ABSTRACT
Background: Emergence agitation is a reformed state of mindfulness, which starts with a sudden form of anesthesia and progresses through the early repossession age. Thus, the purpose of this study is to evaluate 1:3 ketofol performance on children 3–15 years old undergoing adenotonsillectomy.Methods: A total of 60 children aged 3–15 years undergoing adenotonsillectomy were randomly allocated to receive low-dose ketamine 0.15 mg/kg followed by propofol 0.45 mg/kg i.v. ketofol (1:3) about 10 min before the end of surgery in comparison to 60 children aged 3–15 years who received only normal saline and dextrose. Anesthesia was induced and maintained with sevoflurane. Postoperative pain and EA were assessed with objective pain score (OPS) and the Pediatric Anesthesia Emergence Delirium (PAED) scale, respectively. EA was defined as a PAED 10 points. Recovery profile and postoperative complications were also recorded.Results: The incidence and severity of EA were found significantly lower in the ketofol group in comparison to the control group with a percentage of (13.33% vs 48.33%) (8% vs 15%) respectively (P < 0.05). Also, the time for interaction from anesthetic tainted to extubating in the ketofol set was significantly less than in the control group (P < 0.05). Interestingly, there are no opposing events such as nausea, laryngospasm, bronchospasm, hypotension, bradycardia, bleeding, or postoperative respiratory depression (respiratory rate: <16) were noticed in the ketofol supervision (P > 0.05). Moreover, the heart rate was meaningfully higher in the control group starting at the time of tracheal extubating in comparison to the children undergone ketofol (P < 0.05). Alert score and time from painkilling tainted till liberation from PACU showed substantial significant changes at ketofol set (P < 0.05).Conclusion: Ketofol (1:3) shows significant performance to reduce postoperative agitation in the children undergone adenotonsillectomy.
1. Introduction
Emergence agitation (EA) is a postoperative uncomfortable phenomenon that increases with common use of sevorane [Citation1]. Several drugs such as propofol, fentanyl, ketamine, clonidine, and precedex have been used to manage postoperative pains and uncomfortable phenomenon [Citation2,Citation3]. Although there are various anesthetic drugs showed interesting findings to reduce postoperative side effects, in this study, we focused on the ketofol performance because of the missing of ketofol optimum mixing ratio and its performance to reduce EA in the children regarding the age variable. To date, ketofol gains increasing interest as an agent for procedural sedation and analgesia. It is a mix of ketamine and propofol in different mix ratios [Citation4,Citation5]. Routinely, ketamine serves as a most operative analgesic in combination with a low-dose opioid. Although it has been used as a sole analgesic agent, pain fitting measurements presented notable advanced serious side effects [Citation6]; for instance, psychological disorders could appear as ketamine common side effects [Citation7,Citation8]. As known, it was classified as a receptor antagonist of N-methyl-D-aspartate (NMDA). However, its crucial mechanism remains unclear [Citation9,Citation10]. Also, its side effects may include confusion, tension, or delirium [Citation11]. Besides, propofol is a short-acting medication used for general anesthesia induction and maintenance. It has a strong effect to decrease consciousness and lack of memory [Citation12]. Certainly, propofol widely recruited as an alternative of sodium thiopental for opening anesthesia, because regaining from propofol is quicker. Recently, propofol was used as a well cast-off for technical sedation [Citation13]. It was also far and wide used for sedation of newborns and kids undergoing MRI [Citation14]. However, its blend with ketamine significantly reduced server side effects [Citation15]. Truly, propofol has been projected to have several dynamics of action, through potentiation of the GABA receptor, thereby slowing the channel-closing time. Propofol similarities also showed acting as sodium channel blockers [Citation16,Citation17].
Table 1. Demographic characteristics, intraoperative managements, and recovery times
Table 2. Scores and analgesic requirements of the sets
Table 3. Paediatric Anesthesia Sudden Delirium (PAED) score
Table 4. Paediatric Anesthesia Sudden Delirium scale and pain score
On the other hand, propofol-ketamine combinations have been used for practical sedation [Citation18] in comparison to ketamine and propofol with propofol alone in the emergency department by many projects, but the optimum mixing concentrations for children’s sedation yet have not been judged. Ketofol advantages could be reduced or giving reverse interaction if the effect of ketamine and propofol does not well balanced. According to the previously published literature, we noticed that ketamine to propofol 1:3 ratio was reduced emergency agitation’s serious complications and provide better results in children in comparison to other mixing ratios [Citation19]. Thus, we supposed that using a 1:3 mixing ratio could be the best choice for our sedation applications. However, until now children’s age groups that have been documented showed a range between 6 months and 10 years, since most of the children undergo adenotonsillectomy operations are school-aged children more than documented ages. Therefore, in this study we report children from 3 to 15 years old underwent emergency adenotonsillectomy those were received ketofol (1:3) induced and maintained with sevoflurane. We also discussed the clinical significance of ketofol for this category of children in comparison to the non-ketofol sedated group.
2. Methodology and procedure
2.1. Ethical approval
In accordance with Huazhong University of Science & Technology’s research ethics committee, the form of patient’s consent has been reviewed, discussed, and approved according to the Wuhan union hospital’s rules and regulations. The relatives (parents) of enrolled children in this study were listened to the researcher’s explanation and freely signed the form of consent to use ketofol.
2.2. Enrollment of participants
Children aged 3–15 years undergoing adenotonsillectomy surgery were enrolled in this study according to parents' consent and eligibility. We have nominated children for whom the handling physician designated ketofol for practical sedation and analgesia. In brief, the inclusion criteria of children eligible for the study were as follows:
Children aged 3 to 15 years with signed written informed consent by parents
Children undergoing adenotonsillectomy surgery
Normal weight and physical examinations
Children having no immunological, neurological, or hematological disorders
Children not having any drug allergy or asthma
Children not having any vascular disorders
While the exclusion criteria were as follows:
Children having any drug allergy to either reading medication or preoperative medication were omitted from the study
Children having any abnormal disorders
2.3. Sample size
The sample size has been decided according to the number of children underwent adenotonsillectomy operations in 2016 in our group at the Department of Anaesthesia, Union Hospital, Wuhan city. Sample size calculation was performed by using Cochran’s formula (90% confidence level and 10% margin of error). Thereafter, the prior power analysis has been calculated for our null hypothesis (10%) to achieve 90% power. Thus, the sample size of this study showed enough confidence to perform clinical trials.
2.4. Procedures
2.4.1. Ketofol preparation and administration
We have prepared ketofol as a 1:3 blend of 0.15 mg/kg ketamine and 0.45 mg/kg propofol mixed in one syringe fitted with a blind tip conduit. We then performed procedural sedation and analgesia using ketofol by the intravenous administration of 1 to 3 mL aliquots titrated at the discretion of the physician administering the medication. Sixty Children received opioid analgesia before the procedure at the discretion of the treating physician. The dose and timing of medication administration, together with the ultimate depth of sedation achieved were intentionally not standardized to more accurately reflect the variability in physician preference, patient response, and the differing procedural sedation and analgesia requirements for various procedures in emergency medicine practice.
2.4.2. Checking process
Children were abstained for 6 h before the process. Then we made standard checking (electrocardiography (ECG), non-invasive blood pressure (NIBP), pulse oxygen inundation (SpO2), and Bispectrality index (BIS)) associated with children in the surgical treatment room. All enrolled children were not premeditated before the process. Anesthesia was encouraged via a face-mask with oxygen–air combination and sevoflurane with increments of 1% at each breath up to 8%. Once mindfulness was missing, intravenous access was recognized and an infusion of balanced salt solution was directed according to standard fluid management Guidelines. Thereafter, tracheal intubation was done after realizing the adequate depth of anesthesia and without the use of neuromuscular blocking drugs. After the orientation of anesthesia and before the surgical opening, children were allocated to one of the two sets according to a computer-produced randomization database, through double-blind process. We used an internet site program http://www.random.org to produce sequenced random numbers. Next, to preserve the BIS score among 40 and 60 the inhalation agent was titrated, while ventilation was measured to sustain the end-tidal carbon dioxide (EtCO2) between 35 ± 4 mmHg. Furthermore, baseline hemodynamic variations were kept within a ±20% range. Also, to prevent postoperative nausea, dexamethasone (0.2 mg/kg) was intravenously directed. Yet, children were established 1:3 ketofol (0.15 mg/kg ketamine to 0.45 mg/kg propofol) about 10 min before the end of surgery or volume-matched normal saline (Control set, n = 60).
2.4.3. Measuring procedure
Later, we settled the procedure, sevoflurane was unobtainable, and the O2 flow rate was amplified to 10 L/min. The following time intermissions were detailed: The duration of anesthesia (was measured from the start of sevoflurane induction until endotracheal tube (EET) removal), interval of surgery (was defined as the time between the insertion and removal of the Boyle-Davis mouth gag), time to remove ETT (defined as time from discontinuation of sevoflurane to tracheal extubation), interface time (was defined as spontaneous eye opening or on vocal appreciation from sevoflurane discontinuation), and duration of PACU stay (was measured from arrival to PACU until discharge). Endotracheal tube was uninvolved when breathing was steady and adequate in rate and depth. Study drugs were arranged and hidden behind drapes and controlled by a self-governing anesthesiologist who was blinded to the patient set allocation.
2.4.4. Blood oxygen saturation level (SpO2)
Intraoperative hemodynamic data, level of anesthesia and SpO2 were verified by the same anesthesiologist every 5 min. The anesthesiologists who achieved and preserved the anesthesia did not participate in any of the postoperative evaluations.
2.5. Observations
The primary outcome of this study was the Pediatric Anesthesia Sudden Delirium (PAED) scale in the post anesthesia care unit (PACU). Postoperative pain and EA were assessed for 60 min by a blinded care unit nurse. Because most of the EA incidents ensued within 30 min of PACU arrival [Citation20], EA was appraised interval 5 min for the first 30 min and then every 10 min for the outstanding 30 min. It includes five objects (eye contact with the caregiver, purposeful action, and awareness of surroundings, restlessness, and inconsolability). Each item was scored by five scores (0 to 4) permitting to its degree, for a maximum of 20 points. Anxiety scores ≥10 were observed as presence of tension, and scores ≥15 were viewed as severe nervousness. Postoperative pain was gauged by independent pain score (OPS), a test used to judge pain in children, at the same time intermissions. Postoperative Vomiting (POV) was appraised using a numeric rank score (0–2), where a score of 0 = no vomiting, 1 = vomited once, and 2 = vomited twice or more. Throughing up was not recorded because it was difficult to be evaluated in the children. HR, NIBP, RR, and SpO2 were noticed in the PACU each 5 min for the first 15 min, then at 15-min intermissions for the remaining 45 min. Any desaturation episode with SpO2 below 95% was well known. Difficulties during the appearance period and in the PACU, such as laryngospasm, bronchospasm bradycardia, respiratory downheartedness, hypotension, and nausea were logged and achieved suitably. Children were cleared from the PACU when they were serene and had an Aldrete score of ≥9.
2.6. Statistical analysis
Statistical analysis was performed by using SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA). Data were analyzed by using SD and paired t-test for associated factors. All P-values were 2-tailed and P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Enrolled case selection
Initially, 128 children were enrolled in this study; 8 children were later excluded from the study, because 2 of them were pre-injected with intravenous midazolam (40 μg/kg), 2 due to crying, and 2 due to an upper respiratory tract infection (URI). Meanwhile, there are two cases were omitted because of allergy. Thus, a total of 120 children completed the study.
3.2. Demographic characteristics and intraoperative management
Enrolled children were allocated in two sets: one is the control group in which children were not receiving ketofol (set A), and another set was the children who received 1:3 ketofol (set B). Statistical analysis of patient’s demographic data showed no significant differences between children in both sets regarding the number of children, age, sex, weight, ASA physical status, and duration of ketofol infusion as seen in . Also, the duration of mental orientation, tension score, and duration of surgery represented no statistical differences among the tested groups.
Furthermore, statistical analysis of recovery times, anesthetics consumptions, and consumption of antiemetic revealed that ketofol significantly improved time recovery, anesthetics, and antiemetic consumptions, which indicated that ketofol at 1:3 ratios significantly, improves postoperative symptoms.
These findings confirmed by analysis of ambulation and discharge time that also presented a significant reduction of ambulation and actual discharge times in the ketofol-treated individuals.
3.3. Scores and analgesic requirements
Evaluation of postoperative pain scores and analgesic requirements was extensively searched. Several techniques and treatments were implemented to reduce pain sufferings. Ketofol was mainly used to achieve this issue (pain limitations). Interestingly, we noticed that ketofol significantly reduced oculocardiac reflex time in comparison to ketofol untreated group (P = 0.001) as shown in ; this is indicating a protective effect of ketofol (1:3) against OCR. Also, the preoperative tension score represented no statistical differences between both tested sets, since the postoperative tension score exhibited notable differences from 5.7 to 4.8 score as presented in Table 2. Moreover, Faces Pain Scale (FPS) during awakening presented significant effect of ketofol in comparison to control group (P = 0.001) from five scores of set A to two scores in the ketofol set B. Also, the observation of Ramsay Sedation Score during awakening and postoperative 60th min showed good sedation quality in the ketofol group (P = 0.01) in comparison to control group. Interestingly the numeric rating of postoperative pain presented a significant reduction of postoperative pain in the ketofol group that provided a comfortable awake up. Furthermore, the satisfaction score of surgeons during the procedure showed significant satisfaction of ketofol 1:3 administration in comparison to the control group. These results suggest that ketofol efficiently improves pain reduction, comfortable and stability score, which eliminates postsurgical pain therapy.
3.4. The effect of ketofol on the pediatric anesthesia sudden delirium scores
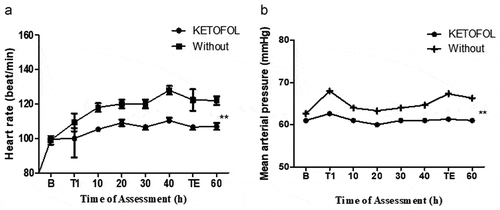
Intraoperative data presented that the Paediatric Anesthesia Sudden Delirium (PEAD) scale/peak score in ketofol preserved group was meaningfully abolished as shown in . We noticed that the time for interaction from anesthetic tainted to eye opening and while from anesthetic tainted to extubation in ketofol group in comparison to non-ketofol were significantly less than in ketofol group (P < 0.05). Also, Aldert score and time from painkilling tainted till liberation from PACU showed substantial changes at Ketofol set (P < 0.05). In addition, tension PEAD score, tension severe score, and OPS score were significantly improved at ketofol set in comparison to untreated set as seen in . Further analysis of heart (beat/min) and arterial pressure (mmHg) represented significant performance of ketofol (1:3) on the cardiovascular signs as seen in .
3.5. Ketofol performance and postoperative complications
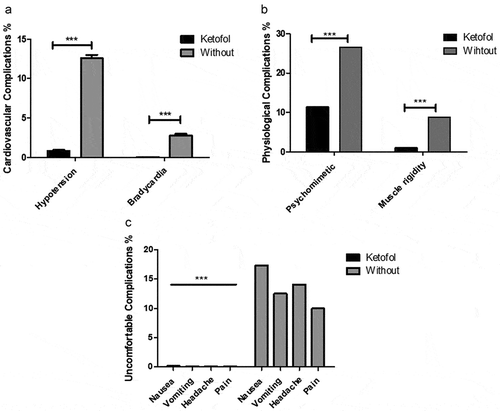
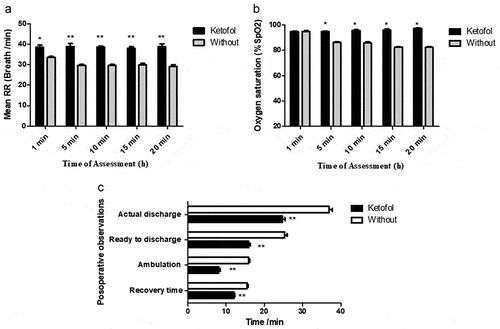
Furthermore, we noticed that ketofol significantly improved postoperative criteria in comparison to ketofol untreated group. Postoperative cardiovascular and physiological criteria showed significant improvement in the ketofol-treated group in comparison to the untreated group as presented in ). Moreover, there were no opposing events such as nausea, laryngospasm, bronchospasm, hypotension, bradycardia, bleeding, or postoperative respiratory depression (respiratory rate: <16) ). Consequently, we have recorded the mean of respiratory rate RR and percentage of oxygen saturation rate (SpO2%) at 5-min intermissions after the induction of anesthesia as shown in (a, b). We observed that the ketofol-treated set significantly showed stable respiratory rate in comparison to untreated set 39 ± 2 and 29 ± 3, respectively ()). Oxygen overload percentage was also meaningfully improved at ketofol set in comparison to untreated set 97 ± 3 and 83 ± 5, respectively ()). RR and SpO2% of untreated set decreased over time due to increased depth of anesthesia, with a significant (P < 0.05) difference noticed between the RR at 10 and 20 min. Further investigation of postoperative observations represented significantly improved recovery, ambulation, and discharge times in comparison to untreated set ()). We found that ketofol performance exhibited good quality circumstances to reduce anesthesia side effects. It efficiently eliminated postoperative analgesic needs and provides comfortable conditions at (1:3) blend rate.
4. Discussion
Usually, practical sedation is performed to deliver a satisfactory level of sedation besides diminishing pain, anxiety, and diminishing drug-associated adverse events [Citation21]. It is also used to regulate adverse behavior and preserving a stable cardiovascular and respiratory rank. A number of readings have proven that the blend of ketamine and propofol (ketofol) for sedation is safe and actual [Citation22,Citation23]. It looks to decrease the side effects of each medication used alone, and allows for a quick recovery time [Citation4,Citation5]. Therefore, in this study we performed ketofol 1:3 (ketamine/iprivan) to diminish practical sedation worse side effects. The statistical analysis offered momentous enhancements at ketofol cured set. They displayed early recovery and short discharge times in contrast to unprocessed set; this is directed that ketofol efficiently diminishes anesthetic long-time sedation. These findings confirm the conclusion of Ebru TK and Resul K, 2019 that described ketofol efficacy. They revealed that ketofol significantly reduces postoperative complications and shorts a recovery time [Citation24]. Furthermore, ketofol 1:3 blend ratio displayed an anticipated depth of sedation and abort pain impression which was due to less ketamine content in such distillation. This mixture (1:3) has been reported by Oh et al. (2019) for improving sedation quality among adults who underwent loop electrosurgical excision procedure (LEEP) because it reduces procedural interference during LEEPs due to hemodynamic and respiratory stability [Citation25]. Our outcomes are dependable with Furuya et al. [Citation26] and Lee et al. [Citation27] who proposed that the negligible change witnessed in arterial burden may be dose linked to and also as sympathomimetic actions of ketamine were effective in counter-acting the hemodynamic despair of propofol. Akin et al. [Citation28] printed a trial of 60 children amongst 1 month and 13 years of age suffering cardiac catheterization who established sedation with diprivan or diprivan plus ketamine (3:1). They found an important reduction in MAP in 11 children in the diprivan monotherapy set and three children in the ketofol set. They determined that the calculation of low-dose ketamine to diprivan well-preserved MAP deprived of extending recovery or cumulative the frequency of adversative events. There were no post-procedural psychotomimetic symptoms recorded in set B. In accumulation, the patient’s mood was suggestively healthier in the recovery room and intellectual function recovered more rapidly in such set than those untreated set A. this is confirmed the same results were reported by Daabiss et al. [Citation29]. In our study, we noticed that a low dose of ketamine in the ketofol blend showed a significant improvement in the postoperative tension score, Ramsay sedation score, and faces pain scale during awakening, which significantly reduced postoperative analgesic needs in the ketofol-treated set in comparison to untreated set. These findings ensured a similar conclusion of Willman and Andolfatto [Citation5]. Interestingly, ketamine low dose has significantly eliminated postoperative (numeric rank score) and sore throat. Recently, the meta-analysis study of Ghojazadeh et al. (2019) on the adults in the emergency department concluded that ketofol administration showed less respiratory adverse effects than propofol alone in emergency department procedural [Citation30]. On the other hand, our results represented that age, sex, and body mass did not show any statistical differences between both sets, which matches with most of the previous studies [Citation31,Citation32]. Ketofol (1:3) suggestively decreases the incidence and severity of post sevorane EA in children undergoing adenotonsillectomy. Likewise, the pain scale shows a lower OPS score in the ketofol set in comparison to the untreated set. This indicated a promising effect of ketofol (1:3) to reduce postoperative severe tension. As known, sudden tension is a considerable side effect post-sevorane anesthesia in pediatric. Some of the previous literature conducted that tension incidence after sevorane anesthesia could rise up to 80% [Citation33,Citation34]. The ketamine small measures showed less respiratory depression, little effect on the blood pressure and heart rate. So, the ratio of 0.16 mg/kg ketamine to 0.5 mg/kg propofol (1:3) presented the optimum blend to reduce postoperative side effects in the wide range of children undergoing adenotonsillectomy. Interestingly, we did not use fentanyl or any other intraoperative analgesic, which could affect result interpretation; meanwhile, we found that ketofol successfully reduced the incidence of postoperative pain or any other compilations in the treatment set, with no adverse effects. Cardiovascular, physiological, and other uncomfortable complications were significantly restricted in comparison to untreated set.
Furthermore, hemodynamic changes are serious complications during anesthetic induction with intravenous anesthesia. Therefore, many reports explained the need for ketofol to provide hemodynamic stability and prevent postoperative agitation [Citation4,Citation18,Citation22]. As known, propofol injection usually leads to hypotension that increases heartbeats. It increases the main arterial pressure and present instability. Besides postoperative agitation was noticed in the individuals were received propofol [36]. Moreover, ketamine sympathomimetic properties result in an increase in blood pressure and heart rate. It has not superior to reduce postoperative agitation. Interestingly a combination of propofol and ketamine showed high efficiency to reduce postoperative agitation with different hemodynamic scores upon the mixing ration of these drugs. Our results interestingly report the satisfactory hemodynamic stability by a blend of 0.15 mg/kg ketamine and 0.45 mg/kg propofol, as well as significant elimination of postoperative agitation in the children. Heart beating and arterial pressure, as well as breathing ratio all, showed convenient hemodynamic stability in comparison to ketofol untreated group. Similar to our findings, Hosseinzadeh et al. noticed that using ketamine and propofol decreases the trend of HR in patients. However, other articles reported an increase in HR after ketofol administration which can be explained on the basis of cardio stimulant effect of ketamine and stress response during intubation. But, in our study, the stability of HR may be a dependent dose of ketamine used and gentle intubation that would prevent stress response.
In conclusion, these findings showed significant efficiency of ketofol (1:3) to reduce postoperative pain and complications for children who underwent anesthesia. The relevant limitation of our study is that no children received any premedication before surgery. This may be the reason why more children in the control set experienced a high incidence of postoperative EA.
Data availability
Authors declare that experimental data are available for interested researchers by sending email to the corresponding author.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81671890). We would specially thank Shihai Zhang and Shujun Sun for helpful assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Marzullo LR, Laurie R. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269–9.
- Huddy N. Emergence agitation in children. Br J Anaesth. 2010;105(1):95–96.
- Kwak KH. Emergence agitation/delirium: we still don’t know. Korean J Anesthesiol. 2010;59(2):73–74.
- Arora S. Combining ketamine and propofol (“Ketofol”) for emergency department procedural sedation and analgesia: a review. West J Emerg Med. 2008;9(1):20–23.
- Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (Ketamine/Propofol Combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49(1):23–30.
- Elia N, Tramer MR. Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain. 2005;113(1):61–70.
- Alhasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363–1381.
- Schonenberg M, Reichwald U, Domes G, et al. Ketamine aggravates symptoms of acute stress disorder in a naturalistic sample of accident victims. J Psychopharmacol. 2008;22(5):493–497.
- Paslakis G, Gilles M, Meyer-Lindenberg A, et al. Oral administration of the NMDA receptor antagonist S-ketamine as add-on therapy of depression: a case series. Pharmacopsychiatry. 2010;43(01):33–35.
- Dinis-Oliveira RJ. Metabolism and metabolomics of ketamine: a toxicological approach. Forensic Sci Res. 2017;2(1):2–10.
- Lalanne L, Nicot C, Lang J-P, et al. Experience of the use of Ketamine to manage opioid withdrawal in an addicted woman: a case report. BMC Psychiatry. 2016;16(1):395.
- Miner JR, Burton JH. Clinical practice advisory: emergency department procedural sedation with propofol. Ann Emerg Med. 2007;50(2):182–187.e1.
- McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67(6):910–923.
- Machata AM, Willschke H, Kabon B, et al. Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging. Br J Anaesth. 2008;101(2):239–243.
- Yan JW, McLeod SL, Iansavitchene A. Ketamine-propofol versus propofol alone for procedural sedation in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2015;22(9):1003–1013.
- Trapani G, Altomare C, Sanna E, et al. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000;7(2):249–271.
- Kotani Y, Shimazawa M, Yoshimura S, et al. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14(2):95–106.
- Phillips W, Anderson A, Rosengreen M, et al. Propofol versus propofol/ketamine for brief painful procedures in the emergency department: clinical and bispectral index scale comparison. J Pain Palliat Care Pharmacother. 2010;24(4):349–355.
- de Moraes AG, Africano CJ, Hoskote SS, et al. Ketamine and propofol combination (“Ketofol”) for endotracheal intubations in critically Ill patients: a case series. Am J Case Rep. 2015;16:81–86.
- Dunn T, Mossop D, Newton A, et al. Propofol for procedural sedation in the emergency department. Emer Med J. 2007;24(7):459–461.
- Upadhyay SP, Tripathy A, Mallick PN. A practical guide to sedation and analgesia in Paediatric Intensive Care Unit (ICU). J Anim Sci. 2017;4(1).
- Alletag MJ, Auerbach M, Baum CR. Ketamine, propofol, and ketofol use for pediatric sedation. Pediatr Emerg Care. 2012;28(12):1391–1395.
- Gokcinar D, Ergin V, Cumaoglu A, et al. Effects of ketamine, propofol, and ketofol on proinflammatory cytokines and markers of oxidative stress in a rat model of endotoxemia-induced acute lung injury. Acta Biochim Pol. 2013;60(3):451–456.
- Tarıkı Kılı Ebru KR. Comparison of ketamine-propofol mixture (ketofol) and midazolam-meperidine in endoscopic retrograde cholangiopancreatography (ERCP) for oldest old patients. Ther Clin Risk Manag. 2019;15:755–763.
- Oh C, Kim Y, Eom H, et al. Procedural sedation using a propofol–ketamine combination (Ketofol) vs. propofol alone in the Loop Electrosurgical Excision Procedure (LEEP): a randomized controlled trial. J Clin Med. 2019;8(7):943.
- Furuya A, Matsukawa T, Ozaki M, et al. Intravenous ketamine attenuates arterial pressure changes during the induction of anaesthesia with propofol. Eur J Anaesthesiol. 2001;18(2):88–92.
- Lee J, Park H-P, Jeong M-H, et al. Efficacy of ketamine for postoperative pain following robotic thyroidectomy: A prospective randomised study. J Int Med Res. 2018;46(3):1109–1120.
- Akin A, Esmaoglu A, Guler G, et al. Propofol and propofol–ketamine in pediatric patients undergoing cardiac catheterization. Pediatr Cardiol. 2005;26(5):553–557.
- Daabiss M, Elsherbiny M, Alotibi R. Assessment of different concentration of ketofol in procedural operation. Br J Med Pract. 2009;2(1):27–31.
- Ghojazadeh M, Sanaie S, Paknezhad SP, et al. Using ketamine and propofol for procedural sedation of adults in the emergency department: a systematic review and meta-analysis. Adv Pharm Bull. 2019;9(1):5–11.
- Mortero RF, Clark LD, Tolan MM, et al. The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesthesia Analg. 2001;92(6):1465–1469.
- Akin A, Guler G, Esmaoglu A, et al. A comparison of fentanyl-propofol with a ketamine-propofol combination for sedation during endometrial biopsy. J Clin Anesth. 2005;17(3):187–190.
- Mohamed AA. Prevention of sevoflurane agitation in children undergoing congenital hernia repair, impact of adding dexmedetomidine to caudal analgesia. Egypt J Anaesth. 2015;31(3):227–231.
- Vlajkovic G, Sindjelic R. Emergence delirium in children: many questions, few answers. Anesthesia Analg. 2007;104(1):84–91.