Abstract
Introduction
This work aims to assess lower limb free flaps spontaneous sensory recovery by comparing and analyzing a single standardized reconstructive procedure, namely the free noninnervated anterolateral thigh (ALT) flap in order to evaluate which flap or patient-related factors may predict flap reinnervation.
Methods
Between January 2010 and March 2018 all nonreinnervated ALT flaps for lower limb coverage performed at our institution were screened. We excluded from the study flaps with less than 18 months of follow-up time, neurotized flaps, and those from patients who missed the last follow up. Sensory modalities that were evaluated included the two-point discrimination (2PD) test, measured in mm; and the Semmes–Weinstein monofilament (SWM) test, measured in gram. The sensory parameter results were compared and analyzed according to flap size (two groups; <160 cm2 vs. > 160 cm2), and post-op time of testing (two groups; <18–28 months vs. > 28 months).
Results
Twenty-one ALT free flaps were finally retained by this study. Our findings showed that flaps of smaller surface area showed a significantly better return in sensory discrimination 2PD and in sensory cutaneous pressure perception SWM testing.
Conclusion
This work establishes for the first time some key quantitative data that can help predict free flap spontaneous reinnervation outcomes when using the same ALT flap. In our series, flaps surface remains the main discriminant value for a better sensory recovery.
Introduction
Trauma, oncologic surgery, chronic wounds and burn sequelae may require soft tissue coverage. In the lower limb, where few local options exist, especially in large and distal defects, free tissue transfer remains an essential tool for reconstruction. In this regard, the anterolateral thigh (ALT) flap becomes the workhorse for lower limb reconstruction [Citation1]. In addition to the coverage of defects, the surgeon’s aim should maximize functional reconstruction to improve the quality of life for those patients after the procedure [Citation2] by replacing like-with-like, and restoring tissue properties.
Nevertheless, sensory recovery could play an important role in re-gaining functional skills and may facilitate the return to normal life. Moreover, the absence of sensory feedback in free tissue transfer may increase the probability of flap breakdown on the long term, especially in weight-bearing zones [Citation3–5]. Despite this, the need for sensory reinnervation at the recipient site remains debatable in literature. Even though sensory nerve coaptation should theoretically lead to superior sensory recovery [Citation6], there is limited evidence that this would finally lead to better clinical functional outcomes [Citation7]. Several authors described spontaneous recovery of sensation in noninnervated flaps through neural infiltration and nerve regeneration from the edges and the base of the healthy recipient site [Citation8–10].
In this work, a multimodal comparison was used to analyze a single standardized reconstructive procedure (free noninnervated ALT flap). The comparison was also used to assess whether different patient-related and flap-related variables could influence, in a predictable way, collateral flap reinnervation on a long-term follow up, and how this could influence reconstructive outcomes and the daily life of patients.
Materials and methods
Patients
We conducted a retrospective study by retrieving records of all lower limbs ALT free flap reconstructions performed on patients who were operated at our institution, between January 2010 and January 2018. The study excluded flaps with short follow-up time (less than 18 months after reconstruction), weight-bearing neurotized flaps, reconstructions in patients with injured nerve, reconstruction after radiotherapy of the reconstructed area, reconstructions where patients’ flap details were either not clearly specified or were unavailable in the operative notes and patients who suffered from diabetes mellitus. After the application of the exclusion criteria, 35 patients were initially identified. Since 14 patients did not return for their last follow-up to perform a full sensory examination (five premature deaths (i.e. mainly Sarcoma patients) and nine lost to follow up (refused the appointment or changed their address)), only the remaining 21 patients were enrolled in the study. These included 14 (66.7%) men, and 7 (33.3%) women with a mean age of 54 years (median 56, range 21–87). The mean follow-up time was 34 months (median 28, range 18–108).
Methods
Reconstruction included 16 fasciocutaneous and 5 musculocutaneous ALT free flaps. Flaps dimensions and complications were recorded. Post-operative radiotherapy and patients’ comorbidities were also reported, using information from discharge letters, medical records and anesthesiology charts. The patients were divided into subgroups based on the length of follow-up time and the size of the harvested flap, with median values used as cut-offs for both follow-up time (≥28 months) and flap surfaces (≥160 cm2). Patient’s demographics and reconstructed areas are summarized in and .
Figure 1. Diagram of lower limb representing percentage distribution of the different reconstructed areas.
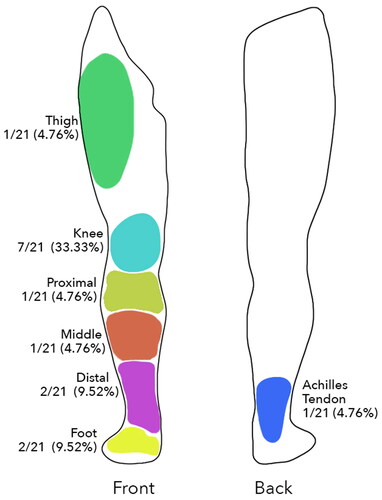
Table 1. Patients demographics.
Methods used to evaluate outcomes
Evaluations for sensory function
Two sensory modalities were used in the evaluation of sensory functions. These were the two-point discrimination (2PD) test, measured in mm, and the Semmes–Weinstein monofilament (SWM) test, measured in gram (North Coast Medical, Inc., Gilroy, CA, USA). Each flap was divided into two sectors: one central and one peripheral (within 3 cm from the scar top, bottom, right and left directions). This approach is similar to the sensory evaluation on the Nipple areola complex when evaluating sensory areolar recovery [Citation11].
Sensory recovery was tested during the last follow-up visit by a plastic surgery resident blind to the study until full recruitment of data was made. In that sense, the examiner knew the type of reconstruction performed, but was not aware nor about the study design (parameter involved) nor the exclusion criteria (tested and recorded neurotized and non-neurotized flaps). Patients were asked to keep their eyes closed during the examination. Recorded values from the flap periphery and center were averaged together. The 2PD test was measured with a caliper, setting the two tips at a distance of 2.5 cm, and placing them simultaneously on the flap, while reducing the distance gradually until the patient felt only a single point. The SWM test was used to measure pressure perception (kit containing 20 filaments from 0.008 to 300 g) [Citation12].
Statistical methods
Median-split separation was performed to divide the study population into subgroups (e.g. greater or less than 28 months follow up, and greater or less than 160 cm2 of flap surface). Comparison between subgroups was made using independent two-sided t tests for means, and Mann–Whitney U tests for medians. ANOVA multiple comparison test was used to investigate potential differences between multiple subgroups. We verified the assumption of normality using the Shapiro–Wilk test. Statistical significance was set at a p < 0.05. Flap locations were homogeneously distributed between the evaluated groups, reducing the potential bias due to flap location.
Statistical analysis was performed using GraphPad Prism (version 8.0, GraphPad software, La Jolla, CA, USA).
Results
Functional outcomes
The sensory parameter results were compared and analyzed according to flap size (two groups; <160 cm2 vs. > 160 cm2) and to post-op time of testing (two groups; <18–28 months vs. > 28 months).
Considering a potential bias on sensitive recovery due to different flap thickness, we performed a comparative analysis of all subgroups which showed no statistical significance in terms of BMI: (27.36; 28.2 in <160 cm2 subgroup vs. 25.19; 24.70 in >160 cm2 subgroup) and (26.55;27.60 in < 28 months subgroup vs. 25.87; 25.70 in > 28 months subgroup); values expressed as average; median. The groups resulted therefore relatively homogeneous, allowing for further investigation of sensory recovery.
Two-point discrimination test outcome
In order to test surface skin discrimination, the average 2PD values were evaluated according to the flap size and post-op time of testing. When considering flap size, a significant difference was present in comparing average 2PD values (*3.48 ± 0.37 in <160 cm2 flap size group vs. *4.41 ± 0.22 in >160 cm2 flap size group; values expressed as average ± SEM). When considering average 2PD values according to follow-up time (<28 m and >28 m), no significant difference was found.
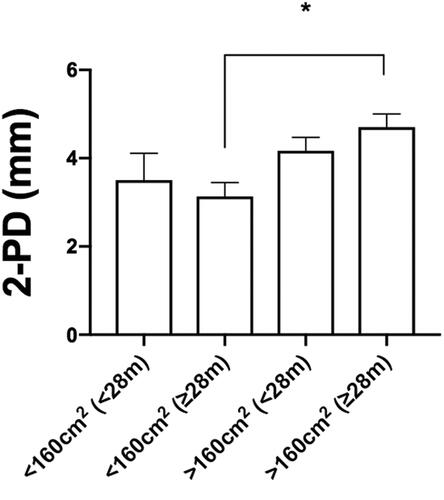
When performing a multiple subgroup analysis ((<28 months, >28 months, <160 cm2 and >160 cm2), the best sensory 2PD values were found in the sub-group of patients having the smaller flap surface (<160 cm2) and longer post-op time (>28 months); and the worst in term of sensory return in the sub-group of patients with the larger flap size (>160 cm2) and shorter post-op time (<28 m) (*3.13 ± 0.31 in <160 cm2 and > 28 m subgroup vs. *4.17 ± 0.31 in >160 cm2 and <28 m subgroup; values expressed as average ± SEM ( and )).
Figure 2. Subgroup analysis according to size flap (<160 cm2 and >160 cm2) for the two-point discrimination (2PD) recorded values. Values are expressed as average ± SEM (*p < 0.05).
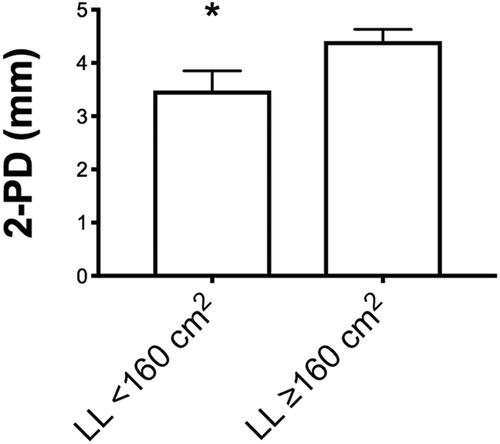
Figure 3. Multiple subgroup analysis (<28 months, > 28 months, <160 cm2 and >160 cm2) for the Semmes–Weinstein pressure recorded values. Values are expressed as average ± SEM (*p < 0.05).
In other terms, flaps of smaller surface area with longer postoperative time showed a significantly better return in sensory discrimination (2PD) than their larger surface counter parts with shorter postoperative time.
Semmes–Weinstein monofilament test outcome
In order to test for skin pressure perception, average SWM values of flaps were evaluated according to the flap size and post-op time of testing, as previously described for 2PD values. The average values showed better sensory return in the smaller flaps (<160 cm2) group (*4.78 ± 0.87 in <160 cm2 flap size group vs. *7.55 ± 0.89 in >160 cm2 flap size group; values expressed as average ± SEM). Again, when considering average SWM values according to follow-up time (< 28 months and > 28 months), no significant difference was shown, even if a strong trend was seen towards better sensory recovery at longer follow-up times (p = 0.15).
Comparative analysis of all subgroups (<28 months, >28 months, <160 cm2 and >160 cm2) showed best sensory return in terms of SWM in the subgroup of patients having the smallest flap size (<160 cm2) and longer post-op time (>28 months); and the worst in term of sensory return in the subgroup of patients with the largest flap size (>160 cm2) regardless of post-op time (both <28 months and >28 months) (*3.43 ± 1.26 in <160 cm2 and >28 months subgroup vs. *8.17 ± 1.6 in >160 cm2 and <28 months subgroup; values expressed as average ± SEM ( and )).
Figure 4. Subgroup analysis according to size flap (<160 cm2 and >160 cm2) for the two-point discrimination (2PD) recorded values. Values are expressed as average ± SEM (*p < 0.05).
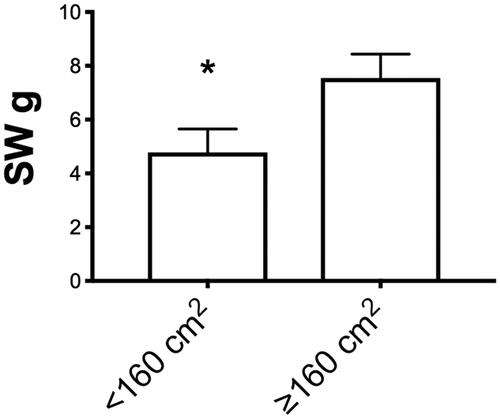
Figure 5. Multiple subgroup analysis (<28 months, > 28 months, <160 cm2 and >160 cm2) for the Semmes–Weinstein pressure recorded values. Values are expressed as average ± SEM (*p < 0.05).
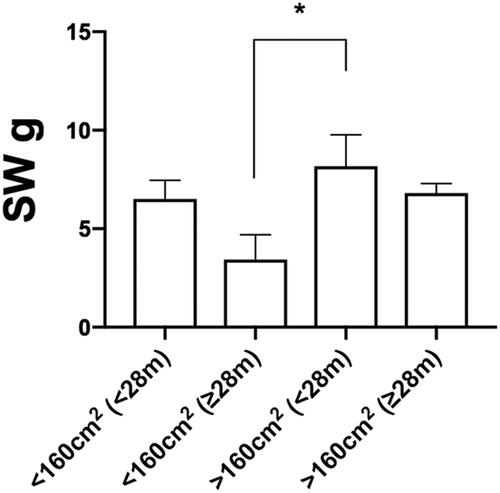
In other terms, flaps of smaller surface area showed a better return in sensory cutaneous pressure perception (SWM) than their larger surface counter parts, without a critical influence on follow-up time.
Discussion
According to literature, several authors have investigated nerve regrowth in transplanted tissue [Citation13,Citation14]. The spontaneous reinnervation is considered a progressive process occurring after free flap tissue transfer [Citation14]. However, this belief remains unclear, and no concrete answers exist in literature as to which factors (patients-related, flap-related and/or recipient site-related) influence sensory recovery. Histochemical studies of human skin grafts and flaps provide basis for understanding the mechanism of sensory recovery in noninnervated flaps [Citation9]. Dykes et al. obtained incisional biopsies from nine patients who had undergone skin grafting and found significant histochemical evidence of regenerating nerves at the bed and margins of the skin grafts 3 weeks after surgery [Citation15]. Other authors underlined that the most important factor for natural recovery of noninnervated flaps appeared to be the axonal sprouting from the periphery to the center [Citation13]. The size of defects was found to be important, since the degree of natural sensory recovery was the greatest when flaps were used for smaller defects because spontaneous reinnervation depended on residual nerve population.
In lower extremities, sensory recovery after reconstruction may become an important issue, since a protective sensation could avoid unintentional injuries resulting from pressure sores or burns. Although sensory recovery was extensively studied in various fields such as autologous breast reconstruction [Citation16,Citation17], mammoplasty [Citation18], digital repair [Citation19], burn scars [Citation20], oral reconstruction [Citation3,Citation21] and lower lip reconstruction [Citation22], only little is known about this issue in free flap extremity reconstruction. Our work establishes for the first time some key quantitative data that can help predict free flap spontaneous reinnervation outcomes. Potential biases were reduced by using the same ALT flap with the same harvest technique (subfascial dissection according to Fu Chan Wei) [Citation23], all the time.
At the level of the foot, literature suggests a mean SW-evaluator size of 3.63 (± 0.0075 SEM), as physiological corresponding to 0.4 g target force when using the Aesthesio® data evaluator chart [Citation24]. Concerning other parts of the lower limb, when tested on a healthy volunteer in our department, we found that the threshold sensation at the level of the knee was 0.6 g and 1.4 g at the level of the ankle. Several studies have determined SW value of 5.07 (corresponding to 10 g target force) to be the threshold for protective sensation [Citation25]. Our best-measured value considering all the leg (small flaps in longer follow up) was on average 3.43 g, largely reaching protective sensation.
Some limitations to our study have to be acknowledged. First, the retrospective investigation (even if based on a prospectively maintained database), and the relatively small number of cases (n = 21) have to be highlighted. As mentioned previously, we only included patients who had a proper and complete follow up (more than 18 months). Moreover, despite the long follow-up time, we could not obtain intermediate values of sensory recovery at different time points. Another limitation that needs to be highlighted is about the flap homogeneity (flap thickness and different recipient sites). However, as already stated, flap locations were homogeneously distributed between the evaluated groups (i.e. thigh, knee, proximal 1/3, middle 1/3, distal 1/3, ankle and foot) (), reducing the potential bias due to flap location. Also, as previously mentioned flap thickness was shown to be similar based on similar BMI between subgroups. Considering the negligeable proportion of female population this implied similar mid/distal thigh thickness and therefore a similar flap thickness distribution. In addition, in this series, no TFL perforator flaps were present, therefore excluding the thicker upper part of the anterolateral thigh and thus reinforcing our assumption of a similar flap thickness.
Finally, only noninnervated ALT flaps were tested against healthy tissue, and therefore an additional group with neurotized flap could have been of theoretic scientific interest. On the other hand, only non-weight-bearing flaps were included in this study, without a real clinical need for neurotization. Some reports highlighted how nonsensate reconstruction of the heel maintained the property for stable weight-bearing without pressure sore appearance [Citation26]. We could argue whether neurotization should be still applied in limited surface flaps. In such cases, the process of spontaneous reinnervation of fasciocutaneous flaps guaranteed indeed sensory recovery above the protective sensation threshold. As reported by Hong et al., nonsensate flaps will eventually recover protective sensation around 12 months [Citation27].
Further studies are needed to compare neurotized and non-neurotized flaps in homogeneous cohorts to assess the real benefit of neurotization in the long run.
However, the results of our study are in line with the general physiological principles of reinnervation, namely that smaller flaps regain faster and better sensation than their larger counterparts. Our results were consistent with the Rothenberger et al. study that found a negative correlation between the flap size and sensory recovery potential [Citation28].
Moreover, new intriguing aspects of free flap spontaneous reinnervation were revealed. This is explained by the fact that in our series, follow-up time has definitely played a supporting role after the cut-off period of 28 months, flap surface remained the main discriminant value, as larger flaps, regardless of follow-up time, did not reach significantly better recovery values when compared to their smaller counterparts. Such a result is interesting as it reveals a limited potential to spontaneous reinnervation. This could be explained by the restricted power of peripheral collateral sensory sprouting, which may get progressively lost if very large areas of the flaps need to be crossed by regenerating fibers. This concept recalls, in a way, the gap limitations in peripheral nerve injury and regeneration, where nerve gaps over 3 cm show significantly worse regeneration outcomes, even when nerve is directed into nerve guides.
With regard to other potentially influencing parameters, our study did not find any statistical correlation between the flap components (fasciocutaneous or musculocutaneous), or age of the patient, and the degree of spontaneous reinnervation.
Future studies are needed to prove if target-derived neurotrophic factors that preexist at the recipient site could provide successful target-specific regeneration across a considerable surface or distance [Citation29], as is the case with what happens in peripheral nerve regeneration [Citation30]. In this sense, the recipient area and its nerve afferences could be a supportive niche influencing the potential for spontaneous sensory reinnervation in different anatomical sites.
Conclusion
In studying the same ALT flap, our data shed some light on free flap spontaneous reinnervation outcomes. Despite the fact that in our series follow-up time (> 28 months) seemed to play a positive role in recovery (for sensory cutaneous pressure perception in particular), flap surface remains the main discriminant value. Larger flaps will still show spontaneous recovery in longer follow-up time despite it being less evident when compared with smaller flaps.
Author contributions
W.W.: concept of study and writing; G.S.: literature review and data extraction; F.T.: statistics and table; C.M.O.: data extraction and methodology; M.C.: figures and statistics; D.C.: data, discussion; W.R.: proof-reading; P.D.S.: conceptualization, writing, idea and proof-reading.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kozusko SD, Liu X, Riccio CA, et al. Selecting a free flap for soft tissue coverage in lower extremity reconstruction. Injury. 2019;50(Suppl 5):S32–S39.
- Kerawala CJ, Newlands C, Martin I. Spontaneous sensory recovery in non-innervated radial forearm flaps used for head and neck reconstruction. Int J Oral Maxillofac Surg. 2006;35(8):714–717.
- Boyd B, Mulholland S, Gullane P, et al. Reinnervated lateral antebrachial cutaneous neurosome flaps in oral reconstruction: are we making sense? Plast Reconstr Surg. 1994;93(7):1350–1359.
- Nordgren M, Hammerlid E, Bjordal K, et al. Quality of life in oral carcinoma: a 5-year prospective study. Head Neck. 2008;30(4):461–470.
- Bordianu A, Gheorghiu-Branaru M, Marinescu S. ALT flap and reverse sural flap for simultaneous soft tissue coverage of both medial and lateral calf wounds in a diabetic patient. Injury. 2020;4:S31–S33. doi: 10.1016/j.injury.2020.02.103.
- Kim JH, Rho YS, Ahn HY, et al. Comparison of sensory recovery and morphologic change between sensate and nonsensate flaps in oral cavity and oropharyngeal reconstruction. Head Neck. 2008;30(8):1099–1104.
- Baas M, Duraku LS, Corten EM, et al. A systematic review on the sensory reinnervation of free flaps for tongue reconstruction: does improved sensibility imply functional benefits? J Plast Reconstr Aesthet Surg. 2015;68(8):1025–1035.
- Lahteenmaki T, Waris T, Asko-Seljavaara S, et al. Recovery of sensation in free flaps. Scand J Plast Reconstr Surg Hand Surg. 1989;23(3):217–222.
- Turkof E, Jurecka W, Sikos G, et al. Sensory recovery in myocutaneous, noninnervated free flaps: a morphologic, immunohistochemical, and electron microscopic study. Plast Reconstr Surg. 1993;92(2):238–247.
- Close LG, Truelson JM, Milledge RA, et al. Sensory recovery in noninnervated flaps used for oral cavity and oropharyngeal reconstruction. Arch Otolaryngol Head Neck Surg. 1995;121(9):967–972.
- Watfa W, Martineau J, Giordano S, et al. Long-term evaluation of Nipple-Areolar complex changes in inferior versus superomedial pedicle reduction mammoplasty: a comparative study. J Plast Reconstr Aesthet Surg. 2022;75(3):1179–1186.
- Levin S, Pearsall G, Ruderman RJ. Von Frey’s method of measuring pressure sensibility in the hand: an engineering analysis of the Weinstein-Semmes pressure aesthesiometer. J Hand Surg Am. 1978;3(3):211–216.
- Shindo ML, Sinha UK, Rice DH. Sensory recovery in noninnervated free flaps for head and neck reconstruction. Laryngoscope. 1995;105(12):1290–1293.
- Stromps JP, Bozkurt A, Grieb G, et al. Spontaneous reinnervation of deep inferior epigastric perforator flaps after delayed breast reconstruction. J Reconstr Microsurg. 2016;32(3):169–177.
- Dykes RW, Terzis JK, Strauch B. Sensations from surgically transferred glabrous skin; central versus peripheral factors. Can J Neurol Sci. 1979;6(4):437–439.
- Beugels J, Cornelissen AJM, Spiegel AJ, et al. Sensory recovery of the breast after innervated and non-innervated autologous breast reconstructions: a systematic review. J Plast Reconstr Aesthet Surg. 2017;70(9):1229–1241.
- Santanelli F, Longo B, Angelini M, et al. Prospective computerized analyses of sensibility in breast reconstruction with non-reinnervated DIEP flap. Plast Reconstr Surg. 2011;127(5):1790–1795.
- Chiari A, Jr., Nunes TA, Grotting JC, et al. Breast sensitivity before and after the L short-scar mammaplasty. Aesthetic Plast Surg. 2012;36(1):105–114.
- Bulut T, Akgun U, Citlak A, et al. Prognostic factors in sensory recovery after digital nerve repair. Acta Orthop Traumatol Turc. 2016;50(2):157–161.
- Meirte J, Moortgat P, Truijen S, et al. Interrater and intrarater reliability of the Semmes Weinstein aesthesiometer to assess touch pressure threshold in burn scars. Burns. 2015;41(6):1261–1267.
- Kimata Y, Uchiyama K, Ebihara S, et al. Comparison of innervated and noninnervated free flaps in oral reconstruction. Plast Reconstr Surg. 1999;104(5):1307–1313.
- Ayhan Oral M, Zeynep Sevim K, Gorgu M, et al. Sensory recovery with innervated and noninnervated flaps after total lower lip reconstruction: a comparative study. Plast Surg Int. 2013;2013:643061.
- Wei FC, Jain V, Celik N, et al. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg. 2002;109(7):2219–2226. discussion 27–30.
- Jeng C, Michelson J, Mizel M. Sensory thresholds of normal human feet. Foot Ankle Int. 2000;21(6):501–504.
- Rith-Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabetes Care. 1992;15(10):1386–1389. PMID: 1425105.)
- Park JH, Choi IC, Hong TC, et al. Reconstruction of the weight- bearing heel with nonsensate reverse sural artery flaps. Injury. 2021;52(7):1993–1998.
- Hong JP, Kim EK. Sole reconstruction using anterolateral thigh perforator free flaps. Plast Reconstr Surg. 2007;119(1):186–193.
- Rothenberger J, Ramms EM, Medved F, et al. Comparison of spontaneous sensory recovery of noninnervated anteromedial thigh flap, latissimus dorsi flap, and gracilis muscle flap in lower extremity reconstruction: a prospective comparative study. Microsurgery. 2019;39(4):297–303.
- Seckel BR, Ryan SE, Gagne RG, et al. Target-specific nerve regeneration through a nerve guide in the rat. Plast Reconstr Surg. 1986;78(6):793–800.
- Tremp M, Waldkircher NJ, Wang W, et al. Sensory assessment of meshed skin grafts over free gracilis muscle flaps without nerve coaptation for lower extremity reconstruction. Arch Plast Surg. 2021;48(2):224–230. doi: 10.5999/aps.2019.00584.

