704 at 2.90 a resolution. 2013.
- Joint Center for Structural Genomics. RCSB PDB - 4L8O: crystal structure of a bile-acid 7-alpha dehydratase (CLOHYLEM_06634) from Clostridium hylemonae DSM 15053 at 2.20 a resolution. 2013.
- Joint Center for Structural Genomics. RCSB PDB - 4N3V: crystal structure of a bile-acid 7-alpha dehydratase (CLOHIR_00079) from Clostridium hiranonis DSM 13275 at 1.89 a resolution with product added. 2013.
- Joint Center for Structural Genomics (JCSG). PDB - 4L8P: crystal structure of a bile-acid 7-alpha dehydratase (CLOHIR_00079) from Clostridium hiranonis DSM 13275 at 1.60 a resolution. 2013.
- Bhowmik S, Chiu HP, Jones DH, Chiu HJ, Miller MD, Xu QP, Farr CL, Ridlon JM, Wells JE, Elsliger MA. et al. Structure and functional characterization of a bile acid 7 alpha dehydratase BaiE in secondary bile acid synthesis. Proteins. 2016;84(3):316–331. doi:10.1002/prot.24971.
- Mallonee DH, Adams JL, Hylemon PB. The bile acid-inducible baiB gene from Eubacterium sp. strain VPI 12708 encodes a bile acid-coenzyme a ligase. J Bacteriol. 1992;174(7):2065–2071. doi:10.1128/jb.174.7.2065-2071.1992.
- Dawson JA, Mallonee DH, Björkhem I, Hylemon PB. Expression and characterization of a C24 bile acid 7 alpha-dehydratase from Eubacterium sp. strain VPI 12708 in Escherichia coli. J Lipid Res. 1996;37(6):1258–1267. doi:10.1016/S0022-2275(20)39155-0.
- Bhowmik S, Jones DH, Chiu HP, Park IH, Chiu HJ, Axelrod HL, Farr CL, Tien HJ, Agarwalla S, Lesley SA. Structural and functional characterization of BaiA, an enzyme involved in secondary bile acid synthesis in human gut microbe. Proteins. 2014;82(2):216–229. doi:10.1002/prot.24353.
- Mallonee DH, Lijewski MA, Hylemon PB. Expression in Escherichia coli and characterization of a bile acid-inducible 3 alpha-hydroxysteroid dehydrogenase from Eubacterium sp. strain VPI 12708. Curr Microbiol. 1995;30(5):259–263. doi:10.1007/BF00295498.
- Kang DJ, Ridlon JM, Moore DR, Barnes S, Hylemon PB. Clostridium scindens baiCD and baiH genes encode stereo-specific 7 alpha/7 beta-hydroxy-3-oxo-Delta(4)-cholenoic acid oxidoreductases. Biochim Biophys Acta. 2008;1781(1–2):16–25. doi:10.1016/j.bbalip.2007.10.008.
- Ye HQ, Mallonee DH, Wells JE, Björkhem I, Hylemon PB. The bile acid-inducible baiF gene from Eubacterium sp. strain VPI 12708 encodes a bile acid-coenzyme a hydrolase. J Lipid Res. 1999;40(1):17–23. doi:10.1016/S0022-2275(20)33335-6.
- Harris SC, Devendran S, Alves JMP, Mythen SM, Hylemon PB, Ridlon JM. Identification of a gene encoding a flavoprotein involved in bile acid metabolism by the human gut bacterium clostridium scindens ATCC 35704. Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids. 2018;1863(3):276–283. doi:10.1016/j.bbalip.2017.12.001.
- Ye L, Guo J, R-S G. Chapter thirteen - environmental pollutants and hydroxysteroid dehydrogenases. Vitam Horm. 2014;94:349–390. doi:10.1016/B978-0-12-800095-3.00013-4.
- Doden HL, Ridlon JM. Microbial hydroxysteroid dehydrogenases: from alpha to omega. Microorganisms. 2021;9(3):469. doi:10.3390/microorganisms9030469.
- Bennett MJ, McKnight SL, Coleman JP. Cloning and characterization of the NAD-dependent 7 alpha-hydroxysteroid dehydrogenase from Bacteroides fragilis. Curr Microbiol. 2003;47(6):475–484. doi:10.1007/s00284-003-4079-4.
- Ji S, Pan Y, Zhu L, Tan J, Tang S, Yang Q, Zhang Z, Lou D, Wang B. A novel 7α-hydroxysteroid dehydrogenase: Magnesium ion significantly enhances its activity and thermostability. Int J Biol Macromol. 2021;177:111–118. doi:10.1016/j.ijbiomac.2021.02.082.
- Lu Q, Jiang Z, Wang Q, Hu H, Zhao G. The effect of Tauroursodeoxycholic acid (TUDCA) and gut microbiota on murine gallbladder stone formation. Ann Hepatol. 2021;23:100289. doi:10.1016/j.aohep.2020.100289.
- Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante S. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581(7809):475–479. doi:10.1038/s41586-020-2193-0.
- Paik D, Yao L, Zhang Y, Bae S, D’Agostino GD, Zhang M, Kim E, Franzosa EA, Avila-Pacheco J, Bisanz JE. et al. Human gut bacteria produce ΤΗ17-modulating bile acid metabolites. Nature. 2022;603(7903):907–912. doi:10.1038/s41586-022-04480-z.
- Li Q, Zhou S, Wang Y, Cong J. Changes of intestinal microbiota and microbiota-based treatments in IBD. Arch Microbiol. 2022;204(7):442. doi:10.1007/s00203-022-03069-4.
- Lou D, Wang B, Tan J, Zhu L. Carboxyl-terminal and Arg38 are essential for activity of the 7α-hydroxysteroid dehydrogenase from Clostridium absonum. Protein Pept Lett. 2014;21(9):894–900. doi:10.2174/0929866521666140507160050.
- Edenharder R, Schneider J. 12 beta-dehydrogenation of bile acids by Clostridium paraputrificum, C. tertium, and C. difficile and epimerization at carbon-12 of deoxycholic acid by cocultivation with 12 alpha-dehydrogenating Eubacterium lentum. Appl Environ Microbiol. 1985;49(4):964–968. doi:10.1128/aem.49.4.964-968.1985.
- Takei H, Narushima S, Suzuki M, Kakiyama G, Sasaki T, Murai T, Yamashiro Y, Nittono H. Characterization of long-chain fatty acid-linked bile acids: a major conjugation form of 3β-hydroxy bile acids in feces. J Lipid Res. 2022;63(10):100275. doi:10.1016/j.jlr.2022.100275.
- Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20(4):461–472. doi:10.1007/s11154-019-09512-0.
- Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. 2014;3(1):14–24. doi:10.3390/pathogens3010014.
- Alnouti Y. Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108(2):225–246. doi:10.1093/toxsci/kfn268.
- Guzior DV, Okros M, Shivel M, Armwald B, Bridges C, Fu Y, Martin C, Schilmiller AL, Miller WM, Ziegler KM. et al. Bile salt hydrolase acyltransferase activity expands bile acid diversity. Nature. 2024;626(8000):852–858. doi:10.1038/s41586-024-07017-8.
- Rimal B, Collins SL, Tanes CE, Rocha ER, Granda MA, Solanki S, Hoque NJ, Gentry EC, Koo I, Reilly ER. et al. Bile salt hydrolase catalyses formation of amine-conjugated bile acids. Nature. 2024;626(8000):859–863. doi:10.1038/s41586-023-06990-w.
- Xu XW, Ocansey DKW, Hang SH, Wang B, Amoah S, Yi CX, Zhang X, Liu LQ, Mao F. The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. 2022;14(1):26. doi:10.1186/s13099-022-00499-9.
- Li N, Zhan S, Tian Z, Liu C, Xie Z, Zhang S, Chen M, Zeng Z, Zhuang X. Alterations in bile acid metabolism associated with inflammatory bowel disease. Inflamm Bowel Dis. 2021;27(9):1525–1540. doi:10.1093/ibd/izaa342.
- Lenicek M, Duricova D, Komarek V, Gabrysova B, Lukas M, Smerhovsky Z, Vitek L. Bile acid malabsorption in inflammatory bowel disease: Assessment by serum markers. Inflamm Bowel Dis. 2011;17(6):1322–1327. doi:10.1002/ibd.21502.
- Battat R, Scherl EJ, Lukin D, Charilaou P, Mahtani P, Gerber J, Gandara JA, Dündar F, Zumbo P, Betel D. et al. Increased primary bile acids with ileocolonic resection impact ileal inflammation and gut microbiota in inflammatory bowel disease. J Crohns Colitis. 2023;17(5):795–803. doi:10.1093/ecco-jcc/jjac173.
- Chiang JYL. Bile acids: regulation of synthesis: thematic Review Series: Bile Acids. J Lipid Res. 2009;50(10):1955–1966. doi:10.1194/jlr.R900010-JLR200.
- Das P, Marcisauskas S, Ji B, Nielsen J. Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genomics. 2019;20(1):157. doi:10.1186/s12864-019-5899-3.
- Heinken A, Ravcheev DA, Baldini F, Heirendt L, Fleming RMT, Thiele I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019;7(1):75. doi:10.1186/s40168-019-0689-3.
- Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain E, Thomas G, Barbu V, Humbert L, Despras G. et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–539. doi:10.1136/gutjnl-2012-302578.
- Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi:10.1038/s41586-019-1237-9.
- Sinha SR, Haileselassie Y, Nguyen LP, Tropini C, Wang M, Becker LS, Sim D, Jarr K, Spear ET, Singh G. et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe. 2020;27(4):659–670.e5. doi:10.1016/j.chom.2020.01.021.
- Yang ZH, Liu F, Zhu XR, Suo FY, Jia ZJ, Yao SK. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J Gastroenterol. 2021;27(24):3609–3629. doi:10.3748/wjg.v27.i24.3609.
- Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, Vatanen T, Hall AB, Mallick H, McIver LJ. et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4(2):293–305. doi:10.1038/s41564-018-0306-4.
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci Usa. 2007;104(34):13780–13785. doi:10.1073/pnas.0706625104.
- Kruis W, Kalek HD, Stellaard F, Paumgartner G. Altered fecal bile acid pattern in patients with inflammatory bowel disease. Digestion. 1986;35(4):189–198. doi:10.1159/000199367.
- Connors J, Dunn KA, Allott J, Bandsma R, Rashid M, Otley AR, Bielawski JP, Van Limbergen J. The relationship between fecal bile acids and microbiome community structure in pediatric Crohn’s disease. Isme J. 2020;14(3):702–713. doi:10.1038/s41396-019-0560-3.
- Diederen K, Li JV, Donachie GE, de Meij TG, de Waart DR, Hakvoort TBM, Kindermann A, Wagner J, Auyeung V, Te Velde AA. et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci Rep. 2020;10(1):18879. doi:10.1038/s41598-020-75306-z.
- Murakami M, Iwamoto J, Honda A, Tsuji T, Tamamushi M, Ueda H, Monma T, Konishi N, Yara S, Hirayama T. et al. Detection of gut dysbiosis due to reduced clostridium subcluster XIVa using the fecal or serum bile acid profile. Inflamm Bowel Dis. 2018;24(5):1035–1044. doi:10.1093/ibd/izy022.
- Wang Y, Gao X, Zhang X, Xiao F, Hu H, Li X, Dong F, Sun M, Xiao Y, Ge T. et al. Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn’s disease. Gut Microbes. 2021;13(1):1865708. doi:10.1080/19490976.2020.1865708.
- Vaughn BP, Kaiser T, Staley C, Hamilton MJ, Reich J, Graiziger C, Singroy S, Kabage AJ, Sadowsky MJ, Khoruts A. A pilot study of fecal bile acid and microbiota profiles in inflammatory bowel disease and primary sclerosing cholangitis. Clin Exp Gastroenterol. 2019;12:9–19. doi:10.2147/CEG.S186097.
- Torres J, Palmela C, Brito H, Bao X, Ruiqi H, Moura-Santos P, da Silva JP, Oliveira A, Vieira C, Perez K. et al. The gut microbiota, bile acids and their correlation in primary sclerosing cholangitis associated with inflammatory bowel disease. United European Gastroenterol J. 2018;6(1):112–122. doi:10.1177/2050640617708953.
- Lavelle A, Nancey S, Reimund JM, Laharie D, Marteau P, Treton X, Allez M, Roblin X, Malamut G, Oeuvray C. et al. Fecal microbiota and bile acids in IBD patients undergoing screening for colorectal cancer. Gut Microbes. 2022;14(1):2078620. doi:10.1080/19490976.2022.2078620.
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi:10.1126/science.284.5418.1362.
- Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14(7):676–684. doi:10.1038/ni.2640.
- Iliev ID. Mycobiota-host immune interactions in IBD: coming out of the shadows. Nat Rev Gastro Hepat. 2022;19(2):91–92. doi:10.1038/s41575-021-00541-2.
- Wilson A, Wang Q, Almousa AA, Jansen LE, Choi Y-H, Schwarz UI, Kim RB. Genetic variation in the farnesoid X-receptor predicts Crohn’s disease severity in female patients. Sci Rep. 2020;10(1):11725. doi:10.1038/s41598-020-68686-9.
- Shin D-J, Wang L. Bile acid-activated receptors: a review on FXR and other nuclear receptors. Handb Exp Pharmacol. 2019;256:51–72. doi:10.1007/164_2019_236.
- Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen ECL, Renooij W, Murzilli S, Klomp LWJ, Siersema PD, Schipper MEI, Danese S. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. GUT. 2011;60(4):463–472. doi:10.1136/gut.2010.212159.
- Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183(10):6251–6261. doi:10.4049/jimmunol.0803978.
- Thibaut MM, Bindels LB. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol Med. 2022;28(3):223–236. doi:10.1016/j.molmed.2021.12.006.
- Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature. 2020;577(7790):410–415. doi:10.1038/s41586-019-1865-0.
- Massafra V, Ijssennagger N, Plantinga M, Milona A, Ramos Pittol JM, Boes M, van Mil SWC. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. Biochim Biophys Acta Mol Basis Dis. 2016;1862(2):166–173. doi:10.1016/j.bbadis.2015.11.001.
- Pan X, Zhu Q, Pan LL, Sun J. Macrophage immunometabolism in inflammatory bowel diseases: From pathogenesis to therapy. Pharmacology & Therapeutics. 2022;238:108176. doi:10.1016/j.pharmthera.2022.108176.
- Biagioli M, Carino A, Cipriani S, Francisci D, Marchianò S, Scarpelli P, Sorcini D, Zampella A, Fiorucci S. The bile acid receptor gpbar1 regulates the m1/m2 phenotype of intestinal macrophages and activation of gpbar1 rescues mice from murine colitis. J Immunol. 2017;199(2):718–733. doi:10.4049/jimmunol.1700183.
- Sorrentino G, Perino A, Yildiz E, El Alam G, Bou Sleiman M, Gioiello A, Pellicciari R, Schoonjans K. Bile acids signal via tgr5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology. 2020;159(3):956–968.e958. doi:10.1053/j.gastro.2020.05.067.
- Chen Y, Le TH, Du Q, Zhao Z, Liu Y, Zou J, Hua W, Liu C, Zhu Y. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int Immunopharmacol. 2019;71:144–154. doi:10.1016/j.intimp.2019.01.021.
- Guo CS, Xie SJ, Chi ZX, Zhang JH, Liu YY, Zhang L, Zheng MZ, Zhang X, Xia DJ, Ke YH. et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45(4):944. doi:10.1016/j.immuni.2016.10.009.
- Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576(7785):143–148. doi:10.1038/s41586-019-1785-z.
- Lee GR. The balance of Th17 versus treg cells in autoimmunity. Int J Mol Sci. 2018;19(3):730. doi:10.3390/ijms19030730.
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J. et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science. 2015;349(6251):993–997. doi:10.1126/science.aaa9420.
- Ishizawa M, Matsunawa M, Adachi R, Uno S, Ikeda K, Masuno H, Shimizu M, Iwasaki KI, Yamada S, Makishima M. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J Lipid Res. 2008;49(4):763–772. doi:10.1194/jlr.M700293-JLR200.
- Cantorna MT, Rogers CJ, Arora J. Aligning the paradoxical role of Vitamin D in gastrointestinal immunity. Trends In Endocrinol Metabolism. 2019;30(7):459–466. doi:10.1016/j.tem.2019.04.005.
- Cheng J, Fang ZZ, Kim JH, Krausz KW, Tanaka N, Chiang JYL, Gonzalez FJ. Intestinal CYP3A4 protects against lithocholic acid-induced hepatotoxicity in intestine-specific VDR-deficient mice. J Lipid Res. 2014;55(3):455–465. doi:10.1194/jlr.M044420.
- Pols TWH, Puchner T, Korkmaz HI, Vos M, Soeters MR, de Vries CJM, Rottenberg ME. Lithocholic acid controls adaptive immune responses by inhibition of Th1 activation through the Vitamin D receptor. PLOS ONE. 2017;12(5):e0176715. doi:10.1371/journal.pone.0176715.
- He L, Liu T, Shi Y, Tian F, Hu H, Deb DK, Chen Y, Bissonnette M, Li YC. Gut epithelial vitamin d receptor regulates microbiota-dependent mucosal inflammation by suppressing intestinal epithelial cell apoptosis. Endocrinology. 2018;159(2):967–979. doi:10.1210/en.2017-00748.
- Huang K, Mukherjee S, Desmarais V, Albanese JM, Rafti E, Draghi A, Maher LA, Khanna KM, Mani S, Matson AP. Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatr Res. 2018;83(5):1031–1040. doi:10.1038/pr.2018.14.
- Mencarelli A, Renga B, Palladino G, Claudio D, Ricci P, Distrutti E, Barbanti M, Baldelli F, Fiorucci S. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur J Pharmacol. 2011;668(1–2):317–324. doi:10.1016/j.ejphar.2011.06.058.
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-κB target gene expression. Am J Physiol-Gastr L. 2007;292(4):G1114–G1122. doi:10.1152/ajpgi.00528.2006.
- Little M, Dutta M, Li H, Matson A, Shi X, Mascarinas G, Molla B, Weigel K, Gu H, Mani S. et al. Understanding the physiological functions of the host xenobiotic-sensing nuclear receptors PXR and CAR on the gut microbiome using genetically modified mice. Acta Pharm Sin B. 2022;12(2):801–820. doi:10.1016/j.apsb.2021.07.022.
- Proctor LM, Creasy HH, Fettweis JM, Lloyd-Price J, Mahurkar A, Zhou W, Buck GA, Snyder MP, Strauss JF, Weinstock GM. et al. The integrative human microbiome project. Nature. 2019;569(7758):641–648. doi:10.1038/s41586-019-1238-8.
- He PD, Yu LL, Tian FW, Zhang H, Chen W, Zhai QX. Dietary patterns and gut microbiota: the crucial actors in inflammatory bowel disease. Adv Nutr. 2022;13(5):1628–1651. doi:10.1093/advances/nmac029.
- Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–184. doi:10.1080/19490976.2017.1290756.
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–573. doi:10.1038/ajg.2011.44.
- Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, Lee D, Bittinger K, Bailey A, Friedman ES, Hoffmann C. et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric Crohn’s disease. Cell Host Microbe. 2015;18(4):489–500. doi:10.1016/j.chom.2015.09.008.
- Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J. et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohn’s Colitis. 2014;8(10):1179–1207. doi:10.1016/j.crohns.2014.04.005.
- Fitzpatrick JA, Melton SL, Yao CK, Gibson PR, Halmos EP. Dietary management of adults with IBD - the emerging role of dietary therapy. Nat Rev Gastro Hepat. 2022;19(10):652–669. doi:10.1038/s41575-022-00619-5.
- Sanderson IR, Bustin SA, Dziennis S, Paraszczuk J, Stamm DS. Age and diet act through distinct isoforms of the class II transactivator gene in mouse intestinal epithelium. Gastroenterology. 2004;127(1):203–212. doi:10.1053/j.gastro.2004.04.014.
- Levine A, Wine E. Effects of enteral nutrition on Crohn’s PLOS ONEisease: Clues to the impact of diet on disease pathogenesis. Inflamm Bowel Dis. 2013;19(6):1322–1329. doi:10.1097/MIB.0b013e3182802acc.
- Leach ST, Mitchell HM, Eng WR, Zhang L, Day AS. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Aliment Pharmacol Ther. 2008;28(6):724–733. doi:10.1111/j.1365-2036.2008.03796.x.
- Ke J, Li Y, Han C, He R, Lin R, Qian W, Hou X. Fucose ameliorate intestinal inflammation through modulating the crosstalk between bile acids and gut microbiota in a chronic colitis murine model. Inflamm Bowel Dis. 2020;26(6):863–873. doi:10.1093/ibd/izaa007.
- Reddy BS, Engle A, Simi B, Goldman M. Effect of dietary fiber on colonic bacterial enzymes and bile acids in relation to colon cancer. Gastroenterology. 1992;102(5):1475–1482. doi:10.1016/0016-5085(92)91704-8.
- Makki K, Brolin H, Petersen N, Henricsson M, Christensen DP, Khan MT, Wahlström A, Bergh PO, Tremaroli V, Schoonjans K. et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut. 2023;72(2):314–324. doi:10.1136/gutjnl-2021-326541.
- Reuter MA, Tucker M, Marfori Z, Shishani R, Bustamante JM, Moreno R, Goodson ML, Ehrlich A, Taha AY, Lein PJ. et al. Dietary resistant starch supplementation increases gut luminal deoxycholic acid abundance in mice. Gut Microbes. 2024;16(1):2315632. doi:10.1080/19490976.2024.2315632.
- Kuang R, Binion DG. Should high-fiber diets be recommended for patients with inflammatory bowel disease? Curr Opin Gastroenterol. 2022;38(2):168–172. doi:10.1097/mog.0000000000000810.
- Limdi JK, Aggarwal D, McLaughlin JT. Dietary practices and beliefs in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(1):164–170. doi:10.1097/mib.0000000000000585.
- Cox SR, Prince AC, Myers CE, Irving PM, Lindsay JO, Lomer MC, Whelan K. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11(12):1420–1429. doi:10.1093/ecco-jcc/jjx073.
- Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A, Colombel JF, Grinspan A, Clemente JC, Merad M, Faith JJ. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology. 2018;154(4):1037–1046.e1032. doi:10.1053/j.gastro.2017.11.030.
- Bretin A, Zou J, San Yeoh B, Ngo VL, Winer S, Winer DA, Reddivari L, Pellizzon M, Walters WA, Patterson AD. et al. Psyllium fiber protects against colitis via activation of bile acid sensor farnesoid x receptor. Cell Mol Gastroenterol Hepatol. 2023;15(6):1421–1442. doi:10.1016/j.jcmgh.2023.02.007.
- Armstrong HK, Bording-Jorgensen M, Santer DM, Zhang Z, Valcheva R, Rieger AM, Sung-Ho Kim J, Dijk SI, Mahmood R, Ogungbola O. et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology. 2023;164(2):228–240. doi:10.1053/j.gastro.2022.09.034.
- Bonazzi E, Bretin A, Vigué L, Hao F, Patterson AD, Gewirtz AT, Chassaing B. Individualized microbiotas dictate the impact of dietary fiber on colitis sensitivity. Microbiome. 2024;12(1):5. doi:10.1186/s40168-023-01724-6.
- Jia YQ, Yuan ZW, Zhang XS, Dong JQ, Liu XN, Peng XT, Yao WL, Ji P, Wei YM, Hua YL. Total alkaloids of Sophora alopecuroides L. ameliorated murine colitis by regulating bile acid metabolism and gut microbiota. J Ethnopharmacol. 2020;255:112775. doi:10.1016/j.jep.2020.112775.
- Dong S, Zhu M, Wang K, Zhao X, Hu L, Jing W, Lu H, Wang S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol Res. 2021;171:105767. doi:10.1016/j.phrs.2021.105767.
- Yu ZT, Li DG, Sun HX. Herba origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. Biomed Pharmacother. 2023; 161. doi:10.1016/j.biopha.2023.114409.
- O’Mahony C, Amamou A, Ghosh S. Diet-microbiota interplay: an emerging player in macrophage plasticity and intestinal health. Int J Mol Sci. 2022;23(7):3901. doi:10.3390/ijms23073901.
- Serrano-Moreno C, Brox-Torrecilla N, Arhip L, Romero I, Morales A, Luisa Carrascal M, Cuerda C, Motilla M, Camblor M, Velasco C. et al. Diets for inflammatory bowel disease: What do we know so far? Eur J Clin Nutr. 2022;76(9):1222–1233. doi:10.1038/s41430-021-01051-9.
- Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastro Hepat. 2010;7(9):503–514. doi:10.1038/nrgastro.2010.117.
- Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16(10):605–616. doi:10.1038/s41575-019-0173-3.
- Wang Y, Guo Y, Chen H, Wei H, Wan C. Potential of Lactobacillus plantarum ZDY2013 and Bifidobacterium bifidum WBIN03 in relieving colitis by gut microbiota, immune, and anti-oxidative stress. Can J Microbiol. 2018;64(5):327–337. doi:10.1139/cjm-2017-0716.
- Xiao F, Dong F, Li X, Li Y, Yu G, Liu Z, Wang Y, Zhang T. Bifidobacterium longum CECT 7894 improves the efficacy of infliximab for DSS-Induced colitis via regulating the gut microbiota and bile acid metabolism. Front Pharmacol. 2022;13:902337. doi:10.3389/fphar.2022.902337.
- Wong WY, Chan BD, Sham TT, Lee MML, Chan CO, Chau CT, Mok DKW, Kwan YW, Tai WCS. Lactobacillus casei strain shirota ameliorates dextran sulfate sodium-induced colitis in mice by increasing taurine-conjugated bile acids and inhibiting NF-kappa B signaling via stabilization of I kappa B alpha. Front Nutr. 2022;9:816836. doi:10.3389/fnut.2022.816836.
- Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm MA, Weismueller J, Beglinger C, Stolte M. et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. GUT. 2004;53(11):1617–1623. doi:10.1136/gut.2003.037747.
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA. et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105(10):2218–2227. doi:10.1038/ajg.2010.218.
- Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104(2):437–443. doi:10.1038/ajg.2008.118.
- van der Lelie D, Oka A, Taghavi S, Umeno J, Fan T-J, Merrell KE, Watson SD, Ouellette L, Liu B, Awoniyi M. et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat Commun. 2021;12(1):3105. doi:10.1038/s41467-021-23460-x.
- Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 2022;106(2):505–521. doi:10.1007/s00253-021-11646-8.
- Scott KP, Martin JC, Duncan SH, Flint HJ. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87(1):30–40. doi:10.1111/1574-6941.12186.
- Alexander C, Cross T-W, Devendran S, Neumer F, Theis S, Ridlon JM, Suchodolski JS, De Godoy MRC, Swanson KS, de Godoy MRC. Effects of prebiotic inulin-type fructans on blood metabolite and hormone concentrations and faecal microbiota and metabolites in overweight dogs. Br J Nutr. 2018;120(6):711–720. doi:10.1017/S0007114518001952.
- Li Z, Li Z, Zhu L, Dai N, Sun G, Peng L, Wang X, Yang Y. Effects of xylo-oligosaccharide on the gut microbiota of patients with ulcerative colitis in clinical remission. Front Nutr. 2021;8:778542. doi:10.3389/fnut.2021.778542.
- He L, Zhang F, Jian Z, Sun J, Chen J, Liapao V, He Q. Stachyose modulates gut microbiota and alleviates dextran sulfate sodium-induced acute colitis in mice. Saudi J Gastroenterol: Off J Saudi Gastroenterol Assoc. 2020;26(3):153–159. doi:10.4103/sjg.SJG_580_19.
- Valcheva R, Koleva P, Martínez I, Walter J, Gänzle MG, Dieleman LA. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes. 2019;10(3):334–357. doi:10.1080/19490976.2018.1526583.
- Kelly CR, Yen EF, Grinspan AM, Kahn SA, Atreja A, Lewis JD, Moore TA, Rubin DT, Kim AM, Serra S. et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT national registry. Gastroenterology. 2021;160(1):183–192.e183. doi:10.1053/j.gastro.2020.09.038.
- Verdier C, Denis S, Gasc C, Boucinha L, Uriot O, Delmas D, Dore J, Le Camus C, Schwintner C, Blanquet-Diot S. An oral FMT capsule as efficient as an enema for microbiota reconstruction following disruption by antibiotics, as assessed in an in vitro human gut model. Microorganisms. 2021;9(2):358. doi:10.3390/microorganisms9020358.
- Mullish BH, McDonald JAK, Pechlivanis A, Allegretti JR, Kao D, Barker GF, Kapila D, Petrof EO, Joyce SA, Gahan CGM. et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. GUT. 2019;68(10):1791–1800. doi:10.1136/gutjnl-2018-317842.
- Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149(1):102–109.e106. doi:10.1053/j.gastro.2015.04.001.
- Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, Paramsothy R, Walsh AJ, van den Bogaerde J, Samuel D. et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019;156(5):1440–1454.e1442. doi:10.1053/j.gastro.2018.12.001.
- Haifer C, Paramsothy S, Kaakoush NO, Saikal A, Ghaly S, Yang T, Luu LDW, Borody TJ, Leong RW. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2022;7(2):141–151. doi:10.1016/s2468-1253(21)00400-3.
- Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, Bourrier A, Le Gall G, Lalande V, De Rougemont A. et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome. 2020;8(1):12. doi:10.1186/s40168-020-0792-5.
- Maharshak N, Ringel Y, Katibian D, Lundqvist A, Sartor RB, Carroll IM, Ringel-Kulka T. Fecal and mucosa-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Dig Dis Sci. 2018;63(7):1890–1899. doi:10.1007/s10620-018-5086-4.
- Keely SJ, Steer CJ, Lajczak-McGinley NK. Ursodeoxycholic acid: a promising therapeutic target for inflammatory bowel diseases? Am J Physiol Gastrointest Liver Physiol. 2019;317(6):G872–G881. doi:10.1152/ajpgi.00163.2019.
- Van den Bossche L, Hindryckx P, Devisscher L, Devriese S, Van Welden S, Holvoet T, Vilchez-Vargas R, Vital M, Pieper Dietmar H, Vanden Bussche J. et al. Ursodeoxycholic acid and its taurine- or glycine-conjugated species reduce colitogenic dysbiosis and equally suppress experimental colitis in mice. Appl Environ Microb. 2017;83(7):e02766–02716. doi:10.1128/AEM.02766-16.
- He Q, Wu J, Ke J, Zhang Q, Zeng W, Luo Z, Gong J, Chen Y, He Z, Lan P. Therapeutic role of ursodeoxycholic acid in colitis-associated cancer via gut microbiota modulation. Mol Ther. 2023;31(2):585–598. doi:10.1016/j.ymthe.2022.10.014.
- Pearson T, Caporaso JG, Yellowhair M, Bokulich NA, Padi M, Roe DJ, Wertheim BC, Linhart M, Martinez JA, Bilagody C. et al. Effects of ursodeoxycholic acid on the gut microbiome and colorectal adenoma development. Cancer Med. 2019;8(2):617–628. doi:10.1002/cam4.1965.
- Van den Bossche L, Borsboom D, Devriese S, Van Welden S, Holvoet T, Devisscher L, Hindryckx P, De Vos M, Laukens D. Tauroursodeoxycholic acid protects bile acid homeostasis under inflammatory conditions and dampens Crohn’s disease-like ileitis. Lab Invest. 2017;97(5):519–529. doi:10.1038/labinvest.2017.6.
- Martínez-Moya P, Romero-Calvo I, Requena P, Hernández-Chirlaque C, Aranda CJ, González R, Zarzuelo A, Suárez MD, Martínez-Augustin O, Marín JJG. et al. Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis. Int Immunopharmacol. 2013;15(2):372–380. doi:10.1016/j.intimp.2012.11.017.
- Eaton JE, Silveira MG, Pardi DS, Sinakos E, Kowdley KV, Luketic VAC, Harrison EM, McCashland T, Befeler AS, Harnois D. et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106(9):1638–1645. doi:10.1038/ajg.2011.156.
- Jia BL, Park D, Hahn Y, Jeon CO. Metagenomic analysis of the human microbiome reveals the association between the abundance of gut bile salt hydrolases and host health. Gut Microbes. 2020;11(5):1300–1313. doi:10.1080/19490976.2020.1748261.
- Parasar B, Zhou H, Xiao XY, Shi QOJ, Brito IL, Chang PV. Chemoproteomic profiling of gut microbiota-associated bile salt hydrolase activity. ACS Cent Sci. 2019;5(5):867–873. doi:10.1021/acscentsci.9b00147.
- Smith K, Zeng XM, Lin J, Zhou H. Discovery of bile salt hydrolase inhibitors using an efficient high-throughput screening system. PLoS One. 2014;9(1):e85344. doi:10.1371/journal.pone.0085344.
- Adhikari AA, Ramachandran D, Chaudhari SN, Powell CE, Li W, McCurry MD, Banks AS, Devlin AS. A gut-restricted lithocholic acid analog as an inhibitor of gut bacterial bile salt hydrolases. ACS Chem Biol. 2021;16(8):1401–1412. doi:10.1021/acschembio.1c00192.
- Su Y, Zhu Q, Hong X, R-S G. Taxifolin inhibits neurosteroidogenic rat steroid 5α-reductase 1 and 3α-hydroxysteroid dehydrogenase. Pharmacology. 2019;105(7–8):397–404. doi:10.1159/000504057.
- Binli F, İnan İ, Büyükbudak F, Gram A, Kaya D, Liman N, Aslan S, Fındık M, Ay SS. The efficacy of a 3β-hydroxysteroid dehydrogenase inhibitor for the termination of mid-term pregnancies in dogs. Animals. 2022;12(18):2475. doi:10.3390/ani12182475.
- Shen H, Aggarwal N, Wun KS, Lee YS, Hwang IY, Chang MW. Engineered microbial systems for advanced drug delivery. Adv Drug Deliv Rev. 2022;187:114364. doi:10.1016/j.addr.2022.114364.
- Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4(6):754–759. doi:10.1016/j.cgh.2006.03.028.
- Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, Van Huysse J, Demetter P, Steidler L, Remaut E. et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3(1):49–56. doi:10.1038/mi.2009.116.
- Praveschotinunt P, Duraj-Thatte AM, Gelfat I, Bahl F, Chou DB, Joshi NS. Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut. Nat Commun. 2019;10(1):5580. doi:10.1038/s41467-019-13336-6.
- Gadaleta RM, Cariello M, Crudele L, Moschetta A. Bile salt hydrolase-competent probiotics in the management of IBD: Unlocking the “Bile Acid Code”. Nutrients. 2022;14(15):3212. doi:10.3390/nu14153212.
- Streidl T, Karkossa I, Segura Muñoz RR, Eberl C, Zaufel A, Plagge J, Schmaltz R, Schubert K, Basic M, Schneider KM. et al. The gut bacterium Extibacter muris produces secondary bile acids and influences liver physiology in gnotobiotic mice. Gut Microbes. 2021;13(1):1–21. doi:10.1080/19490976.2020.1854008.
- Kim KH, Park D, Jia B, Baek JH, Hahn Y, Jeon CO, Chu H. Identification and characterization of major bile acid 7α-dehydroxylating bacteria in the human gut. mSystems. 2022;7(4):e0045522. doi:10.1128/msystems.00455-22.
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A. et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517(7533):205–208. doi:10.1038/nature13828.
- Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190(7):2505–2512. doi:10.1128/jb.01765-07.
- Koh E, Hwang IY, Lee HL, De Sotto R, Lee JWJ, Lee YS, March JC, Chang MW. Engineering probiotics to inhibit Clostridioides difficile infection by dynamic regulation of intestinal metabolism. Nat Commun. 2022;13(1):3834. doi:10.1038/s41467-022-31334-z.