ABSTRACT
Background: The claustrum (CLA) has been discussed as central to integrated conscious percepts, although recent evidence has emphasized a role in detecting sensory novelty or in amplifying correlated cortical inputs.
Objective: We report that many neurons in the macaque CLA are ensheathed in perineuronal nets (PNNs), which contribute to synaptic stability and enhance neuronal excitability, among other properties.
Design: We visualized PNNs by Wisteria floribunda agglutinin (WFA) immunohistochemistry, and quantified these in comparison these to parvalbumin+ (PV) subsets and total neurons.
Results: PNNs ensheath about 11% of the total neurons. These are a range of large, medium, and small neurons, likely corresponding to PV+ and/or other inhibitory interneurons. The PNNs were themselves heterogeneous, consisting of lattice-like, weakly labeled, and diffuse subtypes, and showed some regional preference for the medial CLA.
Conclusion: The abundant neuronal labeling by PNNs in the CLA suggests an important and nuanced role for inhibition, consistent with recent physiological studies of claustrocortical circuitry. For comparison, diversified inhibition in the reticular nucleus of the thalamus (a pan-inhibitory nucleus, with extensive cortical input) exerts a spectrum of control at different local and global spatiotemporal scales. Further investigation of PNN+ neurons in the macaque CLA offers a potentially important new approach to CLA function, relevant to the human brain both in normal and diseased conditions.
Introduction
Multiple studies have reported the neuronal composition of the claustrum (CLA) and the species-specific and regional distribution of excitatory and inhibitory cell types (Citation1–Citation3). Neurons have been grouped on the basis of soma size (large:30–50 µm; medium: 15–30 µm; and small:10–15 µm, longest axis] and dendritic shape, in addition to neurochemistry.
In this communication, we report that some neurons in the macaque CLA are associated with perineuronal nets (PNNs), as visualized by staining for the lectin Wisteria floribunda agglutinin (WFA). PNNs are aggregates of extracellular matrix molecules that surround a subset of neuronal somata and, often, the proximal dendrites (Citation4–Citation7). The appearance of PNNs has been implicated in ending critical periods of synaptic plasticity in early development (Citation8,Citation9). Their widespread and persistent occurrence throughout the brain, however, supports wider roles; for example, in the modulation of inhibitory responses (Citation7). This would be compatible with proposals that the CLA can function in detecting novel sensory stimuli, directing attention, and setting behavioural states (see references in 10). Thus, the identification of PNNs in the macaque CLA is potentially important for further considerations of CLA function, as well as for cross species comparisons in the adult and in development. Previous reports list the CLA as containing PNNs in the rat (-I in (Citation11)) and human ( in (Citation12)) but only briefly, as part of a larger survey.
Fig. 1. Coronal sections reacted for NeuN (a) and WFA (b). The black bar in (b) is an artefact from the stitching process. Higher magnification (from *) illustrates the comparative density of neurons as labeled by NeuN (c) and WFA (d). Representative histology sections for NeuN (at left) and WFA (e) indicate the approximate anterior-posterior level (rightmost = more anterior). Scale bars: (c, d) = 100 µm; (e) = 1.0 cm.
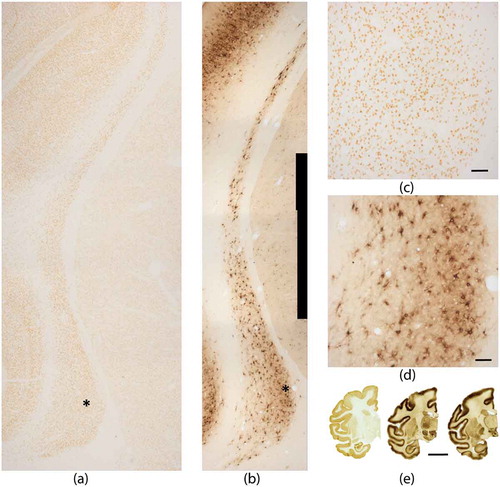
Fig. 2. Three representative ROIs for NeuN (a), WFA (b), and PV (c). Scale bar: 50 µm. The average number of neurons as calculated per mm2 (based on six ROIs per group) is shown in the bar graph (d).
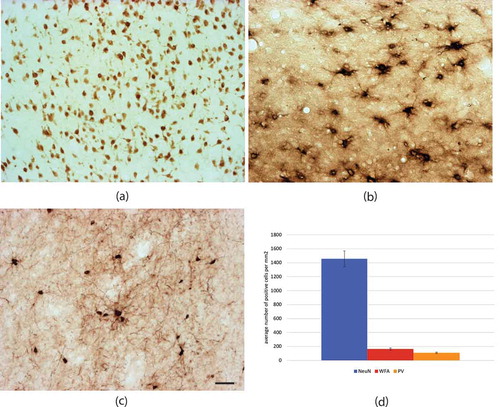
Fig. 3. Differential medial-lateral density of WFA+ neurons in ventral region. (a) Representative histological image, with superimposed counts. (b) Bar plot of number of WFA+ neurons (y-axis) in the medial and lateral sectors, for five sections. The medial sector has a significantly higher number of cells than lateral (p < 0.01). Medial (M) = red; lateral (L) = blue; V = ventral. Scale bar = 50 µm.
Although some excitatory neurons are enwrapped by PNNs, these more commonly are associated with parvalbumin-positive (PV+) and other inhibitory neurons (Citation13–Citation16). A large proportion of the neurons in the reticular nucleus (RT) of the thalamus, for example, are associated with PNNs, so that this structure is one of the most darkly stained regions. Neurons in RT are all inhibitory (Citation17).
Here, we report the occurrence of PNNs in the macaque CLA in relation to the total number of neurons, as visualized by immunohistochemistry for the pan-neuronal label NeuN. Since PNNs have been preferentially associated with PV+ neurons, we further compared morphological parameters between WFA-expressing neurons and PV+ neurons. Correlative comparisons are included for the subpopulation of neurons expressing NADPH-diaphorase (NADPH-d). Results are based on archival tissue.
Materials and methods
Animals
Five young adult rhesus macaques were used in this study. All procedures were in accord with institutionally approved protocols from the University of Iowa (for WFA), RIKEN Brain Science Institute, Japan (for PV), or Boston University School of Medicine (for NeuN). All monkeys were deeply anesthetized with ketamine (11 mg/kg i.m.) and nembutal (overdose, 75 mg/kg, i.p.), and perfused transcardially with saline and 4% paraformaldehyde, subsequently washed out with 0.1M phosphate buffer with 10, 20, and 30% sucrose (Citation18,Citation19). Brains designated for NeuN were postfixed with no wash-out (Citation20). Brains were removed, bisected through the corpus callosum, and left in 30% sucrose until sinking (1–2 d). Brains were subsequently sectioned in the coronal plane at 50 µm on a freezing microtome (but: 30 µm for NeuN), and collected in a repeating series of 3 for WFA, PV, or NADPHd, and a repeating series of 10 for NeuN (respectively, 150 µm or 300 µm inter-section interval).
We designated the WFA material (n = 1 monkey) as the reference series. This series included the posterior CLA through to the level of the mid-amygdala (16 sections or 2.4 mm). NeuN reacted sections (n = 2 monkeys), PV reacted sections (n = 1 monkey), and NADPH-d reacted sections (n = 1 monkey) were matched to approximately the equivalent anterior-posterior level, using the hippocampal-amygdala transition and other landmarks. As all material was archival, used in previous studies (18–20], we were not able to carry out further screens or double labels.
Histochemistry
For WFA
Sections were collected in 0.05M Tris buffered saline (TBS), preincubated in 0.05M TBS with 0.5% Triton-X (TBS-X) (90–120 min, with rocking), and incubated overnight at room temperature in biotinylated WFA (Sigma, St. Louis, MO) in TBS-X (1:200). After rinsing, sections were transferred to avidin biotinylated horseradish peroxidase solution (ABC; Vector Labs, Burlingame, CA) for 3–5 h (room temperature), and, after further rinsing, finally reacted in 0.55 mM 3-3ʹ diaminobenzidine (DAB; Sigma, St. Louis, MO) with H2O2 (26 µl per 80 ml).
For NeuN
Sections were collected and rinsed in 0.05M TBS, incubated for 48 h at 4°C in a mouse anti-NeuN IgG (1:10,000; MAB 377, Chemicon, Temecula, CA) in 0.05M TBS containing 2% normal goat serum and 0.1% Triton-X. With further rinses, this was followed by a 2-h incubation in secondary antibody (goat anti-mouse, 1:600, Vector, Burlingame, CA), a 1-h incubation in ABC elite kit (Vector, Burlingame, CA), and a final visualization in 0.55 mM DAB (Sigma, St. Louis, MO) with 0.01% H202 (Citation20).
For PV
This was closely similar to reactions for NeuN, except that we used 0.1M phosphate buffered saline, with 0.5% Triton-X. PV neurons were visualized by sequential incubations in anti-PV monoclonal mouse antibody (1:50,000; Swant, Marly, Switzerland), anti-mouse IgG polyclonal goat antibody (1:200, Vector, Burlingame, CA), ABC elite kit (Vector, Burlingame, CA), and DAB with 0.01% H2O2 and 0.03% nickel ammonium sulfate (see 18).
For NADPH-d
Substrate solution contained, per 50 ml of TB (pH 7.4), 400 µl Triton-X, 40 mg NADPH (Sigma, St. Louis, MO), 35 mg Nitroblue tetrazolium (Sigma), and 60 mg sodium malate (Fisher Scientific). Free-floating tissue sections were incubated in eight-compartment circular holders at 37° until the desired intensity of staining was obtained, usually about 45–90 min. Degree of staining was evaluated by viewing wet tissue under a dissecting microscope (see 19).
Once reactions were complete, sections were mounted onto glass slides, dried, and subsequently dehydrated and coverslipped.
Identification
The borders of the CLA were determined by contrast with the low signal or background staining of the adjoining white matter (WM; ). Nissl stained sections from other brains were available for further guidance. In the case of ambiguity (for example, a small isolated cell island ventral to the main portion of the CLA), we adopted a conservative standard and restricted counts only to the main body of the CLA.
Quantification
1) Computer-assisted quantitative analyses were performed using ImageJ/FIJI software. The total number of WFA+ neurons throughout the CLA was manually tallied from 12 sections. These were digitized at low magnification (5x objective), and then images were stitched using the ImageJ Grid/Collection plugin (Citation21). Images were zoomed to about 3x further magnification for better visualization. 2) Comparative neuronal densities were obtained by sampling from three ROIs in the wider, ventral third of the CLA, from two sections in each series (NeuN, WFA, PV). ROIs (measuring 0.52 mm × 0.70 mm) were outlined at low magnification (5X objective), and re-photographed with a 20x objective for quantification. We followed general stereological principles, in that neurons that were touching lower and right edges of ROIs were not counted. Neurons were counted at one focal plane, set at the mid portion of the section. NeuN stained sections were used to obtain total neuron density, to which we compared the WFA and PV subpopulations.
In a further quantitative measure, 3) we compared the distribution of WFA+ cells in the medial and lateral portions of the CLA. Medial and lateral zones were distinguished by reference to density of WFA+ cells. All WFA+ cells were counted (n = 5 sections), and the area of the two ROIs was calculated using ImageJ. To establish the density of WFA+ cells in each portion, we divided the scored number of cells by the area.
Photomicrography
Sections were digitalized using a Zeiss Axioskop 2plus microscope with an AxioCam HRc camera. Images were assembled in Zeiss Zen 2.0, ImageJ/FIJI 1.5, Adobe Photoshop CC 2018, and Adobe Illustrator CC 2018.
Results
Proportion of WFA+ neurons
Total counts of WFA+ neurons were obtained from 12 sections; namely 181 (level of the anterior lateral geniculate nucleus), 209, 279, 305, 359, 388, 406, 415, 422, 505, 589, and 597 (level of mid-amygdala). Qualitatively, the WFA+ population appeared to be much less than the total number of neurons, as visualized by NeuN (). To obtain a quantitative estimate of the proportion of WFA+ neurons, we counted neurons in three equivalent sampling boxes (0.52 mm × 0.70 mm) from two sections for each population (). With this protocol, for NeuN, we obtained 487, 587, 544 neurons (section 1) and 483, 518, and 561 neurons (section 2), or an average of 530 neurons +/-42.0. The equivalent numbers for WFA+ neurons were 56, 57, 69 (section 1); 56, 64, 55 (section 2), or an average of 60 neurons +/-6.0. On this basis, WFA+ neurons are about 11% of the total neuronal population. This is in accord with previous findings, that ~10% of cortical neurons in the macaque are surrounded by PNN (Citation10).
To assess the proportion of WFA+ neurons in relation to PV+ neurons, we carried out counts for three equivalent sampling boxes in two sections reacted for PV. Numbers were 41, 34, 40 (section 1); 42,41, 39 (section 2); or an average of 40 neurons +/- 3.0, slightly less than the number of WFA+ neurons. We did not quantify NADPH-d neurons, since these are only sparsely distributed.
Qualitative observation suggested a differential distribution of WFA+ neurons, with a preference for the medial CLA ( and ). To confirm this bias, we counted WFA+ neurons in five sections. Consistently, the lateral region had fewer neurons per mm2 (lateral 83 cells, medial 117; lateral 74, medial 133; lateral 99, medial 170; lateral 124, medial 214; lateral 126, medial 231, p < 0.01). A similar bias was not evident in NeuN in our material. Both labels, however, showed local inhomogeneities, with small, relatively cell-free gaps (; ), which could be further analysed in serial sections without gaps or in thicker, 90–100 µm tissue slabs.
Morphometrics
Soma
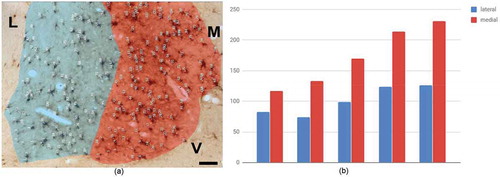
On the basis of soma size, large, medium, and small neurons have consistently been distinguished (Citation1–Citation3). Consistent with these reports, our WFA material had large (30–50 µm longest axis), medium (15–30 µm longest axis), and small (10–15 µm longest axis) neurons. In addition, a very small (‘tiny’) subpopulation was identified (<10 µm longest axis). A similar range was ascertained for PV+ neurons, except that there may have been more small and ‘tiny’ neurons ( and ).
Fig. 4. Further examples of WFA+ neurons to show heterogeneity of size, shape, and type of PNNs. Arrow in (a) and, at higher magnification, (b) indicates a cluster of six small neurons. The asterisk in (a) indicates a WFA+ neuron in the white matter between the CLA and caudate. (c) Small and medium neurons are intermingled. Arrows in (d) indicate two of the four visible WFA-ensheathed dendrites from a medium size neuron. Scale bars: 50 µm.
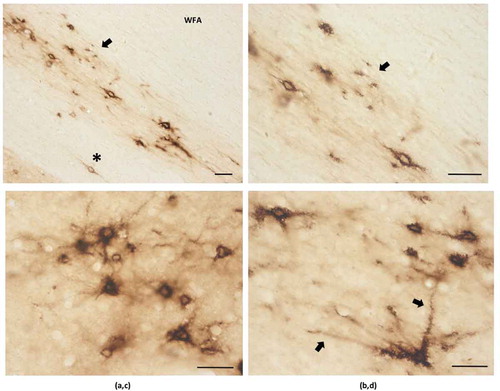
Fig. 5. Immunohistochemical staining for WFA (a–c), PV (d–f), and NADPH-d (g–i). Dorsal is at the top, except for the WFA images, which have been rotated with dorsal to the right. Examples of small neurons are indicated by arrows, including (d) a closely adjacent pair. CD = caudate. Scale bars: (a, d, and g) = 200 µm; otherwise, 50 µm.
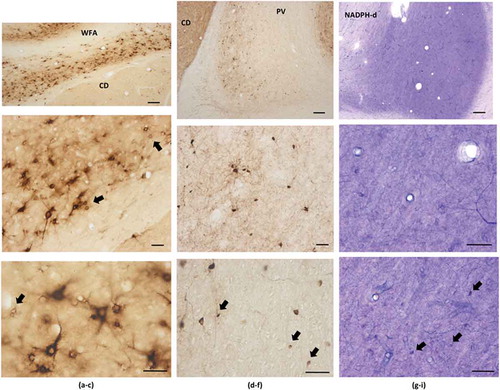
Previous screens for NADPH-d or calretinin have also reported a range of large, medium, and small neurons (Citation22,Citation23). In accord, our NADPH-d material had a few large neurons (>20 µm longest axis), as well as a larger number of small (d = 10 µm or less) faintly stained neurons. Solid ‘Golgi-like’ filling has been defined as type 1 while fainter, more diffuse staining has been considered type 2. Both types were found in our material, although the small to tiny neurons were mainly type 2 ().
Dendritic extent and morphology of PNNs
For a subset of neurons, WFA staining extended into the proximal dendrites. This was for at least 150 µm from the soma (as assessed in single sections). For PV+ neurons, dendritic visualization of 300–500 µm from the soma were common ( and ).
PNNs have been subdivided as lattice-like, weakly labeling, and diffuse (Citation24). Examples of these were easily found in our material ( and ). Small, round neurons were predominantly associated with rim-like PNNs, while medium and large neurons had PNNs that were often sculptured, but could also be rim-like or diffuse. We did not find PNNs in association with thin, narrow CLA neurons, although these could be indistinguishable from WFA+ dendritic segments. WFA+ neurons were typically in isolation, but clusters of two or more closely adjacent neurons could also be found ( and ).
Discussion
Several conclusions follow from the presence of PNN+ neurons in macaque CLA. 1) WFA+ neurons are morphologically heterogeneous; 2) As WFA+ neurons are likely to be inhibitory, the abundance of PNNs hints at a special role for inhibitory processes; 3) Since PNNs have also been reported in rodent CLA, this feature is phylogenetically conserved (Citation1–Citation3,Citation11); the first two statements support the general conclusion that PNNs add an important level of complexity to the operation of CLA circuitry.
PV and PNN
Multiple studies demonstrate that PNNs are preferentially associated with fast-spiking neurons immunoreactive for the Kv3.1b potassium channel (Citation25,Citation26). PNNs are discussed as rapid local buffers of excess cation changes in the extracellular space (Citation25–Citation28), in accord with the idea that PNNs provide protection against oxidative stress (Citation7).
Quantitative data from other brain regions suggest there will be only a partial correspondence of claustral PV+ neurons with PNNs. In the rat basolateral nucleus of the amygdala, all PNN ensheathed neurons were PV+; but that population, of PNN+PV+ neurons, comprised only 60% of all PV+ interneurons. Similar results were obtained for calbindin+ neurons in the amygdala (Citation16). In the mouse hippocampus, PNNs surround 90% of PV+ basket cells, 25–50% of PV+ bistratified cells, but only minor proportions of other PV+ cell types (Citation6,Citation29). In the human reticular nucleus of the thalamus, 63.4% of WFA/PNNs are associated with PV+ neurons; but only 20.5% of PV+ neurons are enwrapped by WFA/PNNs (Citation17).
NADPH-d and PNNS
Neurons positive for NADPH-d have been previously reported in the CLA (Citation1–Citation3,Citation22,Citation23). Consistent with these studies, our material showed densely stained, type 1 neurons together with small, lightly stained type 2 neurons. The small neurons were similar in size to the small WFA enwrapped neurons and small PV+ neurons, but are likely to be a distinct non-PV+ population. Additional material is needed to determine if the small NADPH-d neurons are associated with PNNs.
Significance for CLA circuitry
Do these results tell us anything about the still mysterious functioning of the CLA? At the level of biophysics, PNNs have been discussed in the context of differential excitability, and fast and precise sensory transmission (Citation28), possibly related to activity-dependent modulation (Citation8). Non-synaptic effects also might be considered, such as stabilization of gap junctions. There could be influences on transmitter diffusion, such as might interact with spatial diffusion of nitric oxide. None of these are necessarily CLA-specific, but would serve to enhance circuit complexity.
At the cellular level, PNNs have provided an additional, functionally significant way to subdivide and characterize diverse PV+ (and similarly, calbindin+ or calretinin+) neurons. Significantly, PV+ basket cells in the mouse hippocampus that express brevican receive more excitatory synapses and are also distinguishable on the basis of distinctive intrinsic properties (Citation15). Those positive for brevican were less excitable and showed lower input resistance. They also seemed better tuned to operate at higher spiking frequencies, displaying higher maximum firing frequency, less spike frequency adaptation, narrower action potential half-width, and an earlier fast after-hyperpolarization (Citation15). Again, these properties are not CLA-specific but might enhance circuit complexity in a CLA-specific way.
The differential medial bias of WFA+ neurons in our material is of interest in relation to the topographic arrangement of its connections, i.e. prefrontal and association cortices are reported to receive connections largely from anterior and medial CLA (in capuchin (Citation30):; marmoset (Citation31):; macaque (Citation32):). Comparable cytoarchitectonic non-uniformity has not so far been reported (Citation1–Citation3), but some indication of a medial bias in the ventral enlargement of CLA was noted after in situ hybridization for Netrin-G2 ( in (Citation33)). Miyashita et al. (Citation33), in contrast with the present results, illustrate a NeuN preparation with greater overall neuronal density medially in the ventral enlargement (). This may reflect a species difference between M. mulatta (present report) and M. fuscata (Citation33). At a finer scale, the issue of internal compartments or local heterogeneity in the CLA remains to be investigated.
What we have called the ‘ventral enlargement’ of the CLA is commonly accepted as an integral part of the nucleus in primates (Citation34). This contrasts with the more ambiguous situation in rodents, where there is considerable discussion concerning the dorsal CLA and the ventrally contiguous but, on various grounds, separate endopiriform nucleus (Citation3,Citation34).
At the connectivity level, several recent papers on rodents highlight the particular importance of inhibition in CLA processing. In (Citation35), the authors concluded that cortical inputs from the anterior cingulate cortex to the mouse CLA encode a behaviorally relevant anticipatory top-down signal and that cortical input is importantly re-shaped by complex CLA microcircuitry. Cortical inputs monosynaptically target both glutamatergic projection neurons and inhibitory interneurons, with the interneurons thought to constrain a glutamate-mediated amplification. CLA inputs to frontal cortex were associated with strong and long-lasting feedforward inhibition of cortical activity (Citation36). Kim et al. (Citation37) report stronger responsivity to cortical input from PV+ neurons in the CLA, as well as a high degree of PV-PV interconnectivity by both chemical and electrical synapses.
Crick and Koch (Citation38) emphasized that the CLA might act as a ‘Cartesian theatre,’ with an especially major role in consciousness. They based this conjecture largely on the pattern of abundant and reciprocal claustral-cortical connections, a specialization permitting ‘information to travel widely within [the] anterior-posterior and ventral-dorsal extent to synchronize different perceptual, cognitive, and motor modalities’ [see also (Citation39,Citation40) The inhibitory web of intrinsic connectivity – with gap junctional couplings, diffusion of nitric oxide, and differentially positioned PNNs (Citation41,Citation42) – is another intricate layer of complex processing. This is not incompatible with ideas that the CLA figures in the detection of sensory novelty or in the amplification and coordination of correlated cortical inputs [as summarized in (Citation10)]. As has been proposed for RT (Citation43–Citation45), diversified inhibitory systems could dynamically enable multiple scales of action in a wide variety of functions.
Summary and future directions
The identification of PNNs in the macaque CLA potentially provides a new avenue for investigating CLA function at the cellular and network level. The distinction of two broad categories (PV+PNN+ or PV+PNN-), likely to correlate with a distinguishable physiological profile [as per (Citation15), allows for finer dissection of microcircuitry as well as the exploration of changes under different developmental, pathological, or experimental conditions.
Among the obvious next steps would be 1) a more comprehensive survey, by double labeling, of PNNs in relation to PV, CB, CR, NADPH-d, and glutamatergic populations, across the full extent of the CLA; and 2) a comparison of different types of PNNs. As a functional gauge, one thinks of combined experiments with cFOS activation or, conversely, enzymatic degradation of PNNs in slice (e.g. 28) or in vivo (Citation46).
Acknowledgments
The authors declare they have no competing interests. MP and KSR designed the experiment, collected data, and discussed the results. MP constructed the figures and KSR wrote the manuscript. We would like to thank Michael Masset for help with figure preparation, and Dr. Farzad Mortazavi for sharing Neu-N reacted tissue. We thank the NIH for partial funding support (MH107456).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Edelstein LR, Denaro FJ. The claustrum: A historical review of its anatomy, physiology, cytochemistry and functional significance. Cell Mol Biol. 2004;50:1–9.
- Baizer SJ. The Neurochemical organization of the claustrum (Chapter 3). In: Smytheis J, Edelstein LR, Ramachandran V, eds. The claustrum. Amsterdam: Elsevier, Academic Press; 2014. p. 85–118.
- Baizer JS, Sherwood CC, Noonan M, et al. Comparative organization of the claustrum: what does structure tell us about function? Front Syst Neurosci. 2014;8:article 117.
- Morawski M, Bruckner G, Arendt T, et al. Aggrecan: beyond cartilage and into the brain. Int J Biochem Cell Biol. 2012;44:690–693.
- Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–160.
- Yamada J, Jinno S. Subclass-specific formation of perineuronal nets around parvalbumin-expressing GABAergic neurons in Ammon’s horn of the mouse hippocampus. J Comp Neurol. 2015;523:790–804.
- Mueller AL, Davis A, Sovich S, et al. Distribution of N-acetylgalactosamine-positive perineuronal nets in the macaque brain: anatomy and implications. Neural Plast. 2016;2016:1–19.
- Lorenzo Bozzelli P, Alaiyed S, Kim E, et al. Proteolytic remodeling of perineuronal nets: effects on synaptic plasticity and neuronal population dynamics. Neural Plast. 2018;2018:1–13.
- Pizzorusso T, Medini P, Berardi N, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251.
- Brown SP, Mathur BN, Olsen SR, et al. New breakthroughs in understanding the role of functional interactions between the neocortex and the claustrum. J Neurosci. 2017;37:10877–10881.
- Bertolotto A, Manzardo E, Guglielmone R. Immunohistochemical mapping of perineuronal nets containing chondroitin unsulfated proteoglycan in the rat central nervous system. Cell Tissue Res. 1996;283:283–295.
- Morawski M, Bruckner G, Jager C, et al. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer’s disease. Neuroscience. 2010;169:1347–1363.
- Alpar A, Gartner U, Hartig W, et al. Distribution of pyramidal cells associated with perineuronal nets in the neocortex of rat. Brain Res. 2006;1120:13–22.
- Rossier J, Bernard A, Cabungcal JH, et al. Cortical fast-spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry. 2015;20:154–161.
- Favuzzi E, Marques-Smith A, Deogracias R, et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron. 2017;95:639–655 e610.
- McDonald AJ, Hamilton PG, Barnstable CJ. Perineuronal nets labeled by monoclonal antibody VC1.1 ensheath interneurons expressing parvalbumin and calbindin in the rat amygdala. Brain Struct Funct. 2018;223:1133–1148.
- Steullet P, Cabungcal JH, Bukhari SA, et al. The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry. 2017;1–9. DOI:10.1038/mp.2017.230.
- Ichinohe N, Rockland KS. Region specific micromodularity in the uppermost layers in primate cerebral cortex. Cereb Cortex. 2004;14:1173–1184.
- Rockland KS, Nayyar N. Association of type I neurons positive for NADPH-diaphorase with blood vessels in the adult monkey corpus callosum. Front Neural Circuits. 2012;6:article 4.
- Mortazavi F, Wang X, Rosene DL, et al. White matter neurons in young adult and aged rhesus monkey. Front Neuroanat. 2016;10:article 15.
- Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–1465.
- Hinova-Palova DV, Edelstein L, Landzhov B, et al. Topographical distribution and morphology of NADPH-diaphorase-stained neurons in the human claustrum. Front Syst Neurosci. 2014;8:article 96.
- Landzhov B, Hinova-Palova D, Edelstein L, et al. Comparative investigation of neuronal nitric oxide synthase immunoreactivity in rat and human claustrum. J Chem Neuroanat. 2017;86:1–14.
- Wegner F, Hartig W, Bringmann A, et al. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184:705–714.
- Hartig W, Derouiche A, Welt K, et al. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29.
- Cabungcal JH, Steullet P, Morishita H, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–9135.
- Faralli A, Dagna F, Albera A, et al. Modifications of perineuronal nets and remodelling of excitatory and inhibitory afferents during vestibular compensation in the adult mouse. Brain Struct Funct. 2016;221:3193–3209.
- Balmer TS. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro. 2016;3:1–13.
- Yamada J, Jinno S. Molecular heterogeneity of aggrecan-based perineuronal nets around five subclasses of parvalbumin-expressing neurons in the mouse hippocampus. J Comp Neurol. 2017;525:1234–1249.
- Reser DH, Richardson KE, Montibeller MO, et al. Claustrum projections to prefrontal cortex in the capuchin monkey (Cebus apella). Front Syst Neurosci. 2014;8:123.
- Reser DH, Majka P, Snell S, et al. Topography of claustrum and insula projections to medial prefrontal and anterior cingulate cortices of the common marmoset (Callithrix jacchus). J Comp Neurol. 2017;525:1421–1441.
- Gamberini M, Passarelli L, Bakola S, et al. Claustral afferents of superior parietal areas PEc and PE in the macaque. J Comp Neurol. 2017;525:1475–1488.
- Miyashita T, Nishimura-Akiyoshi S, Itohara S, et al. Strong expression of NETRIN-G2 in the monkey claustrum. Neuroscience. 2005;136:487–496.
- Smith JB, Alloway KD, Hof PR, et al. The relationship between the claustrum and endopiriform nucleus: a perspective towards consensus on cross-species homology. J Comp Neurol. 2018. DOI:10.1002/cne.24537
- White MG, Panicker M, Mu C, et al. Anterior cingulate cortex input to the claustrum is required for top-down action control. Cell Rep. 2018;22:84–95.
- Jackson J, Karnani MM, Zemelman BV, et al. Inhibitory control of prefrontal cortex by the claustrum. Neuron. 2018;99:1029–1039.e4.
- Kim J, Matney CJ, Roth RH, et al. Synaptic organization of the neuronal circuits of the claustrum. J Neurosci. 2016;36:773–784.
- Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci. 2005;360:1271–1279.
- Smythies J, Edelstein L, Ramachandran V. Hypotheses relating to the function of the claustrum. Front Integr Neurosci. 2012;6:53.
- Goll Y, Atlan G, Citri A. Attention: the claustrum. Trends Neurosci. 2015;38:486–495.
- Bitanihirwe BK, Woo TU. Perineuronal nets and schizophrenia: the importance of neuronal coatings. Neurosci Biobehav Rev. 2014;45:85–99.
- Pantazopoulos H, Berretta S. In sickness and in health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plast. 2016;2016:1–23.
- Halassa MM, Acsady L. Thalamic Inhibition: diverse sources, diverse scales. Trends Neurosci. 2016;39:680–693.
- Hou G, Smith AG, Zhang ZW. Lack of intrinsic GABAergic connections in the thalamic reticular nucleus of the mouse. J Neurosci. 2016;36:7246–7252.
- Clemente-Perez A, Makinson SR, Higashikubo B, et al. Distinct thalamic reticular cell types differentially modulate normal and pathological cortical rhythms. Cell Rep. 2017;19:2130–2142.
- Lasek AW, Chen H, Chen WY. Releasing addiction memories trapped in perineuronal nets. Trends Genet. 2018;34:197–208.

