 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
While poor inhaler technique in asthma and chronic obstructive pulmonary disease (COPD) can compromise the effectiveness of inhaled medications, identifying and quantifying these errors may suggest ways to improve inhalation technique and patient outcomes. The objective of this international, multicentre care improvement programme was to investigate errors in inhaler use (handling errors and inhalation errors) made by patients in handling two dry powder inhalers; DuoResp® Spiromax® and Symbicort® Turbuhaler®. Patients with asthma or COPD aged between 18 and 80 years attending the allergology/pneumology departments of 14 hospitals in Spain and Portugal were included. All assessments were performed during one regular scheduled visit to the study clinic. Among 161 eligible patients (138 with asthma; 23 with COPD), inhalation errors were the most common type of error, with no significant difference between devices in overall total error rate, handling error rate or inhalation error rate. Significantly fewer total errors per patient (1.4 vs. 1.9; p < 0.001) and handling errors per patient (0.5 vs. 0.8; p < 0.001) were observed with DuoResp® Spiromax® compared with Symbicort® Turbuhaler®. The mean number of attempts for patients using DuoResp® Spiromax® to perform two correct procedures was 1.9 (0.6) compared with 2.1 (0.9) attempts for patients using Symbicort® Turbuhaler® (p = 0.016). Compared with Symbicort® Turbuhaler®, DuoResp® Spiromax® was found to be easy to learn how to use (p < 0.001), easy to prepare (p < 0.001), easy to use (p < 0.001), comfortable in terms of weight and size (p = 0.001), and patients felt that they were using the device correctly (p < 0.001). Overall, 79.5% of patients stated that they preferred DuoResp® Spiromax® as their first option over Symbicort® Turbuhaler®. The findings of this study may be useful in developing effective inhaler training programmes and thus improve outcomes in asthma and COPD.
Introduction
With an increasing prevalence since 1990 [Citation1], both asthma and chronic obstructive pulmonary disease (COPD) continue to impose a significant economic, humanistic and clinical burden on patients, families and healthcare systems [Citation2,Citation3,Citation4,Citation5]. Improved strategies are needed to address the major public health problems associated with asthma and COPD and to improve outcomes for individual patients.
The recommended and accepted way of managing asthma and COPD is through the use of inhaled medications delivered via an appropriate inhaler device [Citation4,Citation5]. However, the effectiveness of inhaled medications can be substantially diminished by poor inhaler technique [Citation4,Citation5,Citation6,Citation7,Citation8]. In particular, correct inhaler technique can be compromised by a number of factors including patient age [Citation9,Citation10,Citation11,Citation12], level of training [Citation9, Citation13, Citation14,Citation15], gender [Citation9] and educational level [Citation9]. The design of the inhalation device itself can also impact on the likelihood of patient error [Citation15,Citation16,Citation17]. For this reason, various types of inhaler are available, each incorporating specific features designed to make them more precise, practical and easy to use.
DuoResp® Spiromax® and Symbicort® Turbuhaler® are two dry powder inhalers that are commonly available to asthma and COPD patients. Symbicort® Turbuhaler® (AstraZeneca R&D, Lund, Sweden) was one of the first metered-dose powder delivery systems to be developed [Citation18], and was created to deliver an inhaled corticosteroid (ICS; budesonide) and a long-acting β2 agonist (LABA; formoterol fumarate dihydrate) with good deposition when sufficient inspiratory flow is achieved [Citation17,Citation19]. The more recently developed DuoResp® Spiromax® (Teva Pharmaceutical Industries, Petach Tikva, Israel) was designed to deliver budesonide and formoterol with high-dose uniformity and maximum ease of use [Citation20,Citation21].
Because errors in inhalation technique are typically evaluated in clinical practice by an observer only, with no standardisation, the MasterScope® Inhalation Manager tool was developed to improve rigor in this regard and was utilised in the current study.
The objective of the current study was to identify, evaluate and quantify errors in inhaler use (including handling errors and inhalation errors) made by patients with asthma or COPD in handling DuoResp® Spiromax® and Symbicort® Turbuhaler®. The findings of this study may then be used to correct these errors, train the patient and assess efficiency in the long-term handling of both devices.
Materials and methods
Study design and population
This study was part of an international, multicentre care improvement programme in patients with asthma or COPD aged between 18 and 80 years attending the allergology/pneumology departments of 14 hospitals (10 in Spain and four in Portugal). Specific inclusion criteria were a diagnosis of asthma or COPD according to the Global Initiative for Asthma (GINA), Global Initiative for Obstructive Lung Disease (GOLD), or any equivalent local guidelines including the Spanish Asthma Management Guidelines [Citation22] or Spanish COPD Guidelines (GesEPOC) [Citation23]; eligibility for combined ICS/LABA; previous prescription for one of the inhalers being evaluated in the project (up to 6 months without using either of the inhalers was permitted). All patients voluntarily gave their informed consent. The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use and Good Clinical Practice guidelines, and the ethics committee at each participating site. The study was classified as a post-authorisation study and is therefore not included in a clinical trial registry.
Study objectives
The primary objective of the study was to identify, evaluate and quantify inhaler errors (including errors in handling and errors in inhalation) made by asthma or COPD patients using the DuoResp® Spiromax® and Symbicort® Turbuhaler® devices. Handling errors were defined on the basis of the information in the approved Patient Information Leaflets for each of the devices. Study objectives and endpoints are listed in .
Table 1. Study objectives and endpoints
Study assessments
All assessments were performed during one visit to the study clinic, which was scheduled according to the normal clinical practice of each participating centre. At this visit, sociodemographic and clinical data were recorded, handling and inhalation tests were performed for both devices and questions were asked about the participant’s experience with each device, as described below.
Error rates
Total error rate
The patient was provided with one of the inhalers, and was asked to read the accompanying instructions. The patient was then asked to perform an inhalation using a demonstration device, and any critical errors were recorded by the investigator. The same procedure was then carried out with the second device.
Critical errors were recorded by the investigator using a checklist of questions (). A total of 13 possible errors for DuoResp® Spiromax® and 17 possible errors for Symbicort® Turbuhaler® were included in the checklist. Patients were individually assessed on their use of the two devices against the full set of questions.
Table 2. Questions used to identify errors in the use of DuoResp® Spiromax® and Symbicort® Turbuhaler®
The total number of possible errors for each device was then calculated by multiplying the number of error-defining questions (13 or 17) by the number of patients in the study. The overall total error rate for each device was then calculated as follows:
Total number of errors was also stratified by age and diagnosis.
Handling error rate
Five of the 13 DuoResp® Spiromax® error questions and nine of the 17 Symbicort® Turbuhaler® error questions referred specifically to errors in inhaler handling (). The handling error rate was calculated for each device as described above for total errors.
Inhalation error rate
Eight of the 13 DuoResp® Spiromax® error questions and eight of the 17 Symbicort® Turbuhaler® error questions referred specifically to errors in inhalation technique (). The percentage of inhalation errors was calculated for each device as described above for total errors.
Time spent with each device
Three separate measures of the time spent with each device were recorded. Time 1 was the time that the patient took to read the instructions and perform the technique; Time 2 was the time spent by the healthcare professional (HCP) on the training process; and Time 3 was the time spent performing at least two correct inhalation manoeuvres with the Inhalation Manager.
Inhalation technique
Inhalation parameters, comprising peak inspiratory flow at 1.5 s of inhalation (PIF1.5), final peak inspiratory flow (PIF), inhalation volume, initial flow acceleration, inhalation time after reaching 30 l/min (Ti30) and peak expiratory flow (PEF) were measured during the study visit using the MasterScope® Inhalation Manager tool. The necessary inhalation manoeuvres were recorded until the patient performed a minimum of two correct manoeuvres. As more than one result for each assessment could be included for each inhaler, any given patient may have more than one recorded reading per inhaler.
Patient-reported outcomes
At the end of the study visit, patients were asked to evaluate the two devices across the following domains: 1) Easy to learn how to use; 2) Easy to prepare; 3) Easy to use; 4) Comfortably adapts to the lips; 5) Comfortable in terms of weight and size; 6) Feeling of using the device correctly. Each domain was scored on a five-point Likert scale (1 = very; 2 = quite; 3 = somewhat; 4 = not very; 5 = not at all).
Patient preference for DuoResp® Spiromax® or Symbicort® Turbuhaler® inhalers was recorded using a scoring system of 1 to 2 points to indicate the order of priority (2 = first choice; 1 = second choice).
Sample size calculation
As the DuoResp® Spiromax® inhaler has only recently been launched to market, real-world data relating to errors made by patients when using the device are not yet available. The sample size calculation was therefore based on the work of Voshaar et al. [Citation24] which compared the NEXThaler® with other dry powder inhalers such as Turbuhaler®. This approach is valid because the NEXThaler® inhaler is similar to Spiromax® in that it is easy to use in three steps (open, inhale and close). In the Voshaar et al. study, 54% of patients did not commit any critical errors upon the first use of the NEXThaler® inhaler, compared with 30% with Turbuhaler®. It was conservatively assumed that the difference between the two devices in the current study would be smaller than the 24 percentage-point difference observed in the NEXThaler® reference study. Thus, we estimated a difference of 9 percentage points between Spiromax® and Turbuhaler®. With this hypothesis, accepting an alpha risk of 0.05 for 80% accuracy in a bilateral contrast and with a patient loss no greater than 5%, a required population sample of 509 patients was calculated. However, alternative scenarios utilising less conservative assumptions for the percentage-point difference indicated that statistical significance could be demonstrated with data from much smaller samples. For instance, with a difference of 16 percentage points, the required population sample would be 159 patients. With a still less conservative assumption of a percentage-point difference of 24, equivalent to that between NEXThaler® and Turbuhaler®, only 69 patients would be needed to demonstrate significance.
Data analysis
Absolute and relative frequency distributions of qualitative variables was prepared, as well as central trend and dispersion measures (mean, standard deviation [SD], median, minimum and maximum) of quantitative variables. 95% confidence intervals (CI) were calculated for the main quantitative variables of results associated with the primary objective and main secondary variables.
Missing data were not imputed and were treated as lost. The subgroups defined in the protocol (age, comorbidities and social status) were analysed in line with the analyses for the general population.
Version 18.0 of the Statistical Package for the Social Sciences (SPSS) software was used for all statistical analyses.
Primary endpoints
The nature and characteristics of inhaler errors (including handling errors and inhalation errors) was described for both inhalers. Similar errors were summarised by type of mistake, and a categorical analysis was performed of the frequency of prevalent handling errors for both inhalers. The frequencies were compared using a Chi-squared test to assess the statistical significance of the differences between devices.
Secondary endpoints
The categorical analysis of prevalent handling errors for both inhalers was stratified according to age, asthma or COPD diagnosis, and the presence of dexterity problems due to a history of arthritis, osteoarthritis, etc. The number of handling errors was presented for each of these categories, and were compared using a Chi-squared test to assess the statistical significance of the differences between devices.
The categorical analyses of inhalation technique, based on the assessed inhalation parameters: PIF1.5, final PIF, inhalation volume, initial flow acceleration, Ti30 and PEF with DuoResp® Spiromax® and Symbicort® Turbuhaler® were stratified according to age and asthma or COPD diagnosis. The observed parameters were compared using a Mann-Whitney-Wilcoxon test to assess the statistical significance of the differences between devices.
The mean time spent with each device was estimated and, using the Wilcoxon hypothesis test for differences between means, an assessment was made regarding whether there were statistically significant differences between both devices.
The distribution of absolute and relative frequencies of data collected using the 5-point Likert scale were prepared, and the statistical difference between both inhalers was compared using the McNemar test.
Descriptive statistics for patient preference findings are presented.
Results
Study population
At the time of database cut-off (31 March 2017), 175 patients had been enrolled at 14 hospitals in Spain and Portugal. In total, 14 patients were excluded from the analysis for not meeting the selection criteria (two patients were >80 years of age, while for 12 patients, <6 months had passed between the last time they used Symbicort® Turbuhaler® and their inclusion in the study).
In total, 161 patients (54.7% female), with an overall mean (SD) age of 49.2 (17.5) years, were included in the study (). The majority (97.5%) were white, with a level of education ranging from primary education only (36.6%), to secondary education (31.7%) and higher education (29.2%). Of the 161 evaluable patients, 138 (85.7%) were diagnosed with asthma and 23 (14.3%) with COPD. The mean (SD) length of time between initial diagnosis and entry into this study was 11.9 (13.2) years for asthma patients and 8.8 (10.4) years among COPD patients. Most patients (78.3%) were naïve to treatment with Symbicort® Turbuhaler® at the time of inclusion. No patients had prior experience with DuoResp® Spiromax® due to the recent commercialisation of this device at the time the study was conducted.
Table 3. Baseline and demographic data
Sixty-seven (41.6%) patients had at least one comorbidity, with the most common coexisting conditions being hypertension (18 patients, 11.2%), dyslipidaemia (seven patients, 4.3%), hypercholesterolaemia (six patients, 3.7%) and hypothyroidism (six patients, 3.7%). Of 103 comorbidities overall, 95 were active at the start of the study. Twenty-five patients (15.5%) were smokers at the start of the study, 52 (32.3%) were ex-smokers and 84 (52.2%) were non-smokers.
Error rates (total errors, handling errors and inhalation errors)
In terms of rates of errors made by the participants in this study overall, inhalation errors were the most common type of error made with both devices (; ). There was no statistically significant difference between DuoResp® Spiromax® and Symbicort® Turbuhaler® in terms of overall total error rate, handling error rate or inhalation error rate ().
Table 4. Total error rate, handling error rate and inhalation error rate for DuoResp® Spiromax® and Symbicort® Turbuhaler®
Figure 1. Error rates (total errors, handling errors, inhalation errors) for DuoResp® Spiromax® and Symbicort® Turbuhaler®
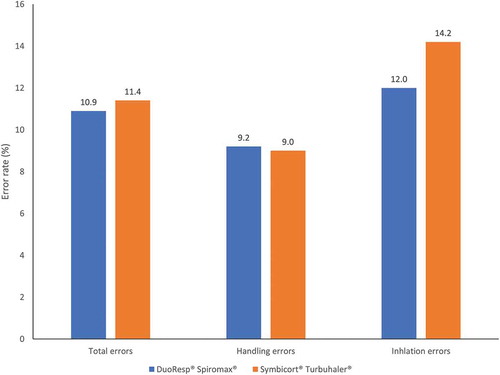
In an analysis of mean errors per patient, significantly fewer total errors per patient (1.4 vs. 1.9; p < 0.001) and significantly fewer handling errors per patient (0.5 vs. 0.8; p < 0.001) were observed with DuoResp® Spiromax® compared with Symbicort® Turbuhaler®. There was no statistically significant difference in the mean number of inhalation errors per patient between devices (1.0 for DuoResp® Spiromax® vs. 1.1 for Symbicort® Turbuhaler®; p = 0.107).
Total number of errors stratified by age
When the total number of errors made using each device was stratified by age (Supplementary Table 1), it was found that patients aged 41–65 years made fewer errors on average with DuoResp® Spiromax® compared with Symbicort® Turbuhaler® (mean 1.2 errors per patient for DuoResp® Spiromax® vs. 1.7 for Symbicort® Turbuhaler®; p = 0.038). A similar result was found for patients aged >65 years (mean 1.9 errors for DuoResp® Spiromax® vs. 2.8 for Symbicort® Turbuhaler®; p = 0.007). There was no difference in the number of errors made with DuoResp® Spiromax® compared with Symbicort® Turbuhaler® in patients aged 18–40 years.
Total number of errors stratified by diagnosis
When the total number of errors made using each device was stratified by diagnosis (Supplementary Table 2), asthma patients made fewer errors on average with DuoResp® Spiromax® compared with Symbicort® Turbuhaler® (mean 1.3 errors per patient for DuoResp® Spiromax® vs. 1.8 for Symbicort® Turbuhaler®; p = 0.001). There was no difference in the number of errors made with DuoResp® Spiromax® compared with Symbicort® Turbuhaler® in patients with COPD (p = 0.081).
Time spent with each device
Mean time taken by patients to read the instructions and perform the technique (Time 1) was significantly longer with Symbicort® Turbuhaler® compared with DuoResp® Spiromax® (3.0 vs. 3.5 min; p < 0.001). Time spent by the HCP on the training process (Time 2) was also significantly longer with Symbicort® Turbuhaler® compared with DuoResp® Spiromax® (1.5 vs. 1.7 min; p = 0.023). There was no statistically significant difference in time spent performing at least two correct inhalation manoeuvres (Time 3) between the two devices (2.2 vs. 2.5 min; p = 0.195).
Inhalation technique
Patients were assessed to determine their ability to perform two independent correct inhalations. The numbers of attempts for each correct inhalation were recorded separately and then averaged. The mean number of attempts for patients using DuoResp® Spiromax® to perform two correct procedures was 1.9 (0.6) compared with 2.1 (0.9) attempts for patients using Symbicort® Turbuhaler® (p = 0.016). A greater PIF1.5 (115.4 [37.8] vs. 105.3 [34.6] l/min; p < 0.001) and a greater final PIF was achieved with DuoResp® Spiromax® compared with Symbicort® Turbuhaler® across all patients (116.3 [36.0] vs. 104.3 [35.9] l/min; p < 0.001).
No difference between Symbicort® Turbuhaler® and DuoResp® Spiromax® was observed in inhalation volume, initial flow acceleration, Ti30 or PEF (p > 0.05 in all cases) (Supplementary Table 1).
Patient-reported outcomes
Patient responses to questionnaires relating to the experience of using each device are presented in . Compared with Symbicort® Turbuhaler®, DuoResp® Spiromax® was found to be easy to learn how to use (p < 0.001), easy to prepare (p < 0.001), easy to use (p < 0.001), comfortable in terms of weight and size (p = 0.001), and patients felt that they were using the device correctly (p < 0.001). Overall, 79.5% of patients stated that they preferred DuoResp® Spiromax® as their first option, while 20.5% (95% CI: 14.7–27.7) preferred Symbicort® Turbuhaler® as their first choice.
Discussion
This study showed that inhalation errors were the most common type of error made with both devices, with no statistically significant difference between DuoResp® Spiromax® and Symbicort® Turbuhaler® in overall total error rate, handling error rate or inhalation error rate. However, the mean numbers of total errors (handling plus inhalation errors) and the mean number of handling errors made by each patient, were lower with DuoResp® Spiromax® compared with Symbicort® Turbuhaler®. In addition, 79.5% of patients in this study preferred DuoResp® Spiromax® as their inhaler of first choice, finding it easy to learn how to use, easy to prepare and to use and comfortable in terms of weight and size. Patients also felt confident that they were using the device correctly.
DuoResp® Spiromax® seemed particularly suited to older patients, with patients aged ≥41 years making fewer errors with DuoResp® Spiromax® compared with Symbicort® Turbuhaler®. Patients with asthma made few device errors than those with COPD. Although we did not investigate the reasons for this difference, it is likely to be because COPD patients are on average older than asthma patients, and therefore may be less manually dextrous [Citation25,Citation26].
DuoResp® Spiromax® also performed better than Symbicort® Turbuhaler® in a number of other measures, including a significantly shorter time required to use the device and statistically higher values for PIF1.5 and final PIF achieved. In addition, inhalation with DuoResp® Spiromax® needed to be repeated fewer times in order to achieve a minimum of two correct procedures compared with Symbicort® Turbuhaler®.
Previous studies have indicated that implementation of inhaler training achieves a measurable improvement in inhaler technique [Citation27,Citation28,Citation29].
Multiple studies have shown that correct inhaler technique leads to improved patient adherence and patient outcomes, and the importance of correct inhaler technique has been highlighted by GINA and GOLD [Citation4,Citation5,Citation8,Citation30,Citation31].
Investigation and analysis of errors in the use of inhalation devices in respiratory disease is therefore essential to allow these errors to be corrected through the implementation of training and education strategies, and to ultimately improve patient outcomes.
The current study has a number of limitations. First, the study population was very homogenous, although this reflects the population of the areas where the study was conducted. In addition, there was a mix of patients who were naïve to treatment with Symbicort® Turbuhaler® (14.3%) versus those who had previously used Symbicort® Turbuhaler® (78.3%), which means that patients were bringing different levels of experience with this device to the study. A strength of this study is that all patients tested both devices, meaning that they were able to compare devices directly through their own experience.
Although the effect of compromised dexterity on handling errors was included as a secondary objective in this study (), it was not possible to conduct this analysis because variables that define this population were not captured in the case report form.
Finally, the results of this study may be skewed by fact that some patients had prior experience with Symbicort® Turbuhaler® but were unlikely to have used DuoResp® Spiromax® because it only became available in the region just before the start of this study. This discrepancy may have favoured Symbicort® Turbuhaler® as the most familiar device.
Conclusion
Inhalation errors were the most common type of error made with both devices, with no statistically significant difference between DuoResp® Spiromax® and Symbicort® Turbuhaler® in the overall rate of errors made. However, the number of total errors and handling errors per patient was lower with DuoResp® Spiromax® compared with Symbicort® Turbuhaler®. Overall, 79.5% of patients in this study preferred DuoResp® Spiromax® over Symbicort® Turbuhaler® as their first option, finding it easy to learn how to use, easy to prepare and to use and comfortable in terms of weight and size.
Disclosure of interest
JGD has received fees and support for presentations from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, Teva Pharmaceuticals, and Menarini. VP in the last 3 years received honoraria for speaking at sponsored meetings from AstraZeneca, Chiesi and Novartis. Received travel support from AstraZeneca, Chiesi and Novartis. Acted as a consultant for ALK, AstraZeneca, Boehringer Ingelheim, Mundipharma and Sanofi. And received funding/grant support for research projects from a variety of Government agencies and not-for-profit foundations, as well as AstraZeneca, Chiesi and Menarini. MVC and JAS are employees of Teva Pharmaceuticals Spain. OP is an employee of Teva Pharmaceuticals. LCH is an employee of Dynamic Science.
Supplemental Material
Download MS Word (29.7 KB)Acknowledgments
We thank Dynamic Science (Spain) for its participation in the project management, monitoring, data management and statistical analysis of this study.
Supplementary data
Supplemental data for this article can be accessed here.
Data availability statement
The data that support the findings of this study are available from Teva Pharmaceuticals Spain upon reasonable request. Please contact [email protected] with any enquiries.
Additional information
Funding
Notes on contributors
Jordi Giner
Jordi Giner, RN, MsC in Respiratory Medicine, PhD. working in the Respiratory Medicine Service of the Santa Creu i Sant Pau Hospital in Barcelona (Spain); Author of the Spanish Asthma Guide (GEMA); GEMA inhalers coordinator; Coordinator of the Social Communication Committee of the Spanish Respiratory Society (SEPAR).
Marta Villarnovo Cerrillo
Marta Villarnovo, BSc, MSc, PhD, is currently Medical Manager at Teva Pharma Spain. Before that she held several positions in the pharmaceutical industry. She earned her Master´s Degree in Biotechnology from International Business School Aliter, Madrid, Spain and her doctoral degree with the field of respiratory research at the University of A Coruña (Spain).
Jaime Aboín Sierra
Jaime Aboín Sierra, BSc, MSc, is currently Medical Manager at Teva Pharma Spain. Before that he held several positions in the pharmaceutical industry. He earned his Master´s Degree in Biotechnology from International Business School Aliter, Madrid, Spain.
Laura Casas Herrero
Laura Casas Herrero, BSc., is currently data analysis and biostatistician at the Clinical Research Organizations Dynamic for 10 years. Earlier she has worked as a biostatistician at the Clinical Research Organizations Biometrica. She earned his Degree from Complutense University, Spain.
Oliver Patino
Oliver Patino, MPH, MA, is currently the Director of Scientific Communications and respiratory area lead of Teva Pharmaceuticals, Europe. He has held various positions in the pharmaceutical industry in R&D and Medical Affairs for the last 20 years. Obtained his Master’s degree in Public Health at the Université Libre de Bruxelles (ULB), Brussels, Belgium and Master in Health Social Science at De La Salle University, Manila. He has held teaching positions in Behavioural Sciences in universities and as an associate researcher.
Vicente Plaza
Vicente Plaza, MD, PhD is currently: Director of the Medicine Respiratory Department of the Santa Creu i Sant Pau Hospital in Barcelona (Spain); Professor of Medicine at Universitat Autonoma de Barcelona (UAB); Coordinator of the Executive Committee of the Spanish Asthma Guidelines (GEMA); and Director of the Teach and Learning Committee of the Spanish Respiratory Society (SEPAR).
References
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5:691–10.
- Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18: 1269–1278.
- Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11:404–406.
- Global Initiative for Asthma. Global strategy for asthma management and prevention, 2018. [cited 2019 Jul 4]. Available from: www.ginasthma.org
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2018. [cited 2019 Jul 4]. Available from: www.goldcopd.org/gold-reports/
- Al-Jahdali H, Ahmed A, Al-Harbi A. Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy Asthma Clin Immunol. 2013;9: 8.
- Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the aerosol drug management improvement team. Respir Med. 2006;100:1479–1494.
- Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10.
- Aydemir Y. Assessment of the factors affecting the failure to use inhaler devices before and after training. Respir Med. 2015;109:451–458.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938.
- van Beerendonk I, Mesters I, Mudde AN, et al. Assessment of the inhalation technique in outpatients with asthma or chronic obstructive pulmonary disease using a metered-dose inhaler or dry powder device. J Asthma. 1998;35:273–279.
- Chapman KR, Love L, Brubaker H. A comparison of breath-actuated and conventional metered-dose inhaler inhalation techniques in elderly subjects. Chest. 1993;104:1332–1337.
- Chrystyn H, Haahtela T. Real-life inhalation therapy – inhaler performance and patient education matter. Eur Respir Dis. 2012;8(1):11–18.
- Giraud V, Allaert FA, Roche N. Inhaler technique and asthma: feasibility and acceptability of training by pharmacists. Respir Med. 2011;105:1815–1822.
- Price D, Bosnic-Anticevich S; Briggs A (Inhaler Error Steering Committee). Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107:37–46.
- Brocklebank D, Ram F, Wright J. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess. 2001;5:1–149.
- O’Connor BJ. The ideal inhaler: design and characteristics to improve outcomes. Respir Med. 2004;98(Suppl 1):S10–S16.
- Atkins PJ. Dry powder inhalers: an overview. Respir Care. 2005;50:1304–1312.
- Symbicort turbohaler 200/6 inhalation powder summary of product characteristics. Revised 2018 [cited 2019 Jul 4]. Available from: https://www.medicines.org.uk/emc/product/1327/smpc
- Canonica GW, Arp J, Keegstra JR. Spiromax, a new dry powder inhaler: dose consistency under simulated real-world conditions. J Aerosol Med Pulm Drug Deliv. 2015;28:309–319.
- DuoResp Spiromax Summary of product characteristics. Revised 2017 [cited 2019 Jul 4]. Available from: https://www.medicines.org.uk/emc/product/3324/smpc
- GEMA. Spanis Asthma Guidelines. Guía Española para el Manejo del Asma (GEMA 4.0). Arch Bronconeumol. 2015 [cited 2019 Jul 4];51(S1):1–68. Available from: http://www.gemasma.com
- GesEPOC. Spanish COPD guidelines (GesEPOC). 2017. Pharmacological treatment of stable chronic obstructive pulmonary disease. [cited 2019 Jul 4]. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0300289617300844
- Voshaar T, Spinola M, Linnane P, et al. Comparing usability of NEXThaler® with other inhaled corticosteroid/long-acting beta2-agonist fixed combination dry powder inhalers in asthma patients. J Aerosol Med Pulm Drug Deliv. 2014;27:363–370.
- Armitage JM, Williams SJ. Inhaler technique in the elderly. Age Ageing. 1988;17:275–278.
- Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients; time for re–evaluation? Age Ageing. 2007;36:213–218.
- Nguyen TS, Nguyen TLH, Van Pham TT, et al. Pharmacists’ training to improve inhaler technique of patients with COPD in Vietnam. Int J Chron Obstruct Pulmon Dis. 2018;13:1863–1872.
- Murray B, O’Neill M. Supporting self-management of asthma through patient education. Br J Nurs. 2018;27:396–401.
- Geryk LL, Roberts CA, Carpenter DM. A systematic review of school-based interventions that include inhaler technique education. Respir Med. 2017;132:21–30.
- Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5:1071–1081.e9.
- Capanoglu M, Dibek Misirlioglu E, Toyran M, et al. Evaluation of inhaler technique, adherence to therapy and their effect on disease control among children with asthma using metered dose or dry powder inhalers. J Asthma. 2015;52:838–845.


![Figure 2. Patient-reported outcomes (patient responses to questions relating to the experience of using each device [%])](/cms/asset/c2353806-a8c5-46c6-8b38-33b74ad89e67/zecr_a_1833411_f0002_oc.jpg)