ABSTRACT
Growth factor receptor-bound protein 2 (Grb2) have been proved by a lot of studies playing a major role in cell proliferation and cell differentiation. However, the regulation of Grb2 expression by microRNAs (miRNAs) in chicken breast muscle still remains unknown. The expression profile of Grb2 was checked based on our previous RNA sequencing data and the Grb2 relative expression level in breast muscle of aged hens (55-week-old) was validated significantly higher than juvenile hens (20-week-old) using qRT-PCR. miRNAs that interact with Grb2 have been predicted in chicken and the relationship between the potential miRNA and Grb2 was verified using dual luciferase reporter assay in chicken DF1 cells. Dual-luciferase reporter assays results demonstrated that the expression of luciferase reporter gene linked with part sequence of the 3′UTR of chicken Grb2 gene was down-regulated by the overexpression of gga (Gallus Gallus)-miR-200a-3p in the DF1 cells, and the down-regulation behavior was abolished when the gga-miR-200a-3p binding site in 3′UTR of Grb2 was mutated, indicating that gga-miR-200a can suppress the expression level of its target gene Grb2. Therefore, we concluded that the significantly increased expression level of Grb2 in the breast muscle of aged chicken can (at least partly can) be explained by the decreased expression of miR-200a, which reduced the inhibitory effect on Grb2. Taken together, these findings suggest that gga-miR-200a can suppress the expression level of its target gene Grb2 and might be involved in the cell differentiation and proliferation of chicken breast muscle through binding with the 3’UTR of Grb2.
Introduction
Growth factor receptor-bound protein 2 (Grb2) was originally discovered for its major role in cell proliferation, cell survival, angiogenesis and cell differentiation (Ward et al. Citation1999). Grb2 is a key adapter protein in intracellular signal transduction and Erk signaling pathways, in which it links activated cell surface receptors to downstream targets by binding to specific phosphotyrosine-containing and proline-rich sequence motifs (Y. Lowenstein et al. Citation1992; Rozakis-Adcock et al. Citation1993; Liu et al. Citation2013).
Recent reports have proved the Grb2 as a crucial member of immune system in the T cell development and TH cell differentiation (Radtke et al. Citation2016). Positive and negative regulatory roles of the Grb2 in receptor tyrosine kinase signaling makes it a double-edged sword (Belov and Mohammadi Citation2012). Signaling via Grb2 is essential to the segregation of epiblast and primitive endoderm progenitors (Chazaud et al. Citation2006). Grb2 is proved necessary for the growth and transformation of mouse embryo cells(D’Ambrosio et al. Citation1996). Deletion of the Grb2 gene leads to preimplantation embryonic lethality in vivo (Cheng et al. Citation1998; Chazaud et al. Citation2006). It was found that growth factor-specific can couple of Grb2 to the Ras pathway, which is also called MAPK pathway (Klint et al. Citation1995). The Ras/mitogen activated protein kinase (MAPK) pathway is one of the most important and intensively studied signaling pathways that governs the growth, proliferation, differentiation and survival of many, if not all, cell types, which are critical to normal development. It is also deregulated in various diseases, ranging from cancer to immunological, inflammatory and degenerative syndromes (Orton et al. Citation2005; Tidyman and Rauen Citation2009). The MAPK pathway includes many proteins, including MAPK (mitogen-activated protein kinases, originally called ERK, extracellular signal-regulated kinases), ERK signaling pathway is a major determinant in the control of diverse cellular processes such as proliferation, survival, differentiation and motility(Kohno and Pouyssegur Citation2006). Grb2 can link integrin engagement to the activation of the Ras/MAPK signal transduction pathway(Schlaepfer et al. Citation1994). Disrupting the consensus for Grb2 binding reveals complex roles in muscle development, it allowed development to proceed but caused a significant reduction in limb muscle and a generalized deficit of secondary fibers(Maina et al. Citation1996). Grb2-associated binder 1 is an essential signaling molecule in mediating axonal neuregulin-1 signaling for the development of both extrafusal and intrafusal muscle fibers on mice (Park et al. Citation2017). In addition, a wide range of documentation have reported the broad involvement of Grb2 in progression and development of multiple systemic malignancies including breast cancer, chronic myelogenous leukemia and lung cancer invasion and metastasis etc. (Pendergast et al. Citation1993; Watanabe et al. Citation2000; Yu et al. Citation2009; Zhang et al. Citation2013; Zuberi et al. Citation2015) (Menju et al. Citation2016).
MicroRNAs (miRNAs) are short, single-stranded RNA molecules. Mature miRNAs are 19- to 25-nucleotide-long molecules cleaved from 70- to 100-nucleotide hairpin pre-miRNA precursors(Bartel Citation2004). miRNA can interact with the 3’ untranslated region (UTR) of mRNAs to regulate gene expression. This usually occurs by repression of protein translation via a mechanism that involves incomplete base pairing with the 3’UTR of target mRNAs, or by causing target sequences to become unstable and degraded sooner (Hendrickson et al. Citation2009; Jiang et al. Citation2010), thereby causing protein expression to be down regulated. Genetic, biochemical, and computational studies have implicated essential and diverse roles of miRNAs in multicellular organisms including development, cell proliferation and differentiation, apoptosis, glucose metabolism, stress resistance, and have been implicated in human cancer development (Ambros Citation2004; Croce and Calin Citation2005; Mizuno et al. Citation2008; Busskamp et al. Citation2014), for instance, the let-7 microRNA was proved to be a master regulator of cell proliferation pathways(Johnson et al. Citation2007).
A lot of studies have proved that Grb2 playing a major role in cell proliferation, cell survival, angiogenesis and cell differentiation. As a signal transducer, Grb2 is related to pathways and genes involved in aging such as INS/IGF1 signaling and SHC1 (Tatar et al. Citation2003) and was observed to have the highest strength score (used to measure the number of aging related diseases the protein involved in) of 75.76 in the human ‘aging network’(Reja et al. Citation2009), it is probable that Grb2 plays a vital role in aging. Chicken breast muscle mainly composed of muscle fibers, and it is the perfect tissue for the study of muscle cell growth and differentiation. The expression profile of Grb2 in breast muscle of aged hens (55-week-old) and juvenile hens (20-week-old) was previous obtained using RNA sequencing (data unpublished) and the Grb2 was found up-regulated in breast muscle of aged hens (55-week-old). Taken together, these previous findings provided the basis for the increased expression level of Grb2 in aged chicken muscle. However, the regulation of Grb2 expression by microRNAs (miRNAs) in chicken breast muscle still remains unknown. Our research aimed at studying how miRNAs involved in chicken breast muscle cell differentiation and proliferation through targeting Grb2. These findings will contribute to further understanding of the regulatory mechanisms of miRNAs in chickens and other avian.
Materials and methods
Ethics statements
The entire procedure for all the laboratorial animals was performed in strict accordance with the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Henan Agricultural University and the Canadian Council on Animal Care (CCAC) guidelines.
Chicken breast tissue sample collection
The experimental animals used were one strain of the Chinese domestic breed laying hens, GuShi chicken. All the chickens were raised in cages under the same environment with ad libitum conditions. Three juvenile hens and three aged hens were selected randomly from two different physiological stages. The juvenile and aged hens were sacrificed when they were 20 weeks old (20w) and 55 weeks old (55w), respectively. After fainting by shock, the chickens were bled within 10–15 s by cutting the throat from the side of the muscles. Breast muscle tissues were harvested immediately after slaughter. The collected samples were immediately snap-frozen in liquid nitrogen and stored at −80°C for further use.
RNA extraction and cDNA synthesis
Total RNA was extracted from the breast muscle tissues of chicken using TRIzol® reagents following the manufacturer's manual (Invitrogen, Carlsbad, CA). RNA integrity and degradation were detected on 1.5% agarose gels. The concentration and purity of total RNA were determined by Nano Photometer® spectrophotometer (IMPLEN, CA). The PrimeScriptTM RT reagent Kit with gDNA Eraser was used to reverse-transcribe RNA samples into cDNA (TaKaRa, China), the cDNA was stored at - 20°C until use.
Grb2 Quantitative RT–PCR
In order to find the role of Grb2 in growth and development process of chicken breast muscle, we detect the Grb2 relative expression level in breast muscle at two different physiological stages: juvenile stage (20-week-old) and aged stage (55-week-old). β-actin were used as endogenous control. Real-time PCR was performed with Power SYBR Green PCR Master Mix (TaKaRa, China) using the LightCycler 96 Real-Time PCR System (Roche, Switzerland). The QPCR amplification procedure for mRNA was as follows: 95°C for 3 min; 35 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 20 s, and an extension for 10 min at 72°C. Relative expression quantity of Grb2 were determined using the 2−ΔΔCt method as provided by the manufacturer (Kenneth and Livak Citation2001). The sequences of gallus gullus Grb2 RT–PCR primers were shown in .
Table 1. The primers used for PCR or qPCR
Computational prediction of target miRNAs of Grb2
To further understand the function of miRNAs, we choosing chicken as the species predicted target miRNAs that interacting with Grb2 using TargetScan Human 7.0 program (Wang X and Naqa Citation2008) (http://www.targetscan.org/vert_71/) and miRDB(X. Wang Citation2008), respectively.
gga-miR-200a Quantitative RT–PCR
We detect the miR-200a relative expression level in breast muscle at two different physiological stages: juvenile stage (20-week-old) and aged stage (55-week-old) by stem-loop real-time QPCR. The stem-loop primers used for the RT–PCR were purchased from GenePharma (Shanghai, China). Real-time PCR was performed in triplicate with Power SYBR Green PCR Master Mix (TaKaRa, China) using the LightCycler 96 Real-Time PCR System (Roche, Switzerland). Relative quantities of mature miRNAs (gga-miR-200a, U6) were determined using the Hairpin-KitTM miRNAs RT–PCR Quantitation Kit (Gene Pharma, China), chicken small nuclear RNA U6 was used as the internal control for miRNA. The RT–PCR amplification procedure for miRNA was as follows: 95°C for 3 min; 40 cycles of 95°C for 12 s, 60°C for 40 s, 72°C for 30 s, and an extension for 10 min at 72°C. Relative expression quantity of gga-miR-200a were determined using the 2−ΔΔCt method as provided by the manufacturer (Kenneth and Livak Citation2001).
DNA plasmids
To generate pcDNA3.1-miR-200a vectors, the gga-miR-200a precursor sequences and the approximate 150 bp flanking region on each side were amplified using the Gushi-Anka F2 chicken resource population (Han et al. Citation2010, Citation2011) genomic DNA. The PCR products were cloned into the pcDNA3.1-EGFP vector (InvitrogenTM, USA) using XhoI and XbaI restriction sites. The correct sample was sent to The Beijing Genomics Institute (BGI) for the sequence identification. The 3’UTR, which contain the potential target gga-miR-200a binding sites of Grb2, were amplified from F2 chicken genomic DNA by a standard PCR method. The PCR products were cloned into the multiple cloning regions of psiCHECK-2 vector (Promega, USA) using XhoI and NotI restriction sites. The reconstructed vectors were sequenced to identify whether the insertion was correct or not. The mutant Grb2 3’UTR reporter, designated as psiCHECK-2-Grb2 3’UTR-mut, was created by knockout the sequences of the gga-miR-200a seed region using overlap PCR. The primers used were shown in .
Cell culture and dual-luciferase report assay
Chicken DF-1 cells (ACTT®, USA) were grown in Dulbecco's modified Eagle's medium (GIBCO, USA) supplemented with 10% FBS and penicillin/streptomycin. Before transfection, DF-1 cells were seeded in 12-well plate at a density of 3.0 × 105 cells/ well, and cultured in a humidified incubator at 37°C with 5% CO2. When the cells reached 80% confluence, they were serum-starved for 12 h and the cells were co-transfected with the luciferase reporters vector (psiCHECK-2 or recombinant psiCHECK-2-Grb2 3’UTR or psiCHECK-2-Grb2 3’UTR-mut, 1000 ng) and gga-miR-200a expression vector (pcDNA3.1-EGFP or pcDNA3.1-miR-200a, 1000 ng) in serum-free DMEM medium using TurboFect transfection reagent (Invitrogen), after 6 h of transfection, the medium was changed. The transfection experiment was performed in triplicate wells and repeated at least three times. About 48 h post-transfection, cells were harvested and lysed in passive lysis buffer (Promega, USA). Lysates was assayed for reporter gene activity using the Dual-Luciferase® Report Assay System (Promega) on a Fluoroskan Ascent FL instrument (Thermo Fisher Scientifc, Shanghai, China) according to the manufacturer's instruction. The renilla luciferase signal was normalized to the firefly luciferase signal.
Statistical analysis
The IBM SPSS Statistics 21 software was used for the association analysis. All data are presented as mean ± SD. Two-tailed unpaired Student's t tests and ANOVA were used for statistical evaluation of the data, a p value < 0.05 was considered significant.
Results
Relative expression level of Grb2 in chicken breast muscle at different stages
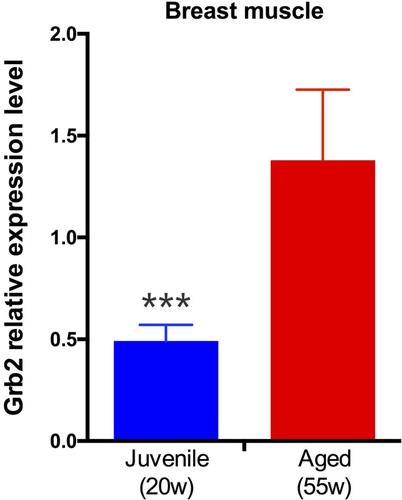
We performed Grb2 expression analysis on breast muscle tissues of juvenile (20-week-old) and aged (55-week-old) chicken. As shown in , in breast muscle, Grb2 relative expression level in aged hens are significantly higher than in juvenile hens (p < 0.001).
Computational prediction of target miRNAs of Grb2
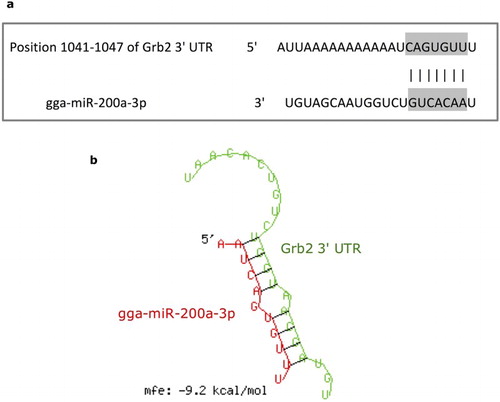
We predicted 3 miRNAs, which are gga-miR-200a-3p, gga-miR-27b-3p, gga-miR-128-3p, that might be target of Grb2 3’UTR with conserved sites through TargetScan Human 7.0 (http://www.targetscan.org/vert_71/) programs. miR-200a were previously proved to play an important role during cell differentiation and proliferation in other species and miR-200a can suppress the differentiation of mouse embryonic stem cells by directly targeting growth factor receptor-bound protein 2, which reminds us gga-miR-200a might play a vital role in the regulation of cell differentiation, growth and proliferation by targeting the target gene Grb2. On the basis of high scorers and previous researches, we selected miR-200a as the potential targets miRNA of Grb2 for further study ((a)). The mFE (minimum free energy) of the RNA duples between miR-200a-3p and Grb2 3’-UTR was −9.2 kCal/mol, which indicates that those two pairs of sequences are highly stabled ((b)).
Figure 2. Prediction of targeting relationship between gga-miR-200a-3p and Grb2. (a) Sequence alignments of gga-miR-200a-3p and the target site in the 3’ UTR of Grb2. (b) Secondary structure of the RNA duplex of gga-miR-200a-3p targeting Grb2 3’ UTR target site respectively (Red: Target sequence; Green: gga-miR-200a-3p).
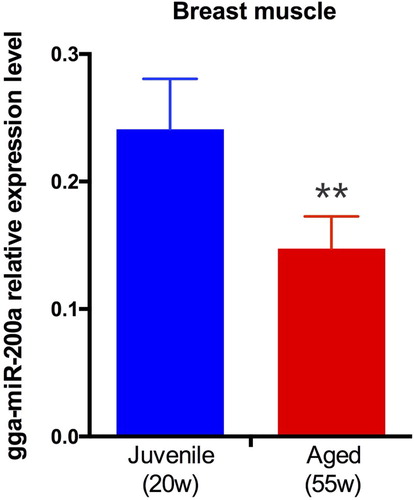
gga-miR-200a expression profile in chicken breast muscle at different stages
We performed gga-miR-200a expression analysis on breast muscle tissues of juvenile (20-week-old) and aged (55-week-old) chicken. As shown in , in breast muscle, gga-miR-200a relative expression level in aged hens are significantly lower than juvenile hens (p < 0.01), which is contrary to the expression trend of Grb2.
Verification the targeting relation between gga-miR-200a and Grb2 in DF-1 cell line
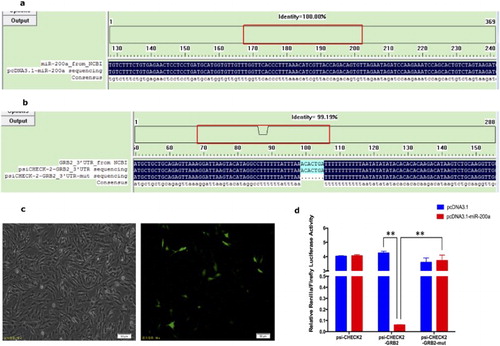
Sequence alignment showed that, the gga-miR-200a precursor sequences were successfully cloned into pcDNA3.1-EGFP vector ((a)), the wild and the mutant 3’UTR of Grb2 were respectively successfully cloned into psiCHECK-2 vector ((b)). Chicken DF-1 cells were cultured and co-transfected with the luciferase reporter and gga-miR-200a expression vector. About 24 h post-transfection, cells were observed under microscope, the green fluorescence observed under the microscope inferred that the vectors were successfully transfected into DF-1 cells ((c)). The luciferase activity of cells co-transfected with pcDNA3.1-miR-200a vector and psiCHECK-2 empty vector was unchanged in comparison to the empty vector group (co-transfected with pcDNA3.1 empty vector and psiCHECK-2 empty vector). However, a significantly decreased luciferase activity was observed in cells co-transfected with pcDNA3.1-miR-200a vector and psiCHECK-2-Grb2 3’UTR vector, comparing with the empty vector control group (co-transfected with pcDNA3.1 empty vector and psiCHECK-2-Grb2 3’UTR vector) (P < 0.01), and the cell luciferase activity was recovered after co-transfected with pcDNA3.1-miR-200a vector and psiCHECK-2-Grb2 3’UTR-mut vector (P < 0.01) ((d)). Dual-luciferase reporter assays results demonstrated that the expression of luciferase reporter gene linked with part sequence of the 3′UTR of chicken Grb2 gene was down-regulated by the overexpression of gga-miR-200a-3p in the DF1 cells, and the down-regulation behavior was abolished when the gga-miR-200a-3p binding site in 3′UTR of Grb2 was mutated, indicating that gga-miR-200a directly target Grb2 through binding to 3’UTR of Grb2, which proved that gga-miR-200a is the positive target miRNA of Grb2.
Figure 4. Verification of the targeting relation between gga-miR-200a and Grb2. (a) Eukaryotic expression vector and dual fluorescence report vector construction. Sequence alignment of pcDNA3.1-miR-200a recombinant expression vector with miR-200a precursor sequence obtain from NCBI. (b) Sequence alignment of psiCHECK 2-Grb2 3’UTR and psiCHECK 2-Grb2 3’UTR-mut (knocked out the miR-200a binding sites) recombinant expression vector with Grb2 3’UTR sequence obtained from NCBI. (c) DF1 cell before (left) and after (right) transfection of the vectors. (d) Dual-luciferase report assays illuminated gga-miR-200a is the positive target of Grb2 and can suppress the expression level of Grb2.
Discussion
Many studies have proved that Grb2 playing a major role in cell proliferation, cell survival, angiogenesis and cell differentiation (Ward et al. Citation1999). While there has been no research on Grb2 and the target miRNAs in chicken breast muscle yet. The expression of Grb2 was previously verified increased with age using western blot, being most abundant in skeletal muscle from aged (30-month-old) ad libitum rats, and high GAB1 (GRB2 associated binding protein 1) and GAB2 levels were exclusively seen in aged (30-month-old) ad libitum rats (Edström et al. Citation2006). Grb2 was found up-regulated in breast muscle of aged hens (55-week-old) comparing with juvenile hens (20-week-old) according to our previous RNA sequencing data (data unpublished). Here, we validated the Grb2 expression level in breast muscle tissues of juvenile (20-week-old) and aged (55-week-old) chicken, and the Grb2 relative expression level in aged hens was found significantly higher than in juvenile hens (p < 0.01), which is consistent with previous studies. Here comes up a doubt that under what molecular mechanism does Grb2 increased its expression level in breast muscle of aged chicken and given rise to a variety of biological functions.
MicroRNAs (miRNAs) represent a class of naturally occurring small noncoding RNA molecules, distinct from but related to small interfering RNAs. Since the initial discovery of miRNAs as essential regulators of development in the nematode Caenorhabditis elegans, microRNAs have been identified playing pivotal posttranscriptional regulatory roles by interacting with the 3’ untranslated region (UTR) of mRNAs to regulate gene expression in animals and other multicellular organisms (Pasquinelli Citation2012). A hypothesis was coming up that miRNA may play a role in the change of Grb2 expression level in breast muscle. We predicted 3 miRNAs (gga-miR-200a-3p, gga-miR-27b-3p, gga-miR-128-3p) that might be the target of Grb2 3’UTR with conserved sites. The anti-adipogenic effect of miR-27b was found in human multipotent adipose-derived stem cells (Karbiener et al. Citation2009) and PPARα was found regulated by miR-27b in human liver(Kida et al. Citation2011). miR-27b is involved in the regulation of cardiac hypertrophy, and was validated as an efficient therapeutic target for cardiac diseases(J. Wang et al. Citation2012), and the aberrantly up-regulate of miR-27b may be one of the critical factors that contribute to malignancy in human gliomas. The serum levels of miR-27b may serve as potential biomarkers for early stage arteriosclerosis obliterans(Li et al. Citation2011). miR-128 was previously revealed as a potentially important negative regulator of prostate cancer cell invasion(Khan et al. Citation2010), and contributes to colorectal cancer progression by posttranscriptionally regulating Galectin-3(Lu et al. Citation2017). Findings also suggest that miR-128-3p might be a potential target against metastasis and chemoresistance in non-small cell lung cancer(Cai et al Citation2017). Thus, miR-27b and miR-128 both are key factors that involved in many human diseases.
miR-200 is a family of tumor suppressor miRNAs consisting of five members (miR-200a, -200b, -200c, -141 and -429) that are expressed as two separate polycistronic pri-miRNA transcripts, which are significantly involved in inhibition of epithelial-to-mesenchymal transition (EMT), repression of cancer stem cells (CSCs) self-renewal and differentiation, modulation of cell division and apoptosis(Feng et al. Citation2014). Previous studies show that miR-200 family members are emerging as important regulators of cell proliferation, differentiation and metastasis (Dykxhoorn Citation2010; Leskelä et al. Citation2010; Peng et al. Citation2012). miR-200 family members were shown to promote the mesenchymal-epithelial transition (MET) and to activate the differentiation of pancreatic, colorectal and breast cancer cells into epithelial cells. miR-200 can regulate oligodendrocyte and oligodendrocyte precursor cells differentiation by repressing expression of SRF (serum response factor) (Buller et al. Citation2012). miR-200a, miR-200b, and miR-200c overexpressions are associated with the aggressive tumor progression and be recognized as reliable markers to predict the prognosis and survival in epithelial ovarian cancer patients(Zuberi et al. Citation2015). miR-200a has also been identified as a prognostic marker for advanced ovarian (Xiaoxia Hu et al. Citation2009) and cervical cancers in women (X. Hu et al. Citation2010). miR-200b has been previously found involved in regulating mammalian body size and mouse embryonic development process (Ren et al.Citation2013) (’The Expression Profile and Target Gene Analysis of Mouse(Mus musculus) Body Size Regulation related microRNA-200b(miR-200b)’ 2013). Previous study elucidates that miR-200a was found inhibit nasopharyngeal carcinoma cell growth, migration and invasion and involving in the regulation of the mouse placental development(Saha et al. Citation2015). However, the biological function of miR-200a in chicken has not been reported. miR-200a were proved to play an indispensable role during cell differentiation and proliferation in other species and miR-200a can suppress the differentiation of mouse embryonic stem cells by directly targeting growth factor receptor-bound protein 2 (Grb2), which reminds us gga-miR-200a might play a vital role in the regulation of cell differentiation, growth and proliferation by targeting the target gene Grb2. On the basis of high scorers and previous researches, we selected miR-200a as the potential targets miRNA of Grb2 for further study.
We evaluated gga-miR-200a expression profile in chicken breast muscle, according to the result of our study, the gga-miR-200a relative expression level in breast muscle of juvenile hens (20 weeks old) are significantly higher than aged hens (55 weeks old), which is contrary to the expression trend of Grb2. Therefore, we hypothesized that the expression of Grb2 might be inhibited by gga-miR-200a. In mouse, previous studies show that miR-200a can suppress the differentiation of embryonic stem (ES) cells into endoderm and mesoderm by directly targeting Grb2 and can control cell fate decisions affecting the early endoderm and mesoderm layers in a manner that is partly dependent on Erk signaling, by regulating Grb2 expression levels (Yang Liu et al. Citation2013), and the up-regulation of miR-200a can down-regulate the expression of Grb2 in rat brain progenitor cells (Santra et al. Citation2016). In the present research, dual-luciferase reporter assays results demonstrated that the expression of luciferase reporter gene linked with part sequence of the 3′UTR of chicken Grb2 gene was down-regulated by the overexpression of gga-miR-200a-3p in the DF1 cells, and the down-regulation behavior was abolished when the gga-miR-200a-3p binding site in 3′UTR of Grb2 was mutated, indicating that gga-miR-200a directly target Grb2 through binding to 3’UTR of Grb2. This comprehensive analysis provides a solid basis for exploring the regulation mechanisms of gga-miR-200a in cell differentiation, growth and proliferation process.
Conclusion
We concluded that the significantly increased expression level of Grb2 in the breast muscle of aged chicken can (at least partly can) be explained by the decreased expression of miR-200a, which reduced the inhibitory effect on Grb2. Taken together, these findings suggest that gga-miR-200a can suppress the expression level of its target gene Grb2 and might be involved in the cell differentiation and proliferation of chicken breast muscle through binding to the 3’UTR of Grb2.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Keren Jiang http://orcid.org/0000-0002-8141-8278
Additional information
Funding
References
- Ambros V. 2004. The functions of animal microRNAs. Nature. 431:350–355. doi: 10.1038/nature02871
- Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. doi:10.1016/S0092-8674(04)00045-5.
- Belov AA, Mohammadi M. 2012. Grb2, a double-edged sword of receptor tyrosine kinase signaling. Sci Signal. 5:pe49. doi: 10.1126/scisignal.2003576
- Buller B, Chopp M, Ueno Y, Zhang L, Zhang RL, Morris D, Zhang Y, Zhang ZG. 2012. Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation. Gila. 60:1906–1914.
- Busskamp V, Krol J, Nelidova D, Daum J, Szikra T, Tsuda B, Jüttner J, Farrow K, Scherf B, Alvarez C, Genoud C, Sothilingam V, Tanimoto N, Stadler M, Seeliger M, Stoffel M, Filipowicz W, Roska B, et al. 2014. miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron. 83:586–600. doi: 10.1016/j.neuron.2014.06.020
- Cai J, Fang L, Huang Y, Li R, Xu X, Hu Z, Zhang L, Yang Y, Zhu X, Zhang H, Wu J, et al. 2017. Simultaneous overactivation of Wnt/ß-catenin and TGFß signalling by miR-128-3p confers chemoresistance-associated metastasis in NSCLC. Nat Commun. 8:15870. doi:10.1038/ncomms15870.
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. 2006. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Devel Cell. 10:615–624. doi: 10.1016/j.devcel.2006.02.020
- Cheng AM, Saxton TM, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice CG, Cardiff RD, Cross JC, Muller WJ, Pawson T, et al. 1998. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 95:793–803. doi: 10.1016/S0092-8674(00)81702-X
- Croce CM, Calin GA. 2005. miRNAs, cancer, and stem cell division. Cell. 122:6–7. doi: 10.1016/j.cell.2005.06.036
- D’Ambrosio C, Hongo A, Li S, Baserga R. 1996. The role of Grb2 in the growth and transformation of mouse embryo cells. Oncogene. 12:371–378.
- Dykxhoorn DM. 2010. MicroRNAs and metastasis: little RNAs go a long way. Cancer Res. 70:6401–6406. doi: 10.1158/0008-5472.CAN-10-1346
- Edström E, Altun M, Hägglund M, Ulfhake B., et al. 2006. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol. 61:663–674 doi: 10.1093/gerona/61.7.663
- Feng X, Wang Z, Fillmore R, Xi Y., et al. 2014. MiR-200, a new star miRNA in human cancer. Cancer Lett. 344:166–173 doi: 10.1016/j.canlet.2013.11.004
- Han RL, Lan XY, Zhang LZ, Ren G, Jing YJ, Li MJ, Zhang B, Zhao M, Guo YK, Kang XT, Chen H, et al. 2010. A novel single-nucleotide polymorphism of the visfatin gene and its associations with performance traits in the chicken. J Appli Genet. 51:59–65. doi: 10.1007/BF03195711
- Han RL, Li ZJ, Li MJ, Li JQ, Lan XY, Sun GR, Kang XT, Chen H. 2011. Novel 9-bp indel in visfatin gene and its associations with chicken growth. Brit Poultry Sci. 52:52–57. doi: 10.1080/00071668.2010.537310
- Hendrickson DG, Hogan DJ, McCullough HL, Myers JW, Herschlag D, Ferrell JE, Brown PO. 2009. Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol. 7:e1000238. doi: 10.1371/journal.pbio.1000238
- Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN, Wang X, et al. 2009. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 114:457–464. doi: 10.1016/j.ygyno.2009.05.022
- Hu X, SchwarzJrJK, Huettner PC, Rader JS, Deasy JO, Grigsby PW, Wang X. 2010. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 70:1441–1448. doi: 10.1158/0008-5472.CAN-09-3289
- Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. 2010. MicroRNAs and the regulation of fibrosis. FEBS J. 277:2015–2021. doi: 10.1111/j.1742-4658.2010.07632.x
- Johnson CD, Esquelakerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J. 2007. The let-7 MicroRNA represses cell proliferation pathways in human cells. Cancer Res. 67:7713–7720 doi: 10.1158/0008-5472.CAN-07-1083
- Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri E-Z, Scheideler M, et al. 2009. RNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun. 390:247–251. doi: 10.1016/j.bbrc.2009.09.098
- Kenneth J, Livak TD. 2001. Analysis of relative gene expression data using rea l—time quantitative PCR and the 2 –△△CT method. Method. 25:402–408 doi: 10.1006/meth.2001.1262
- Khan AP, Poisson LM, Bhat VB, Fermin D, Zhao R, Kalyana-Sundaram S, Michailidis G, Nesvizhskii AI, Omenn GS, Chinnaiyan AM, Sreekumar A, et al. 2010. Quantitative proteomic profiling of prostate cancer reveals a role for miR-128 in prostate cancer. Mol Cell Proteomics. 9:298–312. doi: 10.1074/mcp.M900159-MCP200
- Kida K, Nakajima M, Mohri T, Oda Y, Takagi S, Fukami T, Yokoi T. 2011. PPARα is regulated by miR-21 and miR-27b in human liver. Pharmaceut Res. 28:2467–2476. doi: 10.1007/s11095-011-0473-y
- Klint P, Kanda S, Claessonwelsh L. 1995. Shc and a novel 89-kDa component couple to the Grb2-Sos complex in fibroblast growth factor-2-stimulated cells. J Biol Chem. 270:23337–23344. doi: 10.1074/jbc.270.40.23337
- Kohno M, Pouyssegur J. 2006. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 38:200–211. doi: 10.1080/07853890600551037
- Leskela S, Leandro-Garcia LJ, Mendiola M, Barriuso J, Inglada-Perez L, Munoz I, Martinez-Delgado B, Redondo A, de Santiago J, Robledo M, et al. 2010. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr Relat Cancer. 18:85–95. doi: 10.1677/ERC-10-0148
- Li T, Cao H, Zhuang J, Wan J, Guan M, Yu B, Li X, Zhang W, et al. 2011. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clinica Chimica Acta. 412:66–70. doi: 10.1016/j.cca.2010.09.029
- Liu Y, Liu Q, Jia W, Chen J, Wang J, Ye D, Guo X, Chen W, Li G, Wang G, et al. 2013. MicroRNA-200a regulates Grb2 and suppresses differentiation of mouse embryonic stem cells into endoderm and mesoderm. PloS one. 8:295–298.
- Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. 1992. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 70:431–442. doi: 10.1016/0092-8674(92)90167-B
- Lu W, Wang J, Yang G, Yu N, Huang Z, Xu H, Li J, Qiu J, Zeng X, Chen S, et al. 2017. Posttranscriptional regulation of galectin-3 by miR-128 contributes to colorectal cancer progression. Oncotarget. 8:15242–15251.
- Maina F, Casagranda F, Audero E, Simeone A, Comoglio PM, Klein R, Ponzetto C. 1996. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 87:531–542. doi: 10.1016/S0092-8674(00)81372-0
- Menju T, Nishikawa S, Takahashi K, Neri S, Nakanishi T, Cho H, Shikuma K, Sowa T, Sonobe M, Feller SM, et al. 2016. Abstract 1587: GEP100-Arf6 pathway enhanced by Grb2 expression plays important roles for node-metastasis of lung cancer. Cancer Res. 76:1587–1587. doi: 10.1158/1538-7445.AM2016-1587
- Mizuno Y, Yagi K, Tokuzawa Y, Kanesaki-Yatsuka Y, Suda T, Katagiri T, Fukuda T, Maruyama M, Okuda A, Amemiya T, et al. 2008. miR-125b inhibits osteoblastic differentiation by down-regulation of cell proliferation. Biochem Biophys Res Commun. 368:267–272. doi: 10.1016/j.bbrc.2008.01.073
- Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W. 2005. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem J. 392:249–261. doi: 10.1042/BJ20050908
- Park SY, Jang SY, Shin YK, Yoon BA, Lee HJ, Park HT., et al. 2017. Grb2-associated binder-1 is required for extrafusal and intrafusal muscle fiber development. Neuroreport. 28:604–609 doi: 10.1097/WNR.0000000000000807
- Pasquinelli AE. 2012. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 13:271–282.
- Pendergast AM, Quilliam LA, Cripe LD, Bassing CH, Dai Z, Li N, Batzer A, Rabun KM, Der CJ, Schlessinger J, Gishizky ML. 1993. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 75:175–185. doi: 10.1016/S0092-8674(05)80094-7
- Peng C, Li N, Ng Y-K, Zhang J, Meier F, Theis FJ, Merkenschlager M, Chen W, Wurst W, Prakash N. 2012. A unilateral negative feedback loop between miR-200 microRNAs and Sox2/E2F3 controls neural progenitor cell-cycle exit and differentiation. J Neurosci. 32:13292–13308. doi: 10.1523/JNEUROSCI.2124-12.2012
- Radtke D, Lacher SM, Szumilas N, Sandrock L, Ackermann J, Nitschke L, Zinser E., et al. 2016. Grb2 Is important for T cell development, Th cell differentiation, and induction of experimental autoimmune encephalomyelitis. J Immunol. 196:2995–3005 doi: 10.4049/jimmunol.1501764
- Reja R, Venkatakrishnan AJ, Lee J, Kim B-C, Ryu J-W, Gong S, Bhak J, Park D, et al. 2009. Mitointeractome: mitochondrial protein interactome database, and its application in ‘aging network’ analysis. Bmc Genomics. 10:S20–8. doi: 10.1186/1471-2164-10-S3-S20
- Ren HY, Liu N, Tao C, Zheng JW, Li K. 2013. The expression profile and target gene analysis of mouse(Mus musculus) body size regulation related microRNA-200b(miR-200b). J Agric Biotechnol. 21:47–54.
- Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D., et al. 1993. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 363:83–85 doi: 10.1038/363083a0
- Saha S, Choudhury J, Ain R. 2015. MicroRNA-141-3p and miR-200a-3p regulate insulin-like growth factor 2 during mouse placental development. Mol Cell Endocrinol. 414:186–193. doi: 10.1016/j.mce.2015.07.030
- Santra M, Chopp M, Santra S, Nallani A, Vyas S, Zheng GZ, Morris DC., et al. 2016. Thymosin beta 4 up-regulates miR-200a expression and induces differentiation and survival of rat brain progenitor cells. J Neurochem. 136:118–132 doi: 10.1111/jnc.13394
- Schlaepfer DD, Hanks SK, Hunter T, Van d. 1994. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 372:786–791. doi: 10.1038/372786a0
- Tatar M, Bartke A, Antebi A., et al. 2003. The endocrine regulation of aging by insulin-like signals. Science. 299:1346–1351 doi: 10.1126/science.1081447
- Tidyman WE, Rauen KA. 2009. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 19:230–236. doi: 10.1016/j.gde.2009.04.001
- Wang X. 2008. miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA. 14:1012–1017. doi: 10.1261/rna.965408
- Wang X, Naqa IME. 2008. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics. 24:325–332. doi: 10.1093/bioinformatics/btm595
- Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, Guo S, Wang Y, Fan K, Zhan D, Zha L, Cao Y, Li Z, Cheng X, Zhang Y, Yang X, et al. 2012. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 22:516–527. doi: 10.1038/cr.2011.132
- Ward AC, Smith L, de Koning JP, Van AY, Touw IP. 1999. Multiple signals mediate proliferation, differentiation, and survival from the granulocyte colony-stimulating factor receptor in myeloid 32D cells. J Biol Chem. 274:14956–14962. doi: 10.1074/jbc.274.21.14956
- Watanabe T, Shinohara N, Moriya K, Sazawa A, Kobayashi Y, Ogiso Y, Takiguchi M, Yasuda J, Koyanagi T, Kuzumaki N, Hashimoto A. 2000. Significance of the Grb2 and Son of sevenless (Sos) proteins in human bladder cancer cell lines. Iubmb Life. 49:317–320. doi: 10.1080/15216540050033195
- Yu GZ, Chen Y, Wang JJ. 2009. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 135:1331–1339. doi: 10.1007/s00432-009-0574-8
- Zhang Y, Li Z, Yang M, Wang D, Yu L, Guo C, Guo X, Lin N. 2013. Identification of GRB2 and GAB1 coexpression as an unfavorable prognostic factor for hepatocellular carcinoma by a combination of expression profile and network analysis. Plos One. 8:e85170. doi: 10.1371/journal.pone.0085170
- Zuberi M, Mir AR, Das J, Ahmad I, Javid J, Yadav P, Masroor M, Ahmad S, Ray PC, Saxena A. 2015. Expression of serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological features. Cli Trans Oncol. 17:1–10. doi: 10.1007/s12094-014-1207-5