ABSTRACT
β-Citronellol is a monoterpene alcohol found in essential oils of various aromatic plant species. The physiological effects of β-citronellol inhalation on the central nervous system remain unclear. We investigated the effects of β-citronellol inhalation on mouse behavior. First, we examined whether the odor of β-citronellol was attractive or repellent to mice. Then, following 30 minutes of β-citronellol inhalation, a series of behavioral tests (elevated plus maze, open field, Y-maze, tail suspension, and forced swim tests) were performed. Mice were neither attracted to nor repelled by β-citronellol. Mice that inhaled β-citronellol showed an increase in anxiety-like behavior in the elevated plus maze and open field tests. Performance in the Y-maze and forced swim tests was not affected. These results indicate that β-citronellol acts on the central nervous system of mice following inhalation and increases anxiety. Essential oils and cosmetics containing β-citronellol should be used with caution.
1. Introduction
Existing treatments for various diseases including central nervous system disorders and inflammatory diseases are not always effective [Citation1,Citation2]. There is a growing need for therapeutic options that are more effective and have fewer side effects. In recent years, interest in alternative medicine approaches, including aromatherapy, has increased. Aromatherapy is a type of complementary and alternative medicine widely used in the management of chronic pain, depression, anxiety, insomnia and stress-related disorders [Citation4,Citation5]. However, scientific evidence for the effects of many essential oils used in aromatherapy is lacking [Citation5,Citation6]. Further, the mechanisms underlying the action of inhaled odor molecules on the central nervous system are unknown.
Several studies have reported that essential oils used in aromatherapy have neurophysiological effects [Citation7–Citation9]. These effects are attributed to monoterpenes, which are the main chemical components of essential oils [Citation10]. Monoterpenes are also the main chemical constituents of the aromatic components obtained by steam distillation or solvent extraction from a wide variety of aromatic plants. Indeed, they are also found in edible plants and those that have established therapeutic properties [Citation11].
Citronellol, a monoterpene alcohol, is a naturally occurring monoterpene compound widely found in essential oils of various aromatic plant species, such as Cymbopogon citratus [Citation12], C. winterianus [Citation13], and Lippia alba [Citation14]. β-citronellol (3,7-dimethyl-6-octen-1-ol; CAS number 106–22-9) is a volatile non-cyclic monoterpene found naturally in the essential oils of several plant species worldwide. β-citronellol is naturally abundant as a volatile component responsible for the pleasant aroma and flavor of fruits. β-citronellol has odor properties that render it useful in the perfume industry [Citation15]. It is generally recognized as an edible compound. The acceptable daily intake of β-citronellol is 0.5 mg/kg body weight [Citation3]. Some pharmacological actions of citronellol have been studied [Citation16,Citation17], including antibacterial, antifungal, antihypertensive, vasorelaxant and anticonvulsant effects [Citation10,Citation18,; Citation19].
β-citronellol is found in the environment, in cosmetics, softeners, and other scented products. β-citronellol in essential oils is absorbed into the olfactory and respiratory systems by inhalation or enters the body percutaneously by massage [Citation20]. However, the biological effects of β-citronellol inhalation are not well established. The purpose of this study was to clarify what changes in emotion and behavior are caused by inhalation of β-citronellol in mice. This study describes some actions of β-citronellol and suggests a physiological basis of essential oils as a function of aromatherapy.
2. Materials and methods
2.1. Animals
Male C57BL/6 mice, aged 11 weeks, were used for these experiments. We used male mice for these studies to eliminate the effects of the estrous cycle in females. Mice were randomly divided into two groups; one group was exposed to β-citronellol (n = 10) and the other was not (n = 10). We purchased the animals from Charles River Laboratories (Kanagawa, Japan) and housed them in cages with ad libitum access to food and water in a 12-/12-h light/dark cycle and a temperature range of 23°C–26°C. All efforts were made to minimize the suffering and number of animals used. These experiments complied with the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised in 1996) and were approved by the Committee for Animal Experiments at Kawasaki Medical School Advanced Research Center.
2.2. Inhalation of β-citronellol
The inhalation apparatus was the same as that used previously [Citation21]. Inhalation was carried out in a sealed container. β-citronellol occurs widely in nature and animals may be exposed when breathing in air. No other information about potential health effects in animals inhaled β-citronellol was reported. We have chosen 2 mL of β-citronellol for inhalation. A piece of absorbent cotton (4 × 4 cm) impregnated with 2 mL of β-citronellol (or saline control) was placed in a stainless-steel container (60 × 60 × 35 mm) capped by a lid with holes. The mice were unable to lick or touch the cotton. The container was placed in a clean breeding cage (235 × 325 × 170 mm) surrounded by two larger cages (292 × 440 × 200 mm). Approximately 20 min after cotton placement, mice were placed into the internal cage for 30 min, prior to behavioral testing.
2.3. Behavioral tests
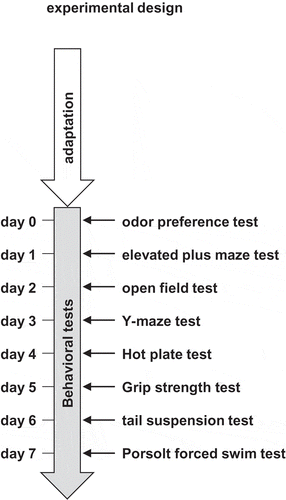
All behavioral experiments were performed during the light cycle (9:00–16:00). Each behavioral test was separated from the next by at least one day (). Mice were tested in random order. After completion of each test, the apparatus was cleaned with 70% ethanol and water containing superoxidized hypochlorous acid to prevent bias due to olfactory cues.
2.4. Odor preference test
β-citronellol was acquired from FUJIFILM Wako Pure Chemical Corporation (325–53052, Osaka, Japan). Saline was used as a control. The testing apparatus was rectangular (30 × 60 × 40 cm) and contained two transparent cages (7.5 × 7.5 × 10 cm, with several holes 1 cm in diameter) placed at both ends of the apparatus. Each mouse was placed in the box for 6 min and allowed to freely explore, to habituate. Exposure to olfactory stimuli was performed by gently impregnating a piece of absorbent cotton (4 × 4 cm) with 200 μL of saline or β-citronellol. The absorbent cotton was placed in one of the transparent cages located in the corners of each lateral compartment. Approximately 1 min after cotton placement, mice were placed into the box. The subject was placed in the center of the box and allowed to explore for 6 min. One side of the rectangular area was identified as the β-citronellol area and the other as the control area. The amount of time spent in and around each cage during the 6 min session was measured. The preference index for β-citronellol was calculated as follows: time spent around β-citronellol/[time spent around control + time spent around β-citronellol]. After completion of each test, the apparatus was cleaned with water containing superoxidized hypochlorous acid to prevent bias due to olfactory cues. All components of the apparatus were cleaned after each phase of this test. After 10 min of ventilation, each mouse was individually placed into the test box. The test was conducted prior to other behavioral experiments. Data were video recorded and analyzed using video tracking software (ANY-MAZE, Stoelting Co., Wood Dale, IL, USA).
2.5. Hot plate test
The hot plate test was used to evaluate nociception (sensitivity to a painful stimulus) [Citation22]. Mice were placed on a plate heated to 55.0°C ± 0.3°C, and the latency to the first paw response was recorded. Paw responses included foot shakes or paw licks. A latency period of 30 s was defined as complete analgesia and used as the cut-off time to prevent tissue injury.
2.6. Neuromuscular strength evaluation
Neuromuscular strength was examined just once using the grip strength test. A grip strength meter was used to assess forelimb strength. Mice were lifted and held by the tail such that their forepaws could grasp a wire grid; they were then pulled back gently until they released the grid. The peak force applied by the forelimbs was recorded in Newton (cN).
2.7. Elevated plus maze test
Anxiety-like behavior was examined using the elevated plus maze. The apparatus consisted of two open arms (8 × 25 cm) and two closed arms of the same size, with 30 cm high transparent walls. The arms were constructed from white plastic plates and elevated to a height of 40 cm above the floor. Arms of the same type were located opposite each other. Each mouse was placed in the central square of the maze, facing one of the closed arms, and could move freely between the four arms for 10 min. The mice were video recorded and the number of arm entries, distance traveled (m), and time spent in the open arms were analyzed using ANY-MAZE software.
2.8. Open field test
Exploratory behavior, anxiety-like behavior, and general locomotor activity were examined using the open field test. Each mouse was placed in the center of the apparatus consisting of a square area surrounded by walls (45 × 45 × 40 cm). The total distance traveled (m) and the time spent in the central area (s) were recorded. The central area was defined as the middle 20 × 20 cm area of the field. The test chamber was illuminated at 100 lux. Data were collected over a 30 min period. Data analysis was performed using ANY-MAZE software.
2.9. Y-maze test
Spatial working memory was measured using a Y-maze apparatus (arm length: 40 cm, arm bottom width: 3 cm, arm upper width: 10 cm, height of wall: 12 cm). Mice were placed at the center of the Y-maze for 10 min. Visual cues were placed around the maze in the testing room and were constant throughout the testing sessions. Mice were tested with no previous exposure or habituation to the maze. The total distance traveled (m), the number of entries, and the number of alternations were recorded and analyzed using ANY-MAZE software.
2.10. Tail suspension test
Depression-like behavior was examined using the tail suspension test. Each mouse was suspended by the tail at 60 cm above the floor in a white plastic chamber using adhesive tape placed <1 cm from the tip of the tail. The resultant behavior was recorded for 6 min. Images were captured via a video camera, and immobility time was measured. In this test, the ‘immobile period’ was defined as the period when the animals stopped struggling for ≥1 s. Data acquisition and analysis were performed using ANY-MAZE software.
2.11. Porsolt forced swim test
The Porsolt forced swim test (PFST) was also used to examine depression-like behavior. The apparatus consisted of four Plexiglas cylinders (20-cm height × 10-cm diameter). The cylinders were filled with water (23°C) to a depth of 7.5 cm, based on previous studies [Citation23,Citation24]. The mice were placed into the cylinders for 6 min and recorded. As in the tail-suspension test, immobility time was evaluated using ANY-MAZE software.
3. Statistical analyses
Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test, two-way repeated measures ANOVA followed by Fisher’s LSD test, Student’s t-tests, or paired t-tests. Differences with a p-value <0.05 were regarded as statistically significant. Data are presented as box plots.
4. Results
4.1. β-Citronellol odor preference
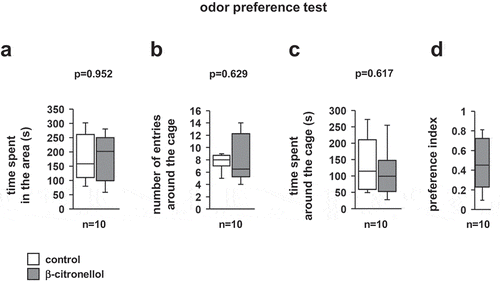
We examined whether mice found β-citronellol repulsive or attractive. No differences were observed in the time spent in different areas of the apparatus, the number of entries around the cage, or time spent around the cage (, t = −0.061, p = 0.952; ), t = −0.498, p = 0.629; , t = 0.516, p = 0.617). The β-citronellol preference index was approximately 0.5 ().
4.2. Effect of β-citronellol on neuromuscular strength and pain response
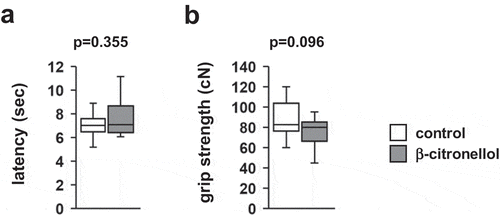
To evaluate the acute effect of β-citronellol on thermal pain, we used the hot plate test. Mice were placed on a hot plate to assess nociception. There were no significant differences in the pain threshold between mice exposed to β-citronellol and control mice (, t = −0.949, p = 0.355).
We compared the neuromuscular strength of mice exposed to β-citronellol and control mice. There were no significant differences in grip strength between the two groups (, t = 1.750, p = 0.096).
4.3. Effect of β-citronellol in the elevated plus maze test
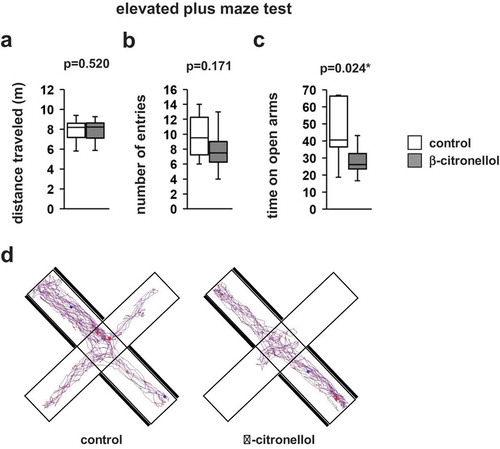
Using the elevated plus maze, we evaluated anxiety-like behavior in mice after β-citronellol inhalation. No differences were observed in the total distance traveled (, t = 0.655, p = 0.520) or the total number of entries into open arms (, t = 1.426, p = 0.170). The time spent in the open arms was significantly lower in mice exposed to β-citronellol than in control mice (, t = 2.456, p = 0.024).
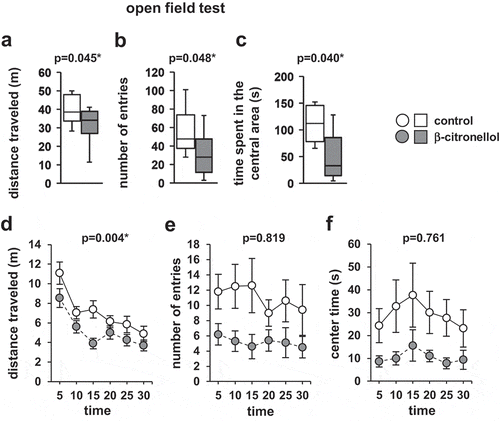
4.4. Effect of β-citronellol in the open field test
In the open field test, we observed significant difference in the total distance traveled (, t = 2.150, p = 0.045, D, F5,90 = 1.924, p = 0.004), the number of total entries into the central area (, t = 2.111, p = 0.048, E, F5,90 = 0.744, p = 0.761), or the time spent in the central area (, t = 2.219, p = 0.040, F, F5,90 = 0.324, p = 0.819) between the two groups.
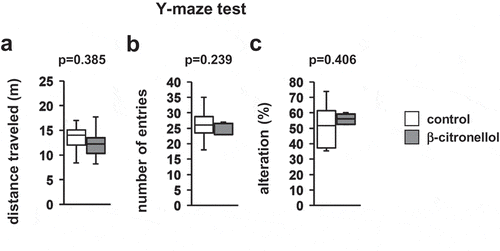
4.5. Effect of β-citronellol in the Y-maze test
We examined the effect of β-citronellol on short-term spatial working memory by monitoring spontaneous alternation in the Y-maze test. There were no significant differences between the groups in the total distance traveled (, t = 0.888, p = 0.385), number of arm entries (, t = 1.215, p = 0.239), or alternation percentage (, t = −0.850, p = 0.406).
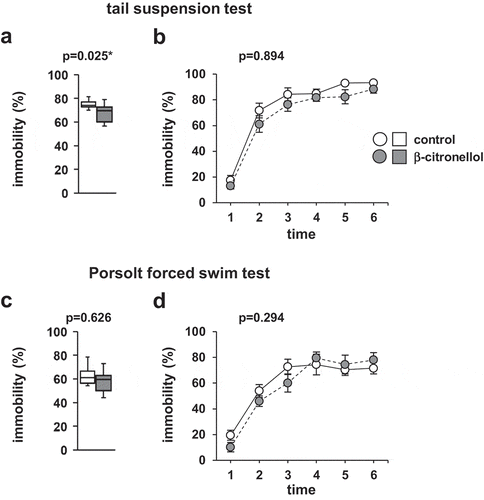
4.6. Effect of β-citronellol on depressive-like behavior in mice
We evaluated depression-like behavior in mice after β-citronellol inhalation. In the tail-suspension test, the percentage of time spent immobile was significantly lower in mice exposed to β-citronellol than in control mice (, t = 2.436, p = 0.025). There were no significant differences between the groups in the proportion of time spent immobile in each 1 min period (, F5,90 = 0.318, p = 0.893). In the PFST, no significant differences were observed between the groups (, t = 0.495, p = 0.626; , F5,90 = 0.293, p = 0.293).
5. Discussion
In this study, we show that inhalation of β-citronellol increases anxiety-like behavior in mice, as determined by the lower time spent in open arms of the elevated plus maze, and the decreased activity in the open field. Inhalation of β-citronellol caused no clear changes in several other behavioral tests compared to control mice.
Mice exhibited neither favorable nor repellent behavior to β-citronellol. This is consistent with previous reports of interest in citronellol in mice [Citation25]. Furthermore, this result indicates that repulsive odors like those of predators do not increase anxiety in mice.
Using the hot plate test, we found that inhalation of β-citronellol showed no analgesic effect. However, citronellol has previously been reported to have central analgesic effects at several doses [Citation26]. These effects, as well as similar ones observed following administration of monoterpenes such as citronellal [Citation27,Citation28], α-terpineol [Citation29], and linalool [Citation30], suggest that the opioid system is involved in the analgesic action of citronellol. The discrepancy between those results and the ones of the present study may be due to differences in the dose administered or the route of administration. To further elucidate any analgesic effect of β-citronellol by inhalation, it would be useful to investigate varying the inhaling time and dose.
We found no effect of β-citronellol inhalation on motor function. This is in line with a previous report showing that intraperitoneal administration of citronellol has no effect on motor function [Citation26], and inhalation of citronellol had no effect on forelimb grip strength.
To determine whether inhalation of β-citronellol affected cognitive function, which has not been previously explored in the literature, we performed the Y-maze test. We found no effect of β-citronellol in this test.
Our results showed that following β-citronellol inhalation, mice exhibited reduced depression-like behavior in the tail suspension test. This is the first report of this finding to our knowledge, and it aligns with other reports that essential oils containing citronellol reduce depression-like behavior [Citation31]. However, in the forced swimming test, mice that inhaled β-citronellol did not exhibit reduced depression-like behavior. Thus, the influence of β-citronellol on depression-like behavior in the tail suspension test may be due to an anxiety-increasing effect. Further studies are needed to clarify the effects of β-citronellol on depression-like behavior.
This study showed that mice that inhaled β-citronellol exhibited increased anxiety-like behavior in the elevated plus maze and open field test. There are reports of the anxiolytic effects of citronellol using the Geller and Vogel competition tests in mice [Citation32]. To the best of our knowledge, this is the only report that has shown the effect of citronellol on anxiety-like behavior. However, it is difficult to compare the results of behavioral experiments performed in mice using intraperitoneal injections, which is highly stressful compared to administration by inhalation. Furthermore, intraperitoneal administration of scent molecules is an unrealistic administration method in humans. Nonetheless, these results show that citronellol is likely to affect anxiety.
In addition, it has been reported that inhalation of odorant molecules such as linalool [Citation33], limonene [Citation34], linalool oxide [Citation35], and α-pinene [Citation36] results in anxiolytic action. It is interesting to note that although there are reports of anxiolytic effects of odor molecules, there are no reports showing anxiety effects to the best of our knowledge. While there are essential oils that have a sedation effect, there are also essential oils that have a stimulant effect. It is plausible that there exist similar increases and decreases regarding anxiety. The results of this study are the first showing the neuropsychological effects of β-citronellol inhalation as an odorant in mice.
In recent years, symptoms including headaches, allergic symptoms, and discomfort are increasingly common due to scent molecules contained in perfumes, detergents, and softeners. It has also been reported that activation of the Transient Receptor Potential A1 (TRPA1) Cation Channel, a receptor for odor molecules, may be involved in inflammation, pain, and neurotoxicity [Citation37]. Anxiety and fear are stressful for animals. An increase in anxiety due to inhalation of β-citronellol is also presumed to be stressful. The results of this study show that inhalation of β-citronellol may exacerbate fragrance pollution and aggravate neuropsychiatric disorders. Further studies are needed to determine the effects of long-term inhalation.
Anxiety disorders are the most common class of mental disorders. About 5.3% of adults in Japan and 18.2% of adults in the USA have met at least one diagnostic criterion for anxiety disorder within the last 12 months [Citation38]. Because of these high morbidities, the development of effective treatments and tools to treat anxiety disorders is a pressing issue in the psychiatric field. To that end, it is necessary to clarify the mechanisms by which anxiety occurs.
In addition to anti-anxiety drugs, aromatic compounds derived from plant extracts have been used in traditional medicine as a treatment for anxiety [Citation39]. However, the neural mechanisms underlying the reported anxiolytic effects of odorant compounds have not yet been fully elucidated. This study indicates that essential oils including β-citronellol may have an anxiety-enhancing effect. The results may not be true for humans, as mice have a sharper sense of smell and smaller body size than humans. However, the use of essential oils, including β-citronellol, needs to be carefully considered.
6. Conclusions
Inhalation of β-citronellol acts on the central nervous system of mice and increases anxiety-like behavior. However, inhalation of β-citronellol did not affect activity, sensory function, or cognitive function. This study provides evidence that essential oils including β-citronellol have neuropsychological effects.
Author contributions
All authors had full access to all study data and take full responsibility for the integrity of the data and accuracy of data analysis. Study concept and design: H.U., A.S., and M.O. Data acquisition: H.U., S.S., and Y.T. Data analysis and interpretation: H.U., A.S., and S.S. Drafting of the manuscript: H.U., A.S., Y.T., and M.O. Critical revision of the manuscript for important intellectual content: A.S., S.M., N.K., K.W., Y.T., Y.M., M.O., and T.I. Statistical analyses: H.U and S.S. Study supervision: M.O. and T.I.
Acknowledgments
We thank Kawasaki Medical School Central Research Institute for providing instruments to support this study. The authors would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- D’Angelo S, Carriero A, Gilio M, et al. Safety of treatment options for spondyloarthritis: a narrative review. Expert Opin Drug Saf. 2018;17(5):475–9.
- Dubash S, McGonagle D, Marzo-Ortega H. New advances in the understanding and treatment of axial spondyloarthritis: from chance to choice. Ther Adv Chronic Dis. 2018;9(3):77–87.
- World Health Organization.Evaluation of certain food additives and contaminants: Eightieth report of the Joint FAO/WHO Expert Committee on Food Additives. (2016). World Health Organization, Food and Agriculture Organization of the United Nations & Joint FAO/WHO Expert Committee on Food Additives. Meeting (80th: 2015, Rome, Italy).
- Lv XN, Liu ZJ, Zhang HJ, et al. Aromatherapy and the central nerve system (CNS): therapeutic mechanism and its associated genes. Curr Drug Targets. 2013;14(8):872–879.
- Zhang Y, Wu Y, Chen T, et al. Assessing the metabolic effects of aromatherapy in human volunteers. Evid Based Complement Alternat Med. 2013;2013:356381.
- Lis-Balchin M. Essential oils and ‘aromatherapy’: their modern role in healing. J R Soc Health. 1997;117(5):324–329.
- de Sousa DP, de Almeida Soares Hocayen P, Andrade LN, et al. A systematic review of the anxiolytic-like effects of essential oils in animal models. Molecules. 2015;20(10):18620–18660.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Sánchez-Vidaña DI, Ngai SP, He W, et al. The effectiveness of aromatherapy for depressive symptoms: a systematic review. Evid Based Complement Alternat Med. 2017;2017:5869315.
- de Sousa DP, Gonçalves JC, Quintans-Júnior L, et al. Study of anticonvulsant effect of citronellol, a monoterpene alcohol, in rodents. Neurosci Lett. 2006;401(3):231–235.
- Gomes-Carneiro MR, Felzenszwalb I, Paumgartten FJ. Mutagenicity testing (±)-camphor, 1,8-cineole, citral, citronellal, (-)-menthol and terpineol with the Salmonella/microsome assay. Mutat Res. 1998;416(1–2):129–136.
- Abegaz B, Yohannes PG, Dieter RK. Constituents of the essential oil of Ethiopian cymbopogon citratus stapf. Journal of Natural Products. 1983;46(3):424–426.
- Quintans-Júnior LJ, Souza TT, Leite BS, et al. Phythochemical screening and anticonvulsant activity of Cymbopogon winterianus Jowitt (Poaceae) leaf essential oil in rodents. Phytomedicine. 2008;15(8):619–624.
- Tavares ES, Julia˜o LS, Lopes D, et al. Ana´lise do o´leo essencial de folhas de quimiotipos de Lippia alba (Mill.) N. E. Br. (Verbenaceae) cultivados em condic¸o˜es semelhantes. Braz J Pharmacog. 2005;15:1–5.
- Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010;101(1):372–378.
- Hierro I, Valero A, Pérez P, et al. Action of different monoterpenic compounds against Anisakis simplex s.l. L3 larvae. Phytomedicine. 2004;11(1):77–82.
- Viollon C, Chaumont JP. Antifungal properties of essential oils and their main components upon Cryptococcus neoformans. Mycopathologia. 1994;128(3):151–153.
- Bastos JF, Moreira IJ, Ribeiro TP, et al. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin Pharmacol Toxicol. 2010;106(4):331–337.
- Santos MRV, Moreira FV, Fraga BP, et al. Cardiovascular effects of monoterpenes: a review. Rev Bras Farmacogn. 2011;21(4):764–771.
- Cavanagh HM, Wilkinson JM. Biological activities of lavender essential oil. Phytother Res. 2002;16(4):301–308.
- Ueno H, Shimada A, Suemitsu S, et al. Anti-depressive-like effect of 2-phenylethanol inhalation in mice. Biomed Pharmacother. 2019;111:1499–1506.
- Vogel HG, Maas J, Gebauer A. Drug discovery and evaluation: methods in clinical pharmacology 2011th edition. Springer; 2011 edition (February 3, 2011).
- Hagihara H, Horikawa T, Nakamura HK, et al. Circadian gene circuitry predicts hyperactive behavior in a mood disorder mouse model. Cell Rep. 2016;14(12):2784–2796.
- Ohashi R, Takao K, Miyakawa T, et al. Comprehensive behavioral analysis of RNG105 (Caprin1) heterozygous mice: reduced social interaction and attenuated response to novelty. Sci Rep. 2016;6:20775.
- Saraiva LR, Kondoh K, Ye X, et al. Combinatorial effects of odorants on mouse behavior. Proc Natl Acad Sci USA. 2016;113(23):E3300–6.
- Brito RG, Guimarães AG, Quintans JS, et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J Nat Med. 2012;66(4):637–644.
- Melo MS, Sena LC, Barreto FJ, et al. Antinociceptive effect of citronellal in mice. Pharm Biol. 2010;48(4):411–416.
- Quintans- Júnior LJ, Melo MS, De Sousa DP, et al. Antinociceptive effects of citronellal in formalin-, capsaicin-, and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. J Orofac Pain. 2010;24:305–312.
- Quintans-Júnior LJ, Oliveira MG, Santana MF, et al. a-Terpineol reduces nociceptive behavior in mice. Pharm Biol. 2011;49(6):583–586.
- Peana AT, D’Aquila PS, Chessa ML, et al. -)-Linalool produces antinociception in two experimental models of pain. Eur J Pharmacol. 2003;460(1):37–41.
- Leite, Mariana P; Fassin Júnior, Jaime; Baziloni, Eliane M. F; Almeida, Reinaldo N; Mattei, Rita; Leite, José R. Rev. bras. farmacogn ; 18(supl): 661–666, Dec. 2008.
- Umezu T, Ito H, Nagano K, et al. Anticonflict effects of rose oil and identification of its active constituents. Life Sci. 2002;72(1):91–102.
- Harada H, Kashiwadani H, Kanmura Y, et al. Linalool odor-induced anxiolytic effects in mice. Front Behav Neurosci. 2018;12:241.
- Lima NG, De Sousa DP, Pimenta FC, et al. Anxiolytic-like activity and GC-MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol Biochem Behav. 2013;103(3):450–454.
- Souto-Maior FN, Carvalho FLD, de Morais LCSL, et al. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol Biochem Behav. 2011;100:259–263.
- Satou T, Kasuya H, Maeda K, et al. Daily inhalation of α-pinene in mice: effects on behavior and organ accumulation. Phytother Res. 2014;28(9):1284–1287.
- Workshop Session. (2016) Transient receptor potential A1 (TRPA1) cation channels: fluttering hearts, headaches and hot flashes—can one “environmental sensor” be the cause of all the pain?
- WHO World Mental Health Survey Consortium, Demyttenaere K, Bruffaerts R, Posada-Villa J, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World health organization World Mental health surveys. JAMA. 2004;291(21):2581–2590.
- Connor, K. M., & Vaishnavi, S. (2009). Complementary and alternative approaches to treating anxiety disorders. In M. M. Antony & M. B. Stein (Eds.), Oxford library of psychology. Oxford handbook of anxiety and related disorders (p. 451–460). Oxford University Press.