ABSTRACT
Fetal bovine serum (FBS) is the most commonly used supplement in studies involving cell-culture experiments. However, FBS contains large numbers of bovine extracellular vesicles (EVs), which hamper the analyses of secreted EVs from the cell type of preference and, thus, also the downstream analyses. Therefore, a prior elimination of EVs from FBS is crucial. However, the current methods of EV depletion by ultracentrifugation are cumbersome and the commercial alternatives expensive. In this study, our aim was to develop a protocol to completely deplete EVs from FBS, which may have wide applicability in cell-culture applications. We investigated different EV-depleted FBS prepared by our novel ultrafiltration-based protocol, by conventionally used overnight ultracentrifugation, or commercially available depleted FBS, and compared them with regular FBS. All sera were characterized by nanoparticle tracking analysis, electron microscopy, Western blotting and RNA quantification. Next, adipose-tissue mesenchymal stem cells (AT-MSCs) and cancer cells were grown in the media supplemented with the three different EV-depleted FBS and compared with cells grown in regular FBS media to assess the effects on cell proliferation, stress, differentiation and EV production. The novel ultrafiltration-based protocol depleted EVs from FBS clearly more efficiently than ultracentrifugation and commercial methods. Cell proliferation, stress, differentiation and EV production of AT-MSCs and cancer cell lines were similarly maintained in all three EV-depleted FBS media up to 96 h. In summary, our ultrafiltration protocol efficiently depletes EVs, is easy to use and maintains cell growth and metabolism. Since the method is also cost-effective and easy to standardize, it could be used in a wide range of cell-culture applications helping to increase comparability of EV research results between laboratories.
Introduction
The supplementation of basal culture media with serum is essential for cell growth, metabolism and stimulation of proliferation. The most widely used supplements are bovine sera of adult or newborn animals, or of fetal origin. FBS contains factors required for cell attachment and proliferation, and is thus used as a universal growth supplement for most types of human and animal cells [Citation1]. One such factor in FBS is the extracellular vesicles (EVs), the importance of which has become evident only recently [Citation2,Citation3]. EVs, secreted by all cells, play a key role in cell-to-cell communication by shuttling protein, lipids, RNA and other molecules between cells with diverse functional consequences in health and various diseases including cancer [Citation4–Citation7]. For example, therapeutic activities in adipose-derived mesenchymal stem cells (AT-MSCs) are mediated by EVs along with other paracrine signalling routes [Citation8,Citation9]. The MSC EVs have a unique capability to induce regeneration of damaged tissues offering a paradigm shift towards cell-free therapy [Citation10,Citation11].
However, EV research is currently hampered by the fact that it relies on cell-culture experiments using FBS, which contains a large amount of EVs that are morphologically, and largely also contentwise, similar to the EVs from the cultured cells [Citation12]. Importantly, FBS EVs are taken up by the cultured cells causing substantial physiological effects, including altered viability and migration [Citation2]. They are also co-isolated with cell-culture-derived EVs, causing bias and misinterpretation of results. FBS-derived EVs are thus considered as common contaminants in experiments aiming to study the EVs released by the cultured cells or to elucidate the effects of exogenous EVs added to the medium. Since cell-culture-based EV studies rely heavily on serum-derived supplements, such as FBS, development of a cost-effective, standardized, simple and efficient method for EV depletion of FBS is of utmost importance.
Currently, there are no standardized protocols for eliminating EVs from FBS. Ultracentrifugation (UC) at 100,000–200,000 g for 2–19 h is commonly used for depleting FBS EVs [Citation7]. However, UC-based EV depletion only partially depletes EVs from FBS [Citation3,Citation13]. Furthermore, it is a time-consuming, difficult-to-standardize and relatively expensive method. Recently, several commercial alternatives have also emerged. However, they are costly and may also contain residual bovine EVs. Thus, it is necessary to develop standardized protocols for EV depletion from FBS in order to minimize the effect of FBS EVs on cell phenotype and downstream analysis of EVs. In this study, we developed a novel protocol based on ultrafiltration (UF) to deplete EVs from FBS, and addressed the effects of this ultrafiltration EV-depleted FBS (UF-dFBS) on proliferation, stress, differentiation and EV production of AT-MSCs and cancer cell lines in comparison with regular FBS, ultracentrifugation EV-depleted FBS (UC-dFBS), commercial EV-depleted FBS (SBI-dFBS) and serum-free media.
Materials and methods
Preparation of EV-depleted FBS
Ultrafiltration EV-depleted FBS (UF-dFBS) was obtained by centrifuging regular FBS in Amicon ultra-15 centrifugal filters (ref: UFC910024, 100kDa Merk Millipore Ltd., Tullagreen, Carrigtwohill, Co. Cork, Ireland) for 55 min at 3,000 g. The liquid concentrated by the ultrafilter was denoted as UF-dFBS retentate and the flow-through as UF-dFBS. UC-dFBS was prepared by 19 h ultracentrifugation of regular FBS at 26 000 rpm (121 896 gmax) using an SW28 rotor (k-factor 284.7, Beckmann-Coulter). Only the light-coloured top layers of the supernatant (approx. 9/10) were retained and used in the subsequent analyses. Further, UC-dFBS was filtered with a 0.22 µm filter (Millipore Stericup-GP, 0.22 µm, polyethersulfone filter) before addition to cell-culture medium. Commercially available EV-depleted FBS (SBI-dFBS), Exo-FBS™ (System Biosciences, EXO-FBS-50A-1, lot: 082715, Mountain View, CA, USA), was used as a control. Details of the FBS and dFBS used are described in .
Table 1. Method for preparing depleted FBS. Prices are without working hour’s costs for hands-on work.
Isolation of FBS-derived EVs for characterization
For EV-RNA isolation and a part of electron microscopy samples, EVs were extracted from regular FBS, different dFBS or UF-dFBS retentate using the miRCURY exosome isolation kit (Exiqon, Vedbaek, Denmark) according to the manufacturer’s instructions. For all other characterization analyses, EVs were extracted using UC at 26 000 rpm (121 896 gmax) for 2 h at 4°C with SW28 rotor to collect the EV pellet, which was washed by filtered PBS (0.1 µm filter), and then the UC was repeated. The final EV pellets were suspended in filtered PBS and stored in Protein LoBind microcentrifuge tubes (Eppendorf) at −80°C.
Nanoparticle tracking analysis (NTA)
The EVs isolated from regular and different dFBS by UC were analysed by NTA to determine vesicle concentration and size distribution using Nanosight model LM14 (NanoSight Technology, Salisbury, UK, http://www.malvern.com) equipped with blue (404 nm, 70 mW) laser and CMOS camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan). The samples were diluted in filtered (0.1 µm) PBS to obtain the optimal detection concentration of 106–109 particles/ml, and three 60 s videos were recorded using camera level 13. In total, three biological replicates were measured from each sample. The data were analysed using NTA software 3.0 with the detection threshold 5 [Citation14].
Transmission electron microscopy (EM)
EV samples isolated by UC or miRCURY exosome isolation kit from each type of dFBS or regular FBS were prepared for EM and imaged as described previously [Citation15]. Briefly, after loading to 200 mesh copper grids and fixation with 2% PFA in 0.1 M NaPO4 buffer (pH 7.0), samples were washed with the 0.1 M NaPO4 buffer and deionized water, negatively stained with 2% neutral uranyl acetate and embedded in methyl cellulose uranyl acetate mixture (1.8/0.4%). Samples from two or three independent EV isolations, each with two or more technical replicates, of all sample types were viewed with transmission EM using Tecnai 12 (FEI Company, Eindhoven, the Netherlands) or Jeol JEM-1400 (Jeol Ltd, Tokyo, Japan) operating at 80 kV. Images were taken with Gatan Orius SC 1000B CCD-camera (Gatan Inc., USA) with an image size of 4008 × 2672 px and no binning.
Western blotting and silver staining
Western blotting was performed as described previously [Citation15] using an antibody against Hsp70 (no. 554243, BD Biosciences) and anti-transferrin receptor/CD71 H68.4 (no. 13–6800, Thermofisher Scientific) at 1:1000 dilution. EVs isolated by UC from equal volumes (0.5 mL) of each dFBS were loaded to gels. As controls, 30 µg of protein from AT-MSC lysates measured by BCA assay (Pierce BCA Protein Assay Kit) and EVs isolated by UC from 0.25 ml FBS and from UF-dFBS retentate (corresponding to approximately 1.5 ml of original FBS) were loaded to gels. EV samples were denatured at 95°C for 5 min in reducing Laemmli sample buffer, separated using Mini-PROTEAN® TGX™ 4–20% gradient SDS-PAGE gel (Bio-Rad, Hercules, CA, USA) with page ruler prestained protein ladder (Thermo Scientific, Rockford, IL) as a standard and blotted on Immobilon-P PVDF membrane (Millipore, Bedford, MA). Blocking and antibody incubations were performed in Odyssey blocking buffer (LI-COR) without or with 0.1% Tween-20, respectively. Membranes were subsequently probed with IRDye® 800CW Goat anti-Mouse (Li-COR) at 1:15,000 2 hours at RT. After incubation, membranes were washed three times in TBS-T for 10 minutes at RT and imaged on an Odyssey FC Imager (Li-COR). For silver staining, EVs isolated by UC from UF-dFBS retentate (as above) and equal volumes (0.3 mL) of FBS and the different dFBS were mixed with loading buffer and heated at 95°C for 5 min. The gel was stained using the Pierce® Silver Stain Kit (Thermo Scientific) following the manufacturer’s instructions and imaged by Gel Doc XR imager (Bio-Rad).
Total RNA extraction and quality control
EVs from equal volumes (5 ml) of regular FBS and different dFBS, except for the UF-dFBS retentate (0.5 ml derived from 15 ml of regular FBS) owing to its consistency, were extracted using the miRCURY exosome isolation kit, followed by total RNA extraction using the miRCURY RNA isolation kit, cell and plant (Exiqon, Denmark) according to the manufacturer’s instructions. RNA was also extracted directly from 0.2 ml of the different dFBS and regular FBS or 0.1 ml of the UF-dFBS retentate (derived from approximately 3 ml of regular FBS) using the miRNEasy Serum/Plasma kit (Qiagen). Three replicate samples of each type were analysed with the Bioanalyzer 2100 using the Small RNA Kit (all Agilent Technologies, Santa Clara, CA, USA).
Isolation and culture of cells
The study was carried out under the approval of the ethical committee of Helsinki and Uusimaa Hospital District and with informed consent from the donors. Ethical approval has been granted for the use of adipose tissue (DNro 217/13/03/02/2015). AT-MSCs were obtained from water-assisted lipotransfer liposuction aspirates [Citation16] from four donors using mechanical and enzymatic isolation as described previously [Citation17]. All donors were female, with an age range of 33–47 years and a BMI range of 22.7–25.4. Cells were cultured in media consisting of Dulbecco’s modified Eagle’s medium/Ham’s Nutrient Mixture F-12 with 1% l-alanyl-l-glutamine (DMEM/F-12 1:1 GlutaMAX; Gibco ref. 31331–028, lot. 1765,999), 1% antibiotics (100 U/ml penicillin, 0.1 mg/ml streptomycin; Lonza ref. DE 17–602 E, lot. 5MB 068) and 10% FBS, qualified, EU-approved, South America origin, Gibco) at 37°C and 5% CO2 [Citation18]. Once AT-MSCs had adhered to the culture flask, non-adherent populations were washed away with PBS, and fresh culture media was added. Isolation and culturing of prostate and renal cancer patient-tissue-derived cells and mouse 3T3 cells was carried out as described [Citation19]. The patient-derived cultures were established in regular FBS, after which the cells were washed three times with PBS before culturing in the media containing 10% of different dFBS or in serum-free media for up to 96 h. Details of the used culture media, i.e. the test media, are described in . Osteosarcoma cell line HOS143b (ATCC® CRL-8303™) and prostate cancer cell line PC-3 (ATCC® CRL-1435™) were purchased from ATCC. Oral cancer cell line HSC3 was kindly provided by Prof. Tuula Salo (University of Helsinki).
Table 2. Culture media formulation overview.
Flow cytometry of immunophenotype of adipose-tissue mesenchymal stem cells
AT-MSCs cultured in FBS media (n = 3) were characterized using BD Accuri C6 flow cytometer (Becton Dickinson, Franklin Lakes, NJ) to confirm the mesenchymal origin of the cells. Allophycocyanin (APC)-conjugated monoclonal antibodies against CD14 (clone: M5E2), CD19 (clone: HIB19), CD34 (clone: 581), CD45RO (clone: UCHL1), CD54 (clone: HA58), CD73 (clone: AD2), CD90 (clone: 5E10), CD105 (clone: 266) and HLA-DR (clone: G46-6) (BD Pharmingen, Becton Dickinson) were used. Further, to assess whether AT-MSCs retained their immunophenotype after being cultured in media with UC-dFBS or UF-dFBS compared with FBS for 48 h, AT-MSCs (n = 3) were analysed for surface markers CD34 (clone: 581), CD45RO (clone: UCHL1), CD54 (clone: HA58), CD73 (clone: AD2) and CD105 (clone: 266). A total of 100 000 cells per sample were analysed, and positive expression was defined as the level of fluorescence greater than 99% of the corresponding unstained cell sample [Citation20].
Proliferation assays
AT-MSCs
Cells (n = 4) were plated at 7500 cells/cm2 in triplicates per condition in 24-multiwell plates to analyse proliferation in the test media (). Morphology of the cells was observed in T25 flasks (7500 cells/cm2) with 4× and 10× magnification using a Nikon Eclipse TS100 inverted phase-contrast microscope (Nikon Corporation, Tokyo, Japan) equipped with a Nikon DS-Fi2 camera. Once cell cultures reached 70% confluency, they were washed carefully with PBS and test media () were added. Cells were examined at 48 h and 96 h time points to analyse morphology, metabolic activity (CCK8) and total DNA (CyQuant).
Cancer cells
Cancer cell lines PC3, HSC3 and HOS143b were plated at 2500 cells/cm2 in triplicates for maintenance in Dulbecco’s modified Eagle’s medium (DMEM/F-12 1:1 GlutaMAX) supplemented with 10% FBS until they reached 70% confluency. Then, the cells were washed with PBS and cultured in the test media (). Cells were examined at 48 h and 96 h for metabolic activity (CCK8) and total DNA (CyQuant).
CCK8
The metabolic activity of live cells was analysed using the Cell Counting Kit-8 (CCK8) (Dojindo, US). The principle of the assay is based on the dehydrogenase activity in viable cells cleaving the tetrazolium salt into water-soluble coloured formazan dye. The dehydrogenase activity is directly proportional to the number of living cells. At 48 h and 96 h after adding the test media () to the AT-MSCs, the assay was performed according to the manufacturer’s instructions, and measured at wavelength 450 nm using a microplate reader (PerkinElmer VICTOR™ X4 Multilabel Microplate Reader 2030, Turku, Finland).
CyQuant
The total cellular DNA was determined using the CyQUANT™ Cell Proliferation Assay Kit (CyQUANT; Molecular Probes, Invitrogen, Paisley, UK) on the same cells analysed by CCK8. Briefly, at 48 and 96 h time points, the cells were lysed with 0.1% Triton-X 100 buffer (Sigma-Aldrich, St. Louis, MO, USA) and analysed after a freeze–thaw cycle. Fluorescence was measured with a microplate reader (PerkinElmer VICTOR™ X4 Multilabel Microplate Reader 2030, Turku, Finland) at 480/520 nm.
ROS-Glo H2O2 assay
Cells from three different AT-MSC donors were plated at 2500 cells/cm2 in triplicates per condition in 96-multiwell plate to analyse concentration-dependent reactive oxygen species (ROS) accumulation from cells in FBS, UF-dFBS, UC-dFBS and serum-free media () after 24 and 48 h exposure. Cells were first plated in the FBS medium. After 24 h, cells were washed carefully with PBS, and then the test media were added. After 24 h and 48 h, cells were washed carefully with PBS, and the ROS-GLO H2O2 Assay (Promega Cat. no. G8820/1, lot. no. 0000264704, Fitchburg, WI, USA) was performed according to the manufacturer’s instructions. For the luminescence signal detection, a microplate reader was used (PerkinElmer VICTOR™ X4 Multilabel Microplate Reader 2030, Turku, Finland).
Osteogenic differentiation
Cells from three different donors were plated at 2500 cells/cm2 in duplicates per condition in a 24-multiwell plate to analyse whether the AT-MSCs could retain their osteogenic potential after 48 h exposure to FBS and dFBS media. Cells were first plated in the FBS medium. After 24 h, cells were washed carefully with PBS, and then the test media were added. After 48 h, cells were washed carefully with PBS, and osteogenic differentiation was induced using osteogenic medium (FBS medium supplemented with 50 µM l-ascorbic acid 2-phosphate, 10 mM β-glycerophosphate disodium salt hydrate and 5 nM dexamethasone (all Sigma-Aldrich)). After 7 days of osteogenic differentiation, cells were lysed, and RNA extraction was performed using the Nucleospin RNA kit (Macherey-Nagel, Ref. no. 740955.50, Düren, Germany) according to the manufacturer’s instructions. The concentration and purity of RNA was measured using NanoDrop-1000 (Thermo Fisher Scientific, Waltham, MA, USA).
Quantitative reverse transcription PCR (qRT-PCR)
Gene expression was analysed from AT-MSCs from three donors. Total RNA was converted into cDNA by reverse transcription using a SuperScript™ IV VILO™ reaction mixture (Thermo Fisher Scientific). Gene expression was quantified using TaqManR ©assays (Thermo Fisher Scientific). RUNX2 (Hs01047973_m1) expression was measured as an early indicator of osteogenesis, and three housekeeping genes, TBP (Hs00427620_m1), RPLP0 (Hs99999902_m1) and YWHAZ (Hs03044281_g1), were used to normalize the data [Citation21]. The PCR reactions were conducted in triplicates using the Applied BiosystemsR© 7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Data were analysed using the 2ΔΔCt method to quantify the relative gene expression [Citation22].
Cell adhesion assays
Fibronectin supplementation
AT-MSCs were plated at 7500 cells/cm2 in triplicates on a normal 24-well plate in the FBS medium. After 24 h, cells were washed carefully with PBS, and then the test media were added (). UF-dFBS media were supplemented with 20 µg/ml Native Fibronectin Human Protein (Gibco/Thermofisher Scientific, cat no. PHE0023). After 48 h and 96 h, cells were washed carefully with PBS, and CCK8 and CyQuant analyses were performed.
Carboxyl-coated plates
Carboxyl plates are enhanced attachment surfaces produced through vacuum-gas plasma carboxyl group polymerization treatment. They are chemically defined and free of any animal-derived substances supporting attachment and proliferation of cells. AT-MSCs were plated at 2500 cells/cm2 in triplicates on carboxyl coated 24-multiwell plates (Corning™ PureCoat™ Carboxyl Cat. no. 356773, Lot. no. 6,237001, New York, NY, USA) and on regular tissue culture treated 24-multiwell plates to analyse cell proliferation. Cells were plated in the FBS medium to allow uniform and equal attachment of cells. After 24 h, cells were washed carefully with PBS, and then the media supplemented with UF- or UC-dFBS () were applied. Cells cultured in both plate types were analysed for metabolic activity (CCK8) at 48 h.
Assessment of EV production by AT-MSCs and cancer cell lines
NTA analysis was performed on EVs isolated from AT-MSCs (n = 2) and PC-3 cells (n = 1) cultured in the test media (). Briefly, cells were plated at 7500 cells/cm2 in triplicates on a normal 24-well plate in FBS medium. After 24 h, cells were washed carefully with PBS, and then the test media were added. After 48 h, the media were collected and centrifuged at 2500 g for 20 min at +4°C, followed by EV extraction by UC (121 896 gmax, for 1.5 h). EV pellets were resuspended in 200 µl of PBS and analysed with NTA as described. Particle numbers of similar UC preparations from all of the fresh test media were subtracted from particle numbers derived from the harvested media at the 48 h time point to assess the number of particles produced by the cells.
Statistical analysis
As biological replicates, three or four donor cell samples of AT-MSCs and three different cancer cell lines were analysed using three technical replicates of each in all assays. The graphs (, , , and Supplemental Figure 2) show the biological and/or technical replicates with means. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc., CA) statistical software. For CCK8/CyQuant assays, ROS-Glo assays and flow cytometry, statistical analyses were performed using ANOVA two-way analysis of variance. Bonferroni post-hoc tests were used to determine individual significant differences. The results were considered significant when the Bonferroni corrected p-value was below 0.05.
Results
Analysis of the different EV-depleted FBS
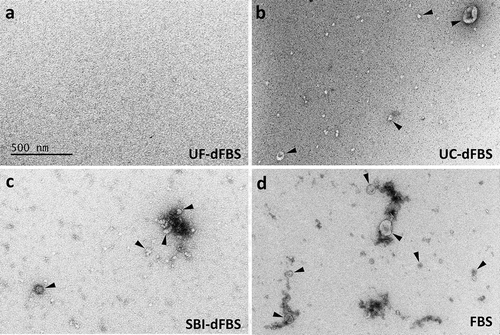
absence of EVs in our UF-dFBS and other dFBS preparations/absence of EVs in our UF-dFBS and other dFBS preparations, we isolated EVs remaining in the dFBS by ultracentrifugation or using the miRCURY exosome isolation kit and characterized them using NTA, EM, Western blotting and RNA analyses. The NTA results showed that the UF-dFBS had similar low amounts of particles to that of SBI-dFBS, whereas only partial depletion of EVs was observed in UC-dFBS (). Characterization of the EV samples by EM mostly supported the NTA results (). While the UF-dFBS samples were essentially EV-free, UC-dFBS and SBI-dFBS still contained some EVs or other EV-like particles that were mainly small (<200 nm). As expected, regular FBS contained both large and small EVs. The results were similar when EV isolation was carried out using either UC () or the commercial kit (Supplementary Figure 1).
Figure 1. EV concentration and size distribution by nanoparticle tracking analysis (NTA). Concentrations (particles/ml of original FBS) are shown in the y-axis and the different EV-depleted FBS and regular FBS samples on the x-axis. The UF-dFBS and commercial EV-depleted FBS (SBI-dFBS) contained fewer particles than the UC-dFBS or regular FBS. The dots depict measurements from technical replicates, and bars show means. FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), SBI (System Biosciences), dFBS (EV-depleted FBS).
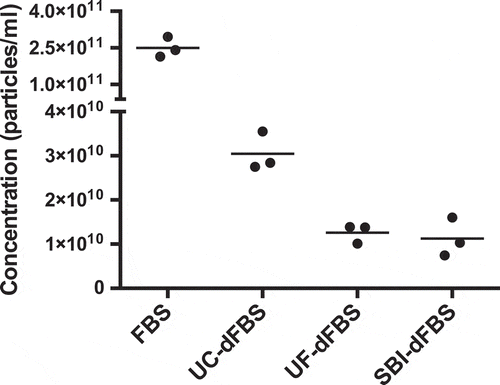
Figure 2. Electron microscopy of EV-depleted and regular FBS. Electron microscopy revealed that EVs were absent only in the UF-dFBS. EVs were isolated by UC from the different EV-depleted FBS and regular FBS. EV preparations were derived from (a) UF-dFBS lacked EVs, whereas (b) UC-dFBS and (c) commercial dFBS (SBI-dFBS) preparations showed mainly small EVs or EV-like particles. (d) Regular FBS contained both small and large EVs. Arrowheads mark examples of the EVs detected in the samples. The scale bar (500 nm) applies to all images. FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), SBI (System Biosciences), dFBS (EV-depleted FBS).
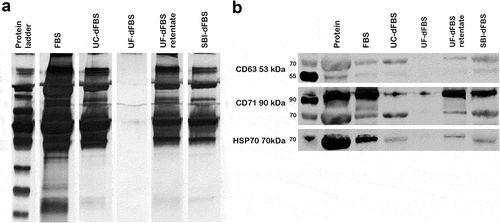
Next, we performed a total protein analysis by silver staining. When analysing EVs, UF-dFBS was the only dFBS that showed an almost complete removal of EV proteins (. Indeed, EV preparations of all other dFBS, FBS and the UF-dFBS retentate had a similar kind of pattern of protein bands. Furthermore, in Western blotting, the EV marker Hsp70 was absent, and only a faint band of CD71 protein was detected in the UF-dFBS sample ()). Both EV markers gave faint bands from the UC-dFBS sample, whereas the corresponding bands were clearer in the SBI-dFBS and UF-dFBS retentate samples. The strongest bands for Hsp70 and CD71 were detected in the regular FBS sample.
Figure 3. Silver staining and Western blotting of EV proteins. Analysis of total EV-protein and EV-marker proteins Hsp70 and CD71. EVs were isolated by UC from the different EV-depleted FBS and regular FBS. (a) Silver staining of EV proteins derived from regular FBS shows a distinct protein pattern that can also be detected in UC-dFBS, commercial dFBS (SBI-dFBS) and UF-dFBS retentate, but not in the UF-dFBS. (b) Western blotting detected Hsp70 and CD71 in EV preparations derived from regular FBS, UC-dFBS, SBI-dFBS and UF-dFBS retentate. In contrast, only a faint band of CD71 and no Hsp70 could be detected in EV preparations from UF-dFBS. FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), SBI (System Biosciences), dFBS (EV-depleted FBS).
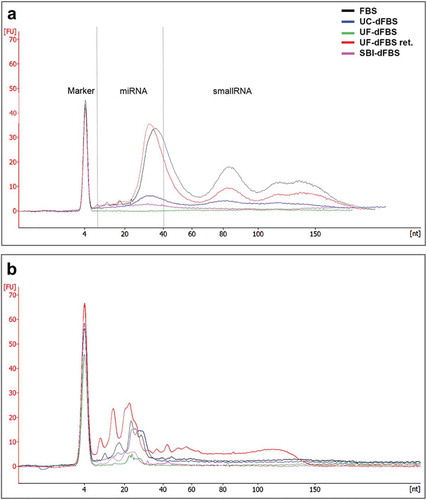
We further verified the EV depletion efficiency of the UF protocol by RNA extraction from EVs isolated from all dFBS preparations. RNA analysis with the Bioanalyzer Small RNA kit showed no EV-RNA peaks from UF-dFBS samples, whereas small amounts of EV-RNA were obtained from SBI-dFBS and UC-dFBS (). EVs from regular FBS contained the largest amount of small RNA. EVs from the UF-dFBS retentate served as a control and contained EV-RNA as well.
Since the FBS contains both intra- and extra-vesicular RNA, we also investigated the amount of total RNA present in the different dFBS and regular FBS (without prior EV isolation) ()). Bioanalyzer results indicated that all the FBSs, including UF-dFBS, contained some RNA. The amount of total RNA was lowest in the UF-dFBS followed by SBI-dFBS, UC-dFBS and FBS. UF-dFBS retentate, included as a control, also contained RNA. The results therefore suggest that the filtration protocol removed all the EV-RNA, but still retained some soluble RNA in the UF-dFBS. Together, these results indicate that UF was the only method to completely deplete EVs from the FBS.
Figure 4. Bioanalyzer profiles of small RNA (miRNA and small RNA, 6–150 nt) from different EV-depleted FBS. (a) RNA was extracted from the EVs isolated from the FBS, dFBS and UF-dFBS retentate. (b) RNA was extracted from the total FBS and dFBS (without prior EV isolation) or from UF-dFBS retentate. As compared with the other dFBS, the UF-dFBS showed no EV-RNA signal, whereas some RNA was detected from the total UF-dFBS, indicating its non-vesicular origin. All other samples contained EV- and total FBS-RNA. FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), SBI (System Biosciences), dFBS (EV-depleted FBS).
Effect of EV-depleted FBS on cell proliferation
In order to analyse the effects of the different dFBS-supplemented media on cell adhesion and proliferation, the media were tested on AT-MSCs and compared with regular FBS medium (). The AT-MSC proliferation tests ()) were performed on cell lines from four donors, with assays run twice on two cell lines in order to reduce donor variability.
According to the standards defined by the International Society for Cellular Therapy (ISCT), MSCs should be adherent to plastic under normal culturing conditions, have fibroblast-like morphology and show a specific immunophenotype measured by flow cytometry [Citation23,Citation24]. In concordance with these guidelines, AT-MSC donor cell lines grown in the FBS-supplemented medium expressed surface markers CD73, CD90 and CD105, and lacked the expression of CD14, CD19, CD45 and HLA-DR (). In addition, CD34 was only moderately expressed, as previously reported for AT-MSCs cultured in FBS media [Citation25]. CD54 expression also conformed to the previous reports of AT-MSCs in FBS media [Citation25]. Further, when we assessed the surface marker expression in AT-MSCs after their culture in the different dFBS media vs. the FBS medium, we detected no statistically significant differences. Yet, a trend towards a higher expression of CD34 and CD54 in UF-dFBS was seen (). We also observed the cell morphology via light microscopy after culturing in all the test media () for 48 h and 96 h (). In all conditions, we detected a characteristic mesenchymal stem cell morphology, i.e. a small spindle-shaped cell body with a few long and thin cell processes, during the 96 h follow-up period.
Table 3. Surface marker expression (%) of undifferentiated AT-MSCs analysed by flow cytometric analysis.
Proliferation was measured in AT-MSCs grown in all the test media (, ) and Supplemental Figure 2). Metabolic activity normalized to total DNA, i.e. cell proliferation, in FBS and UC-dFBS media was comparable at both time points of 48 h and 96 h with significant donor variability, particularly at 96 h. At the 48 h time point, proliferation of the cells grown in UF-dFBS was similar to proliferation of cells grown in FBS, UC-dFBS or SBI-dFBS. However, at 96 h, the cells grown in the UF-dFBS and SBI-dFBS proliferated more slowly than the cells grown in media supplemented with other sera, although these differences were not statistically significant ()). Cell proliferation was the lowest in the serum-free medium at each time point. In summary, all media except for serum-free media were able to maintain AT-MSC proliferation until 96 h.
Figure 5. Morphological characterization of adipose-tissue derived mesenchymal stem cells by light microscopy after culturing in the test media for 48 h and 96 h. No differences could be detected in the size or shape between the different culturing conditions when monitored for up to 96 h. The spindle-shaped body of cells could be observed in all images and time points. Scale bars are 100 µm. FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), SBI (System Biosciences), dFBS (EV-depleted FBS).
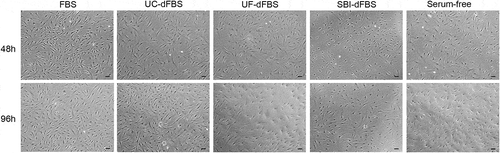
Figure 6. Cell proliferation and metabolic activity of AT-MSCs and cancer cell lines in test media. Metabolic activity per cell, i.e. cell proliferation, was calculated by the ratio of CCK8/CyQuant. (a) Adipose-tissue mesenchymal stem cells (AT-MSCs) showed a similar proliferation rate in FBS and UC-dFBS media between 48 h and 96 h, which was somewhat higher than in UF-dFBS and SBI-dFBS media. No marked proliferation was detected in the serum-free conditions. Each dot represents a biological replicate, and bars show means. (b) Proliferation rate of three different cancer cell lines (HSC3, PC-3, HOS143b) in FBS and dFBS media were assessed at 48 h and 96 h. UF-dFBS had a stimulatory effect on the proliferation of all three cancer cell lines. Each dot represents a technical replicate, and bars represent means. FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), SBI (System Biosciences), dFBS (EV-depleted FBS).
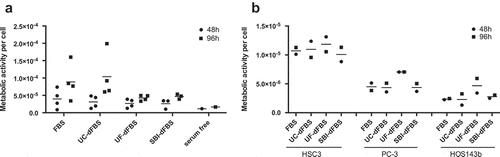
We also assessed cell proliferation of three different cancer cell lines (HSC3, PC-3, HOS143b) in the test media at 48 h and 96 h ()). Here, we observed a stimulating effect on cell proliferation in all three cancer cell lines when they were cultured in the UF-dFBS media in comparison with the other test media. Metabolic activity and total DNA count for each cell type are shown in the Supplement Figure 2. In addition, we tested UF-dFBS for culturing of mouse 3T3 cells and conditionally reprogrammed cells [Citation26] derived from prostate (as described in [Citation19]) and renal cancer [Saeed et al., unpublished results] patient tissue samples. All cell types cultured for up to 72h (n = 12) exhibited no apparent arrest in the proliferation (data not shown). In all, the cell proliferation assays showed that UF-dFBS medium supported cell proliferation, indicating that it can be used for a range of cell types including AT-MSCs.
Evaluation of ROS levels and stem cell differentiation of AT-MSCs grown in dFBS
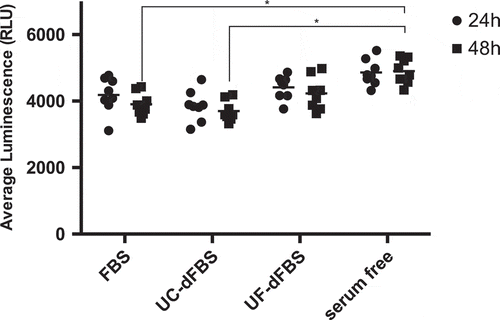
It is known that the stress levels of cells correspond to the levels of ROS released. Therefore, we analysed the ROS levels after 24 h and 48 h of cell culture in UF-dFBS, UC-dFBS, FBS or serum-free media. The AT-MSCs grown in the serum-free media released significantly more ROS than the cells grown in all the other media already at 24 h (p < 0.05, Figure 7). There was no statistically significant difference in the ROS levels after 24 h among the UF-dFBS, UC-dFBS or regular FBS conditions. After 48 h, there was a significant difference in the ROS accumulation between the serum-free and regular FBS media, as well as between the serum-free and UC-dFBS media (both p < 0.05, ).
Figure 7. Evaluation of H2O2 formation in AT-MSCs grown in FBS, UC-dFBS, UF-dFBS and serum-free culture conditions after 24 h and 48 h. After 48 h, a significant difference was seen between the serum-free and regular FBS media (*p < 0.05), as well as between the serum-free and UC-dFBS media (*p < 0.05). Dots represent biological and technical replicates, and bars represent means. AT-MSCs (adipose-tissue mesenchymal stem cells), FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), dFBS (EV-depleted FBS).
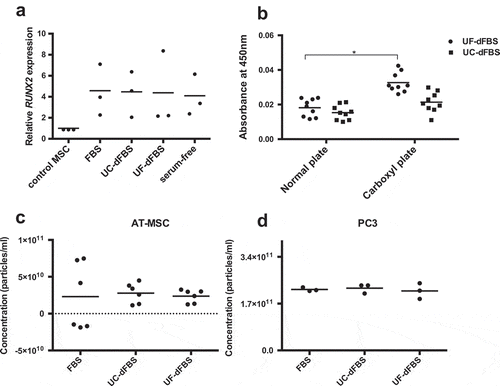
Next, after being cultured for 48 h in the dFBS media and serum-free conditions, AT-MSCs were induced for 7 days in the osteogenic differentiation media (containing regular FBS) to evaluate whether the AT-MSCs retained their capacity to differentiate into osteoclasts. RT-PCR analysis of early osteogenic marker RUNX2 showed that the osteogenic differentiation capacity of AT-MSCs was not affected by the UF-dFBS, UC-dFBS or serum-free media (Figure 8(a)). In summary, none of the dFBS media induced elevated ROS levels or altered the differentiation capacity of the AT-MSCs.
Improvement of cell proliferation in the dFBS media with carboxyl plates
To test if the cell proliferation rate of AT-MSCs grown in the UF-dFBS media could be increased, we compared different means of improving cell adhesion: supplementation of an extracellular matrix protein, fibronectin and carboxyl plates. First, we tested fibronectin supplementation into medium in combination with UF-dFBS. Proliferation in this medium was compared with the proliferation in the other dFBS and regular FBS media. However, we repeated this study with only one donor cell line, as we detected no improvement in cell proliferation (data not shown). Next, we cultured AT-MSCs for 48 h in UF-dFBS or UC-dFBS media on carboxyl plates compared with normal cell-culture plates. Cells proliferated significantly faster on the carboxyl plates than on regular plates ()). We observed a similar trend for cells grown in both UF- and UC-dFBS media, but the increase in proliferation rate was the highest in cells grown in the UF-dFBS medium.
Figure 8. Differentiation capacity and improvement of metabolic activity of AT-MSCs cultured in EV-depleted FBS media. (a) Real-time quantitative PCR analysis of RUNX2 expression of AT-MSCs cultured in dFBS media followed by osteogenic differentiation showed that the cells retained their osteogenic differentiation capacity. Dots represent biological replicates, and bars represent means. (b) Compared with normal cell-culture plates, carboxyl plates enhanced the metabolic activity, CCK8 of cells cultured in UF-dFBS (*p < 0.05) and in UC-dFBS up to 48 h. Dots represent biological and technical replicates and bars represent mean. (c) Nanoparticle tracking analysis showed an equal production of EVs by AT-MSCs and (d) PC-3 cells cultured in the FBS and dFBS media. Dots represent biological and technical replicates, and bars represent the means. AT-MSCs (adipose-tissue mesenchymal stem cells), FBS (fetal bovine serum), UC (ultracentrifugation), UF (ultrafiltration), dFBS (EV-depleted FBS).
NTA analysis of EV production in dFBS
Finally, we studied whether EV production of AT-MSCs and PC-3 cells was affected by the UF-dFBS and UC-dFBS media in comparison with the regular FBS medium. AT-MSCs ()) and PC-3 cells ()) cultured in all the media produced equal numbers of particles as measured by NTA. Thus, the EV production by the cultured cells was not affected by either dFBS.
Discussion
As FBS is the most commonly used supplement in cell cultures, the removal of EVs from FBS is of high importance for in vitro EV studies. FBS EVs have been reported to be internalized by the recipient cells and to affect them physiologically, for example by inducing cell migration [Citation2,Citation3]. Therefore, studies focusing on the functions of EVs released by the cultured cells, or of supplemented EVs from another cell type, become compromised if FBS-derived EVs are also present. Furthermore, contamination of the media with FBS EVs [Citation12,Citation13] may confound potential markers in studies aiming to identify EV-derived biomarkers from cultured cells.
Recently, an RNA sequencing analysis by Wei and co-workers revealed that FBS contains protein-coding and regulatory RNAs including miRNA, rRNA, snoRNA and Y-RNA, which are indistinguishable from human and mouse transcripts, suggesting that FBS-derived RNA could potentially influence extracellular RNA analysis from both human and mice [Citation13]. FBS-derived RNA has also been detected in publicly available extracellular RNA sequencing datasets from several cell cultures, indicating that bovine RNA is a common contaminant among extracellular RNA [Citation27]. Notably, mir-122, mir-451a and mir1246 previously reported to be abundant in cell-culture-derived EVs have now been shown to actually originate from the FBS EVs [Citation13]. Overall, previous studies clearly suggest that the removal of FBS EV-derived RNA is of high priority, as it competes with or confounds the EV-RNA in focus [Citation13,Citation27].
Despite the obvious need, there are currently no standardized protocols for eliminating EVs from FBS. UC is commonly used for obtaining EV-depleted FBS. However, UC even up to 24 h only partially removes the EVs or EV-RNA [Citation3,Citation13,Citation27], and the protocols are very heterogeneous [Citation28]. Removal of FBS EVs is sensitive to multiple equipment, protocol or handler-dependent parameters (steps before pelleting, viscosity/dilution/volume of FBS, centrifugation time and speed, used supernatant fraction as well as the tube and rotor type determining for example angle of sedimentation, maximum radius, usable g-force and pelleting efficiency), and unfortunately, the details about the parameters are often not provided in publications. Inevitably, this produces variability in the results from different laboratories. Commercial alternatives of EV-depeleted sera are produced using other methods and may still suffer from incomplete removal of FBS EVs. Thus, lack of details and harmonization of the dFBS production renders comparison of the EV research results from different laboratories very difficult.
Our UF-based method for EV depletion from FBS appears to be an effective alternative for the existing methods. First, it can be standardized and performed in any laboratory using common, non-expensive equipment. Second, according to our NTA, EM, protein and RNA results, the UF-dFBS did not contain any detectable EVs. As no EV-RNA signal was detected from the UF-dFBS, and it still supported EV production from the cultured cells, its use offers the possibility of obtaining only pure cellular EVs and their RNA for downstream analyses. Third, compared with cell cultures grown in serum-free media, cells grown in the media supplemented with UF-dFBS had a higher viability and proliferation rate and lower stress levels. Thus, the UF-based method yields truly EV-free serum, which is crucial for cell-culture-based EV research.
The measurement of total EV-protein from all the EV-depleted FBS showed that our UF-dFBS contained very small amounts of EV proteins. The UF filter used in this study retains proteins larger than 100 kDa, allowing smaller proteins to pass into the filtrate. This is likely to enable cell growth and potentially extracellular matrix production (ECM) by the cells [Citation29,Citation30]. However, it has been reported that the lack of large molecules, such as glycoproteins, may have adverse effects on cell differentiation and proliferation [Citation31]. In addition, the stimulating effect of FBS-derived EVs on cell proliferation and differentiation has been shown to affect the biological outcome of the cells [Citation3]. Although not observed in our cancer cell cultures, the lack of large proteins and EVs could potentially be the cause of the slower cell proliferation of AT-MSCs in the UF-dFBS-supplemented media compared with FBS supplemented media during longer cultures (up to 96 h) on normal cell culture plates. Similarly to the UF-dFBS, UC-dFBS has also been reported to induce decreased growth rate, migration rate and differentiation capacity in some cell types, such as lung epithelial cells (A549) [Citation3], cardiac progenitor cells [Citation32], human U87 glioblastoma cells, human HEK293t cells, human SH-SY5Y neuroblastoma cells and mouse N2a neuroblastoma cells [Citation2]. However, with UF-dFBS media, the proliferation rate still increased between 48 and 96 h, which is a sign of adjustment to the low-nutrient conditions, i.e. the cells in UF-dFBS were viable and proliferating. Importantly, although the cell proliferation was lower in UF-dFBS media than in UC-dFBS and regular FBS, a large heterogeneity in the proliferation rates in UC-dFBS and FBS media was detected compared with in UF-dFBS media. Since there was less variation between the test repeats in UF-dFBS supplemented medium than other media, UF-dFBS appears to offer enhanced reproducibility.
The flow cytometry data pointed towards a tendency for a higher expression of CD34 [Citation33] and CD54 expression in AT-MSCs cultured in UF-dFBS on normal cell-culture plates. Both of these markers relate to cell adhesion, suggesting that UF-dFBS medium may support less cell adhesion than FBS and UC-dFBS media. This is potentially due to the lack of large molecules such as glycoproteins in the UF-dFBS media causing adaptation of the cells via upregulation of adhesion molecule expression. First, as an attempt to rescue cell attachment, and second, to enhance proliferation, we employed fibronectin and enhanced attachment plates (carboxyl plates). Although no effect was obtained by fibronectin-supplemented medium, carboxyl plates successfully improved the proliferation rates. Thus, by optimization of cell attachment, the UF-FBS may also support higher proliferation rates. Further, since EV production or differentiation capacity of cells was not compromised by the UF-dFBS media even on normal cell-culture plates, our results support wide applicability of the UF protocol for EV research.
The clinical use of MSC EVs requires a scale-up of the EV production, good manufacturing practice (GMP) protocols and numerous other regulatory considerations [Citation34]. One of the safety considerations is the use of animal free/bovine EV-free media. In that regard, our UF depletion protocol producing EV-free serum, which still supports EV production, could be applied to any sera including human serum, thus providing a good option for the future GMP production of EVs for clinical applications in regenerative medicine and therapy [Citation10].
In conclusion, we have shown for the first time that completely EV-free dFBS can be easily prepared in any research laboratory. Our UF-dFBS method is cost-effective and simple to standardize, and supports cell proliferation for up to 96 h, which covers the duration of most published EV experiments. Utilization of the UF-dFBS protocol by the EV research community will help the researchers to obtain pure cellular EVs with high confidence, thus improving the quality of their research. Thus, UF-dFBS offers an attractive alternative for serum-free conditions or UC-dFBS for future EV research including clinical applications.
Supplemental_data.zip
Download Zip (11.1 MB)Acknowledgements
The authors thank Biomedicum Functional Genomics Unit (FUGU), Biomedicum Flow Cytometry Core Facility, EV Core Facility and Electron Microscopy Unit of the Institute of Biotechnology (all University of Helsinki) as well as Heidi Husu for technical assistance and urological teams from Helsinki University Central Hospital, Helsinki Urological Biobank project and Olli Kallioniemi’s research group in the Institute for Molecular Medicine Finland FIMM includingKhalid Saeed, Mari-Liina Arjama and Piia Mikkonen for conditionally reprogrammed cell cultures. This research was supported by University of Helsinki project funding (WBS490302, WBS73714112), Helsinki University Hospital State funding for university-level health research (Y1014SUL05, TYH2016130), Paulo Foundation, the Finnish Dental Society Apollonia, TEKES the Finnish Funding Agency for Innovation; TekesHealth grant CraMaxS 5773/31/16 and new generation biobanking grant 40294/11 and SalWe Research Programme Personalized Diagnostics and Care (GET IT DONE, TEKES) grant 3986/31/2013, Academy of Finland programme grant no. 287089, Evald and Hilda Nissi Foundation and Selma ja Maja-Lisa Selander foundation. Chancellor’s travel grant was provided for doctoral candidates in the Doctoral Programme in Oral Sciences (FINDOS Helsinki).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Brunner D, Frank J, Appl H, et al. Serum-free cell culture: the serum-free media interactive online database. Altex. 2010;27(1):1–14.
- Eitan E, Zhang S, Witwer KW, et al. Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth. J Extracell Vesicles. 2015;4:26373.
- Shelke GV, Lasser C, Gho YS, et al. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014;3. DOI:10.3402/jev.v3.24783.eCollection2014.
- Camussi G, Deregibus MC, Bruno S, et al. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848.
- Ratajczak J, Wysoczynski M, Hayek F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–1495.
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593.
- Théry C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006; 30:3.22:3.22.1–3.22.29.
- Doorn J, Moll G, Le Blanc K, et al. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev. 2012;18(2):101–115.
- Bruno S, Collino F, Tetta C, et al. Dissecting paracrine effectors for mesenchymal stem cells. Adv Biochem Eng Biotechnol. 2013;129:137–152.
- Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973.
- Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92(4):387–397.
- Lasser C, Alikhani VS, Ekstrom K, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9-5876–9-5879.
- Wei Z, Batagov AO, Carter DR, et al. Fetal bovine serum RNA interferes with the cell culture derived extracellular RNA. Sci Rep. 2016;6:31175.
- Kerkelä E, Laitinen A, Rabina J, et al. Adenosinergic immunosuppression by human mesenchymal stromal cells requires co-operation with T cells. Stem Cells. 2016;34(3):781–790.
- Puhka M, Nordberg ME, Valkonen S, et al. KeepEX, a simple dilution protocol for improving extracellular vesicle yields from urine. Eur J Pharm Sci. 2017;98:30–39.
- Peltoniemi HH, Salmi A, Miettinen S, et al. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: A prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66(11):1494–1503.
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260.
- Lindroos B, Aho KL, Kuokkanen H, et al. Differential gene expression in adipose stem cells cultured in allogeneic human serum versus fetal bovine serum. Tissue Eng Part A. 2010;16(7):2281–2294.
- Saeed K, Rahkama V, Eldfors S, et al. Comprehensive drug testing of patient-derived conditionally reprogrammed cells from castration-resistant prostate cancer. Eur Urol. 2017;71(3):319–327.
- Lindroos B, Boucher S, Chase L, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11(7):958–972.
- Ragni E, Viganò M, Rebulla P, et al. What is beyond a qRT-PCR study on mesenchymal stem cell differentiation properties: how to choose the most reliable housekeeping genes. J Cell Mol Med. 2013;17(1):168–180.
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
- Hematti P. Mesenchymal stromal cells and fibroblasts: A case of mistaken identity? Cytotherapy. 2012;14(5):516–521.
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317.
- Patrikoski M, Juntunen M, Boucher S, et al. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013;4(2):27.
- Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180(2):599–607.
- Tosar JP, Cayota A, Eitan E, et al. Ribonucleic artefacts: are some extracellular RNA discoveries driven by cell culture medium components? J Extracell Vesicles. 2017;6(1):1272832.
- Taylor D, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87(1):3–10.
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423(6942):876–881.
- Kleinman HK, Luckenbill-Edds L, Cannon FW, et al. Use of extracellular matrix components for cell culture. Anal Biochem. 1987;166(1):1–13.
- Hynes RO, Yamada KM. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982;95(2 Pt 1):369–377.
- Angelini F, Ionta V, Rossi F, et al. Foetal bovine serum-derived exosomes affect yield and phenotype of human cardiac progenitor cell culture. Bioimpacts. 2016;6(1):15–24.
- Sidney L, Branch M, Dunphy S, et al. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32(6):1380–1389.
- Riazifar M, Pone EJ, Lotvall J, et al. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–154.

