?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to assess the kinetics of antibodies against the SARS-CoV-2, following natural infection in a cohort of employees of the Institut Pasteur de Tunis (IPT) and to assess the risk of reinfection over a 12-months follow-up period. A prospective study was conducted among an open cohort of IPT employees with confirmed SARS-CoV-2 infection that were recruited between September 2020 and March 2021. Sera samples were taken at 1, 3, 6, 9 and 12 months after confirmation of COVID-19 infection and tested for SARS-CoV-2-specific immunoglobulin G (IgG) antibodies to the spike (S-RBD) protein (IgG anti-S-RBD) and for neutralizing antibodies. Participants who had an initial decline of IgG anti-S-RBD and neutralizing antibodies followed by a subsequent rise in antibody titers as well as those who tested positive for SARS-CoV-2 by RT-PCR after at least 60 days of follow up were considered as reinfected. In total, 137 individuals were included with a mean age of 44.7 ± 12.3 years and a sex-ratio (Male/Female) of 0.33. Nearly all participants (92.7%) were symptomatic, and 2.2% required hospitalization. Among the 70 participants with three or more prospective blood samples, 32.8% were reinfected among whom 11 (47.8%) reported COVID-19 like symptoms. Up to 12 months of follow up, 100% and 42.9% of participants had detectable IgG anti-S-RBD and neutralizing antibodies, respectively. This study showed that humoral immune response following COVID-19 infection may persist up to 12 months after infection despite the potential risk for reinfection that is mainly explained by the emergence of new variants.
1. Introduction
After the span of more than one century, the COVID-19 pandemic stands out as the most important public health crisis that the world has faced [Citation1]. As of 25 October 2023, COVID-19 has caused an estimation of 771 million cases leading to nearly 7 million deaths [Citation2]. Since the beginning of the SARS-CoV-2 circulation, efforts have been deployed to understand the immune response induced by the infection aiming to developing effective vaccines.
The SARS-CoV-2 humoral immune response plays a key role in antiviral defense. Specifically, the production of specific antibodies in response to SARS-Cov-2 antigens control viral replication through seroneutralization [Citation3]. The speed of viral elimination and the duration of protection against the virus depend on the dynamics of humoral immunity. Measuring the kinetics of SARS-CoV-2 antibodies is thus essential to better understand the durability of humoral response after natural infection and to guide vaccine strategy especially in developing countries where the management and prevention of COVID-19 is more challenging due to limited resources [Citation4]. In addition, adhesion to barrier measures has considerably waned all over the world. Thus, the control of disease spread is essentially based on vaccination coverage but also on the protection conferred by natural infection. This protection relies on the degree to which the immunity is sustained over time [Citation5].
Several studies investigating antibody kinetics have been conducted worldwide [Citation6–10]. They were primarily focused on blood donors and hospitalized individuals potentially introducing selection bias. Hospital-based recruitment may exclude asymptomatic or mildly affected COVID-19 cases, while including blood donors might exclude patients with acute or long-term COVID-19 illnesses [Citation11]. Also, discrepant results were reported. Some studies found a persistence of high levels of IgG antibodies over one year following COVID-19 infection [Citation11,Citation12], while others noted a rapid decline of antibodies after 90 days [Citation13,Citation14].
In Tunisia, a SARS-CoV-2 seroprevalence study was previously conducted in the capital city of Tunisia in March-April 2021 revealing a 71.6% seroconversion rate among participants who were previously diagnosed with COVID-19 [Citation15]. However, we do not have an idea about the durability of SARS-CoV-2 antibodies in this country. We thus aimed to assess the kinetics of antibodies against the SARS-CoV-2, following natural infection in a cohort of employees of the Institut Pasteur de Tunis (IPT). Additionally, we aimed to assess the risk of reinfection over a 12-month follow-up period.
2. Materials and methods
This prospective study was conducted among an open cohort of IPT employees. We included all workers at IPT who has been infected by SARS-CoV-2 with confirmed RT-PCR testing. Individuals who were unable to comply with the principle of serological follow-up due to personal or professional constraints, those who did not give their informed consent to participate to this study and participants who were vaccinated against COVID-19 were not included. Participants were recruited between September 2020 and March 2021. They underwent evaluation at 1, 3, 6, 9 and 12 months after confirmation of COVID-19 infection. Those who received the COVID-19 vaccine during the study were subsequently discontinued from follow-up.
Included subjects were interviewed face-to-face by trained interviewers to fill out a paper questionnaire. The latter covered participants’ socio-demographic details, comorbidities, and COVID-19 related data (date of symptoms onset, clinical form and progression of the disease).
During each follow-up visit, blood samples of approximately 2–5 ml, were collected from each participant. Sera samples were tested using the Elecsys anti-SARS-CoV-2 S assay (Roche Diagnostics, Mannheim) to measure the level of antibodies against the receptor binding domain of the SARS-CoV-2 spike protein (IgG anti-S-RBD). This electrochemiluminescence assay has a measuring range from 0.4 UI/mL to 250 UI/mL. Samples with an antibody concentration greater than 1 UI/mL were considered positive. When antibody levels were above 250 UI/mL, the specimen was retested after a 10-fold dilution using the Elecsys Diluent Universal as recommended by the manufacturer [Citation16].
The detection of neutralizing antibodies against SARS-CoV-2 RBD in sera of participants with at least three prospective blood samples were performed using the GenScript SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) kit. The inhibition rate for SARS-CoV-2 neutralizing antibodies was calculated using the following formula [Citation17].
The used cutoff values for SARS-CoV-2 neutralizing antibodies detection was 30% according to manufacturer’s specifications [Citation18].
During each visit, participants were asked about the presence of symptoms suggestive of COVID-19 reinfection and whether they were tested for SARS-CoV-2 by RT-PCR.
Participants who had an initial decline of IgG anti-S-RBD and neutralizing antibodies followed by a subsequent rise in antibody titers as well as those who tested positive for SARS-CoV-2 by RT-PCR after at least 60 days of follow up were considered as reinfected.
Qualitative variables were expressed in terms of frequencies and percentages. Quantitative variables were summarized in terms of means and standard deviation if the variable has a normal distribution and in terms of median with its interquartile range if not. The Shapiro–Wilk test was used to test the normal distribution of variables. We used a linear regression model with mixed-effects to model the evolution of antibody titers following natural infection.
Paired samples t-test was used to compare antibodies response at each time point with that in the first month. As four comparisons were made, a Bonferroni adjustment of the p value was performed in order to avoid the increase of the probability of obtaining significant results due to chance (Type I error). The difference was thus considered statistically significant if the p value<; (0.05/4 = 0.0125).
Chi-square test or Fisher exact test, when applicable, were used to determine factors associated with reinfection.
3. Results
In total, 137 individuals with confirmed COVID-19 infection were included. They were mainly female (75.2%), and the mean age was equal to 44.7 ± 12.3 years. Notable, the included participants were predominantly affiliated to the administrative staff (63.5%) while 24.8% were scientists. Among the participants, 46.0% had at least one comorbidity and 11.7% were tobacco users as detailed in . The most frequent comorbidities in our study were obesity (16.8%), diabetes (8.8%) and hypertension (8.8%). No participant had a history of immune diseases or immune deficiency.
Table 1. Characteristics of participants (n = 137).
Regarding COVID-19 infection, nearly all subjects (92.7%) were symptomatic, and a small percentage (2.2%) required hospitalization. The most frequent reported symptoms were headache (79.5%), anosmia (73.2%) and muscle pain (70.1%). At baseline, 58.9% of participants had detectable anti-S-RBD antibodies.
After retrospectively excluding participants with fewer than three prospective blood samples, the final cohort comprised 70 individuals. In the conclusive cohort and at each sampling time, we found a significant strong correlation between neutralizing and anti-S-RBD antibodies ().
Table 2. Correlation between neutralizing and anti-S-RBD antibodies.
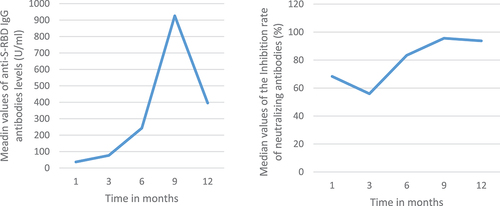
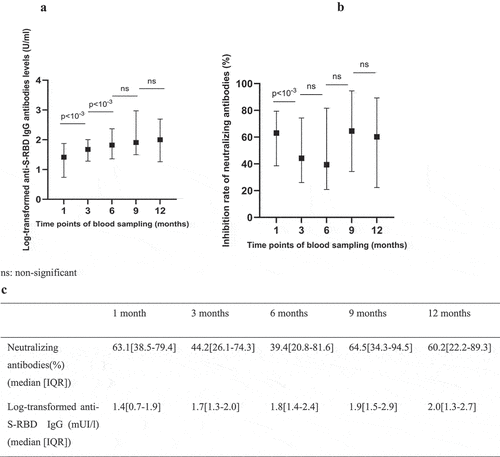
The median levels of anti-S-RBD antibodies exhibited a progressive increase over time between the first and the sixth month of follow up then remained relatively stable. Conversely, for the neutralizing antibodies, there has been a decrease in the median inhibition rate of neutralizing antibodies between the first and the sixth time point (63.1% vs 39.4%). In the ninth month of follow up, an increase in neutralizing antibody titer was observed exceeding that of the first month. Significant differences were found, for the log-transformed anti S-RBD antibodies, between the third and the first month as well as between the sixth and the third. Notably, significant differences were observed only between the first and third months (63.1% vs 44.2%) for neutralizing antibodies during the follow-up period ().
Figure 1. Antibody response over time following natural infection: a and c: illustrate the median value of the log-transformed anti-S-RBD IgG levels with the interquartile range; b and c: illustrate the median value of neutralizing antibodies with the interquartile range.
Throughout the follow-up period, 22 participants exhibited an important increase of antibodies mainly between the third and the ninth month of follow-up suggesting a potential reinfection and one tested positive for SARS-CoV-2 by RT-PCR in the fourth month of follow-up. The cumulative incidence of reinfection was then equal to 32.8%. Among reinfected participants, 11 (47.8%) reported COVID-19 like symptoms. The kinetics of neutralizing and anti-S-RBD antibodies over time is illustrated in .
Reinfection was more frequent among women, older groups, those who have comorbidities, as well as those who reported a rare/occasional mask wearing and hand hygiene. However, no difference was statistically significant ().
Table 3. Factors associated with reinfection: results of univariate analysis.
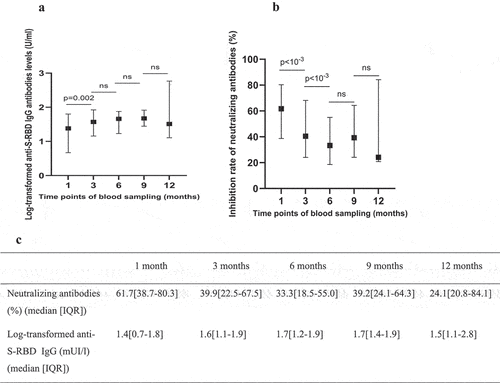
Upon excluding the reinfected participants from the analysis, we noted a decrease in the median level of neutralizing antibodies between the first and the third month of follow-up, followed by relative stability between the third and the ninth months, and a subsequent decrease in antibody levels. The estimated decay rate of the neutralizing antibodies in the first sixth months of follow up was equal to −3.4%, 95% confidence interval [−5.7% to −1.2%], p = 0.03. For anti-S-RBD IgG antibodies, the median level of antibodies increased between the first and the third month, remained relatively stable between the third and the ninth month and then decreased ().
Figure 3. Antibody response over time following natural infection among non-reinfected participants: a and c: illustrate the median value of the log-transformed anti-S-RBD IgG levels with the interquartile range; b and c: illustrate the median value of neutralizing antibodies with the interquartile range.
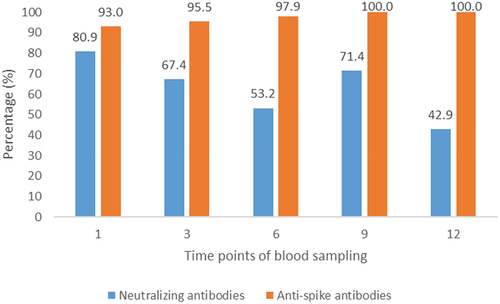
The proportion of detectable antibodies (exceeding the cut offs set by manufacturers) consistently surpassed 50% throughout the first nine months of follow-up. By the end of the 12-month observation period, all participants exhibited detectable anti-S-RBD IgG antibodies, while 42.9% retained detectable levels of neutralizing antibodies. ().
4. Discussion
The present study aimed to elucidate the dynamics of anti-SARS-CoV-2 antibodies among convalescent, non-vaccinated IPT employees in Tunisia. Among the 70 participants with three or more prospective blood samples, a significant decrease in neutralizing antibodies’ inhibition rate and a significant increase in anti-S-RBD IgG levels were detected between the first and the third month of follow-up. Up to 12 months of follow up, 100% and 42.9% of participants had detectable IgG anti-S-RBD and neutralizing antibodies respectively. Twenty-two participants had an important increase of antibodies mainly at the sixth month compared to the third month of follow up suggesting a reinfection.
The significant decrease of neutralizing antibodies’inhibition rate within the third month post infection was also reported by previous studies and was explained by the rapid decay of IgA and IgM titers occurring during the first two months post infection [Citation19]. However, the significant higher titer of anti-S-RBD IgG in the third month compared to the first month of follow up is in discordance with previous studies results revealing that anti-S-RBD IgG titers attended their peak at one month post infection and tended to decrease after that point of time [Citation20,Citation21]. Deshpande et al. [Citation22] reported that the average of IgG anti-SRBD continued to rise even after one month after the onset of symptoms to reach a peak at the sixth week. However, the comparison of our results with those of previous studies must be cautious given that certain factors, notably the severity of the disease, could influence the humoral response in the short term. Indeed, earlier and stronger antibody response was found to be associated with severe forms of COVID-19 [Citation23,Citation24] while a delay in seroconversion and in reaching the peak of anti- SARS-CoV-2 antibodies’ titers could be seen in less severe forms. Notably, our study population predominantly consisted of individuals with mild to moderate forms of COVID-19, which could account for the differences observed compared to earlier surveys.
Unlike previous studies [Citation7,Citation25,Citation26] that reported a decline in antibody titers between the third and the sixth months post infection, our findings revealed a significant increase in anti-S-RBD IgG titers at the 6th month of follow-up compared to the 3rd month. Likewise, an increase, although not significant, was found for neutralizing antibodies titers between the ninth and the sixth months of follow-up. Indeed, 22 individuals among the 70 participants with three or more prospective blood samples had an important increase of antibodies mainly between the third and the ninth month of follow up. This could be linked to the occurrence of reinfections in these participants that may be explained by a drop in antibody titers mentioned in previous studies between the third and the sixth month post infection [Citation25–28]. Furthermore, the presence of SARS-CoV-2 variants (Beta, Delta, and Omicron) during the study period, known for their association with high transmissibility and/or reduced susceptibility to antibodies, could also contribute to these reinfections. It is important to note that the immunological response following natural infection involves the generation of immunological memory that yields to a more robust and rapid immune response after reinfection.
Up to 12 months of follow-up, all participants had detectable anti-S-RBD IgG antibodies and 42.9% had detectable neutralizing antibodies. Similar results were found by Gallais et al. [Citation29] who concluded to the persistence of circulating anti-SARS-CoV-2 up to 13 months after infection. Yang et al. [Citation27] and Choe et al. [Citation26] also found that the neutralizing antibody response after natural infection persists for up to 16 months and 18 months, respectively.
It is important to mention that although the immune response following infection seems durable and natural infection and vaccination of COVID-19 naïve individuals were found to show equivalent protection against new SARS-CoV-2 infection [Citation28], vaccination without prior natural infection seems to induce a higher IgG response but more rapid waning of IgG antibodies compared to natural infection alone [Citation29] and vaccination after infection showed a greater humoral immune response than natural infection alone [Citation29].
While the short-term kinetics of anti-SARS-CoV-2 antibodies have been extensively studied during the acute phase of the pandemic [Citation30–32]our study stands out as one of the few assessing the long-term kinetics. Another notable strength of the present survey is the assessment of neutralizing antibodies testing recognized as the ‘best surrogate of immunity’ [Citation33]. Our study has an implication for vaccination strategies as it could suggest the best timing for vaccination post natural infection, considering both immunity longevity and the interval until reinfection. Nonetheless, our study had some limitations. The relatively small sample size with only 70 participants having three or more prospective blood samples and only 9 were followed until one-year post-infection may compromise the statistical power and affect the robustness of our results. Notably, Tunisian health authorities recommended COVID-19 vaccination for persons previously infected by SARS-CoV-2 starting from three months’ post-infection and this vaccination was considered as exclusion criteria in our study. Additionally, confirmation of reinfection via RT-PCR for all suspected cases was not feasible. Finally, we were not able to study the immune response according to the presence or not of underlying immune system diseases as all participants were immunocompetent. Indeed, in contrast to immunocompetent individuals, immunocompromised persons had insufficient immune response against SARS-CoV-2 [Citation34].
5. Conclusions
The present study showed that immune response against SARS-CoV-2 may persist up to 12 months after infection although potential for reinfection, likely prompted by the emergence of new variants, starting around three months post-infection. To refine vaccination strategies, particularly in terms of vaccination frequency, further research should monitor the immune response to immunization, either naturally or by vaccination.
Informed consent statement
A written informed consent was obtained from participants.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Biomedical Ethics Committee (CEBM) of Institut Pasteur de Tunis (IPT) (reference 2021/01/I/LR16IPT).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Miyah Y, Benjelloun M, Lairini S, et al. COVID-19 impact on public health, environment, human psychology, global socioeconomy, and education. Sci World J. 2022 Jan 11;2022:1–8. doi: 10.1155/2022/5578284
- WHO Coronavirus (COVID-19) dashboard [internet]. [cited 2022 Jan 16]. Available from: https://covid19.who.int
- Zheng J, Deng Y, Zhao Z, et al. Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects. Cell Mol Immunol. 2022 Feb;19:(2):150–157.
- Pasquale S, Gregorio GL, Caterina A, et al. COVID-19 in low- and middle-income countries (LMICs): a narrative review from prevention to vaccination strategy. Vaccines. 2021 Dec;9:(12):1477.
- Stein C, Nassereldine H, Sorensen RJD, et al. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023 Mar 11;401(10379):833–842. doi: 10.1016/S0140-6736(22)02465-5
- Sjaarda CP, Moslinger E, Tozer K, et al. Distinct age-specific SARS-CoV-2 IgG decay kinetics following natural infection. medRxiv [Internet]. 2021 [cited 2022 Feb 3]; 2021.08.05.21259465. https://www.medrxiv.org/content/10.1101/2021.08.05.21259465v1
- Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Dec 17;71(10):2688–2694. doi: 10.1093/cid/ciaa721
- Wu J, Liang B, Chen C, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021 Mar 22;12(1):1813. doi: 10.1038/s41467-021-22034-1
- Sun B, Feng Y, Mo X, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020 Jan 1;9(1):940–948. doi: 10.1080/22221751.2020.1762515
- Jayathilaka D, Jeewandara C, Gomes L, et al. Kinetics of immune responses to SARS-CoV-2 proteins in individuals with varying severity of infection and following a single dose of the AZD1222. Clin Exp Immunol. 2022 Jan 27;208(3):323–331. doi: 10.1093/cei/uxac009
- Amellal H, Assaid N, Charoute H, et al. Kinetics of specific anti-SARS-CoV-2 IgM, IgA, and IgG responses during the first 12 months after SARS-CoV-2 infection: a prospective longitudinal study. PLOS ONE. 2023;18(7):e0288557. doi: 10.1371/journal.pone.0288557
- Serwanga J, Ankunda V, Sembera J, et al. Rapid, early, and potent spike-directed IgG, IgM, and IgA distinguish asymptomatic from mildly symptomatic COVID-19 in Uganda, with IgG persisting for 28 months. Front Immunol. 2023;14:1152522. doi: 10.3389/fimmu.2023.1152522
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med. 2020 Sep 10;383(11):1085–1087. doi: 10.1056/NEJMc2025179
- Yousefi Z, Taheri N, Dargahi M, et al. Long-term persistence of anti-SARS-COV-2 IgG antibodies. Curr Microbiol. 2022;79(4):96. doi: 10.1007/s00284-022-02800-0
- Cherif I, Kharroubi G, Chaabane S, et al. COVID-19 in Tunisia (North Africa): seroprevalence of SARS-CoV-2 in the General population of the Capital City Tunis. Diagn Basel Switz. 2022 Apr 13;12(4):971. doi: 10.3390/diagnostics12040971
- Elecsys® Anti-SARS-CoV-2 [Internet]. Diagnostics [cited 2024 Jan 24]. Available from: https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html
- Technical Support [Internet]. [cited 2023 Jul 4]. Available from: https://www.genscript.com/product/documents?cat_no=L00847-A&catalogtype=Document-PROTOCOL
- COVID-19 detection SARS-CoV-2 neutralization antibody detection kit (RUO) GenScript [Internet]. [cited 2024 Jan 19]. Available from: https://www.genscript.com/covid-19-detection-svnt.html
- Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021 Jun;21:(6):395–404.
- Madanat L, Sager M, O’Connor D, et al. Prognostic value of SARS-CoV-2 anti-RBD IgG antibody quantitation on clinical outcomes in hospitalized COVID-19 patients. Int J Gen Med. 2022 Jun 18;15:5693–5700.
- Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of Neutralizing Antibody Titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021 Feb 3;223(2):197–205. doi: 10.1093/infdis/jiaa618
- Deshpande GR, Kaduskar O, Deshpande K, et al. Longitudinal clinico-serological analysis of anti-nucleocapsid and anti-receptor binding domain of spike protein antibodies against SARS-CoV-2. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2021 Nov;112:103–110. doi: 10.1016/j.ijid.2021.09.024
- Plūme J, Galvanovskis A, Šmite S, et al. Early and strong antibody responses to SARS-CoV-2 predict disease severity in COVID-19 patients. J Transl Med. 2022 Apr 15;20(1):176. doi: 10.1186/s12967-022-03382-y
- Lee N, Chan PKS, Ip M, et al. Anti-SARS-CoV IgG response in relation to disease severity of severe acute respiratory syndrome. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2006 Feb;35:(2):179–184.
- Feng C, Shi J, Fan Q, et al. Protective humoral and cellular immune responses to SARS-CoV-2 persist up to 1 year after recovery. Nat Commun. 2021 Aug 17;12(1):4984. doi: 10.1038/s41467-021-25312-0
- Choe PG, Kang CK, Kim KH, et al. Persistence of neutralizing antibody response up to 1 year after asymptomatic or symptomatic SARS-CoV-2 infection. J Infect Dis. 2021 Sep 17;224(6):1097–1099. doi: 10.1093/infdis/jiab339
- Yang Y, Yang M, Peng Y, et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol. 2022 Mar;7:(3):423–433.
- Shenai MB, Rahme R, Noorchashm H. Equivalency of protection from natural immunity in COVID-19 recovered versus fully vaccinated persons: a Systematic Review and pooled analysis. Cureus. 2021;13(10):e19102. doi:10.7759/cureus.19102
- Epsi NJ, Richard SA, Lindholm DA, et al. Understanding “hybrid immunity”: comparison and predictors of humoral immune responses to severe acute respiratory syndrome coronavirus 2 infection (SARS-CoV-2) and coronavirus disease 2019 (COVID-19) vaccines. Clin Infect Dis. 2023 Feb 1;76(3):e439–49. doi: 10.1093/cid/ciac392
- Beaudoin-Bussières G, Laumaea A, Anand SP, et al. Decline of humoral responses against SARS-CoV-2 Spike in convalescent individuals. MBio. 2020 Oct 16;11(5) doi: 10.1128/mBio.02590-20
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med. 2020 Sep 10;383(11):1085–1087. doi: 10.1056/NEJMc2025179
- Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021 Feb 19;12(1):1162. doi: 10.1038/s41467-021-21444-5
- Gerhards C, Thiaucourt M, Kittel M, et al. Longitudinal assessment of anti-SARS-CoV-2 antibody dynamics and clinical features following convalescence from a COVID-19 infection. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2021 Jun;107:221–227. doi: 10.1016/j.ijid.2021.04.080
- Antinori A, Bausch-Jurken M. The burden of COVID-19 in the immunocompromised patient: implications for vaccination and needs for the future. J Infect Dis. 2023 Aug 4;228(Suppl 1):S4–12.