Abstract
The purpose of this study was to examine the effect of amygdalin on cell growth and telomerase activity in human cancer and MRC-5 fibroblast cell lines. The level of β-glucosidase activity for releasing cyanide was significantly (P < .05) higher in cancer cell lines (A-549, MDA-MB-231, MCF-7 and U87-MG) than in MRC-5 fibroblasts. Growth rate of cancer cells was apparently inhibited in concentrations above 10 mg/ml amygdalin with senescent-like abnormal morphology. Whereas the effects were absent or marginally detected in MRC-5 fibroblasts. High incidence of β-galactosidase activity was observed in amygdalin-treated cancer cells, compared with that of untreated control while no difference was observed between the control and amygdalin-treated MRC-5 fibroblasts. Furthermore, level of telomerase activity was significantly (P < .05) higher (∼8–13 fold) in cancer cell lines along with high expression of telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC) than in MRC-5 fibroblasts which did not expressed TERT and TERC. However, telomerase activity was significantly (P < .05) down-regulated in amygdalin-treated cancer cells with the decreased expression of TERT and TERC compared with control cancer cells. There were no difference in the telomerase activity between control and amygdalin-treated MRC-5 fibroblasts. Based on these observations, we concluded that amygdalin treatment offers a valuable option for the cancer treatment, causing inhibition of cell growth and down-regulation of telomerase activity in human cancer cell lines by increasing β-glucosidase activity.
Introduction
Cancer, known as a malignant tumor, is characterized by uncontrolled cell growth with the potential to invade into other tissues of the body. Along with chemotherapy, radiotherapy and surgery, immunotherapy also holds a promising approach for cancer therapy; however, development of an effective and efficient method is one of the most interesting subject in the modern research area (Nazarkina & Laktionov Citation2015). Amygdalin, sometimes known as laetrile or vitamin B17, is one of the natural cyanogenic diglucoside existing in the fruits and nuts, such as cherries, apricots kernels, apples and almonds (Holzbecher et al. Citation1984; Santos Pimenta et al. Citation2014). It has been previously reported that amygdalin treatment in human cancer cells exerts a potential cytotoxic and anti-cancer effects by inducing apoptotic cell death (Syrigos et al. Citation1998; Chang et al. Citation2006; Chen et al. Citation2013), arrest cell cycle through down-regulation of cell cycle-related genes (Park et al. Citation2005; Makarević et al. Citation2014a) and blocking metastasis that can lead to the spread of cancer to other tissues (Makarević et al. Citation2014b). Primarily, cancer has been treated by using conventional therapies; however, amygdalin is also used as an alternative medicine and was found one of the most useful and effective alternative in cancer therapy (Milazzo et al. Citation2007; Sauer et al. Citation2015).
The cytotoxic effects of amygdalin against cancer cells are related with the release of hydrogen cyanide (HCN), which is an antitumor compound, decomposing carcinogenic substances in the body, inhibiting cancer growth and blocking nutrient source of tumor cells (Syrigos et al. Citation1998; Chang et al. Citation2006). The release of HCN from amygdalin glycoside is also related with activity of β-glucosidase enzyme that catalyzes the hydrolysis of covalent bonds linking two glucose or glucose-substituted molecules (Syrigos et al. Citation1998). Therefore, level of β-glucosidase expressed in cancer cells against amygdalin should be considered as a very important factor that has an influence on cell viability or cytotoxicity. However, the β-glucosidase activity in cancer and normal somatic cells has not been fully investigated.
The end of each eukaryotic chromosome, known as telomere, consists of repetitive DNA sequences, GGTTAGn. The telomeric repeats in normal cells are gradually shortened by the incomplete replication problem that occurs at the end of each chromosome along with each cell division. Meanwhile, the length of telomeric repeats in the embryonic stem cells and cancer cells (∼90%) was continuously maintained or extended by up-regulated telomerase activity, which adds up repetitive DNA sequences at the end of each eukaryotic chromosome as compared with normal somatic cells (Campisi & Yaswen Citation2009; Artandi & DePinho Citation2010). The cells with fully shorted telomere subsequently lead to DNA damage and enter into the senescent status with irreversible cell arrest at the G1 phase of the cell cycle and apoptotic cell death (Hwang et al. Citation2009; Rudolph et al. Citation2009; Jeon et al. Citation2011a). It is well known that the telomerase activity is composed of telomerase reverse transcriptase (TERT) catalytic subunit, RNA-dependent polymerases and telomerase RNA component (TERC), and is mainly regulated by expression of TERT and TERC transcripts (Toouli et al. Citation2002; Cao et al. Citation2008). The important feature that distinguishes a cancer cell from a normal cell is its ability to grow indefinitely by high level of telomerase activity, so by reducing telomerase activity and shortening telomeric repeats should be one of the important factors in cancer treatment. Moreover, telomere-targeting medicines have been successfully applied for cancer chemotherapy (Phatak et al. Citation2008; Jeon et al. Citation2011b).
In normal somatic and many cancer cell lines, the cytotoxic effect of amygdalin on cell growth and telomerase activity which is linked with β-glucosidase activity is not yet fully investigated. The fundamental molecular mechanisms which underlie the interaction of amygdalin with the cancer and somatic cells are not fully understood, although the growth pattern with senescence-associated-β-galactosidase activity and level of telomerase activity along with TERT and TERC expression have been considered as general markers for cellular aging or senescence in cancer cells. Therefore, in the present study, we evaluated the β-glucosidase activity in human cancer and normal cell lines, and then the pattern of cell growth with senescence-associated-β-galactosidase activity was subsequently analyzed in the human cancer and normal cell lines treated with amygdalin. Furthermore, we examined the level of telomerase activity along with expression of TERT and TERC genes.
Materials and methods
Chemicals and media
All chemicals were purchased from Sigma (Sigma, USA) and media from Gibco (Invitrogen), unless otherwise specified. For all the media, the pH was adjusted to 7.4 and the osmolality to 280 mOsm/kg.
Culture and treatment of cells
A-549 lung adenocarcinoma, MDA-MB-231, MCF-7 breast adenocarcinoma, U87-MG brain glioblastoma and MRC-5 normal fetal lung fibroblasts were purchased from the American Type Culture Collection (USA). The cells were maintained in advanced Dulbecco's modified Eagle's medium (A-DMEM) supplemented with 3% fetal bovine serum (FBS) and 1.0% penicillin–streptomycin (10,000 IU and 10,000 μg/ml, respectively) at 37.5°C in a humidified atmosphere of 5% CO2 in air and sub-cultured upon confluence (70–80%), while culture media were changed twice a week. For the analysis of growth curve, cancer and normal cells (1 × 103/per well) were seeded in A-DMEM containing 0 (control), 2.5, 5.0, 10, 20 and 30 mg/ml amygdalin (d-mandelonitrile-β-gentiobioside) into a 6-well plate for 7 days with change of media every 3 days after treatment. The cells were counted with a hemocytometer at 3 and 7 days interval after treatment. Furthermore, cancer and normal cells were treated in A-DMEM containing 10 mg/ml amygdalin for 7 days. On confluent, cells were dissociated using 0.25% trypsin–EDTA solution and pelleted at 500×g for 5 min. Cells were cryopreserved or re-propagated for further analysis.
Analysis of β-glucosidase activity
The β-glucosidase activity was analyzed in cancer and normal cells using QuantiChrom™. β-Glucosidase Assay Kit (BioAssay Systems, USA) was according to the manufacturer's protocols with minor modifications. Briefly, the cells were lysed by repeated cycles of freezing and thawing in PBS and then centrifuged at 12,000×g for 20 min at 4°C. The protein concentration was determined using a spectrophotometer (Mecasys, Korea) and 10 µg of total protein was analyzed for β-glucosidase activity using a 96 well plate. The working solution was freshly prepared by mixing 200 μl assay buffer and 8 μl p-NPG substrate. Furthermore, 200 μl of working solution and 20 μl of sample (10 µg proteins) was added to each well, and the plate was slightly tapped to mix the samples briefly. The optical density was measured using an ELISA reader (Bio-Tek, USA) with a wavelength of 405 nm immediately and 12 h later, respectively. The activity of β-glucosidase was assessed as unit per 10 μg of protein in three replicates.
Analysis of senescence-associated β-galactosidase
The senescence-associated β-galactosidase assay was performed in cancer and normal cells treated with 0 (control) and 10 mg/ml amygdalin using the cell senescence assay kit (Cell Signaling Technology, USA) according to the manufacturer's protocols. Briefly, cells were washed in PBS, fixed in 0.2% glutaraldehyde for 15 min at room temperature. The cells were then incubated with senescence-associated β-galactosidase staining solution at 37°C for overnight. The stained cells were observed under an inverted microscope (Nikon, Japan) equipped with a CCD camera.
Analysis of telomerase activity by relative-quantitative telomerase repeat amplification protocol (RQ-TRAP)
The RQ-TRAP assay was modified from a conventional TRAP assay for its use on the LightCycler 3.0 (Roche, Germany), as previously described by Betts et al. (Citation2006) and Jeon et al. (Citation2011a). Briefly, 1 × 105 cells were lysed in 400 µl of 0.5% (v/v) 1·3-[(3-cholamidopropyl) dimethylam-monio] propanesulfonic acid (CHAPS) lysis buffer (pH 7.5) supplemented with 10 mM Tris–HCl, 1 mM MgCl2, 1 mM EGTA, 0.1 mM benzamidine, 5 mM 2-mercaptoethanol and 10% glycerol for 30 min on ice. Following lysis, samples were centrifuged for 20 min at 12,000×g at 4°C. Protein concentration was measured by a spectrophotometer (Mecasys, Korea) and 5 µg of total proteins were used for RQ-TRAP. The RQ-TRAP was optimized using the PCR reagent LightCycler FastStart DNA Master SYBR Green 1 (Roche, Germany) according to the manufacturer's protocol, containing 2.5 mM MgCl2, 0.02 µg of primer TS (5′-AAT CCG TCG GAG CAG AGT T-3′), 0.04 µg of primer ACX (5′-GCG CGG CTT ACC CTT ACC CTT ACC CTA ACC-3′), total volume was adjusted up to 20 µl with sterile H2O. The program consisted of 30 min incubation at 30°C, followed by a denaturing cycle of 10 min at 94°C, and 40 cycles of PCR (94°C for 30 s and 60°C for 90 s). All samples were quantified using the LightCycler Quantification Software's (Roche, Germany) second derivative method of crossing point (Cp) determination, and the telomerase activity of control MRC-5 fibroblasts was considered as 100% for comparison with the treatment groups in five replicates.
Analysis of transcripts by reverse transcription-polymerase chain reaction (RT-PCR)
To analyze expression level of TERT and TERC transcripts, total RNA was isolated as per the protocol provided in the RNeasy Micro kit (Qiagen, GmbH, Hilden, Germany) with on-column DNase I treatment. The concentration of total RNA was determined by a spectrophotometer (Mecasys, Korea). cDNA was synthesized using Omniscript reverse transcription kit (Qiagen) with 1 µg total RNA and each of cDNA reactions contained 2 µl of 10 µM random hexamer (Invitrogen), 1 µl of 10 U/µl RNase Inhibitor (Invitrogen), 2 µl RT buffer, 2 µl dNTP, and 1 µl Omniscript (Qiagen, USA), adjusted to a total volume of 20 µl. The cDNA samples were then incubated in a thermal cycler (TaKaRa, Japan) at 42°C for 1 h, followed by 5 min at 95°C to inactivate the enzyme. A total of three reverse transcription reactions were used for each RNA sample. The PCR amplification was carried out in an thermal cycler (TaKaRa, Japan) using the Maxime-PCR PreMix Kit (iNtRON Biotechnology, Korea) in 30 cycles with each cycle consisting of an initial denaturation step at 94°C for 30 s, annealing step at 58–60°C for 30 s and elongation step at 72°C for 90 s and 10 min final extension at 72°C. The PCR products were fractionated by 1% agarose gel electrophoresis. For each sample of cDNA, three replicates of PCRs were carried out. The primers used in the study were for reference genes, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), sense: GAAGGTGAAGGTCGGAGTC, antisense: GAAGATGGTGATGGGATTTC; for telomerase reverse transcriptase (TERT), sense: CGGAAGAGTGTCTGGAGCAA, antisense: GGATGAAGCGGAGTCTGGA; for telomerase RNA component (TERC), sense: TCTAACCCTAACTGAGAAGGGCGTAG, antisense: GTTTGCTCTAGAATGAACGGTGGAAG, and the length of PCR products was 228, 198 and 126 bp, respectively. The relative quantification of transcripts was calculated to ratio based on the level of GAPDH in each cDNA samples using a Gelviewer image processing software (Innogene, Korea).
Statistical analysis
Differences among the cell groups were analyzed by using one-way analysis of variance. Differences in the β-glucosidase, telomerase activity and transcript level of genes were analyzed using a Student's t-test. Significance was set at P < .05.
Results
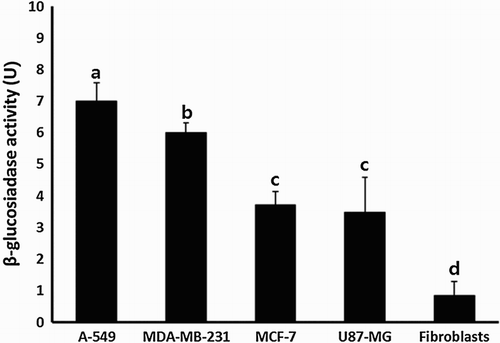
Activity of β-glucosidase in cancer and normal cells
The β-glucosidase activity was assessed as unit per 10 μg of protein derived from cancer and normal cell lines. The activity was 7.0 ± 0.57, 5.6 ± 0.30, 3.7 ± 0.41, 3.5 ± 1.11 and 0.8 ± 0.44 Unit/10 ug in A-549, MDA-MB-231, MCF-7, U87-MG and MRC-5 fibroblasts, respectively. The activity of β-glucosidase in all of cancer cell lines was significantly (P < .05) higher than in MRC-5 normal fibroblasts (). Furthermore, the activity level tended to be interestingly increased in A-549 and MDA-MB-231 cancer cell lines with high proliferation capacity (), compared with those in MCF-7 and U87-MG cancer cell lines.
Figure 1. β-Glucosidase activity in A-549, MDA-MB-231, MCF-7 and U87-MG cancer cell lines and MRC-5 normal fibroblasts. Values indicated unit per 10 µg of protein extract and the mean β-glucosidase activity (mean ± SEM) of three replicates. a, b, c and d indicate significant (P < .05) difference among each cell lines.
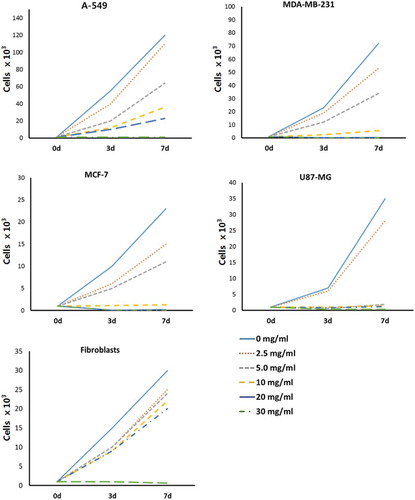
Growth curve in cell lines treated with amygdalin
To examine proliferation capacity, the cell growth curve was analyzed in cell lines treated with 0 (control), 2.5, 5, 10, 20, 30 mg/ml. After 7 days of culturing the cells (1 × 103 cells), high proliferation capacity was observed in A-549 and MDA-MB-231 cancer cell lines. However, the proliferation capacity of MCF-7 and U87-MG cancer cell lines was almost similar to MRC-fibroblasts, as shown in . The average cell number in 0, 10 and 30 mg/ml amygdaline-treated A-549 and MDA-MB-231 cancer cells was 1.2 × 105 and 7.2 × 104; 3.6 × 104 and 5.5 × 103; 9.1 × 102 and 1.9 × 102 cells, respectively. In 0, 10 and 30 mg/ml amygdaline-treated MCF-7 and U87-MG cancer cells, the average of cell number was 2.3 × 104 and 3.5 × 104; 1.3 × 103 and 1.4 × 103; 8.6 × 101 and 3.1 × 102 cells, respectively. The cell growth in most of cancer cell lines was efficiently inhibited at 10 mg/ml concentration of amygdalin. Whereas the average of cell number in 0, 10 and 30 mg/ml amygdaline-treated MRC-5 fibroblasts was 3.0 × 105, 2.3 × 105 and 6.5 × 102 cells, respectively. Moreover, the cell growth was considerably low in all cell lines at 30 mg/ml of amygdalin.
The morphological changes in control and 10 mg/ml amygdalin-treated cells were observed under a phase contrast microscope (). The morphology of amygdalin-treated cell became enlarged and star shaped as compared with the control untreated cancer cells, indicating an increase in cellular senescence. However, there was no change observed in the morphology of control and 10 mg/ml amygdalin-treated MRC-5 fibroblasts ().
Figure 3. Changes of cell morphology and senescence-associated-β-galactosidase activity in A-549, MDA-MB-231, MCF-7 and U87-MG cancer cell lines, and MRC-5 normal fibroblasts treated with 0 (control) and 10 mg/ml amygdalin for 7 days, respectively ( × 100). Cell alterations with enlarged size and star-like shape were observed in 10 mg/ml amygdalin-treated cancer cell lines, but morphological changes were not observed in MRC-5 fibroblasts. And 10 mg/ml amygdalin-treated cancer cell lines displayed a higher expression of senescence-associated-β-galactosidase (blue) than that of 10 mg/ml amygdalin-treated MRC-5 fibroblasts. Scale bars: 50 μm.
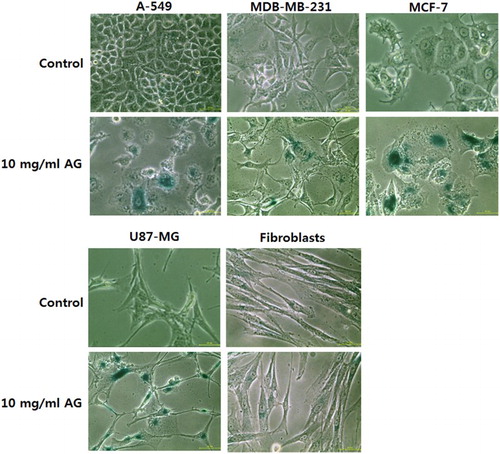
Activity of senescence-associated-β-galactosidase in cancer and normal cells
The activity of senescence-associated-β-galactosidase was analyzed in the control and 10 mg/ml amygdalin-treated cell lines, as shown in . The frequency of cells with senescence-associated-β-galactosidase activity was higher in control MRC-5 fibroblasts, compared with control cancer cell lines; however, the frequency in amygdalin-treated MRC-5 fibroblasts was similar to control MRC-5 fibroblasts. Whereas the frequency of cells with β-galactosidase activity was apparently higher in amygdalin-treated cancer cells, compared with that of control cancer cells, implying that cancer cells reached the senescent status more quickly than MRC-5 fibroblasts after amygdalin treatment.
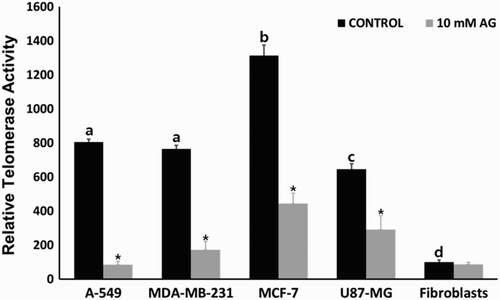
Telomerase activity in cancer and normal cells
The RQ-TRAP assay was conducted to analyze the telomerase activity in control and amygdalin- (10 mg/ml) treated cancer and normal cells. The telomerase activity in control MRC-5 fibroblasts was considered as 100% for comparison with other cell lines. Telomere activity was 805 ± 12.1%, 764 ± 23.3%, 1313 ± 62.6%, 645 ± 32.1% and 100 ± 12.3% in control A-549, MDA-MB-231, MCF-7, U87-MG and MRC-5 fibroblasts, respectively (). Telomerase activity in cancer cells was significantly (P < .05) higher than that in normal MRC-5 fibroblasts, reaching the levels almost ∼8–13 fold that of MRC-5 fibroblasts. However, telomerase activity in amygdalin- (10 mg/ml) treated cells, that is, A-549, MDA-MB-231, MCF-7, U87-MG and MRC-5 fibroblasts was 85.5 ± 20.3%, 172 ± 50.1%, 445 ± 60.5%, 292 ± 80.4% and 88 ± 10.3%, respectively. The telomerase activity was significantly (P < .05) decreased in amygdalin- (10 mg/ml) treated cancer cells. Interestingly, apparent down-regulation of telomerase activity was observed in A-549 cells with high β-glucosidase activity following amygdalin treatment. Moreover, no changes were observed between amygdalin-treated and untreated fibroblasts (MRC-5).
Figure 4. Changes of telomerase activity analyzed by the RQ-TRAP method in A-549, MDA-MB-231, MCF-7 and U87-MG cancer cell lines and MRC-5 normal fibroblasts treated with 0 (control) and 10 mg/ml amygdalin for 7 days, respectively. Values indicated the mean telomerase activity (mean ± SEM) of five replicates and the telomerase activity in control MRC-5 fibroblasts was considered as 100% for comparison with other cell lines. a, b, c and d indicate significant (P < .05) difference among untreated control cell lines, * indicates significant (P < .05) difference between control and 10 mg/ml amygdalin-treated cell lines, respectively.
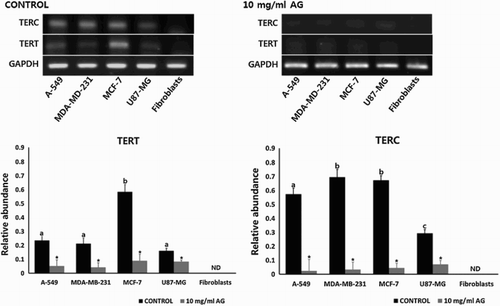
Expression of TERT and TERC in cancer and normal cells
The quantitative expression of TERT and TERC transcripts-related telomerase activity in control and amygdalin-treated (10 mg/ml) A-549, MDA-MB-231, MCF-7, U-87 MG and MRC-5 fibroblasts was measured (). GAPDH was used as reference genes. Transcript level of TERT and TERC was up-regulated in A-549, MDA-MB-231, MCF-7 and U-87 MG cancer cell lines, compared with normal MRC-fibroblasts which did not express TERT and TERC. As compared with other cancer cell lines, a higher expression of TERT and TERC was detected in MCF-7 cells (). However, amygdalin (10 mg/ml) treatment resulted in low expression of TERT and TERC in all cancer cells as compared with their untreated counterparts.
Figure 5. Expression of TERT and TERC transcripts related to telomerase activity by RT-PCR in A-549, MDA-MB-231, MCF-7 and U87-MG cancer cell lines and MRC-5 normal fibroblasts treated with 0 (control) and 10 mg/ml amygdalin for 7 days, respectively. Values indicated the mean transcript levels (mean ± SEM) of three replicates and were calculated as the ratio based on the level of GAPDH. a, b, c and d indicate significant (P < .05) difference among untreated control cell lines, * indicates significant (P < .05) difference between control and 10 mg/ml amygdalin-treated cell lines, respectively.
Discussion
The present study was conducted to investigate the effect of amygdalin treatment on cell growth and telomerase activity in cancer (A-549, MDA-MB-231, MCD-7 and U87-MG) and normal MRC-5 fibroblasts cells. Furthermore, β-glucosidase activity related with cellular cytotoxicity, effect of amygdalin on cell growth, senescence-associated-β-galactosidase activity and telomerase activity were evaluated. Our results show that cancer cell lines exhibit higher β-glucosidase activity than that of normal MRC-5 fibroblasts because of release of cyanide compound from amygdalin. Moreover, senescence-associated-β-galactosidase activity was found higher along with morphological changes in cancer cell lines. The telomerase activity and expression of related genes were also down-regulated in cancer cell lines without exerting any deleterious effects on normal MRC-5 fibroblasts. The differential cytotoxic effects of amygdalin should be correlated by the high level of β-glucosidase activity in cancer cell lines than that of normal somatic cells.
The β-glucosidase activity is not yet fully investigated in normal and cancer cell lines. It has been reported that rat and human small intestinal mucosa exhibit high level of glucosidase activity; however, release of glucose from amygdalin by β-glucosidase activity was not detected in any of the human neoplastic tissues (Newmark et al. Citation1981). Our results clearly demonstrated that cancer cell lines contain high level of β-glucosidase activity as compared with normal somatic cells (MRC-5 fibroblast), which is in accordance with the findings of Kwon et al. (Citation2003), that Persicae semen contains amygdalin with β-glucosidase activity and induces the anti-proliferative cytotoxicity effects and apoptotic cell death in promyelocytic leukemia HL-60 cells.
Amygdalin (C20H27NO11, d-Mandelonitrile 6-O-β-d-glucosido-β-d-glucoside, d-Mandelonitrile-β-gentiobioside) was initially isolated from the seeds of tree almonds, but the compound is a natural plant substance found in the seeds of various fruits (apples, cherries, peaches, apricots and others), and also present in plants, such as lima beans or clover. The amygdalin is not toxic itself; however, the release of HCN from amygdalin make it toxic (Shragg et al. Citation1982; Syrigos et al. Citation1998). It has been reported that HCN is one of the most rapidly acting poisons and can kill within seconds or minutes to hours depending on the dose and length of exposure (Shragg et al. Citation1982; Nelson Citation2006). Cytochrome oxidase generally converts oxygen to water using electron with low energy at the end of the electron transport chain in the inner membrane of mitochondria. Oxidative phosphorylation is responsible for creating large amounts of adenosine triphosphate (ATP), using it as the primary source of cellular energy (Mégarbane et al. Citation2003). However, cyanide compounds, such as HCN, is spontaneously changed to the negatively charged cyanide ion (CN−). The cyanide ion prevents cells from using oxygen by inhibiting the oxidative function of mitochondrial cytochrome oxidase, terminal enzyme in the electron transport chain which helps in the production of ATP (Mégarbane et al. Citation2003). It has been reported that the reduced production of ATP leads to cellular dysfunction, inhibition of cell growth and apoptotic cell death (Mégarbane et al. Citation2003). Furthermore, the toxic effect of cyanide ion on neural system has also been reported (Persson et al. Citation1985).
Previously several reports have shown the anti-tumor effect of amygdalin in cancer cells by inhibiting the cell growth. Cell growth and proliferation were dose-dependently induced in UMUC-3, RT112 and TCCSUP bladder cancer cell lines treated with amygdalin, resulting in arrest of cell cycle progression by down-regulation of cdk2 and cyclin A (Makarević et al. Citation2014a), and amygdalin treatment to SNU-C4 human colon cancer cells also displayed anticancer effect via down-regulation of cell cycle-related genes, implying that the amygdalin compound is induced to incomplete DNA synthesis (Park et al. Citation2005). Furthermore, it has been demonstrated that apoptosis is induced by up-regulating caspase-3 activity and pro-apoptotic Bax protein, whereas down-regulating anti-apoptotic protein Bcl-2 in the amygdalin-treated HeLa cells (Chen et al. Citation2013), human DU145 and LNCaP prostate cancer cells (Chang et al. Citation2006). In another study, it has been shown that amygdalin influences the decrease in adhesion and migratory properties of UMUC-3, TCCSUP and RT112 bladder cancer cells by modulating β1 or β4 integrin expression (Makarević et al. Citation2014b). These results suggest that amygdalin induces cell cytotoxicity by interfering with the progression of the cell cycle and inducing apoptosis.
Supporting these observations, our results also found that the amygdalin treatment leads to the effective inhibition of the cell growth and senescent status of the cells. Moreover, our results showed that the amygdalin is efficiently induced to the inhibition of cell growth at ∼10 mg/ml concentration as reported in the previous studies (Shragg et al. Citation1982; Chen et al. Citation2013; Makarević et al. Citation2014a, Citation2014b). However, we interestingly found that the normal somatic cells (MCR-5 fibroblasts) show considerable resistance to the cell growth and senescence at 10 mg/ml concentration of amygdalin. Meanwhile, the present results have showed that A-549 and MDA-MB-231 cancer cells with relatively high proliferation capacity possess high β-glucosidase activity, but their inhibition of cell growth at 10 mg/ml concentration of amygdalin is decreased as compared with those of MCF-7 and U87-MG cancer cells. Both MDA-MB-231 and MCF-7 cancer cells are types of adenocarcinoma tumors derived from breast epithelial cells, but it is generally known that MCF-7 cancer cells exhibit characterizations of relatively less malignant tumor cells with weak proliferation capacity than that in MDA-MB-231 cancer cells. It has been previously demonstrated that cell proliferations of MCF-7 cancer cells are more effectively inhibited by treating anticancer drug than those of MDA-MB-231 and A-549 cancer cells (Ghate et al. Citation2013; Abdullah et al. Citation2014). The differential cytotoxic effect on cell growth is probably related with cellular characterizations of malignant tumor cells among each cancer cell lines. Even though highly malignant tumor cells, such as A-549 and MDA-MB-231 cancer cells, exhibit high β-glucosidase activity, these cells should display higher resistance against amygdalin than MCF-7 and U87-MG cancer cells lines.
The cells with high frequency of senescent status generally exhibited an increased G0/G1 phase of cell cycle as well as high activity of senescence-associated-β-galactosidase and change into senescence-like morphology with enlarged and irregular cell shape (Funayama & Ishikawa Citation2007). As shown in our results, amygdalin treatment of the cancer cells at the 10 mg/ml concentration displayed higher senescence-associated-β-galactosidase activity with morphological alterations, whereas the normal MRC-5 fibroblasts displayed lower activity of senescence-associated-β-galactosidase without any morphological alterations.
Despite the fact that the effect of amygdalin treatment has not still been reported in normal somatic cells, our results showed that normal somatic cells treated with amygdalin possess more tolerance against senescence and aging by analyzing cell growth curve and senescence-associated-β-galactosidase activity with morphological alterations.
Telomere is a repetitive DNA sequence (TTAGGG)n which protect the linear DNA and maintain genomic integrity at the end of eukaryotic chromosomes. However, the telomeric DNA sequences in normal somatic cells (except cancer, germ and stem cells) become gradually shortened by loss of the terminal repeats, resulting in incomplete DNA replication during each cell division. The normal somatic cells with fully shortened telomeric repeats immediately enter cellular aging and replicative senescence that undergo a growth arrest with morphologic and metabolic changes. Telomere length should reflect the replicative history of the cells. It has been demonstrated that capacity for cell division before reaching replicative senescence is related with the telomere length in human fibroblasts (Allsopp et al. Citation1992). Moreover, the replicative senescence of normal somatic cells is thought to play an important role in aging and to help in tumor suppression without promoting tumorgenesis (Smith & Kipling Citation2004; Falandry et al. Citation2014).
Telomerase enzyme that adds DNA sequences to the 3′ end of the linear eukaryotic DNA strands can continually maintain or extend the length of telomeric DNA repeats (Falandry et al. Citation2014). High levels of telomerase activity are detected in germ, stem and most malignant transformed cells and their telomere length is stably maintained and extended during cell division. Therefore, the cells conserve the capacity of limitless replication and high proliferation. However, the low level of telomerase activity is expressed in most of the differentiated somatic cells, and their telomeric repeats are gradually shortened during each cell division (Falandry et al. Citation2014) which subsequently leads to cellular senescence. Our results have also demonstrated the level of telomerase activity is markedly up-regulated in cancer cell lines, that is, A-549, MDA-MB-231, MCF-7 and U87-MG (∼8–13 fold), as compared with that of normal MRC-5 fibroblasts. Previously, the higher telomerase activity in cancer cell lines has been reported (Kim et al. Citation1994; Cao et al. Citation2008; Jeon et al. Citation2011b), and our results also demonstrated higher telomerase activity in cancer cell lines as compared with normal fibroblasts. Even though, telomerase activity is not detected in the normal somatic cells (Cao et al. Citation2008), but we detected telomerase activity at a low level in the MRC-5 fibroblasts by using the real-time RQ-TRAP method and compared with the conventional TRAP assay using the gel image system. Considering the reliability, accuracy and sensitivity of the real-time RQ-TRAP assay employed in the present study, it seems to be a suitable method for the detection of telomerase activity at a low level, such as MRC-5 fibroblasts (Betts et al. Citation2006; Jeon et al. Citation2011a, Citation2011b).
Telomerase enzyme consists of several molecules, including human telomerase reverse transcriptase (TERT), telomerase RNA component (TERC), and nucleoprotein and others. It has been showed that telomerase activity is closely regulated by the expression of TERT and TERC. Furthermore, it has been reported that expression of TERT seems to be the rate-limiting factor for telomerase activity rather than TREC (Cao et al. Citation2008). In the present study, MCF-7 cancer cells with high expression of TERT transcript also showed a high level of telomerase activity, as compared with other cancer cell lines. However, the level telomere length in MCF-7 cancer cells was very similar to those of MDA-MB-231 and U87-MG cancer cell lines and continually maintained, as shown in our previous report (Jeon et al. Citation2011b), implying that the high level of telomerase activity exhibited in MCF-7 cancer cells is not directly related with extension of telomere length. However, our results demonstrated that amygdalin-treated cancer cell lines show low telomerase activity while the normal fibroblasts remain unchanged. As the cell growth and division were markedly inhibited in the amygdaline-treated cancer cells, which might be one reason due to which the length of telomeric repeats remains unchanged in these cells. It is an obvious that telomere length is shortened by progression of cell division. The down-regulation of telomerase activity was probably culminated from the down-regulation of TERT and TERC, which also was thought to be a consequence of cytotoxicity due to β-glucosidase activity in cancer cells. As mentioned above, cyanide ion released from amygdalin by β-glucosidase activity should be induced to the down-regulation of ATP production in cellular respiration and the deficiency of ATP presumed to induce limitation of RNA transcription, protein translation and others metabolites. Namely, we presumed that the inhibition of cell growth and induction of cellular senescence is caused by the cytotoxicity of cyanide ion rather than shortening of telomeric repeats in amygdalin-treated cancer cells; however, the length of telomeric repeats in amygdalin-treated cancer cells remains to be investigated.
Although any intrinsic analysis related to cellular cytotoxicity was not carried out in amygdalin-treated cancer cells, still our results have clearly demonstrated that amygdalin can be used as a potential alternative chemotherapeutic drug for human cancer cells by effecting cell growth and telomerase activity of cancer cells. These cancer cells possess high level of β-glucosidase activity rendering them more susceptible against amygdalin when compared with those of normal somatic cells. Therefore, in cancer cells amygdalin treatment induced cellular senescence, down-regulated telomerase activity and transcript level of TERT and TERC without causing any repressive effects on normal somatic cells. However, even though amygdalin exhibits definitely anticancer effects in vitro, this compound should be considered as a potential toxic material in vivo. As we earlier discussed in this article that the toxic effect of amygdalin is due to the release of HCN. In animal models, the lethal dose of amygdalin has been reported, that is, 880 mg/kg body weight by oral administration in rats and 25 g/kg by intravenous injections in mice (Park et al. Citation2013). It seems that the toxic effect through oral administration is higher than the intravenous injections, which is due to the release of more HCN upon hydrolysis of amygdalin by intestinal microbes (Carter et al. Citation1980). Because of such toxicity, there is a need for further in-depth studies to investigate its effects in normal somatic cell lines in vivo. Moreover, the cytotoxic effects of amygdalin in high concentration demand a careful consideration, that is, specific dosage and route of administration before using them for therapeutic applications.
Disclosure
No potential conflict of interest was reported by the authors.
Funding
This study was supported by grants from Korean Foundation for the Advancement of Science and Creativity.
References
- Abdullah AS, Mohammed AS, Abdullah R, Mirghani ME, Al-Qubaisi M. 2014. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complement Altern Med. 14:199. doi: 10.1186/1472-6882-14-199
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. 1992. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA. 89:10114–10118. doi: 10.1073/pnas.89.21.10114
- Artandi SE, DePinho RA. 2010. Telomeres and telomerase in cancer. Carcinogenesis. 31:9–18. doi: 10.1093/carcin/bgp268
- Betts DH, Perrault S, Harrington L, King WA. 2006. Quantitative analysis of telomerase activity and telomere length in domestic animal clones. Methods Mol Biol. 325:149–180.
- Campisi J, Yaswen P. 2009. Aging and cancer cell biology. Aging Cell. 8:221–225. doi: 10.1111/j.1474-9726.2009.00475.x
- Cao Y, Bryan TM, Reddel RR. 2008. Increased copy number of the TERT and TERC telomerase subunit genes in cancer cells. Cancer Sci. 99:1092–1099. doi: 10.1111/j.1349-7006.2008.00815.x
- Carter JH, McLafferty MA, Goldman P. 1980. Role of the gastrointestinal microflora in amygdalin (laetrile)-induced cyanide toxicity. Biochem Pharmacol. 29:301–304. doi: 10.1016/0006-2952(80)90504-3
- Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, Kim J, Kim KH, Kim CJ. 2006. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 29:1597–1602. doi: 10.1248/bpb.29.1597
- Chen Y, Ma J, Wang F, Hu J, Cui A, Wei C, Yang Q, Li F. 2013. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol. 35:43–51. doi: 10.3109/08923973.2012.738688
- Falandry C, Bonnefoy M, Freyer G, Gilson E. 2014. Biology of cancer and aging: a complex association with cellular senescence. J Clin Oncol. 32:2604–2610. doi: 10.1200/JCO.2014.55.1432
- Fulda S, Kögel D. 2015. Cell death by autophagy: emerging molecular mechanisms and implications for cancer therapy. Oncogene. 10:1038.
- Funayama R, Ishikawa F. 2007. Cellular senescence and chromatin structure. Chromosoma. 116:431–440. doi: 10.1007/s00412-007-0115-7
- Ghate NB, Chaudhuri D, Sarkar R, Sajem AL, Panja S, Rout J, Mandal N. 2013. An antioxidant extract of tropical lichen, Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. PLoS One. 8:e82293. doi: 10.1371/journal.pone.0082293
- Holzbecher MD, Moss MA, Ellenberger HA. 1984. The cyanide content of laetrile preparations, apricot, peach and apple seeds. J Toxicol Clin Toxicol. 22:341–347. doi: 10.3109/15563658408992565
- Hwang ES, Yoon G, Kang HT. 2009. A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci. 66:2503–2524. doi: 10.1007/s00018-009-0034-2
- Jeon BG, Kumar BM, Kang EJ, Maeng GH, Lee YM, Hah YS, Ock SA, Kwack DO, Park BW, Rho GJ. 2011b. Differential cytotoxic effects of sodium meta-arsenite on human cancer cells, dental papilla stem cells and somatic cells correlate with telomeric properties and gene expression. Anticancer Res. 31:4315–4328.
- Jeon BG, Kwack DO, Rho GJ. 2011a. Variation of telomerase activity and morphology in porcine mesenchymal stem cells and fibroblasts during prolonged in vitro culture. Anim Biotechnol. 22:197–210. doi: 10.1080/10495398.2011.624651
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science. 266:2011–2015. doi: 10.1126/science.7605428
- Kwon HY, Hong SP, Hahn DH, Kim JH. 2003. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch Pharm Res. 26:157–161. doi: 10.1007/BF02976663
- Makarević J, Rutz J, Juengel E, Kaulfuss S, Reiter M, Tsaur I, Bartsch G, Haferkamp A, Blaheta RA. 2014b. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS One. 9:e105590. doi: 10.1371/journal.pone.0105590
- Makarević J, Rutz J, Juengel E, Kaulfuss S, Tsaur I, Nelson K, Pfitzenmaier J, Haferkamp A, Blaheta RA. 2014a. Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS One. 9:e110244. doi: 10.1371/journal.pone.0110244
- Mégarbane B, Delahaye A, Goldgran-Tolédano D, Baud FJ. 2003. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 66:193–203.
- Milazzo S, Lejeune S, Ernst E. 2007. Laetrile for cancer: a systematic review of the clinical evidence. Support Care Cancer. 15:583–595. doi: 10.1007/s00520-006-0168-9
- Nazarkina ZK, Laktionov PP. 2015. Preparation of dendritic cells for cancer immunotherapy. Biomed Khim. 61:30–40. doi: 10.18097/pbmc20156101030
- Nelson L. 2006. Acute cyanide toxicity: mechanisms and manifestations. J Emerg Nurs. 32:S8–S11. doi: 10.1016/j.jen.2006.05.012
- Newmark J, Brady RO, Grimley PM, Gal AE, Waller SG, Thistlethwaite JR. 1981. Amygdalin (Laetrile) and prunasin beta-glucosidases: distribution in germ-free rat and in human tumor tissue. Proc Natl Acad Sci USA. 78:6513–6516. doi: 10.1073/pnas.78.10.6513
- Ozturk S, Sozen B, Demir N. 2014. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 20:15–30. doi: 10.1093/molehr/gat055
- Park HJ, Yoon SH, Han LS, Zheng LT, Jung KH, Uhm YK, Lee JH, Jeong JS, Joo WS, Yim SV, Chung JH, Hong SP. 2005. Amygdalin inhibits genes related to cell cycle in SNU-C4 human colon cancer cells. World J Gastroenterol. 11:5156–5161.
- Park JH, Seo BI, Cho SY, Park KR, Choi SH, Han CK. 2013. Single oral dose toxicity study of prebrewed armeniacae semen in rats. Toxicological. 29:91–98. doi: 10.5487/TR.2013.29.2.091
- Persson SA, Cassel G, Sellström A. 1985. Acute cyanide intoxication and central transmitter systems. Fundam Appl Toxicol. 5:S150–S159. doi: 10.1016/0272-0590(85)90124-1
- Phatak P, Dai F, Butler M, Nandakumar MP, Gutierrez PL, Edelman MJ, Hendriks H, Burger AM. 2008. KML001 cytotoxic activity is associated with its binding to telomeric sequences and telomere erosion in prostate cancer cells. Clin Cancer Res. 14:4593–4602. doi: 10.1158/1078-0432.CCR-07-4572
- Rudolph KL, Hartmann D, Opitz OG. 2009. Telomere dysfunction and DNA damage checkpoints in diseases and cancer of the gastrointestinal tract. Gastroenterology. 137:754–762. doi: 10.1053/j.gastro.2009.07.037
- Santos Pimenta LP, Schilthuizen M, Verpoorte R, Choi YH. 2014. Quantitative analysis of amygdalin and prunasin in Prunus serotina Ehrh. using (1) H-NMR spectroscopy. Phytochem Anal. 25:122–126. doi: 10.1002/pca.2476
- Sauer H, Wollny C, Oster I, Tutdibi E, Gortner L, Gottschling S, Meyer S. 2015. Severe cyanide poisoning from an alternative medicine treatment with amygdalin and apricot kernels in a 4-year-old child. Wien Med Wochenschr. 165:185–188.
- Shragg TA, Albertson TE, Fisher CJ Jr. 1982. Cyanide poisoning after bitter almond ingestion. West J Med. 136:65–69.
- Smith SK, Kipling D. 2004. The role of replicative senescence in cancer and human ageing: utility (or otherwise) of murine models. Cytogenet Genome Res. 105:455–463. doi: 10.1159/000078219
- Syrigos KN, Rowlinson-Busza G, Epenetos AA. 1998. In vitro cytotoxicity following specific activation of amygdalin by beta-glucosidase conjugated to a bladder cancer-associated monoclonal antibody. Int J Cancer. 78:712–719. doi: 10.1002/(SICI)1097-0215(19981209)78:6<712::AID-IJC8>3.0.CO;2-D
- Toouli CD, Huschtscha LI, Neumann AA, Noble JR, Colgin LM, Hukku B, Reddel RR. 2002. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene. 21:128–139. doi: 10.1038/sj.onc.1205014