ABSTRACT
Archaeological research at Hoeke, a Late Medieval outer harbour of Bruges (Belgium), has revealed large quantities of iron slags, fuel and other remains of iron working. Archaeometrical study has provided an enhanced insight into the historic iron working process, a craft activity which had up till now remained completely unknown in one of the largest economic hubs of medieval Europe. Several petrological, mineralogical and geochemical analytical methods have been applied for this purpose. The metallographic analysis was performed using reflected light optical microscopy, while the mineralogical composition of the slags was characterized X-ray Diffraction (XRD) Spectrometry. Macroscopic identification of plano-convex bottom slag and hammerscales, combined with the geochemical data pointed out that the examined slag is indicating smithing activity, while no traces for iron ore melting were discovered.
Introduction
Slags are the main by-products of metal production and can be produced during both smelting and smithing processes (Serneels and Perret Citation2003). The first step of historical iron production was bloomery smelting, in which the iron compounds from the ore were reduced to metallic iron (Erb-Satullo and Walton Citation2017). This reaction took place in a furnace where the temperature was around 1100 °C, far below the melting point of iron. The product of this smelting procedure was a spongy, consolidated mass of iron and slag, called bloom. One of the characteristic features of smelting operations is the occurrence of tap slag. Tap slag was formed by material flowing out from the furnace through a hole at the side and normally it appears to have an oxidized thin layer of iron oxides on the surface exposed to air (Erb-Satullo and Walton Citation2017). In the further steps, iron bloom was reheated to high temperatures so that iron was transformed to the austenitic state (at around 900 °C) and fayalitic slag became molten (1200–1300 °C) (Pleiner Citation2000). The product thus obtained was subsequently subjected to further processing, such as hammering to expel the excess of slag, and shaped into a finished object (Buchwald and Wivel Citation1998). These two processes of reheating and hammering might have been repeated many times to ensure maximal expulsion of slag. The gangue minerals in the ore (mainly quartz and lime), reacted predominately with iron oxide and consequently formed slag (Blakelock Citation2009; Joosten, Jansen, and Kars Citation1998).
Since the chemical characteristics of a slag are closely related to the conditions in which iron was produced, the analysis of slags can provide crucial information on the technological aspects of the metal production, even when no evidence of mineral processing has been found on site.
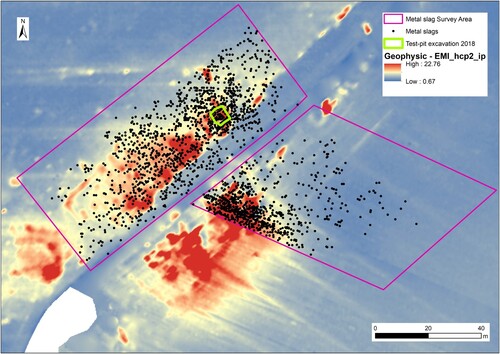
The iron slags studied here were retrieved during a test-excavation at Hoeke, one of the outer harbours of Bruges, located along the Zwin tidal inlet. The Zwin inlet was the artery which connected the medieval metropolis with the rest of maritime Europe (). Bruges’ economy and cultural thriving was only viable owing to a unique chain of small harbour towns, each of those taking up specific functions within the harbour system. Next to shipping and commercial activities, the small towns like Hoeke were economically also active on the production level. Geophysical survey within the territory of the lost harbour area of Hoeke which has been carried out over 12 ha by an Electromagnetic Induction sensor (EMI), followed by artefact accurate fieldwalking (Trachet et al. Citation2017) revealed the presence of large-scale iron working activities at the site, possibly related to the historically attested repair of ships at the site. Over an area of more than 1 ha, strong geophysical signals have been observed which spatially correlated with the find during fieldwalking of large quantities of iron slags on the surface (). The excavation of a 5 × 5 m test-pit positively validated the observations with in situ data by yielding large quantities of iron slag, hammer scales and other waste, found in layers deposited on the flank of a medieval embankment, corresponding with the geophysical signal. Historical sources also mention the presence of ironworking and the import of coal, in medieval times locally called “zeecoolen” (coal from the sea) or “smedecoolen” (smithy coal), at the site (Clercq et al. Citation2020). Although the outer harbour region of medieval Bruges can be considered as an important commercial, cultural and technological node in late medieval Europe, so far little in-depth analytical research has been conducted to examine metal production activities at this site and remarkably even to a wider extent, in the late medieval historic County of Flanders as a whole. The literature has mainly focused on the study of other archaeological remains, such as pottery and settlement remnants, neglecting a full archaeometallurgical study of metal wastes like slags. However, Windey (Citation2013) in his review on medieval iron working activities in Flanders, suggested a foremost small scale marginal activity (refinement and/or smithing) rather than large-scale iron production. The objective of the archaeometrical work at Hoeke, therefore, was to determine the chemical and mineralogical composition, and physical properties of the iron slag and to establish the type of iron-working (chaîne opératoire) represented. This paper presents the first results that can help to fill the gap in our knowledge on historical iron production in the medieval Harbour at Hoeke and in Flanders in general, and sets the background for future study.
Figure 1. The medieval harbour town of Hoeke was located in the centre of the Bruges outer harbour system, situated along the tidal inlet “Zwin”. (Copyright: Ghent University).
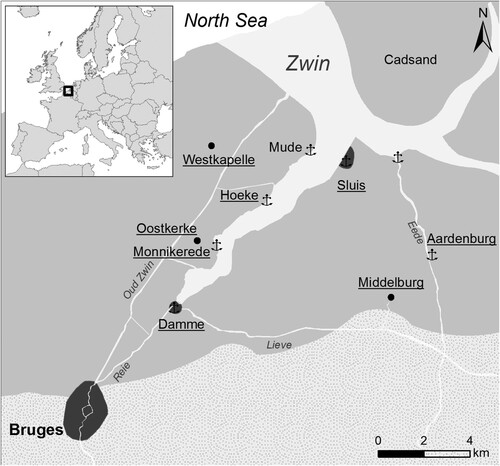
Figure 2. Location of the test-pit within the concentration of high electric values in geophysical EMI-survey and related to the scattering of iron slags found at the surface of the site. (Copyright: Ghent University).
Sample sets
The iron slags studied here were retrieved from the waste layers found in the 5 × 5 m test-pit at Hoeke. In total 250 slags were found, representing a total weight of 150 kg. The archaeological iron slags were first examined macroscopically, followed by different analyses using microscopic and geochemical methods applied to a selection of samples.
The slags were categorized according their morphological features and investigated as groups. Initially, the findings were categorized into three groups: iron-working slag (c. 95% of the total), Plano Convex-Bottom (PCB) slag (c. 2%) and slags rich in technical ceramic (c. 3%). Within the group consisting of the iron-working slags, two additional subgroups could be distinguished. The slags from the first sub-group (1) are more compact and dense ((A)), and are predominantly yellow to orange, without clear evidence of vesicles. They represent c. 46% of the total iron-working slag samples. The second sub-group (2) is characterized by samples with a dark brown colour with a slightly reddish tint and a porous surface ((B)), sometimes with an indication of vesicular structure ((F)). They make up c. 54% of the total number of iron-working slag samples. Most of the samples in the second sub-group have small inclusions of partially melted sand (quartz) on the surface ((E)). The samples from the subgroups have been labelled with number and letter annotations, hence the first sub-group (1) was divided into seven smaller groups, and in the second sub-group (2) nine smaller groups were distinguished ().
Figure 3. Photographs of iron production debris found at the Hoeke site. (A) is a sample representing the first (1) microscopical group, (B), is a sample representing the second (2) group, (C) represents a PCB slag, and (D) is an iron slag sample with technical ceramics attached to the surface. (E) shows an example of a slag with imprints of partially melted sand on the surface. The vesicular texture of the slag from the second (2) group is shown in F.
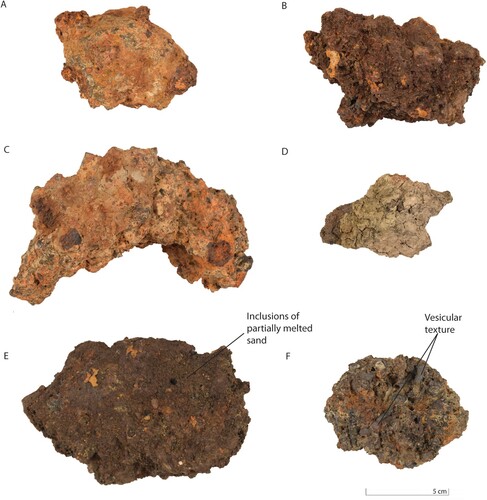
Besides the two sub-groups of iron-working slags, there were slags with technical ceramics (FL) attached to the surface (8 pieces) and Plano-Convex Bottom (PCB) slags (5 pieces). The slag pieces which were in contact with clay are usually vitrified, porous and irregularly shaped. The PCB slags are large and dense pieces, ranging in colour from grey/brown to yellow ((C)). They show localized magnetism. The average weight of a PCB slag is c. 600 g, with the largest one found in this study, weighing 1536 g. PCB slag is a very common by-product found at archaeological iron working sites, directly correlated to smithing activities. This type of material is usually produced by an accumulation of fused materials at the bottom of the hearth (Serneels and Perret Citation2003). (D) shows a sample with technical ceramics attached to one side of a slag.
Serneels and Perret Citation2003 distinguish three types of PCB slag. The first type SGD (scorie grise dense – dense grey slag) is mainly dominated by the presence of fayalite and a variable amount of iron oxide, and its composition is related to low-temperature melts of SiO2-FeOx. The second type of PCB slag is a material rich in silica compounds, but low in iron content and is labelled SAS for scorie argilo-sableuse (sand-clayey slag). These specimens are usually vitrified and may contain some grains of quartz (sand), which indicate the use of a flux. The last type, scorie ferreuse rouille or SRF (iron-rich rusty slag), is characterized by a high content of iron in different forms, such as metal particles, Fe-oxide and Fe-oxide-hydroxide particles. Occasionally, fayalite may be observed. Each of these types of PCB slag can be related to a specific stage of the smithy work. SGD material is produced in hot oxidation processes, for instance during heating of the iron piece for hot forging. SFR is created at very high temperature near to the melting point of iron. The silica-rich material (SAS) is, by contrast, produced by adding a large amount of flux during welding or after fashioning an object. Quartz is hereby used to minimize the occurrence of oxidation. Depending on the type of work, one piece of PCB can also be the result of a combination or a sequence of activities (Serneels and Perret Citation2003).
Additionally, other remains, such as stones, nails, and hammer scales (c. 0.5 cm in diameter), were found during the excavation. Hammerscales have been separated from the soil samples by using a handheld magnet. Nails and other small metal remnants were too rusty to be examined and gave no significant results. Overall, visual analysis did not provide an unambiguous answer as to at which step in the chaîne opératoire the slags have been involved. Macroscopically, several characteristics, such as the presence of PCB slag, the absence of evidence of ore, the occurrence of hammer scales on the site, may suggest that the slags are products related to smithing activity rather than to the smelting process (Dunster and Dungworth Citation2012). Further geochemical analysis was applied to evaluate this hypothesis.
Methods
The mineralogical composition of the slag samples and other debris was determined by using X-ray Diffraction (XRD) analysis. Additionally, metallographic analysis was performed on polished sections of the material by using reflected light optical microscopy.
A total of 34 samples were analysed by XRD, 16 of which were taken from subgroup 1, 14 from subgroup 2, 2 represented slags with attached technical ceramics to the surface and 2 belonged to the PCB group. A total of 12 samples were used for metallographic analysis, 5 of which from group 1 and 7 from group 2.
For the XRD analysis, the selected slag material was first cleaned with water and then subsamples were broken off in order to collect material from a fresh surface, which did not show traces of post-depositional processes and superficial weathering. Afterwards, the slags were crushed to gravel size by using a hammer and subsequently grinded to a fine powder by using a Retsch planetary ball mill for about 20 min. The powder was sieved at 100 µm (Retsch sieve) and placed in glass tubes.
XRD analyses were performed using a Philips PW3710 X-Ray diffractometer (current 30 mA, voltage 40 kV), equipped with a cobalt anode X-ray tube to minimize the effect of fluorescence, at a 2θ angle of 3° to 70°. The step size was set at 0.020° with a residence time per step of 2.5 s. The spectra obtained were analysed using the X’Pert Highscore software of PANalytical.
Mineralogical identification was also carried out using reflected light optical microscopy. A Nikon Eclipse Ni-E motorized microscope equipped with a Nikon DS-Ri2 camera was used. This approach provides information on the distribution of the mineral phases formed in the material and supplies details of the mineralogical composition and the internal textures. The samples were first cut using a circular saw blade and subsequently embedded in epoxy resin. Afterwards, the sections were polished using wet SiC abrasive papers of 1200, 2000 and 4000 grit (sequentially) and subsequently with 3 and 1 µm diamond paste. Etching with 14 M nitric acid and methanol (1:10 ratio) was applied for one sample with a clear band of iron.
Results
Mineralogical composition
The mineralogical composition was examined using both XRD and optical microscopy. XRD results () show that the main phases of the material studied are quartz (SiO2), which is omnipresent in all materials examined, and fayalite Fe2SiO4. This is followed by a complex mixture of iron oxides (wüstite FeO and mainly magnetite Fe3O4), silicates (ferrosilite Fe2Si2O6, laihunite Fe2+Fe3+2(SiO4)2), and other compounds, such as goethite α-FeO(OH) and lepidocrocite γ-FeO(OH). Representative examples of XRD patterns are displayed in . All XRD data are provided in supplemental Table S1. On the external surface of the slags, the main minerals dominantly observed were goethite, lepidocrocite and quartz. The samples with yellow patches on the surface have additional detectable amounts of iron oxide (wüstite) with a small amount of goethite, whereas samples with a dark brown colour mainly contain considerable amounts of goethite and lepidocrocite. These minerals are generally assumed to be alteration/weathering products of the original iron compounds (Dungworth Citation2015); (Kramar et al. Citation2015).
Figure 4. XRD patterns of three selected slags from Hoeke. Fa – fayalite, FeAl – iron aluminium silicate, W – wüstite, M – magnetite, Q – quartz, J – jarosite, An – anorthoclase.
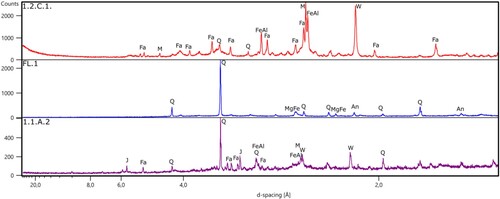
Table 1. Mineralogical composition based on the XRD results for the iron slag samples from Hoeke. S – surface, FL – clay attached, PCB – Plano Convex Bottom slag, HS – Hammer Scales.
The metallographic analysis indicated that all iron slag samples examined are very heterogenous. Based on microscopic observations, the slags were dominated by a combination of wüstite and magnetite, iron silicates (mainly fayalite) and organic matter unevenly dispersed within the matrix of the samples. This matrix is composed of siliceous minerals and amorphous material. Wüstite is present both as dendritic and/or subhedral crystals ((D, E)) and often as cotectic intergrowths with fayalite. Magnetite was observed as idiomorphic crystals, exsoluted from the siliceous matrix, and usually surrounded by wüstite or fayalite ((B, D)). Fayalite occurs in the shape of needles and skeletal shape and often forms spinifex textures ((C)) (Portillo-Blanco et al. Citation2020; (Hauptmann Citation2021, chap. 5); Hauptmann Citation2007). In most cases, these spinifex textures were randomly oriented and did not show any preferably direction of crystallization. Furthermore, some clusters of iron oxides are probably relict structures of flakes of hammerscales incorporated in the slag (Dunster and Dungworth Citation2012; Eekelers, Degryse, and Muchez Citation2016). Another characteristic feature of the slag samples is the presence of inclusions of metallic iron ((A)), as well as of corroded iron metal. An example of a large band of metallic iron in the slag is shown in (A). Here, the metallic iron is incorporated into the siliceous matrix with a big lump of coal in the middle. For this polished section, etching was applied and it revealed the presence of relic steel structures of ferrite iron ((B–D)). The microstructure is characterized by acicular ferrite iron with some volume of pearlite grains. The heterogeneity within this sample is very distinctive. The areas with high and low carbon content occurred randomly. In this case, fayalite and iron oxides were only sparsely present. Occasionally, metallic iron particles and corroded metal were observed. Iron ore or partially reacted inclusions of iron have not been found in any of the materials examined. Hammer scales were mainly composed of quartz and magnetite.
Figure 5. Optical photomicrographs of polished sections in reflected light. (A) is characterized by long laths of fayalite (Fa) in a siliceous matrix. The white dots are inclusions of metallic iron (Fe). At the bottom of the photo, there are some traces of organic matter – coal (org). (B) is generally composed of fayalite (Fa) and magnetite (Ma), accompanied by the presence of metallic iron inclusions (Fe). Texture (C) demonstrates elongated fayalite forming a spinifex texture that is associated with a fast cooling rate. (D) is characterized by wüstite (Wu) and magnetite crystals (Ma). The presence of wüstite both in a dendrite and globular form indicates variable cooling conditions, with faster cooling in the areas within which the dendrites are formed. Sample (E) is mainly dominated by well-developed wüstite dendrites (Wu). (F) is representing a fluidal texture (MS), which suggests the application of a high temperature. All the photomicrographs were taken under plane-polarized light unless otherwise stated.
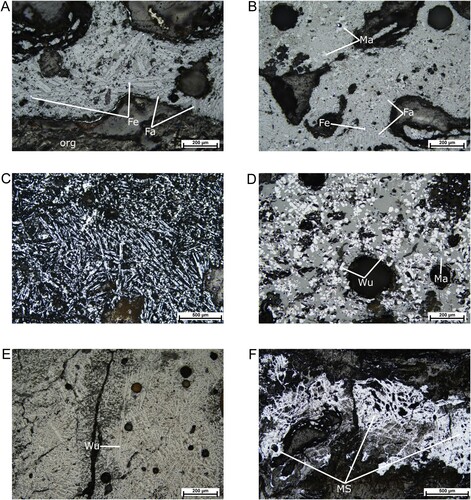
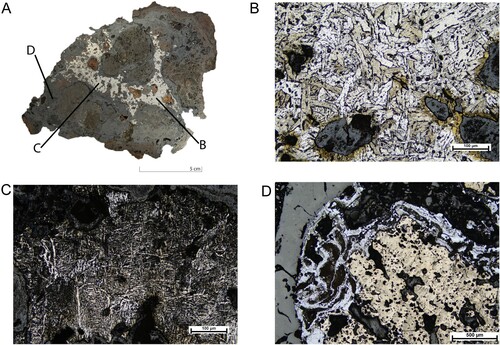
Figure 6. Photograph and microphotograph of a sample with a large metallic band (A). The sample is etched with 2% nital. The microstructure of this specimen is characterized by the occurrence of a large amount of iron spread throughout the sample. The letters on the photograph indicate the following photomicrographs. In (B) and (C), the uneven distribution of ferrite iron with pearlite grains within one sample can be observed. (D) presents a partial melting structure with traces of coal.
In every analysed polished section, organic matter such as coal was found, either non-altered or altered by temperature ((A)). The detailed identification of the carboneous materials is beyond the scope of this paper. Nevertheless, it is important to note that two types of coal have been identified based on petrographic observation: anisotropic and isotropic. The coal occurred randomly in the samples and did not show any particular pattern.
In many cases, the slag textures demonstrate the solidification of liquified melts, which indicates that the material has been exposed to a sufficiently high temperature ((F)). These areas show flow structures with a layered colour caused by presence of inclusions of organic matter oriented along this flow direction. No minerals could be identified within these layers. Because the melted areas are not present in each examined sample and are not equally distributed, it suggests slag has been only partially liquified.
The polished sections are characterized by a high variability that does not follow the grouping based on macroscopic appearance. Therefore, new types have been established based on microscopical differences. The first type (I) is distinguished by the presence of euhedral magnetite crystals. Fayalite and wüstite are present in scarce amounts. The samples are porous and show a lot of voids under the microscope. The matrix is vitreous and contains melting textures in some areas. The samples in the second type (II) are dominated by large, feathery spinifex-textured fayalite, and the presence of wüstite. Wüstite appears mainly as globular grains embedded within the olivine. Magnetite was not distinguishable. Slags of the third type (III) are a mixture between the first and second. They contain both wüstite and fayalite and a low amount of magnetite. Within this type, both structures of wüstite were observed: sub-rounded, globular crystals and dendrites. And similarly, fayalite appears in the form of both laths and spinifex texture. Occasionally, small metallic iron particles were observed. The last type (IV) contains samples with technical ceramics. For these, mainly laihunite and anorthoclase, were identified. Olivine and iron oxides were detected in lower amounts. All of the slags studied show quartz regardless of the group.
Discussion
Diverse microscopical and mineralogical characteristics, i.e. the presence of PCB slags, the occurrence of hammer scales, both in the soil samples and partially dissolved in the slag samples, and traces of steel structures support the hypothesis that the material studied was related to an iron smithing process rather than to the earlier smelting stage.
Although no preserved hearth structure has been found so far, some of the slag pieces have technical ceramics attached to the surface, which points towards hearth structures being used in the production process. Iron smelting slags usually have remains of partially-reacted ore fragments, fuel ashes and furnace material (Erb-Satullo and Walton Citation2017); (Gordon Citation1997). Moreover, one of the most distinguishing features of iron smelting is the presence of tap slag (Blakelock Citation2009). Neither of these characteristic features could be observed in the material from Hoeke.
The volume of the discovered material is related to the kind of work done by a blacksmith. For the production of larger objects larger amounts of iron are required, thus giving origin to more slags (Dunster and Dungworth Citation2012). Furthermore, the duration of the activity, the temperature in the hearth, as well as the iron content at the beginning of the process also contribute to a higher total volume of slags. Taking into account that c. 250 iron slag fragments were recovered from the 5 × 5 m pit, it can be assumed that the scale of the iron production at Hoeke was quite extensive. Hoeke was a harbour city where boats were repaired, hence the presence of pronounced iron working activity on that site (Trachet Citation2016).
The groups and types of slags distinguished both macroscopically and mineralogically possibly represent different stages or various operations in smithing process. Since the composition of slags depends mainly on the type of work that took place (Eekelers, Degryse, and Muchez Citation2016), it can be assumed that the slags of the first (I) mineralogical type were produced in a more oxidizing atmosphere (because of the presence of magnetite) at high temperatures. The slags of the second type (II) were formed in more reducing conditions. The presence of both spinifex texture and globular wüstite indicate that the cooling rate was unequal. The samples of the third type (III) were exposed to very heterogenous conditions. The presence of large grains of wüstite, as well as the spinifex fayalite do not provide a straightforward answer concerning the possible activity carried out on the site. The specimens of the fourth (IV) type provide evidence for the use of a ceramic construction, possibly a smithing hearth, because they are mainly composed of quartz and contain considerable amounts of refractory oxides such as Al2O3, while iron oxides and fayalite were detected in scarce amounts only.
Elements such as silicon and aluminium forming some minerals (for example iron aluminium silicate and ferrosilite) might have been introduced in the system during the superficial reaction between the slag and the walls of the hearth. The presence of quartz in each group can also be explained either by solidified slag at the bottom of the hearth, where quartz adhered to the wall, or as a result of using a flux. Flux prevents metal oxidation while hammering and it might consist of calcite or quartz (Serneels and Perret Citation2003); (Selskienė Citation2007). In this case, the flux used was definitely not composed of calcium as calcium minerals were not detected by XRD, nor by microscopic analysis. The absence of high-temperature polymorphs of quartz (cristobalite, tridymite) suggests that silica was added at the later stage of the process and did not have the time to recrystallize to these polymorphs which might indicate that quartz was taken up from hearth during the formation of slag. The presence of quartz as a post-depositional contamination is excluded, based on the careful sample preparation and the absence in XRD spectra of any peaks related to other minerals typically occurring in these sediments, like clay minerals.
The occurrence of PCB slags corresponds to the accumulation of fused materials in the hearth and is the result of repeated heating and hammering of the material (Portillo et al. Citation2018). The biggest PCB slags found are rich in iron oxides and fayalite, and mostly have a rusty appearance. They could be classified into the group of iron-rich rusty slags (SFR) according to (Serneels and Perret Citation2003). This type of PCB is related to high-temperature processes, for instance welding. The other small pieces of PCB are rather a combination of different PCB types of slags. They contain a moderate amount of quartz and fayalite, are variable in colour, and contain inclusions of coal. These materials could be a result of a sequence of activities, such as hot forging, welding and fashioning the object. In this study, it is not possible to indicate unambiguously during which exact smithy activity the PCB slags were produced. This question needs to be addressed by examination and analysis of more materials.
The chemistry of the slags is useful to evaluate some technological parameters of the smithing process (Portillo et al. Citation2018). The occurrence of certain minerals is associated with the temperature at which slag has been formed. Iron oxides and fayalite are typical examples. Because of the large amount of quartz as probable flux, the melting point of a mixture of iron oxide and silica was lowered, which allows the iron oxides to react with silica and form a molten ferrosilicate slag at a temperature around 1200 °C (Charlton et al. Citation2010). If the concentration of iron oxides is too high, iron oxides can crystallize as magnetite or wüstite, depending on the redox conditions (Muralha, Rehren, and Clark Citation2011). The presence of magnetite could, however, also be due to thermal decomposition of wüstite at 570 °C, with the formation of metallic iron particles (Portillo et al. Citation2018) or be the result of an oxidation process. Additionally, the air caught in pores of the slags could lead to oxidation of wüstite into magnetite (Portillo-Blanco et al. Citation2020). The local oxidation of iron oxides can make the samples ferro-magnetic, which explains the magnetic properties of some specimens (Tylecote Citation1987, chap. 8). It is also relevant to consider the behaviour of fayalite. Fayalite is stable above 1040°C (Ströbele et al. Citation2010), which suggests that the silicate liquid was formed at least at that temperature and subsequently supercooled, which led to rapid growth of the fayalite spinifex texture. The presence of textures relating to a liquid phase in some samples may be an indication of reheating of the bloom to a temperature at which the slag (partially) melts, making it easy to expel. By striking the slag with a hammer, the iron starts to consolidate and subsequently, it is subjected to further smithing processes leading to the forging of artefacts (Sim Citation1998). The relic melt textures could potentially be easier to achieve by the use of a flux (quartz). Since quartz was observed to be present in each slag sample, the hypothesis of the use of quartz as a flux seems very plausible. All things considered, the correlations of fayalite and iron oxides indicate a plausible hearth temperature varying from 570–1100 °C.
Unfortunately, the mineralogical and microscopical results are inconclusive in determining the atmospheric redox condition in which the slag has been formed. The amount of iron oxides present in the slags can depend on the skills of the craftsmen, more specifically on how efficiently the slag was separated from the metal during smelting and smithing (Liu et al. Citation2019). The presence of wüstite and magnetite may provide insight into the atmospheric conditions at which the slag was formed. Smelting furnaces work in more reducing conditions, whereas smithing hearths are more oxidizing. Slag with a small amount of iron oxides is assumed to be formed under reducing conditions, and is indicative of a highly efficient smithing process (Portillo et al. Citation2018). Nevertheless, this concept could be potentially misleading because redox conditions might vary within the hearth and furnace leading to the formation of very heterogeneous slags (Charlton et al. Citation2010). Exemplary is the presence of both wüstite dendrites and magnetite in one polished section, suggesting more reducing conditions for the wüstite-rich area, and more oxidizing for the magnetite parts. Additionally, the occurrence of iron metal particles especially with magnetite and wüstite are also indicative of variable redox conditions (Rehren, Boscher, and Pernicka Citation2012). It is difficult to assess in which atmospheric conditions each sample has been formed as the variability in single pieces was too high. However, this kind of heterogeneity occurs more often in smithing activities than in smelting (Serneels and Perret Citation2003).
The shape, habit and distribution of crystals can provide information on the cooling process (Portillo et al. Citation2018). The dendritic texture of wüstite is associated with a rapid cooling, while the globular, compacted crystals are the results of slow cooling (Jagou, Citation2020). The presence and texture of fayalite also depends on the cooling rate and oxygen fugacity of the slag (Hauptmann Citation2021, chap. 5). A slow cooling rate produces granular and idiomorphic crystals, while upon very rapid cooling fayalite acquires a spinifex texture. The longer the cooling, the more elongated the crystals can become (Donaldson Citation1976). Cracks and voids indicate that the samples have been affected by a high temperature and were cooled down quickly. Interestingly, in the third group, both spinifex fayalite and large grains of wüstite were observed, thus indicating variable cooling conditions during slag formation.
In some polished sections, textural patches of wüstite were observed under the microscope ((D)). These structures sometimes display partly reabsorbed hammer scale textures. By hammering the material, the scales might have been repelled from the object and partially dissolved in the slag (Eekelers, Degryse, and Muchez Citation2016). This hypothesis is supported by the fact that also hammer scales were found on the site.
The presence of metallic iron particles in the slag may be interpreted as a result of bloom/semi-finished product that has been left in the hearth too long and liquified pieces of iron being trapped in the slag. Also, the large bands of metallic iron may be part of refined bloom (Roberts and Thornton Citation2014, chap. 2). Interestingly, relic steel is noticed in some samples – α-iron particles are observed as well as acicular ferrite iron with some volume of pearlite grains (). These features were not commonly found within the assemblages. It is possible that steel was accidentally formed during forging as no other similar structures have been found on the site so far.
The occurrence of the sulphate mineral jarosite, and the later observed widespread organic inclusions lead to the conclusion that coal was used during the iron-making process. Jarosite, which was also detected as a minor phase by XRD, is an iron-hydroxysulphate mineral that can be formed during the reaction between certain elements in slags (Al, K, Si and S) and coal (Jagou, Citation2020); (Augustithis Citation1979). The use of coal instead of charcoal did not significantly alter the iron making process. However, it provided a much higher temperature than obtained upon combustion of charcoal (Mathieson et al. Citation2015). This can explain the occurrence of partial melt textures and signs of vitrification. During the metallographic investigation, two types of coal have been identified. The first type consisted of isotropic and weakly anisotropic coal. Coals of this type usually have large pores and fissures, produce highly volatile charge material, and are indicative of poor coal quality. A second kind of coal consisted of strong anisotropic types of carbon, indicating material with low volatility and thus better coal quality (Flores et al. Citation2017). The fuel is an important factor in metal production, as it provides both the required temperature and a reducing atmosphere. It is possible that the blacksmiths used two different kinds of coals for obtaining a technological advantage: one type of coal as a reducing agent (low-sulphur), and the second type as a fuel (cheaper coal).
Conclusions
The study of iron slags found at the location of the late medieval Harbour at Hoeke (Belgium) demonstrated that wide-scale metal-working activities took place. This confirms archaeological and historical indications. The mineralogical composition of the slags supported by a microscopical study, confirms the primary hypothesis that these materials were formed during the smithing process. Distinctive features include the presence of PCB slags, the occurrence of hammerscales and the usage of flux (quartz) during the operation. At this point it is clear, however, that no smelting of iron ore was attested in the material studied. The iron must probably have been imported as bloom or ingots.
The materials examined provided information on the cooling rates and the possible atmospheric conditions within the hearth. Mineralogical analysis carried out using optical microscopy showed a complex texture with the presence of both skeletal fayalite and dendritic wüstite, indicating a rapid cooling rate, while the large grains of olivine and wüstite suggest rather a slow cooling process. Additionally, the spinifex texture observed within samples of the second (II) and third (III) mineralogical type showed that some samples were exposed to a very rapid cooling or even to quenching. These conclusions imply that during the smithy activity different techniques, such as hot forging and fashioning an object, were applied at variable temperatures.
XRD analysis revealed that the atmospheric conditions were variable. The occurrence of magnetite, wüstite, as well as iron particles suggest that the slags could have been related to different smithing activities such as fashioning, welding or hot forging. Moreover, the geochemical results point out the usage of a flux (i.e. most probably quartz sand), which decreased the melting point of a mixture of iron oxide and silica and facilitated the smithing process.
An interesting technological aspect of the iron production at Hoeke is the use of coal as charged material. This change in forging technique was most likely accelerated by the scarcity of forest resources, which has spurred the transition from charcoal to coal. The use of coal allowed the craftsmen to operate at higher temperature (and maintain it), perhaps modernizing the metal-working process. However, further research needs to be done in this direction to provide additional insight into the use of coal at Hoeke.
At this moment, it is challenging to unequivocally deduce from which step of smithing the slags originate from because the results are ambiguous, thus further work needs to be performed to determine the exact steps in the chaîne opératoire.
It is worth mentioning that the excavated trench does not cover the whole area of investigation. The next step will be to excavate and study a larger area at Hoeke and to examine more materials found on site related to historical iron production.
Geolocation
As explained in the article, our study area is located at Hoeke, nowadays in the province of West Flanders, Belgium.
Supplemental Material
Download MS Word (129.1 KB)Acknowledgements
Excavations at Hoeke were done in the framework of the Ghent University GOA-project “High tide, low tide. Bruges’ late-medieval harbour system as a maritime cultural landscape”. PB thanks Veerle Vandenhende for assistance with XRD.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Paulina Biernacka
Paulina Biernacka is a Ph.D. student at the Department of Chemistry and the Department of Archaeology of Ghent University (Belgium). Her research focuses on the archaeometallurgical study of the chaîne opératoire of medieval iron smithing technology.
Wim De Clercq
Wim De Clercq is a Professor of Historical Archaeology at Ghent University (Belgium). He specialises in the archaeology of the historical periods in NW-Europe: provincial Roman and (Post-) Medieval Archaeology.
Stijn Dewaele
Stijn Dewaele is a Professor at the Department of Geology of Ghent University (Belgium) and leads Mineralogy & Petrology Research Unit (MinPet). Major interests include natural resources and geochemistry.
Frank Vanhaecke
Frank Vanhaecke is Senior Full Professor at the Department of Chemistry of Ghent University (Belgium), where he leads the “Atomic & Mass Spectrometry - A&MS” research unit that studies the determination, speciation and isotopic analysis of (trace) elements using various forms of ICP-mass spectrometry (ICP-MS).
Johan De Grave
Johan De Grave is a Professor at the Department of Geology of Ghent University (Belgium) and leads Mineralogy & Petrology Research Unit (MinPet). Major interests include thermochronology and tectonics.
References
- Augustithis, S.-S. 1979. Atlas of the Textural Patterns of Basic and Ultrabasic Rocks and their Genetic Significance, Atlas of the Textural Patterns of Basic and Ultrabasic Rocks and their Genetic Significance. De Gruyter. doi:10.1515/9783110867466
- Blakelock, E. 2009. “Slag Inclusions in Iron Objects and the Quest for Provenance: An Experiment and a Case Study.” Journal of Archaeological Science 36 (8): 1745–1757. doi:10.1016/j.jas.2009.03.032.
- Buchwald, V. F., and H. Wivel. 1998. “Slag Analysis as a Method for the Characterization and Provenancing of Ancient Iron Objects.” Materials Characterization 40: 73–96. doi:10.1016/s1044-5803(97)00105-8.
- Charlton, M. F., et al. 2010. “Explaining the Evolution of Ironmaking Recipes – An Example from Northwest Wales.” Journal of Anthropological Archaeology 29 (3): 352–367. doi:10.1016/j.jaa.2010.05.001.
- Clercq, W., et al. 2020. “Een archeologische evaluatie waardering en ruimtelijke afbakening van de verdwenen zwinhavens Hoeke en Monnikerede (gemeente Damme, provincie West-Vlaanderen).” Evaluatie- en waarderingsonderzoeken archeologie, 43. Accessed 14 August 2021. https://oar.onroerenderfgoed.be/publicaties/STUA/43/STUA043-001.pdf.
- Donaldson, C. H. 1976. “An Experimental Investigation of Olivine Morphology.” Contributions to Mineralogy and Petrology 57 (2): 187–213. doi:10.1007/BF00405225.
- Dungworth, D. 2015. Archaeometallurgy: Guidelines for Best Practice. Historic England.
- Dunster, Joanna, and D. Dungworth. 2012. Blacksmiths’ Fuel: The Analysis of Slags from Archaeological and Contemporary Iron-Working. Portsmouth: English Heritage.
- Eekelers, K., P. Degryse, and P. Muchez. 2016. “Petrographic Investigation of Smithing Slag of the Hellenistic to Byzantine City of Sagalassos (SW-Turkey).” American Mineralogist 101 (5): 1072–1083. doi:10.2138/am-2016-5390.
- Erb-Satullo, N. L., and J. T. Walton. 2017. “Iron and Copper Production at Iron Age Ashkelon: Implications for the Organization of Levantine Metal Production.” Journal of Archaeological Science: Reports 15: 8–19. doi:10.1016/j.jasrep.2017.06.006.
- Flores, B. D., et al. 2017. “How Coke Optical Texture Became a Relevant Tool for Understanding Coal Blending and Coke Quality.” Fuel Processing Technology 164: 13–23. doi:10.1016/j.fuproc.2017.04.015.
- Gordon, R. B. 1997. “Process Deduced from Ironmaking Wastes and Artefacts.” Journal of Archaeological Science 24 (1): 9–18. doi:10.1006/jasc.1995.0092.
- Hauptmann, A. 2007. ‘The Archaeometallurgy of Copper’, (1964), p. 402. doi:10.1007/978-3-540-72238-0_6.
- Hauptmann, A. 2021. Archaeometallurgy – Materials Science Aspects.
- Jagou, B. 2020. “La place du charbon de terre dans les chaines opératoires dans le Nord de la France de l’Antiquité jusqu’au second Moyen Âge.” Artisanat et savoir-faire : archéologie des techniques. 14 . doi:10.4000/books.psorbonne.62532.
- Joosten, I., J. B. H. Jansen, and H. Kars. 1998. “Geochemistry and the Past: Estimation of the Output of a Germanic Iron Production Site in the Netherlands.” Journal of Geochemical Exploration 62 (1-3): 129–137. doi:10.1016/S0375-6742(97)00043-5.
- Kramar, S., et al. 2015. “Mineralogical and Geochemical Characterization of Roman Slag from the Archaeological Site Near Mošnje (Slovenia).” Materiali in Tehnologije 49 (3): 343–348. doi:10.17222/mit.2013.299.
- Liu, Y., et al. 2019. “Iron Decarburisation Techniques in the Eastern Guanzhong Plain, China, During Late Warring States Period: An Investigation Based on Slag Inclusion Analyses.” Archaeological and Anthropological Sciences 11 (12): 6537–6549. doi:10.1007/s12520-019-00921-5.
- Mathieson, J. G., et al. 2015. “Utilization of Biomass as an Alternative Fuel in Ironmaking.” In Iron Ore: Mineralogy, Processing and Environmental Sustainability, 581–613. Elsevier Ltd. doi:10.1016/B978-1-78242-156-6.00019-8
- Muralha, V. S. F., T. Rehren, and R. J. H. Clark. 2011. “Characterization of an Iron Smelting Slag from Zimbabwe by Raman Microscopy and Electron Beam Analysis.” Journal of Raman Spectroscopy 42 (12): 2077–2084. doi:10.1002/jrs.2961.
- Pleiner, R. 2000. Iron In Archaeology: The European Bloomery Smelters. Praha: Archeologický ústav AV ČR.
- Portillo-Blanco, H., et al. 2020. “Mineralogical Characterization of Slags from the Oiola Site (Biscay, Spain) to Assess the Development in Bloomery Iron Smelting Technology from the Roman Period to the Middle Ages.” Minerals 10 (4), doi:10.3390/min10040321.
- Portillo, H., et al. 2018. “XRD, SEM/EDX and Micro-Raman Spectroscopy for Mineralogical and Chemical Characterization of Iron Slags from the Roman Archaeological Site of Forua (Biscay, North Spain).” Microchemical Journal 138: 246–254. doi:10.1016/j.microc.2018.01.020.
- Rehren, T., L. Boscher, and E. Pernicka. 2012. “Large Scale Smelting of Speiss and Arsenical Copper at Early Bronze Age Arisman, Iran.” Journal of Archaeological Science 39 (6): 1717–1727. doi:10.1016/j.jas.2012.01.009.
- Roberts, B. W., and C. P. Thornton. 2014. “Archaeometallurgy in Global Perspective.” Methods and Syntheses: 1–868. doi:10.1007/978-1-4614-9017-3.
- Selskienė, A. 2007. “Examination of Smelting and Smithing Slags Formed in Bloomery Iron-Making Process.” Chemija 18 (2): 22–28.
- Serneels, V., and S. Perret. 2003. “Quantification of Smithing Activities Based on the Investigation of Slag and Other Material Remains.” Archaeometallurgy in Europe-Proceedings of the International Conference (Milano, 24–26 septembre 2003), 1(January 2014), 469–478.
- Sim, D. 1998. Beyond the Bloom, Bloom Refining and Iron Artefact Production in the Roman World. Edited by I. Ridge. British Archaeological Report.
- Ströbele, F., et al. 2010. “Mineralogical and Geochemical Characterization of High-Medieval Lead-Silver Smelting Slags from Wiesloch Near Heidelberg (Germany) – An Approach to Process Reconstruction.” Archaeological and Anthropological Sciences 2 (3): 191–215. doi:10.1007/s12520-010-0039-7.
- Trachet, J. 2016. Inland Outports: An Interdisciplinary Study of Medieval Harbour Sites in the Zwin Region. Ghent: Ghent University.
- Trachet, J., et al. 2017. “Reassessing Surface Artefact Scatters. The Integration of Artefact-Accurate Fieldwalking with Geophysical Data at Medieval Harbour Sites Near Bruges (Belgium).” Archaeological Prospection 24 (2): 101–117. doi:10.1002/arp.1552.
- Tylecote, R. F. 1987. The Early History of Metallurgy in Europe. London: Longman.
- Windey, S. 2013. Archeometallurgische studie van metaalslakken uit drie Oost-Vlaamse sites. Ghent: Universiteit Gent.