ABSTRACT
Background: Economic models are broadly used in the economic evaluation of antipsychotics in schizophrenia. Our objective was to summarize the structure of these models.
Methods: Model-based economic evaluations of antipsychotics in schizophrenia were identified through Medline and Embase. General information was extracted including analysis type, model type, perspective, population, comparator, outcome, and timeframe. Model-specific structures for decision tree (DT), cohort- and patient-level Markov model (CLMM, PLMM), and discrete-event simulation (DES) models were extracted.
Results: A screen of 1870 records identified 79 studies. These were mostly cost-utility analyses (n = 48) with CLMM (n = 32) or DT models (n = 29). They mostly applied payer perspective (n = 68), focused on general schizophrenia for relapse prevention (n = 73), compared pharmacotherapies as first-line (n = 71), and evaluated incremental cost per quality-adjusted life year (QALY) gained (n = 40) with a 1-year (n = 32) or 5-year (n = 26) projection. DT models progressed with the branching points of response, relapse, discontinuation, and adherence. CLMM models transitioned between disease states, whereas PLMM models transitioned between adverse event states with/without disease state. DES models moved forward with times to remission, relapse, psychiatrist visit, and death.
Conclusions: A pattern of pharmacoeconomic models for schizophrenia was identified. More subtle structures and patient-level models are suggested for a future modelling exercise.
Introduction
Schizophrenia imposes a great burden on both economics and quality of life. Its estimated societal cost ranges from 37% to 214% of GDP per capita [Citation1], and it is ranked the sixteenth highest cause of years lived with disability out of the 25 most common diseases worldwide [Citation2]. Many treatments are available for schizophrenia, with very different benefit/risk profiles. A network meta-analysis found that out of 15 antipsychotics, clozapine ranked first in symptom control, but lower than tenth in safety [Citation3].
Economic evaluation produces data incorporating the benefits and risks of treatments. This approach to the evaluation of atypical treatments for schizophrenia has been used since 1990 [Citation4]. In the current literature, model-based economic evaluations outweigh clinical trial-based analyses [Citation5,Citation6]. However, model-based studies may generate inconsistent conclusions on cost-effectiveness for the same comparison, even when the same modelling technique is used [Citation7].
A comprehensive review of models used in the evaluation of treatment for schizophrenia can provide useful information for future model-based economic evaluations. Recent systematic reviews have not presented current models of pharmacotherapy for schizophrenia, as they have narrowed their search to long-acting/extended-release antipsychotics [Citation5], newer second-generation agents [Citation6], and the relationship between modelling technique and reported outcomes [Citation7]. Although Nemeth et al [Citation8]. did address models of pharmacotherapy, their study missed lots of model-based studies discussed by Scheele et al [Citation7]., such as the NICE models from 2009 [Citation9] (updated in 2014 [Citation10]) and 2011 [Citation11].
In view of the limitations in existing studies, we aimed to identify economic models of pharmacotherapy for schizophrenia and to present the structures of these models.
Methods
This systematic review considered all model-based economic evaluations of pharmacotherapy for schizophrenia published after 2000. The search was conducted using Medline and Embase via Ovid in April 2016. Search terms combining ‘schizophrenia’, ‘economic evaluation’, and ‘model’ were used as free term and medical subject heading (MeSH) term. The search strategy is presented in Supplement 1 and Supplement 2. Studies were excluded if they discussed aspects not related to treatment effect, the full text was unavailable, the study was later updated, or the study was not in English. Systematic reviews identified through the screening process were reviewed for manuscript inclusion. References in the studies identified were searched manually to find studies which might have been missed in the initial search.
To present the model structure, both general and model-specific information were extracted. General information included type of analysis, type of model, country/region, perspective, patient population, comparator, economic outcome, and timeframe. Model-specific information included branching points for decision tree (DT) models, health states and cycle length for cohort-level (CL) and patient-level (PL) Markov models (MM), and time approach methods for discrete event simulation (DES) models. For patient-level models, PLMM, and DES models, patient characteristics to be simulated were also extracted.
Within general information, type of analysis included cost-benefit analysis, cost-effectiveness analysis (CEA), cost-minimization analysis, and cost-utility analysis (CUA) [Citation12]. CEA in this review referred to CEA using health outcomes other than quality of life, to differentiate these analyses from CUA, which was considered a special form of CEA using quality of life as health outcome. Cost analysis was also considered as a type of economic analysis, since while early economic evaluations primarily compared costs, they also presented health outcomes. Type of model included DT, CLMM, PLMM, DES model [Citation7,Citation13], and others. Perspective was classified into societal, payer, and patient perspective [Citation14]. Patient population was classified into early, general and treatment resistant schizophrenia. According to NICE guidelines, there are 4 major areas of treating schizophrenia with antipsychotic drugs: initial treatment for early schizophrenia, treatment for acute schizophrenia, relapse prevention for stable schizophrenia, and treatment for resistant schizophrenia [Citation10]. In this study, acute and stable schizophrenia were classified as general schizophrenia, since the timeframe of over 1 year covers both the acute and maintenance phase. Outcomes were classified into incremental cost per positive outcome gained, incremental cost per negative outcome avoided, and others. For positive and negative outcomes, 3 categories were considered: time spent in status, number of patients with an outcome, and number of outcomes per patient.
To present the general information, the studies that applied each category of each area considered under general information were summarized. Further information was presented, including the definition of perspective and patient population, and comparator and outcome options. Comparator options were the pharmacotherapy to be compared and the number of lines for switch.
To present the model-specific information, the statistics and options of the structures in the DT and CLMM models were summarized. For patient-level models, PLMM, and DES models, only the structural options in the original models were taken into account, since the number of original patient-level models was small, and the models adapting these original models would not involve a large change in the model structure.
Results
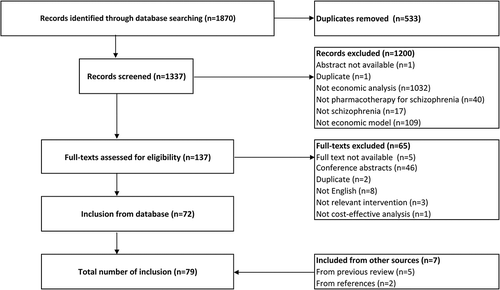
A screen of 1870 records identified 72 studies. By checking the references in these studies and the models included in previously published reviews, a further 7 studies were identified (). The characteristics of the 79 studies [Citation10,Citation11,Citation15–Citation91] are presented in Supplement 3.
General information
A summary of study characteristics is presented in . The studies included were conducted in 28 countries/regions (mostly in United States: n = 17), usually applying CUA (n = 48) with cohort-level models (29 DT and 32 CLMM models). They mostly compared pharmacotherapy as first-line treatment (n = 71) for general schizophrenia (n = 73) in terms of incremental cost per quality-adjusted life years (QALY) gained (n = 40) over a 1-year (n = 32) or 5-year (n = 26) timeframe from the payer perspective (n = 68).
Table 1. Summary of studies included in the analysis.
Perspective: definition
Different terms were used for payer perspectives, including terms related to payer (payer, third-party payer, health care payer, government payer), insurance (health insurance, general insurance), health service (ministry of health, health care system, health care service, health care provider) and others (health sector, direct cost).
Population: definition
In most studies, the term ‘general schizophrenia’ referred to non-treatment-resistant schizophrenia with a history of more than one relapse. Half the models specified the phase of disease at entry: acute phase (n = 15) and stable phase (n = 21). Twenty-seven studies further defined the patient population according to current setting (n = 14; outpatient, inpatient), treatment status (n = 13; accept long-acting injection/oral treatment, naive to atypical antipsychotics, partially responsive), adherent status (n = 10; high risk of non-adherent, adherent), other disease status (n = 3; moderate severity, residual state, young schizophrenia with duration < 5 year). Treatment-resistant schizophrenia was classified as moderate/moderate to severe, stabilized on clozapine, or progressive deterioration.
Comparator options
Olanzapine and risperidone were the most common pharmacotherapies compared (n = 51 and n = 50). Groups of typical and atypical antipsychotics were considered in 9 and 8 studies. Only half of the studies on typical/atypical antipsychotics provided detailed information about the drugs included (typical treatment – haloperidol, chlorpromazine; atypical treatment – olanzapine, risperidone) using the weighted mean of market share. Treatment switch was allowed in most studies (n = 49), with 3 treatment lines most commonly used (n = 26), followed by 4, 2, and 5 treatment lines (n = 12, n = 8, n = 2 respectively).
Outcome options
The following positive outcomes were considered: QALY, life years, and being stable, compliant, or employable. Different terms for stable were applied: response, non-relapse, symptom-controlled. The following negative outcomes were considered: disability-adjusted life years (DALY) and occurrence of relapse and side effects. Relapse was further classified into inpatient and/or outpatient relapse in 6 studies. Side effects considered were extrapyramidal symptoms (EPS), metabolic syndrome, and diabetes as primary outcome. Other outcomes were a decrease in incremental cost per Positive and Negative Syndrome Scale (PANSS) score and difference in cost, remaining on first-line treatment, tardive dyskinesia, coronary heart disease, agranulocytosis, and death.
Other than QALY, the most commonly used outcome of time spent in state was the duration of stable phase (n = 9). Others were DALY, life-year, time spent in relapse, EPS, and duration of first-line treatment. For number of patients with outcome, the most commonly used outcome was relapse (n = 14), either requiring (n = 5) or not requiring hospitalization (n = 4). Others were number of patients being stable, experiencing relapse, metabolic syndrome, or diabetes, and remaining on first-line treatment or employable. For number of outcomes per patient, only relapse was considered.
Model-specific information
DT
DT models evaluated 2 to 64 outcomes and 1 to 12 branching points with increasing model complexity. Most DT models applied branching points at relapse level (72%), response level (41%), discontinuation option (62%), or adherence level (38%).
The relapse branch was composed of no relapse (or stable) and relapse. Around half of the studies (57%) further classified relapse into inpatient and outpatient relapse. The response branch was composed of response and no response. The discontinuation branch was composed of continuation, discontinuation, and sometimes dose increase (n = 7). Discontinuation could be followed by a medication switch or just discontinuation. The adherence branch was composed of adherent, non-adherent, and sometimes partially adherent (n = 2).
CLMM
Twenty-nine (91%) CLMMs reported their structure, considering from 2 to 33 Markov health states. Common health states were stable and relapse (n = 22). Stable phase was replaced with response level (PANSS improvement: > 30%, 30%–20%, < 20%), more detailed symptom (mix/positive/negative/no symptom), or residual symptoms state in 2, 1, and 1 studies. The other elements of states were setting of care (inpatient, outpatient), treatment (lines of treatments and no treatment), adherence level (adherent, partially adherent, non-adherent), presence of side effect, and death.
The following cycle lengths were applied: 6 weeks, 18 weeks, 1 month, 3 months, 6 months, and 1 year. A 1-year cycle was most often used, with a lifetime (n = 7) and 5-year timeframe (n = 4). A 3-month cycle with 5-year timeframe was also used (n = 6). All these cycle lengths were used in the models using schizophrenia disease status as Markov states. For models using Markov states with long-term side effects, only a 1-year cycle length was used. For models using Markov states with only treatment sequences, 1-year (n = 2) and 18-week (n = 1) cycle lengths were used.
PLMM
Two PLMM models were original [Citation75,Citation77]. The other models adapted the model of Furiak et al [Citation75]., with minor changes to the model structure (adding 2 side effects: hyperprolactinaemia and tardive dyskinesia). The model of Vera-Llonch et al [Citation77]. had 6 health states (no chronic side effects, diabetes, prolactin-related disorders, both of these, discontinuation, and death) with a 1-month cycle. They set the baseline body weight and body mass index to patients individually, and simulated the changes in body weight and possible adverse events (AE). The model of Furiak et al [Citation75]. had 3 health states (stable with or without side effects, and relapse requiring hospitalization) and 3 adherence levels, with a cycle length of 3 months. They initialized the baseline adherence level for patients individually and simulated changes in their adherence level, disease progression, and possible AEs (weight gain, EPS, diabetes, hyperlipidaemia) and treatment sequence.
DES
Two DES models were original [Citation80,Citation81]. The other models adapted the model of Heeg et al [Citation81]. by adding another compliance level and a line for treatment switch. The model of Heeg et al [Citation81]. moved forward by times to remission, relapse, and psychiatrist visit, and ended at completion of timeframe or death. They initialized the following characteristics: age (on entering the model and at death), gender, disease severity, risk of self-harming or harming others, possibility of AEs, and social and environmental factors with impact on their treatment location. The model of Dilla et al [Citation80]. moved forward with time to remission, relapse, AE, psychiatrist visit, and death. They did not report on the simulation of patient characteristics.
Discussion
Similarities between models
The pharmacoeconomic models analysed in this review addressed similar research questions. They primarily focused on the comparative cost-effectiveness from the payer perspective of different pharmacotherapies in terms of QALYs and relapse in general schizophrenia patients who required relapse prevention.
Among the models identified, DT and CLMM dominated. Within DT or CLMM, the models shared similar structures. Most DT models defined response as an outcome for acute phase treatment and relapse as an outcome for maintenance treatment, as well as discontinuation (switch or dropout) and adherence level as factors to adjust outcome probability. Most CLMM models transitioned between stable phase and relapse, with treatment status and adherence level as factors that may change transition probabilities, and with AE and setting/severity that may change QALY.
Differences between models
Some models addressed non-common research questions, such as patient population (e.g. early schizophrenia or treatment-resistant schizophrenia), comparison (e.g. different treatment regimen, compliance levels, and brand–generic switch), and outcome (e.g. long-term AEs and discontinuation). Some models also employed non-common structures. For example, some DT models included the additional branch points of suicide, AE, and employability. Some CLMM models did not have disease states. Instead, they focused on AEs (e.g. transition between no AE and long-term AE, short-term AE and/or death due to AE), settings (e.g. transition between outpatient and inpatient care), or treatment (e.g. transition between treatment lines).
The definition of framework in models with similar research questions and structure was another source of heterogeneity. Defining general schizophrenia across studies was associated with subtle differences in initial disease severity, acute or maintenance phase of disease, settings, and adherence. Pharmacotherapy comparisons included models with different subsequent treatments in terms of the number of lines and treatments available for switching. Calculation of QALYs was based on different ranges of disease status and AEs. Different definitions also existed for other common outcomes, such as relapse and response. There were sometimes differences in the methods of evaluation (PANSS scale or Brief Psychiatric Rating Scale) and the thresholds to determine response or relapse (20% or 30% changes).
Many DT models shared similar branching points with single-choice, binary, or multiple options, e.g. a patient could be classified as a responder or a non-responder, but in some models, the option of a partial response existed for patients with residual symptoms. There were also models which assumed that patients who received the last line treatment would respond to the treatment. The majority of CLMM models had similar health states and relations between them; however, important factors, such as current treatment, AEs, and adherence level were excluded from some models.
Limitations
The major limitation of this review is the necessity of making assumptions in cases where studies did not provide sufficient information. For example, in studies that lacked a description of the patient population, general schizophrenia was assumed. Secondly, this review aimed to identify and present economic models of pharmacotherapy for schizophrenia, and so does not attempt to account for differences between schizophrenia models that could be rooted in different requirements existing in different countries, such as the preferences of different health technology assessment institutions, different treatment guidelines, and the availability of comparators and treatment lines.
Suggestion for future studies
Analysis on modelling studies
It would be interesting to conduct further studies to investigate whether the choice of model structure is influenced by the research question and if particular structures are chosen for particular treatments and/or patient populations, such as the antipsychotic treatments to be compared or patient populations with special features.
It would also be interesting to investigate the impact of structural choices on modelling results. Developing economic models to test this impact is needed. The impact will be difficult to identify through review of existing economic models, as they are different not only in model structure but also in the inputs and assumptions used. Our review summarizes the options for model structures, and this may help future modelling studies to identify alternative modelling options that could be tested.
Modelling studies
We have also identified several areas that need further research for modelling. First, modelling for treatment-resistant schizophrenia should be addressed, due to the limited number of studies focusing on this area. Many studies have involved long-term simulations that inevitably reached the late stage of the disease, but these studies used assumptions instead of more subtle modelling techniques.
Another area relates to treatment sequence. Despite the fact that some models allowed treatment switch, most of them did not consider the reasons for switching. In addition, only 3 studies considered treatments with better attributes had a higher probability of being switched to.
Future models should also consider more detailed outcomes. Due to the variety of benefit and risk profiles among available treatment options, economic models that do not take account of residual states in natural disease progression, detailed adherence levels, or long-term AEs are prone to bias.
Finally, patient-level models are recommended, as the complexity of schizophrenia limits the use of cohort-level models. By increasing the complexity of the model, DT models can have tree structures with 64 outcomes, and CLMM models can have Markov structures with 33 health states, making them inconvenient as a model. Additionally, cohort models cannot take into account patient heterogeneity, which may possibly make their results less precise and their uncertainty analysis less complete. However, patient-level models require more data and better modelling skills.
Conclusions
Current patterns and structures for modelling pharmacotherapy in schizophrenia focus on general schizophrenia using cohort-level models. Future modelling should concentrate on rare cases, such as treatment-resistant schizophrenia, and on details of treatment sequences and outcomes. Employing patient-level models may provide the complexity needed for schizophrenia modelling.
Supplemental Material
Download Zip (751.7 KB)Acknowledgments
The authors would like to thank Malgorzata Biernikiewicz for medical writing support.
Disclosure statement
The authors have no conflict of interest.
Supplementary material
Supplementary data present in this article can accessed here.
Additional information
Funding
References
- Jin H, Mosweu I. The societal cost of schizophrenia: A systematic review. Pharmacoeconomics. 2017;35:1–10.
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. The Lancet. 2012;380:2163–2196.
- Leucht S, Cipriani A, Spineli L, et al. Comparative effi cacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. The Lancet. 2013;382:951–962.
- Revicki DA, Luce BR, Weschler JM, et al. Cost-effectiveness of clozapine for treatment-resistant schizophrenic patients. Hospital and Community Psychiatry. 1990;41:850–854.
- Achilla E, McCrone P. The cost effectiveness of long-acting/extended-release antipsychotics for the treatment of schizophrenia: a systematic review of economic evaluations. Appl Health Econ Health Policy. 2013;11:95–106.
- Kruse G, Wong BJO, Duh MS, et al. Systematic literature review of the methods used to compare newer second-generation agents for the management of schizophrenia: A focus on health technology assessment. Pharmacoeconomics. 2015;33:1049–1067.
- Scheele B, Mauskopf J, Brodtkorb T-H, et al. Relationship between modeling technique and reported outcomes: case studies in models for the treatment of schizophrenia. Expert Rev Pharmacoecon Outcomes Res. 2014;14:235–257.
- Németh B, Fasseeh A, Molnár A, et al. A systematic review of health economic models and utility estimation methods in schizophrenia. Expert Rev Pharmacoecon Outcomes Res. 2018; 18(3): 267–275.
- National Institute for Health and Clinical Excellence. Psychosis and schizophrenia: management. NICE Guideline CG82; 2009; Available from: https://www.nice.org.uk/guidance/cg82. Last accessed 2018 Apr 04
- National Institute for Health and Clinical Excellence. Psychosis and schizophrenia in adults: prevention and management. NICE Guideline CG178; 2014; Available from: https://www.nice.org.uk/guidance/cg178. Last accessed 2018 Apr 04
- National Institute for Health and Clinical Excellence. Aripiprazole for schizophrenia in people aged 15 to 17 years. NICE Technology apprasial guidance TA213; 2011; Available from: www.nice.org.uk/guidance/TA213. Last accessed 2018 Apr 04
- Kobelt G. Health economics: an introduction to economic evaluation. London: Office of Health Economiics; 2013.
- Heeg BMS, Damen J, Buskens E, et al. Modelling approaches: the case of schizophrenia. Pharmacoeconomics. 2008;26:633–648.
- Roberts M, Russell LB, Paltiel AD, et al. Conceptualizing a model: a report of the ISPOR-SMDM modeling good research practices task force–2. Value Health. 2012;15:804–811.
- Alexeyeva I, Mauskopf J, Earnshaw SR, et al. Comparing olanzapine and ziprasidone in the treatment of schizophrenia: a case study in modeling. J Med Econ. 2008;4:179–192.
- Bagnall AM, Jones L, Ginnelly L, et al. A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess. 2003;7:1–193.
- Bernardo M, Azanza JR, Rubio-Terres C, et al. Cost-effectiveness analysis of the prevention of relapse of schizophrenia in the longitudinal study Ziprasidone Extended Use in Schizophrenia (ZEUS). Actas Esp Psiquiatr. 2007;35:259–262.
- Bounthavong M, Okamoto MP. Decision analysis model evaluating the cost-effectiveness of risperidone, olanzapine and haloperidol in the treatment of schizophrenia. J Eval Clin Pract. 2007;13:453–460.
- Citrome L, Kamat SA, Sapin C, et al. Cost-effectiveness of aripiprazole once-monthly compared with paliperidone palmitate once-monthly injectable for the treatment of schizophrenia in the USA. J Med Econ. 2014;17:567–576.
- Colombo GL, Caruggi M, Di Matteo S, et al. An economic evaluation of aripiprazole vs olanzapine adapted to the Italian setting using outcomes of metabolic syndrome and risk for diabetes in patients with schizophrenia. Neuropsychiatr Dis Treat. 2008;4:967–976.
- De Graeve D, Smet A, Mehnert A, et al. Long-acting risperidone compared with oral olanzapine and haloperidol depot in schizophrenia: a belgian cost-effectiveness analysis. Pharmacoeconomics. 2005;23(Suppl 1):35–47.
- Edwards NC, Locklear JC, Rupnow MF, et al. Cost effectiveness of long-acting risperidone injection versus alternative antipsychotic agents in patients with schizophrenia in the USA. Pharmacoeconomics. 2005;23(Suppl 1):75–89.
- Edwards NC, Pesa J, Meletiche DM, et al. One-year clinical and economic consequences of oral atypical antipsychotics in the treatment of schizophrenia. Curr Med Res Opin. 2008;24:3341–3355.
- Edwards NC, Muser E, Doshi D, et al. The threshold rate of oral atypical anti-psychotic adherence at which paliperidone palmitate is cost saving. J Med Econ. 2012;15:623–634.
- Einarson TR, Pudas H, Zilbershtein R, et al. Cost-effectiveness analysis of atypical long-acting antipsychotics for treating chronic schizophrenia in Finland. J Med Econ. 2013;16:1096–1105.
- Einarson TR, Zilbershtein R, Skoupa J, et al. Economic and clinical comparison of atypical depot antipsychotic drugs for treatment of chronic schizophrenia in the Czech Republic. J Med Econ. 2013;16:1089–1095.
- Einarson TR, Vicente C, Zilbershtein R, et al. Pharmacoeconomic analysis of paliperidone palmitate versus olanzapine pamoate for chronic schizophrenia in Norway. Acta Neuropsychiatr. 2013;25:85–94.
- Einarson TR, Geitona M, Chaidemenos A, et al. Pharmacoeconomic analysis of paliperidone palmitate for treating schizophrenia in Greece. Ann Gen Psychiatry. 2012;11:18.
- Einarson TR, Vicente C, Zilbershtein R, et al. Pharmacoeconomics of depot antipsychotics for treating chronic schizophrenia in Sweden. Nord J Psychiatry. 2014;68:416–427.
- Einarson TR, Pudas H, Goswami P, et al. Pharmacoeconomics of long-acting atypical antipsychotics for acutely relapsed chronic schizophrenia in Finland. J Med Econ. 2016;19:111–120.
- Ganguly R, Miller LS, Martin BC. Future employability, a new approach to cost-effectiveness analysis of antipsychotic therapy. Schizophr Res. 2003;63:111–119.
- Garcia-Ruiz AJ, Perez-Costillas L, Montesinos AC, et al. Cost-effectiveness analysis of antipsychotics in reducing schizophrenia relapses. Health Econ Rev. 2012;2:8.
- Geitona M, Kousoulakou H, Ollandezos M, et al. Costs and effects of paliperidone extended release compared with alternative oral antipsychotic agents in patients with schizophrenia in Greece: a cost effectiveness study. Ann Gen Psychiatry. 2008;7:16.
- Graham CN, Mauskopf JA, Lawson AH, et al. Updating and confirming an industry-sponsored pharmacoeconomic model: comparing two antipsychotics in the treatment of schizophrenia. Value Health. 2012;15:55–64.
- Greenhalgh J, Knight C, Hind D, et al. Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies. Health Technol Assess. 2005;9:iii–iv. 1–156.
- Jukic V, Jakovljevic M, Filipcic I, et al. Cost-utility analysis of depot atypical antipsychotics for chronic schizophrenia in croatia. Value in Health Regional Issues. 2013;2:181–188.
- Kongsakon R, Leelahanaj T, Price N, et al. Cost analysis of the treatment of schizophrenia in Thailand: a simulation model comparing olanzapine, risperidone, quetiapine, ziprasidone and haloperidol. J Med Assoc Thai. 2005;88:1267–1277.
- Layton S, Barbeau M. Generic replacement of clozapine: a simple decision model from a Canadian perspective. Curr Med Res Opin. 2004;20:453–459.
- Obradovic M, Mrhar A, Kos M. Cost-effectiveness of antipsychotics for outpatients with chronic schizophrenia. Int J Clin Pract. 2007;61:1979–1988.
- Oh PI, Iskedjian M, Addis A, et al. Pharmacoeconomic evaluation of clozapine in treatment-resistant schizophrenia: a cost-utility analysis. Can J Clin Pharmacol. 2001;8:199–206.
- Oh PI, Lanctôt KL, Mittmann N, et al. Cost-utility of risperidone compared with standard conventional antipsychotics in chronic schizophrenia. J Med Econ. 2001;4:137–156.
- Yang YK, Tarn YH, Wang TY, et al. Pharmacoeconomic evaluation of schizophrenia in Taiwan: model comparison of long-acting risperidone versus olanzapine versus depot haloperidol based on estimated costs. Psychiatry Clin Neurosci. 2005;59:385–394.
- Yang L, Li M, Tao LB, et al. Cost-effectiveness of long-acting risperidone injection versus alternative atypical antipsychotic agents in patients with schizophrenia in China. Value Health. 2009;12(Suppl 3):S66–9.
- Almond S, O’Donnell O. Cost analysis of the treatment of schizophrenia in the UK. A Simulation Model Comparing Olanzapine, Risperidone and Haloperidol. Pharmacoeconomics. 2000;17:383–389.
- Anh NQ, Linh BN, Ha NT, et al. Schizophrenia interventions in Vietnam: primary results from a cost-effectiveness study. Glob Public Health. 2015;10(Supppl 1):S21–39.
- Beard SM, Maciver F, Clouth J, et al. A decision model to compare health care costs of olanzapine and risperidone treatment for schizophrenia in Germany. Eur J Health Econ. 2006;7:165–172.
- Bobes J, Canas F, Rejas J, et al. Economic consequences of the adverse reactions related with antipsychotics: an economic model comparing tolerability of ziprasidone, olanzapine, risperidone, and haloperidol in Spain. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1287–1297.
- Davies A, Vardeva K, Loze JY, et al. Cost-effectiveness of atypical antipsychotics for the management of schizophrenia in the UK. Curr Med Res Opin. 2008;24:3275–3285.
- Druais S, Doutriaux A, Cognet M, et al. Cost effectiveness of paliperidone long-acting injectable versus other antipsychotics for the maintenance treatment of schizophrenia in France. Pharmacoeconomics. 2016;34:363–391.
- Emsley R, Booysen F. Cost-effectiveness of an atypical conventional antipsychotic in South Africa: an economic evaluation of quetiapine versus haloperidol in the treatment of patients partially responsive to previous antipsychotics. South Afr J Psychiatry. 2004;10:7.
- Frey S, Linder R, Juckel G, et al. Cost-effectiveness of long-acting injectable risperidone versus flupentixol decanoate in the treatment of schizophrenia: a markov model parameterized using administrative data. Eur J Health Econ. 2014;15:133–142.
- Hansen K, Francois C, Toumi M, et al. A pharmacoeconomic evaluation of zuclopenthixol compared with haloperidol and risperidone in the treatment of schizophrenia. Eur J Health Econ. 2002;3:173–179.
- Kasteng F, Eriksson J, Sennfalt K, et al. Metabolic effects and cost-effectiveness of aripiprazole versus olanzapine in schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2011;124:214–225.
- Kim B-R-M, Lee T-J, Lee H-J, et al. Cost-effectiveness of sertindole among atypical antipsychotics in the treatment of schizophrenia in South Korea. Value in Health Regional Issues. 2012;1:59–65.
- Lachaine J, Beauchemin C, Mathurin K, et al. Cost-effectiveness of asenapine in the treatment of schizophrenia in Canada. J Med Econ. 2014;17:296–304.
- Lecomte P, De Hert M, Van Dijk M, et al. A 1-year cost-effectiveness model for the treatment of chronic schizophrenia with acute exacerbations in Belgium. Value Health. 2000;3:1–11.
- Lin L, Zhao YJ, Zhou HJ, et al. Comparative cost-effectiveness of 11 oral antipsychotics for relapse prevention in schizophrenia within Singapore using effectiveness estimates from a network meta-analysis. Int Clin Psychopharmacol. 2016;31:84–92.
- Lindner LM, Marasciulo AC, Farias MR, et al. Economic evaluation of antipsychotic drugs for schizophrenia treatment within the Brazilian healthcare system. Rev Saude Publica. 2009;43(Suppl 1):62–69.
- Lindstrom E, Eberhard J, Fors BM, et al. A pharmacoeconomic analysis of sertindole in the treatment of schizophrenia in Sweden. Nord J Psychiatry. 2011;65:403–413.
- Lubinga SJ, Mutamba BB, Nganizi A, et al. A cost-effectiveness analysis of antipsychotics for treatment of schizophrenia in Uganda. Appl Health Econ Health Policy. 2015;13:493–506.
- Magnus A, Carr V, Mihalopoulos C, et al. Assessing cost-effectiveness of drug interventions for schizophrenia. Aust N Z J Psychiatry. 2005;39:44–54.
- McIntyre RS, Cragin L, Sorensen S, et al. Comparison of the metabolic and economic consequences of long-term treatment of schizophrenia using ziprasidone, olanzapine, quetiapine and risperidone in Canada: a cost-effectiveness analysis. J Eval Clin Pract. 2010;16:744–755.
- Mehnert A, Nicholl D, Pudas H, et al. Cost effectiveness of paliperidone palmitate versus risperidone long-acting injectable and olanzapine pamoate for the treatment of patients with schizophrenia in Sweden. J Med Econ. 2012;15:844–861.
- Mortimer A, Williams P, Meddis D. Impact of side-effects of atypical antipsychotics on non-compliance, relapse and cost. J Int Med Res. 2003;31:188–196.
- O’Day K, Rajagopalan K, Meyer K, et al. Long-term cost-effectiveness of atypical antipsychotics in the treatment of adults with schizophrenia in the US. Clinicoecon Outcomes Res. 2013;5:459–470.
- Palmer CS, Brunner E, Ruiz-Flores LG, et al. A cost-effectiveness clinical decision analysis model for treatment of Schizophrenia. Arch Med Res. 2002;33:572–580.
- Park T, Kuntz KM. Cost-effectiveness of second-generation antipsychotics for the treatment of schizophrenia. Value Health. 2014;17:310–319.
- Perlis RH, Ganz DA, Avorn J, et al. Pharmacogenetic testing in the clinical management of schizophrenia: a decision-analytic model. J Clin Psychopharmacol. 2005;25:427–434.
- Phanthunane P, Vos T, Whiteford H, et al. Cost-effectiveness of pharmacological and psychosocial interventions for schizophrenia. Cost Eff Resour Alloc. 2011;9:6.
- Rajagopalan K, Hassan M, O’Day K, et al. Cost-effectiveness of lurasidone vs aripiprazole among patients with schizophrenia who have previously failed on an atypical antipsychotic: an indirect comparison of outcomes from clinical trial data. J Med Econ. 2013;16:951–961.
- Tilden D, Aristides M, Meddis D, et al. An economic assessment of quetiapine and haloperidol in patients with schizophrenia only partially responsive to conventional antipsychotics. Clin Ther. 2002;24:1648–1667.
- Wang PS, Ganz DA, Benner JS, et al. Should clozapine continue to be restricted to third-line status for schizophrenia?: a decision-analytic model. J Ment Health Policy Econ. 2004;7:77–85.
- Zeidler J, Mahlich J, Greiner W, et al. Cost effectiveness of paliperidone palmitate for the treatment of schizophrenia in Germany. Appl Health Econ Health Policy. 2013;11:509–521.
- Ascher-Svanum H, Furiak NM, Lawson AH, et al. Cost-effectiveness of several atypical antipsychotics in orally disintegrating tablets compared with standard oral tablets in the treatment of schizophrenia in the USA. J Med Econ. 2012;15:531–547.
- Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness model comparing olanzapine and other oral atypical antipsychotics in the treatment of schizophrenia in the USA. Cost Eff Resour Alloc. 2009;7:4.
- Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the USA: a micro-simulation economic decision model. Curr Med Res Opin. 2011;27:713–730.
- Vera-Llonch M, Delea TE, Richardson E, et al. Outcomes and costs of risperidone versus olanzapine in patients with chronic schizophrenia or schizoaffective disorders: a markov model. Value Health. 2004;7:569–584.
- Chue PS, Heeg B, Buskens E, et al. Modelling the impact of compliance on the costs and effects of long-acting risperidone in Canada. Pharmacoeconomics. 2005;23(Suppl 1):62–74.
- Damen J, Thuresson PO, Heeg B, et al. A pharmacoeconomic analysis of compliance gains on antipsychotic medications. Appl Health Econ Health Policy. 2008;6:189–197.
- Dilla T, Moller J, O’Donohoe P, et al. Long-acting olanzapine versus long-acting risperidone for schizophrenia in Spain - a cost-effectiveness comparison. BMC Psychiatry. 2014;14:298.
- Heeg B, Buskens E, Knapp M, et al. Modelling the treated course of schizophrenia: development of a discrete event simulation model. Pharmacoeconomics. 2005;23(Suppl 1):17–33.
- Heeg B, Buskens E, Botteman M, et al. The cost-effectiveness of atypicals in the UK. Value Health. 2008;11:1007–1021.
- Heeg BM, Antunes J, Figueira ML, et al. Cost-effectiveness and budget impact of long-acting risperidone in Portugal: a modeling exercise. Curr Med Res Opin. 2008;24:349–358.
- Hensen M, Heeg B, Lothgren M, et al. Cost effectiveness of long-acting risperidone in Sweden. Appl Health Econ Health Policy. 2010;8:327–341.
- Laux G, Heeg B, Van Hout BA, et al. Costs and effects of long-acting risperidone compared with oral atypical and conventional depot formulations in Germany. Pharmacoeconomics. 2005;23(Suppl 1):49–61.
- Treur M, Heeg B, Moller HJ, et al. A pharmaco-economic analysis of patients with schizophrenia switching to generic risperidone involving a possible compliance loss. BMC Health Serv Res. 2009;9:32.
- Treur M, Baca E, Bobes J, et al. The cost-effectiveness of paliperidone extended release in Spain. J Med Econ. 2012;15(Suppl 1):26–34.
- Chisholm D. Choosing cost-effective interventions in psychiatry: results from the CHOICE programme of the World Health Organization. World Psychiatry. 2005;4:37–44.
- Chisholm D, Gureje O, Saldivia S, et al. Schizophrenia treatment in the developing world: an interregional and multinational cost-effectiveness analysis. Bull World Health Organ. 2008;86:542–551.
- Chisholm D, Saxena S. Cost effectiveness of strategies to combat neuropsychiatric conditions in sub-Saharan Africa and South East Asia: mathematical modelling study. BMJ. 2012;344:e609.
- Strand KB, Chisholm D, Fekadu A, et al. Scaling-up essential neuropsychiatric services in Ethiopia: a cost-effectiveness analysis. Health Policy Plan. 2016;31:504–513.