Abstract
Different factors have to be considered and weighted in the treatment algorithm of lower extremity reconstruction. A combination of both clinicians’ and patients’ perspectives is necessary to provide a conclusive picture. Currently, there aren’t any standardized and validated measurement data sets for lower extremity reconstructions. This makes it necessary to identify the relevant domains. We, therefore, performed a systematic review and metanalysis of outcome measurements and evaluated their ability to measure outcomes after lower extremity reconstruction. A systematic review and metanalysis according to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ protocol were performed for studies reporting at least one structured outcome measurement of lower extremity reconstruction. Both Patient (PROMs)- and Clinician reported outcome measurements (CROMs)were analyzed. Of the 2827 identified articles, 102 were included in the final analysis. In total 86 outcome measurements were identified, 34 CROMs, 44 PROMs and 8 (9.3%) outcome measurements that have elements of both. Twenty-four measure functional outcome, 3 pain, 10 sensations and proprioception, 9 quality of life, 8 satisfaction with the result, 5 measure the aesthetic outcome, 6 contours and flap stability and 21 contain multidomain elements. A multitude of different outcome measurements is currently used in lower extremity reconstruction So far, no consensus has been reached on what to measure and how. Validation and standardization of both PROMs and CROMs in plastic surgery is needed to improve the outcome of our patients, better meet their needs and expectations and eventually optimize extremity reconstruction by enabling a direct comparison of studies’ results.
Introduction
The reconstruction of complex tissue loss in the lower extremity is often challenging. Many different factors have to be considered and weighted in the treatment algorithm, with the best possible functional recovery of the limb as the ultimate goal. Clinical results however are not enough anymore to describe treatment results, in fact, a lot more is necessary to really understand patients’ needs with the overall goal to improve outcomes. Lower extremity reconstruction is complex as it often has to address multiple components, both bone and soft tissues. Different outcome measurement tools were developed to assess this complexity but are not able to describe the outcome both for the point of view of the clinician and that of the patient [Citation1]. Surgical outcomes focused on complications and function from the physician’s viewpoint provide valuable information but do not provide a complete picture. To this end, as in other areas of healthcare, the patient-reported outcomes have become an integral part of assessing the quality and efficacy of care delivered [Citation2]. Only a combination of both clinicians’ and patients’ perspectives can help to provide a conclusive picture of the outcome. Currently, however there aren’t any standardized and validated measurement data sets for lower extremity reconstruction. Therefore, it is necessary to start from the foundation and identify the domains which are really relevant for the different stakeholders, first of all, patients and surgeons. An additional challenge is the small size of study cohorts in reconstructive plastic surgery that leads in many cases to studies with a relative low level of evidence. This is also the reason why physicians rarely have sufficiently reliable data about expected results and potential complications that can be easily explained to non-experts with misunderstandings and eventually unmet patients’ expectations as a consequence [Citation3]. Some surgeons have tried to address this lack of evidence by creating their own outcome measurement tools, which furthermore increases the difficulty to compare study outcomes and makes apparent the necessity to build a minimal data set. Choosing the appropriate outcome measurements is crucial to understand the patient’s needs and accordingly choose the right treatment. Also, it is pivotal to plan and to carry out studies, to enable comparison between similar studies and finally to draw conclusions with a clinical impact as inappropriate outcome measurements can generate misleading results [Citation4]. For these reasons, we decided to perform a systematic review and metanalysis of the outcome measurements currently available (PROMs and CROMs) and to evaluate their effectiveness to measure outcomes after lower extremity reconstruction.
Materials and methods
Overview
A systematic review was carried out to identify any studies reporting PROMs and CROMs to assess the results of lower extremity reconstructions. Title and abstract screening, full-text review and data extraction were handled independently by two reviewers (ISB and FEZ), and disagreements at any stage were resolved by discussion and consensus. Persisting disagreements were resolved by discussion with a third reviewer (MC). We followed the Preferred Reporting Items for Systematic Reviews (PRISMA) protocol [Citation5]. This review was registered on PROSPERO (www.crd.york.ac.uk/prospero, Record ID: 42021219425).
Search strategy
The PubMed/MEDLINE, EMBASE, Cochrane and Web of Science database were searched to identify eligible articles. The search strategy included combinations of the following terms: lower extremity; reconstruction; leg; lower limb; foot; knee; tight; heel; toe; tibia; flap and microsurgery (see Table S1, Supplementary Material). Word variations and exploded medical subject headings were searched for whenever feasible. Additionally, reference lists were hand-searched to identify further studies of interest. The last systematic search was conducted on 27th November 2020.
Study selection
Only in-vivo clinical studies enrolling adults over sixteen were considered. As a small number of controlled trials was anticipated, prospective and retrospective single-arm cohort studies and case series of more than five individuals were also included. Inclusion criteria were studies on patients with flap-based soft tissue reconstruction of the lower extremity and at least one outcome measurement, whether functional, sensation and proprioception, pain sensation, aesthetic, patient satisfaction or overall quality of life and whether a CROM or PROM. Flap survival itself and complications were not considered as outcome measurements. Questionnaires and scores, created by authors for a specific study, often an assembly of various clinician and patient-reported outcome measurements, were considered and listed as a separate outcome measurement.
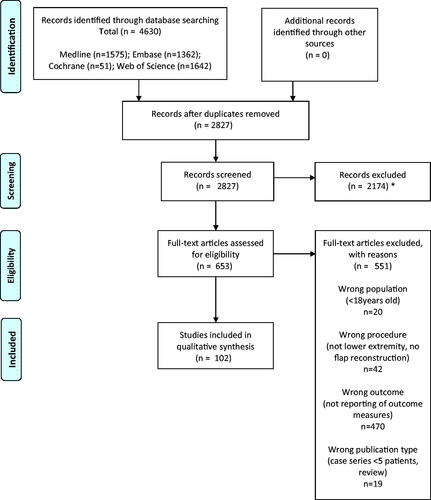
Exclusion criteria were: all animal studies, studies reporting only on outcomes of bone flaps without soft tissue transfer, studies where outcome measurements were used only to analyze flap donor-site outcome. Furthermore, articles older than 10 years or not in English were excluded. Exact cohort duplicates were excluded, although we did include updates of previously published cohorts with a sample size increase of at least 50%. We report our review process in the flow diagram following the four stages of the PRISMA statement () [Citation5,Citation6].
Data extraction and quality assessment
We extracted the following information, if available, from all included publications: study design and year of publication, country of study conduction, number of patients, mean age, gender distribution, flap type, indication, defect size, donor site, recipient site, number of outcome measurements and follow up time. To assess the identified outcome measurements we described each outcome measure qualitatively and extracted the following data for each of them if available: outcome measure type (PROM, CROM), mode of administration, assessment requirements, range of scores and instrument validity. We defined outcome measure validity according to the consensus-based standards for the selection of health measurement instruments (COSMIN) guidelines [Citation7,Citation8]. Validity refers to the degree to which an instrument measurements the outcome that it is meant to measure, content validity (relevancy, comprehensiveness and comprehensibility), construct validity (including structural validity, hypotheses testing and cross-cultural validity\measurement invariance) and criterion validity are different components [Citation7,Citation8].
Results
Our search resulted in 4630 studies, 1575 were found in Pubmed/MEDLINE, 1362 studies in EMBASE, 51 studies were found in the Cochrane database and 1642 in Web of science. After the removal of duplicates, the titles and abstracts of 2827 studies were manually screened. Eventually, 102 studies from 23 different countries, referring to 86 different outcome measurements were included [Citation1,Citation9–109]. The review process is shown in and an overview of included studies is given in .
Table 1. Overview over included studies and population characteristics.
The included studies reported on lower extremity reconstruction with any of the following flaps, either free or pedicled: anterolateral thigh (ALT), gracilis, latissimus dorsi, anterior serratus, gastrocnemius, sural, medial plantar, tibial, fibula, deep inferior epigastric perforator (DIEP), superficial circumflex iliacal artery perforator (SCIP), parascapular, scapular and radial forearm. Forty-three (42.2%) studies reported on outcomes of free flaps, 38 (37.3%) on pedicelled flaps outcomes and 14 (13.7%) report outcomes of both. In 7 (6.9%) studies the flap type was not reported. Mean follow-up in the studies assessed ranged from 12 to 80 months.
Thirty-four (39.5%) identified outcome measurements are CROMs and 44 (51.1%) are PROMs.
Eight outcome measurements were a combination of CROMs and PROMs. The function of the reconstructed extremity was measured with 16 different CROMs and 8 PROMs. The aesthetic outcome was measured with 3 CROMs and 2 PROMs, sensation and proprioception with 9 clinician-reported instruments and one PROM and pain with 3 PROMs. The general quality of life was assessed with 9 PROMs and satisfaction with 8 PROMs. Thirteen PROMs consisted of elements from multiple domains. However, many measurements were not performed according to uniform and standardized protocols and instructions given to participants, test protocols and analysis of results profoundly varied across studies for many identified measurements. An overview of all outcome measurements and their brief description and characteristics is listed in .
Table 2. Overview over the characteristic of the included outcome measures.
Validated CROMs
The most frequently applied validated CROM was the static two-point discrimination (s2PD) test (n = 18 publications; 17.6%) with a cumulative number of 464 reported subjects. Other frequent applied CROMs were the Semmes–Weinstein monofilament (SWM) test (n = 11 publications; 10.8%; n = 271 patients) and Range of Motion (ROM) (n = 15 publications; 14.8%, n = 587 patients). For the full list refer to .
Validated PROMs
The most frequently applied validated PROM was the Lower Extremity Functional Scale 15 (LEFS) (n = 13 publications; 12.7%) with a cumulative number of 859 reported subjects. Other frequent applied PROMs were the 36-Item Short-Form Health Survey (n = 12 publications; 11.8%) with 420 reported subjects and the visual analogue pain scale (VAS) (n = 8 publications; 7.8%), n = 135 patients). For the full list refer to .
Non-validated outcome measurements
Seventeen patient-reported questionnaires and scores, 25 CROMs and 5 clinician- and patient-reported outcome measurements were found, that are not currently validated for lower extremity reconstruction. Non-validated questionnaires individually assembled for a specific study sometimes based on or a combination of other validated outcome measurements were frequently found in the literature ().
Table 3. Overview over the characteristic of the included non-validated outcome measures and outcome measures validated for other intentions.
Discussion
Reconstruction of complex defects of the lower limb using free or pedicled flaps is a routine procedure [Citation110]. Evidence on these complex procedures however is scarce [Citation111]. In order to compare outcomes it is essential to rely on defined outcome measurements [Citation112]. The goal of this systematic review was thus to identify the most commonly used outcome measurements in lower limb reconstruction to aid clinicians with the choice of appropriate outcome measurements to best follow up their patients with the overall goal to improve patients’ outcomes. Our review yielded several noteworthy findings.
Our review showed that there is a wide variety of outcome measurements currently in use, more than 60. This makes it difficult to compare outcomes among different studies. The most frequently used outcome measurements are those that have been in use over the last decades (s2PD, SWM and ROM) and are not exclusively used in lower extremity reconstruction [Citation113,Citation114]. The CROM which is most frequently used in studies is the static two-point discrimination (s2PD) test, used in 16 of the included publications with a cumulative number of 274 reported subjects. Range of motion ROM Testing was only used in 13 publications but with a cumulative population of 417.
Secondly, the majority of outcome measurements used were CROMs (38%). To realistically describe the outcome a combination of different types of outcome measurements is needed to capture at the same time the surgical result and the complications [Citation115]. Well-known clinician-reported outcome measurements are amongst others flap survival, donor- and recipient-site complications, range of motion, grip and pinch strength, ability to bear weight, sensation, length of surgery and length of hospital stay [Citation116,Citation117]. They are used in determining cost-effectiveness and quality assurance [Citation118].
Additionally to CROMS different PROMs were used in 31 studies. The importance of PROMs steadily increases and new PROMs are currently being developed [Citation119,Citation120]. PROMs allow surgeons and researchers to quantify otherwise intangible outcomes like form, function and quality of life from a patient’s perspective [Citation121]. PROMs can be deployed across the patient care journey, to support diagnosis, disease severity determination, referral pathways, treatment decision-making and post-operative care [Citation119]. To address the discrepancy between the patient’s expectations and the clinicians definition of a successful procedure PROMs are crucial [Citation119]. The most commonly used PROM was the Lower Extremity Functional Scale, a self-report condition-specific measurement that yields reliable and valid results and is appropriate for use as a clinical and research tool [Citation118]. The fact that despite its introduction only in 1999, it has already been used to rate results of 793 patients illustrates the growing appreciation for this tool and more generally the growing awareness for the importance of PROMs use.
Certain outcome measurements (e.g. Vancouver Scar Scale) can be used as PROMs and CROMs. A comparison of the rating of outcomes shows that clinicians tend to overrate outcomes compared to patients [Citation122]. CROMs are well accepted by patients and show high reliability [Citation123]. It is argued whether PROM results are more challenging to interpret than objective CROMs due to a higher inter- and intraobserver variability and subjective PROM scoring for cultural and other individual reasons [Citation124].
Most studies included only a smaller number of patients and are retrospective, demonstrating a need for larger studies, ideally prospective and with some sort of reliable comparison (randomized, matched pairs, or similar). Many advances in outcome research have been achieved in the past two decades enabled by new information technologies, data sharing and collaborative efforts but the need for improvement in outcome measurement methodology used in plastic surgery research remains urgent [Citation125,Citation126].
There is a tendency toward self-created scores, which are often used without validation and/or cultural adaptation. Some authors also assembled their own questionnaire using parts of already existing outcome measurements [Citation1,Citation79]. We advocate for the use of validated scores only and hopefully for an internationally recognized standard set that could be used for all studies and make outcomes comparable across the world. This is true not only for CROMs but also for PROMs. Pusic et al. showed in a systematic review the increasing importance of validated PROMs, the group found that only few PROMs (in this case used in breast surgery studies), were validated and had evidence to support their use [Citation127]. Validation and standardization of PROMs but also CROMs in plastic surgery is needed [Citation127].
Our review shows that there isn’t any accepted ‘gold standard’ in outcome measurements in lower extremity reconstructions, neither what to measure, nor how or when. In particular, the most striking finding is that there isn’t any agreement about which domains are important and relevant for clinicians and patients, that should be included in the analysis. This is the first step for every reliable analysis and we are convinced that an expert group should identify these domains together with the instruments to measure them and, not less important, the time points when to perform the measurements. based on the literature no single ‘gold standard’ for outcome measurements in lower extremity reconstruction exists regarding which outcome measurement should be used or how the outcome measurement should be assessed and analyzed exactly [Citation126]. To optimize surgical treatment and rehabilitation in extremity reconstruction it is crucial to record and evaluate standardized outcomes. The identification of the relevant domains should then become the basis for the establishment of outcomes registries.
Our systematic review only evaluated studies within the last 10 years. This time interval was found to be representative of extremity reconstruction in its current form. Additionally, older studies would have been unlikely to use PROMs but it is possible that relevant outcome measures, that might be considered less ‘popular’ these days could have been missed using this time interval.
Also, some of the excluded papers (small series, non-structured outcome) might have analyzed interesting aspects, relevant to patients and clinicians, but in a way that prevents comparison. This study combines data from a wide range of lower extremity defects and reconstructions and lists the outcome measures used overall. Indeed for clinical application sub-analysis of certain groups might be more intuitive for analysis rather than as a whole. This need however could not be satisfactorily addressed due to the heterogeneous nature of most publications assessed.
Conclusion
A big number of different outcome measurements is currently used in lower extremity reconstruction and while there are many different measurements, there is no validation study exploring the needs of the patients. We, therefore, advocate for international action to address this shortcoming. The literature shows that, unlike in other fields of medicine, no consensus has been reached on what to measure and how. We need to analysis the relevant domains and need to put them through validation studies. There is a need for registries that will allow for studies with significant cohort sizes. This has to be done in order to improve results and put the needs of our patients first.
Supplemental Material
Download MS Word (354.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Heidekrueger PI, Ehrl D, Prantl L, et al. Microsurgical reconstruction of the plantar foot: long-term functional outcomes and quality of life. J Reconstr Microsurg. 2019;35(5):379–388.
- Momoh AO, chung KC. Measuring outcomes in lower limb surgery. Clin Plast Surg. 2013;40(2):323–329.
- Kash BA, McKahan M, Tomaszewski L, et al. The four Ps of patient experience: a new strategic framework informed by theory and practice. Health Mark Q. 2018;35(4):313–325.
- Ioannidis JPA, Greenland S, Hlatky MA, et al. Increasing value and reducing waste in research design, conduct, and analysis. The Lancet. 2014;383(9912):166–175.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Vu-Ngoc H, Elawady SS, Mehyar GM, et al. Quality of flow diagram in systematic review and/or meta-analysis. PLOS One. 2018;13(6):e0195955.
- Terwee CB, Prinsen CAC, Chiarotto A, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a delphi study. Qual Life Res. 2018;27(5):1159–1170.
- Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–1157.
- Riccio M, Zingaretti N, Verdini F, et al. Functional donor-site morbidity after soleus muscle-flap procedure in the treatment of lower limb severe injuries. Handchir Mikrochir Plast Chir. 2019;51(6):453–463.
- Rajkumar R, Sharma HK, Dash S, babu VS. Comparative analysis of propeller flaps vs. modified perforator-based flaps in foot and ankle reconstruction. Eur J Plast Surg. 2021;44(2):243–248.
- Pototschnig H, Schaff J, Kovacs L, et al. The free osteofasciocutaneous fibula flap: clinical applications and surgical considerations. Injury. 2013;44(3):366–369.
- Mojallal A, Shipkov CD, Braye F, et al. Distally based adipofascial sural flap for foot and ankle reconstruction. J Am Podiatr Med Assoc. 2011;101(1):41–48.
- Gu H, Xiong Z, Xu J, et al. Clinical and anatomical study of the distally based lesser saphenous veno-lateral sural neurocutaneous flap for lower extremity coverage. J Orthop Sci. 2013;18(5):740–748.
- Unluer Z, Al-Ajam Y, Al-benna S. Functional outcome after reconstruction of traumatic soft tissue defects in the lower half of the leg with distally based medial hemisoleus muscle flaps: a case series and literature review. Ann Plast Surg. 2018;81(4):468–471.
- Yang C, Geng S, Fu C, et al. A minimally invasive modified reverse sural adipofascial flap for treating posttraumatic distal tibial and calcaneal osteomyelitis. Int J Low Extrem Wounds. 2013;12(4):279–285.
- Luo Z, Dong Z, Ni J, Wei J, Peng P, Lv G. Distally based peroneal artery perforator-plus fasciocutaneous flap to reconstruct soft tissue defect combined with chronic osteomyelitis in the lateral malleolus. Clin Med Surg. 2022;21(4):464–470.
- Abula A, Yushan M, Ren P, et al. Reconstruction of soft tissue defects and bone loss in the tibia by flap transfer and bone transport by distraction osteogenesis: a case series and our experience. Ann Plast Surg. 2020;84(5S Suppl 3): S202–S207.
- Akdag O, Karamese M, Yıldıran GU, et al. Foot and ankle reconstruction with vertically designed deep inferior epigastric perforator flap. Microsurgery. 2018;38(4):369–374.
- Al-Himdani S, Din A, Wright TC, et al. The medial sural artery perforator (MSAP) flap: a versatile flap for lower extremity reconstruction. Injury. 2020;51(4):1077–1085.
- Bhullar DS, Karuppiah SV, Aljawadi A, et al. Local flaps vs. free flaps for complex lower limb fractures: effect of flap choice on patient-reported outcomes. J Orthop. 2020;17:91–96.
- Bigdeli AK, Gazyakan E, Schmidt VJ, et al. Long-term outcome after successful lower extremity free flap salvage. J Reconstr Microsurg. 2019;35(4):263–269.
- Brunetti B, Barone M, Tenna S, et al. Pedicled perforator-based flaps: risk factor analysis, outcomes evaluation and decisional algorithm based on 130 consecutive reconstructions. Microsurgery. 2020;40(5):545–552.
- Buono P, Castus P, Dubois-Ferrière V, et al. Muscular versus non-muscular free flaps for soft tissue coverage of chronic tibial osteomyelitis. World J Plast Surg. 2018;7(3):294–300.
- Cang ZQ, Ni XD, Xu Y, et al. Reconstruction of the distal lower leg and foot sole with medial plantar flap: a retrospective study in one center. J Plast Surg Hand Surg. 2020;54(1):40–46.
- Cherubino M, Stocco C, Ronga M, et al. Comparisons of fascio-cutaneous anterolateral thigh and sandwich fascial ALT free flap in the distal extremity reconstruction. Microsurgery. 2020;40(4):452–459.
- Chou CY, Chiao HY, Wang CY, et al. Functional results of free tissue transfer for complex heel-calcaneal defects. Microsurgery. 2018;38(4):381–387.
- Corten K, Struelens B, Evans B, et al. Gastrocnemius flap reconstruction of soft-tissue defects following infected total knee replacement. Bone Joint J. 2013;95-B(9):1217–1221.
- Dai J, Chai Y, Wang C, et al. Comparative study of two types of distally based sural neurocutaneous flap for reconstruction of lower leg, ankle, and heel. J Reconstr Microsurg. 2013;29(2):125–130.
- Dai J, Chai Y, Wang C, et al. Distally based saphenous neurocutaneous perforator flap for reconstructive surgery in the lower leg and the foot: a long-term follow-up study of 70 patients. J Reconstr Microsurg. 2013;29(7):481–485.
- DeFazio MV, Han KD, Iorio ML, et al. Combined Free tissue transfer for the management of composite Achilles defects: functional outcomes and patient satisfaction following thigh-based vascularized reconstruction with a neotendon construct. J Reconstr Microsurg. 2014;30(6):431–440.
- Demiralp B, Ege T, Kose O, et al. Amputation versus functional reconstruction in the management of complex hind foot injuries caused by land-mine explosions: a long-term retrospective comparison. Eur J Orthop Surg Traumatol. 2014;24(4):621–626.
- Summa P D, Sapino G, Cherubino M, et al. Reconstruction of complex soft tissue defects including tendons with anterolateral thigh flap extended to fascia lata: long term recovery and functional outcomes. Microsurgery. 2019;39(5):405–415.
- Xuan DK, Xu YQ, Yu FX, et al. Perforator pedicled propeller flaps for soft tissue coverage of lower leg and foot defects. Orthop Surg. 2014;6(1):42–46.
- Doukas CW, Hayda CR, Frisch HM, et al. The military extremity trauma amputation/limb salvage (METALS) study: outcomes of amputation versus limb salvage following major Lower-extremity trauma. J Bone Joint Surg Am. 2013;95(2):138–145.
- Egeler SA, de Jong T, Luijsterburg AJM, et al. Long-term patient-reported outcomes following free flap lower extremity reconstruction for traumatic injuries. Plast Reconstr Surg. 2018;141(3):773–783.
- Ehrl D, Heidekrueger PI, Schmitt A, et al. The anterolateral thigh flap for Achilles tendon reconstruction: functional outcomes. Plast Reconstr Surg. 2019;143(6):1772–1783.
- Elgohary H, Nawar AM, Zidan A, et al. Functional and aesthetic outcomes of reconstruction of soft-tissue defects of the heel with free flap. JPRAS Open. 2019;19:35–44.
- Ellington JK, Bosse MJ, Castillo RC, et al. The mangled foot and ankle: results from a 2-year prospective study. J Orthop Trauma. 2013;27(1):43–48.
- Falola RA, Lakhiani C, Green J, et al. Assessment of function after free tissue transfer to the lower extremity for chronic wounds using the lower extremity functional scale. J Reconstr Microsurg. 2018;34(5):327–333.
- Fu D, Zhou L, Yang S, et al. Surgical technique: repair of forefoot skin and soft tissue defects using a lateral tarsal flap with a reverse dorsalis pedis artery pedicle: a retrospective study of 11 patients. Clin Orthop Relat Res. 2013;471(1):317–323.
- Gill D, Bruce DJ, Ponsford MJ, et al. Long-term follow-up of patients undergoing free tissue transfer to the lower limb following trauma. Eur J Plast Surg. 2013;36(7):431–442.
- Goil P, Sharma AK, Gupta P, et al. Comparison of the outcomes of adipofascial and two–staged fasciocutaneous reverse sural flap in patients with lower leg trauma. J Clin Orthop Trauma. 2021;14:113–120.
- Grauberger JN, Gibreel WO, Moran SL, et al. Long-term clinical and patient-reported outcomes in free flap reconstruction of the weight-bearing heel pad and non-weight-bearing Achilles tendon regions. Microsurgery. 2020;40(8):835–845.
- Grinsell D, Di Bella C, choong PFM. Functional reconstruction of sarcoma defects utilising innervated free flaps. Sarcoma. 2012;2012:315190.
- Gu J X, Shi HA, Chen ZN, et al. Reconstruction of heel soft tissue defects using medial plantar artery island pedicle flap: clinical experience and outcomes analysis. J Foot Ankle Surg. 2017;56(2):226–229.
- Halim AS, Chai SC, Wan Ismail WF, et al. Long-term outcome of free fibula osteocutaneous flap and massive allograft in the reconstruction of long bone defect. J Plast Reconstr Aesthet Surg. 2015;68(12):1755–1762.
- Houdek MT, Wagner ER, Wyles CC, et al. Long-term outcomes of pedicled gastrocnemius flaps in total knee arthroplasty. J Bone Joint Surg Am. 2018;100(10):850–856.
- Izadi D, Paget JTEH, Haj-Basheer M, et al. Fasciocutaneous flaps of the subscapular artery axis to reconstruct large extremity defects. J Plast Reconstr Aesthet Surg. 2012;65(10):1357–1362.
- Jandali Z, Lam MC, Aganloo K, et al. The free medial sural artery perforator flap: versatile option for soft tissue reconstruction in small-to-moderate size defects of the foot and ankle. Microsurgery. 2018;38(1):34–45.
- Jeon BJ, Lim SY, Pyon JK, et al. Secondary extremity reconstruction with free perforator flaps for aesthetic purposes. J Plast Reconstr Aesthet Surg. 2011;64(11):1483–1489.
- Jeon BJ, Lee KT, Lim SY, et al. Plantar reconstruction with free thoracodorsal artery perforator flaps. J Plast Reconstr Aesthet Surg. 2013;66(3):406–413.
- Jiga LP, Jandali Z, Merwart B, et al. The free vastus lateralis muscle flap. A smart less used flap for soft tissue reconstruction of the weight-bearing foot. Injury. 2020;51:S34–S40.
- Kapoor T, Banuelos J, Adabi K, et al. Analysis of clinical outcomes of upper and lower extremity reconstructions in patients with soft-tissue sarcoma. J Surg Oncol. 2018;118(4):614–620.
- Kask G, Barner-Rasmussen I, Repo J, et al. Functional outcome after lower extremity soft tissue sarcoma treatment: a pilot study based on translated and culturally adapted measures. Scand J Surg. 2019;108(2):164–171.
- Khai Luen K, wan sulaiman WA. Functional outcomes after heel pad reconstruction: a review of 7 cases. J Foot Ankle Surg. 2017;56(5):1114–1120.
- Khan MAA, Jose RM, Taylor C, et al. Free radial forearm fasciocutaneous flap in the treatment of distal third tibial osteomyelitis. Ann Plast Surg. 2012;68(1):58–61.
- Kneser U, Brockmann S, Leffler M, et al. Comparison between distally based peroneus brevis and sural flaps for reconstruction of foot, ankle and distal lower leg: an analysis of donor-site morbidity and clinical outcome. J Plast Reconstr Aesthet Surg. 2011;64(5):656–662.
- Kotsougiani D, Platte J, Bigdeli AK, et al. Evaluation of 389 patients following free-flap lower extremity reconstruction with respect to secondary refinement procedures. Microsurgery. 2018;38(3):242–250.
- Kwasnicki RM, Hettiaratchy S, Jarchi D, et al. Assessing functional mobility after lower limb reconstruction: a psychometric evaluation of a sensor-based mobility score. Ann Surg. 2015;261(4):800–806.
- Lee HI, Ha SH, Yu SO, et al. Reverse sural artery island flap with skin extension along the pedicle. J Foot Ankle Surg. 2016;55(3):470–475.
- Lee MJ, Yun IS, Rah DK, et al. Lower extremity reconstruction using vastus lateralis myocutaneous flap versus anterolateral thigh fasciocutaneous flap. Arch Plast Surg. 2012;39(4):367–375.
- Lin J, Zhou F, Sun YD, et al. Modified anterior tibial artery perforator-pedicled propeller flap for soft-tissue coverage of the ankle and heel. World J Surg. 2020;44(7):2237–2242.
- Liu S, Tan J, Tao S, et al. Reconstruction of a distal foot skin defect using an intermediate dorsal neurocutaneous flap. Orthop Surg. 2020;12(2):442–449.
- Lu S, Chai Y, Wang C, et al. Complex heel reconstruction with a sural fasciomyocutaneous perforator flap. J Reconstr Microsurg. 2014;30(2):83–90.
- Lu J, DeFazio MV, Lakhiani C, et al. Limb salvage and functional outcomes following free tissue transfer for the treatment of recalcitrant diabetic foot ulcers. J Reconstr Microsurg. 2019;35(2):117–123.
- Luo Z, Lv G, Wei J, et al. Comparison between distally based peroneal and posterior tibial artery perforator-plus fasciocutaneous flap for reconstruction of the lower extremity. Burns. 2020;46(1):225–233.
- Maruccia M, Fallico N, Cigna E, et al. Suprafascial versus traditional harvesting technique for free antero lateral thigh flap: a case-control study to assess the best functional and aesthetic result in extremity reconstruction. Microsurgery. 2017;37(8):851–857.
- Meyer-Marcotty MV, Sutmoeller K, Kopp J, et al. Postoperative insole-paedobarographic gait analysis for patients with flap coverages of weight-bearing and non-weight-bearing areas of the foot. J Plast Reconstr Aesthet Surg. 2012;65(4):482–488.
- Nanda D, Sahu SA, Karki D, et al. Adipofascial perforator flaps: its role in reconstruction of soft-tissue defects of lower leg and ankle. Indian J Plast Surg. 2018;51(2):216–221.
- Oh SJ, Moon M, Cha J, et al. Weight-bearing plantar reconstruction using versatile medial plantar sensate flap. J Plast Reconstr Aesthet Surg. 2011;64(2):248–254.
- Olivan MV, Busnardo FF, Faria JC, et al. Chimerical anterolateral thigh flap for plantar reconstruction. Microsurgery. 2015;35(7):546–552.
- Ovaska MT, Madanat R, Tukiainen E, et al. Flap reconstruction for soft-tissue defects with exposed hardware following deep infection after internal fixation of ankle fractures. Injury. 2014;45(12):2029–2034.
- Patel KM, Economides JM, Franklin B, et al. Correlating patient-reported outcomes and ambulation success following microsurgical lower extremity reconstruction in comorbid patients. Microsurgery. 2014;34(1):1–4.
- Philandrianos C, Moullot P, Gay AM, et al. Soft tissue coverage in distal lower extremity open fractures: comparison of free anterolateral thigh and free latissimus dorsi flaps. J Reconstr Microsurg. 2018;34(2):121–129.
- Qing L, Wu P, Yu F, et al. Use of dual-skin paddle anterolateral thigh perforator flaps in the reconstruction of complex defect of the foot and ankle. J Plast Reconstr Aesthet Surg. 2018;71(9):1231–1238.
- Repo JP, Barner-Rasmussen I, Roine RP, et al. Role of free iliac crest flap in foot and ankle reconstruction. J Reconstr Microsurg. 2016;32(5):386–394.
- Repo JP, Barner-Rasmussen I, Roine RP, et al. Treatment of compound tibia fracture with microvascular latissimus dorsi flap and the ilizarov technique: a cross-sectional study of long-term outcomes. J Plast Reconstr Aesthet Surg. 2016;69(4):524–532.
- Rothenberger J, Ramms EM, Medved F, et al. Comparison of spontaneous sensory recovery of noninnervated anteromedial thigh flap, latissimus dorsi flap, and gracilis muscle flap in lower extremity reconstruction: a prospective comparative study. Microsurgery. 2019;39(4):297–303.
- Schmidt K, Jakubietz MG, Gilbert F, et al. Quality of life after flap reconstruction of the distal lower extremity: is there a difference between a pedicled suralis flap and a free anterior lateral thigh flap? Plast Reconstr Surg Glob Open. 2019;7(4):e2114.
- Schmidt K, Jakubietz M, Djalek S, et al. The distally based adipofascial sural artery flap: faster, safer, and easier? A long-term comparison of the fasciocutaneous and adipofascial method in a multimorbid patient population. Plast Reconstr Surg. 2012;130(2):360–368.
- Seyidova N, Anderson K, abood A. Comparison of patients satisfaction with aesthetic outcomes following lower extremity reconstruction: muscle vs. fasciocutaneous free flaps. J Plast Reconstr Aesthet Surg. 2021;74(1):65–70.
- Sofiadellis F, Liu DS, Webb A, et al. Fasciocutaneous free flaps are more reliable than muscle free flaps in lower limb trauma reconstruction: experience in a single trauma center. J Reconstr Microsurg. 2012;28(5):333–340.
- Song B, Chen J, Han Y, et al. The use of fabricated chimeric flap for reconstruction of extensive foot defects. Microsurgery. 2016;36(4):303–309.
- Soni A, Tzafetta K, Knight S, et al. Gustilo IIIC fractures in the lower limb. J Bone Joint Surg Br. 2012;94(5):698–703.
- J S AR, R M, Laj M, et al. Reconstruction of defects involving the Achilles tendon and local soft tissues. Bone Jt J. 2015;97-B(2):215–220.
- Struckmann V, Hirche C, Struckmann F, et al. Free and pedicled flaps for reconstruction of the weightbearing sole of the foot: a comparative analysis of functional results. J Foot Ankle Surg. 2014;53(6):727–734.
- Lu S, Wang C, Zhong W, et al. Amputation stump revision using a free sural neurocutaneous perforator flap. Ann Plast Surg. 2016;76(1):83–87.
- Mahmoud WH. Foot and ankle reconstruction using the distally based sural artery flap versus the medial plantar flap: a comparative study. J Foot Ankle Surg. 2017;56(3):514–518.
- Suh YC, Suh HP, Lee JS, et al. Reconstruction using a perforator free flap after malignant melanoma resection of the ankle and foot. J Surg Oncol. 2017;116(7):862–869.
- Tan O, Aydin OE, Demir R, et al. Neurotized sural flap: an alternative in sensory reconstruction of the foot and ankle defects. Microsurgery. 2015;35(3):183–189.
- Tekin L, Zor F, Akarsu S, et al. Quality of life and functionality of patients with heel reconstruction after landmine explosions. PM R. 2013;5(7):591–595.
- Theil C, Stock ME, Gosheger G, et al. Gastrocnemius muscle flaps for soft tissue coverage in periprosthetic knee joint infection. J Arthroplasty. 2020;35(12):3730–3736.
- Trevatt AEJ, Filobbos G, Ul Haq A, et al. Long-term sensation in the medial plantar flap: a two-centre study. Foot Ankle Surg. 2014;20(3):166–169.
- Myung Y, Yim S, kyu KB. A comparison of axial circumference between superficial circumflex iliac artery perforator flap and other workhorse flaps in dorsal foot reconstruction. J Plast Surg Hand Surg. 2017;51(6):381–386.
- Vallier HA, Fitzgerald SJ, Beddow ME, et al. Osteocutaneous pedicle flap transfer for salvage of transtibial amputation after severe lower-extremity injury. J Bone Joint Surg Am. 2012;94(5):447–454.
- Wang SJ, Kim YD, Huang H h, et al. Lateral calcaneal artery perforator-based skin flaps for coverage of lower-posterior heel defects. J Plast Reconstr Aesthet Surg. 2015;68(4):571–579.
- Yang Y F, Xu Z h, Zhang G M, et al. Modified classification and single-stage microsurgical repair of posttraumatic infected massive bone defects in lower extremities. J Reconstr Microsurg. 2013;29(9):593–600.
- Zhang X, Bai G, Zhang Z, et al. Treatment of the secondary defect on the first metatarsophalangeal joint using the medial plantar hallucal artery dorsal perforator flap. Ann Plast Surg. 2016;76(5):536–540.
- Zheng X, Zhan Y, Li H, et al. Emergency repair of severe limb injuries with free flow-through chimeric anterolateral thigh perforator flap. Ann Plast Surg. 2019;83(6):670–675.
- Zheng L, Zheng J, dong ZG. Reverse sural flap with an adipofascial extension for reconstruction of soft tissue defects with dead spaces in the heel and ankle. Eur J Trauma Emerg Surg. 2016;42(4):503–511.
- Zhong W, Lu S, Chai Y, et al. One-stage reconstruction of complex lower extremity deformity combining ilizarov external fixation and sural neurocutaneous flap. Ann Plast Surg. 2015;74(4):479–483.
- Rounds AD, Burtt KE, Leland HA, et al. Functional outcomes of traumatic lower extremity reconstruction. J Clin Orthop Trauma. 2019;10(1):178–181.
- Asʼadi K, Salehi SH, shoar S. Early reconstruction of distal leg and foot in acute high-voltage electrical burn: does location of pedicle in the zone of injury affect the outcome of distally based sural flap? Ann Plast Surg. 2017;78(1):41–45.
- Klinkenberg M, Fischer S, Kremer T, et al. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast Reconstr Surg. 2013;131(2):293–302.
- Sapino G, Zaugg P, Cherix S, et al. ALT flap with vascularized fascia lata for one-stage functional patellar tendon reconstruction. J Plast Reconstr Aesthet Surg. 2019;72(3):467–476.
- Löfstrand JG, lin CH. Reconstruction of defects in the weight-bearing plantar area using the innervated free medial plantar (instep) flap. Ann Plast Surg. 2018;80(3):245–251.
- Pappalardo M, Jeng SF, Sadigh PL, et al. Versatility of the free anterolateral thigh flap in the reconstruction of large defects of the weight-bearing foot: a single-center experience with 20 consecutive cases. J Reconstr Microsurg. 2016;32(7):562–570.
- Kim DY, Choi JH, Moon SK, et al. Feasibility and aesthetic results of small bilateral V-Y advancement flaps in the extremities and back. Arch Aesthetic Plast Surg. 2017;23(3):127.
- Fischer S, Diehm Y, Hirche C, et al. Comparison of sub‐ versus suprafascially raised anterolateral thigh free flaps with regard to donor‐site morbidity, function and aesthetics. Microsurgery. 2017;38(5):444–449.
- Bigdeli AK, Thomas B, Falkner F, et al. Microsurgical reconstruction of extensive lower extremity defects with the conjoined parascapular and latissimus dorsi free flap. Microsurgery. 2020;40(6):639–648.
- Azoury SC, Stranix J, Othman S, et al. Outcomes following soft-tissue reconstruction for traumatic lower extremity defects at an orthoplastic limb salvage center: The need for lower extremity guidelines for salvage (L.E.G.S.). Orthop Surg. 2021;3:1–7.
- Smith PG, Morrow RH, Ross DA, editors. Field Trials of health interventions. 3rd ed. Oxford (UK): Oxford University Press; 2015.
- Helen ER, David JM, Ross HE, Murray DJ, editors. E.H. Weber on the tactile senses. 2nd ed. London: Psychology Press; 2018.
- Feng Y, Schlösser FJ, sumpio BE. The semmes weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50(3):675–682.e1.
- Patel KK, Veenstra DL, patrick DL. A review of selected patient-generated outcome measures and their application in clinical trials. Value Health. 2003;6(5):595–603.
- Brown MT, Couch ME, huchton DM. Assessment of donor-site functional morbidity from radial forearm fasciocutaneous free flap harvest. Arch Otolaryngol Head Neck Surg. 1999;125(12):1371–1374.
- Boku T, Yokoyama K, Nakamura K, et al. Functional outcome and quality of life of gustilo iiib open tibial fractures requiring free tissue transfers. Microsurgery. 2005;25:532–537.
- Wormald JCR, rodrigues JN. Outcome measurement in plastic surgery. J Plast Reconstr Aesthet Surg. 2018;71(3):283–289.
- Thoma A, Jansen L, sprague S. Outcomes in microsurgery. Plast Reconstr Surg. 2009;124(6 Suppl):e303–e312.
- Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353.
- Weldring T, smith SMS. Article commentary: patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6: 61–68.
- Janssen SJ, van Rein EAJ, Paulino Pereira NR, et al. The discrepancy between patient and clinician reported function in extremity bone metastases. Sarcoma. 2016;2016:e1014248.
- Joswig H, Stienen MN, Smoll NR, et al. Patients’ preference of the timed up and go test or patient-reported outcome measures before and after surgery for lumbar degenerative disk disease. World Neurosurg. 2017;99:26–30.
- Gautschi OP, Corniola MV, Schaller K, et al. The need for an objective outcome measurement in spine surgery—the timed-up-and-go test. Spine J. 2014;14(10):2521–2522.
- Karakiewicz PI, Briganti A, Chun FKH, et al. Outcomes research: a methodologic review. Eur Urol. 2006;50(2):218–224.
- Cano SJ, Browne JP, lamping DL. Patient-based measures of outcome in plastic surgery: current approaches and future directions. Br J Plast Surg. 2004;57(1):1–11.
- Pusic AL, Chen CM, Cano S, et al. Measuring quality of life in cosmetic and reconstructive breast surgery: a systematic review of patient-reported outcomes instruments. Plast Reconstr Surg. 2007;120(4):823–837.
- Gideroğlu K, Cakici H, Yildirim S, et al. Önkol ve eldeki geniş ve kompleks yumuşak doku kayıplarının anterolateral uyluk flebi ile fonksiyonel onarımı [Functional reconstruction in large and complex soft tissue defects of forearm and hand with multifunctional anterolateral thigh flap]. Eklem Hastalik Cerrahisi. 2009;20(3):149–155.
- Paley D, Catagni MA, Argnani F, et al. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989;241:146–165.
- Wang CY, Olson SL, protas EJ. Test-retest strength reliability: hand-held dynamometry in community-dwelling elderly fallers. Arch Phys Med Rehabil. 2002;83(6):811–815.
- Prieto TE, Myklebust JB, Hoffmann RG, et al. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 1996;43(9):956–966.
- Heffernan G, Khan F, Awan N, et al. A comparison of outcome scores in os calcis fractures. Ir J Med Sci. 2000;169(2):127–128.
- Weber O, Müller MC, Goost H, et al. The articular fracture of the lower limb. Z Orthop Unfall. 2009;147(3):298–305.
- Zhang H, Zhang X, Yu D, et al. Reconstruction of skin and soft tissue defects by pedicle skin flaps. Chin J Orthop. 2012;32(3):260–264.
- Strasser EJ. An objective grading system for the evaluation of cosmetic surgical results. Plast Reconstr Surg. 1999;104(7):2282–2285.
- Baryza MJ, Baryza GA. Vancouver scar scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16(5):535–538.
- American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine. Muscle Nerve. 1992;15(2):229–253.
- Ridehalgh C, Sandy-Hindmarch OP, schmid AB. Validity of clinical small-fiber sensory testing to detect small–nerve fiber degeneration. J Orthop Sports Phys Ther. 2018;48(10):767–774.
- Meirte J, Moortgat P, Truijen S, et al. Interrater and intrarater reliability of the semmes weinstein aesthesiometer to assess touch pressure threshold in burn scars. Burns. 2015;41(6):1261–1267.
- Hale SA, hertel J. Reliability and sensitivity of the foot and ankle disability index in subjects with chronic ankle instability. J Athl Train. 2005;40(1):35–40.
- Leigheb M, Rava E, Vaiuso D, et al. Translation, cross-cultural adaptation, reliability, and validation of the italian version of the foot and ankle disability index (FADI). Acta Biomed. 2020;91(4-S):160–166.
- Repo JP, Ponkilainen VT, Häkkinen AH, Ylinen J, Bergman P, Kyrölä K. Assessment of construct validity of the Oswestry disability index and the scoliosis research society-30 questionnaire (SRS-30) in patients with degenerative spinal disease. Spine Deform. 2019;7(6):929–936.
- Budiman-Mak E, Conrad KJ, roach KE. The foot function index: a measure of foot pain and disability. J Clin Epidemiol. 1991;44(6):561–570.
- Binkley JM, Stratford PW, Lott SA, et al. The lower extremity functional scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371–383.
- Turhan E, Demirel M, Daylak A, et al. Translation, cross-cultural adaptation, reliability and validity of the turkish version of the olerud-molander ankle score (OMAS). Acta Orthop Traumatol Turc. 2017;51(1):60–64.
- Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–205.
- Simpson K. Validity and reliability of the Paffenbarger physical activity questionnaire among healthy adults [master’s theses]. University of Connecticut; 2011
- Davis AM, Wright JG, Williams JI, et al. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5(5):508–516.
- Gur G, Turgut E, Dilek B, et al. Validity and reliability of visual analog scale foot and ankle: the Turkish version. J Foot Ankle Surg. 2017;56(6):1213–1217.
- Richter M, Zech S, Geerling J, et al. A new Foot and Ankle Outcome Score: questionnaire based, subjective, visual-analogue-scale, validated and computerized. Foot Ankle Surg. 2006;12(4):191–199.
- Langley GB, sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int. 1985;5(4):145–148.
- Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101.
- Rosas S, Paço M, Lemos C, et al. Comparison between the visual analog scale and the numerical rating scale in the perception of esthetics and pain. Int Orthod. 2017;15(4):543–560.
- Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain. 1992;50(2):133–149.
- Smith BH, Penny KI, Purves AM, et al. The chronic pain grade questionnaire: validation and reliability in postal research. Pain. 1997;71(2):141–147.
- Carlsson I, Cederlund R, Höglund P, et al. Hand injuries and cold sensitivity: reliability and validity of cold sensitivity questionnaires. Disabil Rehabil. 2008;30(25):1920–1928.
- Drixler K, Morfeld M, Glaesmer H, et al. Validation of the short-form-health-survey-12 (SF-12 version 2.0) assessing health-related quality of life in a normative german sample. Z Psychosom Med Psychother. 2020;66(3):272–286.
- Cash TF, Jakatdar TA, Williams EF. The body image quality of life inventory: further validation with college men and women. Body Image. 2004;1(3):279–287.
- Henrich G, herschbach P. Questions on life satisfaction (FLZM): a short questionnaire for assessing subjective quality of life. Eur J Psychol Assess. 2000;16(3):150–159.
- Janke KH, Raible A, Bauer M, et al. Questions on life satisfaction (FLZM) in inflammatory bowel disease. Int J Colorectal Dis. 2004;19(4):343–353.
- Ware JE, sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483.
- Ware JE, Kosinski M, keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233.
- Singh A, Gnanalingham K, Casey A, et al. Quality of life assessment using the short form-12 (SF-12) questionnaire in patients with cervical spondylotic myelopathy: comparison with SF-36. Spine. 2006;31(6):639–643.
- Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–336.
- Rabin R, de Charro F. EQ-SD: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343.
- Bergner M, Bobbitt RA, Carter WB, et al. The sickness impact profile: development and final revision of a health status measure on JSTOR. Medical Care. 1981;19:787–805.
- Boyden EM, Kitaoka HB, Cahalan TD, et al. Late versus early repair of Achilles tendon rupture. Clin Orthop Relat Res. 1995;317:150–158.
- Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788–794.
- Mousavi SJ, Parnianpour M, Mehdian H, et al. The Oswestry Disability Index, the Roland-Morris Disability Questionnaire, and the Quebec Back Pain Disability Scale: translation and validation studies of the iranian versions. Spine. 2006;31:E454–E459.
- Erh BXY, Gu HH, Carter KF, et al. Validation of the chinese Manchester foot pain and disability index (C-MFPDI) among patients with inflammatory arthritis. J Foot Ankle Res. 2019;12(1):6.
- Wada T, Kawai A, Ihara K, et al. Construct validity of the Enneking score for measuring function in patients with malignant or aggressive benign tumours of the upper limb. J Bone Joint Surg Br. 2007;89(5):659–663.
- Gerrand CH, rankin K. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. In: Banaszkiewicz PA, Kader DF, editors. Classic papers in orthopaedics. London: Springer Verlag London; 2014. 489–490.
- Williams DP, Blakey CM, Hadfield SG, et al. Long-term trends in the oxford knee score following total knee replacement. Bone Joint J. 2013;95-B(1):45–51.
- Williams N. The short musculoskeletal function assessment (SMFA) questionnaire. Occup Med. 2016;66(9):757.
- Ebrahimzadeh MH, Makhmalbaf H, Birjandinejad A, et al. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) in persian speaking patients with knee osteoarthritis. Arch Bone Jt Surg. 2014;2(1):57–62.
- Johner R, wruhs O. Classification of tibial shaft fractures and correlation with results after rigid internal fixation. Clin Orthop. 1983;178:7–25.
- Cöster MC, Rosengren BE, Bremander A, et al. Comparison of the self-reported foot and ankle score (SEFAS) and the American Orthopedic foot and ankle society score (AOFAS). Foot Ankle Int. 2014;35(10):1031–1036.
- Scuderi GR, Bourne RB, Noble PC, et al. The new knee society knee scoring system. Clin Orthop Relat Res. 2012;470(1):3–19.
- The Knee Society Score. [cited 2021 June 16]. Available from: https://www.kneesociety.org/the-knee-society-score